Abstract
Background
Hydrogen peroxide (H2O2) has a variety of actions in skin wounds but has been rarely studied in deep muscle tissue. Based on response to the Transient Receptor Potential Ankyrin 1 (TRPA1) antagonists after plantar incision, we hypothesized that H2O2 exerts nociceptive effects via the TRPA1 in muscle.
Methods
Nociceptive behaviors in rats (n=269) and mice (n=16) were evaluated after various concentrations and volumes of H2O2 were injected into the gastrocnemius muscle or subcutaneous tissue. The effects of H2O2 on in vivo spinal dorsal horn neuronal activity and lumbar dorsal root ganglia (DRG) neurons in vitro were evaluated from 26 rats and 6 mice.
Results
Intramuscular (mean ± SD: 1436 ± 513 seconds), but not subcutaneous (40 ± 58 seconds), injection of H2O2 (100 mM, 0.6 ml) increased nociceptive time. Conditioned place aversion was evident after intramuscular (−143 ± 81 seconds), but not subcutaneous (−2 ± 111 seconds), injection of H2O2. These H2O2-induced behaviors were blocked by TRPA1 antagonists. Intramuscular injection of H2O2 caused sustained in vivo activity of dorsal horn neurons, and H2O2 activated a subset of DRG neurons in vitro. Capsaicin nerve block decreased guarding after plantar incision, and reduced nociceptive time after intramuscular H2O2. Nociceptive time after intramuscular H2O2 in TRPA1 knockout mice was shorter (173 ± 156 seconds), compared with wild-type mice (931 ± 629 seconds).
Conclusions
The greater response of muscle tissue to H2O2 may help explain why incision that includes deep muscle, but not skin incision alone, produces spontaneous activity in nociceptive pathways.
Introduction
Acute postoperative pain management continues to be an important perioperative problem.1 We have examined mechanisms for incisional injury in order to better understand the etiology of postoperative pain.2 Previously we have shown that incised deep tissue rather than skin has importance in the genesis of spontaneous activity in pain transmitting pathways and non-evoked guarding behavior after plantar incision in rats.3–5 We have shown that treatments that reduce clinical postoperative pain reduce this guarding behavior.6–8 In addition, capsaicin treatments, acid sensing ion channel subunit 3 (ASIC3) blockers and the Transient Receptor Potential Ankyrin 1 (TRPA1) antagonist HC-030031 also reduce this unprovoked nociceptive behavior.9–12
The TRPA1 channel is activated by noxious cold, reactive oxygen species and mechanical stimuli.13–15 TRPA1 is expressed in small-and medium-sized nociceptive neurons of the dorsal root, trigeminal and nodose ganglia and TRPA1 antagonists have been studied as analgesic drugs.13 We recently demonstrated that the TRPA1 antagonist HC-030031 inhibited guarding behavior after plantar incision but did not affect exaggerated heat or mechanical responses.12
We also demonstrated reactive oxygen species, such as Hydrogen peroxide (H2O2), were increased early after incision in both skin and muscle.12 H2O2 is the major reactive oxygen species that functions as an oxidant with antibacterial and cytotoxic effects and is an early signal of incision tissue injury.16 H2O2 has several roles in wounds including recruiting leukocytes, promoting neovascularization and healing.17,18 Endogenously, H2O2 is generated through oxidative phosphorylation in mitochondria, produces Ca2+ influx in a subset of dorsal root ganglia (DRG) neurons19 and can act as a TRPA1 agonist.20
The actions of H2O2 as a nociceptive mediator of incisional pain, especially its role in the deep muscle tissue have not been studied. Because nociceptive behaviors that were caused by deep muscle incision were affected by HC-030031 whereas those largely produced by skin incision were not, we hypothesized that H2O2 exerts nociceptive effects in muscle tissue. Therefore, we performed a detailed evaluation of the actions of H2O2 on nociception of deep muscle tissue in rats and mice.
Material and Methods
All procedures in this study were approved by The University of Iowa Animal Care and Committee (Approval number: 5011267), Iowa City, Iowa, USA and conformed to the NIH guide for the Care and Use of Laboratory Animals. Adult male and female Sprague-Dawley rats (200–300 g, Harlan, Indianapolis, IN, USA) were housed in groups of 2 in clear plastic cages (40 × 60 × 30 cm) on fresh bedding with free access to food and water. The environment was controlled with a 12-hour light-dark cycle and a room temperature of 22.0 ± 2.0 °C. The physical conditions of the animals were carefully monitored every weekday for clinical signs of illness during the experiments. The animals did not show any signs of stress (except nociception-related behavior) or illness throughout the experiment. Based on our previous experience, we used 6–9 rats for the behavioral studies, and collected 6 neurons per group for the in vivo extracellular dorsal horn neuron (DHN) recording. Altogether, 288 rats and 22 mice were used in this study: one hundred and eighty five rats were assigned for nociceptive behavior after injection of H2O2 or cinnamaldehyde, 58 rats for conditioned place aversion (CPA), 11 rats for DHN recording, 27 rats for nociceptive behavior after capsaicin nerve block, and 8 rats were assigned for Ca2+ imaging. Sixteen mice were assigned for nociceptive time after H2O2 injection, and 6 mice were utilized for Ca2+ imaging.
Measurement of nociceptive behaviors
Measurement of nociceptive time
Rats were acclimated individually on a small plastic mesh floor covered with a clear plastic cage top for 1 hour per day for at least 2 days before testing. Rats were acclimated to being held in a towel. H2O2 was made daily from a 30% solution (Sigma-Aldrich, St. Louis, MO, USA) by diluting into synthetic interstitial fluid (SIF; in mM: 107.8 NaCl, 3.5 KCl, 1.5 CaCl22H2O, 0.7 MgSO47H2O, 1.7 Na2HPO42H2O, 26.2 NaHCO3, 9.6 C6H11NaO7, 7.6 sucrose; pH 7.4; equilibrated with 95% O2/5% CO2). On testing day, rats were gently held after wrapped in a towel in the same way as the acclimation session. Various concentrations (10–100 mM) and volumes (0.4–1.0 ml) of H2O2 were injected into the left gastrocnemius muscle or subcutaneous tissue overlying the gastrocnemius muscle using a 1-ml syringe with a 30-gauge needle. Rats were returned to the testing cage immediately after injection, and observed for 60 minutes. Data were collected in 5-minute bins after H2O2 injection. Equivalent volumes of SIF were injected as a control. H2O2-induced nociceptive behavior in rats was recorded as total time spent flinching, lifting and licking of the hind leg during the 60-minute observation period. In our preliminary experiments, Methylene blue dye was injected into the gastrocnemius muscle in various volumes (0.4–1.0 ml) to trace its distribution. After euthanasia, it was observed that the 0.4–0.8 ml of injectate was confined within the muscle, while leakage outside of the muscle was observed after injection of 1.0 ml of dye.
The effects of local injection of TRPA1 antagonist AP-18 (Sigma-Aldrich) on nociceptive behavior elicited by H2O2 injections were also evaluated. Sequential injections of AP-18 (50 mM, 0.3 ml) or vehicle [10% dimethyl sulfoxide (DMSO; Molecular Probes, Eugene, OR, USA) in phosphate buffered saline (PBS; Gibco, Grand Island, NY, USA)], followed by H2O2 (100 mM, 0.3 ml), were made into the gastrocnemius muscle. Therefore, the total injection volume was 0.6 ml, and the final concentration of H2O2 was 50mM. Immediately after injection, nociceptive behavior was recorded for 60 minutes as described above. The effect of systemic administration of sodium azide (Sigma-Aldrich, dissolved in PBS; 10 mg/kg, intraperitoneal), a catalase inhibitor, on nociceptive behavior after injection of H2O2 (10 or 30 mM, 0.6ml) into the gastrocnemius muscle or subcutaneous tissue overlying the gastrocnemius muscle was also tested. We used systemic administration of sodium azide (10 mg/kg) to reduce peroxide catabolism and potentially increase the nociceptive behavior evoked by H2O2 injection.
Nociceptive behavior induced by injection of 30 mM cinnamaldehyde (Sigma-Aldrich, dissolved in PBS, 0.6 ml), a TRPA1 agonist,21 was also evaluated in rats. Cinnamaldehyde was injected into the left gastrocnemius muscle or subcutaneous tissue overlying the gastrocnemius muscle. Nociceptive behavior protocol was similar to that described above for H2O2. For all behavioral experiments, a second person randomized and blinded the observer to drug and dose.
Conditioned place aversion
For CPA experiments, we used a biased approach.22 All animals were handled by a single researcher and housed in a separate area from animals used for other experiments. Bedding and food pellets were changed by the same researcher. One and 2 days before the preconditioning day, the animals underwent a 5-minute handling session each day. Following each handling, the animals were placed in a fresh cage and transported to the animal care unit.
The CPA apparatus consisted of 3 chambers, two larger end chambers (46 × 32 × 32 cm) and one smaller center-connecting chamber (46 × 21.5 × 13 cm). The center chamber was lined with white walls. One lateral chamber had grey walls, a rough black floor, and a lemon scent. The other lateral chamber had black and white striped walls, a smooth black floor, and a vanilla scent. Baseline data were collected on the preconditioning day. Rats were placed in the center of the CPA apparatus, with free access to all three chambers. The position of the rat was determined by the position of its head; a rat was considered to be in a specific chamber when its head including both pinnae were in that chamber. The time spent in each chamber was recorded for 15 minutes to determine the preconditioning baseline, and the preferred and non-preferred chambers were identified. Rats spending greater than 80% or less than 20% time in a chamber were excluded from further testing. Rats underwent a single conditioning trial the following day as described below.23,24
We conducted two series of CPA experiments. In the first CPA experiment, in the morning of the conditioning day, rats received intra-gastrocnemius muscle injection of SIF (0.6 ml) as a vehicle. Immediately after SIF injection, rats were placed into the non-preferred chamber for 45 minutes, with no access to the other chambers. In the afternoon approximately 4 hours later, the rats received one of the following injections: (1) SIF (0.6 ml) intramuscularly, (2) H2O2 (100 mM, 0.6 ml) intramuscularly, (3) H2O2 (100 mM, 0.6 ml) subcutaneously or (4) co-injection of H2O2 (200 mM, 0.3 ml) and AP-18 (50 mM, 0.3 ml) intramuscularly. Rats were then placed in the preferred chamber for 45 minutes.
The second series of CPA experiments were performed using 30 mM of H2O2, instead of 100 mM. Therefore, on the conditioning day, intra-gastrocnemius muscle injection of SIF (0.6 ml) was paired with the non-preferred chamber in the morning, and one of the following injections were paired with the preferred chamber in the afternoon: (1) SIF (0.6 ml) intramuscularly, (2) H2O2 (30 mM, 0.6 ml) intramuscularly, (3) H2O2 (30 mM, 0.6 ml) subcutaneously or (4) co-injection of H2O2 (60 mM, 0.3 ml) and AP-18 (50 mM, 0.3 ml) intramuscularly.
In the morning of the next day, a place aversion test was performed by placing the animals back into the center of the CPA chambers with free access to all chambers for 15 minutes. The time spent in each chamber was again recorded and analyzed off-line. CPA data were expressed as an absolute measure of preference and aversion, by comparing the total time spent in the initially preferred and non-preferred chambers during preconditioning baseline vs. the total time spent in the same chambers after conditioning. We also used the CPA score, defined as time (seconds) spent in the initially preferred chamber during the test period minus the time spent in that chamber during preconditioning baseline. The person scoring the time spent in each chamber was blinded to the solution injected in the afternoon session.
Guarding behavior
Detailed methods for plantar incision-induced guarding were described previously.4,26 Briefly, rats were first acclimated to the testing environment for 3 days. Then a baseline test was performed 1 day before incision. The next day rats underwent plantar hind paw incision as described below. Guarding nociceptive behaviors were measured up to 10 days after incision using the guarding scores as follows.
To measure guarding behavior, rats were placed individually on a small plastic mesh floor (grid 8 × 8 mm) covered with a clear plastic cage top (21 × 27 × 15 cm). Both incised and non-incised hind paws were closely observed during a 1-minute scoring period, and a score of 0, 1, or 2 was given. Zero was scored when the incised area was touching the mesh, and the area was blanched or distorted by the mesh; 1 was scored when the incised area touched the mesh without blanching or distortion; 2 for the position when the incised area was completely off of the mesh. We scored once every 5 minutes for 1 hour after incision. Therefore, a cumulative score was obtained by adding the 12 scores during the 1-hour testing period (0–24) for each hind paw. The guarding score was then obtained by subtracting the score of the incised hind paw from that of the non-incised hind paw. The person scoring guarding score was blinded to treatment.
In vivo extracellular recording of dorsal horn neurons
The surgical preparation of rats for DHN recording was performed according to previously described methods.25 Briefly, anesthesia was initially induced with 5% isoflurane in air in a plastic box followed by 2% isoflurane via a nose cone. Then a tracheotomy was performed and the tracheal cannula was connected to a ventilator (Harvard Apparatus, Inc., South Natick, MA, USA). End-tidal CO2 was measured in the first two rats to assure ventilator parameters maintained end-tidal CO2 between 36–40 mmHg. The animal was artificially ventilated with 100% oxygen and anesthesia was maintained with 2% isoflurane. The rat was then positioned in a stereotactic frame. The head and vertebral column of the rat were stabilized with ear bars and vertebral clamps, respectively. Limited laminectomies were performed to expose the dorsal spinal cord at the lumbar enlargement between 13th thoracic and 3rd lumbar vertebra. The underlying dura was removed and the spinal cord was covered with mineral oil. During the DHN recording, the body temperature was maintained at approximately at a range of 35–37 °C with a servo-controlled electric heating lamp and an underbody heating pad.
After these surgical preparations, isoflurane was decreased to 1.2% during the subsequent recording period. For DHN recording, small holes were made with fine forceps on the pia mater of the lumbar enlargement between the L3 and L5 segments. A tungsten parylene-coated electrode (1–1.5 mΩ impedance, Microprobe Inc., Clarksburgh, MA, USA) was then driven through the pia mater hole, and the depth was set 0 μm once the electrode entered the surface of the dorsal spinal cord. Then the electrode was advanced into the spinal cord slowly at 10 μm per step via a micropositioner (David Kopf Instruments, Tujunga, CA, USA) until a neuron was encountered or a depth of 1000 μm was reached. Innocuous mechanical search stimuli (tapping and touching) were applied to the gastrocnemius region; gently squeezing of the gastrocnemius was also tested. Neurons were tested for response to pinch of the skin of the gastrocnemius region using a small blunt, curved forceps, and squeezing the gastrocnemius muscle using a large forceps; Neurons were accepted for the further study if they responded to both stimuli. The depth of the neuron recorded was based on the electrode position optimized for maximum action potential amplitude.
Once a DHN with a receptive field for both skin of the gastrocnemius region and the gastrocnemius muscle was identified, baseline activity of the neuron was recorded for 5 minutes. Each neuron was randomly assigned by Research Randomizer (https://www.randomizer.org/) into 3 groups: (1) intramuscular injection of H2O2 (100 mM, 0.4 ml), (2) intramuscular injection of SIF (0.4 ml), or (3) subcutaneous injection of H2O2 (100 mM, 0.4 ml). The injection was made into the receptive field, and DHN activity was recorded for 60 minutes after injection. In some animals, if possible, we made a second injection to the contralateral gastrocnemius muscle or skin while recording from a DHN in the contralateral lumbar spinal cord to reduce the number of rats used. Because the first injection was unlikely to affect the response to the contralateral injection, each neuron was evaluated independently. Eighteen neurons were recorded from 11 rats. We performed two injections, one in each side in 7 rats and one injection in 4 rats. The person performing the recordings was not blinded to drug.
Neuron activities were amplified (Grass Instruments, Quincy, MA, USA), and displayed on an oscilloscope and a computer monitor. All data were stored in a PC computer with a data acquisition system, 1401 Plus Laboratory Interface and Spike2 software (Cambridge Electronic Design, Cambridge, UK).
Plantar hind paw incision
The plantar incision was made as previously described.26 Briefly, a 1-cm longitudinal incision was made and the underlying fascia and the plantar flexor digitorum brevis muscle were incised with a #11 surgical blade under isoflurane anesthesia (1.5–2%). Blunt curved forceps were then inserted through the incision into the muscle to further divide and retract the muscle. The muscle origin and insertion remained intact. The wound was then closed with two subcutaneous mattress sutures with 6-0 nylon on a P-1 needle (Ethicon, Somerville, NJ, USA).
Capsaicin nerve block on nociception-related behavior
We examined the effect of peripheral nerve block with a capsaicin (Sigma-Aldrich) and bupivacaine (Hospira, Lake Forest, IL, USA) mixture on spontaneous guarding behavior after plantar incision, and on nociceptive behavior induced by injection of H2O2 into the gastrocnemius muscle in rats. Briefly, the nerve block was performed with the aid of a nerve stimulator (Tracer III, Smiths Medical, Keene, NH, USA) using a 30-mm, 22-gauge nerve block needle (Teleflex, NC, USA). After brief isoflurane anesthesia, a shaved region of the posterior thigh was palpated, and the groove created by the proximal portion of the hamstring muscles was identified. The stimulating needle was then inserted at this area and aimed toward the proximal femur. Stimulation frequency was at 2 Hz and the pulse duration was 200 microsecond. As the needle approached the sciatic nerve, dorsiflexion and/or plantar flexion of the ankle was observed. Prior to injection of the drug, the stimulating needle was positioned to maximize the response at the ankle to a current of 0.5 to 0.55 mA. Using this combined landmark and stimulation technique for the sciatic nerve block, we were able to observe reversible loss of knee flexion and ankle/hindpaw motor function in rats injected with bupivacaine. Nerve stimulator-guided sciatic nerve block was performed using either 0.05% capsaicin mixed in 0.5% bupivacaine or 0.5% bupivacaine containing vehicle. Capsaicin was first dissolved in ethanol and Tween 80 to yield 2.5% stock solution, and then diluted 50 times with 0.5% bupivacaine to a final concentration of 0.05% capsaicin. For the control group, the injectate consisted of 1% ethanol and 1% Tween 80 diluted in 0.5% bupivacaine. One day after nerve block with the above-mentioned drugs, rats underwent plantar incision. The guarding score was recorded through postoperative day 10.
A separate group of rats underwent nerve block with either 0.05% capsaicin/ 0.5% bupivacaine mixture or 0.5% bupivacaine with vehicle as above. Rats were tested for nociception induced by injection of H2O2 into the gastrocnemius muscle at several time points (1, 3, 5, and 7 days) following the nerve block. On each day of testing, H2O2 (100 mM, 0.6 ml) was injected into the gastrocnemius muscle, and the total time spent flinching, lifting, and licking as nociceptive behavior was recorded for 60 minutes as described above. The person performing the behavioral experiments was blinded to drug used for the nerve block.
In vitro responses of lumbar dorsal root ganglia neurons to H2O2
Rats were anesthetized with isoflurane and then euthanized using increasing concentrations of CO2. Lumbar DRGs (L2-L5) were removed bilaterally, placed into Hank’s Balanced Salt Solution (HBSS, Gibco) and minced. DRGs were enzymatically treated by 40–60 units of activated papain (Worthington Biochemical, Lakewood, NJ, USA) for 20 minutes, and then by collagenase type 4 (2 mg/ml, Worthington Biochemical) and dispase (2.5 mg/ml, Gibco) for 20 minutes in a 37 °C water bath. The dissociated lumbar DRG neurons were placed on poly-lysine-coated glass coverslips (Sigma-Aldrich) inside 35-mm culture dishes, and incubated at 37 °C in 5% CO2 and 90% humidity overnight before Ca2+imaging.
The DRG neurons were incubated in 3 μM Fura-2 AM (Molecular Probes, Eugene, OR, USA) in HBSS containing 0.2% pluronic acid F-127 at room temperature for 1 hour, washed with HBSS, and left in this solution at room temperature in the dark for 1 hour so the cells could stabilize. Ratiometric Ca2+imaging was performed using an inverted fluorescent microscope (Olympus IX71, Olympus, Waltham, MA, USA). Coverslips were placed on epifluorescence microscope and continuously perfused (5 ml/min) with HBSS. Fura-2 was excited alternately with ultraviolet light at 340 and 380 nm; and the fluorescence emission was detected at 510 nm using a computer-controlled monochromator. Fluorescent images and ratios were acquired every 1 second. Wavelength selection, timing of excitation, and the acquisition of images were controlled using the program Slidebook 5.0 (Intelligent Imaging Innovation, Denver, CO, USA) running on a personal computer. Digital images were stored for off-line analysis. One coverslip usually contained 20–30 DRG neurons/microscopic field at 40x magnification. Drugs were diluted in HBSS and delivered via bath application using a gravity-driven system (infusion rate of 5 ml/minute). The drugs used were: H2O2 (1 mM, 20 seconds), capsaicin (0.5 μM, 20 seconds), and allyl isothiocyanate (AITC, Sigma-Aldrich, 100 μM, 20 seconds). Cells were considered responsive if their F340/F380 ratio increased by > 20% during the drug application. All experiments were performed at room temperature. At the end of each protocol, 50 mM KCl extracellular solution was used to depolarize neurons, thereby allowing for identification of viable neurons from non-neuronal cells or non-functioning neurons.
TRPA1 knockout mice
Experiments were conducted on 4–6 month old male and female TRPA1 knockout (TRPA1 −/−) mice (B6.129P-Trpa1tm1Kykw/J; Jackson Laboratories, Bar Harbor, ME, USA) in which the exons essential for the TRPA1 gene function were deleted (Kwan KY et al Neuron 2006).27 The TRPA1 −/− mouse line was created on a C57BL/6J background. Nociceptive behavior after injection of H2O2 (30 mM, 0.05 ml) into the gastrocnemius muscle was measured for 60 minutes in both TRPA1 −/− and +/+ mice, in a similar manner as described above for rats.
Intracellular Ca2+ transient evoked by H2O2 (1 mM, 20 seconds) was examined in the lumbar DRG neurons from TRPA1 −/− and +/+ mice using Ca2+ imaging. The Ca2+ imaging protocols were similar to those described above for rats.
Statistical Analysis
Statistics were conducted using GraphPad Prism (version 5.04, Graphpad Software, Inc., CA, USA). Kolmogorov-Smirnov tests were applied to all continuous data sets to test for normality. Parametric tests were used for normally distributed, continuous data, which were expressed as mean and standard deviation. Nonparametric analyses were used for non-normally distributed and categorical data. Non-normally distributed data were presented as median and interquartile range. All analyses were two-tailed. Nociceptive behavioral data in rats were compared using two-way ANOVA followed by post-hoc t-test with Bonferroni correction, one-way ANOVA with Tukey’s post-hoc test, or unpaired t-test. CPA experimental data were analyzed using paired t-test or one-way ANOVA with Tukey’s post-hoc test. In vivo extracellular recording data were compared using Kruskal-wallis test. The data for behavioral tests after nerve blockade with capsaicin were compared using two-way ANOVA with repeated measured on one factor followed by Bonferroni’s post-hoc test. The data from the TRPA1 −/− mice experiments were compared using unpaired t-test and Chi-square test. Values of P < 0.05 were considered significant.
Results
Nociceptive behavior after injection of H2O2
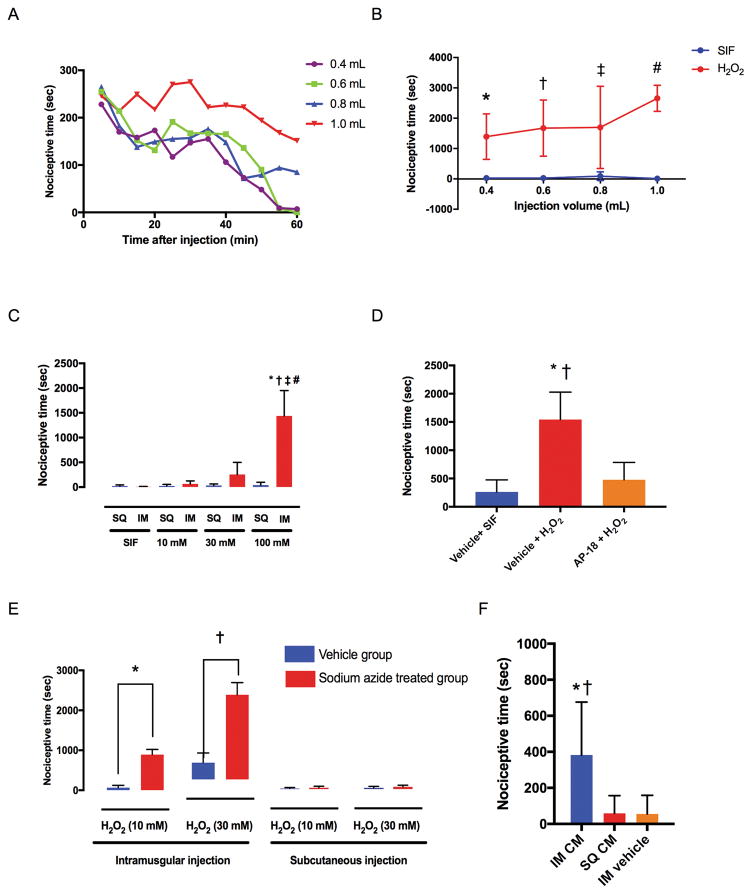
Injection of 100 mM H2O2 into the gastrocnemius muscle produced significantly greater nociceptive behavior at all volumes tested, compared to intramuscular injection of SIF (Fig. 1A and B). Nociceptive behavior was the greatest immediately after injection of H2O2, and subsided by the end of 60-minute period in the 0.4 and 0.6 ml groups, but did not in the 0.8 and 1.0 ml groups (Fig. 1A). Total time of nociceptive behavior (summarized data from Fig. 1A) was 1393 ± 750 (P = 0.0035 vs. SIF), 1675 ± 926 (P = 0.0004 vs. SIF), 1698 ± 1353 (P = 0.0005 vs. SIF), and 2658 ± 430 seconds (P < 0.0001 vs. SIF) respectively, after injection of 0.4, 0.6, 0.8, and 1.0 ml of 100 mM H2O2 (Fig. 1B). Nociceptive time after injection of 0.6 ml of H2O2 into the gastrocnemius muscle was 7 ± 7, 64 ± 60, 255 ± 245, and 1436 ± 513 seconds for SIF, 10, 30, and 100 mM of H2O2, respectively, (Fig. 1C). Intramuscular injection of 100 mM H2O2 produced significantly greater nociceptive behavior compared to the lower concentrations of H2O2 (P < 0.0001 vs. 30 mM group and P < 0.0001 vs. 10 mM group) or the SIF group (P < 0.0001). Nociceptive time after injection of H2O2 into the subcutaneous tissue was 21 ± 23, 24 ± 32, 32 ± 34, and 40 ± 58 seconds respectively, for SIF, 10, 30, and 100 mM of H2O2 (Fig. 1C). Nociceptive time after subcutaneous injection of H2O2 (10, 30, and 100 mM) was not significantly different from that after SIF injection (P > 0.9999 vs. subcutaneous SIF for all concentration groups). Intramuscular injection of H2O2 produced greater nociceptive behavior compared to subcutaneous injection in the 100 mM group (P < 0.0001), but not in the 10 (P > 0.9999) or 30 (P > 0.9999) mM groups. Pre-treatment with locally injected TRPA1 antagonist AP-18 significantly reduced nociceptive behavior induced by intramuscular injection of H2O2, compared with vehicle (P = 0.0001) (Fig. 1D). Nociceptive time after intramuscular injection of 10 and 30 mM of H2O2 was significantly greater in sodium azide-treated (10 mg/kg) rats, compared to that in the vehicle-treated rats (P = 0.0286 vs. vehicle group for 10 mM H2O2 and P = 0.0286 vs. vehicle group for 30 mM H2O2) (Fig. 1E). Nociceptive time after subcutaneous injection of 10 and 30 mM of H2O2 was not different between the sodium azide group and the vehicle group (Fig. 1E). Nociceptive time after intramuscular injection of 30 mM of cinnamaldehyde was significantly greater compared with the subcutaneous injection of cinnamaldehyde or intramuscular injection of vehicle (Fig. 1F).
Fig. 1. Nociceptive behavior in rats as total time spent flinching, lifting and licking of the hind leg during a 60-minute period.
(A) Time-course of the nociceptive behavior after various volumes of 100 mM H2O2 were injected into the gastrocnemius muscle. Data were collected in 5-minute bins from six animals in each group. Data points show the average nociceptive time in 5-minute bins, and error bars were omitted for clarity. (B) Total time of nociceptive behavior over 60 minutes after various volumes of 100 mM H2O2 were injected into the gastrocnemius muscle (summarized data from Fig. 1A). Each group contained six rats. * P = 0.0035, † P = 0.0004, ‡ P = 0.0005, # P < 0.0001 compared with the SIF injection group by two-way ANOVA (interaction factor: F3, 40 = 2.227, P = 0.0999, Injection volume factor: F3, 40 = 2.022, P = 0.1263, Group factor: F1, 40 = 91.61, P < 0.0001) followed by post-hoc t-test with Bonferroni’s correction. (C) Spontaneous nociceptive behavior after various concentrations of 0.6 ml H2O2 were injected subcutaneously (SQ) or intramuscularly (IM). Each group contained six rats. * P < 0.0001 compared with the IM SIF injection group, † P < 0.0001 compared with IM 10 mM H2O2 injection group, ‡ P < 0.0001 compared with IM 30 mM H2O2 injection group, # P < 0.0001 compared with SQ 100 mM H2O2 injection group by one-way ANOVA (F7, 40 = 34.92, P < 0.0001) followed by post-hoc Tukey’s test. (D) Effects of local pre-injection of a TRPA1 antagonist AP-18 (50 mM, 0.3 ml) on nociceptive behavior caused by intramuscular injection of H2O2 (100 mM, 0.3 ml). Therefore, the total injection volume was 0.6 ml, and the final concentration was 25 mM for AP-18, and 50 mM for H2O2. Vehicle + SIF group (n=7); Vehicle + H2O2 group (n=7); AP-18 + H2O2 group (n=6). * P < 0.0001 compared with vehicle + SIF injection group, † P = 0.0001 compared with AP-18 + H2O2 injection group by one-way ANOVA (F2, 17 = 25.65, P < 0.0001) followed by post-hoc Tukey’s test. Vehicle + SIF and Vehicle + H2O2 control groups are the same control data presented in Fig. 5B in our previous study.12 (E) Total nociceptive time during the 60-minute period after injection of H2O2 into the gastrocnemius muscle or subcutaneous tissue overlying gastrocnemius muscle in sodium azide-treated rats. Sodium azide (10 mg/kg) was administered intraperitoneally 30 minutes before the injection of H2O2. Each group contained six rats. *, † P < 0.0001 compared with vehicle group by unpaired t-test. All data are expressed as means ± SEM. (F) Total nociceptive time during the 60-minute period after cinnamaldehyde (CM, 30 mM, 0.6 ml) injection into the gastrocnemius muscle (IM CM) or subcutaneous tissue (SQ CM) overlying gastrocnemius muscle in rats. Each group contained seven rats. * P = 0.0129 compared with the SQ CM group, † P = 0.0121 compared with IM vehicle group by one-way ANOVA (F2, 18 = 6.923, P = 0.0059) followed by post-hoc Tukey’s test. All data are expressed as means ± SD.
CPA after H2O2 injection
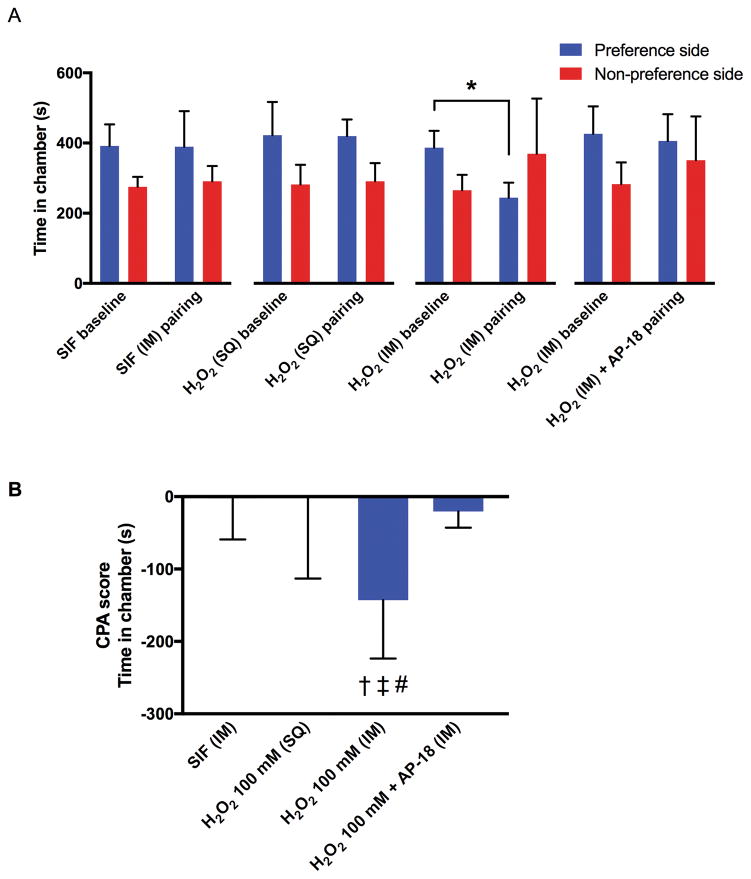
In the first series of CPA experiments, one rat that spent more than 80% time in one chamber during the preconditioning baseline session was excluded. Rats spent significantly less time in their initially preferred chamber after conditioning with intramuscular injection of 0.6 ml of 100 mM H2O2 (P = 0.0075), but not after conditioning with intramuscular injection of SIF, subcutaneous injection of H2O2 or intramuscular co-injection of AP-18 and H2O2 (Fig. 2A). The CPA score of the intramuscular H2O2 group (−143 ± 81 seconds) was significantly greater than those of the intramuscular SIF group (−3 ± 57 seconds; P = 0.0199), subcutaneous H2O2 group (−2 ± 111 seconds; P = 0.0194), and the co-injection of AP-18 and H2O2 group (−21 ± 22 seconds; P = 0.0479) (Fig. 2B).
Fig. 2. The effects of intramuscular (IM) or subcutaneous (SQ) injection of H2O2 (100 mM, 0.6 ml) on conditioned place aversion (CPA) in rats.
Each column represents the time spent in the preferred and non-preferred chambers during the pre- and post-conditioning sessions (A), and the CPA scores (B). For the intramuscular co-injection of H2O2 and AP-18, sequential injections of AP-18 (50 mM, 0.3 ml) followed by H2O2 (200 mM, 0.3 ml), were made into the gastrocnemius muscle. Therefore, the total injection volume was 0.6 ml, and the final concentration of H2O2 was 100 mM. Each group contained six rats. All data are expressed as means ± SD. * P = 0.0075 by paired t-test. † = 0.0199 compared with the SIF group, ‡ P = 0.0194 compared with the subcutaneous injection of H2O2 group, # P = 0.0479 compared with the intramuscular co-injection of H2O2 and AP-18 group by one-way ANOVA (F3, 20 = 4.884, P = 0.0104) followed by post-hoc Tukey’s test.
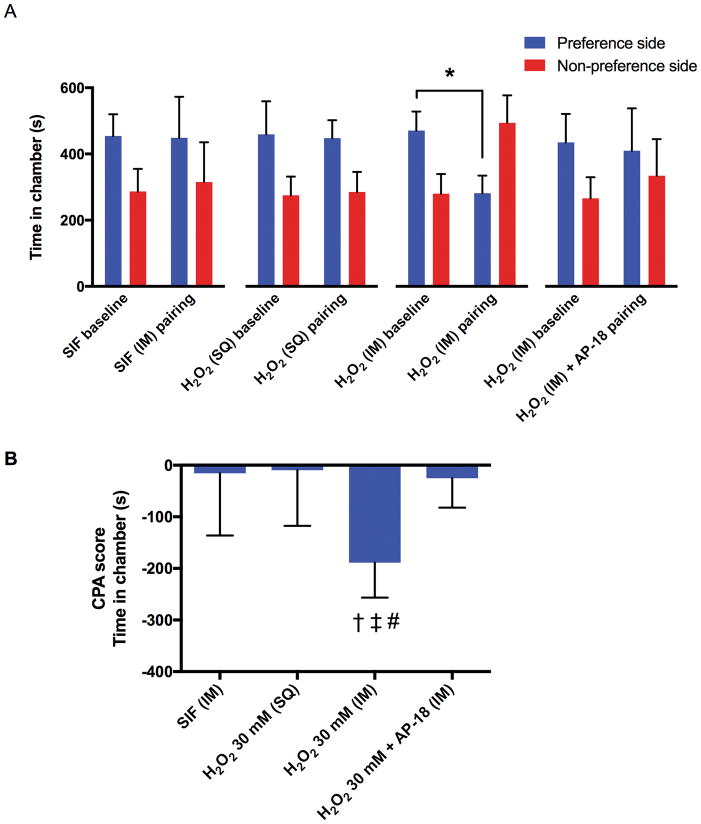
The second series of CPA experiments used a lower concentration of H2O2 (30 mM) that did not cause significant flinching or lifting. Compared to the baseline, rats spent less time in their initially preferred chamber after conditioning with intramuscular injection of H2O2 (P = 0.0011), but not after conditioning with intramuscular injection of SIF, subcutaneous injection of H2O2 or intramuscular co-injection of AP-18 and H2O2 (Fig. 3A). The CPA score of the intramuscular H2O2 group (−189 ± 68 seconds) was significantly greater compared with the intramuscular SIF group (−15 ± 121 seconds; P = 0.0036), subcutaneous H2O2 group (−9 ± 108 seconds; P = 0.0026), and the co-injection of AP-18 and H2O2 group (−25 ± 57 sec seconds; P = 0.0048) (Fig. 3B).
Fig. 3. Effects of intramuscular (IM) or subcutaneous (SQ) injection of 30 mM H2O2 on conditioned place aversion (CPA) in rats.
Each column represents the time spent in the preferred and non-preferred chambers during the pre- and post-conditioning sessions (A), and the CPA scores (B) of the intramuscular injection of SIF group (n = 8), subcutaneous injections of H2O2 (30 mM, 0.6 ml) group (n = 8), and intramuscular injection of H2O2 (30 mM, 0.6 ml) group (n = 8), intramuscular sequential-injection of AP-18 (50 mM, 0.3 ml) followed by H2O2 (60 mM, 0.3 ml) group (n = 9). All data are expressed as means ± SD. * P = 0.0011 by paired t-test. † = 0.0036 compared with the SIF group, ‡ P = 0.0026 compared with the subcutaneous injection of H2O2 group, # P = 0.0048 compared with the intramuscular co-injection of H2O2 and AP-18 group by one-way ANOVA (F3, 29 = 7.212, P = 0.0009) followed by post-hoc Tukey’s test.
In vivo extracellular DHN recording
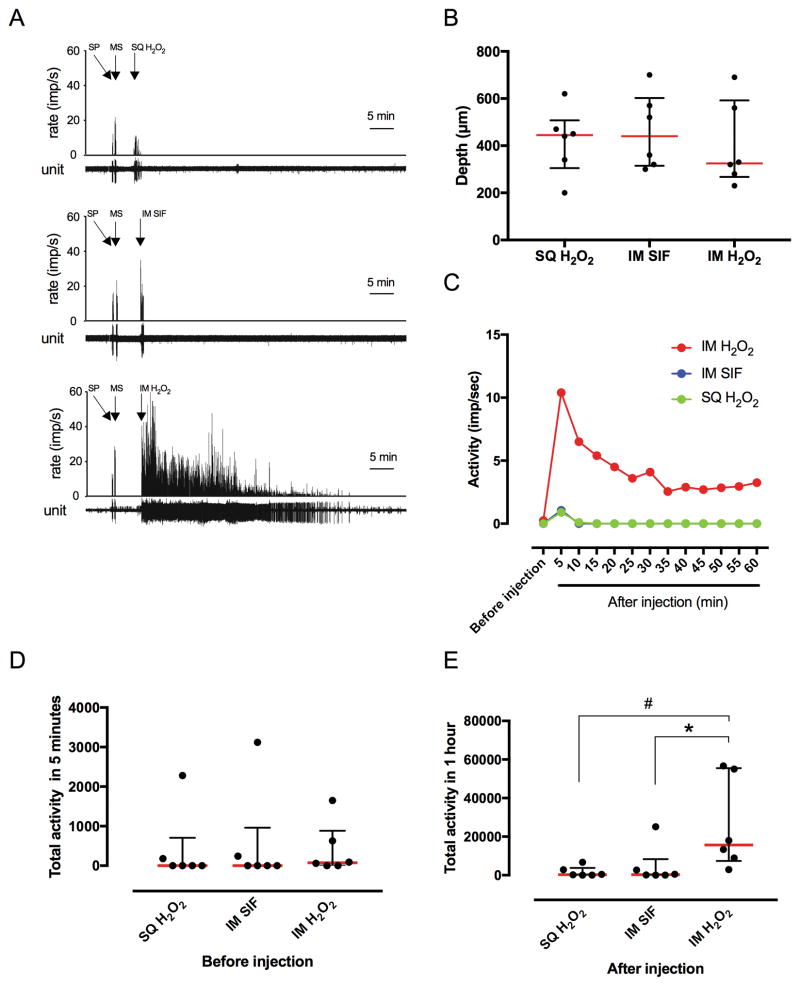
Extracellular single-unit activities were recorded from18 DHNs; an example of 3 neurons’ responses are shown in Fig 4A. All the neurons had receptive fields in both the skin overlying the gastrocnemius region and in the gastrocnemius muscle based on response to pinch. There was no difference in depth of DHNs among groups (Fig. 4B). The time-course trend of changes in activity of the DHNs after injection of H2O2 are shown in Fig. 4C. Activities of the DHNs were greatly increased in the intramuscular H2O2 injection (100 mM, 0.6 ml) group during the 60-minute period after injection (Fig. 4C). Activity in the intramuscular SIF and the subcutaneous H2O2 injection groups was transiently increased early after injection, and then returned to the pre-injection level (Fig. 4C). There was no difference in total activity of DHNs among groups before injection (Fig. 4D). Total activity of DHNs for 60 minutes after injection in intramuscular H2O2 group was greater compared with the intramuscular SIF group (P = 0.0482) and the subcutaneous H2O2 group (P = 0.0261) (Fig. 4E). Similar to the nociceptive behaviors, DHN activities were greatest early after injection and abated towards the end of the 60-minute period.
Fig. 4. Effects of intramuscular injection of H2O2 on activity of dorsal horn neurons (DHNs).
(A) Example recordings of neurons after subcutaneous (SQ) injection of H2O2 (upper panel), intramuscular (IM) injection of SIF (middle panel), and IM injection of H2O2 (lower panel). Arrows represent pinching the skin (SP), squeezing gastrocnemius muscle (MS), and the time of injections. Bin width = 1 second. Unit represents each single action potential. (B) The average depth from the surface of spinal cord in which DHNs were recorded in each groups. Data are expressed as median with interquartile range. (C) Time-course trend of changes in activity of the DHNs after injection of H2O2 or vehicle. Data points show the median impulse/second for six neurons in each group in 5-minute bins. (D) Total activity in 5 minutes of the DHNs prior to injection. Data are expressed as median with interquartile range. Each group contained 6 neurons. (E) Total activity of the DHNs during 60 minutes after injection. Data are expressed as median with interquartile range. Each group contained 6 neurons. * P = 0.0482, # P = 0.0261 by Kruskal-Wallis test.
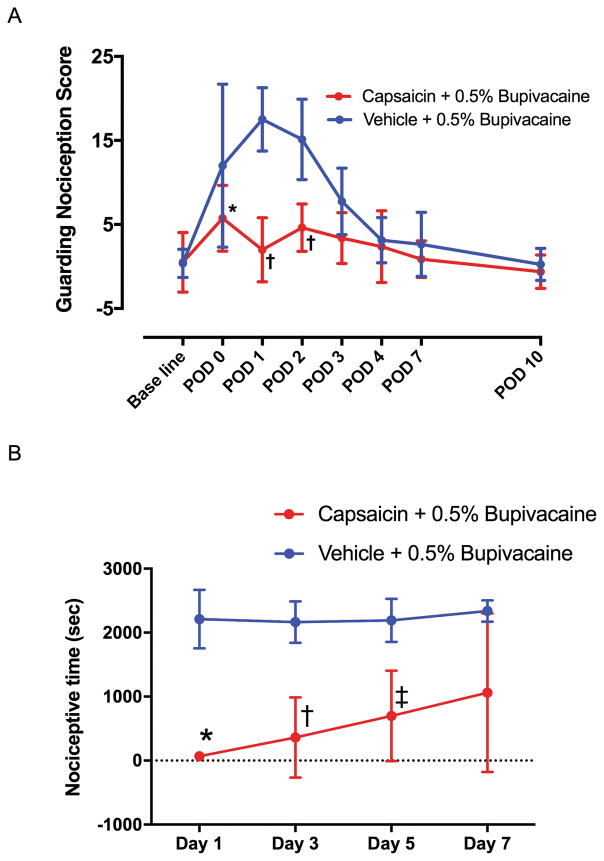
Sciatic nerve block with capsaicin
Capsaicin 0.05% in 0.5% bupivacaine applied to the sciatic nerve the day before incision decreased the guarding score on postoperative day 0 (P = 0.0202), day 1 (P < 0.0001) and day 2 (P < 0.0001) compare with vehicle + 0.5% bupivacaine group (Fig. 5A), in agreement with previous studies.10 In vehicle + 0.5% bupivacaine group, H2O2 injection evoked nociceptive behaviors when injected 1, 3, 5 and 7 days after nerve block (Fig. 5B). Injections of H2O2 on alternating days elicited the same response, thus not producing tachyphylaxis. Nerve block with 0.05% capsaicin in 0.5% bupivacaine significantly reduced nociceptive behaviors induced by H2O2 injection into the gastrocnemius muscle through 5 days (Fig. 5B). Recovery of responses to injections of H2O2 was evident by the seventh day after capsaicin nerve block.
Fig. 5. Effect of sciatic nerve block with capsaicin on behavior after skin + deep tissue plantar incision and intramuscular injection of H2O2.
(A) Effect of sciatic nerve block with capsaicin on guarding behavior after skin + deep tissue plantar incision. Percutaneous sciatic nerve block was performed using 0.05% capsaicin plus 0.5% bupivacaine or 0.5% bupivacaine in vehicle. The results are presented as means ± SD for eight rats in each group. Two-way ANOVA with repeated measures on one factor (interaction factor: F7, 98 = 7.913, P < 0.0001) followed by Bonferroni’s post hoc test was used to compare the cumulative pain score at each time point between the groups. * P = 0.0202, † P < 0.0001 compared with the vehicle + 0.5% bupivacaine group at each time point. POD = postoperative day. (B) Effect of peripheral nerve block with 0.05% capsaicin on nociceptive behavior induced by injection of H2O2 into the gastrocnemius muscle. H2O2 (100 mM, 0.6 ml) was injected into the gastrocnemius muscle at various time points (1, 3, 5, and 7 days) following the nerve block, and the total time spent flinching, lifting, and licking was recorded for 60 minutes. Data are presented as mean ± SD. Capsaicin (0.05%) + 0.5% Bupivacaine group contained 5 rats and vehicle group contained 6 rats. * P = 0.0004, † P = 0.0029, ‡ P = 0.0168 compared with the vehicle + 0.5% bupivacaine group by two-way ANOVA with repeated measured on one factor (interaction factor: F3, 27 = 0.9405, P = 0.4348, Time factor: F3, 27 = 1.554, P = 0.2233, Group factor: F1, 9 = 22.56, P = 0.0010) followed by Bonferroni’s post-hoc test.
Ca2+ imaging
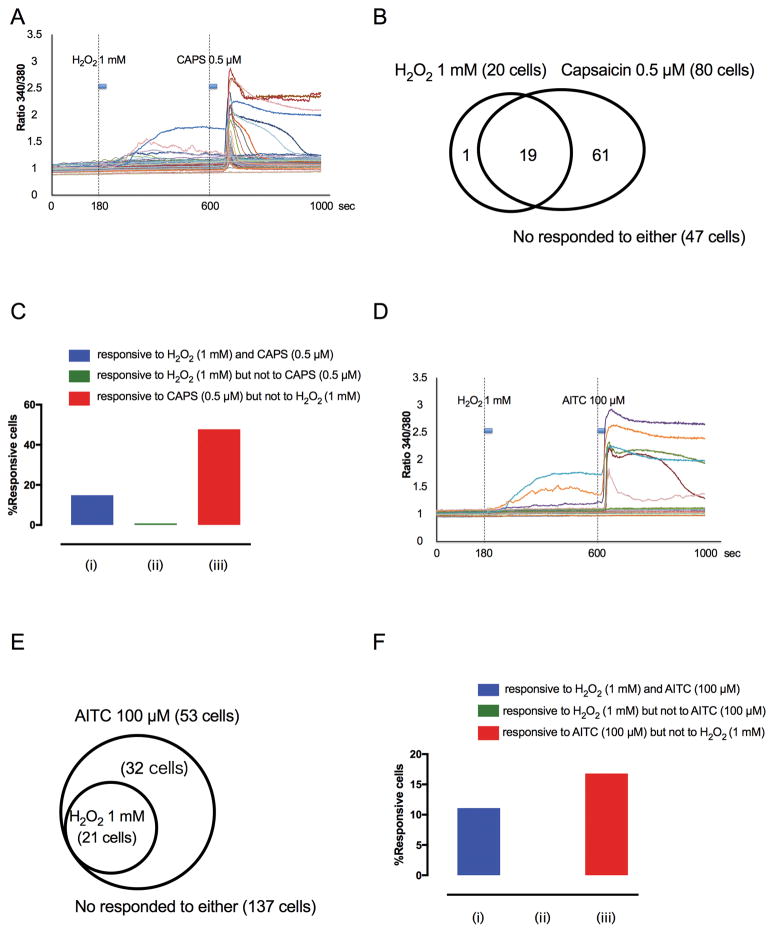
After evaluating the hindlimb responses to H2O2 injection, Ca2+ transients in 128 lumbar DRG neurons were recorded during the sequential application of 1 mM H2O2 and 0.5 μM capsaicin. The example traces are shown in Fig. 6A. Twenty of 128 total neurons (16%) responded to 1 mM H2O2, and most of these H2O2-responsive neurons (95%, 19 of 20) also responded to 0.5 μM capsaicin (Fig. 6B and C). Sixty one of 128 total neurons (48%) responded to 0.5 μM capsaicin, but not to 1 mM H2O2 (Fig. 6B and C).
Fig. 6. DRG neuronal responses to H2O2 (1 mM), capsaicin (0.5 μM) and allyl isothiocyanate (AITC; 100 μM).
(A) Example traces of response to H2O2 and capsaicin (CAPS; 20-second application of each) of individual, dissociated lumbar 3–5 DRG neurons during Fura-2 Ca2+ imaging. (B) Venn diagrams illustrating the overlap of cells exhibiting Ca2+ transients in response to H2O2 and capsaicin. (C) Percentage of cells that responded both to H2O2 and capsaicin (blue column), that responded to H2O2 but not to capsaicin green column), and that responded to capsaicin but not to H2O2 (red column). (D) Example traces in response to H2O2 and AITC (20-second duration of each) of individual, dissociated lumbar 3–5 DRG neurons during Fura-2 Ca2+ imaging. (E) Venn diagrams illustrating the overlap of cells exhibiting Ca2+ transients in response to H2O2 and AITC. (F) Percentage of cells that responded to both H2O2 and AITC (blue column), that responded to H2O2 but not to AITC (green column), and that responded to AITC but not to H2O2 (red column).
Ca2+ transients were recorded during the sequential application of 1 mM H2O2 and 100 μM of the TRPA1 activator, AITC, in 190 neurons from 4 rats. The example traces are shown in Fig. 6D. Twenty one out of 190 DRG neurons (11%) responded to both 1 mM H2O2 and 100 μM AITC, and all the H2O2-responsive lumbar DRG neurons also responded to AITC (100%, 21 of 21) (Fig. 6E). Thirty two of 190 neurons (17%) responded to AITC but not to H2O2 (Fig. 6E and F).
TRPA1 knockout mice
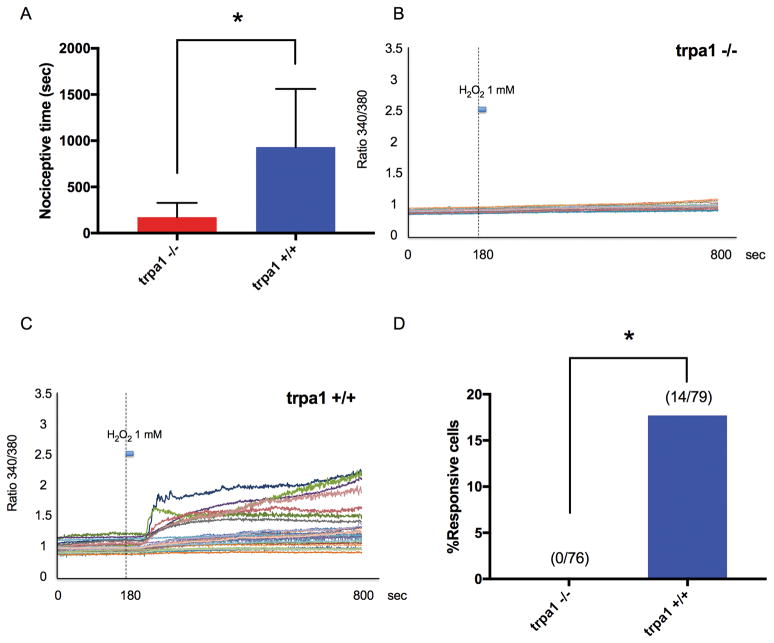
Total time of nociceptive behavior after intramuscular injection of H2O2 was less in TRPA1 −/− mice, compared with TRPA1 +/+ mice (P = 0.0051, Fig. 7A). Ca2+ transients were recorded from 76 lumbar DRG neurons from 3 TRPA1 −/− mice and 79 lumbar DRG neurons from 3 TRPA1 +/+ mice. The example traces of Ca2+ transients of DRG neurons from TRPA1 −/− and +/+ mice during the application of 1 mM H2O2 are shown in Fig. 7B and 7C, respectively. While 14 or 79 DRG neurons (18%) from TRPA1 +/+ mice were activated by 1 mM H2O2, none of 76 neurons from TRPA1 −/− mice were activated by 1 mM H2O2 (P < 0.0001, Fig. 7D).
Fig. 7. H2O2-induced nociceptive behavior and Ca2+ transients in dorsal root ganglia (DRG) neurons induced by H2O2 in TRPA1 knockout (TRPA1 −/−) and wild-type (TRPA1 +/+) mice.
(A) Total time of nociceptive behavior over 60 minutes after H2O2 (0.05 ml, 30 mM) was injected into the gastrocnemius muscle. Data were obtained from eight animals in each group. * P = 0.0051 compared with the trpa1 +/+ group by unpaired t-test. Data are expressed as means ± SD. (B) Example traces in response to H2O2 (1 mM; 20-second duration) of individual, dissociated lumbar 3–5 DRG neurons from TRPA1 −/− mice during Fura-2 Ca2+ imaging. (C) Example traces in response to H2O2 (1 mM; 20-second duration) of individual, dissociated lumbar 3–5 DRG neurons from TRPA1 +/+ mice during Fura-2 Ca2+ imaging. (D) Summary of the percentage of cells responding to 1 mM H2O2. Numbers in parentheses represent the number of cells responding and total number of cells tested. * P < 0.0001 compared with the TRPA1 +/+ mice group by Chi-square test.
Discussion
In the present study, we have demonstrated that intramuscular injection of H2O2, but not subcutaneous injection of H2O2, produced nociceptive and aversive behaviors via the TRPA1 receptor. Intramuscular injection of H2O2 caused a sustained increase in activity of DHNs that had a similar time course as the behavioral responses. Using Ca2+ imaging, H2O2 activated a subset of lumbar DRG neurons that also responded to AITC, a TRPA1 agonist, and to capsaicin, a TRPV1 agonist. Capsaicin nerve block inhibited guarding behavior after plantar incision, and the nociceptive response after intramuscular injection of H2O2. The actions of intramuscular H2O2 through the TRPA1 receptor on behavior and on Ca2+ imaging in lumbar DRG neurons were confirmed in TRPA1 knockout mice.
Nociceptive behaviors after intramuscular Injection of H2O2
Several studies have shown that H2O2 activates sensory pathways. H2O2 induces asthma when inhaled20 and emesis when ingested by activating vagal afferent sensory fibers.28 H2O2 may also contribute to nociceptive behaviors in animal models of acute joint pain,29,30 and ischemia-reperfusion injury.31 In previous studies by others, hindpaw injections of H2O2 produced only brief nociceptive responses, and specific subcutaneous versus deep muscle tissue injections were not characterized.32 When applied to the rat skin-saphenous nerve preparation in vitro, H2O2 had only brief and limited excitatory effects on C-fiber terminals.33 In contrast, in vivo injection of H2O2 markedly activated all recorded group IV chemosensitive afferents innervating the rat tibialis anterior muscle.34 Intramuscular injection of H2O2 induced place aversion, which was blocked by a TRPA1 antagonist (Fig. 2 and 3). Recent studies have shown the CPA test to be an effective method to measure spontaneous nociception.8,22 H2O2 produced aversion in doses that did not produce overt nociceptive behaviors (Fig. 1C vs. Fig. 3); thus, CPA appears to be more sensitive towards detecting nociception by intramuscular H2O2 injection in rats.
TRPA1 responses and TRPV1-expressing neurons
We have previously reported that plantar incision-induced guarding nociception is inhibited by pre-treatment with capsaicin infiltration9,10 and with capsaicin application to the branches of the sciatic nerve.10 We also showed that guarding behavior requires deep muscle tissue injury.5 In the present study, similar to our previous studies, guarding behavior was inhibited by sciatic nerve block using a mixture of bupivacaine and capsaicin. The sciatic nerve block using the mixture of bupivacaine and capsaicin also inhibits the nociceptive response to H2O2 injection into the gastrocnemius muscle. The dilute capsaicin solution we have used does not cause sensory fiber degenerative loss; Rather, it depletes neuropeptides and impairs heat and chemosensitivity, but not mechanosensitivity.9 These data suggest that the analgesic effect of capsaicin-induced sensory fiber desensitization is associated with temporary loss of responses chemical stimuli, to both lactic acid and to H2O2.
Our results from Ca2+ imaging of lumbar DRG neurons demonstrating chemosensitivity of TRPV1-expressing DRG neurons to H2O2 via TRPA1 are consistent with a previous studies,35 First, only AITC-responsive neurons responded to H2O2 and responses to H2O2 were absent in TRPA1 knockout mice.19,36 Fewer neurons responded to 1 mM H2O2 than to 100 μM AITC in the present study, but H2O2 concentrations higher than 1 mM will likely produce greater responses.37 Second, all H2O2-responsive lumbar DRG neurons were capsaicin-sensitive.36 Thus, the capsaicin treatment that inhibits guarding nociception is associated with loss of responses to H2O2 and lactic acid both of which are acutely increased in incised muscle.12,38,39 H2O2 is one of the reactive oxygen species with diverse biologic actions40 including wound healing.16,18,41 The greater responses to H2O2 injection into muscle compared to subcutaneous injection may, in part, explain why incision that includes deep muscle produces spontaneous activity in nociceptive pathways but skin incision alone does not.
Conclusion
This study demonstrates that in vivo intramuscular injection of H2O2 into the hindlimb evoked nociceptive behavior and activated lumbar spinal DHNs. H2O2 also increased intracellular Ca2+ in lumbar DRG neurons in vitro via the TRPA1 receptor. Guarding after deep tissue incision and nociceptive behavior after intramuscular injection of H2O2 were reduced by nerve blockade with capsaicin. Together, these findings indicate that reduced guarding after incision of deep plantar tissue by a TRPA1 antagonist like HC-03003112 and capsaicin nerve block may in part be related to reduced responses to reactive oxygen species like H2O2 in incised deep tissue. Incision that includes muscle may be more painful than skin incision because of greater responses to reactive oxygen species like H2O2 in muscle.
Acknowledgments
The authors are grateful to Durga P. Mohapatra Ph.D. (Department of Anesthesiology, Washington University, St. Louis MO, USA) for providing TRPA1 −/− mice. The authors also acknowledge Alberto Subieta B.S. (Department of Anesthesia, University of Iowa, Iowa City, IA, USA), Brandt Uitermarkt M.A. (Department of Anesthesia, University of Iowa, Iowa City, IA, USA), He Gu (Department of Anesthesia, University of Iowa, Iowa City, IA, USA) and John Kennedy M. D. (Department of Anesthesia, University of Iowa, Iowa City, IA, USA) for technical assistance.
Financial support: In part supported by National Institutes of Health, Bethesda, Maryland NS092851 to Y.M.U. In part supported by the Foundation of Anesthesia Education and Research to SK.
Footnotes
Competing Interests: The authors declare no competing interests.
References
- 1.Gan TJ, Habib AS, Miller TE, White W, Apfelbaum JL. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin. 2014;30:149–60. doi: 10.1185/03007995.2013.860019. [DOI] [PubMed] [Google Scholar]
- 2.Brennan TJ. Pathophysiology of postoperative pain. Pain. 2011;152:S33–S40. doi: 10.1016/j.pain.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang S, Brennan TJ. Chemosensitivity and Mechanosensitivity of Nociceptors from Incised Rat Hindpaw Skin. Anesthesiology. 2009;111:155–64. doi: 10.1097/ALN.0b013e3181a16443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu J, Brennan TJ. Comparison of skin incision vs. skin plus deep tissue incision on ongoing pain and spontaneous activity in dorsal horn neurons. Pain. 2009;144:329–39. doi: 10.1016/j.pain.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu J, Brennan TJ. Guarding Pain and Spontaneous Activity of Nociceptors after Skin versus Skin Plus Deep Tissue Incision. Anesthesiology. 2010;112:153–64. doi: 10.1097/ALN.0b013e3181c2952e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu C, Gavva NR, Brennan TJ. Effect of AMG0347, a Transient Receptor Potential Type V1Receptor Antagonist, and Morphine on Pain Behavior after Plantar Incision. Anesthesiology. 2008;108:1100–8. doi: 10.1097/ALN.0b013e31817302b3. [DOI] [PubMed] [Google Scholar]
- 7.Spofford CM, Ashmawi H, Subieta A, Buevich F, Moses A, Baker M, Brennan TJ. Ketoprofen Produces Modality-Specific Inhibition of Pain Behaviors in Rats After Plantar Incision. Anesth Analg. 2009;109:1992–9. doi: 10.1213/ANE.0b013e3181bbd9a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zahn PK, Gysbers D, Brennan TJ. Effect of Systemic and Intrathecal Morphine in a Rat Model of Postoperative Pain. Anesthesiology. 1997;86:1066–77. doi: 10.1097/00000542-199705000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Kang S, Wu C, Banik RK, Brennan TJ. Effect of capsaicin treatment on nociceptors in rat glabrous skin one day after plantar incision. Pain. 2010;148:128–40. doi: 10.1016/j.pain.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamalainen MM, Subieta A, Arpey C, Brennan TJ. Differential effect of capsaicin treatment on pain-related behaviors after plantar incision. J Pain. 2009;10:637–45. doi: 10.1016/j.jpain.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang S, Jang JH, Price MP, Gautam M, Benson CJ, Gong H, Welsh MJ, Brennan TJ. Simultaneous Disruption of Mouse ASIC1a, ASIC2 and ASIC3 Genes Enhances Cutaneous Mechanosensitivity. PLoS ONE. 2012;7:e35225–12. doi: 10.1371/journal.pone.0035225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugiyama D, Kang S, Brennan TJ. Muscle Reactive Oxygen Species (ROS) Contribute to Post-Incisional Guarding via the TRPA1 Receptor. PLoS ONE. 2017;12:e0170410–7. doi: 10.1371/journal.pone.0170410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Julius D. TRP channels and pain. Ann Rev Cell Dev Biol. 2013;29:355–84. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- 14.Sun W, Wang Z, Cao J, Cui H, Ma Z. Cold stress increases reactive oxygen species formation via TRPA1 activation in A549 cells. Cell Stress Chaperones. 2015;21:367–72. doi: 10.1007/s12192-015-0663-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viana F. TRPA1 channels: molecular sentinels of cellular stress and tissue damage. J Physiol. 2016;594:4151–69. doi: 10.1113/JP270935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–9. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lingqiang GM, Scandalios JG. Original Contribution. Free Radic Biol Med. 2000;28:1182–90. doi: 10.1016/s0891-5849(00)00212-4. [DOI] [PubMed] [Google Scholar]
- 18.Roy S, Khanna S, Nallu K, Hunt TK, Sen CK. Dermal Wound Healing Is Subject to Redox Control. Mol Ther. 2005;13:211–20. doi: 10.1016/j.ymthe.2005.07.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawada Y, Hosokawa H, Matsumura K, Kobayashi S. Activation of transient receptor potential ankyrin 1 by hydrogen peroxide. Eur J Neurosci. 2008;27:1131–42. doi: 10.1111/j.1460-9568.2008.06093.x. [DOI] [PubMed] [Google Scholar]
- 20.Bessac BF, Sivula M, Hehn von CA, Escalera J, Cohn L, Jordt S-E. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest. 2008;118:1899–910. doi: 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious Cold Ion Channel TRPA1 Report Is Activated by Pungent Compoundsand Bradykinin. Neuron. 2004;41:849–57. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 22.Kaneko T, Kaneda K, Ohno A, Takahashi D, Hara T, Amano T, Ide S, Yoshioka M, Minami M. Activation of adenylate cyclase-cyclic AMP-protein kinase A signaling by corticotropin-releasing factor within the dorsolateral bed nucleus of the stria terminalis is involved in pain-induced aversion. Eur J Neurosci. 2016;44:2914–24. doi: 10.1111/ejn.13419. [DOI] [PubMed] [Google Scholar]
- 23.King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12:1364–6. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okun A, DeFelice M, Eyde N, Ren J, Mercado R, King T, Porreca F. Transient inflammation-induced ongoing pain is driven by TRPV1 sensitive afferents. Mol Pain. 2011;7:4. doi: 10.1186/1744-8069-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pogatzki EM, Vandermeulen EP, Brennan TJ. Effect of plantar local anesthetic injection on dorsal horn neuron activity and pain behaviors caused by incision. Pain. 2002;97:151–61. doi: 10.1016/s0304-3959(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 26.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 27.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang D-S, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but Is not essential for hair-cell transduction. Neuron. 2006;50:277–89. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 28.Khan SA, Mary MK, Margaret S, Steven H, Stephen Z. Effectiveness and adverse effects of the use of apomorphine and 3% hydrogen peroxide solution to induce emesis in dogs. J Am Vet Med Assoc. 2012;241:1179–84. doi: 10.2460/javma.241.9.1179. [DOI] [PubMed] [Google Scholar]
- 29.Trevisan G, Hoffmeister C, Rossato MF, Oliveira SM, Silva MA, Silva CR, Fusi C, Tonello R, Minocci D, Guerra GP, Materazzi S, Nassini R, Geppetti P, Ferreira J. TRPA1 receptor stimulation by hydrogen peroxide is critical to trigger hyperalgesia and inflammation in a model of acute gout. Free Radic Biol Med. 2014;72:200–9. doi: 10.1016/j.freeradbiomed.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 30.Trevisan G, Hoffmeister C, Rossato MF, Oliveira SM, Silva MA, Ineu RP, Guerra GP, Materazzi S, Fusi C, Nassini R, Geppetti P, Ferreira J. Transient receptor potential ankyrin 1 receptor stimulation by hydrogen peroxide Is critical to trigger pain during monosodium urate-induced inflammation in rodents. Arthritis Rheum. 2013;65:2984–95. doi: 10.1002/art.38112. [DOI] [PubMed] [Google Scholar]
- 31.So K, Tei Y, Zhao M, Miyake T, Hiyama H, Shirakawa H, Imai S, Mori Y, Nakagawa T, Matsubara K, Kaneko S. Hypoxia-induced sensitisation of TRPA1 in painful dysesthesia evoked by transient hindlimb ischemia/reperfusion in mice. Sci Rep. 2016:1–12. doi: 10.1038/srep23261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keeble JE, Bodkin JV, Liang L, Wodarski R, Davies M, Fernandes ES, de Faria Coelho C, Russell F, Graepel R, Muscara MN, Malcangio M, Brain SD. Hydrogen peroxide is a novel mediator of inflammatory hyperalgesia, acting via transient receptor potential vanilloid 1-dependent and independent mechanisms. Pain. 2009;141:135–42. doi: 10.1016/j.pain.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 33.Kress M, Riedl B, Reeh PW. Effects of oxygen radicals on nociceptive afferents in the rat skin in vitro. Pain. 1995;62:87–94. doi: 10.1016/0304-3959(94)00254-C. [DOI] [PubMed] [Google Scholar]
- 34.Delliaux S, Brerro-Saby C, Steinberg JG, Jammes Y. Reactive oxygen species activate the group IV muscle afferents in resting and exercising muscle in rats. Pflugers Arch - Eur J Physiol. 2009;459:143–50. doi: 10.1007/s00424-009-0713-8. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with aδ/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- 36.Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci. 2008;28:2485–94. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pouokam E, Rehn M, Diener M. Effects of H2O2 at rat myenteric neurones in culture. Eur J Pharmacol. 2009;615:40–9. doi: 10.1016/j.ejphar.2009.04.066. [DOI] [PubMed] [Google Scholar]
- 38.Kim TJ, Freml L, Park SS, Brennan TJ. Lactate Concentrations in Incisions Indicate Ischemic-like Conditions May Contribute to Postoperative Pain. J Pain. 2007;8:59–66. doi: 10.1016/j.jpain.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Young WC, Soo PS, Alberto SR, Brennan TJ. Changes in Tissue pH and Temperature after Incision Indicate Acidosis May Contribute to Postoperative Pain. Anesthesiology. 2004;101:468–75. doi: 10.1097/00000542-200408000-00029. [DOI] [PubMed] [Google Scholar]
- 40.Bekeschus S, Kolata J, Winterbourn C, Kramer A, Turner R, Weltmann KD, Bröker B, Masur K. Hydrogen peroxide: A central player in physical plasma-induced oxidative stress in human blood cells. Free Radic Res. 2014;48:542–9. doi: 10.3109/10715762.2014.892937. [DOI] [PubMed] [Google Scholar]
- 41.Balzer J, Heuer K, Demir E, Hoffmanns MA, Baldus S, Fuchs PC, Awakowicz P, Suschek CV, Opländer C. Non-thermal dielectric barrier discharge (DBD) effects on proliferation and diffedentiation of human fibroblasts are primary mediated by hydrogen peroxide. PLoS ONE. 2015;10:e0144968–18. doi: 10.1371/journal.pone.0144968. [DOI] [PMC free article] [PubMed] [Google Scholar]