Abstract
Drug-induced liver injury (DILI) is an important cause of death and indication for liver transplantation (fatality). The role of DILI in these fatalities ispoorly characterized particularly when fatalities occur > 26 weeks after DILI onset. We analyzed patients in the U.S. Drug-Induced Liver Injury Network prospective study having a fatal outcome within 2 years of onset. Each case was reviewed by 8 Network investigators and categorized as DILI having a primary, contributory or no role in the fatality. We subcategorized primary role cases as acute, chronic, acute-on-chronic or acute cholestatic liver failure. For contributory and no role cases, we assigned a primary cause of death. Among 1089 patients, 107 (9.8%) fatalities occurred within 2 years. DILI had a primary role in 68 (64%), a contributory role in 15 (14%) and no role in 22 (21%); 2 had insufficient data. Among primary role cases, 74% had acute, 13% chronic, 7% acute-on-chronic and 6% acute cholestatic failure. For the 15 contributory role cases, common causes of death included sepsis, malignancy and severe cutaneous reactions with multi-organ failure. For the 22 no role cases, malignancies accounted for most fatalities. Higher bilirubin, coagulopathy, leukocytosis and thrombocytopenia were independently associated with DILI fatalities. nR Hy’s Law had a higher positive predictive value for overall fatality (14% vs. 10%) and stronger independent association with DILI fatalities within 26 weeks compared to the original version of Hy’s Law (HR: 6.2, CI 3.4 – 11.1 vs. 2.2, CI 1.3–3.7). DILI leads directly or indirectly to fatality in 7.6% of cases; 40% of these have non-acute liver failure courses. nR Hy’s Law better identifies risk for death compared to the original Hy’s Law.
Keywords: acute liver failure, acute-on-chronic liver failure, liver transplantation, hepatotoxicity
INTRODUCTION
Most patients suffering hepatotoxicity due to medications, herbal or dietary supplements (HDS) recover from the acute liver injury without long-term sequelae. However, a proportion does not survive the injury or require liver transplantation (referred hereafter as a death, mortality, fatality or fatal outcome). Most large registries of drug-induced liver injury (DILI) report a mortality rate of approximately 10% 1–3. However, the contribution of DILI in these fatalities is not always clear, particularly when they occur more than 26 weeks after DILI onset. There are emerging data on chronic or persistent DILI that continues beyond 6 or 12 months.4–7 DILI may also contribute significantly to a fatality without being the primary cause of death. Such cases should be analyzed separately, but counted as part of DILI’s disease burden. Determining the contribution of liver injury to a fatality can be difficult, if patient data are not collected prospectively and assessed systematically. Lastly, accurate classification of DILI related fatalities allows more accurate analyses of predictors of death, such as Hy’s Law and its derivations.
For these reasons, we performed a systematic analysis of all patients who had a fatal outcome within 2 years of onset in the Prospective Study of the U.S. Drug Induced-Liver Injury Network (DILIN). In addition, we categorized the DILI as playing a primary, contributory or no role in the fatality. Using these categories of fatal outcomes, we assessed clinical variables including MELD, Hy’s Law and its modified version using a new R-ratio (nR) proposed recently by Robles-Diaz et al.8
METHODS
Design
The design of the DILIN Prospective Study has been described in detail in previous publications.9,10 Briefly, all patients suspected of having idiosyncratic drug- or herbal/dietary supplement (HDS)-induced liver injury were eligible if they could be enrolled within 6 months of onset of the injury and met specific criteria of laboratory abnormalities. These included serum alanine (ALT) or aspartate aminotransferase (AST) greater than 5 times upper limit of normal (ULN) (or pretreatment baseline if abnormal) on 2 consecutive occasions; or alkaline phosphatase (Alk P) levels greater than 2 times ULN (or pretreatment baseline if abnormal) on 2 consecutive occasions; or total bilirubin at least 2.5 mg/dL or an INR greater than 1.5 accompanied by any elevation in AST, ALT, or Alk P. Acetaminophen overdose cases and cases with certain chronic liver diseases such as autoimmune hepatitis and primary biliary cirrhosis were excluded. Subjects were asked to return 6 months after onset, and if laboratory or clinical abnormalities related to the liver injury were still present, to return at 12 and 24 months. At enrollment, relevant medical information was extracted from the medical records and interim history, physical examination and appropriate laboratory results were obtained.
Causality Assessment and Severity of Injury
The DILIN Prospective Study used a consensus expert opinion method of causality assessment.9,10 The DILIN investigator who enrolled the patient and two investigators from two other centers were provided with a standardized clinical narrative and summary of relevant clinical, laboratory, histologic and imaging results. The three investigators independently assign a causality score representing percent likelihood of attribution, in which 1 = definite or > 95% likelihood of DILI, 2 = highly likely or 75–95% likelihood, 3 = probable or 50–74%, 4 = possible or 25–49%, and 5 = unlikely or < 25%. Consensus on scoring was reached by e-mails and web based conference calls which include all DILIN investigators. Each case was also assigned a severity score of 1 to 5 (5 = fatal or needing transplant) based on bilirubin, INR, hospitalization, need for transplant, or fatality.2
Study Cohort
We identified all cases enrolled in DILI between September 2004 to April 2015 and scored as a 1 (definite DILI), 2 (highly likely) or 3 (probable). We then selected and analyzed all patients who died or underwent liver transplantation during the 2-year period of follow-up. For the purposes of this analysis, we refer to all these cases as fatalities or deaths even though some survived beyond transplantation.
Categorization by Role of DILI in Death or LT
Clinical death narratives and summaries of serial clinical, laboratory, histologic and imaging results were prepared for each case of death or liver transplantation in the DILIN Prospective Cohort study. A Death Review Subcommittee consisting of 8 DILIN hepatologists was established, and each case was assigned randomly to two members for independent review. Each reviewer subjectively categorized the cases for whether the DILI episode played a primary, contributory or no role in the death. We resolved discrepancies between reviewers by conference call discussion that included the entire Death Review Subcommittee. The Subcommittee had the prerogative to ask that any case be sent back for full causality assessment described above, if on review of the fatal outcome, original diagnosis of DILI was called into question.
Pattern of liver failure for primary role cases
Cases where DILI clearly played a primary role in the death or transplant, had to be subcategorized into 1 of 4, mutually exclusive clinical patterns of fatal liver failure:
Acute liver failure (ALF) deaths were defined as acute liver insult with INR ≥1.5, hepatic encephalopathy, no evidence of pre-existing, underlying advanced liver disease or cirrhosis and death occurring in less than 26 weeks.11,12
Acute-on-chronic liver failure (ACLF) deaths were defined as acute liver insult with INR ≥1.5, hepatic encephalopathy, and clinical or histologic evidence of pre-existing advanced liver disease or cirrhosis and death occurring in less than 26 weeks.13
Acute cholestatic liver failure deaths had acute injury with R-value < 2, no pre-existing liver disease, no no hepatic encephalopathy and death occurring in less than 26 weeks (i.e. acute cholestatic failure not meeting ALF criteria).
Chronic liver failure (CLF) deaths were defined as acute liver insult with consequent hepatic decompensation (e.g. ascites, jaundice, encephalopathy, coagulopathy) with or without evidence of pre-existing liver disease and death occurring due to liver failure more than 26 weeks later.
Cause of death for contributory and no role cases
Criteria used to categorize DILI as having a contributory role in the death included the following: (1) DILI left the patient in a weakened or malnourished state which contributed to death by another disease (e.g. cancer), (2) DILI prevented necessary care (e.g. surgery or chemotherapy) of another fatal illness, (3) DILI exacerbated or was part of another concurrent fatal illness (e.g. sepsis, multi-organ failure, DRESS) and (4) the death was due to a complication from a test or procedure done to evaluate the DILI (e.g. bowel perforation from an ERCP).
No role cases lacked any of the above criteria and often had recovered or nearly recovered from the DILI when they died of another illness. Both contributory and no role cases were then assigned 1 of 18 primary causes of death (Supplementary Table 1). We resolved discrepancies between reviewers by conference call discussion that included the entire Death Review Subcommittee of 8 members.
Descriptive data, clinical variables and analysis
Data analyzed included routine demographic, clinical and laboratory test results as well as time from starting the implicated drug or HDS to onset of injury and time from injury onset to death or transplantation. The pattern of injury was categorized as hepatocellular, cholestatic, or mixed based upon the R-ratio from laboratory tests taken at the time of onset: R-ratio = (ALT/ULN) ÷ (Alk P/ULN). R-ratios greater than 5 were considered hepatocellular, less than 2 cholestatic, and 2–5 mixed. We also classified cases by Hy’s Law and the new-R ratio (nR) Hy’s Law criteria at presentation:
Hy’s Law: bilirubin ≥ 2.5 mg/dL, ALT > 3 times ULN and Alk P < 2 times ULN
nR Hy’s Law: bilirubin ≥ 2.5 mg/dL, and [(ALT/ULN) ÷ (Alk P/ULN)] > 5. (AST is substituted for ALT, if the AST yields a greater R-ratio). 8
Using these clinical variables, we described those who survived compared to those who died or underwent liver transplantation. We also compared primary, contributory or no role cases. We determined performance characteristics for MELD, Hy’s Law and nR Hy’s Law for identifying mortality risk due primarily or partially to DILI at 2 years, and DILI related ALF and ACLF on univariate and multivariate analyses.
Statistical Analysis
Standard descriptive methods (e.g. mean, standard deviation, median, quartiles, percentages) were used to describe the total cohort and subgroups. Comparisons between subgroups were performed using standard parametric and non-parametric tests where appropriate. We used Cox proportional hazard ratios to identify clinical variables independently associated with overall DILI related deaths (primary or contributory role) at 2 years. We also determined the performance characteristics for MELD, Hy’s Law and nR Hy’s Law (e.g. positive predictive value, hazard ratios, C-statistic) in predicting deaths from DILI related ALF and ACLF.
Role of Funding Source and Institutional Board Review (IRB)
The DILIN Network is structured as a U01 cooperative agreement with funds provided by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Separate IRB approvals were maintained at each DILIN center throughout the period of this study and protocols were approved and all data monitored by a separate Data Safety and Monitoring Board appointed by the NIDDK.
RESULTS
Subjects
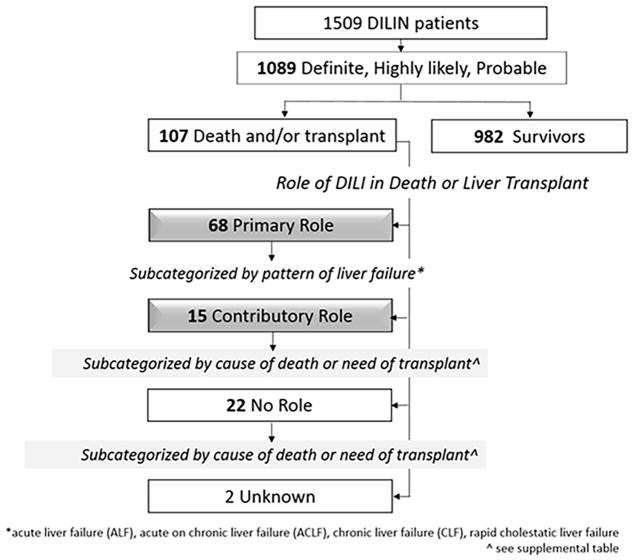
As of April 10, 2015, a total of 1509 patients were enrolled in the DILIN Prospective Study of which 1332 underwent formal causality assessment and 1089 were judged to have definite, highly likely or probable DILI. Among these, 107 (9.8%) died or underwent liver transplantation (all cause mortalities) within 2 years of onset. (Figure 1). The Death Review Subcommittee did not send any of the 107 fatalities back for causality reassessment.
Figure 1.
Comparison of patients who survived with those who died or underwent liver transplantation is shown in Table 1. Patients with a fatal outcome were on average somewhat older than those who survived (mean = 53.1 vs 48.5 years) but were similar in other demographic aspects. Initial laboratory results showed higher levels of ALT, AST, bilirubin and INR in those with fatal outcome, but the R-ratio and distribution of patterns of injury were similar. Fatalities had higher proportions meeting Hy’s Law and nR Hy’s Law criteria but only the latter reached statistical significance.
Table 1.
Characteristics of patients dying or requiring liver transplantation within 2 years of DILI onset (n = 107) compared to those alive at 2 years without liver transplantation.
| Characteristic | Died/LT within 2 years post DILI (n = 107) | Alive at 2 years post DILI (n = 982) | p-value |
|---|---|---|---|
| Mean age, yr. (SD) | 53.1 (16.4) | 48.5 (17.0) | 0.01 |
| Gender, female (%) | 57 (53.3%) | 577 (58.8%) | 0.30 |
| Mean BMI, kg/m2 (SD) | 28.7 (6.82) | 27.4 (6.81) | 0.01 |
| Race/Ethnicity | |||
| White | 76 (72%) | 781 (80%) | 0.10 |
| Black | 17(16%) | 110/ (11%) | |
| Asian | 7(7%) | 30 (3%) | |
| Other and Unknown | 7/(7%) | 62 (6%) | |
| Latino | 6 (6%) | 110 (11%) | 0.10 |
| Alcohol Use | 48/104 (46%) | 501 (52%) | 0.30 |
| Chronic liver disease | 13/62 (21%) | 144/866 (17%) | 0.50 |
| Liver biochemistries at DILI onset—Mean (SD) | |||
| ALT, U/L | 1162 (1326) | 787 (1064) | 0.02 |
| AST, U/L | 1093 (1199) | 634 (993) | <0.01 |
| Alk P U/L | 281 (217) | 280 (249) | 0.36 |
| Bilirubin, mg/dL | 10.1 (8.0) | 6.3 (6.3) | <0.01 |
| INR | 2.2 (1.8) | 1.3 (0.7) | <0.01 |
| R-ratio^ | 14.9 (19.8) | 12.7 (25.16) | 0.43 |
| Pattern of injury (102/925) | |||
| Hepatocellular | 61 (60%) | 497 (54%) | 0.11 |
| Mixed | 15 (15%) | 220 (24%) | |
| Cholestatic | 26 (26%) | 208 (23%) | |
| Causality Category | |||
| Definite | 16 (15%) | 253 (25.8%) | <0.01 |
| Highly likely | 45 (42%) | 523 (53.3%) | |
| Probable | 46 (43%) | 206 (21.0%) | |
| Hy’s Law Δ | 34/101 (33.7%) | 269/913 (29.5%) | 0.42 |
| nR Hy’s Law ∘ | 50/103 (48.5%) | 292/936 (31.2%) | <0.01 |
LT = liver transplantationSD = standard deviation
R-ratio = (ALT/ULN) ÷ (Alk P/ULN).
bilirubin ≥ 2.5 mg/dL, ALT > 3x ULN and Alk P < 2x ULN at presentation
bilirubin ≥ 2.5 mg/dL & R-ratio > 5 at presentation. (AST substituted for ALT, if AST yields greater R-ratio) 8
Categories by Role of DILI in Death or Transplant
DILI was considered to have a primary role in the death or need for transplantation in 68 (64%) patients. (Figure 1) DILI had a contributory role in 15 cases (14%), and no role 22 (21%). The role could not be determined due to insufficient follow-up details for 2 patients. The clinical and laboratory features associated with the three categories are shown in Table 2. Patients in whom DILI was the primary cause of death were younger, more likely female, had higher levels of ALT, AST, bilirubin, INR and R-ratio, and were more likely to have hepatocellular injury than no role cases. Contributory role cases had features more like no role cases, except for female sex, which was similar to primary role cases. A higher proportion of primary role cases met Hy’s Law criteria (40.3%) compared to contributory or no role cases, but the difference was statistically significant only when compared to no role cases. (Table 2) An even higher proportion of primary role cases met nR Hy’s Law (65.6%) and was statistically higher than either contributory or no role cases. Listing and frequency of specific agents are shown in Supplemental Tables 2–5.
Table 2.
Comparison of patients dying or requiring liver transplantation within 2 years of DILI onset (n = 105*) by DILI role in death or need for transplant.
| Characteristic | DILI Role in Death or Need of LT n = 105* |
p-values | |||
|---|---|---|---|---|---|
| Primary n = 68 |
Contributory n = 15 |
No role n=22 |
Primary vs. Contributory | Primary vs. No Role | |
| Mean age, yr. (SD) | 50.7 (16.0) | 58.9 (16.2) | 57.9 (17.2) | 0.06 | 0.08 |
| Gender, female | 42 (62%) | 9 (60%) | 6 (27%) | 0.89 | 0.01 |
| Mean BMI, kg/m2 (SD) | 29.2 (6.7) | 31.3 (9.1) | 25.6 (4.8) | 0.31 | 0.02 |
| Race/Ethnicity | |||||
| White | 46 (69%) | 12 (80%) | 17 (77%) | 0.95 | 0.58 |
| Black | 11 (16%) | 2 (13%) | 3 (14%) | ||
| Asian | 6 (9%) | 1 (7%) | 0 (0%) | ||
| Other | 4 (6%) | 0 (0%) | 2 (9%) | ||
| Latino | 5 (7%) | 1 (7%) | 0 (0.0%) | 1.00 | 0.33 |
| Alcohol Use | 35/66 (53%) | 4/14 (29%) | 9/22 (41%) | 0.10 | 0.33 |
| Chronic liver disease | 11 (16%) | 3 (20%) | 6 (27%) | 0.13 | 0.25 |
| Liver biochemistries at DILI onset | |||||
| ALT, U/L | 1470 (1483) | 587 (694) | 579 (740) | 0.02 | <0.01 |
| AST, U/L | 1422 (1326) | 503 (589) | 446 (547) | 0.01 | <0.01 |
| Alk P U/L | 252 (150) | 343 (326) | 331 (288) | 0.34 | 0.66 |
| Bilirubin mg/dl | 11 (7.5) | 9.6 (8.8) | 6.6 (7.5) | 0.31 | <0.01 |
| INR | 2.2 (1.0) | 2.4 (2.1) | 1.6 (0.9) | 0.43 | 0.17 |
| R-ratio^ | 18.6 (22.4) | 6.5 (6.6) | 9.2 (15.5) | 0.04 | 0.02 |
| Pattern of injury | |||||
| Hepatocellular | 50 (74%) | 6 (40%) | 8 (36%) | <0.01 | 0.01 |
| Mixed | 4 (6%) | 6 (40%) | 5 (23%) | ||
| Cholestatic | 14 (21%) | 3 (20%) | 9 (41%) | ||
| Causality Category | |||||
| Definite | 9 (13%) | 1 (7%) | 5 (23%) | 0.61 | 0.23 |
| Highly likely | 26 (41%) | 5 (33%) | 11 (50%) | ||
| Probable | 31(46%) | 9 (60%) | 6 (27%) | ||
| Hy’s Law Δ | 25/62(40.3%) | 5/15 (33.3%) | 3/22 (13.6%) | 0.62 | 0.02 |
| nR Hy’s Law ∘ | 42/64(65.6%) | 5/15 (33.3%) | 2/22 (9.1%) | 0.02 | <0.01 |
DILI role could not be determined in two patients.
SD = standard deviation
R-ratio = (ALT/ULN) ÷ (Alk P/ULN)
bilirubin ≥ 2.5 mg/dL, ALT > 3x ULN and Alk P < 2x ULN at presentation
bilirubin ≥ 2.5 mg/dL & R-ratio > 5 at presentation. (AST substituted for ALT if AST yields greater R-ratio)
Clinical course of liver failure in DILI Primary Role cases
Clinical features of the patients in which DILI played a primary role in the fatal outcome are shown in Table 3, sub-categorized by their clinical course of liver failure. Of these 68 primary role cases, 50 (74%) had ALF as shown by marked elevations in serum ALT and AST levels with relatively mild increases in Alk P and a median time to death or transplant of 24 days (range 2 to 123 days). Another 5 (7%) patients had acute-on-chronic liver failure with less marked increases in ALT and AST (and AST > ALT). Time to death or transplant ranged from 6 to 135 days. Nine patients (13%) had a DILI related chronic liver failure accompanied by lower median values for ALT and AST and higher values for Alk P. For 7 of these 9, time from DILI onset to death or transplant ranged from 217 to 410 days. The other two had liver injury from methotrexate and amiodarone, both known to cause gradual fibrosis accumulation with only modest enzyme elevations. Therefore a precise onset of DILI for these agents was indeterminable, but the exposure time for the amiodarone was 847 days and for methotrexate 2129 days. Finally, four patients (6%) had a rapidly progressive cholestatic course resulting in liver failure and death in 3 and transplantation in 1. Death or transplant occurred 48 to 171 days of onset in these four, who tended to be older (ages 75, 65, 61 and 48). Acute and acute-on-chronic liver failure cases had significantly higher proportions meeting Hy’s Law and nR Hy’s Law criteria (40–79%), compared to relatively few patients meeting such criteria in the chronic or acute cholestatic groups (0–25%) (Table 3).
Table 3.
Characteristics and course of liver failure in those patients who died or needed liver transplantation due primarily to DILI. Values are means (SD) for continuous variables
| Characteristic | Primary Role Cases n = 68 |
||||
|---|---|---|---|---|---|
| Acute Liver Failure n = 50 |
Acute on Chronic Liver Failure n = 5 |
Chronic Liver Failure n= 9 |
Rapid Cholestatic Liver Failure n=4 |
p-value | |
| Mean age, yr. (SD) | 48.7 (16.9) | 60.1 (11.0) | 51.5 (11.9) | 62.3 (11.2) | 0.19 |
| Gender, female | 31 (62%) | 3 (60%) | 6 (67%) | 2 (50%) | 0.96 |
| Mean BMI, kg/m2 (SD) | 29.2 (7.3) | 27.3 (7.1) | 28.6 (4.2) | 33.0 (2.6) | 0.36 |
| Race/Ethnicity | |||||
| White | 33 (67%) | 2 (40%) | 7 (78%) | 4 (100%) | 0.66 |
| Black | 8 (16%) | 1 (20%) | 2 (22%) | 0 (0%) | |
| Asian | 5 (10%) | 1 (20%) | 0 (0%) | 0 (0%) | |
| Other or unknown | 4 (6%) | 1 (20%) | 0 (0%) | 0 (0%) | |
| Latino | 4 (8%) | 0 (0%) | 0 (0%) | 1 (25%) | 0.49 |
| Alcohol Use** | 28/48 (58%) | 2/5 (40%) | 4 (44%) | 1 (25%) | 0.54 |
| Time (days) from onset to Death/LT | 35 (28) | 59 (50) | 236 (120) | 132 (91) | <0.01 |
| Liver biochemistries at onset | |||||
| ALT, U/L | 1864 (1534) | 451 (449) | 330 (478) | 395 (248) | <0.01 |
| AST, U/L | 1807 (1348) | 699 (717) | 279 (347) | 375 (217) | <0.01 |
| Alk P U/L | 240 (108) | 191 (133) | 226 (106) | 523 (367) | 0.26 |
| Bilirubin, mg/dL | 12.5 (7.2) | 11.3 (7.4) | 7.0 (7.3) | 2.9 (2.3) | 0.01 |
| INR | 2.7 (2.0) | 2.3 (0.7) | 1.7 (1.1) | 1.1 (0.0) | 0.03 |
| R-ratio^ | 24.4 (23.7) | 4.5 (3.4) | 5.5 (11.6) | 3.3 (2.9) | <0.01 |
| Pattern of injury (n) | (45) | (5) | (9) | (4) | |
| Hepatocellular | 38 (84%) | 3 (60%) | 2 (22%) | 2 (50%) | <0.01 |
| Mixed | 3 (7%) | 0 (0%) | 1 (11%) | 0 (0%) | |
| Cholestatic | 4 (9%) | 2 (40%) | 6 (67%) | 2 (50%) | |
| Causality Category | |||||
| Definite | 9 (18%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.33 |
| Highly likely | 19 (38%) | 1 (20%) | 4 (44%) | 3 (75%) | |
| Probable | 22 (44%) | 4 (80%) | 5 (56%) | 1 (25%) | |
| Hy’s Law Δ | 23/45(51.1%) | 2/5 (40.0%) | 0/8 (0.0%) | 0/4 (0.0%) | <0.01 |
| nR Hy’s Law ∘ | 37/47(78.7%) | 3/5 (60.0%) | 1/8 (12.5%) | 1/4 (25.0%) | <0.01 |
R-ratio = (ALT/ULN) ÷ (Alk P/ULN)
bilirubin ≥ 2.5 mg/dL, ALT > 3x ULN and Alk P < 2x ULN at presentation
bilirubin ≥ 2.5 mg/dL, and R-ratio > 5 at presentation. (AST is substituted for ALT if AST yields greater R-ratio)
Gender, race and ethnicity did not differ significantly among the cases with different patterns of liver failure, although those with typical acute liver failure tended to be younger (mean age 48.7 years) than patients with other patterns. The percentage of liver transplantations was low for acute-on-chronic liver failure (1 of 5: 20%) and for acute cholestatic liver failure (1 of 4: 25%) and higher for chronic liver failure (5 of 9: 56%) and acute liver failure (34 of 50: 68%).
Causes of death in Contributory Role cases
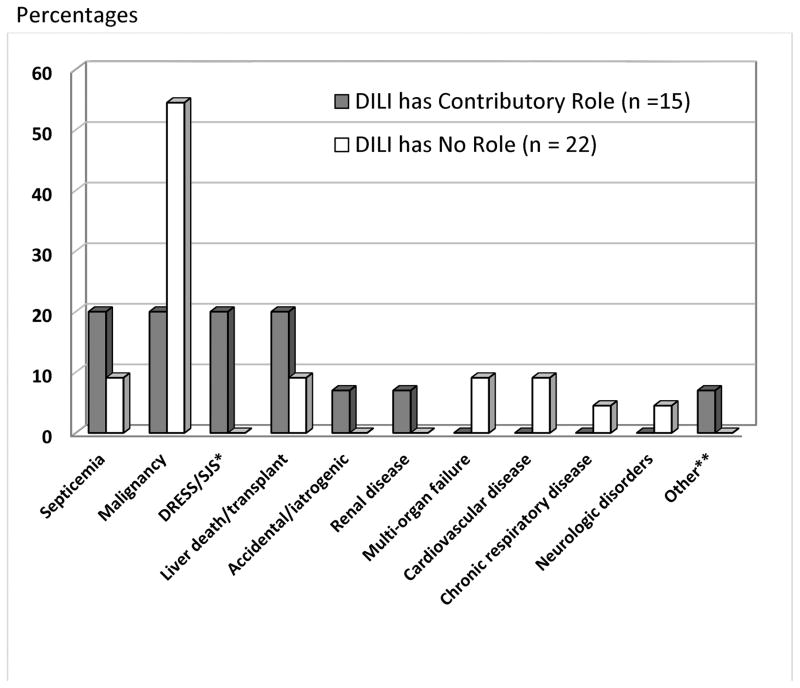
Of the 15 patients whose DILI was considered to play a contributory role, the primary causes of death were judged to be sepsis in 3, malignancy in 3, another unrelated liver disease in 3 (nonalcoholic liver disease in 1 and autoimmune hepatitis in 2) and severe cutaneous reaction with systemic complications in 3. (Figure 2) In many situations, the role of the liver injury was difficult to assess, the underlying co-morbidities being severe and potentially fatal on their own. In these situations, the episode of DILI was believed to hasten the fatal outcomes.
Figure 2.
Causes of death in No Role cases
Of the 22 patients where DILI played no role in the outcome, malignancies accounted for over half of cases (12; 55%). The remaining patients died from a variety of causes shown in Figure 2.
Specific agents leading to fatality
Specific agents and HDS products as a whole are shown in frequency tables by role of DILI in the fatalities. (Supplemental Tables 2–5).
Variables associated with death or liver transplant
We compared patients with DILI related death or liver transplantation (primary and contributory role patients, n = 83) to the rest of the cohort (survivors, no role cases and unknown role cases = 1006) (Figure 1). Variables associated with fatality due primarily or partially to DILI on univariate and multivariate analyses are shown in Tables 4. Higher bilirubin, higher INR, lower platelet count, and lower albumin were associated with mortality.
Table 4.
Variables significantly associated with fatalities due primarily (primary role) or partially (contributory role) to DILI on univariate and multivariate analysis:
| Variable | Cox Regression | |||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | |
| Race | ||||
| Caucasian | Referent | --- | ||
| Black | 1.5 (0.8, 2.8) | 0.16 | ||
| Asian | 3.4 (1.6, 7.5) | <0.01 | ||
| Other | 1.1 (0.4, 3.0) | 0.89 | ||
| BMI (kg/M2) | 1.04 (1.0, 1.1) | 0.01 | ||
| Diabetes | 2.3 (1.5, 3.5) | <0.01 | ||
| Heart failure | 3.3 (1.2, 9.0) | 0.02 | ||
| Chronic Liver disease | 1.9 (1.1, 3.3) | 0.03 | ||
| Neutropenia | 3.6 (1.1, 11.4) | <0.01 | ||
| SJS/DRESS | 5.0 (1.2, 20.5) | 0.02 | ||
| INR at onset +/−14 days | 1.5(1.4, 1.6) | <0.01 | 1.6(1.4, 1.7) | <0.01 |
| ALT (per 50 U/L) | 1.01 (1.0, 1.01) | <0.01 | ||
| Total bilirubin (mg/dL) | 1.1 (1.0, 1.1) | <0.01 | 1.1(1.0, 1.1) | <0.01 |
| Hemoglobin (g/dL) | 0.9 (0.8, 1.0) | 0.05 | ||
| White blood cells (109/L) | 1.1 (1.0, 1.1) | <0.01 | 1.06 (1.02, 1.10) | <0.01 |
| Platelets (1010/L) | 0.99 (0.99, 0.99) | <0.01 | 0.99(0.99,0.99) | <0.01 |
| Albumin (g/dL) | 0.4 (0.3, 0.5) | <0.01 | 0.4(0.3, 0.6) | <0.01 |
| Corticosteroids given | 3.3 (2.2, 5.1) | <0.01 | ||
Hy’s Law, nR Hy’s Law and MELD
Hy’s Law had a lower positive predictive value for deaths primarily or partially due to DILI up to 2 years after onset compared to nR Hy’s Law (10% versus 14%). Specificities were about the same at 71% and 69%, respectively. When limited only to deaths primarily due to DILI, the positive predictive values fell to 8% for Hy’s Law and 12% for nR Hy’s Law. Specificities did not change. Because Hy’s Law and especially MELD were put forth to predict shorter term mortality, we also analyzed their association with DILI fatalities occurring within 26 weeks due to acute liver failure or acute on chronic liver failure. On this multivariate analysis, Hy’s Law was significantly associated with early mortality primarily due to DILI (HR: 2.2, CI 1.3–3.7). However, the association with nR Hy’s Law was stronger (HR: 6.2, CI 3.4 – 11.1) and with MELD stronger still. The HR per MELD point was 1.2 (CI 1.1 – 1.2), and at various MELD cut-offs, the HRs were all over 10. (Supplemental Table 6) C-statistic for a MELD cut-off of 19 was 0.83 compared to 0.73 for nR Hy’s Law and 0.60 for Hy’s Law For the Hy’s Law Cox regression analyses, ALT, AST and bilirubin were excluded as they are part of Hy’s Law and the nR Hy’s Law. INR, creatinine and bilirubin were excluded in the Cox modeling for MELD.
DISCUSSION
It is well known that DILI can lead to death or liver transplant (fatal outcome), but the precise role and course of DILI in these cases is poorly defined particularly when fatalities occur more than 26 weeks after liver injury. In the DILIN prospective cohort, 107 of 1089 patients with definite, highly likely or probable DILI had a fatal outcome within 2 years of onset. Thus, the all-cause fatality rate was 9.8%. (Figure 1). Fatalities tended to be older, have more hepatocellular injury and more underlying chronic liver disease than patients who survived (Table 1). Their DILI causality assessments also tended to be less certain, probably because the recovery, or washout, phase was truncated by the death. The majority of the fatalities were related to DILI, being the primary cause of death in 68 patients and a contributing cause in another 15. The remaining 22 patients had fatal outcomes unrelated to DILI. (Figure 1) Therefore, the DILI fatality rate (primary or contributory) was 7.6% (83/1089). This rate increased to 9.5% (68/712) if the denominator is limited to those presenting with jaundice (bilirubin ≥ 2.5 mg/dL).
Comparing DILI primary, contributory and no role cases revealed important differences. (Table 2) Primary and contributory role cases were predominantly women compared to no role cases who were predominantly men. Primary role cases were younger and usually followed a course typical of acute liver failure (ALF) (50 of 68, 74%). As expected, the majority (83%) of ALF cases presented with a hepatocellular pattern of enzyme elevations (high R-ratios). (Table 3) This predominance of ALF is in line with DILI being a leading cause of ALF in the U.S.14,15 Our cases were similar to those reported by the US ALF Group. Most were young (<45) and women. Antimicrobials, isoniazid in particular, were the most common agents implicated but herbal and dietary supplements were also prominent (Supplemental Tables 2–4). The majority of patients with ALF were listed for liver transplantation (74%), and most of these were transplanted (68%). The remaining ALF patients were not listed due to medical or psychosocial contraindications.
DILI fatality by non-ALF injury patterns has not been well described, though a quarter of the DILIN deaths due directly to DILI followed such non-ALF courses. Five (7%) of the 68 primary role cases had underlying cirrhosis and were categorized as acute-on-chronic liver failure. For this study, we defined acute-on-chronic liver failure as a case with pre-existing cirrhosis and DILI being the primary cause of death or LT within 26 weeks of onset. Our dataset did not have the clinical information to determine more recently developed CLIF (Chronic Liver Failure) classifications and scores.16,17 Of the 5 acute-on-chronic cases, 3 were listed for transplant but only one received a liver. The other two became too ill and were removed from listing. The listed cases may not have met criteria for priority listing (Status 1a) due to their underlying cirrhosis, which could explain the lower rate of transplantation.
Another 9 (13%) of the 68 primary role cases had a fatality due to chronic liver failure. These cases died or needed LT more than 26 weeks after DILI onset. Agents leading to chronic liver failure were varied with 3 of 9 being HDS products and another 3 were antibiotics. (Supplemental Table 4) Amiodarone and methotrexate, both known to cause an insidious fibrosis accounted for one each. Chronic liver injury after DILI has been previously described by our group and others.6,7 While the definition, incidence and significance of chronic DILI is unclear, this study clearly documents a small number of fatalities due to prolonged liver injury from DILI. These 9 cases had lower initial transaminases and R-ratios compared to those with ALF and ACLF, but no other distinguishing features could be gleaned from this small number of cases (Table 3). Larger numbers with robust prospective follow-up will be needed to fully understand these more insidious DILI deaths.
Four (6%) of the 68 primary role patients had a rapid (< 26 weeks) and progressive cholestatic injury. DILI causing such severe cholestatic injury with ductopenia has been described.18–20 These four all died or required transplant within 26 weeks of DILI onset. They tended to be older, three being over 60. Two had a cholestatic pattern of injury from onset to demise, while two presented with a hepatocellular pattern of injury (R ratio just over 5) that quickly transitioned to cholestatic. The R ratios were less than 1 in all four at time of death or transplant. One patient underwent LT and the explant histology showed absence of bile ducts. The remaining 3 had medical or psychosocial contraindications to transplantation. Liver biopsies in these three did not show ductopenia but biopsies may have been obtained too early to capture vanishing bile duct histology. Autopsy liver tissuewere not available for these cases.
DILI as a contributing cause of fatality has been poorly defined and rarely reported. Yet, such contributory role in deaths may be an important part of DILI disease burden. In this series, DILI contributed to death by worsening of an underlying liver disease (20%) or by contributing to a non-liver related death such as DRESS/SJS, sepsis or malignancy (20% each). (Figure 2) In the three patients with cancer, DILI significantly limited subsequent therapeutic options. In one case, evaluation of liver injury included an ERCP that was complicated by a fatal duodenal perforation.
While categorizing DILI as a primary versus contributory cause of death was occasionally difficult, assigning it as having no role was often straightforward. Many of these deaths occurred well after DILI had resolved. The majority of these cases were due to progression of a malignancy. (Figure 2). Certain comorbidities could forebode poor outcome even after apparent liver recovery. Identifying such factors by a comorbidity index (e.g. Charleson, Elixhauser) would be useful, but unfortunately the DILIN does not capture the detailed information on comorbidities needed to complete such indices.
After we had categorized all fatalities, we identified variables associated with DILI-related deaths (primary and contributory role cases). The current analysis differs from our prior studies examining DILI fatalities at just 26 weeks.2,5 In this study, we included fatalities up to 2 years after DILI onset. Also, categorization of DILI’s role in the fatality was done using a systematic approach and by a subcommittee of DILIN investigators, who used a new contributory role category and subcategorization of primary role cases by pattern of liver demise.
Variables independently associated with overall DILI related fatality were all plausible. Higher INR, higher bilirubin and lower albumin all reflect more severe liver dysfunction at onset. Higher WBC could reflect more immune mediated injury or concurrent infection (i.e. sepsis). The association of mortality with lower platelets is in line with a recent study identifying thrombocytopenia with poor outcome for ALF in general.21 The lower platelet count may reflect multi-organ failure, sepsis or disseminated intervascular coagulation. Interestingly, pre-existing chronic liver disease was not associated with mortality on multivariate analysis, though it was on univariate. This finding is similar to our two prior studies that found an association between chronic liver disease and short term mortality on univariate analysis only. Neither study detected an independent association.2,5 With the inclusion of deaths up to 2 years later and DILI contributory role deaths, we thought this variable might now be independently associated. The persistent lack of association could be due too few patients to detect a smaller increased risk (under powered) or the risk may apply only to more advanced liver disease. Our definition of prior chronic liver disease did not specify degree of fibrosis or liver dysfunction.
We show an incremental improvement in Hy’s Law by use of the modified nR form.8 For deaths due primarily or partially to DILI, Hy’s Law had a 10% PPV precisely in line with Dr. Zimmerman’s original suggestion.22 However, the nR modification of Hy’s Law yielded a higher PPV at 14%. The difference lay primarily in the ability to identify those who would die of DILI related ALF: 79% of ALF fatalities met nR Hy’s Law versus only 52% meeting standard Hy’s Law. (Table 3) The Alk P < 2x ULN criteria explained all the ALF deaths which met nR Hy’s Law but not standard Hys’ Law. In Dr. Zimmerman’s text, the limit for alkaline phosphatase was < 1–3x ULN, but a cut-off of 2x ULN is typically used.5,8,22 The ALF fatalities not meeting Hy’s Law had Alk P/ULN ratios ranging from 2.0 to 3.5, low enough to yield an R-ratio > 5, but too high for standard Hy’s Law. Thus, the use of nR Hy’s Law over the traditional Hy’s Law as a positive predictor of increased risk of death due to DILI may be warranted in clinical practice and drug development.
Compared to Hy’s Law or nR Hy’s Law, MELD was a better predictor of acute DILI deaths (< 26 weeks) by ALF and ACLF. (Supplemental Table 6) The hazard ratio for a MELD greater than 19 was 35.6 with a C-statistic of 0.83, compared to a HR of 2.2 and C-statistic of 0.60 for Hy’s Law and 6.2 and 0.73 for nR Hy’s Law. MELD probably had a small advantage of often being calculated later because INR was not always obtained with the first set of elevated liver enzymes. More importantly, Hy’s Law and MELD were put forth for different purposes. Dr. Zimmerman shared his astute observation as a warning to clinicians to be vigilant of a severe outcome when hepatocellular DILI meets certain ALT, Alk P and bilirubin criteria. As such, it was merely a positive predictive value set low at 10% to cast a wide net for patients needing close follow-up. On the other hand, MELD was based on multivariate modeling to predict mortality risk surrounding therapeutic interventions, first TIPS and then transplant, where predictive precision is the goal, and not just a low threshold positive predictive value.
In this systematic examination of fatalities prospectively followed in the DILIN, 7.6% of patients died primarily or partially from the DILI within two years of onset. When DILI was the primary cause of death, typical acute liver failure (ALF) accounted for most fatalities, but one-quarter had non-ALF courses including acute-on-chronic, chronic and progressive cholestatic liver failure. When DILI contributes to death, most fatalities were due to sepsis, malignancy, DRESS/SJS or worsening pre-existing chronic liver disease. However, pre-existing liver disease was not independently associated with fatality from DILI. The nR modification of Hy’s Law improved the positive predictive value for those at risk of death from acute hepatocellular injury by accepting moderately higher alkaline phosphatase elevations into the criteria. The nR modification also has a stronger the association with deaths within 26 weeks directly related to DILI, but neither outperformed MELD in predictive value.
Supplementary Material
Acknowledgments
Funding: The DILIN Network is structured as U01 cooperative agreements funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) under grants: DK065176 (Duke) -DK065201 (UNC), DK065184 (Michigan), -DK065211 (Indiana), DK065193 (UConn), DK065238 (UCSF/CPMC), DK083023 (UTSW), DK083027 (TJH/UPenn), DK082992 (Mayo), DK083020 (USC). Additional funding is provided by CTSA grants: UL1 RR025761 (Indiana), UL1 RR025747 (UNC), UL1 RR024134 (UPenn), UL1 RR024986 (Michigan), UL1 RR024982 (UTSW), UL1 RR024150 (Mayo) and by the Intramural Research Program of the National Cancer Institute, NIH.
Abbreviations
- ACLF
acute on chronic liver failure
- ALF
acute liver failure
- ALT
alanine aminotransferase
- Alk P
Alkaline phosphatase
- AST
aspartate aminotransferase
- BMI
body mass index
- CLF
chronic liver failure
- DILI
drug-induced liver injury
- DILIN
Drug-induced Liver Injury Network
- HDS
herbal and dietary supplements
- MELD
model for end-stage liver disease
- NIH
National Institutes of Health
- nR
new R-ratio
- ULN
upper limit of normal
Footnotes
Disclosures: RJ Fontana: Consultant GSK, Tibotec, Vertex, Gilead; N Kaplowitz, Consultant Pfizer, GSK, Roche, Takeda; N Chalasani: Consultant Merck, Abbvie, Aegerion, Salix, BMS, Lilly; H Barnhart, PH Hayashi, JH Hoofnagle, J Gu, D Rockey, AH Sherker and HL Tillmann: No relevant disclosures or conflicts of interest.
Writing Assistance: No writing assistance beyond the authors listed.
Author contributions:
Concept and design: PHH, DR, NK, RFJ, HLT, AHS, KRR, JHH
Acquisition of data: PHH, DR, NK, AHS, JHH, RFJ, HLT, KRR, JB
Analysis and interpretation: PHH, DR, NK, RFJ, HLT, AHS, JHH
Drafting of the manuscript: PHH, JHH
Critical revision of the manuscript for important intellectual content: PHH, DR, NK, RFJ, HLT, AHS, KRR, JHH
Statistical analysis: JG, HB, PHH
Obtaining funding: PHH, DR, NK, RFJ, HLT
Administrative, technical, material support: Hoss Rostami, JG, HB
Study supervision: JHH, AHS
References
- 1.Andrade RJ, Lucena MI, Fernandez MC, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005 Aug;129(2):512–521. doi: 10.1016/j.gastro.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Bonkovsky HL, Fontana R, et al. Features and Outcomes of 899 Patients With Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology. 2015 Jun;148(7):1340–1352. e1347. doi: 10.1053/j.gastro.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjornsson E, Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology. 2005 Aug;42(2):481–489. doi: 10.1002/hep.20800. [DOI] [PubMed] [Google Scholar]
- 4.Fontana RJ, Gu J, Barnhart H, et al. Subject race and liver biochemical profile are strongly assoicated with clinical outcomes and chronic liver injury: Results from the DILIN Prospective Study. Hepatology. 2012;56(4):837A. [Google Scholar]
- 5.Fontana RJ, Hayashi PH, Gu J, et al. Idiosyncratic drug-induced liver injury is associated with substantial morbidity and mortality within 6 months from onset. Gastroenterology. 2014 Jul;147(1):96–108. e104. doi: 10.1053/j.gastro.2014.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fontana RJ, Hayashi PH, Barnhart H, et al. Persistent liver biochemistry abnormalities are more common in older patients and those with cholestatic drug induced liver injury. Am J Gastroenterol. 2015 Oct;110(10):1450–1459. doi: 10.1038/ajg.2015.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medina-Caliz I, Robles-Diaz M, Garcia-Munoz B, et al. Definition and risk factors for chronicity following acute idiosyncratic drug-induced liver injury. J Hepatol. 2016 Sep;65(3):532–542. doi: 10.1016/j.jhep.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robles-Diaz M, Lucena MI, Kaplowitz N, et al. Use of Hy’s law and a new composite algorithm to predict acute liver failure in patients with drug-induced liver injury. Gastroenterology. 2014 Jul;147(1):109–118. e105. doi: 10.1053/j.gastro.2014.03.050. [DOI] [PubMed] [Google Scholar]
- 9.Fontana RJ, Watkins PB, Bonkovsky HL, et al. Drug-Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Saf. 2009;32(1):55–68. doi: 10.2165/00002018-200932010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rockey DC, Seeff LB, Rochon J, et al. Causality assessment in drug-induced liver injury using a structured expert opinion process: comparison to the Roussel-Uclaf causality assessment method. Hepatology. 2010 Jun;51(6):2117–2126. doi: 10.1002/hep.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee WM, Stravitz RT, Larson AM. Introduction to the revised American Association for the Study of Liver Diseases Position Paper on acute liver failure 2011. Hepatology. 2012 Mar;55(3):965–967. doi: 10.1002/hep.25551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karvellas CJ, Fix OK, Battenhouse H, et al. Outcomes and complications of intracranial pressure monitoring in acute liver failure: a retrospective cohort study. Critical care medicine. 2014 May;42(5):1157–1167. doi: 10.1097/CCM.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olson JC, Wendon JA, Kramer DJ, et al. Intensive care of the patient with cirrhosis. Hepatology. 2011 Nov;54(5):1864–1872. doi: 10.1002/hep.24622. [DOI] [PubMed] [Google Scholar]
- 14.Lee WM. Drug-induced acute liver failure. Clin Liver Dis. 2013;17(4):575–586. doi: 10.1016/j.cld.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostapowicz G, Fontana RJ, Schiodt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002 Dec 17;137(12):947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 16.Jalan R, Saliba F, Pavesi M, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014 Nov;61(5):1038–1047. doi: 10.1016/j.jhep.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Gustot T, Fernandez J, Garcia E, et al. Clinical Course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology. 2015 Jul;62(1):243–252. doi: 10.1002/hep.27849. [DOI] [PubMed] [Google Scholar]
- 18.Anania FA, Rabin L. Terbinafine hepatotoxicity resulting in chronic biliary ductopenia and portal fibrosis. The American journal of medicine. 2002 Jun 15;112(9):741–742. doi: 10.1016/s0002-9343(02)01109-9. [DOI] [PubMed] [Google Scholar]
- 19.Stickel F, Droz S, Patsenker E, Bogli-Stuber K, Aebi B, Leib SL. Severe hepatotoxicity following ingestion of Herbalife((R)) nutritional supplements contaminated with Bacillus subtilis. J Hepatol. 2009 Jan;50(1):111–117. doi: 10.1016/j.jhep.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Martinez MA, Vuppalanchi R, Fontana RJ, et al. Clinical and histologic features of azithromycin-induced liver injury. Clin Gastroenterol Hepatol. 2015 Feb;13(2):369–376. e363. doi: 10.1016/j.cgh.2014.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stravitz RT, Ellerbe C, Durkalski V, et al. Thrombocytopenia Is Associated With Multi-organ System Failure in Patients With Acute Liver Failure. Clin Gastroenterol Hepatol. 2016 Apr;14(4):613–620. e614. doi: 10.1016/j.cgh.2015.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmerman HJ. Drug-Induce Liver Injury. In: Zimmerman HJ, editor. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2. Philadelphia: Lipponcott Williams & Wilkins; 1999. pp. 432–433. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.