Abstract
Hematopoietic cell transplantation (HCT) is effective in the treatment of inherited marrow failure disorders and other non-malignant diseases. Conventional myeloablative conditioning regimens have been associated with high transplant related mortality particularly in patients with co-morbid conditions. Here we report on 14 patients with marrow failure disorders (Shwachman-Diamond syndrome n=3, Diamond Blackfan anemia n=4, GATA2 deficiency n=2, paroxysmal nocturnal hemoglobinuria n=4, and an undefined marrow failure disorder n=1) who underwent HCT on a prospective phase II multi-center clinical trial. Patients were given HLA-matched related (n=2) or unrelated (n=12) grafts following conditioning with treosulfan (42 grams/m2), fludarabine (150 mg/m2), ± thymoglobulin (n=11; 6 mg/kg). All patients engrafted. At a median follow-up of 3 years, 13 patients are alive with complete correction of their underlying disease. These results indicate that the combination of treosulfan, fludarabine, and thymoglobulin is effective at establishing donor engraftment with a low toxicity profile and excellent disease-free survival in patients with marrow failure disorders.
Keywords: one marrow failure, Diamond Blackfan Anemia, Shwachman-Diamond Syndrome, reduced toxicity conditioning in nonmalignant diseases
INTRODUCTION
Hematopoietic cell transplantation (HCT) offers curative treatment for cytopenias in patients with certain bone marrow failure disorders. In contrast to acquired aplastic anemia, some marrow failure disorders may be characterized by normocellular or even hypercellular marrow, which poses additional barriers to engraftment. As a result, these marrow failure disorders may require more aggressive conditioning to establish sustained donor engraftment. However, high intensity myeloablative regimens are not well tolerated in many marrow failure disorders, and there is an increased risk for early transplant related mortality (TRM) from organ dysfunction or comorbidities associated with their underlying disease [1–4]. Therefore, less toxic conditioning approaches are needed.
In 2014 we reported the preliminary results of a U.S. multicenter prospective phase I/II trial of a treosulfan-based reduced intensity conditioning regimen for treatment of life-threatening nonmalignant disorders [5]. We observed a low incidence of both toxicity and TRM, consistent with that reported in a number of large retrospective studies [6–10]. Several European groups have evaluated treosulfan in combination with fludarabine for conditioning of patients with primary immune deficiency disorders [7], hemophagocytic lymphohistiocytosis [8], and thalassemia [11, 12]; however, limited data have been published regarding the use of treosulfan-based conditioning for patients with bone marrow failure disorders. Here we report on a cohort of patients with marrow failure disorders treated on a prospective U.S. study.
METHODS
Patients and Methods
Among 59 patients treated on protocol during the time frame for analysis, 14 had an underlying diagnosis of a marrow failure disorder, including Shwachman Diamond Syndrome (SDS, n=3), Diamond Blackfan Anemia (DBA, n=4), paroxysmal nocturnal hemoglobinuria (PNH, n=4), GATA2 deficiency (n=2), and an undefined marrow failure disorder (n=1). Toxicity and survival data on 8 of the 14 patients with marrow failure were reported previously [5]. The protocol was approved by the Institutional Review Boards of all participating institutions and monitored by an independent Data Safety Monitoring Board (DSMB). Patients or their legal guardians provided written consent. The conditioning regimen consisted of treosulfan 14 g/m2 given once daily IV on days −6 through −4 (total dose 42 g/m2) and fludarabine 30 mg/m2 given once daily IV on days −6 through −2 (total dose 150 mg/m2), as previously reported [5]. Patients were given either marrow or granulocyte-colony stimulating factor mobilized peripheral blood stem cell (PBSC) grafts. Prophylaxis for graft-vs.-host disease (GVHD) included tacrolimus and methotrexate. Tacrolimus was started as an intravenous (IV) continuous infusion on day −1 at a dose of 0.03 mg/kg and was continued until at least day +50 post-HCT followed by a taper of approximately 5% per week if there was no evidence of GVHD and the patient’s graft was stable. Methotrexate was given on day +1 (15 mg/m2/dose) and on days +3, +6, and +11 (10 mg/m2/dose). After the first 3 patients, thymoglobulin (rabbit anti-thymocyte globulin, rATG) was added to the regimen and given once daily IV on days −4 through −2 (total dose 6 mg/kg; n=11)[13, 14]. Supportive care included antibiotic prophylaxis, intravenous immunoglobulin, nutritional support, and weekly polymerase chain reaction (PCR) monitoring for reactivation of cytomegalovirus (CMV), Epstein-Barr virus (EBV), and adenovirus, according to institutional practices.
The pre-HCT co-morbidity score was assessed by the augmented HCT co-morbidity index (HCT-CI) [15–17]. Diagnosis, clinical grading, and treatment of acute and chronic GVHD were performed according to established criteria [18, 19]. Toxicities were defined by the National Cancer Institute’s Common Toxicity Criteria, version 2.0, excluding hematologic toxicities [20]. Disease response was evaluated by hematopoietic recovery, which was assessed by peripheral blood counts, transfusion independence, and bone marrow evaluation. In addition, flow cytometry of peripheral blood for glycosylphosphatidyl inositol (GPI)-anchored extracellular proteins was used to assess PNH. Donor chimerism levels were assessed in flow cytometry sorted CD33+, CD3+, CD19+, and CD56+ subsets by PCR-based analyses of polymorphic microsatellite regions, using methods previously described [21–24]. Primary neutrophil engraftment was defined as absolute neutrophil count ≥ 0.5 × 109/L for 3 consecutive days. The median time to platelet recovery was defined as >50 × 109/L for 5 days.
The method of Kaplan and Meier was used to estimate overall survival, which was defined as the duration from date of transplant to date of death due to any cause. Patients last known to be alive were censored at their date of last contact. Graphical representations of donor chimerism values across time were created by taking the chimerism value closest to day 28, day 80, day 180, and yearly thereafter up to 5 years following HCT. These values are depicted in the figures as occurring at these time points even though individual chimerism values may have occurred at times earlier or later than the listed time.
RESULTS
Patient Characteristics
Between June 2010 and October 2016, 14 patients with an underlying diagnosis of bone marrow failure underwent HCT. Patient characteristics at the time of HCT are shown in Tables 1, 2, 3, and 4. The median age at HCT was 15 (range 2–22) years. The median augmented HCT-CI was 1 (range 0–8). None of the patients had significant underlying cardiac or renal dysfunction pre-HCT. Three patients had underlying pulmonary disease defined as an FEV1 or DLCO 66%-80% (n=1; SDS) or an FEV1 or DLCO ≤65% (n=2; DBA and PNH). In addition, one patient with PNH (patient #9) had mild transaminitis pre-HCT (ALT 2.5 × the upper limit of normal). This patient had received a previous HLA-matched unrelated PBSC HCT following nonmyeloablative conditioning; however, this patient developed recurrent hemolysis and cytopenias roughly 1 year following the first HCT consistent with either recurrent PNH or a dysregulated immune system prompting a second HCT on the current trial.
Table 1.
Shwachman Diamond syndrome
| PRE-HCT CHARACTERISTICS | PATIENT #1 | PATIENT #2 | PATIENT #3 |
|---|---|---|---|
| Age at diagnosis /at HCT (yr) | 12 / 19.4 | 6 / 7.2 | 0.6 / 2.8 |
| Patient sex | Male | Female | Male |
| Genetic mutation | SBDS compound heterozygous: c.183_184TA>CT; c.258+2T>C (splicing) | SBDS compound heterozygous: c.258+2T>C (splicing); c.624+1G>A (splicing) | SBDS compound heterozygous: c.183_184TA>CT; c.258+2T>C (splicing) |
| Pancreatic dysfunction | Yes | Yes | Yes |
| Trypsinogen ng/mL | 11.0 (LLN 16.9) | 8.9 (LLN 10) | 5.4 (LLN 10) |
| Isoamylase (normal >13 U/L) | 3 | 5 | 3 |
| Additional clinical features | − | Cleft-palate, Pierre Robin, Height <5%ile | Height 5–10%ile |
| Augmented HCT/CI at HCT | 3 | 1 | 1 |
| Peripheral blood counts | |||
| ANC (cells/µ.L) | 600 | 170 | 180 |
| Hematocrit | 39% | 19% | 20% |
| Platelet Count (cells/µL) | 58,000 | 7,000 | 76,000 |
| Transfusion dependent | No | Yes (pRBC and platelets) | Yes (pRBCs) |
| Bone marrow evaluation | |||
| Cellularity | Average 7–10% | Average 60% | Average 50% |
| Cytogenetics/FISH | Normal at HCT/h/o deletion 20q | Normal/Normal | +1,der(1;17)(q10;q10)[17]/17p- (33.8%) |
| Indication(s) for HCT | Cytopenias | Cytopenias, pRBC and platelet transfusion dependent | Cytopenias, pRBC transfusion dependent, abnormal FISH |
| HCT Characteristics and Outcomes | |||
| Donor/stem cell source | MURD/marrow | MURD/PBSC | MURD/marrow |
| Follow-up (yr) | Alive, 5.5 | Alive, 4.6 | Alive, 3.1 |
| Donor chimerism (CD3+/CD33+)† | 100%/100% | 100%/100% | 100%/100% |
| GVHD§ | |||
| Acute/Chronic (Yes/No) | No/No | Yes/No | Yes/No |
| Day Immune Suppression Stopped | 242 | 278 | 450 |
| Peripheral blood counts† | |||
| ANC (cells/µL) | 4473 | 2090 | 1460 |
| Hematocrit | 48.3% | 39.4% | 37.4% |
| Platelet count (cells/µL) | 159,000 | 206,000 | 272,000 |
| Bone marrow evaluation† | |||
| Cellularity | 20% | 70% | N/A |
| Cytogenetics/FISH | Normal/Normal | Normal/ND | Normal/Normal |
| Disease response | Normal blood counts, Transfusion Independent | Normal blood counts, Transfusion Independent | Normal blood counts, Transfusion Independent, Normal marrow cytogenetics |
At last time point studied;
Acute grade II-IV GVHD, delayed acute GVHD, or NIH chronic GVHD
Abbreviations: ANC, absolute neutrophil count; HCT, hematopoietic cell transplantation; HCT-CI, hematopoietic cell transplantation comorbidity index; h/o, history of; MURD, matched unrelated donor; N/A, not available; ND, not done; PBSC, peripheral blood stem cells; pRBC, packed red blood cell; R, recipient; SDS, Shwachman-Diamond syndrome; yr, years
Table 2.
Diamond Blackfan anemia
| PRE-HCT CHARACTERISTICS | PATIENT #4 | PATIENT #5 | PATIENT #6 | PATIENT #7 |
|---|---|---|---|---|
| Age at diagnosis/at HCT (yr) | 0.3 / 6.1 | 1.2 / 12.2 | 0.1 / 21.9 | 0.1 / 2.3 |
| Patient sex | Male | Female | Female | Female |
| Genetic mutation | None | None | None | RPS19 Heterozygous: c357-2A>G (splicing) |
| Failed steroids | Yes | Initial steroid response, 10 yr remission | Yes | Yes |
| Clinical features | Autism, Neonatal intracerebral hemorrhage, GH deficiency, Hypogonadotropic hypogonadism, Bicuspid aortic valve | − | Hypothyroidism, Bicuspid aortic valve | Anterior ectopic anus |
| Iron | ||||
| Ferritin ng/mL | 342 | 674 | 1643 | 1215 |
| T2* MRI‡ | Hepatic: 3.36 Myocardial: 16.4 | Hepatic: 10.3 Myocardial: ND | Hepatic: 9.7 Myocardial: 41 | Hepatic: 1.9 Myocardial: 45–69 |
| Liver biopsy | Slight to moderate hemosiderosis, septal fibrosis (Batts & Ludwig, grade 2–3, stage 3) | None | 4+ iron, mild to moderate portal fibrosis | None |
| Augmented HCT/CI | 1 | 0 | 5 | 0 |
| Peripheral blood counts | ||||
| ANC (cells/µL) | 960 | 2540 | 2440 | 430 |
| Hematocrit | 23% | 25% | 24% | 24% |
| Platelet count (cells/µL) | 180,000 | 382,000 | 60,000 | 238,000 |
| Transfusion dependent | Yes (pRBC) | Yes (pRBC) | Yes (pRBC) | Yes (pRBC) |
| Bone marrow evaluation | ||||
| Morphology | Marked erythroid hypoplasia, slight myelofibrosis | Moderate erythroid hypoplasia, mild erythroid atypia | Erythroid aplasia, no dysplasia | Erythroid aplasia, no dysplasia |
| Cellularity | 85% | 60–70% | 20–30% | 40% |
| Cytogenetics | Normal | Normal | Normal | Normal |
| Indication for HCT | pRBC transfusion dependent, iron overload (on Exjade and desferol) | pRBC transfusion dependent | pRBC transfusion dependent, iron overload (on Exjade) | pRBC transfusion dependent, iron overload (on Exjade and desferol), Neutropenic despite G-CSF, frequent infections |
| HCT Characteristics and Outcomes | ||||
| Donor/Stem cell source | MURD/marrow | MURD/marrow | MURD/marrow | MURD/marrow |
| Follow-up (yr) | Alive, 4.5 | Alive, 4.0 | Alive, 3.0 | Alive, 0.3 |
| Donor chimerism (CD3+/CD33+)† | 100%/100% | 39%/34% | 96%/100% | 100%/100% |
| GVHD§ | ||||
| Acute/Chronic (Yes/No) | Yes/Yes | Yes/No | No/No | Yes/No |
| Day Immune Suppression Stopped | 1432 | 392 | 198 | Still on |
| Peripheral blood counts† | ||||
| ANC (cells/µL) | 14,421 | 4570 | 4770 | 7280 |
| Hematocrit | 34.9% | 39% | 42% | 37% |
| Platelet count (cells/µL) | 336,000 | 187,000 | 215,000 | 82,000 |
| Bone marrow evaluation† | ||||
| Morphology / cellularity | Trilineage hematopoiesis, 30–40% | ND/ND | ND/ND | N/A/N/A |
| Cytogenetics | Normal | ND | ND | Normal |
| Disease response | Normal hemoglobin, Transfusion independent | Normal hemoglobin, Transfusion independent | Normal hemoglobin, Transfusion independent | Normal hemoglobin, Transfusion independent, Resolution of neutropenia |
at last time point studied;
Hepatic T2* mg Iron/g dry weight hepatic tissue, Myocardial T2*: msec;
Acute grade II-IV GVHD, delayed acute GVHD or NIH chronic GVHD
Abbreviations: ANC, absolute neutrophil count; DBA, Diamond-Blackfan anemia; GH deficiency, growth hormone deficiency; HCT, hematopoietic cell transplantation; HCT-CI, hematopoietic cell transplantation comorbidity index; MURD, matched unrelated donor; N/A, not available; ND, not done; pRBC, packed red blood cell; R, recipient; vs, versus; yr, years
Table 3.
Paroxysmal nocturnal hemoglobinuria
| PRE-HCT CHARACTERISTICS | PATIENT #8 | PATIENT #9 | PATIENT #10 | PATIENT #11 |
|---|---|---|---|---|
| Age at diagnosis/ at HCT (yr) | 15.4 / 15.9 | 19 / 22.1 | 14.5 / 17.6 | 14 / 14.9 |
| Patient sex | Female | Female | Female | Male |
| GP-1 flow cytometry | GP1-deficient neutrophils (57–60%), monocytes (62%), erythrocytes (1%) | Normal post 1st-HCT | GP1-deficient neutrophils (82%), monocytes (79.2%), and erythrocytes (5.7%) | GP1-deficient neutrophils (27.7%), monocytes (77.6%), and erythrocytes (54.1%) |
| History of eculizumab | NO | NO | Yes; initiated 3 days prior to start of conditioning and continued through engraftment | Yes; initiated 11 months prior to HCT and continued through engraftment |
| Augmented HCT/CI | 1 | 8 | 1 | 0 |
| Peripheral blood counts | ||||
| ANC (cells/µL) | 720 | 700 | 380 | 560 |
| Hematocrit | 25% | 23% | 22% | 32% |
| Platelet count (cells/µL) | 13,000 | 33,000 | 13,000 | 179,000 |
| Transfusion dependent | Yes (pRBCs and platelets) | Yes (pRBCs and platelets) | Yes (pRBCs and platelets) | No |
| Bone marrow evaluation | ||||
| Cellularity | 10%-20% | 70% | 70% | 70% |
| Cytogenetics | Normal | Normal | Normal | Normal |
| Indication for HCT | Cytopenias, G-CSF dependent, pRBC and platelet transfusion dependent | Cytopenias following 1st HCT, pRBC and platelet transfusion dependent. | Cytopenias, pRBC and platelet transfusion dependent | Failed Eculizumab (profound fatigue, continued hemolysis) |
| HCT Characteristics and Outcomes | ||||
| Donor/Stem cell source | MURD/PBSC | MURD/marrow | A-Allele MMURD/PBSC | MRD/BM |
| Follow-up (yr) | Alive, 6.5 | Died GVHD, 0.4 | Alive, 2.3 | Alive, 1.4 |
| Donor Chimerism (CD3+/CD33+)† | 100%/100% | 100%/100% | 97%/100% | 95%/100% |
| GVHD§ | ||||
| Acute/Chronic (Yes/No) | No/No | Yes/No | No/Yes | No/No |
| Day Immune Suppression Stopped | 160 | Died on | 423 | 513 |
| Peripheral blood counts† | ||||
| ANC (cells/µL) | 3300 | 3965 | 4400 | 3460 |
| Hematocrit | 40.9% | 27.9% | 34.5% | 42% |
| Platelet Count (cells/µL) | 289,000 | 53,000 | 275,000 | 231,000 |
| Bone marrow evaluation† | ||||
| Cellularity | N/A | 20% | 70% | 50% |
| Cytogenetics | Normal | Normal | Normal | Normal |
| Disease response | Normal GP-1 expression | Normal GP-1 expression | Normal GP-1 expression | Normal GP-1 expression |
at time point last studied
Acute grade II-IV GVHD, delayed acute GVHD or NIH chronic GVHD
Abbreviations: HCT, hematopoietic cell transplantation; HCT-CI, hematopoietic cell transplantation comorbidity index; MRD, matched related donor; MURD, matched unrelated donor; MM, mismatched; N/A, not available; PBSC, peripheral blood stem cells; pRBC, packed red blood cell; R, recipient; yr, years
Table 4.
GATA2 deficiency and undefined bone marrow failure
| PRE-HCT CHARACTERISTICS | PATIENT #12 | PATIENT #13 | PATIENT #14 |
|---|---|---|---|
| Age at diagnosis/at HCT (yr) | 12.5 / 16.8 | 11.4 / 15.1 | 4.7 / 5.6 |
| Patient sex | Female | Male | Male |
| Genetic mutation | GATA2 Heterozygous: c.1082G>A/G (p.R361H) | GATA2 Heterozygous: c.1072_1074delACC (p.T358del) | None |
| Clinical features | Hashimoto’s thyroiditis, primary EBV | Viral warts | − |
| Iron | |||
| Ferritin ng/mL | 929 | 42 | 550 |
| T2* MRI‡ | Hepatic: 5.11 Myocardial: 36.39 | ND | Hepatic: 9.1–10.1 Myocardial: 33.7–34.2 |
| Augmented HCT/CI | 1 | 0 | 1 |
| Peripheral blood counts | |||
| ANC (cells/µL) | 470 | 187 | 250 |
| Hematocrit | 22% | 33.8% | 25% |
| Platelet count (cells/µL) | 87,000 | 105,000 | 16,000 |
| Transfusion dependent | Yes (pRBC) | No | Yes (pRBC and platelets) |
| Bone marrow evaluation | |||
| Cellularity | Average 40% | Average 40–50% | 40% |
| Cytogenetics / FISH | Normal/+8 (1.6%) | Normal/Normal | Normal/Normal |
| Indication for HCT | Cytopenias, pRBC transfusion dependent, Trisomy 8 (1.6%) | Cytopenias | Cytopenias, pRBC and platelet transfusion dependent |
| HCT Characteristics and Outcomes | |||
| Donor / Stem cell source | MRD/marrow | MURD/marrow | MURD/marrow |
| Follow-up (yr) | Alive, 2.6 | Alive, 0.5 | Alive, 2.4 |
| Donor chimerism (CD3+/CD33+)† | 100%/100% | 99%/100% | 100%/100% |
| GVHD§ | |||
| Acute/Chronic (Yes/No) | Yes/No | Yes/No | Yes/No |
| Day Immune Suppression Stopped | 285 | Still on | 347 |
| Peripheral blood counts† | |||
| ANC (cells/µL) | 4000 | 3519 | 1860 |
| Hematocrit | 41% | 32% | 39% |
| Platelet Count (cells/µL) | 165,000 | 163,000 | 216,000 |
| Bone marrow evaluation† | |||
| Cellularity | 40%-50% | ND | 65% |
| Cytogenetics / FISH | Normal/No evidence of Trisomy 8 | ND/ND | Normal/ND |
| Disease response | Normal blood counts, Transfusion independent, Normal marrow cytogenetics | Resolution of neutropenia and thrombocytopenia, Transfusion independent | Normal blood counts, Transfusion independent |
at last time point studied;
mg Iron/g dry weight hepatic tissue, Myocardial: msec;
Acute grade II–IV GVHD, delayed acute GVHD or NIH chronic GVHD
Abbreviations: HCT, hematopoietic cell transplantation; HCT-CI, hematopoietic cell transplantation comorbidity index; MSD, matched related donor; MURD, matched unrelated donor; ND, not done; pRBC, packed red blood cells; R, recipient; yr, years
Engraftment and Chimerism
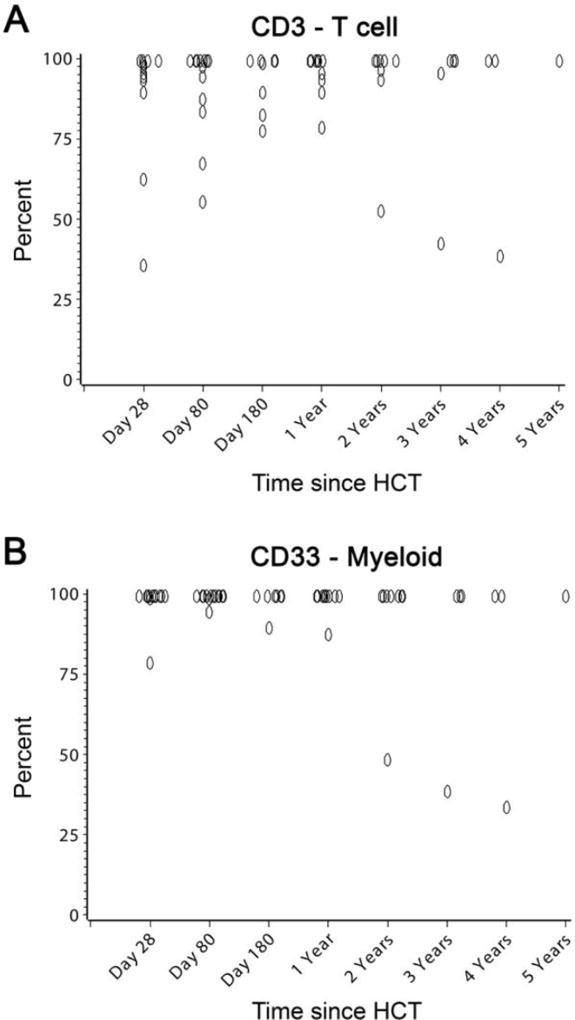
Neutrophil engraftment was observed in all patients at a median of 21 (range, 15–26) days. The median time to platelet recovery was 28 (range, 10–76) days. The median number of red blood cell (RBC) and platelet transfusions was 3 (range, 0–10), and 6 (range, 1–28), respectively. Full donor chimerism, defined as ≥ 95% donor cell origin of peripheral blood CD3+ T-cell and CD33+ myeloid subsets, was established in 13 patients, and mixed donor-host chimerism was present in 1 (patient #5; Tables 1, 2, 3, and 4, and Figure 1).
Figure 1. CD3+ and CD33+ donor chimerism in 14 patients conditioned with treosulfan-based conditioning.
Shown are the percent donor chimerism of sorted peripheral blood CD3+ (panel A) and CD33+ (panel B) subsets according to day after transplant. Overlapping points at days +28, +80, +180, years, and at 100% chimerism, are jittered slightly to improve visibility.
Transplant-Related Complications
All patients were observed for non-hematologic toxicities possibly related to the conditioning regimen through day 30 post HCT. Similar to the previous report, there were few clinically significant toxicities. Of the 14 patients enrolled, 5 developed one or more toxicities that included grade 3 mucositis (n=4), grade 3 skin rash not attributable to infection or GVHD (n=1), grade 3 hypoxia, which was transient in the setting of RSV infection (n=1), grade 3 pancreatitis which resolved (n=1), and grade 4 allergic reaction to rATG which resolved following discontinuation of rATG after the first dose (n=1). None of the patients developed liver toxicity, including the 6 patients with a pre-HCT diagnosis of iron overload (patient #4, #5, #6, #7, #12, and #14). None of the patients developed cardiac or renal toxicity.
Six patients developed grade II acute GVHD and one patient who did not receive rATG developed grade IV acute GVHD. Two patients developed delayed acute skin GVHD at day +161 and + 175 post-HCT, and two patients developed chronic GVHD by NIH consensus criteria. Eleven patients have successfully tapered off immune suppression at a median of 347 (range, 160–1432) days post-HCT. Two patients remain on immune suppression at 3 and 6 months post-HCT and one patient died on immune suppression.
All patients were monitored weekly by PCR for viral reactivation. EBV reactivation was detected in 3 patients; all resolved completely after rituximab therapy. CMV reactivation was detected in 1 of the 4 patients who had positive CMV serology pre-HCT. In addition, one patient had HHV6 reactivation that resolved after foscarnet therapy. Other infections within the first 100 days after HCT included RSV upper respiratory infection on day +5 (n=1), central line associated bacteremia (n=3), and clostridium difficile enteritis (n=3), all resolving with therapy.
Survival and Disease Response
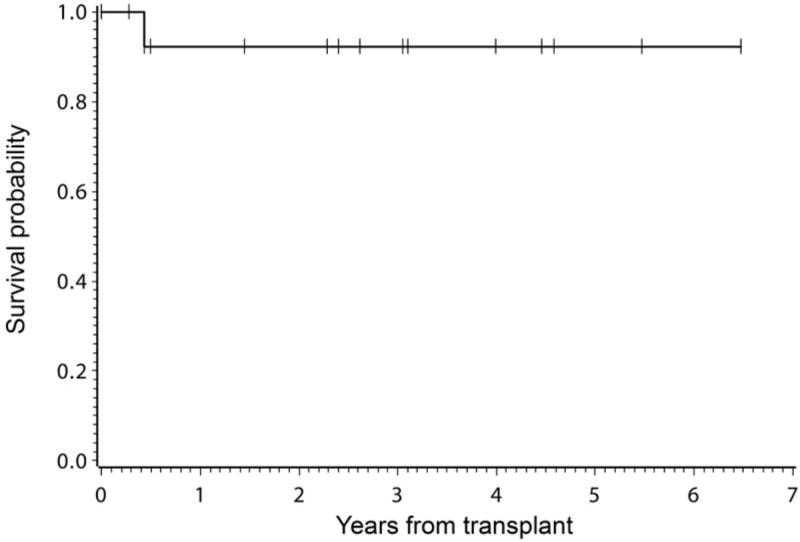
With a median follow up of 3 (range 0.3–6.5) years, 13 of the 14 patients are alive (Figure 2) with restoration of normal marrow function and Lansky/Karnofsky performance scores of 100% at last follow-up. One patient (#9) died of grade IV GVHD on day +158; this patient did not receive rATG. Disease responses at last follow-up are shown in Tables 1, 2, 3, and 4.
Figure 2. Disease-free survival.
Shown is the Kaplan Meir estimate of disease-free survival for 14 patients with bone marrow failure disorders who received treosulfan-based conditioning.
Shwachman Diamond syndrome (SDS, patients #1, #2, and #3)
All three patients have 100% donor multi-lineage engraftment 5.5, 4.6, and 3.1 years, respectively following HCT. All three patients are transfusion independent with resolution of neutropenia (patient #1, #2, and #3), anemia (patient #2 and #3) and thrombocytopenia (patient #1, #2, and #3) post-HCT. Patient #3 had a history of 17p deletion by cytogenetics and FISH pre-HCT. Follow-up marrow evaluation at 1-year post-HCT showed no evidence of 17p deletion by cytogenetics and FISH evaluations.
Diamond Blackfan anemia (DBA, patients #4, #5, #6, and #7)
Of the three patients with >1 year of follow-up (patients #4, #5, and #6), all have normal hemoglobin levels and are transfusion independent 4.5, 4.0, and 3.0 years, respectively post-HCT, including patient #5 who has mixed donor-recipient chimerism. In addition, patient #6 also had resolution of thrombocytopenia. Patient #7 is currently 3 months post-HCT and is transfusion independent with normal hemoglobin levels and resolution of neutropenia.
Before HCT, all patients with DBA had a history of iron overload with either elevated serum ferritin levels or increased hepatic iron on T2* MRI (Table 2). In addition, three patients (patients #4, #6, and #7) were receiving iron chelation therapy pre-HCT. Post-HCT one patient underwent phlebotomy × 18 months with improvement of liver iron to near normal (patient #6). One patient remains on iron chelation therapy post-HCT (patient #4). This patient required transfusions post-HCT due to autoimmune hemolytic anemia. Patient #7 is currently 3 months post-HCT with a serum ferritin of 1690 ng/mL.
Paroxysmal nocturnal hemoglobinuria (PNH, patients #8, #9, #10, and #11)
Of the four patients with PNH, one (#11) received eculizumab for PNH therapy pre-HCT. This patient had a history of debilitating fatigue and continued hemolysis despite 11 months of eculizumab therapy which prompted HCT. The patient continued on eculizumab therapy post-HCT until donor engraftment was confirmed and PNH cells declined to <1%. An additional patient (#10) was started on Eculizumab 3 days prior to the start of conditioning and continued eculizumab through engraftment. Three of the 4 patients are alive 6.5, 2.3, and 1.4 years post-HCT. One patient (#9) died of grade IV gut GVHD on day +158; this patient did not receive rATG. The three living patients all achieved full donor CD3 and CD33 engraftment and had resolution of PNH with normalization of blood counts and normal expression of glycosylphosphatidyl inositol-anchored proteins (Table 3).
GATA 2 deficiency
Patient #12 had a 4-year history of pancytopenia and mild immune dysfunction (Table 4). Genetic testing identified a heterozygous mutation in the GATA2 gene (c.1082G>A/G, p.R361H) approximately 6 months before HCT. The bone marrow showed trisomy 8 in 1.6% of marrow cells, without evidence for myelodysplasia. There was evidence for mild immune dysfunction, with low peripheral blood T and B lymphocyte subsets, but normal lymphocyte function and immunoglobulin production. Immunophenotyping showed B-cell lymphopenia with B cells constituting only 1.8% of the lymphocyte population. There were virtually no immature B cells observed; however, mature-naïve and mature-memory cells were present. Despite the low B cell number, immunoglobulin class-switching appeared normal in the memory cell population and there were both IgG+ and IgA+ switched memory B cells present. BAFF receptor expression was decreased relative to healthy controls, which was likely due to B cell lymphopenia. Post-HCT, the patient achieved full donor multi-lineage engraftment with resolution of anemia, thrombocytopenia, and neutropenia, and normalization of peripheral blood T and B lymphocyte subsets. The bone marrow evaluations at 3 and 12 months post-HCT showed no evidence of trisomy 8. Patient #13 had a 3-year history of progressive neutropenia, mild anemia and thrombocytopenia. An extensive marrow failure work-up was performed that identified a heterozygous mutation in the GATA2 gene (c.1072_1074delACC, p.T358del). This patient also had evidence for mild immune dysfunction, with low peripheral blood T and B lymphocyte subsets and had a history of viral warts pre-HCT. The patient is currently 6 months post-HCT with full donor CD3 and CD33 engraftment and has resolution of neutropenia and thrombocytopenia. This patient continues to have mild anemia.
Undefined marrow failure disorder
Patient #14 was diagnosed with bone marrow failure characterized by neutropenia, thrombocytopenia, macrocytic anemia, and a marrow that was hypocellular for age (cellularity 40%). The patient had no physical anomalies or family history to suggest a genetic disorder. Despite a comprehensive evaluation, no causative genetic mutation could be identified. Post-HCT, full donor chimerism was achieved, resulting in complete resolution of all cytopenias, and transfusion independence. This patient had evidence of iron overload pre-HCT based on a serum ferritin of 550 ng/mL and a hepatic T2* MRI that showed the liver iron content to be elevated at 9.1–10.1 mg Iron/g dry weight. This patient underwent monthly phlebotomy × 3 months post-HCT which resulted in improvement in his serum ferritin to 351 ng/mL as well as decreased hepatic liver iron content to 4.7–4.8 mg iron/g dry weight.
DISCUSSION
Bone marrow failure disorders are rare diseases that pose a challenge for successful HCT, as many patients have pre-existing organ dysfunction that increases the risk for mortality with conventional conditioning agents. Yet in many cases myeloablative conditioning is required to eliminate the abnormal marrow and facilitate sustained donor engraftment; thus there is a significant need to develop less toxic, yet myeloablative regimens. Treosulfan is a pro-drug of an alkylating agent structurally related to busulfan, and has similar myelosuppressive and immunosuppressive properties [20]. However, treosulfan has a different mode of alkylation, is not activated by liver enzymes, and has highly predictable pharmacokinetics. These properties reduce its potential for causing liver toxicity, as well as other tissue damage. We and others have successfully used a treosulfan-based regimen in an effort to reduce the risk for toxicity and mortality associated with allogeneic HCT for treatment of nonmalignant disorders [5]. Here we show that when combined with fludarabine and rATG, a treosulfan-containing regimen is well tolerated and can be highly effective in correcting marrow failure disorders.
Historical experience with conventional myeloablative conditioning regimens for patients with SDS has shown event-free survival to be around 60% [4]. The poor outcome can be partially explained by a delay of HCT until patients developed myelodysplastic syndromes (MDS), in which case mortality from relapse contributed to poor survival. However, patients without MDS also have a high transplant related mortality. Patients with SDS may develop cardiac dysfunction that may not be detected by echocardiogram [25–27], and may explain the increased incidence of cardiac failure after HCT with conventional regimens [1, 4]. Liver failure also has been reported following conventional HCT. Several case series have shown promising results with reduced intensity regimens, including fludarabine, melphalan, and alemtuzumab for HLA-matched grafts, with the main transplant-related complication being infections [28]. Sauer, et al. reported successful outcome in 2 of 3 patients conditioned with treosulfan in combination with fludarabine, melphalan, and alemtuzumab or rATG [29]. Taken together with our results, these studies show very low risk of mortality with reduced intensity regimens and support the use of HCT early after onset of marrow failure or detection of high-risk cytogenetics associated with a progression to MDS, particularly when an HLA matched related or unrelated donor is available. In contrast to SDS, patients with DBA appear to have better outcome with conventional conditioning regimens. The largest series of DBA patients was reported by the Center for International Blood and Marrow Transplant, and included 61 patients with about half of the cohort receiving HLA-matched sibling donor grafts. Most patients were conditioned with cyclophosphamide combined with either busulfan or total body irradiation. Patients who received HLA matched sibling grafts and those with performance scores 90–100 had significantly superior survival at 100 days, 1 year, and 3 years post HCT, approaching 80%. Given the larger experience in patients with other nonmalignant diseases showing very low mortality, further studies of treosulfan-based conditioning in patients with DBA are warranted to determine if equivalent or better survival can be achieved without the potential late effects of busulfan and TBI, such as growth retardation, gonadal toxicity, or secondary malignancies [30–34].
PNH is another rare marrow failure disorder for which HCT is indicated when medical therapy fails to control the disease manifestations. HCT is performed most often for the aplastic phase of PNH, in which case traditional nonmyeloablative regimens are quite successful [35–39]. Our institutional experience in adult patients with PNH showed that nonmyeloablative conditioning was effective in establishing donor engraftment in patients with either marrow aplasia or normocellular marrows [37]; however, G-CSF mobilized peripheral blood stem cells were given, and most of the patients developed GVHD. Conditioning with treosulfan, fludarabine, and rATG, followed by an allogeneic marrow graft, may be an acceptable alternative, particularly for patients with normal marrow cellularity. The two patients treated with eculizumab before HCT were maintained on it through conditioning until documented clearance of the PNH clone, without additional toxicity.
GATA2 deficiency caused by mutations in the GATA2 gene is a more recently described cause of bone marrow failure. In addition to bone marrow failure, patients with GATA2 deficiency can have underlying immune defects and are at risk for developing MDS or acute myelogenous leukemia (AML) [40–42]. Allogeneic HCT is a potentially curative therapy; however, patients often have significant underlying comorbidities that increase the risk of transplant related toxicities and mortality. Grossman et al. reported on 14 patients with GATA2 deficiency who underwent allogenic HCT following nonmyeloablative conditioning consisting of low dose TBI (2 Gy), fludarabine, +/− cyclophosphamide depending on the stem cell source [PBSC (n=8), bone marrow (n=2), or cord blood (n=4)] [42]. The majority of patients had MDS (<5% blasts) at the time of HCT. Six of the 14 patients died from rejection (n=2), infections (n=2), or other causes (n=2). Although HCT using nonmyeloablative conditioning was effective at correcting the underlying hematologic, immunologic and clinical manifestations of GATA2 deficiency, the authors stated that they are currently investigating a more intensive conditioning regimen consisting of busulfan and fludarabine with the goal of decreasing the risk of rejection and relapse. Our results as well as other published studies using treosulfan-based conditioning for patients with MDS/AML support that treosulfan-based conditioning may be another viable option for patients with GATA2 deficiency [43–45].
As previously reported, the initial patients on the prospective trial were conditioned with treosulfan and fludarabine alone. Subsequently, rATG was added to the regimen to reduce the incidence of grades III-IV acute GVHD [5], decreasing the incidence of acute grades III-IV GVHD from 33% to 0%. In the current cohort of 14 patients with marrow failure, only three patients did not receive rATG and one died of grade IV GVHD. Of the 11 patients who received rATG, none developed grades III-IV GVHD, and none died from infectious complications.
We were interested in determining the extent of disease response according to the level of donor chimerism established in each patient. However, all but one patient achieved full donor chimerism, defined as >95% donor cells in both CD3+ and CD33+ subsets. Disease response in the single case of DBA with <95% donor CD33 cells was similar to patients with full chimerism. Thus, the combination of treosulfan with fludarabine and rATG appears to be effective in establishing donor chimerism. The ability to establish full donor chimerism with minimal toxicity is particularly important in those patients with bone marrow failure disorders who have cytogenetic abnormalities with a risk for transformation to leukemia. In summary, the combination of treosulfan, fludarabine, and rATG appears promising as a conditioning regimen for patients with bone marrow failures disorders, which resulted in excellent long-term disease-free survival.
HIGHLIGHTS.
Treosulfan-based conditioning has a low toxicity profile
High level donor engraftment in marrow failure disorders
Excellent disease-free survival
Acknowledgments
The authors would like to thank the nursing and clinical staff, the referring physicians, and the patients who participated in this trial. We also thank Joshua Latos for data management; Michelle Bouvier and Bernie McLaughlin, research nurses; and Helen Crawford, Bonnie Larson, and Eun Ju Lee for assistance with manuscript preparation.
FINANCIAL DISCLOSURE
This study was supported in part by grants P01 HL122173, PO1 HL036444, and K23 HL085288 from the National Heart, Lung, and Blood Institute, NIH, Bethesda, MD, U.S.A., and research funding from medac, GmbH (Hamburg, Germany), which also provided treosulfan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Statement
This study was supported in part by research funding from Medac GmbH (Hamburg, Germany), which also provided treosulfan. In addition, medac GmbH covered travel costs for meetings. Otherwise, the author have no further conflicts of interest in relation to this work.
Author Contributions
L.M.B. and A.E.W. designed the study, analyzed the data and wrote the manuscript. L.M.B., A.S., J.A.T., J.D., and A.E.W. performed the research. A.S., J.A.T., J.D., C.D., H.F., D.M., K.S.B., A.G., K.M., B.M.S., H.J.D., and R.S. critically reviewed the manuscript.
References
- 1.Tsai PH, Sahdev I, Herry A, Lipton JM. Fatal cyclophosphamide-induced congestive heart failure in a 10-year-old boy with Shwachman-Diamond syndrome and severe bone marrow failure treated with allogeneic bone marrow transplantation [erratum appears in Am J Pediatr Hematol Oncol 1991 Summer;13(2):248] American Journal of Pediatric Hematology/Oncology. 1990;12:472–476. doi: 10.1097/00043426-199024000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Okcu F, Roberts WM, Chan KW. Bone marrow transplantation in Shwachman-Diamond syndrome: report of two cases and review of the literature (Review) Bone Marrow Transplantation. 1998;21:849–851. doi: 10.1038/sj.bmt.1701170. [DOI] [PubMed] [Google Scholar]
- 3.Cesaro S, Oneto R, Messina C, et al. Haematopoietic stem cell transplantation for Shwachman-Diamond disease: a study from the European Group for Blood and Marrow Transplantation. British Journal of Haematology. 2005;131:231–236. doi: 10.1111/j.1365-2141.2005.05758.x. [DOI] [PubMed] [Google Scholar]
- 4.Donadieu J, Michel G, Merlin E, et al. Hematopoietic stem cell transplantation for Shwachman-Diamond syndrome: experience of the French neutropenia registry. Bone Marrow Transplantation. 2005;36:787–792. doi: 10.1038/sj.bmt.1705141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burroughs LM, Nemecek ER, Torgerson TR, et al. Treosulfan-based conditioning and hematopoietic cell transplantation for nonmalignant diseases: a prospective multicenter trial. Biology of Blood and Marrow Transplantation. 2014;20:1996–2003. doi: 10.1016/j.bbmt.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greystoke B, Bonanomi S, Carr TF, et al. Treosulfan-cointaining regimens achieve high rates of engraftment associated with low transplant morbidity and mortality in children with non-malignant disease and significant co-morbidities. British Journal of Haematology. 2008;142:257–262. doi: 10.1111/j.1365-2141.2008.07064.x. [DOI] [PubMed] [Google Scholar]
- 7.Slatter MA, Rao K, Amrolia P, et al. Treosulfan-based conditioning regimens for hematopoietic stem cell transplantation in children with primary immunodeficiency: United Kingdom experience. Blood. 2011;117:4367–4375. doi: 10.1182/blood-2010-10-312082. [DOI] [PubMed] [Google Scholar]
- 8.Lehmberg K, Albert MH, Beier R, et al. Treosulfan-based conditioning regimen for children and adolescents with hemophagocytic lymphohistiocytosis. Haematologica. 2014;99:180–184. doi: 10.3324/haematol.2013.094730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beier R, Schulz A, Honig M, et al. Long-term follow-up of children conditioned with Treosulfan: German and Austrian experience. Bone Marrow Transplantation. 2013;48:491–501. doi: 10.1038/bmt.2012.188. [DOI] [PubMed] [Google Scholar]
- 10.Slatter MA, Boztug H, Potschger U, et al. Treosulfan-based conditioning regimens for allogeneic haematopoietic stem cell transplantation in children with non-malignant diseases. Bone Marrow Transplant. 2015;50:1536–1541. doi: 10.1038/bmt.2015.171. [DOI] [PubMed] [Google Scholar]
- 11.Bernardo ME, Piras E, Vacca A, et al. Allogeneic hematopoietic stem cell transplantation in thalassemia major: results of a reduced-toxicity conditioning regimen based on the use of treosulfan. Blood. 2012;120:473–476. doi: 10.1182/blood-2012-04-423822. [DOI] [PubMed] [Google Scholar]
- 12.Bernardo ME, Zecca M, Piras E, et al. Treosulfan-based conditioning regimen for allogeneic haematopoietic stem cell transplantation in patients with thalassaemia major. British Journal of Haematology. 2008;143:548–551. doi: 10.1111/j.1365-2141.2008.07385.x. [DOI] [PubMed] [Google Scholar]
- 13.Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–2068. [PubMed] [Google Scholar]
- 14.Deeg HJ, Storer BE, Boeckh M, et al. Reduced incidence of acute and chronic graft-versus-host disease with the addition of thymoglobulin to a targeted busulfan/cyclophosphamide regimen. Biology of Blood and Marrow Transplantation. 2006;12:573–584. doi: 10.1016/j.bbmt.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 15.Sorror M. How I assess comorbidities prior to hematopoietic cell transplantation. Blood. 2013;121:2854–2863. doi: 10.1182/blood-2012-09-455063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith AR, Majhail NS, MacMillan ML, et al. Hematopoietic cell transplantation comorbidity index predicts transplantation outcomes in pediatric patients. Blood. 2011;117:2728–2734. doi: 10.1182/blood-2010-08-303263. [DOI] [PubMed] [Google Scholar]
- 17.Vaughn JE, Storer BE, Armand P, et al. Design and validation of an augmented hematopoietic cell transplantation-comorbidity index comprising pretransplant ferritin, albumin, and platelet count for prediction of outcomes after allogeneic transplantation. Biology of Blood and Marrow Transplantation. 2015;21:1418–1424. doi: 10.1016/j.bbmt.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplantation. 1995;15:825–828. [PubMed] [Google Scholar]
- 19.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and Staging Working Group report. Biology of Blood and Marrow Transplantation. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 20.The Revised Common Toxicity Criteria: Version 2.0. DCTD, NCI, NIH, DHHS; 1999. [Google Scholar]
- 21.Scharf SJ, Smith AG, Hansen JA, McFarland C, Erlich HA. Quantitative determination of bone marrow transplant engraftment using fluorescent polymerase chain reaction primers for human identity markers. Blood. 1995;85:1954–1963. [PubMed] [Google Scholar]
- 22.Kasai K, Nakamura Y, White R. Amplification of a variable number of tandem repeats (VNTR) locus (pMCT118) by the polymerase chain reaction (PCR) and its application to forensic science. Journal of Forensic Sciences. 1990;35:1196–1200. [PubMed] [Google Scholar]
- 23.Boerwinkle E, Xiong WJ, Fourest E, Chan L. Rapid typing of tandemly repeated hypervariable loci by the polymerase chain reaction: application to the apolipoprotein B 3’ hypervariable region. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:212–216. doi: 10.1073/pnas.86.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bryant E, Martin PJ. Documentation of engraftment and characterization of chimerism following hematopoietic cell transplantation. In: Thomas KG, Blume KG, Forman SJ, editors. Hematopoietic Cell Transplantation. 2. Boston: Blackwell Science; 1999. pp. 197–206. [Google Scholar]
- 25.Ryan TD, Jefferies JL, Chin C, et al. Abnormal circumferential strain measured by echocardiography is present in patients with Shwachman-Diamond syndrome despite normal shortening fraction. Pediatr Blood Cancer. 2015;62:1228–1231. doi: 10.1002/pbc.25456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savilahti E. Frequent myocardial lesions in Shwachman’s syndrome. Eight fatal cases among 16 Finnish patients. Acta Paediatrica Scandinavica. 1984;73:642–651. doi: 10.1111/j.1651-2227.1984.tb09989.x. [DOI] [PubMed] [Google Scholar]
- 27.Hauet Q, Beaupain B, Micheau M, et al. Cardiomyopathies and congenital heart diseases in Shwachman-Diamond syndrome: a national survey. Int J Cardiol. 2013;167:1048–1050. doi: 10.1016/j.ijcard.2012.10.084. [DOI] [PubMed] [Google Scholar]
- 28.Bhatla D, Davies SM, Shenoy S, et al. Reduced-intensity conditioning is effective and safe for transplantation of patients with Shwachman-Diamond syndrome. Bone Marrow Transplant. 2008;42:159–165. doi: 10.1038/bmt.2008.151. [DOI] [PubMed] [Google Scholar]
- 29.Sauer M, Zeidler C, Meissner B, et al. Substitution of cyclophosphamide and busulfan by fludarabine, treosulfan and melphalan in a preparative regimen for children and adolescents with Shwachman-Diamond syndrome. Bone Marrow Transplant. 2007;39:143–147. doi: 10.1038/sj.bmt.1705553. [DOI] [PubMed] [Google Scholar]
- 30.Walters MC, Hardy K, Edwards S, et al. Pulmonary, gonadal and central nervous system status after bone marrow transplantation for sickle cell disease. Biology of Blood and Marrow Transplantation. 2010;16:263–272. doi: 10.1016/j.bbmt.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panasiuk A, Nussey S, Veys P, et al. Gonadal function and fertility after stem cell transplantation in childhood: comparison of a reduced intensity conditioning regimen containing melphalan with a myeloablative regimen containing busulfan. Br J Haematol. 2015;170:719–726. doi: 10.1111/bjh.13497. [DOI] [PubMed] [Google Scholar]
- 32.Bernard F, Auquier P, Herrmann I, et al. Health status of childhood leukemia survivors who received hematopoietic cell transplantation after BU or TBI: an LEA study. Bone Marrow Transplant. 2014;49:709–716. doi: 10.1038/bmt.2014.3. [DOI] [PubMed] [Google Scholar]
- 33.Allewelt H, El-Khorazaty J, Mendizabal A, et al. Late effects after umbilical cord blood transplantation in very young children after busulfan-based, myeloablative conditioning. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2016;22:1627–1635. doi: 10.1016/j.bbmt.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 34.Dietz AC, Duncan CN, Alter BP, et al. The Second Pediatric Blood and Marrow Transplant Consortium International Consensus Conference on Late Effects after Pediatric Hematopoietic Cell Transplantation: Defining the unique late effects of children undergoing hematopoietic cell transplantation for immune deficiencies, inherited marrow failure disorders, and hemoglobinopathies. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2017;23:24–29. doi: 10.1016/j.bbmt.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raiola AM, van Lint MT, Lamparelli T, et al. Bone marrow transplantation for paroxysmal nocturnal hemoglobinuria. Haematologica. 2000;85:59–62. [PubMed] [Google Scholar]
- 36.Woodard P, Wang W, Pitts N, et al. Paroxysmal nocturnal haemoglobinuria: Successful unrelated donor bone marrow transplantation for paroxysmal nocturnal haemoglobinuria. Bone Marrow Transplantation. 2001;27:589–592. doi: 10.1038/sj.bmt.1702827. [DOI] [PubMed] [Google Scholar]
- 37.Hegenbart U, Niederwieser D, Forman S, et al. Hematopoietic cell transplantation from related and unrelated donors after minimal conditioning as a curative treatment modality for severe paroxysmal nocturnal hemoglobinuria. Biology of Blood and Marrow Transplantation. 2003;9:689–697. doi: 10.1016/s1083-8791(03)00264-7. [DOI] [PubMed] [Google Scholar]
- 38.Szer J, Deeg HJ, Witherspoon RP, et al. Long-term survival after marrow transplantation for paroxysmal nocturnal hemoglobinuria with aplastic anemia. Annals of Internal Medicine. 1984;101:193–195. doi: 10.7326/0003-4819-101-2-193. [DOI] [PubMed] [Google Scholar]
- 39.Kawahara K, Witherspoon RP, Storb R. Marrow transplantation for paroxysmal nocturnal hemoglobinuria. American Journal of Hematology. 1992;39:283–288. doi: 10.1002/ajh.2830390409. [DOI] [PubMed] [Google Scholar]
- 40.Spinner MA, Sanchez LA, Hsu AP, et al. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014;123:809–821. doi: 10.1182/blood-2013-07-515528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grossman J, Cuellar-Rodriguez J, Gea-Banacloche J, et al. Nonmyeloablative allogeneic hematopoietic stem cell transplantation for GATA2 deficiency. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2014;20:1940–1948. doi: 10.1016/j.bbmt.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pasquet M, Bellanne-Chantelot C, Tavitian S, et al. High frequency of GATA2 mutations in patients with mild chronic neutropenia evolving to MonoMac syndrome, myelodysplasia, and acute myeloid leukemia. Blood. 2013;121:822–829. doi: 10.1182/blood-2012-08-447367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gyurkocza B, Gutman J, Nemecek ER, et al. Treosulfan, fludarabine, and 2-Gy total body irradiation followed by allogeneic hematopoietic cell transplantation in patients with myelodysplastic syndrome and acute myeloid leukemia. Biology of Blood and Marrow Transplantation. 2014;20:549–555. doi: 10.1016/j.bbmt.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nemecek ER, Guthrie KA, Sorror ML, et al. Conditioning with treosulfan and fludarabine followed by allogeneic hematopoietic cell transplantation for high-risk hematologic malignancies. Biology of Blood and Marrow Transplantation. 2011;17:341–350. doi: 10.1016/j.bbmt.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakellari I, Mallouri D, Gavriilaki E, et al. Survival advantage and comparable toxicity in reduced-toxicity treosulfan-based versus reduced-intensity busulfan-based conditioning regimen in myelodysplastic syndrome and acute myeloid leukemia patients after allogeneic hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2017;23:445–451. doi: 10.1016/j.bbmt.2016.11.023. [DOI] [PubMed] [Google Scholar]