Abstract
BACKGROUND & AIMS
Monitoring serum concentrations of tumor necrosis factor antagonists in patients receiving these drugs as treatment for inflammatory bowel disease (IBD), also called therapeutic drug monitoring, is performed either after patient loss of response (reactive drug monitoring) or in patients in clinical remission whose drug has been titrated to a target concentration (proactive drug monitoring). We compared long-term outcomes of patients with IBD undergoing proactive vs reactive monitoring of serum concentrations of infliximab.
METHODS
We performed a multicenter, retrospective study of 264 consecutive patients with IBD (167 with Crohn’s disease) receiving infliximab maintenance therapy. The subjects received proactive (n = 130) or reactive (n = 134) drug monitoring, based on measurements of first infliximab concentration and antibodies to infliximab, from September 2006 to January 2015; they were followed through December 2015 (median time of 2.4 years). We analyzed time to treatment failure, first IBD-related surgery or hospitalization, serious infusion reaction, and detection of antibodies to infliximab. Treatment failure was defined as drug discontinuation for loss of response or serious adverse event, or need for surgery.
RESULTS
Multiple Cox regression analysis independently associated proactive drug monitoring, compared with reactive monitoring, with reduced risk for treatment failure (hazard ratio [HR], 0.16; 95% confidence interval [CI], 0.09–0.27; P < .001), IBD-related surgery (HR, 0.30; 95% CI, 0.11–0.80; P = .017), IBD-related hospitalization (HR, 0.16; 95% CI, 0.07–0.33; P < .001), antibodies to infliximab (HR, 0.25; 95% CI, 0.07–0.84; P = .025), and serious infusion reaction (HR, 0.17; 95% CI, 0.04–0.78; P = .023).
CONCLUSIONS
In a retrospective analysis of patients with IBD receiving proactive vs reactive monitoring of serum concentration of infliximab, proactive monitoring was associated with better clinical outcomes, including greater drug durability, less need for IBD-related surgery or hospitalization, and lower risk of antibodies to infliximab or serious infusion reactions.
Keywords: CD, Immunogenicity, Monitoring Therapy, Ulcerative Colitis
Anti–tumor necrosis factor (TNF) therapy has revolutionized the care of patients with inflammatory bowel disease (IBD), such as Crohn’s disease (CD) and ulcerative colitis (UC).1 Nevertheless, up to 50% of patients after an initial clinical response need to discontinue therapy for loss of response or a serious adverse event (SAE), including a serious infusion reaction (SIR).2 Mechanisms underlying loss of response include pharmacokinetic or pharmacodynamic issues, characterized by inadequate drug concentrations, due to rapid clearance stemming from severe inflammation and nonimmune mechanisms or the development of antidrug antibodies, or a non–TNF-driven inflammatory process, respectively.3,4 The development of antidrug antibodies may also lead to drug intolerance and treatment failure due to a SIR.5
Several retrospective studies and post hoc analyses of randomized controlled trials regarding anti-TNF therapy in IBD demonstrate an association between high serum drug trough concentration (TC) and favorable objective therapeutic outcomes, such as biomarker or endoscopic remission.6–10 However, current therapeutic drug monitoring (TDM)–based treatment algorithms are usually applied in a reactive setting, when loss of response or an infusion reaction occur, to determine the subsequent therapeutic intervention. Nevertheless, preliminary data suggest that proactive TDM with drug titration to a target concentration in patients with clinical response on maintenance anti-TNF therapy may improve treatment cost and effectiveness, although more data from large prospective studies are certainly needed.11,12 Although both reactive and proactive TDM-based therapeutic strategies appear to be more beneficial in terms of favorable clinical outcomes and cost effectiveness than usual care, there are no data regarding the long-term outcomes of proactive versus reactive TDM for optimizing anti-TNF therapy in IBD.13–16 Thus, the primary aim of this study was to investigate long-term outcomes of proactive compared with reactive TDM for optimizing infliximab therapy in IBD real-life clinical practice. The secondary aim was to investigate the association of infliximab TC at the start of TDM with therapeutic outcomes of interest.
Materials and Methods
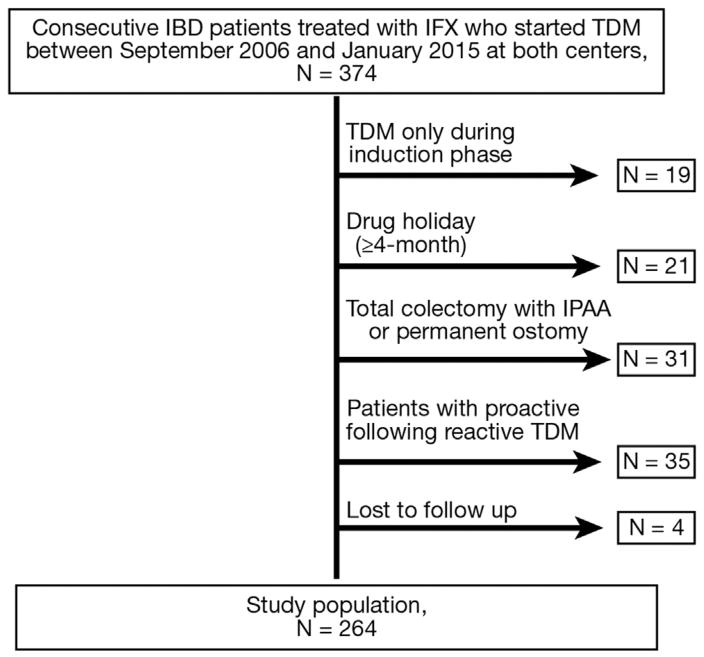
Study Design and Population
This was a multicentre (Beth Israel Deaconess Medical Center and University of Pennsylvania hospitals) retrospective cohort study. Consecutive IBD patients who responded to infliximab induction therapy and subsequently received maintenance therapy and underwent either proactive or reactive TDM, based on the first infliximab concentration or antibodies to infliximab (ATI) measurement from September 2006 to January 2015, were eligible and followed through December 2015. Patients were excluded if they underwent TDM only during the induction phase, subsequent proactive after first reactive TDM, total colectomy with an ileal pouch-anal anastomosis or a terminal ostomy prior to infliximab TDM initiation, had no follow-up visit after the initiation of infliximab TDM, or had a long (≥14 weeks) drug holiday during infliximab maintenance therapy. Demographic and clinical characteristics of the patients were acquired via their electronic medical records. The study was approved by the Institutional Review Boards of Beth Israel Deaconess Medical Center, Harvard Medical School (Boston, MA) and the Department of Medicine, Perelman School of Medicine, University of Pennsylvania (Philadelphia, PA).
Outcomes and Definitions
Therapeutic outcomes of interest included treatment failure, IBD-related surgery, IBD-related hospitalization, SIR, and ATI. Treatment failure was defined either as infliximab discontinuation due to loss of response or SAE, or need for surgery. IBD-related surgery included any intestinal or perianal surgical procedure (eg, bowel resection with or without ostomy or ileal pouch-anal anastomosis, fistulotomy, fistula seton placement, abscess drainage, or strictureplasty).17 IBD-related hospitalization was defined as any hospitalization with IBD either as the primary diagnosis (ie, for disease relapse, symptomatic fistula or abscess, other complications, or surgery) or secondary diagnosis if the primary diagnosis was related to a gastrointestinal symptom (abdominal pain, diarrhea, nausea, vomiting, constipation, or gastrointestinal bleeding) and not associated with infliximab itself or other comorbidities.17 SIR was defined as any acute or delayed infusion reaction necessitating infliximab discontinuation. The study observation time for IBD-related surgery, IBD-related hospitalization, and SIR was defined as the start of TDM until treatment failure (and within 12 weeks of the last infliximab infusion, only if another biologic was not administered) or the end of follow-up.18 The study observation time for ATI was defined as the start of TDM to the last available ATI measurement until treatment failure or the end of follow-up.
Proactive and Reactive TDM Protocols
Proactive TDM was defined as the assessment of infliximab concentration and ATI in patients without any IBD-related symptoms indicative of active disease based on physician’s global assessment, with the aim of prospectively titrating infliximab, typically to a target TC of 5–10 μg/mL, to maintain clinical benefit, as previously described.12 In contrast, reactive TDM was defined as assessment of infliximab concentration and ATI in patients with either gastrointestinal symptoms indicative of suspected loss of response or drug intolerance due to acute or delayed infusion reactions, to guide treatment decisions and optimization, also typically targeting an infliximab TC therapeutic range of 5–10 μg/mL. Changes in the infliximab regimen were made at each physician’s discretion, based also on previously described therapeutic algorithms, reflective of real-life clinical practice.4,19
Infliximab Concentration and ATI Measurement
Serum infliximab concentration and ATI were measured by Prometheus Laboratories (San Diego, CA). A TC was defined as any infliximab measurement performed within 7 days of the next infusion. The study period overlapped with the use of 2 different infliximab assays, a drug-sensitive enzyme-linked immunosorbent assay (ELISA) until July 2012 followed by a drug-tolerant homogeneous mobility shift assay (HMSA).20 Infliximab concentration of <1 and 1.4 μg/mL and ATI <3.1 U/mL and 1.7 μg/mL equivalents were considered as undetectable for the HMSA and ELISA, respectively.
Statistical Analysis
Descriptive statistics were provided as median (interquartile range [IQR]) for continuous variables and frequency and percentage for categorical variables. Continuous and discrete variables between patients undergoing proactive and reactive TDM were compared using the Mann-Whitney U test and the chi-square or Fisher exact test, as appropriate, respectively. The effect of TDM type (proactive vs reactive) on the cumulative probability of therapeutic outcomes of interest was evaluated using time-to-event (survival) methods. Kaplan-Meier estimates were used to draw the cumulative incidence curves, compared by log-rank test.
Univariable and multivariable Cox proportional hazards regression analyses were also performed to determine the independent effects of variables associated with therapeutic outcomes of interest. The following variables were examined: gender, age at diagnosis, age at start of infliximab treatment, duration from infliximab initiation until start of TDM, IBD subtype, UC extension, CD location and behavior, perianal fistulizing disease, ileocolonic resection prior to TDM, smoking ever, immunomodulators at start of TDM, infliximab optimization prior to TDM, prior anti-TNF therapy, infliximab concentration and ATI at the start of TDM, and type of TDM. Only variables with a P value <.1 on univariable analysis entered the multivariable analysis, which was performed using the Wald Backward selection method.
A receiver-operating characteristic (ROC) analysis was performed for infliximab TC at the start of TDM to trace thresholds associated with therapeutic outcomes of interest. Optimal thresholds were chosen using the Youden index, which maximizes the sum of the sensitivity and specificity of the ROC curve.7 Infliximab TC at the start of TDM were also categorized into quartiles. Rates of therapeutic outcomes of interest were compared across infliximab TC using the chi-square test (linear-by-linear association). All analyses were performed using SPSS version 23.0 (IBM, Armonk, NY) and GraphPad Prism version 5.03 for Windows (GraphPad Software, San Diego, CA).
Results
Study Population
The study population consisted of 264 patients (Beth Israel Deaconess Medical Center, n = 149 [56%]; CD, n =167 [63%]) (Figure 1), the great majority of whom (244 [92.4%]) underwent a first TDM after 2010. The median follow-up of the patients was 2.4 (IQR: 1.5–3.3) years. Patient demographic and clinical characteristics are shown in Table 1. Based on their first infliximab concentration or ATI measurement, patients were characterized as having undergone either proactive (n = 130 [49%]) or reactive TDM (n = 134 [51%]). The indication for reactive TDM was gastrointestinal symptoms indicative of suspected loss of response (n = 117 [87%]) or drug intolerance (n = 17 [13%]: acute [n = 9] or delayed [n = 8] infusion reactions).
Figure 1.
Flow chart of study population. IBD, inflammatory bowel disease; IFX, infliximab; IPAA, ileal pouch-anal anastomosis; TDM, therapeutic drug monitoring.
Table 1.
Patient Demographic and Clinical Characteristics
| Patient characteristics | Total cohort (N = 264) | Proactive TDM (n = 130) | Reactive TDM (n = 134) | P value |
|---|---|---|---|---|
| Male | 153 (58) | 78 (60) | 75 (56) | .535 |
| Age at diagnosis, y | 24 (18–35) | 24 (18–34) | 24 (19–37) | .594 |
| Age at start of IFX, y | 31 (25–45) | 31 (23–43) | 31 (25–46) | .312 |
| Duration from IFX initiation until start of TDM, mo | 14 (6–35) | 15 (6–38) | 13 (6–31) | .464 |
| IBD type | .225 | |||
| CD | 167 (63) | 89 (68) | 78 (58) | |
| UC | 90 (34) | 38 (29) | 52 (39) | |
| IBDU | 7 (3) | 3 (3) | 4 (3) | |
| UC extensiona | .559 | |||
| E1 (proctitis) | 3/89 (4) | 2/37 (6) | 1/52 (2) | |
| E2 (left-sided colitis) | 36/89 (40) | 16/37 (43) | 20/52 (38) | |
| E3 (pancolitis) | 50/89 (56) | 19/37 (51) | 31/52 (60) | |
| CD locationa | .217 | |||
| L1 (ileal) | 31/167 (19) | 17/89 (19) | 14/78 (18) | |
| L2 (colonic) | 43/167 (26) | 20/89 (22) | 23/78 (29) | |
| L3 (ileocolonic) | 82/167 (49) | 43/89 (48) | 39/78 (50) | |
| L4 (upper GI disease) | 11/167 (6) | 9/89 (11) | 2/78 (3) | |
| CD behaviora | .029 | |||
| B1 (nonstricturing, nonpenetrating) | 85/167 (51) | 50/89 (56) | 35/78 (45) | |
| B2 (stricturing) | 29/167 (17) | 9/89 (10) | 20/78 (26) | |
| B3 (penetrating) | 53/167 (32) | 30/89 (34) | 23/78 (29) | |
| Perianal fistulizing disease | 59/167 (35) | 26/89 (29) | 33/78 (42) | .104 |
| Ileocolonic resection prior to TDM | 36/167 (22) | 17/89 (19) | 19/78 (24) | .454 |
| Smoking ever | 63 (24) | 35 (27) | 28 (21) | .312 |
| IFX dosing other than 5 mg/kg q8w at start of TDM | 113 (43) | 45 (35) | 68 (51) | .009 |
| Anti-TNF naive | 254 (96) | 127 (98) | 127 (95) | .335 |
| Concomitant IMM at start of TDM | 78 (30) | 42 (32) | 36 (27) | .348 |
| Thiopurines (azathioprine, 6-MP) | 60/78 (77) | 33/42 (79) | 27/36 (75) | |
| Methotrexate | 18/78 (23) | 9/42 (21) | 9/36 (25) |
NOTE. Values are n (%), median (interquartile range), or n/n (%).
CD, Crohn’s disease; IBD, inflammatory bowel disease; IBDU, inflammatory bowel disease unclassified; IFX, infliximab; IMM, immunomodulators; TDM, therapeutic drug monitoring; TNF, tumor necrosis factor; UC, ulcerative colitis; 6-MP, 6-mercaptopurine.
Montreal Classification.
Baseline patient and disease characteristics were comparable between the proactive and reactive TDM group except for CD behavior, although the 2 groups were comparable in terms of complicated (stricturing or penetrating) CD (39 of 89 [44%] vs 42 of 78 [54%], respectively; P = .217) (Table 1). Moreover, the median follow-up time (2.5 [IQR: 1.7–3.3] years vs 2.2 [IQR: 1.3–3.2] years; P = .158), duration from infliximab initiation until start of TDM (15 [IQR: 6–38] vs 13 [IQR: 6–31] months; P = .464), and year of infliximab initiation (2011 [IQR: 2009–2012] vs 2010 [2008–2012]; P = .161) were similar between the proactive and the reactive TDM groups, respectively.
Outcomes
Treatment failure
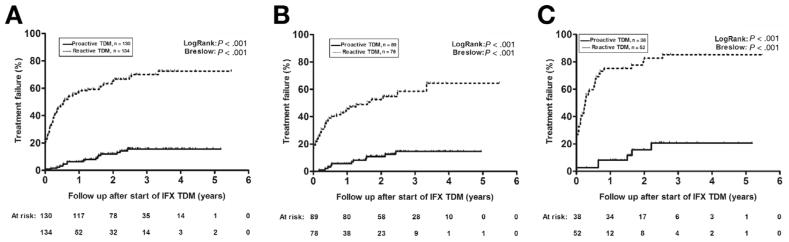
In the entire cohort, 105 patients had a treatment failure during follow-up. Among patients who underwent proactive TDM, only 17 (13%) had a treatment failure, in contrast with 88 (66%) patients in the reactive TDM group. Among patients who underwent reactive TDM, treatment failure was mostly due to loss of response or surgery (n = 73), whereas 15 patients experienced a SAE (SIR, n = 12; acute, n = 8; delayed, n = 4). Among patients who underwent proactive TDM, treatment failure was attributed to loss of response or surgery (n = 13) or SAE (SIR [acute], n = 2; pulmonary infections, n = 2). The first-year cumulative probability of treatment failure was 6% (SE 0.021) for the proactive compared with 57% (SE 0.043) for the reactive TDM group. Kaplan-Meier analysis demonstrated a significantly lower cumulative probability of treatment failure in patients who underwent proactive compared with reactive TDM (log-rank P < .001) (Figure 2A), which as was consistent for both CD (Figure 2B) and UC (Figure 2C). Cox regression identified the use of proactive TDM (hazard ratio [HR], 0.16 [95% confidence interval, 0.09–0.27], P < .001), IBD subtype (UC vs CD) (HR, 1.8 [1.2–2.6], P = .005), and ATI at the start of TDM (HR, 1.8 [1.1–2.9], P = .024) as the only variables independently associated with treatment failure (Table 2).
Figure 2.
Kaplan-Meier cumulative probability curves of treatment failure in patients undergoing either reactive (dotted line) or proactive therapeutic drug monitoring (TDM) (solid line) based on the first infliximab (IFX) concentration measured (A), stratified also by the type of IBD, Crohn’s disease (B) or ulcerative colitis (C).
Table 2.
Variables Associated With Time to Treatment Failure
| Variables | Univariate analysis
|
Multivariate analysis
|
||||
|---|---|---|---|---|---|---|
| P value | HR | 95% CI | P value | HR | 95% CI | |
| Gender | .180 | |||||
| IBD type (UC vs CD) | <.001 | 2.1 | 1.4–3.1 | .005 | 1.8 | 1.2–2.6 |
| CD location | .649 | |||||
| CD behavior | .369 | |||||
| Age at diagnosis | .132 | |||||
| Age at start of IFX | .117 | |||||
| Duration from IFX initiation until start of TDM | .021 | 0.991 | 0.983–0.999 | |||
| UC extension | .359 | |||||
| Perianal fistulizing disease | .050 | |||||
| Ileocolonic resection prior to TDM | .260 | |||||
| Concomitant IMM at start of TDM | .569 | |||||
| Anti-TNF naive | .699 | |||||
| Smoking ever | .150 | |||||
| IFX dosing other than 5 mg/Kg q8w at start of TDM | .051 | |||||
| IFX concentration at start of TDM | <.001 | 0.93 | 0.89–0.97 | |||
| ATI at start of TDM | <.001 | 2.9 | 1.9–4.5 | .024 | 1.8 | 1.1–2.9 |
| Proactive TDM | <.001 | 0.12 | 0.07–0.21 | <.001 | 0.16 | 0.09–0.27 |
ATI, antibodies to infliximab; CD, Crohn’s disease; CI, confidence interval; HR, hazard ratio; IBD, inflammatory bowel disease; IFX, infliximab; IMM, immunomodulators; TDM, therapeutic drug monitoring; TNF, tumor necrosis factor; UC, ulcerative colitis.
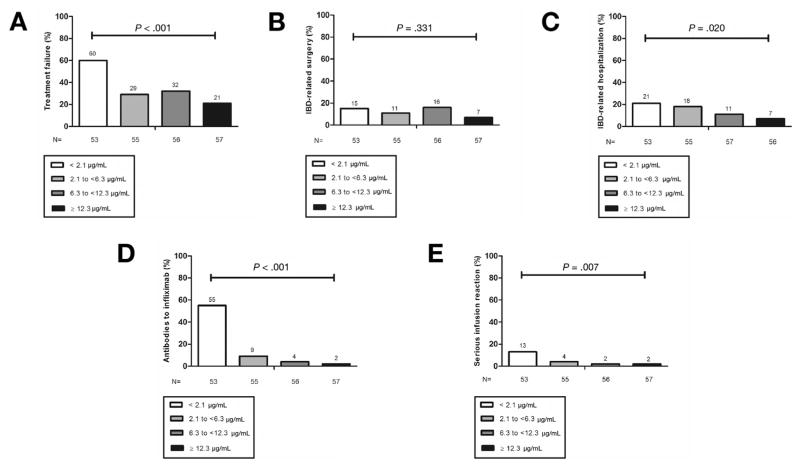
On ROC analysis, an infliximab TC of 3.55 μg/mL was identified as the optimal cutoff for treatment failure (sensitivity 0.75, specificity 0.60) (Supplementary Figure 1A). The relationship between infliximab TC at start of TDM and treatment failure was further analyzed by dividing infliximab TC into quartiles. The lowest infliximab TC quartile was associated with a significant 2–3-fold increased unadjusted rate of treatment failure compared with the other quartiles (Figure 3A).
Figure 3.
Treatment failure (A), inflammatory bowel disease (IBD)–related surgery (B), IBD-related hospitalization (C), antibodies to infliximab (D), and serious infusion reaction (E) by infliximab trough concentration quartiles at start of therapeutic drug monitoring.
IBD-related surgery
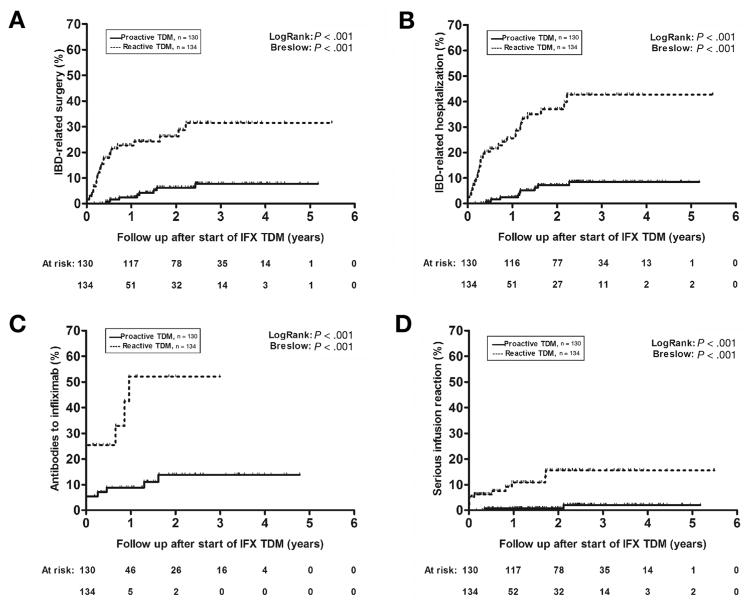
By the end of follow-up, 33 (12.5%) patients underwent an IBD-related surgery (ileocolonic resection or colectomy, n = 25 [76%]). Only 8 (6%) patients who underwent proactive TDM had an IBD-related surgery, compared with 25 (19%) patients in the reactive TDM group. The first-year cumulative probability of IBD-related surgery was 2.4% (standard error 0.014) for the proactive and 22.7% (SE 0.044) for the reactive TDM group. Patients who underwent proactive TDM had a significantly lower cumulative probability of an IBD-related surgery than did those who underwent reactive TDM (log-rank P < .001) (Figure 4A). On multivariable analysis, only the use of proactive TDM (HR, 0.30 [0.11–0.80], P = .017) and perianal fistulizing CD (HR, 3.6 [1.4–9.5], P = .010) were independently associated with an IBD-related surgery (Supplementary Table 1).
Figure 4.
Kaplan-Meier cumulative probability curves of inflammatory bowel disease (IBD)–related surgery (A), IBD-related hospitalization (B), detectable antibodies to infliximab (IFX) (C), and serious infusion reaction (D) in patients undergoing either reactive (dotted line) or proactive therapeutic drug monitoring (TDM) (solid line) based on the first IFX concentration measured.
On ROC analysis, no infliximab TC was identified as the optimal cutoff for IBD-related surgery (Supplementary Figure 1B), and there was no statistically significant association observed between infliximab TC at the start of TDM and IBD-related surgery based on infliximab quartile analysis (Figure 3B).
IBD-related hospitalization
Overall, 42 (16%) patients underwent an IBD-related hospitalization. Among patients who underwent proactive vs reactive TDM, 9 (7%) vs 33 (25%) had an IBD-related hospitalization, with first-year probabilities of IBD-related hospitalization of 2.4% (SE 0.014) vs 25.6% (SE 0.046). The number and days of IBD-related hospitalizations were also lower in patients undergoing proactive than in undergoing reactive TDM (10 days vs 40 days, P < .001; 37 days vs 189 days, P < .001, respectively). The probability of an IBD-related hospitalization was significantly lower among patients who underwent proactive compared with reactive TDM (log-rank P < .001) (Figure 4B). Additionally, the use of proactive TDM (HR, 0.16 [95% confidence interval (CI), 0.07–0.33], P < .001) was the only variable found to be independently associated with IBD-related hospitalization (Supplementary Table 2).
An infliximab TC of 4.65 μg/mL was identified as the optimal cutoff for IBD-related hospitalization (sensitivity 0.63, specificity 0.61) (Supplementary Figure 1C). When examining these data by infliximab TC quartiles, the lowest quartile was associated with highest rate of treatment failure (Figure 3C).
Antibodies to infliximab
During follow-up, ATI were detected in 48 patients (18%), the majority of whom (n = 42) already had detectable ATI at the start of TDM. ATI were seen in 11 (9%) patients undergoing proactive TDM, of whom 7 (5%) had detectable ATI at the start of TDM, compared with 37 (28%) patients undergoing reactive TDM, of whom 35 (26%) had detectable ATI at the start of TDM. Overall, patients monitored proactively had a significantly lower cumulative probability of ATI than did those monitored reactively (log-rank P < .001) (Figure 4C). The only variables found to be independently associated with ATI were the use of proactive TDM (HR, 0.25 [95% CI, 0.07–0.84], P = .025) and infliximab concentration at the start of TDM (HR, 0.7 [95% CI, 0.5–0.8], P < .001) (Supplementary Table 3).
An infliximab TC of 1.85 μg/mL was identified as the optimal cutoff for detectable ATI (sensitivity 0.89, specificity 0.78) (Supplementary Figure 1D). Subdividing the data by infliximab TC quartiles, the lowest quartile was associated with a significant increased rate of ATI compared with the other quartiles (Figure 3D).
Serious infusion reaction
Fourteen (5%) patients developed an SIR, most of whom (n = 10 [71%]) also had ATI. Most SIRs occurred in patients in the reactive TDM group (12 [9%] vs 2 [2%]), and this difference over time was significant (log-rank P = .001) (Figure 4D). The use of proactive TDM (HR, 0.17 [95% CI; 0.04–0.78], P = .023) and infliximab concentration at the start of TDM (HR, 0.78 [95% CI; 0.65–0.94], P = .011) were the only variables found to be independently associated with a SIR (Supplementary Table 4).
An infliximab TC of 6.35 μg/mL was identified as the optimal cutoff for SIR (sensitivity 0.51, specificity 0.91) (Supplementary Figure 1E). On infliximab TC quartile analysis, the lowest quartile was associated with significant increased rate of SIR compared with the other quartiles (Figure 3E).
TDM and Treatment Modifications
The median time from start of infliximab until TDM initiation for the entire population was 14.3 (IQR: 5.6–35.4) months and was comparable between the 2 groups (Table 1). TDM assays were performed on 428 serum samples in total and the number of available infliximab measurements per patient ranged from 1 to 13. Based on initial testing, 151 (57%) samples were evaluated with the HMSA assay. Data regarding the first infliximab TC, ATI, and dosing changes in patients undergoing proactive vs reactive TDM are depicted in Supplementary Table 5. Based on initial testing, patients who underwent proactive TDM had a higher median infliximab TC compared with those who underwent reactive TDM (9.1 μg/mL vs 3 μg/mL; P < .001) and a lower rate of positive ATI (5% vs 26%; P < .001). Moreover, based on target infliximab TC therapeutic ranges of 3–7 μg/mL or 5–10 μg/mL, only 22% or 31%, respectively, of patients who underwent proactive TDM, had a subtherapeutic infliximab concentration, compared with 47% or 59%, respectively, of patients in the reactive TDM group. Based on initial testing, 38 (29%) patients undergoing reactive TDM had to discontinue infliximab mainly due to ATI with low or undetectable infliximab concentrations (n = 25 of 38 [66%]) or (supra)therapeutic infliximab TC (n = 13 of 38 [34%]), the latter implying a possible evolution of the IBD inflammatory process to a non–TNF-driven biology.
Discussion
TDM is becoming more common in the treatment of IBD, but is still typically performed in the setting of loss of response or concern for anti-TNF therapy related adverse events. This is the first study to compare the clinical utility of a proactive versus reactive TDM-based therapeutic strategy for optimizing infliximab in IBD. The use of proactive compared with reactive TDM was found to be associated with significantly better effectiveness and safety, as measured by greater durability on infliximab treatment, less need for IBD-related surgery and hospitalization, and less immunogenicity and SIR. Our results suggest that it is better to optimize infliximab therapy before loss of response or a drug related adverse event happens rather than wait for these undesirable outcomes to occur before testing.
Our results are not surprising for several reasons. Significantly fewer patients who were proactively monitored had a first undetectable or subtherapeutic infliximab TC or detectable ATI than did those monitored reactively. Specifically, approximately 30% of patients in the reactive group either had to discontinue infliximab for loss of response or SIR, or underwent surgery early after the first evaluation of infliximab concentration or ATI, most of whom (approximately two-thirds) had low infliximab concentrations or high titers of ATI. Thus, for many patients who are monitored reactively, the monitoring has occurred too late to receive any kind of treatment optimization, as severe intestinal inflammation and immunogenicity likely exerted an irreversible negative impact on their infliximab pharmacokinetics.
Preliminary data from a randomized controlled trial11 and a small retrospective study12 show that proactive TDM is probably associated with better therapeutic outcomes compared with standard of care, although additional data from large prospective studies are certainly required. The landmark TAXIT (Trough concentration Adapted infliXImab Treatment) study, although not meeting its primary endpoint (clinical and biochemical remission at 1 year after the optimization phase), clearly demonstrated that the infliximab concentration–dosed group needed rescue therapy less frequently and had undetectable infliximab concentrations less often than the clinically dosed group did.11 Additionally, our group was the first to demonstrate the long-term benefit of proactive TDM. In this study, patients undergoing proactive TDM and infliximab dose optimization based on a therapeutic window of 5–10 μg/mL had markedly improved persistence on infliximab when compared with a similar control group of IBD patients receiving standard of care.12
Last, proactive TDM is clinically logical and is standard of care in other clinical situations including UC for cyclosporine and solid organ transplantation for mycophenolate or other immunosuppressants.21,22 In these settings, especially with respect to solid organ transplantation, it is more rational to check drug concentrations before patients reject their organ rather than to wait until after rejection has occurred, when it may be difficult to salvage the organ with additional potentially toxic immunosuppressants and retransplantation may be required.22
Additionally, we identified optimal infliximab TC thresholds of 1.85, 3.55, 4.65, and 6.35 μg/mL for treatment failure, ATI, IBD-related hospitalization, and SIR, respectively. These concentration values are a useful guide for clinicians taking care of patients with IBD and are consistent with prior studies demonstrating that lower infliximab TC are associated with negative therapeutic outcomes including loss of response, higher immunogenicity, and drug discontinuation.23–25
The strengths of this study include the large sample size, long follow-up period, and representation of real-life clinical practice at 2 large referral IBD centers. As TDM is being performed more frequently now than in the past for IBD, the important finding is that testing was shown to be more useful proactively, before symptom onset, rather than reactively, after symptoms have developed and many patients may not benefit from dose optimization. However, our study is inherently limited by the retrospective nature of the design and potential for residual bias. Specifically, the 2 groups being compared are inherently different in that 1 group was clinically asymptomatic (proactive) whereas the other was potentially experiencing a flare (reactive). Moreover, the total population could be a highly selected population as first TDM was performed after a median time of just more than 1 year of infliximab therapy. Nevertheless, confounding by indication is likely limited in this study, as the decision to monitor patients proactively or reactively was largely a matter of physician ideology rather than patient disease activity or other characteristics that could differentially affect the outcomes. Just as importantly, the median follow-up time both prior to and after TDM in each group was similar. An additional limitation of this study is that 2 different TDM assays to detect antibodies to infliximab were used based on evolution of laboratory technology over time. However, the ELISA and HMSA were found to result in similar classifications and interventions in IBD patients treated with infliximab, and with comparable clinical outcomes in prior studies.12,26 Moreover, another limitation could be the lack of a control group that did not undergo TDM.27
In conclusion, this multicenter study reflecting real-life clinical practice demonstrates that optimization of maintenance infliximab prior to loss of response (proactive TDM) is associated with lower risks of treatment failure, IBD-related surgery and hospitalization, ATI, and SIR than is reactive TDM. Given these retrospective results, if physicians are considering testing for infliximab levels or ATI, consideration should be given for doing such testing proactively rather than reactively.
Supplementary Material
Acknowledgments
Mark T. Osterman and Adam S. Cheifetz contributed equally to this article.
Funding
Konstantinos Papamichael received a fellowship grant from the Hellenic Group for the Study of IBD.
Abbreviations used in this paper
- ATI
antibodies to infliximab
- CD
Crohn’s disease
- CI
confidence interval
- ELISA
enzyme-linked immunosorbent assay
- HMSA
homogeneous mobility shift assay
- HR
hazard ratio
- IBD
inflammatory bowel disease
- IQR
interquartile range
- ROC
receiver operating characteristic
- SAE
serious adverse event
- SIR
serious infusion reaction
- TC
trough concentration
- TDM
therapeutic drug monitoring
- TNF
tumor necrosis factor
- UC
ulcerative colitis
Footnotes
To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://dx.doi.org/10.1016/j.cgh.2017.03.031.
Conflicts of interest
The authors disclose the following: Byron P. Vaughn receives research support from Takeda and Genentech and has received compensation from Janssen and AbbVie for speaking and advisory boards. Mark T. Osterman received consultancy fees from Janssen, AbbVie, UCB, Takeda, Pfizer, and Lycera, and received research grant support from UCB. Adam S. Cheifetz received consultancy fees from AbbVie, Janssen, UCB, Takeda, Prometheus, and Pfizer. The remaining authors disclose no conflicts of interest.
References
- 1.Osterman MT, Haynes K, Delzell E, et al. Comparative effectiveness of infliximab and adalimumab for Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:811–817. doi: 10.1016/j.cgh.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-Horin S, Chowers Y. Loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther. 2011;33:987–995. doi: 10.1111/j.1365-2036.2011.04612.x. [DOI] [PubMed] [Google Scholar]
- 3.Papamichael K, Cheifetz AS. Use of anti-TNF drug concentrations to optimise patient management. Frontline Gastroenterol. 2016;7:289–300. doi: 10.1136/flgastro-2016-100685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaughn BP, Sandborn WJ, Cheifetz AS. Biologic concentration testing in inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:1435–1442. doi: 10.1097/MIB.0000000000000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab concentrations in patients with inflammatory bowel disease (IBD): a meta-analysis. Am J Gastroenterol. 2013;108:40–47. doi: 10.1038/ajg.2012.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adedokun OJ, Sandborn WJ, Feagan BG, et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology. 2014;147:1296–1307. doi: 10.1053/j.gastro.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 7.Papamichael K, Van Stappen T, Vande Casteele N, et al. Infliximab concentration thresholds during induction therapy are associated with short-term mucosal healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14:543–549. doi: 10.1016/j.cgh.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough concentration and decrease of C-reactive protein concentration are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut. 2014;63:1721–1727. doi: 10.1136/gutjnl-2012-304094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papamichael K, Baert F, Tops S, et al. Post-induction adalimumab concentration is associated with short-term mucosal healing in patients with ulcerative colitis. J Crohns Colitis. 2017;11:53–59. doi: 10.1093/ecco-jcc/jjw122. [DOI] [PubMed] [Google Scholar]
- 10.Ungar B, Levy I, Yavne Y, et al. Optimizing anti-TNFα therapy: Serum concentrations of infliximab and adalimumab associate with mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2016;14:550–557. doi: 10.1016/j.cgh.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 11.Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148:1320–1329. doi: 10.1053/j.gastro.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 12.Vaughn BP, Martinez-Vazquez M, Patwardhan VR, et al. Proactive therapeutic concentration monitoring of infliximab may improve outcomes for patients with inflammatory bowel disease: results from a pilot observational study. Inflamm Bowel Dis. 2014;20:1996–2003. doi: 10.1097/MIB.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taks M, Pijls PA, Derijks LJ, et al. The effect of implementation of a treatment algorithm for infliximab on remission rates and drug costs in inflammatory bowel disease patients. Eur J Gastroenterol Hepatol. 2017;29:169–173. doi: 10.1097/MEG.0000000000000763. [DOI] [PubMed] [Google Scholar]
- 14.Steenholdt C, Brynskov J, Thomsen OO, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut. 2014;63:919–927. doi: 10.1136/gutjnl-2013-305279. [DOI] [PubMed] [Google Scholar]
- 15.Steenholdt C, Brynskov J, Thomsen OO, et al. Individualized therapy is a long-term cost-effective method compared with dose intensification in Crohn’s disease patients failing infliximab. Dig Dis Sci. 2015;60:2762–2770. doi: 10.1007/s10620-015-3581-4. [DOI] [PubMed] [Google Scholar]
- 16.Velayos FS, Kahn JG, Sandborn WJ, et al. A test-based strategy is more cost effective than empiric dose escalation for patients with Crohn’s disease who lose responsiveness to infliximab. Clin Gastroenterol Hepatol. 2013;11:654–666. doi: 10.1016/j.cgh.2012.12.035. [DOI] [PubMed] [Google Scholar]
- 17.Singh S, Heien HC, Sangaralingham LR, et al. Comparative effectiveness and safety of anti–tumor necrosis factor agents in biologic-naive patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2016;14:1120–1129. doi: 10.1016/j.cgh.2016.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fidder H, Schnitzler F, Ferrante M, et al. Long-term safety of infliximab for the treatment of inflammatory bowel disease: a single-centre cohort study. Gut. 2009;58:501–508. doi: 10.1136/gut.2008.163642. [DOI] [PubMed] [Google Scholar]
- 19.Afif W, Loft us EV, Jr, Faubion WA, et al. Clinical utility of measuring infliximab and human antichimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol. 2010;105:1133–1139. doi: 10.1038/ajg.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang SL, Ohrmund L, Hauenstein S, et al. Development and validation of a homogeneous mobility shift assay for the measurement of infliximab and antibodies-to-infliximab concentrations in patient serum. J Immunol Methods. 2012;382:177–188. doi: 10.1016/j.jim.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Van Assche G, D’Haens G, Noman M, et al. Randomized, double-blind comparison of 4 mg/kg versus 2 mg/kg intravenous cyclosporine in severe ulcerative colitis. Gastroenterology. 2003;125:1025–1031. doi: 10.1016/s0016-5085(03)01214-9. [DOI] [PubMed] [Google Scholar]
- 22.Capron A, Haufroid V, Wallemacq P. Intra-cellular immunosuppressive drugs monitoring: A step forward toward better therapeutic efficacy after organ transplantation? Pharmacol Res. 2016;111:610–618. doi: 10.1016/j.phrs.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 23.Vande Casteele N, Khanna R, Levesque BG, et al. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn’s disease. Gut. 2015;64:1539–1545. doi: 10.1136/gutjnl-2014-307883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yanai H, Lichtenstein L, Assa A, et al. Concentrations of drug and antidrug antibodies are associated with outcome of interventions after loss of response to infliximab or adalimumab. Clin Gastroenterol Hepatol. 2015;13:522–530. doi: 10.1016/j.cgh.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 25.Vande Casteele N, Gils A, Singh S, et al. Antibody response to infliximab and its impact on pharmacokinetics can be transient. Am J Gastroenterol. 2013;108:962–971. doi: 10.1038/ajg.2013.12. [DOI] [PubMed] [Google Scholar]
- 26.Steenholdt C, Bendtzen K, Brynskov J, et al. Clinical implications of measuring drug and antidrug antibodies by different assays when optimizing infliximab treatment failure in Crohn’s disease: post hoc analysis of a randomized controlled trial. Am J Gastroenterol. 2014;109:1055–1064. doi: 10.1038/ajg.2014.106. [DOI] [PubMed] [Google Scholar]
- 27.Billiet T, Cleynen I, Ballet V, et al. Prognostic factors for long-term infliximab treatment in Crohn’s disease patients: a 20-year single centre experience. Aliment Pharmacol Ther. 2016;44:673–683. doi: 10.1111/apt.13754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.