Abstract
Posttraumatic stress disorder (PTSD) has been associated with altered resting-state functional connectivity (rs-FC) of several brain regions within the salience (SN) and default-mode (DMN) networks, including the hippocampus. However, most rs-FC studies have not focused primarily on the hippocampus, nor have they appreciated its structural heterogeneity, despite clear evidence for a dissociation between posterior and anterior hippocampal connectivity. Here, we examine rs-FC of anterior and posterior hippocampus with key regions in the SN (amygdala, insula, and dorsal anterior cingulate cortex/pre-supplementary motor area) and DMN (ventromedial prefrontal cortex, posterior cingulate cortex, and precuneus) previously implicated in PTSD, using a seed-based approach. Resting-state magnetic resonance images were obtained from 48 PTSD patients and 34 trauma-exposed healthy participants (TEHC). Results indicated no group differences when examining the hippocampus as a whole. However, examining the anterior and posterior hippocampus revealed a loss of anterior to posterior connectivity differentiation in PTSD patients. The PTSD group also demonstrated lower negative connectivity of the posterior hippocampus-precuneus pathway compared with the TEHC group. Finally, as differences in anterior and posterior hippocampus connectivity have been also related to age, we performed a secondary analysis exploring the association between age and posterior-and anterior-hippocampus connectivity in both groups. Results showed that among PTSD patients, increased age had the effect of normalizing posterior hippocampus-precuneus and hippocampus-posterior cingulate cortex connectivity, whereas no such effect was noted for the control group. These findings highlight the need for PTSD connectivity research to consider sub-parts of the hippocampus and to account for age-related connectivity differences.
Keywords: PTSD, trauma, resting-state functional connectivity, hippocampus, age
INTRODUCTION
Elucidating neural abnormalities associated with posttraumatic stress disorder (PTSD) is considered a crucial stepping stone toward identifying reliable novel targets for treatment (Patel et al., 2012). The hippocampus is one brain area considered to play an important role in PTSD due to its involvement in memory functions (Brohawn et al., 2010) and fear-related learning processes (Corcoran et al., 2005; Quirk and Mueller, 2008). Research has demonstrated reduced hippocampal volume in PTSD (O'Doherty et al., 2015), which are associated with persistent re-experiencing of the traumatic event (Brewin et al., 2010) and poor treatment response (Rubin et al., 2016). However, task-based functional neuroimaging studies have been less consistent, with some showing reduced or impaired hippocampal activation in PTSD (Etkin and Wager, 2007), while others reporting abnormal hyperactivation (Patel et al., 2012).
Recently, investigations of PTSD-related differences using resting-state functional connectivity (rs-FC) have begun to emerge. Extant PTSD rs-FC studies have primarily focused on key node regions within the salience network (SN) and the default mode network (DMN). SN nodes include the amygdala (Brown et al., 2014; Rabinak et al., 2011; Sripada et al., 2012a), anterior insula (Sripada et al., 2012b), and dorsal anterior cingulate cortex (dACC; (Kennis et al., 2015; Sripada et al., 2012b; Yin et al., 2011), and more specifically the dACC/pre-supplementary motor area (pre-SMA;(Chen and Etkin, 2013; Shirer et al., 2012; Sripada et al., 2012b)). DMN nodes include the hippocampus, ventromedial prefrontal cortex (vmPFC), posterior cingulate cortex (PCC), and the precuneus (Bluhm et al., 2009; DiGangi et al., 2016; Koch et al., 2016; Reuveni et al., 2016). Evidence for altered connectivity between the hippocampus and different SN nodes is mixed at best (Koch et al., 2016), with some reporting reduced connectivity in PTSD (Chen and Etkin, 2013; Sripada et al., 2012a; Sripada et al., 2012b) while others finding no such evidence (Brown et al., 2014; Chen and Etkin, 2013; Rabinak et al., 2011). Research focusing on DMN nodes found reduced hippocampal connectivity with the PCC (Bluhm et al., 2009; Sripada et al., 2012b) and precuneus (Bluhm et al., 2009; Chen and Etkin, 2013) in PTSD patients compared with healthy controls, as well as a positive correlation between the strength of within-DMN connectivity and PTSD and acute stress disorder (ASD) symptom severity (Birn et al., 2014; Cisler et al., 2013; Kennis et al., 2015; Lanius et al., 2010; Sripada et al., 2012b). However, more recent studies comparing within-DMN functional connectivity of PTSD patients and trauma-exposed healthy controls (TEHCs) did not find any evidence for group differences in connectivity (DiGangi et al., 2016; Reuveni et al., 2016).
Though nearly all PTSD seed-based rs-FC studies involving the hippocampus investigated it as a singular structure, research has increasingly recognized three functionally discrete subparts along its longitudinal axis based on gene expression and anatomical connectivity (Chen and Etkin, 2013; Fanselow and Dong, 2010; Zarei et al., 2013). Anterior-posterior hippocampal resting-state connectivity differences have been reported in healthy participants for different brain regions such as the pregenual ACC (pgACC), PCC, precuneus, PFC, and thalamus (Chen and Etkin, 2013; Zarei et al., 2013). Moreover, functional differences between anterior and posterior hippocampus were also found, with the posterior part primarily involved in memory and cognitive functions, and the anterior region in emotion and affect (Fanselow and Dong, 2010; Poppenk et al., 2013; Small et al., 2011). In PTSD, one previous study has shown that compared with healthy controls, PTSD patients demonstrate lower connectivity between the posterior hippocampus and the pgACC, PCC, and precuneus. Connectivity of the anterior hippocampus with dACC/pre-SMA was shown to be reduced in PTSD patients (combined with generalized anxiety disorder (GAD) patients), compared with healthy participants. However, as acknowledged by the authors, this study did not include trauma-exposed healthy participants, which might have yielded different results (Chen and Etkin, 2013).
Age-related anterior and posterior connectivity differences have been noted in previous research. An association between increased age and reduced functional connectivity within the DMN has been found in healthy participants for the posterior but not for the anterior hippocampus (Damoiseaux et al., 2016). Blum et al. (2014) examining connectivity dominance within the hippocampus reported somewhat contradictory results. They found a relative increase in rs-FC of the posterior hippocampus to neocortex areas in older adults, with several brain areas, including the precuneus, identified as demonstrating an age-related shift in connectivity from anterior to posterior hippocampus (Blum et al., 2014). Finally, while numerous neuroimaging studies have examined the hippocampus in PTSD, age-related differences were seldom addressed. The only few neuroimaging studies addressing age mainly focused on volumetric differences between PTSD patients and healthy controls in older adults (Golier et al., 2005; Yehuda et al., 2007). No studies to date have explored the effects of age on hippocampal rs-FC among PTSD patients.
Aiming to address gaps in the above-reviewed research on hippocampal connectivity, we recorded rs-FC of the hippocampus with core pre-defined nodes of the SN (i.e., anterior insula, dACC/pre-SMA, and amygdala) and DMN (i.e., PCC, precuneus, and vmPFC) previously identified as aberrant in PTSD connectivity research (Sripada et al., 2012b). Our primary goal was to examine whether PTSD patients and TEHC participants differ in functional connectivity of the anterior and posterior parts of the hippocampus with these brain regions, in an attempt to clarify mixed results from previous studies. In addition, as research has demonstrated age differences in hippocampal connectivity, we conducted a secondary analysis of specific pathways found to differ between groups in the main analysis by exploring within-group associations between age and rs-FC.
METHODS
Participants
Fifty-three patients with PTSD and 36 TEHC participants were recruited for participation in the study via online advertisement and fliers. PTSD and TEHC participants were matched on age, sex, trauma type, and race/ethnicity. All participants met DSM-IV-TR (American Psychiatric Association., 2000) criterion A for a traumatic event. A psychiatrist determined medical exclusion criteria by conducting a medical history and physical examination. An independent clinical evaluator administered the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) and Clinician-Administered PTSD Scale (CAPS; (Weathers et al., 2001)) to assess psychiatric diagnoses and determine PTSD severity. Moderate PTSD severity was required and indicated by a total CAPS score of >50. Exclusion criteria for the PTSD group included any current comorbid Axis-I diagnosis, including anxiety disorders, except for a moderate level of major depressive disorder (MDD), indicated by 17-item Hamilton Depression Rating Scale (HAM-D; (Hamilton, 1960)) score <25. The final sample included 21 PTSD patients with co-morbid depression. Severe depression (HAM-D score ≥25) was considered an exclusion criterion due to safety concerns of treatment delay. PTSD patients with any history of comorbid Axis-I psychiatric diagnoses were also excluded. Past substance or alcohol dependence was basis for exclusion only when occurring within six months prior to the start of the study (two months for substance abuse). Additional exclusion criteria were any psychotropic medication within four weeks prior to participation in the study (six weeks for fluoxetine). Exclusion criteria for the TEHC group were current or past Axis-I disorders and CAPS score >20. The investigation was carried out in accordance with the latest version of the Declaration of Helsinki. The New York State Psychiatric Institute Institutional Review Board approved all procedures and participants provided written informed consent after receiving explanation of the procedures in accordance with the World Medical Association Code of Ethics.
Image Acquisition and Pre-Processing
A 1.5 T GE Twin Speed MR Scanner operating on the Excite 3 12.0 M4 HD platform equipped with an 8-channel gradient head coil was used. A high-resolution T1-weighted 3D MPRAGE sequence was acquired for each subject (repetition time =7.25ms, echo time =3msec, Flip angle =7°, field of view =25.6cm, 256×256 pixel matrix, slice thickness =1mm). Five-minute functional resting-state images (i.e., blood oxygenation level dependent; BOLD) were acquired using a gradient echo T2*-weighted sequence (repetition time =3sec, echo time =30msec, flip angle =90°, field of view =22.4cm, 64×64 pixel matrix, slice thickness =2.2mm). Subjects were instructed to relax, remain awake, and lie still with their eyes open.
FMRI data were preprocessed using MATLAB version R2016a (The MathWorks, Inc., Natick, MA, USA) and statistical parametric mapping software (SPM12; Wellcome Trust Centre for Neuroimaging, UCL, London, UK), as previously done in our laboratory (Zhu et al., 2016). All functional images were slice time and motion corrected, co-registered to each participant’s T1-weighted structural image and normalized to Montreal Neurological Institute (MNI) canonical template and smoothed with an 8 mm full-width-at-half-maximum (FWHM) Gaussian kernel. Participants who had movement exceeding ±1.5mm, and more than 20% of each subject’s data points having been detected as outliers were excluded from further analysis. Consequently, the final analysis included 82 participants: 48 PTSD and 34 TEHCs.
Seed-Based Functional Connectivity Analyses
Resting-state functional connectivity analyses were carried out using a seed-based approach implicated in the CONN-fMRI Functional Connectivity Toolbox v13 (Whitfield-Gabrieli and Nieto-Castanon, 2012).
ROI-to-ROI connectivity analysis was performed using two seed ROIs, including anterior and posterior hippocampus, and six target ROIs from the SN and DMN, including the amygdala, anterior insula, dACC/pre-SMA, precuneus, PCC, and vmPFC. A similar analysis was also performed using the hippocampus as a whole as a seed ROI. Seed ROIs were derived from the Juelich Histological Atlas from the FSL Harvard-Oxford Atlas maximum likelihood cortical atlas (Desikan et al., 2006). A 70% threshold was applied to these atlases. Any voxels that have any probability of being in the amygdala were excluded for seed ROIs to avoid the possibility that the rs-FC was driven by the amygdala. The hippocampus was divided along the A-P axis into three sections as was performed previously by Chen and Etkin (Chen and Etkin, 2013). Four target ROIs including the entire anatomical structure of amygdala, precuneus, PCC, and vmPFC were derived directly from the Juelich Histological Atlas from the FSL Harvard-Oxford Atlas maximum likelihood cortical and subcortical atlases. Since the FSL Harvard-Oxford Atlas does not provide subdivision of the insula and ACC, anterior insula and dACC/Pre-SMA nodes were derived based on the SN functional network defined by Shirer et al. (Shirer et al., 2012). The mean BOLD time series was computed across all voxels within each ROI. Bivariate-regression analyses were used to determine the linear association of the BOLD time series between each pair of regions for each subject. Both positive and negative correlations were examined. The resultant correlation coefficients were transformed into z-scores using Fisher's transformation to satisfy normality assumptions.
Statistical Analyses
To examine group differences in rs-FC, we performed separate two-way analyses of variance (ANOVAs) for each target ROI, with group (PTSD, TEHC) as a between subjects factor and hippocampus (anterior, posterior) as a within-subject factor. A Bonferroni correction was established for six repeated tests at p<0.008 (p<0.05/6). Fisher LSD was used for posthoc analyses for significant interactions. To examine group differences in rs-FC when considering the hippocampus as a whole, we performed an independent samples t-test for each target ROI. To further clarify significant findings and possible confounding effects of age, gender and education, we performed analysis of covariance (ANCOVA) entering age, sex, and years of education as covariates to the above described main analyses. Finally, we performed a correlation analysis between age and rs-FC only for pathways in which hippocampus (anterior, posterior) was found to interact with group (PTSD, TEHC). This correlation analysis was performed within each group, for anterior and posterior hippocampus separately. A Bonferroni correction was established for two correlation analyses made at p<0.025 (p<0.05/2). To further examine differences between groups in the relationship between age and hippocampal connectivity we performed a moderation analysis of the association between age and rs-FC with group as a moderator using PROCESS macro software (Hayes, 2013), model 1, in SPSS (SPSS Inc., Chicago, Illinois), which allows for bootstrapping of standard errors for indirect effects. We applied 1,000 bootstrap samples.
RESULTS
Demographic and Clinical Characteristics
Participants’ demographic and clinical characteristics are presented in Table 1. As expected, significant differences between groups were noted for PTSD symptom severity (p<.001). Groups differed also in their years of education (p<.001) and depressive symptom severity (P<0.001), but not on age (p=.67), sex (p=.45), ethnicity (p=.70), duration since trauma (p=.96) or trauma type (p=.40).
Table 1.
Demographic and clinical characteristics of the two groups
| PTSD (n=48) | TEHC (n=34) |
Statistics – p value (PTSD vs. TEHC) |
|
|---|---|---|---|
| Sex, N (%) | 0.45 | ||
| Male | 19 (39.6) | 11 (32.4) | |
| Female | 29 (60.4) | 23 (67.6) | |
|
| |||
| Race, N (%) | 0.70 | ||
| Caucasian | 14 (29.2) | 11 (32.4) | |
| African- American | 12 (25.0) | 11 (32.4) | |
| Hispanic | 17 (35.4) | 11 (32.4) | |
| Others | 4 (8.3) | 1 (2.9) | |
|
| |||
| Duration since trauma, mean years (SD) | 12.69 (11.92) | 12.55 (12.94) | 0.96 |
| Trauma type, N (%) | 0.40 | ||
| Sexual assault | 14 (29.2) | 6 (17.6) | |
| Interpersonal violence | 12 (25.0) | 9 (26.5) | |
| Motor vehicle accident | 1 (2.1) | 3 (8.8) | |
| Military-related | 5 (10.4) | 1 (2.9) | |
| Natural disaster | 1 (2.1) | 1 (2.9) | |
| Serious injury (accidental) | 2 (4.2) | 0 | |
| Terror-related | 1 (2.1) | 0 | |
| Witnessing a traumatic event | 4 (8.3) | 4 (11.8) | |
| A traumatic event occurred to a close family member/friend | 8 (16.6) | 10 (29.5) | |
|
| |||
| Age | 0.67 | ||
| Mean (SD) | 36.1 (8.8) | 35.1 (10.6) | |
| Range (minimum – maximum) | 21.9 – 52.5 | 22.2 – 57.5 | |
| Education, mean years (SD) | 14.2 (2.0) | 16.1 (1.9) | <0.001 |
|
| |||
| H-AMD, mean (SD) | 16.2 (5.5) | 2.0 (2.1) | <0.001 |
| CAPS-total score, mean (SD) | 81.3 (15.5) | 3.9 (4.7) | <0.001 |
Note. PTSD = Post-traumatic stress disorder; TEHC = trauma exposed healthy controls; HAM-D = Hamilton Depression Scale; CAPS = Clinician-Administered PTSD Scale.
One PTSD subject lacked ethnicity information.
Functional Connectivity Analysis
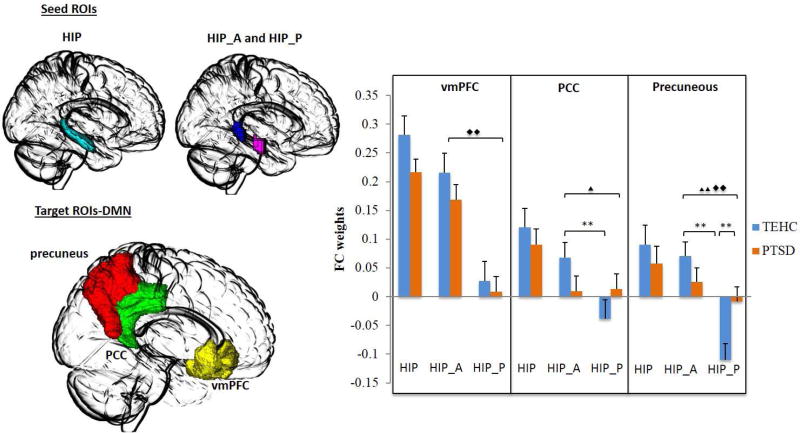
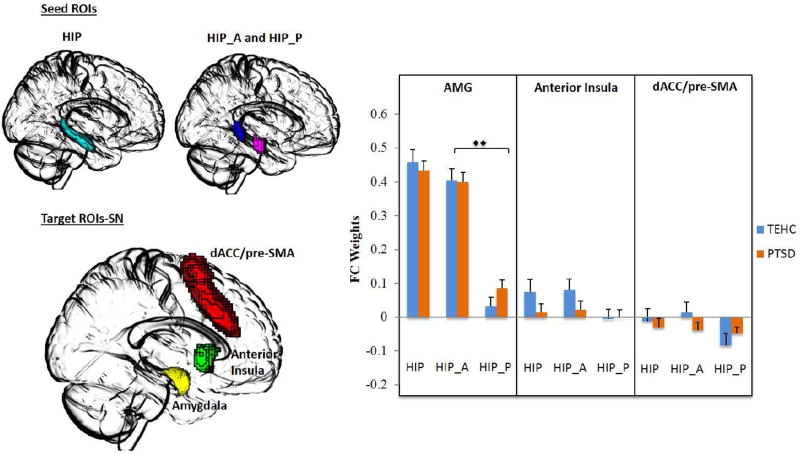
ROI-to-ROI connectivity analysis was performed on a priori defined ROIs (see Figure 1 and 2 for DMN and SN nodes, respectively). No group differences in rs-FC were found for any of the target ROI’s when examining the hippocampus as a single and whole structure (all p’s>.12), reflecting similar rs-FC patterns in the two groups when disregarding the functionally discrete subparts of the hippocampus. However, when examining anterior and posterior hippocampus separately, a significant group (PTSD vs. TEHC) by hippocampus (anterior vs. posterior) interaction effect emerged for the precuneus (F=7.56, p=.006), with a trend interaction found for the PCC (F=3.65, p=.05). Post-hoc Fisher LSD for examining simple effects revealed a significant difference between groups in the connectivity of the posterior hippocampus with the precuneus (t=2.7, p=.007), with the PTSD group showing reduced negative connectivity compared with the TEHC group. No significant difference between groups emerged for the connectivity of the anterior hippocampus and the precuneus (t=1.18, p=.24). In the TEHC group, a significant difference was found between the connectivity of the anterior and posterior subparts of the hippocampus with the precuneus (t=4.4, p<.001), which was not evident in the PTSD group (t=1.0, p=.32), suggesting a loss of dissociation of anterior and posterior hippocampus connectivity with the precuneus in PTSD patients compared with controls. For the PCC, a similar results pattern emerged. A significant difference between the connectivity of the anterior and posterior hippocampus with the PCC was found in TEHC group (t=2.4, p=.016), but again not in the PTSD group (t=0.09, p=.92). There was no significant difference between groups in the connectivity of the posterior hippocampus and the PCC (t=1.27, p>.05). Finally, significant main effects of hippocampus (anterior vs. posterior) were found for the DMN-related nodes of the vmPFC (F=33.0, p<.001) and precuneus (F=7.55, p<.001). For the SN nodes, we found a significant main effect of hippocampus (anterior vs. posterior) only for the amygdala (F=133.07, p<.001).
Figure 1.
Resting-State Functional Connectivity by Group and Hippocampus Region for DMN Nodes.
Note. Seed-based resting-state functional connectivity of the hippocampus (HIP; cyan), anterior hippocampus (HIP_A; violet) and posterior hippocampus (HIP_P; blue) as seed regions of interest (ROIs) with key nodes of the default mode network (DMN), namely, the ventromedial prefrontal cortex (vmPFC, yellow), posterior cingulate cortex (PCC, green), and precuneus (red) as target ROIs in PTSD patients and trauma-exposed healthy controls. The figure on the right depicts the functional connectivity weights in each group. Error bars denote standard error of the mean (SEM). (Two triangles: significant F test; one triangle: trend F test; two diamonds: significant main effect; one diamond: trend main effect; **: Significant Post-hoc Fisher LSD; *: trend Post-hoc Fisher LSD)
Figure 2.
Resting-State Functional Connectivity by Group and Hippocampus Region for SN Nodes.
Note. Seed-based resting-state functional connectivity of the hippocampus (HIP; cyan), anterior hippocampus (HIP_A; violet) and posterior hippocampus (HIP_P; blue) as seed regions of interest with key nodes of the salient network (SN), namely, the amygdala (green), the dorsal anterior cingulate/pre-supplementary motor area (dACC/pre-SMA, yellow), and anterior insula (red) as target ROIs in PTSD patients and trauma-exposed healthy controls. The figure on the right depicts the functional connectivity weights in each group. Error bars denote standard error of the mean (SEM). (Two triangles: significant F test; one triangle: trend F test; two diamonds: significant main effect; one diamond: trend main effect; **: Significant Post-hoc Fisher LSD; *: trend Post-hoc Fisher LSD).
Finally, ANCOVA for significant findings with age, sex, and years of education entered as covariates did not change any of the significant results described above. The group (PTSD vs. TEHC) by hippocampus (anterior vs. posterior) interaction effect remained significant for the precuneus (F=7.92, p=.006), with a trend interaction still evident for the PCC (F=3.64, p=0.05). Post-hoc analyses revealed a significant difference between the TEHC and PTSD groups in the connectivity of the posterior hippocampus and the precuneus (F=4.80, p=.03), with a trend difference for the anterior hippocampus (F=3.57, p=.06), which was not evident in the initial analysis. A significant difference was also found between the anterior and posterior hippocampus in connectivity with the precuneus in the TEHC group (F=23.06, p<.001), but again not in the PTSD group (F=.98, p=.32). For the PCC, a significant difference between anterior and posterior hippocampus in connectivity with the PCC was found in the TEHC group (F=5.99, p=.017), but again not in the PTSD group (F=0.008, p=.93). Finally, main effects of hippocampus remained significant for the vmPFC (F=33.33, p<.001), precuneus (F=17.09, p<.001), amygdala (F=131.42, p<.001) and, unlike the initial analysis, also for the insula (F=5.46, p=.02). Again, no main effects for group emerged.
Relationship between Functional Connectivity of Posterior Hippocampus and Age
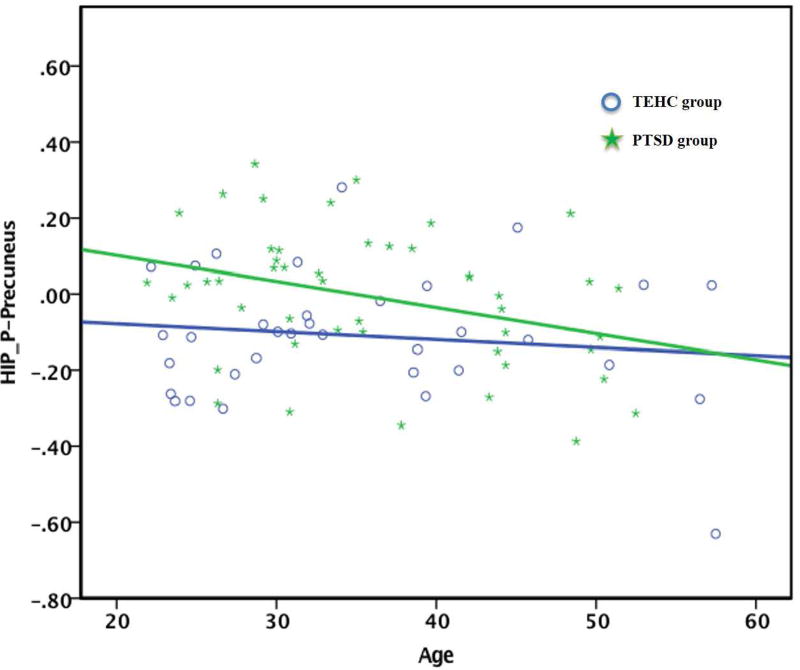
Posterior hippocampus-precuneus was negatively correlated with age (see Figure 3; r=−0.34, p=.018) with a negative correlation also for the PCC but at trend level (r=−0.303, p=.036) in the PTSD group, but not in the TEHC group, suggesting that increased age was associated with normalized posterior hippocampus connectivity with precuneus and PCC in PTSD patients. However, the moderation analysis did not reveal a significant interaction of group and age in predicting the rs-FC of the posterior hippocampus and precuneus or PCC (p=.35, p=.26, respectively). In contrast, the TEHC group showed a significant negative correlation between anterior hippocampus-precuneus connectivity and age (r=−0.377, p=0.028), while no significant relationship was found in PTSD group. Again, the moderation effect of group was found to be non-significant, p=.08. To further ascertain the significant correlations between age and rs-FC for these pathways within the PTSD group, we additionally examined the correlations with duration since trauma (i.e., the time that has passed since the trauma occurred), especially as duration since trauma and age were found to be highly correlated in this sample (r=0.53, p<.001). We found no significant correlation between duration since trauma and rs-FC for the posterior hippocampus-precuneus pathway as well as for the posterior hippocampus-PCC pathway.
Figure 3.
Correlation between Age and Resting-State Functional Connectivity of the Posterior Hippocampus with the Precuneus in the PTSD group.
Note. TEHC = Trauma exposed healthy contols; PTSD = Post traumatic stress disorder; HIP_P-Precuneus = Hippocampus-precuneus resting-state functional connectivity
DISCUSSION
This study examined whether PTSD patients and TEHC participants exhibit different resting-state functional connectivity patterns between anterior and posterior hippocampus and key brain regions of the SN (i.e., amygdala, dACC/pre-SMA, and anterior insula) and the DMN (i.e., precuneus, PCC, and vmPFC) previously implicated in PTSD. A differential rs-FC pattern emerged for the PTSD and TEHC groups regarding anterior and posterior hippocampal connectivity with the precuneus and PCC. In TEHC participants, a significant difference was noted for the connectivity of the anterior and posterior hippocampus with the precuneus and PCC. While the anterior hippocampus demonstrated positive connectivity with the precuneus and PCC, reflecting “co-modulation” of these regions during rest, the posterior hippocampus revealed a negative connectivity pattern with these regions, reflecting a “division of labor” (Uddin et al., 2009). No difference between anterior and posterior hippocampus connectivity was found in the PTSD group, reflecting a potential pathological lack of functional differentiation between anterior and posterior hippocampus. This difference in connectivity patterns between PTSD patients and TEHC participants could be seen as a manifestation of an imbalance between anterior and posterior hippocampus within the DMN in PTSD, especially as the precuneus and PCC are considered key nodes of this network (Utevsky et al., 2014), highly correlating with the rest of the DMN in healthy humans (Bluhm et al., 2009).
The DMN has been found to be involved in inwardly focused mental processes such as self-referential processing, personal introspection, autobiographical memory, and future thinking (Bluhm et al., 2009; Lanius et al., 2015; Reuveni et al., 2016; Sripada et al., 2012b), which all contribute to a coherent sense of self (Lanius et al., 2015). Traumatic events can have long-term effects on one’s sense of self, manifesting in altered core beliefs, disrupted self-referential processing and dissociative symptoms including depersonalization (Lanius et al., 2015). Accordingly, it has been suggested that altered rs-FC within the DMN found in PTSD might be related to dissociative (Bluhm et al., 2009) and depersonalization symptoms (Lanius et al., 2015). While our results are in line with these studies mentioned above, also reporting altered connectivity within key nodes of the DMN in PTSD, they emphasize the need to regard the hippocampus as comprised of discrete sub-regions along its longitudinal axis when examining rs-FC within the DMN.
We also found group differences in posterior hippocampus-precuneus connectivity replicating previous findings (Chen and Etkin, 2013), while comparing PTSD patients to TEHCs, rather than to healthy participants with no previous trauma exposure, thus increasing the generalizability of this finding. The precuneus, considered a core node of the DMN (Utevsky et al., 2014), has been shown to be specifically involved in reflective, self-related processes (Cavanna and Trimble, 2006), integration of past and present information (Fransson and Marrelec, 2008), and different aspects of memory including episodic memory retrieval (Cavanna and Trimble, 2006), autobiographical memory (Addis et al., 2004), and visual-spatial imagery (Cavanna and Trimble, 2006). Previous research has found altered precuneus activation during rest (Yan et al., 2013) and during processing of neutral stimuli (Geuze et al., 2008) in PTSD patients, suggesting the precuneus to be related to patients’ memory (retrieval) impairments and difficulties in relating memories to the present context (Yan et al., 2013). Significant activation of the precuneus in response to trauma-related stimuli has also been demonstrated, implicating the precuneus in memory distortions of traumatic stimuli (Hayes et al., 2011), and intrusive and distressing re-experiencing of the traumatic event (Sartory et al., 2013). Finally, decreased rs-FC of the PCC/precuneus in PTSD has also been linked to PTSD-related dissociative symptoms (Bluhm et al., 2009; Geuze et al., 2008; Lanius et al., 2002). Considering the specific roles of the precuneus and the posterior hippocampus, this finding might reflect lack of differentiation between task- and self-related aspects of processing, possibly contributing to PTSD symptoms such as intrusive traumatic memories, memory distortions, and dissociative and re-experiencing symptoms (Chen and Etkin, 2013).
Results also indicated a preferentially connectivity of the PCC and precuneus with the anterior hippocampus, relative to the posterior hippocampus, in control participants. This finding is surprising as previous research has shown the posterior hippocampus to be preferentially connected to DMN nodes, including the PCC and precuneus, in healthy controls (Chen and Etkin, 2013). However, differences in the nature of the comparison group used in each study might explain this divergence in results. While the current control group comprised of trauma-exposed controls, the latter study utilized healthy participant with no previous trauma exposure. Previous research examining DMN rs-FC in PTSD has shown DMN connectivity to be weakened in veterans with and without PTSD compared to healthy civilian controls (DiGangi et al., 2016), with other studies also demonstrating similar functional and anatomical connectivity patterns in the DMN of PTSD patients and trauma-exposed controls (Reuveni et al., 2016). Taken together, these studies highlight the distinction between trauma-exposed and non-trauma-exposed healthy individuals, a distinction that might reflect a potential resilience marker characterizing trauma-exposed healthy participants. This possibility is further strengthen when considering the exclusion criteria used for the TEHC group in the present study (i.e., any current or past Axis-I disorders and CAPS score above 20) which could reflect an especially resilient sub-group of trauma-exposed healthy controls. Future rs-FC research should examine posterior and anterior hippocampal connectivity patterns while comparing PTSD patients, TEHC and non-trauma-exposed participants to try and elucidate potential resilience factors to PTSD.
Importantly, examining the hippocampus as a whole indicated no group differences in hippocampus connectivity with SN and DMN nodes. With regard to SN nodes (i.e., anterior insula, dACC/pre-SMA and amygdala), our results are in line with previous studies (Brown et al., 2014; Kennis et al., 2015; Rabinak et al., 2011), while being at odds with others (Sripada et al., 2012a; Sripada et al., 2012b). Several possibilities might account for these differences. First, this divergence in results could be related to the nature of the comparison group used in the different studies. As in the present study, some have used TEHC participants (Brown et al., 2014; Rabinak et al., 2011) and found no group differences in connectivity, while others combined TEHC and healthy civilian participants (Sripada et al., 2012b). Second, lack of group differences in hippocampus-amygdala connectivity in the present study might be due to sex-related differential patterns of amygdala functional connectivity at rest (Kilpatrick et al., 2006), as the PTSD sample in the present study included more women than men in both groups (60.4% and 67.6% in the PTSD and TEHC groups, respectively). We also found no group differences in hippocampus connectivity with any of the DMN nodes (i.e., vmPFC, PCC, and precuneus). While this is in contrast to previous studies reporting reduced hippocampal connectivity (Bluhm et al., 2009; Sripada et al., 2012b), once again these studies compared PTSD patients with healthy controls, not previously exposed to a traumatic event. Conversely, our findings support more recent research suggesting a common effect of trauma exposure on DMN resting-state connectivity, unrelated to PTSD per se (DiGangi et al., 2016; Reuveni et al., 2016).
Finally, the connectivity of the precuneus and PCC with the posterior hippocampus showed significant negative correlations with age, which were not evident for the anterior hippocampus. Among PTSD patients, increased age was associated with a more “normalized” posterior hippocampus-precuneus and -PCC connectivity (i.e., increased negativity), whereas no such effect was noted for the control group. Furthermore, while age was significantly correlated with duration since trauma in our PTSD sample, we did not find any association between duration since trauma and PCC- or precuneus-hippocampus connectivity, strengthening the association of rs-FC with the participants’ chronological age, and not their “trauma age”. This somewhat surprising finding is in line with previous research of Blum et al. (2014) who reported the functional connectivity of the posterior hippocampus to be more dominant as we age. According to the authors, while in older individuals there is an overall reduction in functional connectivity of the hippocampus as a whole, a set of brain regions, including the precuneus, is preferentially connected to the posterior hippocampus in older age. The present findings also echo previous studies in older adults with PTSD that reported an absence of hippocampal volume differences in older adults with and without PTSD (Golier et al., 2005; Yehuda et al., 2007). Considering both lines of research may give rise to the speculative possibility that decreased rs-FC dominance of posterior hippocampus to key nodes of the DMN might serve as a possible risk factor in the wake of trauma in younger age groups. Age has been shown to be associated with lower prevalence rates of PTSD among older VA veterans (Frueh et al., 2007), and with lower risks for negative mental and physical health outcomes immediately following trauma (Acierno et al., 2006). However, as the present findings are preliminary, as well as correlative, it should be interpreted with care. Furthermore, our moderation analyses did not support group as a moderator of the relationship between hippocampal connectivity and age, complicating the interpretation of the results. Thus, while we did observe a significant correlation between age and hippocampus-precuneus and -PCC rs-FC in the PTSD group, that was not evident in the TEHC group, we cannot claim for group differences in this regard. Future research should elaborate and clarify the association between age and rs-FC in PTSD by increasing the number of PTSD patients and control participants, as well as by increasing the age-range of participants recruited for research.
The present study has several limitations that deserve consideration. First, it did not include a non-trauma exposed control group, which might explain the lack of group differences in hippocampus connectivity that were previously noted (Sripada et al., 2012a). In addition, our current sample does not allow us to examine the specific role of trauma exposure per se as both groups were similar in this regard. Finally, while our two groups differed on PTSD status they were possibly also different in regard to a resilience factor characterizing TEHC participants but not PTSD patients. Thus, we cannot differentiate these two factors as possible explanation for our results. Future research could include this third group of participants to address this issue. Second, while our exclusion criteria for the PTSD group increases the specificity of our results to PTSD, this also hinders the generalizability of our findings to the general PTSD population as PTSD is highly comorbid with other anxiety disorders such as panic disorders and GAD. To address this shortcoming, future research could employ less stringent inclusion/exclusion criteria for the PTSD group, especially regarding co-morbid anxiety disorders. Third, our analysis of posterior and anterior hippocampus rs-FC is correlative in nature, and as such does not allow inferences about directionality or causality regarding PTSD. Future research could examine longitudinal or task-based changes in connectivity of these pathways. Fourth, the TEHC group differed from the PTSD group on years of education. However, it seems unlikely that this influenced our findings as including years of education as a covariate did not affect the results. Also, while we excluded severely depressed patients from the study, the two groups still differed on depression scores, which might serve as an alternative explanation for the obtained results. However, we did not include depression scores as another covariate as ANCOVA should be avoided when groups are not formed by random assignment (Miller and Chapman, 2001), as was the case in the presented study. Still, as differences between PTSD patients with and without major depressive disorder in rs-FC have been previously reported (Zhu et al., 2016), future studies should attempt to replicate the present finding while including a depression non-PTSD group to enhance the specificity of current results. Finally, our PTSD group ranged in age from 22 to 54 years, which might have limited our ability to find possible moderation effects of group on the relationship between age and rs-FC. Also, we cannot generalize our findings regarding the correlation between age and rs-FC beyond this age group. As changes in connectivity and connectivity dominance of posterior and anterior hippocampus have been noted with normal aging (Blum et al., 2014; Damoiseaux et al., 2016), future research should address this relationship by including older PTSD patients in research.
CONCLUSIONS
The present study provides evidence for the importance of referring to the anterior and posterior parts of the hippocampus as distinct regions with different roles when examining resting-state connectivity in PTSD. While we found no differences in rs-FC between subjects with PTSD and TEHCs when examining the hippocampus as a whole, examining the anterior and the posterior hippocampus revealed a pathologic loss of anterior to posterior connectivity differentiation in PTSD patients. In addition, the PTSD group exhibited a lower negative connectivity of the posterior hippocampus-precuneus pathway compared with the TEHC group. Finally, Among PTSD patients, increased age had the effect of normalizing posterior hippocampus-precuneus and hippocampus-PCC connectivity, whereas no such effect was noted for the control group. Future research concentrating on resting-state functional connectivity in PTSD should acknowledge age-related as well as hippocampus sub-parts-related differences in connectivity.
HIGHLIGHTES.
PTSD is characterized by lack of anterior-posterior connectivity differentiation
PTSD is associated with lower negative posterior hippocampus-precuneus connectivity
No findings emerge when examining the hippocampus as a whole
In PTSD age is associated with normalized posterior hippocampus connectivity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acierno R, Ruggiero KJ, Kilpatrick DG, Resnick HS, Galea S. Risk and protective factors for psychopathology among older versus younger adults after the 2004 Florida hurricanes. The American Journal of Geriatric Psychiatry. 2006;14(12):1051–1059. doi: 10.1097/01.JGP.0000221327.97904.b0. [DOI] [PubMed] [Google Scholar]
- Addis DR, McIntosh AR, Moscovitch M, Crawley AP, McAndrews MP. Characterizing spatial and temporal features of autobiographical memory retrieval networks: a partial least squares approach. Neuroimage. 2004;23(4):1460–1471. doi: 10.1016/j.neuroimage.2004.08.007. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-4 TR. 4. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Birn RM, Patriat R, Phillips ML, Germain A, Herringa RJ. Childhood maltreatment and combat posttraumatic stress differentially predict fear-related fronto-subcortical connectivity. Depress Anxiety. 2014;31(10):880–892. doi: 10.1002/da.22291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm RL, Williamson PC, Osuch EA, Frewen PA, Stevens TK, Boksman K, Neufeld RW, Theberge J, Lanius RA. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J Psychiatry Neurosci. 2009;34(3):187–194. [PMC free article] [PubMed] [Google Scholar]
- Blum S, Habeck C, Steffener J, Razlighi Q, Stern Y. Functional connectivity of the posterior hippocampus is more dominant as we age. Cognitive Neuroscience. 2014;5(3–4):150–159. doi: 10.1080/17588928.2014.975680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin CR, Gregory JD, Lipton M, Burgess N. Intrusive images in psychological disorders: Characteristics, neural mechanisms, and treatment implications. Psychological Review. 2010;117(1):210–232. doi: 10.1037/a0018113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohawn KH, Offringa R, Pfaff DL, Hughes KC, Shin LM. The neural correlates of emotional memory in posttraumatic stress disorder. Biol Psychiatry. 2010;68(11):1023–1030. doi: 10.1016/j.biopsych.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Brown VM, LaBar KS, Haswell CC, Gold AL, McCarthy G, Morey RA. Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology. 2014;39(2):351–359. doi: 10.1038/npp.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: A review of its functional anatomy and behavioural correlates. Brain: A Journal of Neurology. 2006;129(3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chen AC, Etkin A. Hippocampal network connectivity and activation differentiates post-traumatic stress disorder from generalized anxiety disorder. Neuropsychopharmacology. 2013;38(10):1889–1898. doi: 10.1038/npp.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Steele JS, Smitherman S, Lenow JK, Kilts CD. Neural processing correlates of assaultive violence exposure and PTSD symptoms during implicit threat processing: A network-level analysis among adolescent girls. Psychiatry Research: Neuroimaging. 2013;214(3):238–246. doi: 10.1016/j.pscychresns.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. The Journal of Neuroscience. 2005;25(42):8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Viviano RP, Yuan P, Raz N. Differential effect of age on posterior and anterior hippocampal functional connectivity. Neuroimage. 2016;133:468–476. doi: 10.1016/j.neuroimage.2016.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- DiGangi JA, Tadayyon A, Fitzgerald DA, Rabinak CA, Kennedy A, Klumpp H, Rauch SA, Phan KL. Reduced default mode network connectivity following combat trauma. Neurosci Lett. 2016;615:37–43. doi: 10.1016/j.neulet.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage. 2008;42(3):1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Frueh BC, Grubaugh AL, Acierno R, Elhai JD, Cain G, Magruder KM. Age differences in posttraumatic stress disorder, psychiatric disorders, and healthcare service use among veterans in Veterans Affairs primary care clinics. The American Journal of Geriatric Psychiatry. 2007;15(8):660–672. doi: 10.1097/JGP.0b013e3180487cc2. [DOI] [PubMed] [Google Scholar]
- Geuze E, Vermetten E, de Kloet CS, Westenberg HG. Precuneal activity during encoding in veterans with posttraumatic stress disorder. Prog Brain Res. 2008;167:293–297. doi: 10.1016/S0079-6123(07)67026-5. [DOI] [PubMed] [Google Scholar]
- Golier JA, Yehuda R, De Santi S, Segal S, Dolan S, de Leon MJ. Absence of hippocampal volume differences in survivors of the Nazi Holocaust with and without posttraumatic stress disorder. Psychiatry Research: Neuroimaging. 2005;139(1):53–64. doi: 10.1016/j.pscychresns.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Introduction to meditaion, moderation, and conditional process analysis: A regression-based approach. Guilford Press; 2013. [Google Scholar]
- Hayes JP, LaBar KS, McCarthy G, Selgrade E, Nasser J, Dolcos F, workgroup VM-AM, Morey RA. Reduced hippocampal and amygdala activity predicts memory distortions for trauma reminders in combat-related PTSD. J Psychiatr Res. 2011;45(5):660–669. doi: 10.1016/j.jpsychires.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennis M, Rademaker AR, van Rooij SJH, Kahn RS, Geuze E. Resting state functional connectivity of the anterior cingulate cortex in veterans with and without post-traumatic stress disorder. Hum Brain Mapp. 2015;36(1):99–109. doi: 10.1002/hbm.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick LA, Zald DH, Pardo JV, Cahill LF. Sex-related differences in amygdala functional connectivity during resting conditions. Neuroimage. 2006;30(2):452–461. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Koch SBJ, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M. Aberrant resting-state brain activity in posttraumatic stress disorder: A meta-analysis and systematic review. Depression and Anxiety. 2016;33(7):592–605. doi: 10.1002/da.22478. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Bluhm RL, Coupland NJ, Hegadoren KM, Rowe B, Theberge J, Neufeld RWJ, Williamson PC, Brimson M. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiat Scand. 2010;121(1):33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Frewen PA, Tursich M, Jetly R, McKinnon MC. Restoring large-scale brain networks in PTSD and related disorders: a proposal for neuroscientifically-informed treatment interventions. Eur J Psychotraumatol. 2015;6:27313. doi: 10.3402/ejpt.v6.27313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Boksman K, Densmore M, Gupta M, Neufeld RW, Gati JS, Menon RS. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biol Psychiatry. 2002;52(4):305–311. doi: 10.1016/s0006-3223(02)01367-7. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. Journal of Abnormal Psychology. 2001;110(1):40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- O'Doherty DCM, Chitty KM, Saddiqui S, Bennett MR, Lagopoulos J. A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiat Res-Neuroim. 2015;232(1):1–33. doi: 10.1016/j.pscychresns.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Patel R, Spreng RN, Shin LM, Girard TA. Neurocircuitry models of posttraumatic stress disorder and beyond: A meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2012;36(9):2130–2142. doi: 10.1016/j.neubiorev.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 2013;17(5):230–240. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33(1):56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Welsh RC, Kenndy AE, Lyubkin M, Martis B, Phan KL. Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Frontiers in Psychiatry. 2011;2:62. doi: 10.3389/fpsyt.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveni I, Bonne O, Giesser R, Shragai T, Lazarovits G, Isserles M, Schreiber S, Bick AS, Levin N, Id, Schreiber SOhoo. Anatomical and functional connectivity in the default mode network of post-traumatic stress disorder patients after civilian and military-related trauma. Hum Brain Mapp. 2016;37(2):589–599. doi: 10.1002/hbm.23051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin M, Shvil E, Papini S, Chhetry BT, Helpman L, Markowitz JC, Mann JJ, Neria Y, Id, Helpman LOhoo. Greater hippocampal volume is associated with PTSD treatment response. Psychiatry Research: Neuroimaging. 2016;252:36–39. doi: 10.1016/j.pscychresns.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartory G, Cwik J, Knuppertz H, Schurholt B, Lebens M, Seitz RJ, Schulze R. In search of the trauma memory: a meta-analysis of functional neuroimaging studies of symptom provocation in posttraumatic stress disorder (PTSD) PLoS ONE. 2013;8(3):e58150. doi: 10.1371/journal.pone.0058150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012;22(1):158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci. 2011;12(10):585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, Liberzon I, Id, Sripada RKOhoo, Liberzon IOhooX. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci. 2012a;37(4):241–249. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS, Liberzon I, Id, Sripada RKOhoo, King APOhoo, Liberzon IOhooX. Neural dysregulation in posttraumatic stress disorder: Evidence for disrupted equilibrium between salience and default mode brain networks. Psychosomatic Medicine. 2012b;74(9):904–911. doi: 10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30(2):625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utevsky AV, Smith DV, Huettel SA. Precuneus is a functional core of the default-mode network. The Journal of Neuroscience. 2014;34(3):932–940. doi: 10.1523/JNEUROSCI.4227-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety. 2001;13(3):132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain connectivity. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Yan X, Brown AD, Lazar M, Cressman VL, Henn-Haase C, Neylan TC, Shalev A, Wolkowitz OM, Hamilton SP, Yehuda R, Sodickson DK, Weiner MW, Marmar CR. Spontaneous brain activity in combat related PTSD. Neurosci Lett. 2013;547:1–5. doi: 10.1016/j.neulet.2013.04.032. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Golier JA, Tischler L, Harvey PD, Newmark R, Yang RK, Buchsbaum MS. Hippocampal volume in aging combat veterans with and without post-traumatic stress disorder: Relation to risk and resilience factors. J Psychiatr Res. 2007;41(5):435–445. doi: 10.1016/j.jpsychires.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Yin Y, Jin C, Hu X, Duan L, Li Z, Song M, Chen H, Feng B, Jiang T, Jin H, Wong C, Gong Q, Li L. Altered resting-state functional connectivity of thalamus in earthquake-induced posttraumatic stress disorder: A functional magnetic resonance imaging study. Brain Research. 2011:98–107. doi: 10.1016/j.brainres.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Zarei M, Beckmann CF, Binnewijzend MA, Schoonheim MM, Oghabian MA, Sanz-Arigita EJ, Scheltens P, Matthews PM, Barkhof F. Functional segmentation of the hippocampus in the healthy human brain and in Alzheimer's disease. Neuroimage. 2013;66:28–35. doi: 10.1016/j.neuroimage.2012.10.071. [DOI] [PubMed] [Google Scholar]
- Zhu X, Helpman L, Papini S, Schneier F, Markowitz JC, Van Meter PE, Lindquist MA, Wager TD, Neria Y. Altered resting state functional connectivity of fear and reward circuitry in comorbid PTSD and major depression. Depress Anxiety. 2016 doi: 10.1002/da.22594. [DOI] [PMC free article] [PubMed] [Google Scholar]