Abstract
Objective
We aimed to (i) determine reference values for trochlear morphology and patellofemoral (PF) alignment in adults without MRI-defined PF full thickness cartilage damage or knee pain; and (ii) evaluate dose-response patterns for these measures with prevalent MRI-defined PF structural damage and/or knee pain.
Design
The Framingham Community Cohort is a population-based sample of ambulatory adults aged ≥50 years. We evaluated six morphology and alignment measures using MRI (n=985), and reported reference values (mean ±2SD) in a subsample without MRI-defined PF full thickness cartilage damage or knee pain (n=563). With restricted cubic spline Poisson regression, we evaluated dose-response patterns of each of the six measures with prevalent MRI-defined PF structural damage or joint pain. Our primary outcome was full thickness cartilage damage.
Results
For dose-response curves, prevalence ratios increased monotonically for all measures except patellar tilt, which rose with both lateral and medial tilt. Associations were generally strongest in the lateral PF compartment. Prevalence ratios (PR) for the strongest predictors of full thickness cartilage damage reached clinical relevance (PR>1.5) at sulcus angle ≥135.0°; patellar tilt angle at ≤1.0° and ≥15.0°; and bisect offset ≥57.0%. Lateral trochlear inclination achieved PR>1.5 at ≤23.0° for full thickness cartilage damage with pain.
Conclusions
Sulcus angle, patellar tilt, and bisect offset were most strongly associated with full thickness cartilage damage. Lateral trochlear inclination, patellar tilt and bisect offset had stronger associations with the addition of pain. These findings contribute to better identifying a subset of patients who may benefit from mechanically based interventions.
Keywords: patellofemoral osteoarthritis, alignment, morphology, reference values, normative data, dose-response
Introduction
Patellofemoral osteoarthritis (OA) is present in about 65% of people with knee pain aged 50 years or older1. The condition is associated with knee pain and reduced function2–5, and those with patellofemoral OA are at increased risk of both incidence and progression of tibiofemoral OA6–8. Despite the clinical importance of this sub-type of knee OA, the risk factors and features associated with patellofemoral OA are not well understood9–11.
Trochlear morphology and patellofemoral alignment are associated with patellofemoral OA12. In the axial plane, a shallower/wider trochlea and a patella that is more laterally displaced or tilted are associated with more severe patellofemoral OA features12, and trochlear morphology is associated with patellofemoral alignment13, 14. Theoretically, trochlear dysplasia may cause patellar instability and result in malalignment and/or aberrant movement patterns at the patellofemoral joint. Patellofemoral malalignment reduces contact area which increases joint stress during activities of daily living13, 15, potentially leading to OA. To date, our understanding is limited as to what extent patellofemoral alignment and trochlear morphology contribute - either independently or together - to the onset or progression of patellofemoral OA16,17. Given that patellofemoral alignment may be modifiable with conservative treatments (e.g. bracing, taping, targeted exercises)18–20, a better understanding of morphology and alignment as potential risk factors for patellofemoral OA could inform clinical management.
A critical gap in the literature is the absence of robust reference values for common measures of trochlear morphology and patellofemoral alignment in patellofemoral joints without OA or knee pain, as well as an understanding of their dose-response patterns (i.e. prevalence of patellofemoral OA features across the range of morphology or alignment values)21. A recent systematic review reported that only one of 16 studies investigating patellofemoral morphology and alignment recruited an asymptomatic comparison group12, 18. This is in contrast to other important medical conditions (e.g. femoroacetabular impingement, osteoporosis, cardiovascular disease) in which reference values have been defined for healthy or asymptomatic populations, and cut-points established, to guide interventions or identify those at highest risk of an outcome22–30.
Therefore, in a population-based community cohort of adults aged 50 years or older, we aimed to: (i) determine reference values for trochlear morphology and patellofemoral alignment in individuals without MRI-defined features of patellofemoral OA or knee pain; and (ii) evaluate dose-response patterns of each of these morphology and alignment measures with prevalent MRI-defined features of patellofemoral OA and/or knee pain.
Methods
Study Design
We conducted a cross-sectional study within an existing prospective cohort.
Study participants
The Framingham Community Cohort is a population-based sample of ambulatory (with or without walking aids), community-dwelling adults aged 50 or older living in Framingham, Massachusetts. Details of the recruitment and enrollment process have been published31. Briefly, participants were selected irrespective of knee pain or OA status, and were unaware of the purpose of the study. Individuals were excluded if they planned to move out of the area within the next five years, had bilateral total knee replacements, rheumatoid arthritis, dementia, terminal cancer, or had contraindications to magnetic resonance imaging (MRI)31, 32. Within the cohort, 996 participants had MRI images of at least one knee.
Image acquisition
MRI scans were acquired on a 1.5-tesla scanner (Siemens Healthineers, Erlangen, Germany) using an eight-channel phased array knee coil31, 32. To assess axial alignment and morphology, we used the axial reformatted 3D FLASH sequence (repetition time 16.8; echo time 7.64; flip angle 15; slice thickness 1.5 mm; interslice gap 0 mm; matrix 512 x 512) 31, 32. To assess OA features, we used axial, sagittal and coronal fat suppressed, proton density weighted, turbo spin echo images (repetition time 3610; echo time 40; slice thickness 3.5 mm; interslice gap 0 mm; echo train length 7; matrix 256 × 256) and sagittal T1 weighted spin echo images without fat suppression (repetition time 475; echo time 24; slice thickness 3.5 mm; interslice gap 0 mm; matrix 256×256) 31, 32.
Trochlear morphology and patellar alignment
One author (EMM) analyzed all measures of morphology and alignment based on previously established methods33, 34. Prior to analysis, two authors (EMM, JJS) used a random sample of 30 left knee MR images (i.e. contralateral MR images, not read for semiquantitative scoring using the Whole Organ MRI Score [WORMS] – see below for details) from the Framingham data set to standardize our evaluation methods. Once the protocol was established, we calculated inter-rater and intra-rater reliability using a random sample of 20 left knees. For this we used a two-way random effects model intraclass correlation coefficient, ICC(2,1)35. Inter-rater reliability ranged from 0.85 to 0.98, and intra-rater reliability ranged from 0.89 to 0.98.
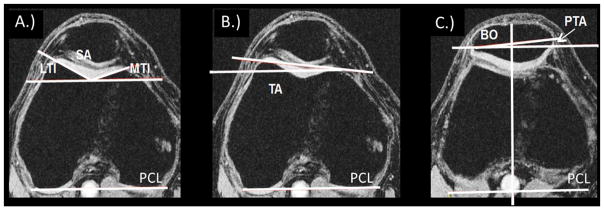
To evaluate morphology and alignment, we first selected the axial MRI slice with the largest posterior femoral condyles (i.e. adjacent slices demonstrated a smaller amount of posterior femoral condyle)(see Figure 1)33. Using this slice we evaluated trochlear morphology with four different measures: sulcus angle, lateral trochlear inclination, medial trochlear inclination, and trochlear angle33 (see descriptions in Figure 1A, B). We then selected the axial slice with the maximum mediolateral patellar diameter and used both slices to evaluate two patellar alignment measures: bisect offset and patellar tilt angle14 (Figure 1C). All measurements were made in OsiriX Lite version 8.0 (Pixmeo SARL, Switzerland).
Figure 1.
Morphology and alignment measures. A. Sulcus angle (SA) is the angle between the medial and lateral trochlear facets. Larger value means wider sulcus. Lateral trochlear inclination (LTI) is the angle between the posterior condylar line (PCL) and the lateral trochlear facet margin. Larger value means more prominent lateral facet. Medial trochlear inclination (MTI) is the angle between the PCL and the medial trochlear facet margin. Larger value means more prominent medial facet. B. Trochlear angle (TA) is the angle between the PCL and the anterior condylar line. Larger positive value means lateral facet protrudes more anteriorly than the medial facet. C. Patellar tilt angle (PTA) is the angle between the PCL (transposed from the slice with the largest PCL) and patellar width line. Larger positive value means more lateral tilt. Bisect offset (BO) is the % of the patella lateral to midline of the trochlea (the midline is transposed from the slice with the largest PCL to the slice with the widest patella). Larger value means more lateral displacement.
MRI-defined patellofemoral OA grading
We defined and graded patellofemoral structural damage using MRI. MRI features were scored by two experienced musculoskeletal radiologists (AG, FWR) using the WORMS36, 37. Images were read for the right knee only when possible (n=983); the left knee (n=13) was read when the right knee images were not acquired or readable. Inter-rater agreement (weighted kappa statistic, κ) for cartilage damage was κ =0.89; for bone marrow lesions was κ= 0.85, and for osteophytes was κ=0.7332. For this study we evaluated four patellofemoral joint sub-regions defined in WORMS: medial and lateral patella, and medial and lateral trochlea. We combined these four sub-regions to provide a single patellofemoral joint outcome measure, and also examined medial and lateral compartments separately. This decision was informed by knowledge that medial and lateral compartmental prevalence of OA features differs, and associations with morphology or alignment also differ by compartment33,37.
Our primary outcome, and operational definition of patellofemoral OA, was full thickness patellofemoral cartilage damage (WORMS scores of 2.5, 5, or 6) irrespective of tibiofemoral joint involvement. As in previous work8, we conducted secondary analyses using additional patellofemoral OA features: (i) ‘any’ cartilage damage (WORMS scores ≥2); (ii) ‘any’ bone marrow lesion (BMLs, WORMS score ≥1), and ‘definite’ osteophyte (defined as at least a small osteophyte, WORMS score ≥2).
Knee pain outcomes
Symptomatic OA features may provide different and clinically relevant information and thus, were also evaluated. Study participants were asked “In the past month, have you had any pain, aching, or stiffness in your knees?” Additional questions were asked to identify those who reported at least ‘mild’ pain on most days of the previous month. These individuals were deemed to have ‘knee pain’. Since this definition of ‘knee pain’ could not necessarily be localized to the patellofemoral joint, we also identified those who also reported at least mild pain when climbing stairs38, an item from the Western Ontario McMaster (WOMAC) scale and a task known to increase the load at the patellofemoral joint. We confirmed allocation to this second group, ‘pain with stairs’ if pain was unilateral affecting the scored knee, or if bilateral that the pain severity reported for the imaged/scored knee was at least as severe as the pain in the contralateral unread knee.
In total we evaluated three regional outcomes (patellofemoral joint as a whole and medial and lateral patellofemoral compartments separately); four structural features (full thickness cartilage damage, our primary outcome measure; and also any cartilage damage, any BMLs, and definite osteophytes), and two pain outcomes (knee pain; pain with stairs) that we evaluated alone and in combination with the structural features.
Statistical analyses
To generate reference values, we identified a subsample of knees with no full thickness cartilage damage on MRI in the patellofemoral joint as well as no knee pain. We then calculated mean and standard deviation (SD) for all morphology and alignment measures, and provided reference values (mean ±two SD) for each measure25, 26. If a measure differed by sex (defined as p<0.05 in a two-tailed t-test), we reported values separately for women and men.
To examine the dose-response relationship between each morphology or alignment measure and MRI-defined structural features of patellofemoral OA, we performed multivariable restricted cubic spline Poisson regression39, 40. We chose this method instead of examining the ranges of exposure in categories (e.g. quintiles) because we expected only individuals with the most extreme values of morphology or alignment would have higher prevalence of OA. Categorizing can result in collapsing those at the extreme ends (who have truely higher prevalence of an outcome) within a broader category (at relatively lower prevalence), which can mask a true significant relationship21. Restricted cubic spline analysis is thus a more robust method for modeling dose-response patterns41. We elected to use three knots for each morphology or alignment variable (10th, 50th and 90th percentile)39 because we expected, based on biology, that prevalence would be higher at one or both extreme ends of morphology or alignment values, but likely would not increase within mid-range values. In each model we adjusted for age, sex and body mass index (BMI). For all models, we compared those with the outcome of interest (e.g. full thickness cartilage damage) to those remaining from the sample who had no patellofemoral joint full thickness cartilage and no pain.
After performing spline Poisson regression, we exported the predicted prevalence ratios for full thickness cartilage damage against the entire range of the independent variable (morphology or alignment measure), using the median value of each variable for the sample with no full thickness cartilage damage and no pain as the referent point28. We illustrated the dose-response patterns using line graphs of the predicted prevalence ratios for each morphology or alignment measure. Finally, we reported values for each variable where predicted prevalence ratios were equal to or greater than 1.5 and 2.0 (and whose confidence interval lower bounds were greater than unity). We reported multiple values in this fashion because their clinical relevance is subjective and may vary with their intended use42.
Using the same approach, we examined each morphology and alignment measure with other symptoms and/or MRI features (i.e. knee pain alone, pain with stairs alone, any cartilage damage, any BMLs, definite osteophytes, plus structural outcomes combined with the two definitions of pain). Finally, we repeated these methods by medial and lateral compartments separately. All statistical analyses were done using Stata Intercooled 12.1 (StataCorp, TX, USA).
Results
Of the 996 participants, one was excluded from this study due to having no patella in the imaged knee; one due to having no axial plane MR image; and nine due to inadequate coverage of the knee joint or poor image quality. This resulted in 985 participants for analysis: mean age was 63.5 (SD 8.8) years old; 562 (57%) were women; and mean BMI was 28.5 (SD 5.6) (see Table 1 for additional demographics). The sample was primarily Caucasian. Compared to those with no patellofemoral full thickness cartilage damage and no pain, the subsample with full thickness cartilage damage was about 3 years older (p<0.001), had 1.7 kg/m2 higher BMI (p<0.001), and had 10% more women (p=0.01).
Table 1.
Participant characteristics. All values are N(%) unless otherwise noted.
| Full sample (n=985) | No full thickness cartilage damage, no pain (n=563) | Full thickness cartilage damage (n=255) | |
|---|---|---|---|
| Women | 562 (57%) | 305 (54.2%) | 164 (64.3%) |
| Age, mean(SD) | 63.5 (8.8) | 62.4 (8.2) | 65.3 (9.1) |
| BMI mean(SD) | 28.5 (5.6) | 27.7 (5.2) | 29.4 (5.6) |
| <25 N | 267 (27.5%) | 178 (31.8%) | 55 (22.0%) |
| 25–30 | 390 (40.16%) | 230 (41.1%) | 98 (39.2%) |
| >30 | 314 (32.34%) | 151 (27.0%) | 97 (38.8%) |
| Knee pain | 222 (23.2%) | 0 (0.0%) | 83 (33.9%) |
| Pain with stairs^ | 164 (17.4%) | 0 (0.0%) | 64 (26.9%) |
| Any cartilage damage | 627 (64.6%) | 274 (48.7%) | 255 (100%) |
| Full thickness cartilage damage | 255 (26.3%) | 563 (0%) | 255 (100%) |
| Any BMLs | 385 (39.3%) | 124 (22.0%) | 200 (78.7%) |
| Definite osteophytes | 603 (61.5%) | 263 (46.7%) | 227 (89.4%) |
WOMAC item #2 regarding pain during stairs, pain rated at least ‘mild’
Reference values
Reference values are reported along with mean (SD) for all morphology and alignment measures (Table 2 for referent sample, i.e. no patellofemoral full thickness cartilage damage or pain; Supplementary Table 1 for patellofemoral full thickness cartilage damage). Only two of the measures, medial trochlear inclination and patellar tilt angle, differed by sex and are reported separately.
Table 2.
Reference values among those with no patellofemoral joint full-thickness cartilage damage or knee pain (n=563).
| Mean (SD) | 2.5 Centile | 97.5 Centile | |
|---|---|---|---|
| Sulcus angle (°) | 128.8(6.5) | 116.0 | 141.5 |
| Lateral trochlear inclination (°) | 27.1(4.4) | 18.6 | 35.7 |
| Medial trochlear inclination (°) | 31.5(5.1) | 21.5 | 41.4 |
|
| |||
| Women (n=305)* | 32.2(5.1) | 22.1 | 42.3 |
| Men (n=258) | 30.6(4.8) | 21.1 | 40.1 |
|
| |||
| Trochlear angle (°) | −0.6(2.7) | −6.0 | 4.7 |
| Patellar tilt angle (°) | 8.6(4.4) | 0.0 | 17.2 |
|
| |||
| Women | 9.1(4.6) | 0.1 | 18.1 |
| Men | 8.1(4.1) | 0.1 | 16.0 |
|
| |||
| Bisect offset (%) | 52.2(4.8) | 42.8 | 61.6 |
Only medial trochlear inclination and patellar tilt angle differed by sex, thus are reported separately here
Dose-response patterns
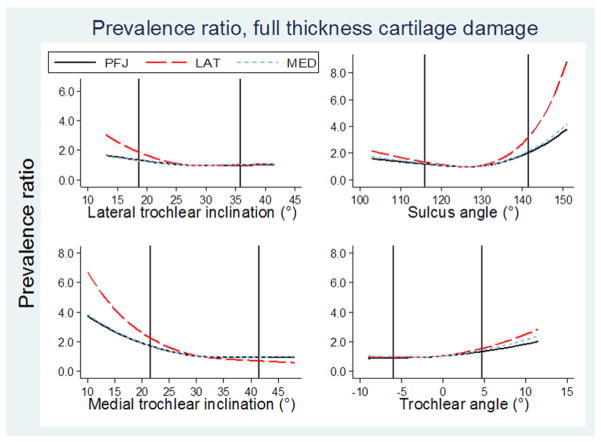
Dose-response patterns were curvilinear for all six predictors (four morphology and two alignment measures)(Figure 2). Prevalence ratios for having patellofemoral full thickness cartilage damage) as a function of morphology or alignment were generally highest in the lateral compartment as compared with the medial compartment or the whole patellofemoral joint. Values below are therefore reported for the lateral compartment unless otherwise specified. Superimposing the reference values from the subsample with no full thickness cartilage damage or pain onto the graphs illustrated the highest prevalence ratios for having patellofemoral full thickness cartilage damage generally occur beyond the reference values.
Figure 2. Dose-response patterns, morphology.
Line graphs showing predicted prevalence ratios for having patellofemoral full thickness cartilage damage against each morphology predictor, compared to the median value of the sample with no full thickness cartilage damage or pain. Two vertical lines indicate reference values for subgroup with no patellofemoral full thickness cartilage damage or pain (reference values reported in Table 2). From top, left to right: lateral trochlear inclination, median 27°; sulcus angle, median 128°;medial trochlear inclination, median 31°;; trochlear angle, median = −1°.
Lateral trochlear inclination had prevalence ratios of at least 1.5 at or below 21.0° (22.5° and 23.0° if combined with knee pain or pain with stairs, respectively)(Table 3). The shape of the dose-response pattern generally persisted for secondary outcomes, with differing thresholds, though relationships were more often significant if pain was included in the outcome (Supplementary Tables 2 and 3). One exception to the dose-response pattern was that it was reversed for knee pain alone, with prevalence ratio of ≥1.5 for inclinations at or above 37.0°.
Table 3.
Thresholds where predicted prevalence ratios (PR) achieve clinical relevance (PR ≥ 1.5 and PR ≥ 2.0) for having full thickness cartilage damage, with or without one of two pain definitions.
| FT cartilage damage only | FT cartilage damage & knee pain | FT cartilage damage & pain with stairs | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| TH | PR≥1.5 (CI) | PR≥ 2.0 (CI) | TH | PR≥ 1.5 (CI) | PR≥ 2.0 (CI) | TH | PR≥ 1.5 (IC) | PR≥2.0 (IC) | |||||
| Lateral trochlear inclination (°) | PFJ | ≤15.5 | 1.5 (1.0,2.2) | - | - | - | - | - | - | ≤20.5 | 1.5 (1.0,2.4) | - | - |
| LAT | ≤21.0 | 1.5 (1.2,1.9) | ≤17.5 | 2.0 (1.4,3.1) | ≤22.5 | 1.5 (1.1,2.1) | ≤19.5 | 2.1 (1.1,4.0) | ≤23.0 | 1.5 (1.1,2.1) | ≤21.0 | 2.0 (1.2,3.4) | |
| MED | - | - | - | - | - | - | - | - | - | - | - | - | |
| Medial trochlear inclination (°) | PFJ | ≤23.5 | 1.5 (1.3,1.7) | ≤19.0 | 2.1 (1.6,2.6) | ≤25.5 | 1.5 (1.2,1.9) | ≤22.5 | 2.1 (1.4, 3.1) | ≤25.5 | 1.5 (1.2,2.0) | ≤22.5 | 2.0 (1.3,3.2) |
| LAT | ≤25.5 | 1.5 (1.3,1.8) | ≤22.5 | 2.1 (1.5,2.7) | ≤25.0 | 1.5 (1.0, 2.3) | ≤21.5 | 2.1 (1.0, 4.4) | ≤25.0 | 1.5 (1.0,2.2) | ≤21.5 | 2.0 (1.0,4.0) | |
| MED | ≤23.5 | 1.5 (1.3,1.8) | ≤19.5 | 2.0 (1.5,2.7) | ≤26.0 | 1.5 (1.2,1.9) | ≤23.5 | 2.0 (1.4,3.0) | ≤26.0 | 1.5 (1.2,1.9) | ≤23.0 | 2.1 (1.3,3.2) | |
| Sulcus angle (°) | PFJ | ≥137 | 1.5 (1.3,1.7) | ≥142 | 2.1 (1.7,2.6) | ≥136 | 1.5 (1.1,2.0) | ≥141 | 2.1 (1.2,3.7) | ≥136 | 1.6 (1.2,2.2) | ≥139 | 2.0 (1.3,3.3) |
| LAT | ≥135 | 1.6 (1.4,1.8) | ≥138 | 2.2 (1.8,2.7) | ≥135 | 1.6 (1.1,2.2) | ≥138 | 2.1 (1.2,3.6) | ≥135 | 1.6 (1.1,2.3) | ≥138 | 2.1 (1.1,3.9) | |
| MED | ≥137 | 1.6 (1.3,1.8) | ≥141 | 2.1 (1.6,2.7) | ≥136 | 1.6 (1.1,2.2) | ≥140 | 2.1 (1.2,3.7) | ≥135 | 1.5 (1.1,2.0) | ≥139 | 2.1 (1.2,3.6) | |
| Trochlear angle (°) | PFJ | - | - | - | - | - | - | - | - | - | - | - | - |
| LAT | - | - | - | - | - | - | - | - | - | - | - | - | |
| MED | - | - | ≥11.5 | 2.4 (1.1,5.4) | ≥3.0 | 1.6 (1.1,2.2) | ≥5.0 | 2.1 (1.1,3.8) | - | - | - | - | |
| Patellar tilt angle (°) | PFJ | ≤1.0 / | 1.6 (1.3,1.9) | ≤ −2.0 / | 2.1 (1.6,2.8) | ≤3.0 / | 1.6 (1.2,2.1) | ≤1.0 / | 2.1 (1.4,3.1) | ≤2.0 / | 1.7 (1.1,2.5) | ≤0.0 / | 2.1 (1.1,3.9) |
| ≥20.0 | 1.5 (1.1,2.1) | ≥27.0 | 2.1 (1.3,3.6) | ≥15.0 | 1.5 (1.2,2.1) | ≥18.0 | 2.1 (1.3,3.2) | ≥15.0 | 1.7 (1.2,2.3) | ≥17.0 | 2.0 (1.3,3,1) | ||
| LAT | ≤2.0 / | 1.6 (1.3, 2.0) | ≤0.0 / | 2.0 (1.5,2.7) | ≤2.0 / | 1.6 (1.0,2.7) | ≤0.0 / | 2.1 (1.0,4.3) | ≥13.0 | 1.7 (1.3,2.0) | ≥15.0 | 2.2 (1.6,3.1) | |
| ≥15.0 | 1.6 (1.3,1.9) | ≥18.0 | 2.1 (1.6,2.7) | ≥13.0 | 1.6 (1.3,1.9) | ≥15.0 | 2.1 (1.6,2.7) | ||||||
| MED | ≤2.0 / | 1.6 (1.3,1.8) | ≤−1.0 | 2.1 (1.6,2.7) | ≤3.0 | 1.6 (1.2,2.2) | ≤1.0 | 2.2 (1.4,3.4) | ≤2.0 / | 1.7 (1.1,2.7) | ≤0.0 / | 2.2 (1.1,4.4) | |
| ≥15.0 | 1.6 (1.0,2.3) | ≥18.0 | 2.1 (1.1,3.9) | ≥14.0 | 1.5 (1.1,2.2) | ≥17.0 | 2.1 (1.2,3.8) | ||||||
| Bisect offset (°) | PFJ | ≥62.0 | 1.5 (1.4,1.7) | ≥68.0 | 2.0 (1.7.2.4) | ≥59.0 | 1.5 (1.3,1.8) | ≥64.0 | 2.1 (1.8,2.5) | ≥58.0 | 1.5 (1.3,1.8) | ≥62.0 | 2.0 (1.7,2.4) |
| LAT | ≥57.0 | 1.6 (1.3,1.9) | ≥61.0 | 2.0 (1.7,2.5) | ≥56.0 | 1.6 (1.2.2.0) | ≥60.0 | 2.2 (1.6,2.9) | ≥56.0 | 1.6 (1.2,2.2) | ≥59.0 | 2.1 (1.5,3.0) | |
| MED | ≥62.0 | 1.5 (1.3,1.7) | ≥68.0 | 2.0 (1.7,2.5) | ≥60.0 | 1.5 (1.3,1.8) | ≥65.0 | 2.1 (1.8,2.6) | ≥59.0 | 1.5 (1.3,1.9) | ≥63.0 | 2.0 (1.7,2.5) | |
FT full thickness; TH threshold; PR prevalence ratio; CI confidence interval; PFJ full patellofemoral joint; LAT lateral compartment only; MED medial compartment only.
- indicates PR not reached or PR not significant at that point estimate
Medial trochlear inclination was the only morphology measure consistently associated with all structural outcomes evaluated in this study (Table 3, Supplementary Tables 2 and 3). The dose-response pattern indicated higher prevalence ratios with a less inclined (i.e. flattened) medial trochlear facet.
For sulcus angle, prevalence ratios for full thickness cartilage damage were equal to or higher than 1.5 at or above 135.0°. Findings were similar for the secondary outcomes with slightly higher angles at each prevalence ratio.
Trochlear angle was only associated with full thickness cartilage damage in the medial compartment with knee pain included in the outcome. Secondary outcomes had similar dose-response curves and were more likely to have higher prevalence ratios with either pain definition included with the structural feature.
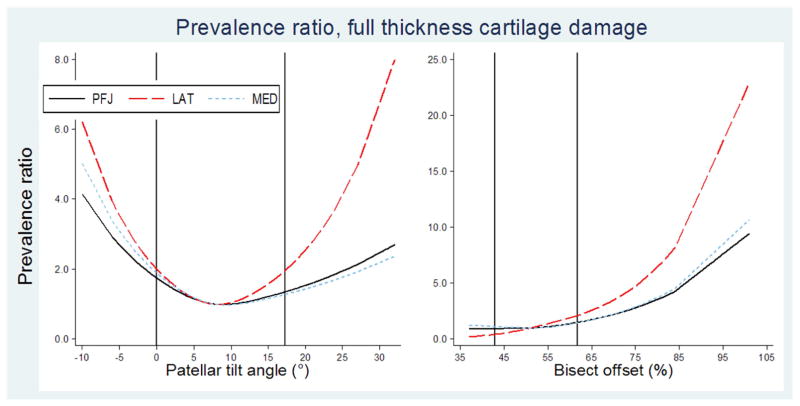
Patellar tilt angle was associated with patellofemoral full thickness cartilage damage both with and without pain (Figure 3). For most outcomes, prevalence ratios rose in two directions, i.e. with both lateral and medial tilt. Prevalence ratios for full thickness cartilage damage rose more steeply when lateral tilt was combined with either pain definition (Supplementary figure 1). As with other measures, the dose-response shape persisted for secondary outcomes, with differing angles for the two prevalence ratios (Supplementary Tables 2 and 3).
Figure 3. Dose-response patterns, alignment.
Line graphs showing predicted prevalence ratios for having patellofemoral full thickness cartilage damage against each alignment predictor, compared to the median predictor value of the sample with no patellofemoral full thickness cartilage damage or pain. Two vertical lines indicate reference values for subgroup with no full thickness cartilage damage or pain (reference values reported in Table 2). Left to right: patellar tilt angle, median 8°; bisect offset, median 51%.
Bisect offset had higher prevalence ratios (≥1.5) at or above 57% for the lateral compartment. Prevalence ratios were higher when pain was included with the outcome (Supplementary figure 1).
Discussion
We compared reference values of participants with and without patellofemoral full thickness cartilage damage and/or pain, and found large within group variability and substantial overlap between groups. This precludes morphology and alignment from being used as diagnostic indicators for patellofemoral OA. In other conditions such as recurrent patellar dislocation43 or femoroacetabular impingement44, morphology cut-points have been identified that distinguish these conditions from their asymptomatic counterparts with reasonable sensitivity and specificity. This was not possible in our study. It may be that morphology and alignment are not strong independent predictors of patellofemoral OA, and rather should be considered in the presence of other risk factors (e.g. BMI, history of trauma) that in combination lead to joint stress that exceeds biological capacity. It may also be that morphology and alignment predict patellofemoral OA longitudinally more powerfully than they do cross-sectionally.
Our study demonstrated a dose-response with morphology or alignment and prevalent MRI-defined patellofemoral structural damage and/or knee pain. Associations were generally stronger when the lateral compartment was evaluated alone, as compared with the medial compartment or the entire patellofemoral joint. This is also the case when evaluating the association of pain with patellofemoral structural damage45. Our study adds to the literature by highlighting the importance of evaluating the lateral compartment separately, as distinct from the entire patellofemoral joint. Evaluating the entire patellofemoral joint may statistically mask actual biological effects. Our study suggests that abnormal morphology and poor alignment may have a more important role in lateral patellofemoral OA than medial.
Sulcus angle had a stronger dose-response relationship with patellofemoral full thickness cartilage damage than the other three morphology measures. Medial trochlear inclination was the only morphology measure that was significantly associated with every pain and OA definition used in this study. When the outcome included pain rather than structure alone (in particular, pain with stairs), lateral trochlear inclination demonstrated a stronger association than medial inclination. Thus, we suggest that both sulcus angle as well as lateral trochlear inclination be used for future studies investigating morphology and patellofemoral OA, as they may be measuring different aspects of morphology that relate to structural features differently. Both alignment measures, on the other hand, showed strong associations (strongest when combined with pain with stairs) and should continue to be used in future studies.
While several studies investigating morphology or alignment have included knee pain in their inclusion criteria17, 18, 46, 47, few have attempted to localize the knee pain to the patellofemoral joint to define symptomatic patellofemoral OA18. This may explain why a relationship between morphology or alignment and pain has not previously been demonstrated in patellofemoral OA17, 46. While the Framingham Community Cohort did not assess the location of knee pain, we used two definitions of pain to see whether pain with tasks known to increase the load at the patellofemoral joint (stairs) would show different results than a more general definition of knee pain. In contrast to previous studies, we showed that morphology and alignment are cross-sectionally associated with symptomatic patellofemoral OA for both pain definitions. The mean morphology and alignment values between the two different pain definitions did not differ, however our results do suggest that how pain is defined may be important. For example, mean (SD) values for bisect offset increased from 52.2% (4.8) for those with no full thickness cartilage damage or pain (Table 2), to 55.6% (8.4) for full thickness cartilage damage alone, to 56.0% (9.2) for cartilage damage plus knee pain, to 56.6% (10.0) for cartilage damage plus pain with stairs (Supplementary Table 1), and the dose response graph for bisect offset clearly shows that combining cartilage damage with pain with stairs results in the highest prevalence ratios (Supplementary Figure 1). This may indicate that greater bisect offset is implicated in symptomatic patellofemoral OA. Future studies should define and localize patellofemoral pain as distinct from general knee pain.
The fact that our study elucidates the association between alignment or morphology and both structural features and knee pain justifies further investigation into conservative treatments that address malalignment18–20 to potentially mitigate pain or reduce/delay structural progression. Specifically, these studies should be targeted towards a subset of patellofemoral OA that may constitute a ‘malalignment phenotype’. Either the reference values or prevalence ratio thresholds reported in the present study can be used to identify those who may benefit most from treatments aimed at addressing malalignment. Reference values may be the simpler approach, and for all four measures recommended in this study, values lying beyond the reference values (sulcus angle ≥141.5°, lateral trochlear inclination ≤19.0°, patellar tilt angle ≥17.0°, and bisect offset ≥61.5%), prevalence ratios will be greater than two for full thickness cartilage damage plus pain with stairs, which combined may be a more clinically important outcome than structural damage alone.
Limitations
Some limitations to our study need mentioning. First, given that this is a cross-sectional study, we were unable to determine if morphology or alignment are etiological risk factors for patellofemoral OA. Second, this study was a secondary analysis of an existing cohort, and we were therefore limited in our ability to accurately define and identify study participants with patellofemoral pain. Another limitation is that there is not yet a gold standard for defining patellofemoral OA using MRI. This leaves a possibility of group misclassification in our comparisons. We operationally defined patellofemoral OA as full thickness cartilage damage. This was in part because any cartilage damage was so highly prevalent in this cohort (65%). We acknowledge that this definition is arbitrary, and resulted in individuals with milder (yet potentially clinically important) patellofemoral structural features being included in our asymptomatic reference group. This may have influenced the results of our study. Osteophytes were also prevalent in this cohort (62%). BMLs were less prevalent in this study (39%) yet are common even in young asymptomatic knees48 and can fluctuate over relatively short time periods49 and thus may not represent a stable indicator of disease state. While we did not report reference values using these other structural features, we did evaluate them as secondary outcomes, and found similar dose-response patterns with thresholds suggesting a similar but somewhat weaker association than full thickness cartilage damage (Table 3, Supplementary tables 2 and 3).
In summary, this study determined reference values and dose-response patterns for morphology and alignment in knees with and without patellofemoral full thickness cartilage damage and pain, in a population-based sample of ambulatory adults aged ≥50 years. Reference values indicate large within group variability and substantial overlap between those with and without patellofemoral full thickness cartilage damage. Dose-response patterns for both morphology and alignment were curvilinear and prevalence ratios for having full thickness cartilage damage (or other MRI features of structural damage) are higher in a single direction with the exception of patellar tilt, which has higher prevalence whether malaligned towards lateral or medial tilt. Of the morphology measures, sulcus angle and lateral trochlear inclination were most strongly associated with patellofemoral full thickness cartilage damage. Both alignment measures had consistently strong associations with patellofemoral full thickness cartilage damage. The definition of pain for describing symptomatic patellofemoral OA is an important consideration that may help better elucidate the clinical relevance of morphology and alignment in the onset and progression of patellofemoral OA.
Supplementary Material
Line graphs showing predicted prevalence ratios for having patellofemoral full thickness cartilage damage (alone = solid black; with knee pain=short dash light blue; with pain when climbing stairs=long dash red) to the lateral compartment only, against each morphology or alignment predictor, compared to the median predictor value of the sample with no patellofemoral full thickness cartilage damage or pain.
Acknowledgments
We thank participants of the Framingham Community Cohort.
Role of the funding source
EM Macri received funding support from an Osteoarthritis Research Society International (OARSI) scholarship and a Vanier Canada Graduate Scholarship (CIHR). The Framingham Community Cohort study was funded by the National Institutes of Health (AG18393 and AR47785). We also acknowledge support from the National Institutes of Health, P60 AR047785 NIAMS Multidisciplinary Clinical Research Center. The funders had no role in the study design, collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Footnotes
Author contributions
All authors contributed to the conception and design of the study, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content, and approved the final version submitted. E.M. Macri and J.J. Stefanik take responsibility for the integrity of the work as a whole, from inception to finished article.
Competing interests
A Guermazi is the president of Boston Imaging Core Lab (BICL), LLC, and a consultant to Merck Serono, TissueGene, Genzyme, AstraZeneca, and OrthoTrophixs. FW Roemer is vice president and shareholder of BICL and is a consultant to Merck Serono and National Institute of Health. F Roemer is CMO and shareholder of BICL.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Erin M Macri, Centre for Hip Health and Mobility, University of British Columbia, Vancouver, Canada; Department of Family Practice, University of British Columbia, Vancouver, Canada 7/F 2635 Laurel Street; Robert HN Ho Research Centre; Vancouver, BC, Canada V5Z 1M9.
David T Felson, Clinical Epidemiology Research and Training Unit, School of Medicine, Boston University, Boston, USA; Division of Musculoskeletal & Dermatological Sciences, University of Manchester, Manchester, UK.
Yuqing Zhang, Clinical Epidemiology Research and Training Unit, School of Medicine, Boston University, Boston, USA; Division of Rheumatology, Allergy, and Immunology, Massachusetts General Hospital, Boston, USA.
Ali Guermazi, Quantitative Imaging Center, Department of Radiology, School of Medicine, Boston University, Boston, USA.
Frank W Roemer, Department of Radiology, University of Erlangen-Nuremberg, Erlangen, Germany; Quantitative Imaging Center, Department of Radiology, School of Medicine, Boston University, Boston, USA.
Kay M Crossley, College of Science Health and Engineering, School of Allied Health, La Trobe University, Melbourne, Australia.
Karim M Khan, Centre for Hip Health and Mobility, University of British Columbia, Vancouver, Canada; Department of Family Practice, University of British Columbia, Vancouver, Canada.
Josh J Stefanik, Department of Physical Therapy, Movement & Rehabilitation Sciences, Northeastern University Bouve College of Health Sciences, Boston, USA; Clinical Epidemiology Research and Training Unit, School of Medicine, Boston University, Boston, USA.
References
- 1.Duncan RC, Hay EM, Saklatavala J, Croft PR. Prevalence of radiographic osteoarthritis - it all depends on your point of view. Rheumatology. 2006;45:757–60. doi: 10.1093/rheumatology/kei270. [DOI] [PubMed] [Google Scholar]
- 2.Duncan R, Peat G, Thomas E, Wood L, Hay E, Croft P. How do pain and function vary with compartmental distribution and severity of radiographic knee osteoarthritis? Rheumatology. 2008;47(11):1704–7. doi: 10.1093/rheumatology/ken339. [DOI] [PubMed] [Google Scholar]
- 3.Kornaat PR, Bloem JL, Ceulemans RY, Riyazi N, Rosendaal FR, Nelissen RG, et al. Osteoarthritis of the Knee: Association between Clinical Features and MR Imaging Findings 1. Radiology. 2006;239(3):811–7. doi: 10.1148/radiol.2393050253. [DOI] [PubMed] [Google Scholar]
- 4.Hunter DJ, March L, Sambrook PN. The association of cartilage volume with knee pain. Osteoarthritis and Cartilage. 2003;11(10):725–9. doi: 10.1016/s1063-4584(03)00160-2. [DOI] [PubMed] [Google Scholar]
- 5.Sharma L, Chmiel JS, Almagor O, Dunlop D, Guermazi A, Bathon JM, et al. Significance of preradiographic magnetic resonance imaging lesions in persons at increased risk of knee osteoarthritis. Arthritis & Rheumatology. 2014;66(7):1811–9. doi: 10.1002/art.38611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duncan R, Peat G, Thomas E, Hay EM, Croft P. Incidence, progression and sequence of development of radiographic knee osteoarthritis in a symptomatic population. Annals of the Rheumatic Diseases. 2011;70(11):1944–8. doi: 10.1136/ard.2011.151050. [DOI] [PubMed] [Google Scholar]
- 7.Mazzuca SA, Brandt KD, Katz BP, Ding Y, Lane KA, Buckwalter KA. Risk factors for progression of tibiofemoral osteoarthritis: an analysis based on fluoroscopically standardised knee radiography. Annals of the Rheumatic Diseases. 2006;65:515–9. doi: 10.1136/ard.2005.039115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stefanik JJ, Guermazi A, Roemer FW, Peat G, Niu J, Segal NA, et al. Changes in patellofemoral and tibiofemoral joint cartilage damage and bone marrow lesions over 7 years: the Multicenter Osteoarthritis Study. Osteoarthritis and Cartilage. 2016;24(7):1160–6. doi: 10.1016/j.joca.2016.01.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silverwood V, Blagojevic-Bucknall M, Jinks C, Jordan JL, Protheroe J, Jordan KP. Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthritis and Cartilage. 2015;23(4):507–15. doi: 10.1016/j.joca.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Schiphof D, van Middelkoop M, de Klerk BM, Oei EH, Hofman A, Koes BW, et al. Crepitus is a first indication of patellofemoral osteoarthritis (and not of tibiofemoral osteoarthritis) Osteoarthritis and Cartilage. 2014;22(5):631–8. doi: 10.1016/j.joca.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Peat G, Duncan RC, Wood LRJ, Thomas E, Muller S. Clinical features of symptomatic patellofemoral joint osteoarthritis. Arthritis Research & Therapy. 2012;14(2):R63. doi: 10.1186/ar3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macri E, Stefanik J, Khan K, Crossley K. Is tibiofemoral or patellofemoral alignment or trochlear morphology associated with patellofemoral osteoarthritis? A systematic review. Arthritis Care & Research. 2016;68(10):1453–70. doi: 10.1002/acr.22842. [DOI] [PubMed] [Google Scholar]
- 13.Van Haver A, De Roo K, De Beule M, Labey L, De Baets P, Dejour D, et al. The effect of trochlear dysplasia on patellofemoral biomechanics: a cadaveric study with simulated trochlear deformities. American Journal of Sports Medicine. 2015;43(6):1354–61. doi: 10.1177/0363546515572143. [DOI] [PubMed] [Google Scholar]
- 14.Stefanik JJ, Zumwalt AC, Segal NA, Lynch JA, Powers CM. Association between measures of patella height, morphologic features of the trochlea, and patellofemoral joint alignment: the MOST study. Clinical Orthopaedics & Related Research. 2013;471(8):2641–8. doi: 10.1007/s11999-013-2942-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Besier TF, Gold GE, Delp SL, Fredericson M, Beaupré GS. The influence of femoral internal and external rotation on cartilage stresses within the patellofemoral joint. Journal of Orthopaedic Research. 2008;26(12):1627–35. doi: 10.1002/jor.20663. [DOI] [PubMed] [Google Scholar]
- 16.Davies-Tuck M, Teichtahl AJ, Wluka AE, Wang Y, Urquhart DM, Cui J, et al. Femoral sulcus angle and increased patella facet cartilage volume in an osteoarthritic population. Osteoarthritis and Cartilage. 2008;16(1):131–5. doi: 10.1016/j.joca.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Hunter DJ, Zhang YQ, Niu JB, Felson DT, Kwoh K, Newman A, et al. Patella malalignment, pain and patellofemoral progression: the Health ABC Study. Osteoarthritis and Cartilage. 2007;15(10):1120–7. doi: 10.1016/j.joca.2007.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crossley K, Marino G, Macilquham M, Schache A, Hinman R. Can patellar tape reduce the patellar malalignment and pain associated with patellofemoral osteoarthritis? Arthritis & Rheumatism. 2009;61(12):1719–25. doi: 10.1002/art.24872. [DOI] [PubMed] [Google Scholar]
- 19.Callaghan M, Guney H, Reeves N, Bailey D, Doslikova K, Maganaris C, et al. A knee brace alters patella position in patellofemoral osteoarthritis: a study using weight bearing magnetic resonance imaging. Osteoarthritis and Cartilage. 2016;24(12):2055–60. doi: 10.1016/j.joca.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Wong YM, Chan ST, Tang KW, Ng GY. Two modes of weight training programs and patellar stabilization. Journal of Athletic Training. 2009;44:264–271. doi: 10.4085/1062-6050-44.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology. 1995:356–65. doi: 10.1097/00001648-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Pasco J, Lane S, Brennan S, Timney E, Bucki-Smith G, Dobbins A, et al. Fracture risk among older men: osteopenia and osteoporosis defined using cut-points derived from female versus male reference data. Osteoporosis International. 2014;25(3):857–62. doi: 10.1007/s00198-013-2561-9. [DOI] [PubMed] [Google Scholar]
- 23.Hall M, Heavens J, Cullum I, Ell P. The range of bone density in normal British women. The British Journal of Radiology. 1990;63(748):266–9. doi: 10.1259/0007-1285-63-748-266. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO study group; Rome. 22 to 25 June 1992; 1994. [PubMed] [Google Scholar]
- 25.Laborie L, Lehmann T, Engesæter I, Sera F, Engesæter L, Rosendahl K. The alpha angle in cam-type femoroacetabular impingement. The Bone & Joint Journal. 2014;96(4):449–54. doi: 10.1302/0301-620X.96B4.32194. [DOI] [PubMed] [Google Scholar]
- 26.Lepage-Saucier M, Thiéry C, Larbi A, Lecouvet FE, Berg BCV, Omoumi P. Femoroacetabular impingement: normal values of the quantitative morphometric parameters in asymptomatic hips. European Radiology. 2014;24(7):1707–14. doi: 10.1007/s00330-014-3171-4. [DOI] [PubMed] [Google Scholar]
- 27.Thomas GE, Palmer AJ, Batra RN, Kiran A, Hart D, Spector T, et al. Subclinical deformities of the hip are significant predictors of radiographic osteoarthritis and joint replacement in women. A 20 year longitudinal cohort study. Osteoarthritis and Cartilage. 2014;22(10):1504–10. doi: 10.1016/j.joca.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 28.Song X, Jousilahti P, Stehouwer C, Söderberg S, Onat A, Laatikainen T, et al. Cardiovascular and all-cause mortality in relation to various anthropometric measures of obesity in Europeans. Nutrition, Metabolism and Cardiovascular Diseases. 2015;25(3):295–304. doi: 10.1016/j.numecd.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Rothman KJ, Moore LL, Singer MR, Nguyen U-SD, Mannino S, Milunsky A. Teratogenicity of high vitamin A intake. New England Journal of Medicine. 1995;333(21):1369–73. doi: 10.1056/NEJM199511233332101. [DOI] [PubMed] [Google Scholar]
- 30.Bellemans J, Colyn W, Vandenneucker H, Victor J. The Chitranjan Ranawat Award: is neutral mechanical alignment normal for all patients?: the concept of constitutional varus. Clinical Orthopaedics and Related Research. 2012;470(1):45–53. doi: 10.1007/s11999-011-1936-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Englund M, Guermazi A, Gale D, Hunter DJ, Aliabadi P, Clancy M, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. New England Journal of Medicine. 2008;359(11):1108–15. doi: 10.1056/NEJMoa0800777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guermazi A, Niu J, Hayashi D, Roemer FW, Englund M, Neogi T, et al. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (Framingham Osteoarthritis Study) British Medical Journal. 2012;345:e5339. doi: 10.1136/bmj.e5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stefanik JJ, Roemer FW, Zumwalt AC, Zhu Y, Gross KD, Lynch JA, et al. Association between measures of trochlear morphology and structural features of patellofemoral joint osteoarthritis on MRI: the MOST study. Journal of Orthopaedic Research. 2012;30(1):1–8. doi: 10.1002/jor.21486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stefanik J, Zhu Y, Zumwalt A, Gross K, Clancy M, Lynch J, et al. Association between patella alta and the prevalence and worsening of structural features of patellofemoral joint osteoarthritis: the multicenter osteoarthritis study. Arthritis Care & Research. 2010;62(9):1258–65. doi: 10.1002/acr.20214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGraw KO, Wong SP. Forming Inferences About Some Intraclass Correlation Coefficients. Psychological Methods. 1996;1(1):30– 46. [Google Scholar]
- 36.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis and Cartilage. 2004;12(3):177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Stefanik JJ, Gross KD, Guermazi A, Felson DT, Roemer FW, Zhang Y, et al. The relation of MRI-detected structural damage in the medial and lateral patellofemoral joint to knee pain: the Multicenter and Framingham Osteoarthritis Studies. Osteoarthritis and Cartilage. 2015;23(4):565–70. doi: 10.1016/j.joca.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stefanik JJ, Neogi T, Niu J, Roemer FW, Segal NA, Lewis CE, et al. The diagnostic performance of anterior knee pain and activity-related pain in identifying knees with structural damage in the patellofemoral joint: the Multicenter Osteoarthritis Study. The Journal of Rheumatology. 2014;41(8):1695–702. doi: 10.3899/jrheum.131555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harrell F. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- 40.Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Medical Research Methodology. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greenland S. Avoiding power loss associated with categorization and ordinal scores in dose-response and trend analysis. Epidemiology. 1995;6(4):450–4. doi: 10.1097/00001648-199507000-00025. [DOI] [PubMed] [Google Scholar]
- 42.Kraemer HC, Morgan GA, Leech NL, Gliner JA, Vaske JJ, Harmon RJ. Measures of clinical significance. Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42(12):1524–1529. doi: 10.1097/00004583-200312000-00022. [DOI] [PubMed] [Google Scholar]
- 43.Carrillon Y, Abidi H, Dejour D, Fantino O, Moyen B, Tran-Minh VA. Patellar instability: assessment on MR images by measuring the lateral trochlear inclination-initial experience. Radiology. 2000;216(2):582–5. doi: 10.1148/radiology.216.2.r00au07582. [DOI] [PubMed] [Google Scholar]
- 44.Sutter R, Dietrich TJ, Zingg PO, Pfirrmann CW. How useful is the alpha angle for discriminating between symptomatic patients with cam-type femoroacetabular impingement and asymptomatic volunteers? Radiology. 2012;264(2):514–21. doi: 10.1148/radiol.12112479. [DOI] [PubMed] [Google Scholar]
- 45.Stefanik JJ, Gross KD, Guermazi A, Felson DT, Roemer FW, Zhang Y, et al. The relation of MRI-detected structural damage in the medial and lateral patellofemoral joint to knee pain: the Multicenter and Framingham Osteoarthritis Studies. Osteoarthritis and Cartilage. 2015;23(4):565–70. doi: 10.1016/j.joca.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalichman L, Zhang Y, Niu J, Goggins J, Gale D, Felson D, et al. The association between patellar alignment and patellofemoral joint osteoarthritis features—an MRI study. Rheumatology. 2007;46(8):1303–8. doi: 10.1093/rheumatology/kem095. [DOI] [PubMed] [Google Scholar]
- 47.Kalichman L, Zhang Y, Niu J, Goggins J, Gale D, Zhu Y, et al. The association between patellar alignment on magnetic resonance imaging and radiographic manifestations of knee osteoarthritis. Arthritis Research & Therapy. 2007;9(2):R26. doi: 10.1186/ar2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van der Heijden RA, de Kanter JL, Bierma-Zeinstra SM, Verhaar JA, van Veldhoven PL, Krestin GP, et al. Structural Abnormalities on Magnetic Resonance Imaging in Patients With Patellofemoral Pain A Cross-sectional Case-Control Study. The American Journal of Sports Medicine. 2016 doi: 10.1177/0363546516646107. 0363546516646107. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Nevitt M, Niu J, Lewis C, Torner J, Guermazi A, et al. Fluctuation of knee pain and changes in bone marrow lesions, effusions, and synovitis on magnetic resonance imaging. Arthritis & Rheumatology. 2011;63(3):691–9. doi: 10.1002/art.30148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Line graphs showing predicted prevalence ratios for having patellofemoral full thickness cartilage damage (alone = solid black; with knee pain=short dash light blue; with pain when climbing stairs=long dash red) to the lateral compartment only, against each morphology or alignment predictor, compared to the median predictor value of the sample with no patellofemoral full thickness cartilage damage or pain.