Abstract
Introduction
Schizophrenia (SCZ) and bipolar disorder (BP) are linked to multiple impairments in everyday functioning which share cognitive and symptom risk factors. Other risk factors for critical aspects of every day functioning (e.g., gainful employment; residential independence) such as physical health have not been evaluated, despite poor health in SCZ and BP.
Methods
We analyzed 20-year follow-up data from the Suffolk County Mental Health Project cohort of consecutive first admissions with a psychotic disorder to 12 psychiatric facilities in Suffolk County, NY, between September 1989 and December 1995. Both 20-year symptom, health, and cognition data, and the 20-year course of weight gain were included as predictors of employment and residence status.
Results
The analysis sample consisted of 122 participants with SCZ ad BP, with SCZ participants less likely to work or live independently. Correlational analyses showed symptoms and cognition predicted vocational outcomes in both samples. The effect of diagnosis was significant for both gainful employment and independence in residence. After consideration of diagnosis, mobility and negative symptoms predicted gainful employment in both samples, but there were no additional predictors of residential independence. Prospective analysis of BMI found that baseline BMI, but not changes during the 20-year follow up, predicted labor force participation.
Discussion
Health status limitations were associated with residential and, particularly, employment status independent from other, previously established predictors of everyday outcomes, including cognition and symptoms. The importance of health status limitations for predicting outcome was confirmed in both SCZ and BP, with schizophrenia representing the more impaired group.
Introduction
Severe and persistent mental illnesses (SPMI), including schizophrenia (SCZ) and bipolar disorder (BP), are associated with high rates of disability in the US and worldwide (Kim et al., 2013; Murray et al., 2013; Whiteford et al., 2010). Despite the striking nature of psychotic and manic symptoms, the costliest impairments include wide-ranging limitations in critical areas of daily functioning, particularly in the ability to live and work independently (Huxley & Baldessarini, 2007; McEvoy, 2007; Jin & McCrone, 2015), which is lower than population norms (Harvey, 2009; Harvey et al., 2010).
Disability is a complex construct attributable to a cascade of multiple interdependent influences. In SPMI, known determinants of disability include cognitive deficits, which are indexed by performance on neuropsychological tests, and other specific symptoms (e.g., enduring negative symptoms, treatment refractory psychosis, or recurrent episodes of mania or depression). However, even in combination with impairments in performance-based measures of functional capacity (“skills deficits”), these predictors account for less than 50% of the total variance, suggesting that other, unexplored factors contribute to the high rates of disability in SPMI (Bowie et al., 2006, 2008, 2010).
Previous studies of BP and SCZ have found that predictors of daily functional outcomes are similar in the two patient populations (Bowie et al., 2010; Mausbach et al., 2010). However, when compared to BP patients, individuals with SCZ have poorer residential outcomes and performance on measures of daily living skills, lower educational achievement, and greater impairment in clinician ratings of everyday outcomes (Mausbach et al., 2010; Meyer et al., 2014; Velthorst et al., 2016). These cross-diagnostic relative levels of impairments hold across different countries and cultures (McIntosh et al, 2011), although the correlations between skills deficits and outcomes seems quite consistent on a cross-national basis.
Given the high prevalence of obesity (De Hert et al., 2010; Allison et al., 2009; Fagiolini et al., 2008) and poor physical functioning in individuals with SPMI (Vancampfort et al., 2017; Strassnig et al., 2014), we hypothesized that physical health limitations may have an even greater influence on disability in SPMI groups than in mentally healthy individuals (Scott et al., 2009) (Figure 1). Individuals with SPMI often have a poor diet, are sedentary, engage in little or no physical exercise, and are persistent smokers (Newcomer & Hennekens, 2007), and these risk factors are exacerbated by cognitive deficits and clinical symptoms (Harvey & Strassnig, 2012), as well as chronic treatment with sedating and obesogenic psychotropic medications.
Fig 1.
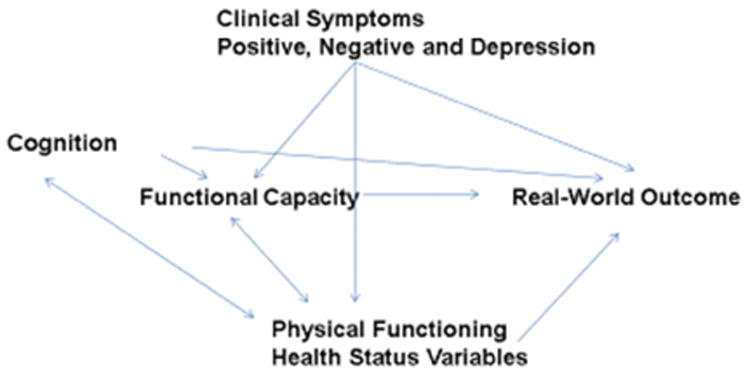
Physical health and functioning predict Disability in SMI
We know of no studies of the SPMI population that have examined the relationships of obesity and physical functioning, together with cognitive impairments and clinical symptom severity, to the ability to live independently or sustain employment. In this paper, we examine these relationships using data from a well-characterized sample that was followed for 20 years after first hospitalization for a psychotic episode. We also used these data to assess whether people with BP and SCZ show similar associations between clinical, cognitive, and physical variables and daily functioning.
Methods
All participants provided written informed consent at the initial and subsequent assessments. Inclusion criteria were age 15-60 years, residence in Suffolk County NY, and psychosis not due to a medical condition. Exclusion criteria were a psychiatric hospitalization more than 6 months before the index admission, more than borderline intellectual disability (IQ < 70), inability to provide informed consent, and being a non-English speaker. The data were collected at 6 months, and 2, 4, 10, and 20 years after the index assessment.
Twenty-year follow up measures of cognitive performance, psychiatric symptoms, and physical performance were analyzed in study participants with a 10-year follow up diagnosis of SCZ or BP.
The Structured Clinical Interview for DSM-III-R (SCID: Spitzer et al., 1990) was administered during the baseline, 6-month, and 2-year follow ups, and the SCID for DSM-IV was administered at year 10 (Bromet et al. 2011). Based on the SCIDs, medical record information, and interviews with significant others, longitudinal DSM-IV consensus diagnoses for each participant was reached (Bromet et al., 1992; Bromet et al., 2011). In this analysis, we used the 10-year follow up diagnosis, which was the most recent available.
Clinical and Health measures
The cross-sectional, 20-year clinical and health correlates included: Scale for Assessment of Negative Symptoms and Scale for Assessment of Positive Symptoms (SANS and SAPS; Andreasen, 1981); cognitive functioning (see below); waist circumference, and physical functioning measured by chair rise ability, a task developed originally to test physical performance in geriatric samples with the Short Physical Performance Battery (Guralnik et al., 1994) for the Centers for Disease Control and Prevention (Guralnik et al., 1996). This battery is used widely in epidemiological research to determine basic physical functioning (Rikli & Jones, 1999). Chair Stand Tests serve as a proxy measure for lower extremity strength that is pivotal for daily functional performance (Brown et al., 1995). Because of concern for potential ceiling effects in our younger, non-geriatric population, we used a modified, expanded test: participants were asked to rise from a chair ten times as fast as possible with their arms folded across their chests rather than five times, as required in the original SPPB. We recorded the total time required to rise from the chair ten times to obtain a continuous score, which was then scaled with a formula (70-T)/70. These Expanded Timed Repeated Chair Stands were completed with 104 (81.3%) of the 128 participants.
To assess cognitive functioning, participants completed a comprehensive battery of cognitive tests at the 20-year assessment that represent eight major ability areas (Mojtabai et al., 2000; Reichenberg et al., 2009). For general verbal ability, we used the Wechsler Adult Intelligence Scale-Revised (WAIS-R) Vocabulary and Information subtest; for verbal declarative memory, the Wechsler Memory Scale-Revised (WAIS-R) Verbal Paired Associates I and II; for visual declarative memory, the WMS-R Visual Reproduction I and II; for executive function, the Stroop Color-Word Test, time on part B of the Trail Making Test, and for working memory, Letter Number Span and digit span. For processing speed, we used the time on part A of the Trail Making Test; for visual processing, the Facial Recognition Test, and for language ability, Letter Fluency. We converted all raw scores on these cognitive tests to standardized (z) scores for the current population sample, and an overall summary measure was computed, as described in the statistical methods.
Longitudinal physical health was assessed with the body mass index (BMI), calculated at each assessment beginning at the 6-month follow up assessment. Weight and height were self-reported except at the 20-year assessment, when they were measured directly. This led to the calculation of a BMI score for each participant at each assessment (kg/m2).
Outcome measures
Two interviewer-coded measures were included: residential independence (defined as living fully independently without any external support, such as group homes or supported settings) and gainful employment (part or full time employment in compensated, non-supportive settings). We chose interviewer-rated outcomes to provide a less biased method for rating outcomes, as interviewers integrated information from interviews with participants, their significant others, and medical records.
Statistical Analyses
We calculated a composite neuropsychological test performance score for the sample by standardizing all cognitive test data available, as described above, and creating a mean z-score. An alternate approach, constructing a single principal component from these scores, showed that the single principal component correlated r=0.92 with the composite z-score, suggesting equivalence of these two potential approaches. We used regression analyses to predict our two functional outcomes. To predict each of the two outcomes, we used a blocked entry regression analysis, entering diagnosis (SCZ/BP) first and then entering the other predictors second.
For our longitudinal analysis, we used structural equation modeling (SEM) growth models using latent intercept, linear slope, and quadratic slope variables. BMI was centered at the first time point (6 months post first episode). Linear change in BMI over time was examined using two models, one with equal residual variances, and one with freely estimated residual variances. Likelihood ratio testing indicated that the model with more parameters was the most appropriate. Subsequently, a model that examined linear and quadratic trends was used to predict labor force participation and independent residence. Exploratory follow up analyses were used to examine the potential mediating effects of SAPS, SANS, chair stands, cognition, and waist circumference at the 20-year endpoint on the relationship between BMI growth and BMI at the onset of illness, and each outcome (residential status and employment).
Results
The final sample included 128 patients with complete data (80 SCZ; 48 BP). Mean age at the 20-year assessment was 47.8±8.6, and 54.2% were male. 62.7% had a diagnosis of schizophrenia or schizoaffective disorder and 37.3% of BP. Participants included 2.6% Asian, 9.4% African American, and 78.5% white, with 9.4% other/unknown, reflecting local population characteristics. In the entire sample, 32.9% were employed, and 53.9% maintained independent residence at the 20-year assessment. There were no statistically significant differences in age or gender distribution between the two groups.
Generally, participants with SCZ exhibited more symptoms and cognitive impairment, had larger waist circumferences, and completed fewer chair stands than did individuals with BP (Table 1). They also were less likely to be living independently (q=51.90, p<0.001) and to be employed (q=26.30, p<0.05).
Table 1. Sample Characteristics: Symptoms, Cognition, Metabolic Parameters, Residential and Employment Status.
| SCZ n=80 | BP n=48 | t | p | |
|---|---|---|---|---|
| SANS | 26±14.8 | 11.6±11.6 | 5.50 | <0.001 |
| SAPS | 10.7±10.9 | 3.8±6.7 | 3.80 | <0.001 |
| Cognitive performance (z-Scores) | -0.11±0.48 | 0.31±0.4 | -4.80 | <0.001 |
| Waist Circumference inches | 45.9±7.4 | 41.5±6.7 | 3.12 | <0.001 |
| Chair Stands** | 55±22% | 67±18% | 3.37 | <0.001 |
| Residential Independence | 21 (27.8%) | 32 (76.7%) | X2(1)=52.0 | <0.001 |
| Gainful Employment | 60 (20.6%) | 23 (53.4%) | X2(1)=26.3 | <0.001 |
Average of three measures
The number of rises to a full standing position in 60 seconds was recorded. We dichotomized chair rise speed into <4 versus 5 or more (%) according to guidelines.
Correlational Analysis
Next, we computed nonparametric correlations between the predictors and outcome variables in the two samples separately (Table 2). We noted first that the correlations between gainful employment and residential independence were small for both groups (0.21 for SCZ and 0.16 for BP). In the SCZ sample, four of the five variables were associated significantly in the directions expected with labor force participation (the exception was waist circumference); the SAPS was the only variable that was associated negatively and significantly with residential independence. In the BP group, all of the predictors but the SANS were associated significantly with employment, but none was associated with residential independence.
Table 2. Intercorrelations of Predictor and Outcome Variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. SAPS total score | -- | 0.16 | 0.04 | -0.15 | -0.05 | 0.03 | -0.21* |
| 2. SANS total score | 0.31* | -- | -0.19 | 0.04 | -0.23* | -0.25* | -0.38* |
| 3. Cognition Composite | 0.09 | -0.16 | -- | -0.14 | 0.14 | 0.02 | -0.21* |
| 4. Waist Circumference | 0.06 | 0.28* | 0.04 | -- | -0.02 | -0.09 | -0.15 |
| 5. Chair stands | 0.05 | -0.14 | 0.37* | -0.22 | -- | 0.04 | 0.27* |
| 6. Residential Independence | 0.07 | -0.14 | 0.12 | -0.10 | 0.06 | -- | 0.21* |
| 7. Labor Force Participation | -0.21 | -0.38* | 0.43* | -0.27* | 0.36* | 0.16 | -- |
Note. Schizophrenia correlations are above the diagonal, bipolar correlations are below. Spearman correlations computed.
We then performed two stepwise logistic regressions to estimate the best combination of variables that explained each outcome. In these models, the diagnosis was entered as the first block and then the other five variables described above were entered in the second block, in a stepwise model. P values for entry were set at <0.05. The model for labor force participation showed that, in the first block, diagnosis accounted for 11% of the variance (p<0.001). In the second block, chair stands entered the equation first, and accounted for 9% of the incremental variance (p<0.001). SANS total scores were entered next and accounted for another 5% of the variance (p<0.005). Thus, after adjusting for diagnosis, impairments in mobility and negative symptoms added 14% of the incremental variance in the final model for labor force participation; the other variables were nonsignificant (all p>0.08).
The modeling for residential independence was performed identically. In block 1, diagnosis accounted for 15.5% of the variance (p<0.001). In block 2, none of the variables entered the equation. To examine whether there were diagnosis-specific predictors of residential independence, we performed separate logistic regression analyses on the two samples. The predictors (e.g., SAPS, SANS, cognition, waist circumference, and chair stands) remained nonsignificant.
Weight Gain over 20 Years as a Predictor of Outcome
When we examined the course of BMI with latent growth models, its changes over time did not predict either of the two outcomes. Specifically, a model with BMI quadratic and linear growth predicting independent residence provided a poor fit to the data (χ2(18)=68.78, p<0.001, RMSEA=0.11 (90% CI [0.08, 0.14]), p<0.001, CFI=0.91). BMI at the first assessment also did not predict independent residence (b=-0.005, p=0.83), nor did the linear (b=-0.56, p=0.12), or quadratic trends (b=-8.62, p=0.32). We ran a model with BMI quadratic and linear growth predicting employment at the 20-year follow up. This model provided an acceptable fit to the data (χ2(18)=42.66, p=0.001, RMSEA=0.077 (90% CI [0.05, 0.11), p=0.07, CFI=0.96). Although the linear and quadratic trends were not significant, BMI at illness onset predicted employment significantly at 20-year follow up (b=-0.05, p=0.03). This finding indicated that the higher the individual's BMI at illness onset, the less likely that individual was to be employed at 20-year follow-up, a finding not seen with residential independence.
Follow-up mediation analyses were run to examine the potential mediating effects of SAPS, SANS, chair stands, cognition, and waist circumference on the relationship between BMI growth and BMI at illness onset, and both residential status and employment. No mediation effects were significant (all p>0.05).
Discussion
We show that health status limitations, recognized recently as pervasive in severe mental illness, predicted employment in a manner independent from other correlates established previously, including cognition and symptoms, and the importance of symptoms and physical limitations in predicting employment outcomes was confirmed in both BP and SCZ. In the multivariable models, a different set of factors predicted the two objective outcome parameters (employment, independent residence). Approximately 30% of the variance in employment was attributable to a combination of physical mobility, assessed by chair stands, and the magnitude of negative symptoms. With the predictive influence of diagnosis controlled, chair stands, followed by negative symptoms, were the most significant predictors of employment. In contrast, and likely because of the major differences in residential outcomes between the groups, none of the variables but diagnosis predicted residential independence. Cognition was not a predictor of employment when physical functioning was considered, but was correlated with the functional outcomes variables and the other predictors, particularly in the BP group.
The results support a closer examination of health status limitations as novel predictors of disability in SPMI, as we have proposed previously (Harvey & Strassnig, 2012). The increased prevalence of abdominal obesity and metabolic derangements in the SPMI population has made providers more aware of health risks and has led to more proactive medical treatment (Janssen et al., 200). Nevertheless, the functional implications for employment-related disability 20 years after first hospitalization suggest a compelling need to focus on controlling BMI early in the disease course. The finding that baseline BMI was a significant longitudinal predictor of employment status at the 20-year mark is understandable in the context of previous research. Metabolic abnormalities, including weight gain and detrimental changes in glucoregulation and lipid metabolism, appear to have the most pronounced effects early in treatment (Strassnig et al., 2015) and in most cases, never remit (De Hert et al., 2011). Perhaps reflecting the persistent environmental challenges faced by people with SPMI, poor physical functioning is exacerbated over time (Strassnig et al., under review), and becomes increasingly relevant later in the course of the illness. However, the predictive importance of baseline BMI (i.e., 6 months after diagnosis) highlights the critical importance of early intervention.
Metabolic abnormalities may affect cognition adversely as well (Depp et al., 2014; Yim et al., 2012; Friedman et al., 2010; Dickinson et al., 2008; Tsai et al., 2009), as in mentally healthy but metabolically compromised people (Willette & Kapogiannis, 2014; Beeri et al., 2009; Panza et al., 2010; McCrimmon et al., 2012; Novak and Haijar, 2010). This may account for some of the variance in cognitive deficits seen in SPMI. It also may explain why the zero-order correlations with cognition and functional outcomes lose their influence when physical functioning factors are considered as well. Better cognitive performance also correlated positively with chair stands. Finally, negative, but not positive symptoms, correlated with waist circumference. Increased waist circumference in patients with higher negative symptom load may simply represent a physical manifestation of the inherent anergia/amotivation that leads to reduced energy expenditure.
The relative inability to predict residential outcomes may be attributable to several factors. First, cognition, and even negative symptoms, may exert much of their influence on residential outcomes through impaired functional capacity (Bowie et al., 2010), measures of which were not included in the current analysis. Several studies have found that performance-based indices of functional capacity (skills deficits) are correlated at least as strongly with real-world functional outcomes as are cognitive performance and negative symptoms (Bowie et al., 2008). Cognitive impairments may be most important in separating individuals unable to perform even minimal functional tasks from those with higher levels of achievement who do not reach the threshold of full time work or residential independence, but are able to perform at least marginally, particularly in BP. Physical health, mobility, and symptoms also may be operative at these lower levels of functioning as well.
A second factor that makes residential independence challenging to predict is the fact that the majority of our SCZ patients also were unemployed. Chronic unemployment has the potential to lead to chronic residential problems, particularly in an area such as Suffolk County with its high cost-of-living compared to the national average. In our sample, 66/80 of the SCZ patients were both unemployed and living dependently, while only 8/48 BP patients failed to achieve both milestones.
On a practical level, our results clarified the differential effects of certain health status variables on outcomes. Successful employment that requires active and sustained physical effort was predicted by chair stands. Moreover, success in employment settings may require not only a desire on the part of patients to seek and sustain work and overcome negative symptoms—the other relevant predictor—but also perhaps, as our data suggested, adequate physical ability to do so and continue to do so. This is particularly the case with many entry-level jobs, which typically require some physical stamina, and patients with a lifelong history of challenges in educational domains may lack the requisite skills to be eligible for less physically demanding jobs. Further, given the stigmas associated with SPMI and obesity, it seems plausible that people with high BMI and SPMI would have more trouble finding work in the first place, and once their employment history became inconsistent, it would become even more difficult to obtain meaningful employment.
Overall, we surmise that physical health limitations have a differential effect on important areas of daily outcomes. These limitations reduce real-world performance further in areas in which the intrinsic limitations of SPMI, such as symptoms and cognition, also are operative. Thus, health status limitations should be considered in the overall prediction of real-world functioning and interventions designed to reduce disability that target health status may be needed to address both obesity and metabolic derangements, as well as improve physical functioning, which will add to the interventions available for cognitive enhancement, skills training, and public advocacy for better services.
Limitations
The initial BMI measures were obtained at month 6 as opposed to the first contact baseline assessment which had a predominantly diagnostic focus. As a result, we were unable to measure the early impact of weight gain on later functioning and disability during a time period where significant treatment induced weight gain tends to occur. However, we have recently described the weight gain trajectories in this sample (Strassnig et al, 2017) and have found that weight gain in both SCZ and BP continues to accumulate over the entire 20 year observation period. While the available data does not allow us to estimate the impact of the magnitude of early weight gain on functional trajectories over 20 years, or estimate the relation between illness severity and early weight gain, the data shows the effects of long-term weight gain on functioning.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature
- Kim SS, Vos T, Flaxmant T, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2010;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJ, et al. U.S. Burden of Disease Collaborators. The state of US health, 1990-2010: Burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford HA, Ferrari AJ, Degenhardt L, Feigin V, Vos T. The global burden of mental, neurological and substance use disorders: An analysis from the Global Burden of Disease Study 2010. PLoS One. 2010;10(2):e0116820. doi: 10.1371/journal.pone.0116820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley N, Baldessarini RJ. Disability and its treatment in bipolar disorder patients. Bipolar Disord. 2007;9(1-2):183–196. doi: 10.1111/j.1399-5618.2007.00430.x. [DOI] [PubMed] [Google Scholar]
- McEvoy JP. The costs of schizophrenia. J Clin Psychiatry. 2007;68(14):4–7. [PubMed] [Google Scholar]
- Jin H, McCrone P. Cost-of-illness studies for bipolar disorder: Systematic review of international studies. Pharmacoeconomics. 2015;33(4):341–353. doi: 10.1007/s40273-014-0250-y. [DOI] [PubMed] [Google Scholar]
- Harvey PD. Functional recovery in schizophrenia: Raising the bar for outcomes in people with schizophrenia. Schizophr Bull. 2009;35(2):299. doi: 10.1093/schbul/sbn186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Wingo AP, Burdick KE, Baldessarini RJ. Cognition and disability in bipolar disorder: lessons from schizophrenia research. Bipolar Disord. 2010;12(4):364–375. doi: 10.1111/j.1399-5618.2010.00831.x. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Reichenberg A, Patterson TL, et al. Determinants of real-world functional performance in schizophrenia subjects: Correlations with cognition, functional capacity, and symptoms. Am J Psychiatry. 2006;163(3):418–425. doi: 10.1176/appi.ajp.163.3.418. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Leung WW, Reichenberg A, et al. Predicting schizophrenia patients' real world behavior with specific neuropsychological and functional capacity measures. Biol Psychiatry. 2008;63:505–511. doi: 10.1016/j.biopsych.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie CR, Depp C, McGrath JA, et al. Prediction of real world functional disability in chronic mental disorders: A comparison of schizophrenia and bipolar disorder. Am J Psychiatry. 2010;167:1116–1124. doi: 10.1176/appi.ajp.2010.09101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mausbach BT, Harvey PD, Pulver AE, et al. Relationship of the Brief UCSD Performance-based Skills Assessment (UPSA-B) to multiple indicators of functioning in people with schizophrenia and bipolar disorder. Bipolar Disord. 2010;1:45–55. doi: 10.1111/j.1399-5618.2009.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EC, Carrión RE, Cornblatt BA, et al. The relationship of neurocognition and negative symptoms to social and role functioning over time in individuals at clinical high risk in the first phase of the North American prodrome longitudinal study. Schizophr Bull. 2014;40(6):1452–1461. doi: 10.1093/schbul/sbt235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velthorst E, Fett AJ, Reichenberg A, et al. The 20-year longitudinal trajectories of social functioning in individuals with psychotic disorders. Am J Psychiatry. 2016:appiajp201615111419. doi: 10.1176/appi.ajp.2016.15111419. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh BJ, Zhang XY, Kosten T, et al. Performance based assessment of functional skills in severe mental illness: Results of a large-scale study in China. J Psychiatr Res. 2011;4:1089–1094. doi: 10.1016/j.jpsychires.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hert M, Schreurs V, Vancampfort D, et al. Metabolic syndrome in people with schizophrenia: A review. World Psychiatry. 2009;8(1):15–22. doi: 10.1002/j.2051-5545.2009.tb00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison DB, Newcomer JW, Dunn AL, et al. Obesity among those with mental disorders: A National Institute of Mental Health meeting report. Am J Prev Med. 2009;36(4):341–350. doi: 10.1016/j.amepre.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Fagiolini A, Chengappa KN, Soreca I, et al. Bipolar disorder and the metabolic syndrome: Causal factors, psychiatric outcomes and economic burden. CNS Drugs. 2008;22(8):655–669. doi: 10.2165/00023210-200822080-00004. [DOI] [PubMed] [Google Scholar]
- Vancampfort D, Rosenbaum S, Schuch F, et al. Cardiorespiratory fitness in severe mental illness: A systematic review and meta-analysis. Sports Med. 2017;47(2):343–352. doi: 10.1007/s40279-016-0574-1. [DOI] [PubMed] [Google Scholar]
- Strassnig M, Signorile J, Gonzalez C, Harvey PD. Physical performance and disability in schizophrenia. Schizophr Res Cogn. 2014;1(2):112–121. doi: 10.1016/j.scog.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott KM, Von Korff M, Alonso J, et al. Mental-physical co-morbidity and its relationship with disability: Results from the World Mental Health Surveys. Psychol Med. 2009;39(1):33–43. doi: 10.1017/S0033291708003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer J, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298:1794–1796. doi: 10.1001/jama.298.15.1794. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Strassnig M. Predicting the severity of everyday functional disability in people with schizophrenia: Cognitive deficits, functional capacity, symptoms, and health status. World Psychiatry. 2012;11(2):73–79. doi: 10.1016/j.wpsyc.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams J, Gibbon M, et al. Structured Clinical Interview for DSM-III-R, Patient Edition/Non-patient Edition, (SCID-P/SCID-NP) Washington, D.C: American Psychiatric Press, Inc; 1990. [Google Scholar]
- Bromet EJ, Schwartz JE, Fennig S, et al. The epidemiology of psychosis: The Suffolk County Mental Health Project. Schizophr Bull. 1992;18(2):243–355. doi: 10.1093/schbul/18.2.243. [DOI] [PubMed] [Google Scholar]
- Bromet EJ, Kotov R, Fochtmann LJ, et al. Diagnostic shifts during the decade following first admission for psychosis. Am J Psychiatry. 2011;168(11):1186–1194. doi: 10.1176/appi.ajp.2011.11010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) Iowa City: University of Iowa; 1984. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) Iowa City: University of Iowa; 1984. [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Fried LP, Salive ME. Disability as a public health outcome in the aging population. Annu Rev Public Health. 1996;17:25–46. doi: 10.1146/annurev.pu.17.050196.000325. [DOI] [PubMed] [Google Scholar]
- Rikli R, Jones C. Functional fitness normative scores for community-residing older adults, ages 60-94. J Aging Phys Activity. 1999;7(2):162–181. [Google Scholar]
- Brown M, Sinacore DR, Host HH. The relationship of strength to function in the older adult. J Gerontol A Biol Sci Med Sci. 1995;Spec No:55–59. doi: 10.1093/gerona/50a.special_issue.55. [DOI] [PubMed] [Google Scholar]
- Mojtabai R, Bromet EJ, Harvey PD, et al. Neuropsychological differences between first-admission schizophrenia and psychotic affective disorders. Am J Psychiatry. 2000;157(9):1453–1460. doi: 10.1176/appi.ajp.157.9.1453. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Harvey PD, Bowie CR, et al. Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophr Bull. 2009;35(5):1022–1029. doi: 10.1093/schbul/sbn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004;79(3):379–384. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- Strassnig M, Clarke J, Mann S, et al. Body composition, pre-diabetes and cardiovascular disease risk in early schizophrenia. Early Interv. Psychiatry. 2015 doi: 10.1111/eip.12225. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- De Hert M, Detraux J, van Winkel R, et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114–126. doi: 10.1038/nrendo.2011.156. [DOI] [PubMed] [Google Scholar]
- Depp CA, Strassnig M, Mausbach BT, et al. Association of obesity and treated hypertension and diabetes with cognitive ability in bipolar disorder and schizophrenia. Bipolar Disord. 2014;16(4):422–431. doi: 10.1111/bdi.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim CY, Soczynska JK, Kennedy SH, et al. The effect of overweight/obesity on cognitive function in euthymic individuals with bipolar disorder. Eur Psychiatry. 2012;27:223–228. doi: 10.1016/j.eurpsy.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Wallenstein S, Moshier E, et al. The effects of hypertension and body mass index on cognition in schizophrenia. Am J Psychiatry. 2010;167(10):1232–1239. doi: 10.1176/appi.ajp.2010.09091328. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Gold JM, Dickerson FB, et al. Evidence of exacerbated cognitive deficits in schizophrenia patients with comorbid diabetes. Psychosomatics. 2008;49:123–131. doi: 10.1176/appi.psy.49.2.123. [DOI] [PubMed] [Google Scholar]
- Tsai SY, Kuo CJ, Chung KH, et al. Cognitive dysfunction and medical morbidity in elderly outpatients with bipolar disorder. Am J Geriatr Psychiatry. 2009;17(12):1004–1011. doi: 10.1097/JGP.0b013e3181b7ef2a. [DOI] [PubMed] [Google Scholar]
- Willette AA, Kapogiannis D. Does the brain shrink as the waist expands? Ageing Res Rev. 2014 doi: 10.1016/j.arr.2014.03.007. S1568-1637(14)00044. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeri MS, Ravona-Springer R, Silverman JM, Haroutunian V. The effects of cardiovascular risk factors on cognitive compromise. Dialogues Clin Neurosci. 2009;11(2):201–212. doi: 10.31887/DCNS.2009.11.2/msbeeri. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panza F, Frisardi V, Capurso C, et al. Metabolic syndrome and cognitive impairment: Current epidemiology and possible underlying mechanisms. J Alzheimers Dis. 2010;21(3):691–724. doi: 10.3233/JAD-2010-091669. [DOI] [PubMed] [Google Scholar]
- McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379(9833):2291–2299. doi: 10.1016/S0140-6736(12)60360-2. [DOI] [PubMed] [Google Scholar]
- Novak V, Hajjar I. The relationship between blood pressure and cognitive function. Nat Rev Cardiol. 2010;7(12):686–698. doi: 10.1038/nrcardio.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassnig M, Kotov R, Cornacchio D, et al. 20-year progression of BMI in a county-wide cohort people with schizophrenia and bipolar disorder identified at their first episode of psychosis. Bipolar Disord. 2017 doi: 10.1111/bdi.12505. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]


