Abstract
IL-4 and IL-13 have been defined as anti-inflammatory cytokines which can counter myelin-reactive T cells and modulate experimental allergic encephalomyelitis (EAE). However, it is not known whether endogenous IL-4 and IL-13 contribute to the maintenance of peripheral tolerance and whether their function is coordinated with T regulatory cells (Tregs). Here, we utilized mice in which the common cytokine receptor for IL-4 and IL-13, namely the IL-4Rα/IL-13Rα1 heteroreceptor (HR), is compromised and determined whether the lack of signaling by endogenous IL-4 and IL-13 through the HR influences the function of effector Th1 and Th17 cells in a Treg-dependent fashion. The findings indicate that mice-deficient for the HR (13R-/-) are more susceptible to EAE than mice sufficient for the HR (13R+/+) and develop early onset and more severe disease. Moreover, Th17 cells from 13R-/- mice had reduced ability to convert to Th1 cells and displayed reduced sensitivity to suppression by Tregs relative to Th17 effectors from 13R+/+ mice. These observations suggest that IL-4 and IL-13 likely operate through the HR and influence Th17 cells to convert to Th1 cells and to acquire increased sensitivity to suppression leading to control of immune-mediated central nervous system inflammation. These previously unrecognized findings shed light on the intricacies underlying the contribution of cytokines to peripheral tolerance and control of autoimmunity.
Introduction
Autoimmunity develops when peripheral tolerance (1) is no longer able to keep self-reactive lymphocytes in check (2). T regulatory cells (Tregs) and anti-inflammatory cytokines are usually adept at containing aggressive lymphocytes and prevent the development of autoimmune diseases. However, whether these forms of tolerance coordinate their function and synergize their action against autoreactive lymphocytes has yet to be determined. IL-4 and IL-13 function as anti-inflammatory cytokines (3-7) and may serve alongside Tregs to preserve peripheral tolerance and prevent autoimmunity. In fact, we have previously shown that neonatal exposure to self-Ag, which induces responses dominated by IL-4 producing Th2 cells, confers resistance to EAE (8, 9). On the other hand, Tregs play a major role in keeping myelin-reactive T cells in check and preventing the development of EAE (10-12). In this study, we asked whether and how endogenous IL-4 and IL-13 synergize with Tregs to restrain myelin-reactive T cells and prevent the development of EAE.
IL-4 and IL-13 share the IL-4Rα/IL-13Rα1 heteroreceptor (HR) (13) and most likely carry out their anti-inflammatory function through its expression on antigen presenting cells (APCs) such as DCs and macrophages, as T cells in adult mice do not express this receptor (14-16). Also IL-4 does not signal through the conventional IL-4R (IL-4Rα/common γ chain) in Th1 cells (17) and the conventional IL-13 receptor (IL-13Rα1/IL-13Rα2) serves rather as a decoy receptor (18). Thus, mice lacking IL-13Rα1 in which the conventional IL-4R is intact but the HR does not form (19-21) provide a suitable model to determine whether anti-inflammatory IL-4/IL-13 synergize with Tregs to maintain peripheral tolerance and contain EAE. This was indeed the case as IL-13Rα1-deficient (13R-/-) mice which lack the HR (HR-/-) are more susceptible to EAE relative to 13R+/+ wild type mice. Specifically, 13R-/- mice develop early onset and severe EAE when induced for disease with myelin oligodendrocyte glycoprotein 35-55 peptide (MOGp). This phenotype has been correlated with effects on Th17 to Th1 conversion (22) as well as interference with the sensitivity of these effectors to suppression by T regulatory cells (Tregs). Indeed, there was limited Th17 to Th1 conversion in 13R-/- mice relative to 13R+/+ animals. Also, while there was no effect on the development of Tregs in 13R-/- mice, both Th1 and Th17 cells displayed differential sensitivity to suppression by Tregs when compared to counterparts from 13R+/+ mice. These findings indicate that endogenous IL-4/IL-13 cytokines synergize their function with Tregs to control peripheral tolerance and restrain autoimmunity.
Materials and Methods
Mice
C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). IL-13Rα1-/- C57BL/6 (13R-/-) mice were previously described (19). IL-17acre mice obtained from Dr. Stockinger (The Francis Crick Institute) were also previously described (22). To generate 13R-/- IL-17acre-eYFP mice we first bred the IL-17acre mice with the 13R-/- mice and then to B6.129×1-Gt(ROSA)26Sortm1(EYFP)Cos/J(ROSA26-YFP) (obtained from Jackson Laboratory). 13R-/-.Foxp3.GFP mice were generated by breeding 13R-/-C56BL/6 to 13R+/+.Foxp3.GFP C57BL/6 mice. Because both 13R and Foxp3 are located on X chromosome the breeding was done by speed congenic technology. Only age matched female mice were used in the experiments. All animals were maintained in our animal care facility for the duration of the experiments. All experimental procedures were performed according to the guidelines of the University of Missouri Animal Care and Use Committee.
MOG peptide and tetramer
MOG peptide (MOGp) encompassing aa residues 35-55 of MOG which is encephalitogenic in C57BL/6 mice (23) was purchased from EZBiolab (Westfield, IN). I-Ab tetramer containing MOG aa 38-49 (MOGtet) was obtained from NIH Core Tetramer Facillity (Emory University, Atlanta, Georgia).
Induction of EAE
EAE was induced with MOGp as described (23, 24). Briefly, female mice (6–8 week-old) were induced for EAE by s.c. injection of a 200μl IFA/PBS (v/v) solution containing 300μg, 100 μg, or 60μg MOG peptide and 200 μg of Mycobacterium tuberculosis H37Ra (Difco) in the footpads and at the base of the limbs. Six hours later, the mice were given 500 ng Bordetella pertussis toxin (List Biological Laboratories) intravenously (i.v.). A second injection of B. pertussis toxin was given after 48 h. The mice were then scored daily for clinical signs of EAE as follows: 0, no clinical score; 1, tail weakness; 2, loss of tail tone; 3, hind limb weakness; 4, hind limb paralysis; 5, forelimb paralysis; and 6, moribund or death. A cumulative disease score (CDS) was calculated by adding the daily scores that the mice received during the monitoring period divided by the number of mice per group.
Detection of cytokines by ELISA
The levels of IFNγ and IL-17 in culture supernatant were determined by ELISA as previously described (25). The antibodies used in this assay were: IFNγ, R4-6A2 (capture) and biotinylated XMG1.2; IL-17, TC11-18H10 and biotinylated TC11-8H4 (BD Biosciences). The OD405 was measured using a SpectraMax 340 microplate reader (Molecular Devices, Menlo Park, CA) and analyzed with SoftMAX Pro software v3.1.1. Graded amounts of IFNγ and IL-17 (Peprotech, Rocky Hill, NJ) were used to generate standard curves. The linear portion of the standard curve was used to calculate the concentration of cytokines in culture supernatants.
Flow cytometry
Cells from SP, LN (axillary, inguinal and popliteal: draining LN), or CNS were first incubated with mouse IgG to block FcγRs. Subsequently, APC-conjugated MOGtet and anti-CD4 (RM4-5, BD biosciences) or isotype control antibody were added for 30 min on ice. The cells were then washed, fixed, and permibealized using the eBioscience Intracellular Fixation & Permeabilization Buffer according to the manufacturer's instructions. For detection of intracellular cytokine the cells were incubated with anti-IL-17A antibody (TC11-18H10), anti-IFNγ (XMG1.2, BD biosciences), or isotype control for 30 min on ice. For detection of transcription factors, the cells were incubated with anti-RoRγt (AFKJS-9), anti-Tbet (4B10), anti- Foxp3 (FJK-16s) antibody or isotype controls (from e-Biosciences) also for 30 min on ice.
Data were collected on a Beckman Coulter CyAn (Brea, CA) and analyzed using FlowJo version 10.1 (Tree Star) or Summit Software version 4.0 (Dako).
For the conversion experiments using IL-17acre-eYFP mice, the cells were prefixed with 1% paraformaldehyde for 15 min prior to the use of the Intracellular Fixation & Permeabilization buffer.
Isolation of CNS mononuclear cells
Mice were perfused through the left cardiac ventricle with 50mL ice cold PBS. The brain was removed and spinal cord was flushed out by hydrostatic pressure and placed in PBS. Tissue was minced and cells were processed through a 70 μM filter. Homogenized tissue was resuspended in 8ml of 37% Percoll, overlaid onto 4ml of 70% Percoll and centrifuged at 1800 rpm for 20 min at RT with no brake. Mononuclear cells were collected from the interphase of the 37% and 70% Percoll gradient and used for subsequent experiments.
Stimulation with PMA and Ionomycin
Isolated draining LN or CNS mononuclear cells (1 × 106/well) were stimulated in vitro with 50 ng/ml PMA and 500 ng/ml ionomycin in the presence of 10 μg/ml Brefeldin A for 2 hours. The cells were then washed and used for intracellular cytokine and transcription factors staining as above.
Isolation of Tregs and T effector cells
Female 13R+/+ or 13R-/- Foxp3.GFP mice were immunized s.c. with 200μl IFA/PBS (v/v) solution containing 100 μg MOG peptide and 200 μg of Mycobacterium tuberculosis H37Ra (Difco) in the footpads and at the base of the limbs.
For isolation of Tregs the LN were harvested on day 10 post immunization. Cells were gated on CD4 and CD25 and sorted on the basis of GFP (Foxp3+) expression (97% purity) using a Beckman Coulter MoFlo XDP.
For isolation of effector Th1 and Th17 cells, SP cells were harvested on day 10 post immunization. CD4+ T cells were isolated from the SP by negative selection of lineage specific cells using a CD4+ T cell isolation kit (Miltenyi Biotech, San Diego, CA). The CD4+ T cells were stimulated with PMA and ionomycin for 3 hours to induce cytokine secretion optimal for isolation of bulk Th17 and Th1 cells by IL-17 and IFN-γ Secretion Assay/Cell Enrichment and Detection Kits, respectively (Miltenyi Biotech, San Diego, CA). Only cells with 98% purity are used for in vitro and in vivo suppression assays.
In vitro Treg suppression assays
Tregs and effector T cells were co-cultured at different Treg:Teff ratios for 3 days and the culture supernatants were used to measure residual IFNγ or IL-17 by ELISA.
Inhibition ratio 50 (IR50) and sensitivity index (SI)
The percent residual cytokine production represents the amount of cytokine produced in the presence of Tregs over the amount of cytokine produced in the absence of Tregs times 100. The inhibition ratio 50 (IR50) is the Treg to T effector ratio at which the residual cytokine production is reduced by 50%. It is calculated by dividing the number of Tregs over the number of T effector cells at the 50% inhibition point. The sensitivity index (SI) is the intrinsic decline in cytokine production potential per effector T cell, which represents the absolute amount of cytokine that an effector T cell could not produce due to suppression by Tregs. It is calculated as the ratio whereby the numerator is the amount of cytokine (fg/ml) produced at the 50% inhibition point and the denominator is the absolute number of T effector cells per ml times the IR50. The SI unit is fg.
Evaluation of the impact of Effector T cell sensitivity to Treg suppression on EAE
Effector Th1 or Th17 cells from 13R+/+ or 13R-/- mice were separately mixed with Tregs isolated from 13R+/+and transferred i.v. into Rag2-/- C57BL/6 mice. Two days later the hosts were induced for EAE with 100μg MOGp and then monitored daily for clinical signs of EAE.
Statistical analysis
Data were analyzed using either an unpaired, two-tailed Student's t-test or Mann-Whitney U test as indicated. All statistical analyses were performed using Prism software v6 (GraphPad, La Jolla, CA).
Results
13R-/- mice develop early onset and more severe EAE
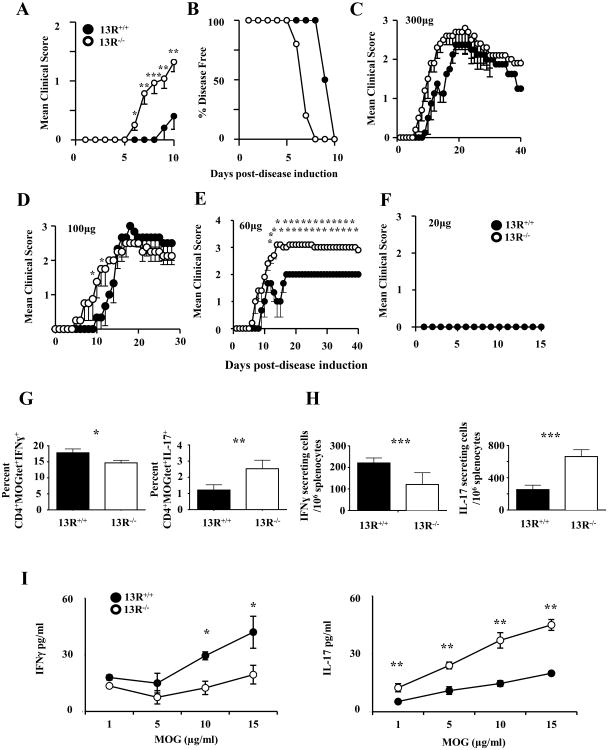
The onset of EAE in C57BL/6 mice induced for disease with 300 μg MOGp usually manifests around day 10 post-immunization (23). However, when a similar disease induction regimen was applied to 13R-/- mice, the clinical signs of paralysis manifested as early as day 6 post-immunization while 13R+/+ control mice had disease onset around day 10 as is typical in our mouse colony (Fig. 1A). Specifically, the average day of onset for 13R-/- mice was 6.6 ± 0.2 compared to 10.7 ± 0.3 for 13R+/+ animals (Fig. 1A). Discrepancies also manifest at disease incidence level, as 100% of 13R-/- mice displayed clinical signs of paralysis at day 8 post-immunization while 13R+/+ mice remained symptom free (Fig. 1B). Despite differences in disease onset both strains had similar clinical scores at the peak of disease and resolved their symptoms in a comparable manner (Fig 1C). This indicates that 13R contributes regulatory mechanisms that influence the onset of disease. To begin exploring the mechanisms by which 13R influences disease manifestation, mice were induced for EAE with graded doses of MOGp and the animals were monitored daily for signs of paralysis. The findings indicate that 100 μg MOGp, while preserving early disease onset in 13R-/- mice, does not significantly influence the maximal severity or resolution of disease relative to 13R+/+ mice (Fig. 1D). However, when the mice were induced for EAE with 60 μg MOGp, the 13R-/- animals developed more severe paralytic signs at the plateau of disease and were unable to resolve their symptoms for the 40 day monitoring period in comparison to 13R+/+ mice (Fig. 1E). Neither strain developed disease with 20 μg MOGp (Fig. 1F). Taken together, these results indicate that 13R serves to control disease manifestation. To determine how 13R influences T cell responses to hasten disease onset we analyzed the frequency of, as well as cytokine production by, Th1 and Th17 effector cells at the initial phase of EAE. Ex vivo analysis of T cell responses indicate that in the 13R-deficient mice there were more Th17 but less Th1 cells among antigen-specific MOGtet+ LN cells relative to 13R-sufficient mice (Fig 1G and H). This is significant whether the results are presented as percentages (Fig. 1G) or absolute number (Fig. 1H). Re-stimulation of LN cells with MOG peptide in vitro shows that there was higher IL-17 production but lower IFNγ secretion by cells from 13R-/- versus 13R+/+ mice (Fig. 1I). These significant differences are Ag-specific as they correlate with the dose of MOG peptide. Together, these data suggest that the increased susceptibility and early onset of EAE in 13R-/- mice is related to increased frequency of Th17 rather than Th1 effector cells.
Figure 1.
13R-/- mice develop early onset and more severe EAE. 13R+/+ and 13R-/- C57BL/6 mice (6-8 per group) were induced for EAE with 300μg MOGp and monitored daily for clinical signs of paralysis. (A, B) Mean clinical score of disease severity ± SD and percentage of mice that remained disease free for the initial 10 day-phase of disease onset. (C) Shows the mean clinical score of disease severity ± SD for the entire 30 day-monitoing phase. (D-F) Show mean clinical score of disease severity ± SD for 13R+/+ and 13R-/- mice (6 mice per group) induced for EAE with 100 (D), 60, (E) or 20 (F) μg of MOGp. *p<0.05, **p<0.01 as determined by Mann-Whitneys U test. (G-I) Draining lymph nodes were harvested at day 10 post-disease induction from mice induced for EAE with 100μg MOGp and the cells were stimulated with PMA and ionomycin (G, H,) or graded concentrations of MOGp (I) and IFNγ and IL-17 responses were measured. (G) Shows the percentages while (H) illustrates the absolute numbers of cytokine secreting CD4+MOGtet+ T cells. (I) Shows cytokine secretion as measured by ELISA. Data is representative of at least 3 independent experiments. *p<0.05, **p<0.01, ***p<0.001 as determined by two-tailed, unpaired Student's t-test.
13R controls Ag-driven Th17 to Th1 conversion
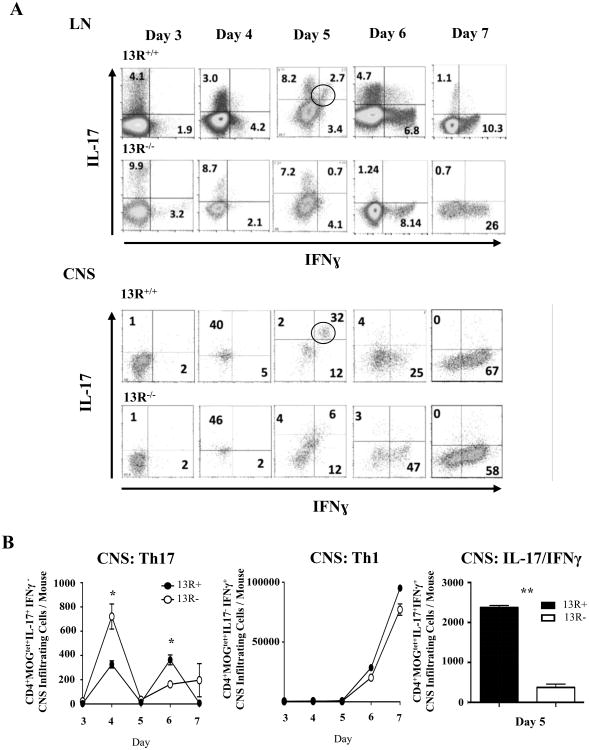
To begin analyzing the mechanism by which 13R controls manifestation of EAE we sought to profile the responses of Th1 and Th17 cells both in the LN and CNS. Accordingly, mice were induced for EAE with MOGp and their LN and CNS ex vivo Ag-specific T cell responses were analyzed daily during the early phase of disease. The data show that in the absence of 13R MOGtet+ Th17 cells were more frequent in the LN and CNS as assessed by percentage (Fig. 2A) and absolute number (Fig 2B, left panel) early during disease onset (day 3 and 4). However, MOGtet+ Th1 cells were present in the LN and CNS at similar percentages (Fig. 2A) and absolute number (Figure 2B, middle panel) in both 13R-/- and 13R+/+ mice. Interestingly, a significant number of MOGtet+ IFNγ/IL-17 double positive cells were observed in 13R+/+ but not 13R-/- mice (Fig. 2B, right panel). In all, these findings indicate that 13R plays a key role in Th17 dynamics and perhaps suggest a process of Ag-driven Th17 to Th1 conversion (22, 26) highlighted by the presence of MOGtet+ IFNγ/IL-17 double positive cells (Fig. 2B). This could explain the lower frequency of Th17 cells in the 13R+/+ mice during the early phase of disease (Fig. 1G and H) as the process of conversion is possibly in motion.
Figure 2. 13R deficiency nullifies transitional IFNγ+/IL17+ double positive cells but increases IL17+ single positive cells.
EAE was induced in 13R+/+ and 13R-/- C57BL/6 mice using 100 μg MOGp, the LN and CNS were harvested at the indicated days post-disease induction, and CD4+MOGtet+ T cells were analysed ex vivo for intracellular IFNγ and IL-17. (A) Shows the frequency of single as well as double cytokine producing CD4+MOGtet+ T cells in both the LN and the CNS. (B) Shows the absolute cell numbers accumulated in the CNS of CD4+MOGtet+ T cells producing IL-17 (left panel), IFNγ (median panel), or both (right panel). The data is compiled from 3 independent experiments. *p<0.05, **p<0.01 as determined by two-tailed, unpaired Student's t-test.
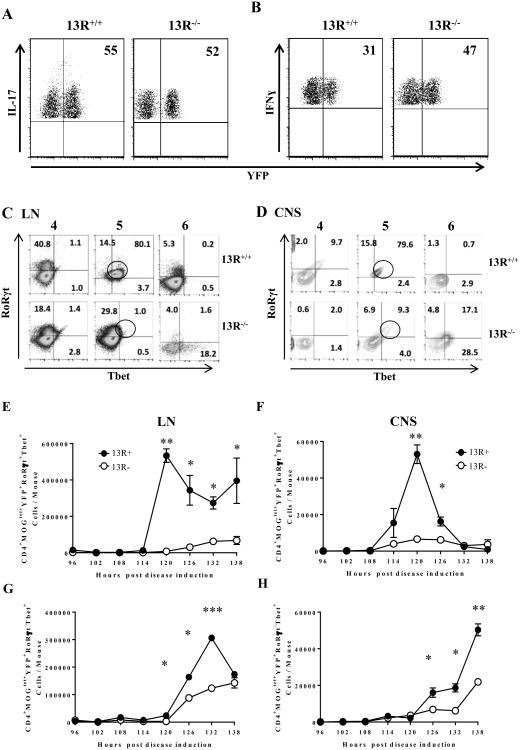
To determine if Ag-driven Th17 to Th1 conversion is occurring and whether 13R plays a role in this process we used IL-17acre (22) and ROSA26-YFP reporter mice to generate 13R+/+ IL-17acre-eYFP and 13R-/- IL-17acre-eYFP strains in which IL-17 production will trigger expression of YFP that persists even when IL-17 expression is terminated as a result of conversion to Th1 cells. In an initial experiment, we assessed the penetrance of the YFP tag to IL-17 production. The results show that in both 13R+/+ and 13R-/- mice approximately half of Th17 cells that were induced upon induction of EAE were tagged with YFP (Fig. 3A), a finding that agrees with the original report in the IL-17acre-eYFP reporter system (22). Furthermore, when Th1 cells arising alongside Th17 cells were tested for YFP expression, a significant number were YFP-positive indicating that conversion is occurring in both strains (Fig. 3B).
Figure 3. 13R deficiency interferes with Th17 conversion leading to reduced Th1 accumulation in the CNS.
(A, B) 13R+/+ and 13R-/- IL-17acre-eYFP mice were induced for EAE with 100 μg MOGp, the LN were harvested on day 7 post-disease induction and the frequency of YFP expressing CD4+IL-17+ (A) and CD4+IFNγ+ (B) T cells was determined by flow cytometry. (C-H) LN and CNS cells were harvested on days 4, 5 and 6 (C, D) or every 6 hours beginning on day 4 as indicated (E-H) and expression of Tbet and RoRγt by CD4+MOGtet+YFP+ cells was measured ex vivo by intracellular staining. (C, D) Show the frequency of CD4+MOGtet+YFP+ cells expressing Tbet and/or RoRγt in the LN (C) and CNS (D). (E-H) show the mean ± SD of the absolute numbers of CD4+MOGtet+YFP+ double positive for RoRγt and Tbet in (E, F) or Tbet single positive (G, H). Data is compiled from 3 independent experiments. *, p<0.05; **, p<0.01 as determined by two-tailed, unpaired Student's t-test.
To correlate the presence of transitional MOGtet+ IFNγ/IL-17 double-positive cells with conversion, the IL-17acre-eYFP reporter mice induced for EAE with 100 μg MOGp were examined for expression of signature transcription factors (RoRγt for Th17 and Tbet of Th1 cells) between day 4 and 6 post-disease induction, a time period when transition is in motion. The data shows that while the majority of CD4+MOGtet+YFP+ cells expressed both RoRγt and Tbet in the LN of 13R+/+ mice on day 5 post-disease induction, no significant percentages of these cells were observed in the 13R-/- mice during the day 4-6 monitoring period (Fig. 3C). Again, in the CNS while the majority (79.6%) of cells were double-positive in the 13R+/+ mice, only 9.3% had both transcription factors in 13R-/- mice at day 5 post disease induction (Fig. 3D). The absolute number of CD4+MOGtet+YFP+ cells expressing both RoRγt and Tbet also were significantly higher in the LN (Fig. 3E) and CNS (Fig. 3F) of 13R+/+ versus 13R-/- peaking at day 5 (120h) post disease induction. Interestingly, CD4+MOGtet+YFP+ T-bet single positive cells begin to accumulate significantly both in the LN (Fig. 3G) and CNS (Fig. 3H) 6 hours past day 5 (120h) as the number of T-bet/ RoRγt double-positive cells decline, indicating that Th17 to Th1 conversion goes through this transitional stage which is under the influence of 13R.
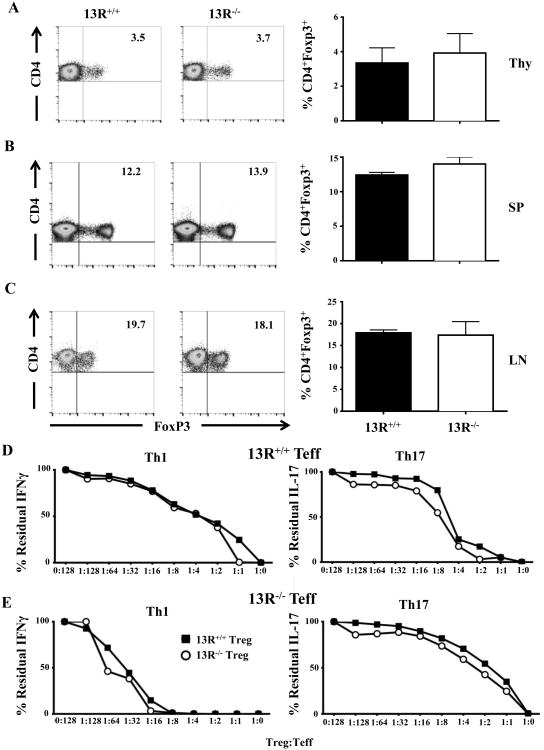
13R does not influence the frequency or function of Tregs
13R seems to foster Th17 to Th1 conversion and accumulation of the latter in the CNS. In light of this observation, however, the early onset and severe EAE observed in 13R-/- mice is puzzling. It is possible, though, that 13R affects the frequency and function of Tregs which would explain the differential disease outcome in the 2 strains. To test this premise, we began by crossing Foxp3-GFP reporter mice to the 13R-/- strain and then analyzed the frequency of CD4+Foxp3+(GFP) Tregs in different lymphoid organs from both 13R+/+- and 13R-/--Foxp3-GFP reporter strains. The results indicate that the frequency of Tregs in the thymus (Thy), SP and LN are similar in both strains and do not show any significant difference (Fig. 4A-C). We then thought that the function of Tregs might be compromised in the 13R-/- mice. To evaluate this postulate, Tregs were sorted from 13R+/+ and 13R-/- mice on day 10 post immunization with MOGp and tested for suppression of Th1 and Th17 effector cells isolated from the same mice. The findings show that Tregs from 13R-/- mice are as effective as Tregs from 13R+/+ mice whether the targets are Th1 or Th17 effector cells from 13R+/+ (Fig. 4D) or 13R-/- (Fig. 4E) mice. These data indicate that there is no defect in Treg function in 13R-/- mice.
Figure 4. 13R deficiency does not affect the frequency or function of Tregs.
Thy (A), SP (B), and LN (C) were harvested from naïve 13R+/+ and 13R-/- mice and the frequency of CD4+Foxp3+ Treg was determined by flow cytometry. (D, E) 13R+/+ and 13R-/- Foxp3-GFP mice were immunized with 100 μg MOGp s.c. in PBS/CFA and on day 10 post-immunization the SP cells were used to isolate effector Th1 and Th17 cells whereas the LN cells were utilized to sort CD4+CD25+Foxp3+(GFP+) Tregs. Effector Th1 and Th17 cells from 13R+/+ (D) and 13R-/- (E) mice were then co-cultured with the LN Tregs from both strains and suppression of effector function was evaluated by measuring residual IFNγ and IL-17 production by ELISA. The percent of residual cytokine represent the ratio of cytokine obtained in the presence of Tregs over cytokine produced in the absence of Tregs multiplied by 100. Data is representative of at least 3 independent experiments.
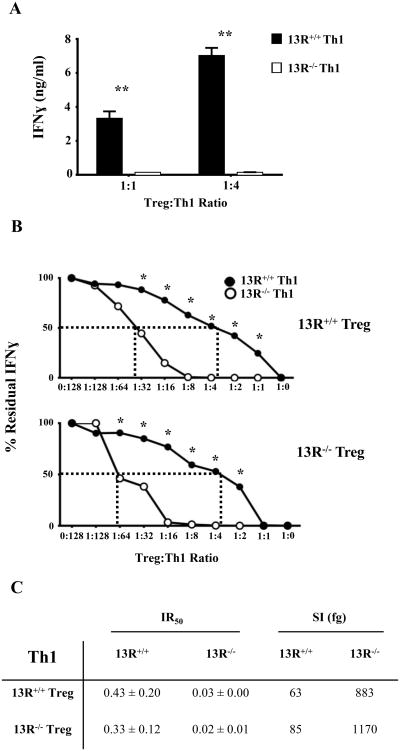
13R promotes diminished sensitivity of Th1 cells to suppression by Tregs
Given that the frequency and function of Tregs is not affected by 13R deficiency one would envision that the early onset and severe EAE observed in 13R-/- mice is related to effects of the receptor on effector T cells. Since both Th1 and Th17 cells contribute to EAE (27-30), the shift in cell frequency with more Th17 and less Th1 cells in 13R-/- mice, while it can be interpreted as compensatory, should not exacerbate the disease. This would logically imply that sensitivity to Treg suppression, rather than frequency, may be influenced by 13R. To test this postulate, splenic Th1 cells were sorted from 13R+/+ and 13R-/- mice on day 10 post immunization with MOGp, and their sensitivity to suppression by LN Tregs was evaluated. The findings indicate that Th1 cells from 13R-/- mice ceased IFNγ secretion at both 1:1 or 1:4 Treg/Th1 ratios while Th1 cells from 13R+/+ mice continued to produce significant levels of the cytokine (Fig. 5A). These findings suggest that Th1 cells from 13R+/+ are less sensitive to Treg suppression than those from 13R-/- mice. To determine the degree of sensitivity to Treg suppression the experiment was carried out with a broad range of Treg to Th1 ratios. Again, the results indicate that Th1 cells from 13R+/+ mice are less sensitive to suppression whether the Tregs are from 13R+/+ or 13R-/- mice (Fig. 5B). In fact, Th1 cells from 13R+/+ mice had their effector cytokine production suppressed by 50% with a ratio of 1:2.3 (Treg to Th1) while Th1 cells from 13R-/- mice reached 50% cytokine suppression with a much lower ratio of 1:33 (Fig. 5C). These 50% inhibition ratios (IR), referred to as IR50 when expressed as numerical values again indicate that the Th1 cells from 13R-/- mice are more sensitive to suppression than Th1 cells from 13R+/+ mice whether the Tregs are from 13R+/+ (compare 0.43 for 13R+/+ to 0.03 for 13R-/- Th1 cells) or 13R-/- (compare 0.33 for 13R+/+ to 0.02 for 13R-/- Th1 cells) mice (Fig 5C). These IR50 values were then used to define the sensitivity index (SI) for suppression of Th1cells by Tregs. The SI was calculated as the ratio whereby the numerator is the amount of cytokine (fg/ml) produced at the 50% inhibition point and the denominator is the absolute number of T effector cells per ml times the IR50. We define the SI as the intrinsic decline in cytokine production potential per effector T cell. In essence, the SI represents the absolute fg amount of cytokine that an effector T cell could not produce due to suppression by Tregs. Accordingly, a Th1 cell from 13R-/- mice was unable to produce 883 fg of IFNγ while a Th1 cell from 13R+/+ mice could not produce 63 fg of IFNγ, indicating that the 13R-/- Th1 cells are much more sensitive to Treg suppression than 13R+/+ Th1 cells (Fig. 5C). Similar sensitivity patterns were observed when the Tregs were from 13R-/- mice (Fig. 5C). Overall, the finding indicate that 13R plays a role in the sensitivity of effector Th1 cells to suppression by Tregs as Th1 cells from 13R+/+ were less sensitive to Treg suppression than Th1 cells from 13R-/- mice.
Figure 5. 13R deficiency increase the sensitivity of Th1 cells to suppression by Tregs.
13R+/+ and 13R-/- Foxp3-GFP mice were immunized with 100 μg MOGp s.c. in PBS/CFA and on day 10 post-immunization splenic effector Th1 cells from both strains were re-stimulated with PMA and ionomycin, isolated based on cytokine secretion and co-cultured with Tregs from the LN of 13R+/+ or 13R-/- mice. (A) Shows IFNγ production in cultures where the Th1 cells from either strain were co-cultured with Tregs from 13R+/+ mice at Treg/Th1 ratios of 1:1 and 1:4. (B) Shows the percent residual IFNγ production obtained at the indicated Treg:Th1 ratios (see Materials and Methods). The dashed lines indicate the inhibition ratios 50 (IR50), which is the Treg to T effector ratio at which the residual cytokine production is reduced by 50% (see Material and Methods). *p<0.05, **p<0.01 as determined by two-tailed, unpaired Student's t-test. (C) Shows the IR50± SD for Th1 effectors obtained with Tregs from 13R+/+ and 13R-/- mice. The SI (sensitivity index) represents the absolute amount of IFNγ that an effector Th1 cell could not produce due to suppression by Tregs (see Material and Methods). Data is compiled from 3 independent experiments.
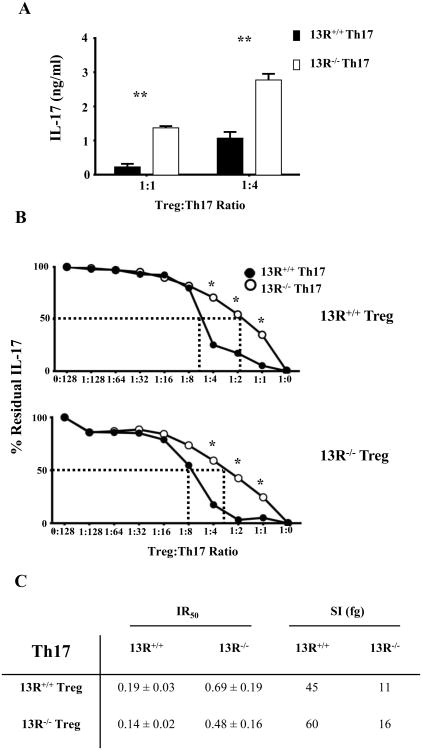
13R promotes increased sensitivity of Th17 cells to Treg suppression
In light of the fact that Th1 cells in 13R-/- mice are highly sensitive to suppression by Tregs, the observation of early onset and severe EAE in 13R-/- mice is perplexing. One likely possibility is that 13R displays a reverse effect on the susceptibility of Th17 cells to Treg suppression. To test this premise, splenic Th17 cells were sorted from 13R+/+ and 13R-/- mice on day 10 post immunization with MOGp and their sensitivity to suppression by LN Tregs was measured. The findings indicate that Th17 cells from 13R-/- mice continue to produce higher levels of IL-17 cytokine at both 1:1 or 1:4 Treg/Th1 ratios while Th17 cells from 13R+/+ mice produce significantly less IL-17 at both ratios (Fig. 6A). These findings indicate that Th17 cells from 13R+/+ mice are more sensitive to Treg suppression than those from 13R-/- mice. Like for Th1 cells, experiments were carried out with a broad range of Treg to Th1 ratios to determine the degree of Th17 sensitivity to Treg suppression. The findings show that Th17 cells from 13R+/+ mice are more sensitive to suppression whether the Tregs are from 13R+/+ or 13R-/- mice (Fig. 6B). In fact, Th17 cells from 13R+/+ mice had their effector cytokine production suppressed by 50% with a ratio of 1:5.2 (Treg to Th1) while Th17 cells from 13R-/- mice reached 50% cytokine suppression with a much higher ratio of 1:1.4. The IR50 for Th17 cells from 13R-/- mice is higher (0.69) than that of Th17 cells from 13R+/+ mice (0.19) indicating that the Th17 cells from 13R-/- are less sensitive to Treg suppression than Th17 cells from 13R+/+ mice (Fig. 6C). Similar IR50 patterns were observed when the Tregs were from 13R-/- mice. Moreover, the SI for Th17 cells from 13R-/- mice was 11 fg while the SI for Th17 cells from 13R+/+ mice was 45 fg, again indicating that the 13R-/- Th17 cells are much less sensitive to Treg suppression than 13R+/+ Th17 cells (Fig. 6C). Similar sensitivity patterns were observed when the Tregs were from 13R-/- mice (Fig. 6C). Overall, the findings indicate that 13R increases the sensitivity of effector Th17 cells to suppression by Tregs.
Figure 6. 13R deficiency diminishes the sensitivity of Th17 cells to suppression by Tregs.
13R+/+ and 13R-/- Foxp3-GFP mice were immunized with 100 μg MOGp s.c. in PBS/CFA and on day 10 post-immunization splenic effector Th17 cells from both strains were re-stimulated with PMA and ionomycin, isolated based on cytokine secretion and co-cultured with Tregs from the LN of 13R+/+ or 13R-/- mice. (A) Shows IL-17 production in cultures where the Th17 cells from either strain were co-cultured with Tregs from 13R+/+ mice at Treg/Th17 ratios of 1:1 and 1:4. (B) Shows the percent residual IL-17 production obtained at the indicated Treg:Th17 ratios. The dashed lines indicate the IR50 at which the residual cytokine production is reduced by 50%. *p<0.05, **p<0.01 as determined by two-tailed, unpaired Student's t-test. (C) Shows the IR50± SD for Th17 effectors obtained with Tregs from 13R+/+ and 13R-/- mice. The SI represents the absolute amount of IL-17 that an effector Th17 cell could not produce due to suppression by Tregs. Data is compiled from 3 independent experiments.
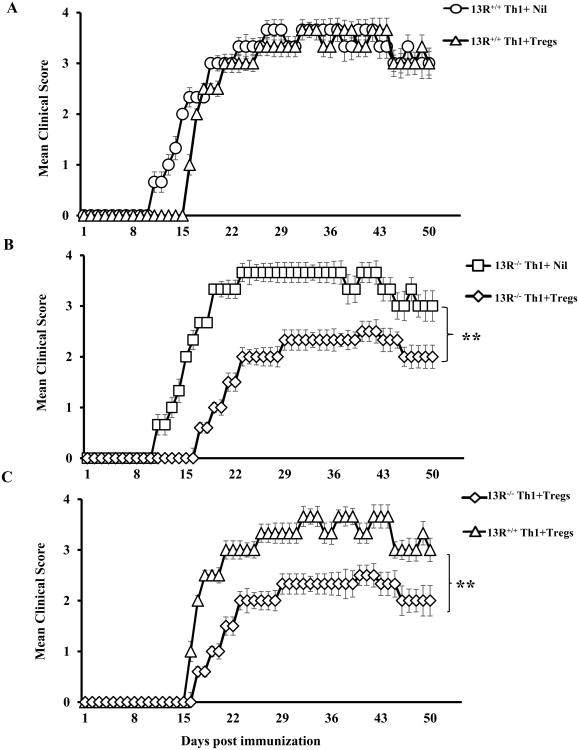
13R-mediated resistance of Th1 effector cells to Treg suppression leads to exacerbation of EAE
Given that in vitro co-culture assays indicate that Th1 cells from 13R+/+ mice are less sensitive to Treg suppression than those derived from 13R-/- mice, one would envision that such differential sensitivity translates into discrepancy in EAE manifestation. To test these premise, effector Th1 cells from MOGp-immunized 13R+/+ or 13R-/- mice were transferred into Rag2-/- C57BL/6 animals alongside LN Tregs from MOGp-immunized 13R+/+ Foxp3-GFP reporter mice. The hosts were then induced for EAE with MOGp and monitored daily for disease progression. Note that transfer of bulk Th1 cells induced weak clinical signs of EAE which would be readily suppressible by Tregs. Immunization with MOGp was therefore used to recall Ag-specific Th1 effectors and maximize disease severity to assess for Treg suppression in a rigorous manner. The results indicate that mice recipient of Th1 effectors from 13R+/+ mice alongside Tregs had delayed disease onset relative to those given effectors without Tregs (Fig. 7A). In contrast, mice recipient of Th1 effectors from 13R-/- mice alongside Tregs had delayed disease onset and more severe paralytic signs of EAE in comparison to hosts recipient of effector Th1 cells without Tregs (Fig. 7B). Thus, Th1 effectors from 13R+/+ mice are less sensitive to Treg suppression and drive severe EAE while Th1 effectors from 13R-/- mice are more sensitive to Treg suppression and drive milder EAE (Fig. 7C). In fact, while the onset of disease was similar for Th1 effectors from 13R+/+ and 13R-/- mice (day 16 and 17, respectively), the cumulative disease score (CDS) was much higher for Th1 effectors from 13R+/+ mice (111.8 ± 7.8) than those from 13R-/- mice (68.7 ± 6.4). Also, the mean maximal disease score (mmds) for Th1 effectors from 13R+/+mice was 3.7 and occurred at 3 episodes (days 32-34, 37-39 and 42-44) which is higher than the mmds for Th1 effectors from 13R-/- mice (2.5) which occurred during one episode only (days 40-43). In all, the 13R supports resistance of Th1 cells to Treg suppression leading to severe EAE.
Figure 7. Absence of 13R increases sensitivity of Th1 effector cells to Tregs.
13R+/+ Foxp3-GFP and 13R-/- mice were immunized with 100μg MOGp s.c. in PBS/CFA and 10 days later the SP were used to isolate effector Th1 cells. Also, the LN from 13R+/+ Foxp3-GFP mice were utilized to sort CD4+CD25+GFP+(Foxp3+) Tregs to serve for suppression. The Th1 cells were then transferred (1 ×106 Th1 cells per mouse) i.v. into Rag2-/- C57BL/6 mice (6 mice per group) with or without Tregs (2.5 ×106 cells per mouse). On day 2 after transfer the hosts were induced for EAE with 100μg MOGp. (A) Shows the mean clinical scores ± SD for hosts recipient of Th1 effector cells from 13R+/+ mice without (13R+/+ + Nil) or with (13R+/+ + Tregs) Tregs. (B) Shows the mean clinical scores ± SD for hosts recipient of Th1 effector cells from 13R-/- mice without (13R-/- + Nil) or with (13R-/- + Tregs) Tregs. (C) Shows comparison of the mean clinical scores ± SD of hosts recipient of Tregs and Th1 effector cells from 13R+/+ versus 13R-/- mice. **p<0.01 as determined by Mann-Whitney U test.
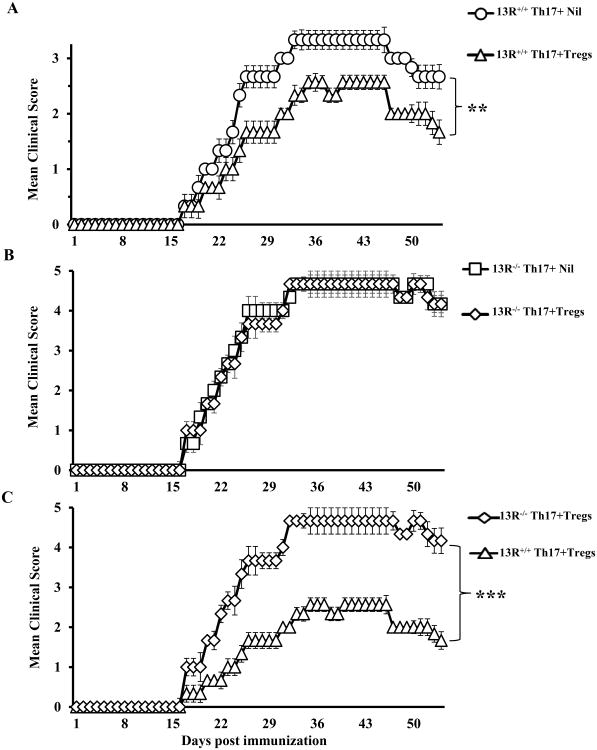
13R-mediated sensitivity of Th17 effector cells to Treg suppression leads to reduced EAE severity
In vitro co-culture suppression assays indicated that Th17 cells from 13R+/+ mice are more sensitive to Treg suppression than those derived from 13R-/- mice. We then set up experiments to test whether such differential sensitivity is operative in vivo and manifests in the severity of EAE Accordingly, effector Th17 cells from MOGp-immunized 13R+/+ and 13R-/- mice were transferred into Rag2-/- C57BL/6 mice alongside LN Tregs from MOGp-immunized 13R+/+ Foxp3-GFP reporter mice. The hosts were then induced for EAE with MOGp and monitored daily for disease progression. Note that transfer of bulk Th17 cells induced weak clinical signs of EAE which would be readily suppressible by Tregs. Immunization with MOGp was therefore used to recall Ag-specific Th17 effectors and maximize disease severity to assess for Treg suppression in a rigorous manner. The results indicate that mice recipient of Th17 effector cells from 13R+/+ mice alongside Tregs had less severe disease relative to those given effectors without Tregs (Fig. 8A). In contrast, mice recipient of Th17 effectors from 13R-/- mice alongside Tregs had a similar disease pattern in comparison to hosts recipient of effector Th17 cells without Tregs (Fig. 8B). Thus, Th17 effectors from 13R+/+ mice are sensitive to Treg suppression and drive milder EAE while Th17 effectors from 13R-/- mice are less sensitive to Treg suppression and drive more severe EAE (Fig. 8C). In fact, while the onset of disease was similar for Th17 effectors from 13R+/+ and 13R-/- mice (day 17), the CDS was lower for Th17 effector from 13R+/+ mice (70.2 ± 9.8) than those from 13R-/- mice (144.8 ± 24.6). Also, the mmds for Th17 effectors from 13R+/+ mice was 2.7 and occurred at 2 episodes (days 35-37, and 41-46) which is lower than the mmds for Th17 effectors from 13R-/- mice (4.6) which occurred for a longer episode (days 32-46). In all, the 13R supports sensitivity of Th17 cells to Treg suppression leading to mild EAE.
Figure 8. Absence of 13R decreases sensitivity of Th17 effector cells to Tregs.
13R+/+ Foxp3-GFP and 13R-/- mice were immunized with 100μg MOGp s.c. in PBS/CFA and 10 days later the SP were used to isolate effector Th17 cells. Also, the LN from 13R+/+ Foxp3-GFP mice were utilized to sort CD4+CD25+GFP+(Foxp3+) Tregs to serve for suppression. The Th17 cells were then transferred (4 ×106 Th17 cells per mouse) i.v. into Rag2-/- C57BL/6 mice (6 mice per group) with or without Tregs (2 ×106 cells per mouse). On day 2 after transfer the hosts were induced for EAE with 100μg MOGp. (A) Shows the mean clinical scores ± SD for hosts recipient of Th17 effector cells from 13R+/+ mice without (13R+/+ + Nil) or with (13R+/+ + Tregs) Tregs. (B) Shows the mean clinical scores ± SD for hosts recipient of Th17 effector cells from 13R-/- mice without (13R-/- + Nil) or with (13R-/- + Tregs) Tregs. (C) Shows comparison of the mean clinical scores ± SD of hosts recipient of Tregs and Th17 effector cells from 13R+/+ versus 13R-/- mice. **p<0.01 and ***p<0.001 as determined by Mann-Whitney U test.
Discussion
Both IL-4 and IL-13 cytokines have been shown to play an anti-inflammatory role in EAE (6, 31). Because both cytokines share the IL-4Rα/IL-13Rα1 HR, IL-4 and IL-13 may exercise such a function through the HR. Indeed, this was the case as mice lacking the HR developed early disease onset and more severe clinical signs of paralysis upon EAE induction with MOGp. This correlates with an increased frequency of Ag-specific Th17 cells in the lymph node and the CNS early on day 4 after disease induction, a phenomenon that bodes well with the notion that these cells migrate to the CNS to attract neutrophils and initiate the inflammatory process (32). Accumulation of Th1 cells within the CNS was comparable to 13R+/+ mice suggesting that the differential disease onset and severity are rather related to Th17 cells. In line with this thought is the observation that there was lower Th17 to Th1 conversion in 13R-/- mice where the onset of disease is early and the severity is more pronounced (Table 1). In contrast, there was greater conversion of Th17 to Th1 cells particularly in the CNS of 13R+/+ mice which developed rather typical EAE (Table I). The process of Ag-driven Th17 to Th1 conversion has previously been described (33) and, like in IL-23R-deficient mice (34), proceeds through a T-bet/RoRγt double-positive transitional intermediate T cells producing both IFNγ and IL-17 cytokines. The HR and its ligands IL-4 and IL-13 likely influence this process as the 13R-/- mice had rather less pronounced Th17 to Th1 conversion. In addition to conversion, there was a differential sensitivity to Treg suppression by the effector T cells in 13R+/+ versus 13R-/- mice which correlates with the type of EAE observed in the two strains (Table 1). Indeed, in 13R-/- mice the Th17 cells which accumulate in the CNS due to limited conversion display little sensitivity to suppression by Tregs further supporting the early onset and severe EAE. In contrast, in 13R+/+ mice where Th17 to Th1 conversion was prominent, the residual Th17 cells were more sensitive to Treg suppression, hence the milder EAE observed in the 13R+/+ strain. Despite the fact that differential sensitivity to Treg suppression was observed among 13R+/+ and 13R-/- derived Th1 cells, it could not influence the disease outcome perhaps because of similar accumulation in the CNS where the disease is impacted by Th17 cells. While sensitivity of effector T cells to Tregs has previously been reported to affect cell proliferation and survival (35, 36), perhaps through regulation of intrinsic factors (37-40), it was not clear whether both Th1 and Th17 effectors display similar patterns of sensitivity. This study however shows that Th1 and Th17 cells display different pattern of sensitivity to Treg suppression in both 13R+/+ and 13R-/- mice. Since Th1 and Th17 cells do not express the HR even in 13R+/+ mice, it is likely that the sensitivity to Treg suppression is guided by APCs whose function had been shaped by the HR (15, 16, 20, 21). It is possible that the interaction of naïve T cells with HR-conditioned APCs not only guides subset differentiation but also prompts expression of surface molecules that control the sensitivity of the effector to Treg suppression (41-44). In addition, since IL-21 has been shown to play a role in effector resistance to Treg suppression (45), it is possible that the differential sensitivity to Treg suppression among Th1 and Th17 cells is related to production of the cytokine by the latter not the former. Overall, both conversion and sensitivity to Treg suppression point to Th17 cells as the culprit of disease severity regulated by the HR. Specifically, Th17 accumulation due to reduced conversion and increased Th17 cell resistance to Treg suppression explain the severity of EAE in 13R-/- mice. The reverse would explain the milder EAE observed in 13R+/+ mice.
Table 1. Summary of effector T cell sensitivity to Treg suppression and its effect on EAE.
| Sensitivity to Treg suppression | Th17 to Th1 conversion | EAE manifestation | ||
|---|---|---|---|---|
|
| ||||
| Th1 | Th17 | |||
| 13R+/+ | * | *** | +++ | typical |
| 13R-/- | ** | * | + | early onset and severe |
No sensitivity to Treg suppression,
sensitive to Treg suppression, and
highly sensitive to Treg suppression as defined by EAE manifestation.
Little or no conversion and
pronounced conversion
In all, the HR and its cytokines IL-4 and IL-13 play a role in Th17 to Th1 conversion as well as the differential sensitivity of the effectors to Treg suppression leading to control of EAE.
Acknowledgments
Funding. This work was supported by grant RO1 NS057194 (to H.Z.) from the National Institute of Neurological Disorders and Stroke by the J. Lavenia Edwards endowment. M.M.M. was supported by T32 Training Grant GM008396 from the National Institute of General Medical Sciences.
Footnotes
Abbreviations. HR, IL-4Rα/IL-13Rα1 heteroreceptor; 13R, IL-13Rα1; DC, dendritic cell; EAE, experimental allergic encephalomyelitis; Treg, T regulatory cell; mmds, mean maximal disease score; MOGp, myelin oligodendrocyte glycoprotein-peptide; MS, multiple sclerosis; SP, spleen; LN, lymph node; Thy, thymus; CDS, cumulative disease score; P. toxin, pertussis toxin; SI, sensitivity index;
References
- 1.Miller JF, Morahan G. Peripheral T cell tolerance. Annu Rev Immunol. 1992;10:51–69. doi: 10.1146/annurev.iy.10.040192.000411. [DOI] [PubMed] [Google Scholar]
- 2.Bluestone JA, Bour-Jordan H, Cheng M, Anderson M. T cells in the control of organ-specific autoimmunity. J Clin Invest. 2015;125:2250–2260. doi: 10.1172/JCI78089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garren H, Ruiz PJ, Watkins TA, Fontoura P, Nguyen LT, Estline ER, Hirschberg DL, Steinman L. Combination of gene delivery and DNA vaccination to protect from and reverse Th1 autoimmune disease via deviation to the Th2 pathway. Immunity. 2001;15:15–22. doi: 10.1016/s1074-7613(01)00171-6. [DOI] [PubMed] [Google Scholar]
- 4.Falcone M, Bloom BR. A T helper cell 2 (Th2) immune response against non-self antigens modifies the cytokine profile of autoimmune T cells and protects against experimental allergic encephalomyelitis. J Exp Med. 1997;185:901–907. doi: 10.1084/jem.185.5.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cash E, Minty A, Ferrara P, Caput D, Fradelizi D, Rott O. Macrophage-inactivating IL-13 suppresses experimental autoimmune encephalomyelitis in rats. J Immunol. 1994;153:4258–4267. [PubMed] [Google Scholar]
- 6.Young DA, Lowe LD, Booth SS, Whitters MJ, Nicholson L, Kuchroo VK, Collins M. IL-4, IL-10, IL-13, and TGF-beta from an altered peptide ligand-specific Th2 cell clone down-regulate adoptive transfer of experimental autoimmune encephalomyelitis. J Immunol. 2000;164:3563–3572. doi: 10.4049/jimmunol.164.7.3563. [DOI] [PubMed] [Google Scholar]
- 7.Ochoa-Reparaz J, Rynda A, Ascon MA, Yang X, Kochetkova I, Riccardi C, Callis G, Trunkle T, Pascual DW. IL-13 production by regulatory T cells protects against experimental autoimmune encephalomyelitis independently of autoantigen. J Immunol. 2008;181:954–968. doi: 10.4049/jimmunol.181.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Min B, Legge KL, Pack C, Zaghouani H. Neonatal exposure to a self-peptide-immunoglobulin chimera circumvents the use of adjuvant and confers resistance to autoimmune disease by a novel mechanism involving interleukin 4 lymph node deviation and interferon gamma-mediated splenic anergy. J Exp Med. 1998;188:2007–2017. doi: 10.1084/jem.188.11.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pack CD, Cestra AE, Min B, Legge KL, Li L, Caprio-Young JC, Bell JJ, Gregg RK, Zaghouani H. Neonatal exposure to antigen primes the immune system to develop responses in various lymphoid organs and promotes bystander regulation of diverse T cell specificities. J Immunol. 2001;167:4187–4195. doi: 10.4049/jimmunol.167.8.4187. [DOI] [PubMed] [Google Scholar]
- 10.Yu P, Haymaker CL, Divekar RD, Ellis JS, Hardaway J, Jain R, Tartar DM, Hoeman CM, Cascio JA, Ostermeier A, Zaghouani H. Fetal exposure to high-avidity TCR ligand enhances expansion of peripheral T regulatory cells. J Immunol. 2008;181:73–80. doi: 10.4049/jimmunol.181.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu P, Gregg RK, Bell JJ, Ellis JS, Divekar R, Lee HH, Jain R, Waldner H, Hardaway JC, Collins M, Kuchroo VK, Zaghouani H. Specific T regulatory cells display broad suppressive functions against experimental allergic encephalomyelitis upon activation with cognate antigen. J Immunol. 2005;174:6772–6780. doi: 10.4049/jimmunol.174.11.6772. [DOI] [PubMed] [Google Scholar]
- 12.Wang D, Ghosh D, Islam SM, Moorman CD, Thomason AE, Wilkinson DS, Mannie MD. IFN-beta Facilitates Neuroantigen-Dependent Induction of CD25+ FOXP3+ Regulatory T Cells That Suppress Experimental Autoimmune Encephalomyelitis. J Immunol. 2016;197:2992–3007. doi: 10.4049/jimmunol.1500411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 14.Li L, Lee HH, Bell JJ, Gregg RK, Ellis JS, Gessner A, Zaghouani H. IL-4 utilizes an alternative receptor to drive apoptosis of Th1 cells and skews neonatal immunity toward Th2. Immunity. 2004;20:429–440. doi: 10.1016/s1074-7613(04)00072-x. [DOI] [PubMed] [Google Scholar]
- 15.Lee HH, Hoeman CM, Hardaway JC, Guloglu FB, Ellis JS, Jain R, Divekar R, Tartar DM, Haymaker CL, Zaghouani H. Delayed maturation of an IL-12-producing dendritic cell subset explains the early Th2 bias in neonatal immunity. J Exp Med. 2008;205:2269–2280. doi: 10.1084/jem.20071371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoeman CM, Dhakal M, Zaghouani AA, Cascio JA, Wan X, Khairallah MT, Chen W, Zaghouani H. Developmental expression of IL-12Rbeta2 on murine naive neonatal T cells counters the upregulation of IL-13Ralpha1 on primary Th1 cells and balances immunity in the newborn. J Immunol. 2013;190:6155–6163. doi: 10.4049/jimmunol.1202207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang H, Paul WE. Impaired interleukin 4 signaling in T helper type 1 cells. J Exp Med. 1998;187:1305–1313. doi: 10.1084/jem.187.8.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly-Welch AE, Hanson EM, Boothby MR, Keegan AD. Interleukin-4 and interleukin-13 signaling connections maps. Science. 2003;300:1527–1528. doi: 10.1126/science.1085458. [DOI] [PubMed] [Google Scholar]
- 19.Haymaker CL, Guloglu FB, Cascio JA, Hardaway JC, Dhakal M, Wan X, Hoeman CM, Zaghouani S, Rowland LM, Tartar DM, VanMorlan AM, Zaghouani H. Bone marrow-derived IL-13Ralpha1-positive thymic progenitors are restricted to the myeloid lineage. J Immunol. 2012;188:3208–3216. doi: 10.4049/jimmunol.1103316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhakal M, Hardaway JC, Guloglu FB, Miller MM, Hoeman CM, Zaghouani AA, Wan X, Rowland LM, Cascio JA, Sherman MP, Zaghouani H. IL-13Ralpha1 is a surface marker for M2 macrophages influencing their differentiation and function. Eur J Immunol. 2014;44:842–855. doi: 10.1002/eji.201343755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhakal M, Miller MM, Zaghouani AA, Sherman MP, Zaghouani H. Neonatal Basophils Stifle the Function of Early-Life Dendritic Cells To Curtail Th1 Immunity in Newborn Mice. J Immunol. 2015;195:507–518. doi: 10.4049/jimmunol.1500027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cascio JA, Haymaker CL, Divekar RD, Zaghouani S, Khairallah MT, Wan X, Rowland LM, Dhakal M, Chen W, Zaghouani H. Antigen-specific effector CD4 T lymphocytes school lamina propria dendritic cells to transfer innate tolerance. J Immunol. 2013;190:6004–6014. doi: 10.4049/jimmunol.1203552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Divekar RD, Haymaker CL, Cascio JA, Guloglu BF, Ellis JS, Tartar DM, Hoeman CM, Franklin CL, Zinselmeyer BH, Lynch JN, Miller MJ, Zaghouani H. T cell dynamics during induction of tolerance and suppression of experimental allergic encephalomyelitis. J Immunol. 2011;187:3979–3986. doi: 10.4049/jimmunol.1100531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bell JJ, Min B, Gregg RK, Lee HH, Zaghouani H. Break of neonatal Th1 tolerance and exacerbation of experimental allergic encephalomyelitis by interference with B7 costimulation. J Immunol. 2003;171:1801–1808. doi: 10.4049/jimmunol.171.4.1801. [DOI] [PubMed] [Google Scholar]
- 26.Kurschus FC, Croxford AL, Heinen AP, Wortge S, Ielo D, Waisman A. Genetic proof for the transient nature of the Th17 phenotype. Eur J Immunol. 2010;40:3336–3346. doi: 10.1002/eji.201040755. [DOI] [PubMed] [Google Scholar]
- 27.Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med. 2008;14:337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Damsker JM, Hansen AM, Caspi RR. Th1 and Th17 cells: adversaries and collaborators. Ann N Y Acad Sci. 2010;1183:211–221. doi: 10.1111/j.1749-6632.2009.05133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-behi M, Rostami A, Ciric B. Current views on the roles of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. J Neuroimmune Pharmacol. 2010;5:189–197. doi: 10.1007/s11481-009-9188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Racke MK, Bonomo A, Scott DE, Cannella B, Levine A, Raine CS, Shevach EM, Rocken M. Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease. J Exp Med. 1994;180:1961–1966. doi: 10.1084/jem.180.5.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J Exp Med. 2008;205:1535–1541. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abromson-Leeman S, Bronson RT, Dorf ME. Encephalitogenic T cells that stably express both T-bet and ROR gamma t consistently produce IFNgamma but have a spectrum of IL-17 profiles. J Neuroimmunol. 2009;215:10–24. doi: 10.1016/j.jneuroim.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O'Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pace L, Pioli C, Doria G. IL-4 modulation of CD4+CD25+ T regulatory cell-mediated suppression. J Immunol. 2005;174:7645–7653. doi: 10.4049/jimmunol.174.12.7645. [DOI] [PubMed] [Google Scholar]
- 36.Pace L, Rizzo S, Palombi C, Brombacher F, Doria G. Cutting edge: IL-4-induced protection of CD4+CD25- Th cells from CD4+CD25+ regulatory T cell-mediated suppression. J Immunol. 2006;176:3900–3904. doi: 10.4049/jimmunol.176.7.3900. [DOI] [PubMed] [Google Scholar]
- 37.Wohlfert EA, Callahan MK, Clark RB. Resistance to CD4+CD25+ regulatory T cells and TGF-beta in Cbl-b-/- mice. J Immunol. 2004;173:1059–1065. doi: 10.4049/jimmunol.173.2.1059. [DOI] [PubMed] [Google Scholar]
- 38.Bopp T, Palmetshofer A, Serfling E, Heib V, Schmitt S, Richter C, Klein M, Schild H, Schmitt E, Stassen M. NFATc2 and NFATc3 transcription factors play a crucial role in suppression of CD4+ T lymphocytes by CD4+ CD25+ regulatory T cells. J Exp Med. 2005;201:181–187. doi: 10.1084/jem.20041538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.King CG, Kobayashi T, Cejas PJ, Kim T, Yoon K, Kim GK, Chiffoleau E, Hickman SP, Walsh PT, Turka LA, Choi Y. TRAF6 is a T cell-intrinsic negative regulator required for the maintenance of immune homeostasis. Nat Med. 2006;12:1088–1092. doi: 10.1038/nm1449. [DOI] [PubMed] [Google Scholar]
- 40.Schneider A, Rieck M, Sanda S, Pihoker C, Greenbaum C, Buckner JH. The effector T cells of diabetic subjects are resistant to regulation via CD4+ FOXP3+ regulatory T cells. J Immunol. 2008;181:7350–7355. doi: 10.4049/jimmunol.181.10.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stephens GL, McHugh RS, Whitters MJ, Young DA, Luxenberg D, Carreno BM, Collins M, Shevach EM. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J Immunol. 2004;173:5008–5020. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 42.Takeda I, Ine S, Killeen N, Ndhlovu LC, Murata K, Satomi S, Sugamura K, Ishii N. Distinct roles for the OX40-OX40 ligand interaction in regulatory and nonregulatory T cells. J Immunol. 2004;172:3580–3589. doi: 10.4049/jimmunol.172.6.3580. [DOI] [PubMed] [Google Scholar]
- 43.Elpek KG, Yolcu ES, Franke DD, Lacelle C, Schabowsky RH, Shirwan H. Ex vivo expansion of CD4+CD25+FoxP3+ T regulatory cells based on synergy between IL-2 and 4-1BB signaling. J Immunol. 2007;179:7295–7304. doi: 10.4049/jimmunol.179.11.7295. [DOI] [PubMed] [Google Scholar]
- 44.Hue S, Kared H, Mehwish Y, Mouhamad S, Balbo M, Levy Y. Notch activation on effector T cells increases their sensitivity to Treg cell-mediated suppression through upregulation of TGF-betaRII expression. Eur J Immunol. 2012;42:1796–1803. doi: 10.1002/eji.201142330. [DOI] [PubMed] [Google Scholar]
- 45.Clough LE, Wang CJ, Schmidt EM, Booth G, Hou TZ, Ryan GA, Walker LS. Release from regulatory T cell-mediated suppression during the onset of tissue-specific autoimmunity is associated with elevated IL-21. J Immunol. 2008;180:5393–5401. doi: 10.4049/jimmunol.180.8.5393. [DOI] [PubMed] [Google Scholar]