Abstract
Immune mechanisms responsible for pathogen clearance from the female reproductive tract (FRT) are incompletely defined and in particular the contribution of lymphocyte trafficking to this process is unclear. CCR7-deficient mice have profoundly altered lymphocyte recirculation and display ectopic formation of lymphocyte aggregates within mucosal non-lymphoid tissues, including the FRT. In this study, we investigated how altered lymphocyte distribution in CCR7-deficient mice would affect host responses to Chlamydia muridarum within the reproductive tract. As expected, CCR7-deficient mice exhibited reduced lymphocyte trafficking to lymph nodes and a corresponding increase in T cells populations within the FRT. After intravaginal infection with Chlamydia, CCR7-deficient mice displayed markedly reduced antigen-specific CD4 T cell responses within the local draining iliac lymph nodes, yet robust Th1 and Th17 responses were prominently detected in the FRT. In addition, Chlamydia-specific antibody responses were disregulated in CCR7-deficient mice, displaying an unexpected increase in the systemic IgA responses. Importantly, prominent mucosal immune responses in CCR7-deficient mice increased the efficiency of bacteria clearance from the FRT while reducing tissue-associated inflammation and pathology. Thus, increased numbers of lymphocytes within the FRT results in pathogen clearance with reduced immune-mediated pathology.
Keywords: CCR7, CD4 T cells, Chlamydia
Introduction
An effective immune response to mucosal infection depends on prompt activation of the immune system within local draining lymphoid tissue and the subsequent directed migration of effector cells to the site of infection (1, 2). Following infection of the female reproductive tract (FRT), pathogen-specific lymphocyte activation and clonal expansion is initiated within local lymph nodes (3–5). Naïve T cells encounter cognate antigen carried to the lymph nodes by antigen presenting cells that have migrated from the inflamed local tissue (4). These activated T cells subsequently undergo functional maturation and acquire the ability to leave the lymph node and enter infected tissues to combat pathogen replication (1, 2). After the primary infection is resolved, the magnitude of the effector T cell population shrinks markedly, leaving memory T cells in lymphoid and non-lymphoid tissues to provide defense against re-encounter with the same pathogen (2, 6). Thus, the immune response to initial FRT infection involves a coordinated series of events where naive lymphocytes within local lymph nodes generate effector populations that mediate pathogen clearance and provide long-lived memory.
Chlamydia trachomatis is the most common bacterial sexually transmitted infection in the US, causing pelvic inflammatory disease and infertility in otherwise healthy women (7, 8). The development of an effective vaccine from Chlamydia is a public health priority and greater understanding of protective immunity in the FRT will be an essential component of this process (9, 10). The mouse model of Chlamydia infection involves intravaginal infection of inbred-mouse strains with the mouse pneumonitis biovar of C. trachomatis, now called C. muridarum (11). C. muridarum rapidly infects vaginal and cervical epithelial cells and ascends the reproductive tract where it causes upper reproductive tract pathology and post-infection infertility that resemble Chlamydia-associated disease sequelae in women (11, 12). Protective immunity to C. muridarum requires CD4 T cells, although antibody and CD8 T cells can contribute to bacterial clearance during secondary infections (5, 13–16). The development of FRT pathology in the mouse model correlates with bacterial burden, the infiltration of neutrophils, and the production of inflammatory mediators downstream of TLR activation (17–19). Thus, an effective Chlamydia vaccine that maximizes CD4-mediated protection and reduces pathology will require greater understanding of Chlamydia-specific T cell biology within the FRT. reproductive tract. In particular, the role of ectopic lymphoid tissues in the FRT is poorly understood. Although the FRT lacks organized lymphoid structures, immune inductive sites are formed in the tissue after the resolution of infection (20, 21). These ectopic structures can contain naïve lymphocytes and are thought to play an important role in secondary immunity (21). Given the absence of organized lymphoid tissues in the FRT, it seems possible that these organized structures are important in accelerating immune responses to heterologous challenge infections. However, the role of ectopic lymphoid tissues in protection against Chlamydia infection has not been carefully examined.
The chemokine receptor, CCR7, allows lymphocytes and dendritic cells to recognize CCL19 and CCL21 and thus sense lymph node-derived chemokine gradients (22, 23). CCR7 expression is induced on dendritic cells following innate activation and plays an essential role in DC homing to the draining lymph node to initiate T cell responses (24). CCR7 is also expressed on lymphocytes and is required for lymph node entry and appropriate anatomical positioning within the lymph node (22, 23). CCR7-deficient mice therefore display defective lymph node architecture and have a reduced number of lymphocytes in LNs (25). In addition, CCR7-deficient mice display ectopic lymphoid structure within mucosal tissues, such as lung, stomach and colon (22, 26). Thus, these mice provide a useful model to examine the importance of lymphoid tissue organization in defense against pathogen challenge. The outcome of infection in CCR7-deficient mice varies considerably, depending on the nature of pathogen studied and the route of challenge infection (27–31). Given recent data suggesting that a protective memory response to Chlamydia infection relies largely upon tissue-resident CD4 T cell populations within the FRT (32), it is of interest to examine how ectopic lymphoid tissues in the FRT of CCR7-deficient mice influence genital Chlamydia infection.
Here, we report that under steady state conditions, CCR7-deficient mice display a marked increase in lymphocytes within the FRT. Following intravaginal Chlamydia infection, CCR7-deficient mice develop disregulated CD4 T cell and antibody responses that involve a reduction in draining lymph node responses combined with enhanced FRT Chlamydia-specific T cell activation and cytokine production. This robust CD4 response in the FRT correlated with rapid bacterial clearance and lower reproductive tract pathology.
Materials and Methods
Mice
C57BL/6 and CCR7-deficient mice (B6.129P2(C)-Ccr7tm1Rfor/J) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All mice used for experiments were 8–16 weeks old, unless specifically noted. Mice were maintained under SPF conditions and all mouse experiments were performed in accordance with University of California Davis Research Animal Resource guidelines.
Bacteria
Chlamydia muridarum strain Weiss was cultured in HeLa 229 cells in Eagle’s minimal essential medium (MEM) (Invitrogen) supplemented with 10% fetal calf serum (FCS). Elementary bodies (EBs) were purified by discontinuous density gradient centrifugation as previously described and stored at −80 degrees (33). Purified EBs were titrated by infection of HeLa 229 cells and enumeration of inclusions that were stained with anti-Chlamydia MOMP antibody (Mo33b) (34). A fresh aliquot was thawed and used for every infection experiment. Heat-killed EBs (HKEBs) were prepared by heating purified EBs at 56°C for 30 min.
Chlamydia infection and enumeration
Mice were synchronized by subcutaneous injection of 2.5mg Depo-provera (Greenstone, NJ), 7 days prior to intravaginal infection. For infection, 1×105 C. muridarum in 5µL SPG buffer were deposited directly into the vaginal vault using a pipet tip. To enumerate bacterial shedding, vaginal swabs were collected, disrupted with glass beads suspended in 1mL SPG buffer, and serial dilutions were then plated on HeLa 229 cells. To enumerate the bacteria burden within tissues, the upper FRT (ovaries, oviducts, upper 1/3 of uterine horn), the lower FRT (vagina, cervix and lower 1/3 of uterine horn), spleen, and draining lymph nodes were homogenized in SPG buffer and the tissue homogenate placed in 2mL tubes with glass beads. After shaking for 5min, samples were centrifuged at 500g for 10 minutes, and supernatants collected and serial dilutions plated on HeLa 229 cells.
Tetramer staining and flow cytometry
Tetramer staining for Chlamydia-specific CD4 T cells was carried out as previously described (5). Spleen and LNs were harvested from naïve or infected mice and single cell suspensions prepared in FACS buffer (PBS with 2% FCS) containing Chlamydia MHC class-II tetramer in Fc block (culture supernatant from the 24G2 hybridoma, 2% mouse serum, 2% rat serum, and 0.01% sodium azide) for 1hr at RT in dark. Cells were washed and tetramer positive cells enriched via magnetic selecting LS MACS columns using anti-fluorochrome magnetic beads (Miltenyi Biotec, Auburn, CA). The resulting bound and unbound fractions were stained using a panel of antibodies (listed below) and analyzed on a FACSCanto or an LSRFortessa flow cytometer (BD Biosciences, San Jose, CA). To stain for intracellular transcription factors and cytokines, surface stained cells were fixed, permeablized and stained using the Foxp3 staining Kit (eBioscience). Antibodies used included FITC-CD11b (M1/70), CD11c (N418), F4/80 (BM8), B220 (RA3-6B2), TNF-α (MP6-XT22), PerCP-eFlour710-CD4 (RM4-5); APC-CCR7 (4B12); eFlour660-T-bet (4B10); Alexa700-CD44 (IM7); eFlour450-CD3 (145-2C11), Foxp3 (FJK-16S), IFN-γ (XMG1.2) (eBioscience, San Diego, CA); FITC-IL-17A (TC11-18H1) and APC-Cy7-CD8 (53-6.7) (BD Biosciences, San Diego, CA). Data were analyzed using FlowJo software (Tree Star, Ashland, OR). Endogenous, tetramer-specific CD4 T cells were identified using a previously described gating strategy (35).
Cytokine ELISA and intracellular staining
Mice were infected with Chlamydia as described above and mononuclear cells from the FRT were isolated as previously described (36). Briefly, the mouse vagina, cervix, uterine horns and oviducts were recovered, minced into small pieces, and digested with collagenase IV (500 mg/L, Sigma-Aldrich) with stirring for 1 h at 37 °C for two rounds. Tissue debris was then filtered and mononuclear cells separated using a Percoll gradient (GE Healthcare). Five million purified cells were incubated in the presence of HKEBs in 96-well round-bottom plates. After 72 h of incubation at 37°C, supernatants were collected and added to 96-well ELISA plates (Costar, Corning, NY) that had been pre-coated with purified anti-IFN-γ (R4-6A2), anti-IL-2, anti-IL-4, anti-IL-6, anti-IL-17A, anti-IL-23 (eBiosciences) or anti-IL-10 (R&D). Cytokine production was detected using biotinylated antibodies specific for each cytokine (eBiosciences), followed by the addition of ExtrAvidin-Peroxidase and TMB substrate (Sigma-Aldrich and BD Biosciences). Developed ELISA plates were analyzed using a spectrophotometer (SpectraMax M5; Molecular Devices), and cytokine concentrations calculated according to standard curves. In other experiments, stimulated mononuclear cells recovered from the FRT were stimulated with HKEBs for 48 hours and stained using antibodies specific for CD4, CD44, IFN-γ, and IL-17A and examined by flow cytometry, as described above.
Chlamydia-specific Antibody ELISA assay
Mice were bled retro-orbitally and vaginal washes collected at 14 days post-infection. Serum and wash fluid was analyzed by ELISA for the presence of Chlamydia-specific antibodies. Briefly, serial dilutions of serum samples were added to HKEB-coated ELISA plates (Costar). Chlamydia-specific Abs were detected using biotinylated isotype-specific Abs (eBioscience and BioLegend) and ExtrAvidin-Peroxidase substrate (Sigma-Aldrich).
Histopathologic scoring
Mice, naïve or infected intravaginally with C. muridarum as described above, were sacrificed at 7 days post-infection by carbon dioxide asphyxiation and cardiac exsanguination. The female reproductive tract (ovary, oviduct, uterus, cervix and vagina) was immersion fixed in 10% neutral buffered formalin. Fixed tissues were processed routinely, embedded in paraffin, sectioned 5 um thick and stained with hematoxylin and eosin. Histopathologic evaluation was performed by a board-certified veterinary anatomic pathologist (DMI). Masking and randomization of samples was done prior to histopathologic scoring. The samples were evaluated for the presence and severity of acute inflammation, chronic inflammation, erosion, dilation and fibrosis. Acute inflammation was defined by neutrophilic infiltration and edema. Chronic inflammation was defined by lymphohistiocytic infiltration. Erosion was defined by the loss of mucosal epithelial cells, typically with breach of the basement membrane. Dilation was defined by distention of the lumen. Fibrosis was defined by either an increase in fibroblasts or an increase in collagenous connective tissue. Parameters were evaluated using an ordinal scoring system based on lesion severity and distribution on a 0–4 point scale. Semi-quantitative histopathologic scores were analyzed for statistically significant differences (Mann-Whitney test) using GraphPad Prism 6.0.
Statistical analysis
Statistical analysis was performed by using an unpaired t test for normally distributed continuous-variable comparisons and a Mann-Whitney U test for nonparametric comparisons (Prism; GraphPad Software, Inc.).
Results
Naïve CCR7-deficient mice exhibit a marked increase in CD4 and CD8 T cells within the female reproductive tract
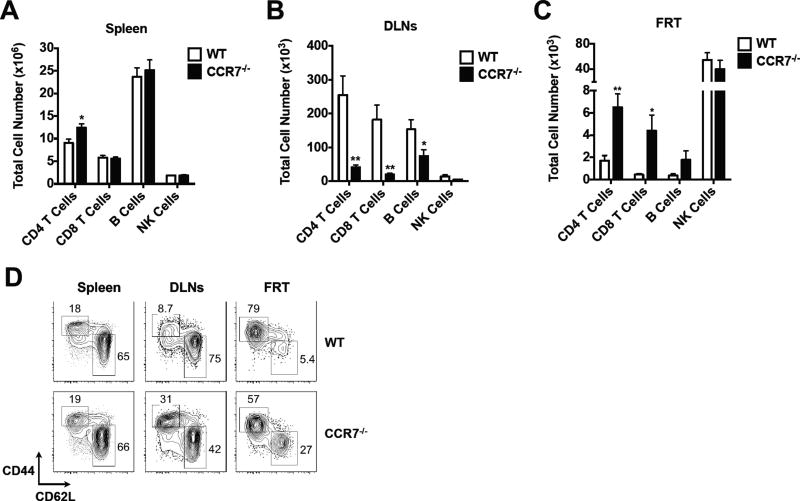
It has been previously demonstrated that CCR7 deficiency results in ectopic formation of lymphoid structures in the mucosal tissues, such as stomach, lung and colon (26). To examine whether lymphocyte aggregates also form in the female reproductive tract under non-inflammatory conditions, we analyzed the frequency of lymphocytes in secondary lymphoid organs (SLOs) and FRTs of naïve wild-type (WT) and CCR7-deficient mice. Comparable numbers of lymphocytes, including B cells, CD8 T cells and NK cells were found in the spleen of CCR7-deficient mice and WT controls, although a slightly higher number of CD4 T cells was detected in CCR7-deficient mice compared with WT controls (Fig. 1A). The draining iliac lymph nodes (DLNs) of FRT in CCR7-deficient mice were smaller (data not shown) and the lymphocyte counts were drastically reduced in these mice compared to WT mice (Fig. 1B). In contrast, higher numbers of CD4 and CD8 T cells were recovered from the FRT of CCR7-deficient mice (Fig. 1C), demonstrating that lymphocyte accumulation occurs in the FRT, as reported for other mucosal tissues (26). Interestingly, while most CD4 T cells in the FRT of WT mice displayed an effector memory T cell phenotype (CD44hiCD62Llo), a significant portion of CD4 T cells in the FRT of CCR7-deficient mice were naïve CD4 T cells (CD44loCD62hi), (Fig. 1D). In contrast, the frequency of naïve CD4 T cells was reduced in the DLNs of CCR7-deficient mice (Fig. 1D), consistent with previous reports showing that memory cells use alternate receptors to enter lymph node high endothelial venules (37, 38). These results demonstrate that CD4 T cells accumulate within the non-lymphoid FRT of uninfected CCR7-deficient mice.
Figure 1.
Elevated numbers of FRT tissue resident T cells in CCR7−/− mice. A. Total splenocytes recovered from the spleen of naïve WT and CCR7−/− mice. B. Total lymphocytes (CD4 T cells, CD8 T cells, B cells and NK cells) recovered from the draining iliac lymph nodes of naïve WT and CCR7−/− mice. C. Total lymphocytes (CD4 T cells, CD8 T cells, B cells and NK cells) recovered from the FRT of naïve WT and CCR7−/− mice. D. The percentages of naïve and effector CD4 T cells in the spleen, DLNs and FRT of naïve WT and CCR7−/− mice. Data shown are representative of three similar experiments with 3–4 mice per group for each experiment. Error bars represent the mean ± SEM, *p < 0.05, **p < 0.01.
CCR7-deficient mice have reduced Chlamydia-specific CD4 T cell responses in the DLNs following infection of the FRT
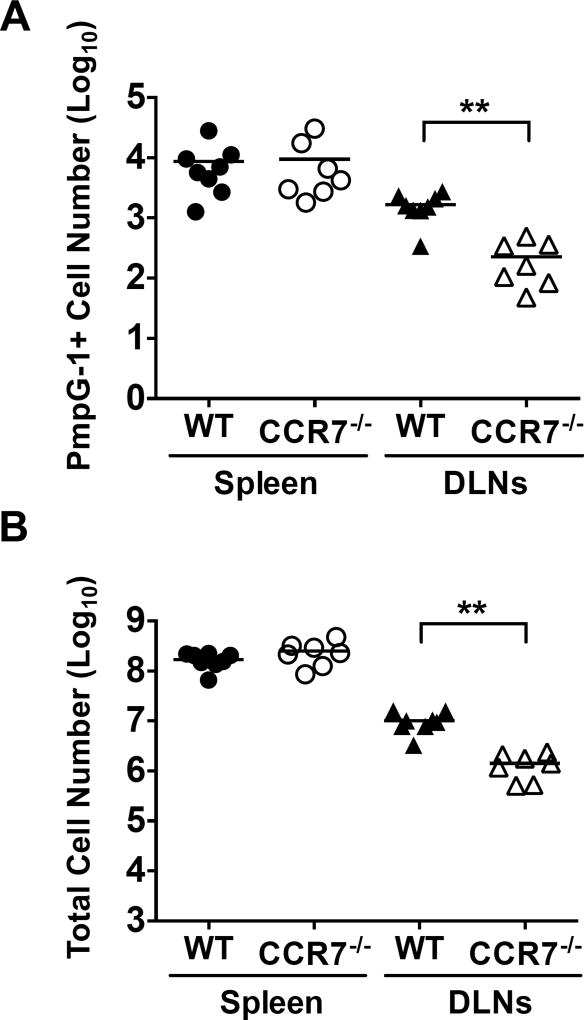
To determine whether the altered lymphocyte distribution in the FRT of CCR7-deficient mice could affect the host immune response, we examined Chlamydia-specific CD4 T cells in lymphoid tissues after Chlamydia muridarum infection. To accomplish this, we utilized a Chlamydia-specific MHC class-II tetramer that directly detects endogenous CD4 T cells specific for C. muridarum polymorphic outer membrane protein G-1 (PmpG-1303–311:I-Ab) (5). At 14 days post intravaginal infection, PmpG-1-specific CD4 T cells had expanded equally in the spleen of WT or CCR7-deficient mice (Fig. 2A). In contrast, clonal expansion of PmpG-1-specific CD4 T cells was markedly reduced in the draining lymph nodes of infected CCR7-deficient mice, compared with WT controls (Fig. 2A). The decreased Chlamydia-specific CD4 T cell response in the lymph nodes of CCR7-deficient mice mirrored an overall reduction in leukocytes within these same lymph nodes (Fig. 2B).
Figure 2.
Reduced antigen-specific CD4 T cell responses in the draining lymph nodes of CCR7−/− mice. A. Total PmpG-1-specific CD4 T cells recovered from the spleen and draining lymph nodes of WT and CCR7−/− mice 14 days post infection. B. Total T cells recovered from the spleenand draining lymph nodes of WT and CCR7−/− mice 14 days post infection. Graph shown are combined data of two independent experiments. Each data point represents individual mouse. Lines represent mean values. **p < 0.01.
CCR7-deficient mice display enhanced effector function of FRT resident T cells
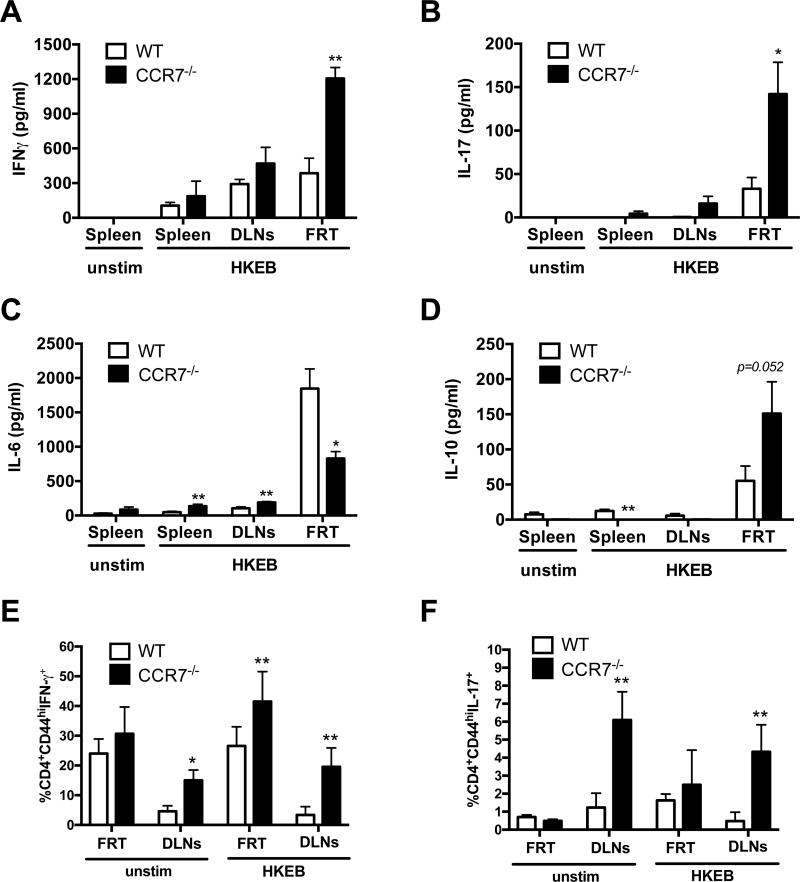
We next examined whether the elevated numbers of CD4 T cells in the FRT of CCR7-deficient mice would enhance the overall response to Chlamydia infection. Total lymphocytes were purified from the FRT of WT and CCR7-deficient mice and stimulated ex vivo with heat-killed elementary bodies (HKEB) to generate an antigen-specific response. Lymphocytes from the spleen and lymph nodes of WT and CCR7-deficient mice produced similar patterns of cytokine production, although a small increase in IL-17 production was detected in CCR7-deficient lymphoid tissues (Fig. 3A–B). However, lymphocytes from the FRT of CCR7-deficient mice produced a more prominent IFN-γ, IL-17, and IL-10 response that WT mice (Fig. 3A–D). When examined by flow cytometry, CD44+ CD4 T cells produced elevated levels of IFN-γ after stimulation with HKEBs (Fig. 3E–F), suggesting that CD4 T cells are more active in the FRT of these mice. Interestingly, the level of IL-6 production was reduced after in vitro stimulation of lymphocytes from CCR7-deficient versus WT mice (Fig. 3C). No IL-4, IL-12, or IL-23 production was detected in lymphocyte cultures from WT or CCR7-deficient mice (data not shown). Thus, FRT lymphocytes from Chlamydia-infected CCR7-deficient mice produce heightened IFN-γ and IL-17 responses but have low IL-6 and high IL-10.
Figure 3.
Higher levels of IFNγ, IL-10 and IL-17 cytokine production in CCR7−/− mice compared to WT controls. WT and CCR7−/− mice were infected with 1×105 C. muridarum intravaginally. A–D. Ten days post infection, cells from FRT were isolated and stimulated ex vivo with HKEB for 72 hrs. Cytokine production (A. IFNγ, B. IL-6, C. IL-10, D. IL-17) in the culture supernatant were measured by cytokine ELISA. Data shown are representative of three similar experiments with 3–4 mice per group for each experiment. E–F. Fourteen days after infection, cells from WT and CCR7−/− FRT were isolated and stimulated ex vivo with HKEB for 48 hrs and stained for cytokine production. Data are representative of one experiment with 4–5 mice per group. Error bars represent mean ± SEM (A–D) or SD (E–F), *p < 0.05, **p < 0.01.
CCR7-deficient mice display reduced FRT inflammation
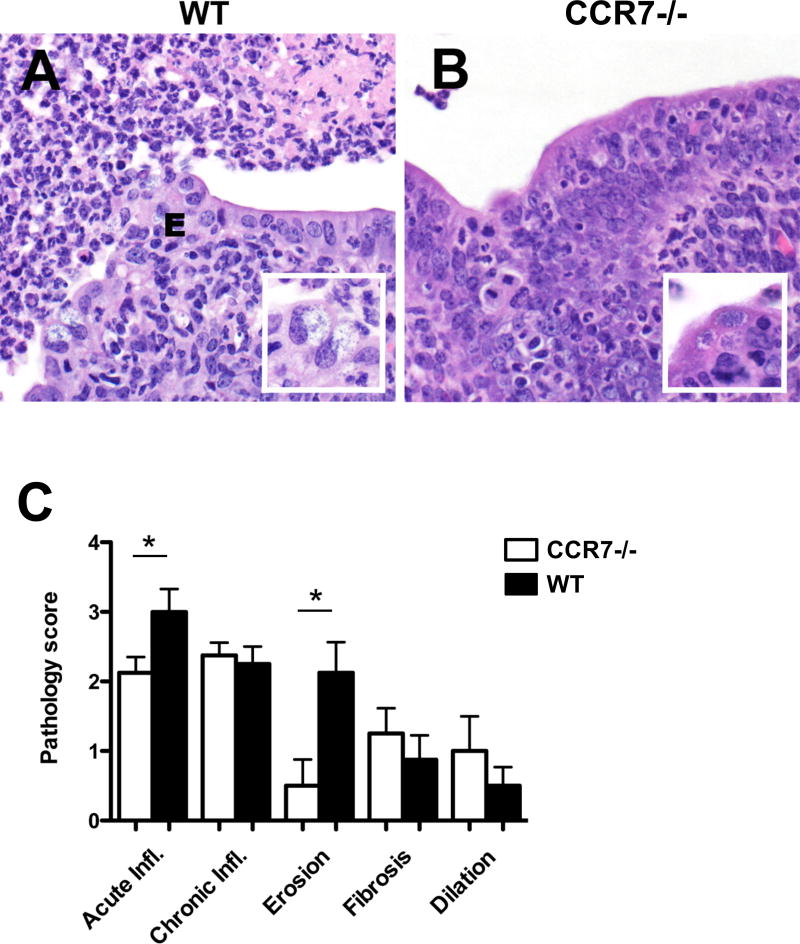
Next we directly examined the pathology scores of histological sections of the FRT recovered from WT and CCR7-deficient mice 7 days post C. muridarum intravaginal infection. This analysis revealed a reduction in acute inflammation and erosion in tissues from CCR7-deficent mice, compared with WT mice (Fig. 4). Thus, the altered production of cytokines from FRT lymphocytes correlates with a reduction in tissue pathology during Chlamydia infection of CCR7-deficient mice.
Figure 4.
Chlamydia-induced uterine inflammation is reduced in CCR7-deficient mice. WT and CCR7−/− mice were infected with 1×105 C. muridarum i.vag.. Seven days post infection, histopathology were analyzed. A. Marked neutrophilic endometritis with luminal exudate (pyometra, left upper field) and mucosal erosions (E) in WT mice inoculated with C. muridarum. B. Mild to moderate neutrophilic endometritis in CCR7−/− mice inoculated with C. muridarum. HE, 400× magnification. Inset, Chlamydia inclusions in epithelial cells. HE, 600× magnification. C. Severity of acute neutrophilic inflammation, chronic lymphohistiocytic inflammation, mucosal erosion, fibrosis and luminal dilation determined by a histopathology scoring system in CCR7−/− (white bars) and WT (black bars) mice inoculated with C. muridarum. Data shown are representative of two similar experiments with 3–4 mice per group for each experiment. Significant differences denoted by asterisk (*), P = 0.0453 and P = 0.0142, respectively.
Chlamydia-specific Antibody production is reduced in CCR7-deficient mice
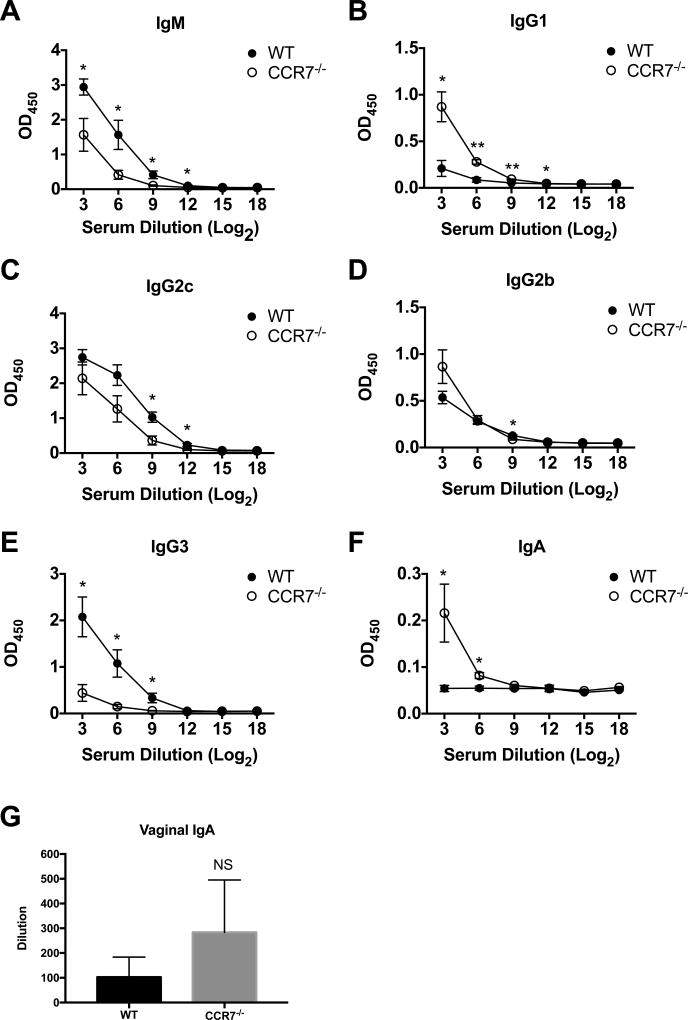
To determine whether the altered CD4 T cell response in the LN and FRT of CCR7-deficient mice also affected antibody production, we examined Chlamydia-specific immunoglobulin isotypes 14 days after intravaginal infection with Chlamydia. Both WT and CCR7-deficient mice developed elevated titers of Chlamydia-specific IgM and IgG isotypes. However, we observed lower levels of IgM, IgG2c and IgG3 in Chlamydia-infected CCR7-deficient mice whereas IgG1 and IgG2b levels were modestly elevated compared to WT mice (Fig. 5A–E). Surprisingly, although Chlamydia-specific IgA was not detected in WT serum, a systemic Chlamydia-specific IgA response was evident in CCR7-deficient mice (Fig. 5F). However, IgA was not elevated in vaginal washes from infected CCR7-deficient mice (Fig. 5G). Overall, these results suggest a heightened immune response occurs at mucosal surfaces in CCR7-deficient mice (Fig. 1–3), while the increased systemic IgA response may indicate colonization of Chlamydia in the intestine (39, 40).
Figure 5.
Serum and vaginal wash antibody levels in WT and CCR7−/− mice 14 days after C. muridarum intravaginal infection. WT and CCR7−/− mice were infected with 1×105 C. muridarum i.vag.. Fourteen days post infection, Chlamydia-specific serum antibodies (A. IgM, B. IgG1, C. IgG2b, D. IgG2c, E. IgG3, F. IgA) and IgA antibody present in vaginal wash (G) were measured by antibody ELISA. Data shown are representative of two similar experiments with 3–4 mice per group for each experiment. Error bars represent the mean ± SEM, *p < 0.05, **p < 0.01; NS, not significant.
CCR7-deficient mice have accelerated Chlamydia clearance from the FRT
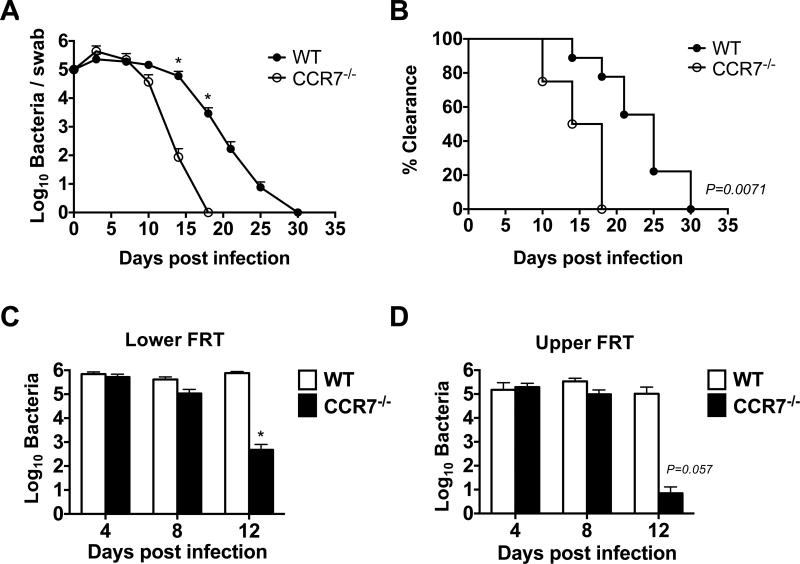
Given the increased number of Chlamydia-specific CD4 T cells and enhanced IFN-γ and IL-17 production observed in CCR7-deficient mice, we hypothesized that this would be beneficial for bacterial clearance from the FRT. We therefore examined the shedding of Chlamydia from vaginally infected WT and CCR7-deficient mice for the course of primary infection. As expected, WT mice shed bacteria for a prolonged period and eventually resolved primary Chlamydia infection around 4–5 weeks post infection (Fig. 6A–B). In marked contrast, all CCR7-deficient mice resolved Chlamydia infection at a faster rate, with all mice clearing bacteria within 20 days of intravaginal infection (Fig. 6A–B). Furthermore, when the bacterial burden in both the upper and lower FRT tissue was directly examined after tissue homogenization, accelerated bacteria clearance was detected in both the upper and lower FRT of CCR7-deficient mice (Fig. 6C–D). In addition, we also monitored the possibility that bacteria could have dissemination from the FRT of CCR7-deficent mice, but did not detect greater numbers of bacteria in systemic sites (data not shown). Thus, CCR7-deficient mice are more capable of resolving primary Chlamydia infection within the FRT.
Figure 6.
Accelerated bacteria clearance in CCR7−/− mice after C. muridarum i.vag. infection. A. Bacteria burden at lower FRT of infected WT and CCR7−/− mice at various time points post infection as measured by vaginal swabs. B. Percentage of mice that cleared the infection as measured by vaginal swabs. C and D. Bacteria burden at lower (C) and upper (D) FRT of infected WT and CCR7−/− mice at day 4, 8 and 12 post infection as measured by tissue grinding. Data shown are representative of three similar experiments. Data shown are representative of two similar experiments with 3–7 mice per group for each experiment. Error bars represent the mean ± SEM, *p < 0.05.
Discussion
A localized bacterial infection causes activation of tissue innate immune cells and initiates dendritic cell homing to the draining lymph node (24). Bacterial antigens are presented to low frequency naïve T cells in the paracortex of the lymph node, initiating rapid T cell activation and clonal expansion (1, 41). These expanded T cells gain relevant effector capabilities and traffic to the infected tissues where bacterial killing can occur (42, 43). In all of these events, the efficiency of the immune response depends on appropriate anatomical positioning of immune cells, which is guided by chemokine gradients (44).
The homeostatic chemokine receptor CCR7 plays multiple roles in the process of immunity to infection by guiding activated dendritic cells to the lymph node T cells area, positioning naïve T cells within this same region, and allowing activated B cells to interact with activated T cells at the follicular border (22, 23). Despite this critical role in host immunity, CCR7 deficiency has variable effects on the immune response to different pathogens. While CCR7-deficient mice display reduced clonal expansion of CD8 T cells to LCMV and Listeria infection, viral and bacterial clearance occurred and protective memory was induced in both cases (27, 28). Thus, CCR7-deficiency modestly impedes the ability of CD8 T cells to eliminate these pathogens. In a mouse model of Mycobacterium tuberculosis infection, CCR7-deficient mice actually had lower bacterial burdens in the spleen at later time points of infection, although this difference was not observed in the lungs (30). A similar reduction in bacterial burden was observed in the mesenteric lymph nodes of CCR7-deficient mice following Salmonella infection (31), although this was attributed to the role of CCR7 in recruiting infected dendritic cells to the lymphoid tissues. Thus, CCR7-deficient mice have profound deficiencies in the generation of T and B cell responses but display only modest deficiencies in the clearance of some pathogens.
Our findings add to these reports by showing that CCR7-deficient mice have accelerated clearance of Chlamydia from the FRT after intravaginal infection. It seems likely that the enhanced efficiency of Chlamydia clearance largely derives from the elevated number of lymphocytes within the FRT prior to infection, although it should be noted that these mice also have a deficiency in DC migration and T regulatory cell function (25, 45). Given impaired bacterial shedding in CCR7-deficient mice, efficient Chlamydia growth may rely upon the absence of organized lymphoid tissues within the FRT of wild-type laboratory mice. The formation of FRT tertiary lymphoid clusters is a hallmark of protective memory (20), suggesting that the anatomical proximity of lymphocytes to infected epithelia is critical for rapid clearance. Recent data suggest an explanation for this observation, since these organized structures likely play an important role in retaining protective tissue-resident memory T cells within the FRT (32, 46). However, our data add to this, by suggesting that lymphocyte accumulation within the FRT is protective prior to the generation of specific memory. It should be noted that this accumulation of lymphocytes is most likely due to the inability of effector or memory cells to egress to draining lymph nodes (47), thus generating a larger pool of effector and memory T cells in the tissue. It will therefore be of interest to determine whether development of tertiary lymphoid tissues by heterologous FRT infection can accelerate Chlamydia clearance.
Our data show that CCR7-deficiency impedes the expansion of Chlamydia-specific CD4 T cells within the local draining lymph nodes. This finding contrasts somewhat with a study of systemic Listeria infection, where antigen-specific CD8 T cells priming was reduced, but CD4 responses were unaffected (28). It is not yet clear whether the reduced Chlamydia-specific CD4 T cell response is due to inefficient dendritic cell migration to the lymph node or to the disrupted lymphoid architecture within secondary lymphoid tissues of CCR7-deficient mice. Either way, this deficiency did not prevent the development of an efficient protective response to Chlamydia in the FRT, a model that is dependent on CD4 T cells for primary clearance (48).
Our data examining effector development of Chlamydia-specific CD4 T cells within the lymph node and local tissue show an elevation in IFN-γ and IL-17 production, consistent with a study reporting higher IFN-γ and IL-17 in the gastric mucosa of CCR7-deficient mice (38). Another study has suggested that CCR7 signaling favors Th2 differentiation and IL-4 production (49), but in the Chlamydia model IL-4 production was barely detectable from FRT lymphocytes. In contrast, a protective role of Chlamydia-specific CD4 Th1 cells has been clearly demonstrated using gene-deficient mice and antibody depletion (14, 15, 50–52) and expanded T-bet+ Th1 cells have been detected in the draining lymph node of vaginally infected mice (5, 53). Our understanding of IL-17 in immunity to Chlamydia is less well-developed and a recent study reported that IL-17 responses were low in seropositive women (54). However, CD4 Th17 cells have been detected in both pulmonary and genital Chlamydia infection models (5, 55, 56). In the pulmonary model, IL-17 neutralization reduced Th1 responses, impeded bacterial clearance, but otherwise enhanced immune pathology (55, 57), suggesting that Th17 cells could be protective by promoting Th1-mediated immunity. Other vaccine studies have noted that IFN-γ/IL-17-double producing cells correlate with protective immunity (58). Thus, the enhancement of Chlamydia-specific IFN-γ and IL-17 detected in CCR7-deficient mice seems likely to explain the rapid bacterial clearance from the FRT of these mice.
An interesting feature of the rapid Chlamydia clearance in CCR7-deficient mice is the fact this occurred with a corresponding reduction in FRT pathology. This also correlated with lower IL-6 production and greater IL-10 production from lymphocytes recovered from the FRT, two cytokines associated with inflammatory and anti-inflammatory responses respectively. The generation of CD4 Treg populations has been reported during Chlamydia infection (59–61), but otherwise the suppression of inflammatory responses is incompletely understood. One possibility is that highly effective bacterial clearance by expanded Th1 and Th17 responses simply reduces the availability of bacterial antigen available to drive innate immune activation.
In summary, our data show that CCR7-deficient mice display a marked increase in naïve lymphocytes within the FRT and that these mice develop disregulated CD4 T cell and antibody responses to genital Chlamydia infection. Surprisingly, these mice develop enhanced adaptive immune responses within the FRT and rapidly clear Chlamydia with reduced reproductive tract pathology. These data suggest that efficient T cell trafficking to the FRT encourages bacterial clearance and should be a key goal of vaccine development,
Acknowledgments
We would like to acknowledge Dr. Richard Morrison and Sandra Morrison for helpful discussions. We thank Dr. Harlan Caldwell for kind gift of C. muridarum monoclonal antibody.
This study was supported by grants from the National Institutes of Health to SJM (AI103422 and AI117303) and to LXL (GM103625).
References
- 1.Jenkins MK, Khoruts A, Ingulli E, Mueller DL, McSorley SJ, Reinhardt RL, Itano A, Pape KA. In vivo activation of antigen-specific CD4 T cells. Annu. Rev. Immunol. 2001;19:23–45. doi: 10.1146/annurev.immunol.19.1.23. [DOI] [PubMed] [Google Scholar]
- 2.Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nature reviews. Immunology. 2013;13:309–320. doi: 10.1038/nri3442. [DOI] [PubMed] [Google Scholar]
- 3.Roan NR, Starnbach MN. Antigen-specific CD8+ T cells respond to Chlamydia trachomatis in the genital mucosa. J Immunol. 2006;177:7974–7979. doi: 10.4049/jimmunol.177.11.7974. [DOI] [PubMed] [Google Scholar]
- 4.Lee HK, Zamora M, Linehan MM, Iijima N, Gonzalez D, Haberman A, Iwasaki A. Differential roles of migratory and resident DCs in T cell priming after mucosal or skin HSV-1 infection. J Exp Med. 2009;206:359–370. doi: 10.1084/jem.20080601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li LX, McSorley SJ. B cells enhance antigen-specific CD4 T cell priming and prevent bacteria dissemination following Chlamydia muridarum genital tract infection. PLoS pathogens. 2013;9:e1003707. doi: 10.1371/journal.ppat.1003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laidlaw BJ, Craft JE, Kaech SM. The multifaceted role of CD4(+) T cells in CD8(+) T cell memory. Nature reviews. Immunology. 2016;16:102–111. doi: 10.1038/nri.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D. o. H. a. H. Services, editor. Prevention, C. f. D. C. a. Sexually Transmitted Disease Surveillance 2012. Altlanta: U.S: 2013. [Google Scholar]
- 8.Miller WC, Ford CA, Morris M, Handcock MS, Schmitz JL, Hobbs MM, Cohen MS, Harris KM, Udry JR. Prevalence of chlamydial and gonococcal infections among young adults in the United States. Jama. 2004;291:2229–2236. doi: 10.1001/jama.291.18.2229. [DOI] [PubMed] [Google Scholar]
- 9.Broutet N, Fruth U, Deal C, Gottlieb SL, Rees H S. T. I. V. T. C. participants of the. Vaccines against sexually transmitted infections: the way forward. Vaccine. 2014;32:1630–1637. doi: 10.1016/j.vaccine.2014.01.053. [DOI] [PubMed] [Google Scholar]
- 10.Hafner LM, Wilson DP, Timms P. Development status and future prospects for a vaccine against Chlamydia trachomatis infection. Vaccine. 2014;32:1563–1571. doi: 10.1016/j.vaccine.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 11.Hafner L, Beagley K, Timms P. Chlamydia trachomatis infection: host immune responses and potential vaccines. Mucosal immunology. 2008;1:116–130. doi: 10.1038/mi.2007.19. [DOI] [PubMed] [Google Scholar]
- 12.Kinnunen A, Molander P, Laurila A, Rantala I, Morrison R, Lehtinen M, Karttunen R, Tiitinen A, Paavonen J, Surcel HM. Chlamydia trachomatis reactive T lymphocytes from upper genital tract tissue specimens. Hum Reprod. 2000;15:1484–1489. doi: 10.1093/humrep/15.7.1484. [DOI] [PubMed] [Google Scholar]
- 13.Ramsey KH, Soderberg LS, Rank RG. Resolution of chlamydial genital infection in B-cell-deficient mice and immunity to reinfection. Infect Immun. 1988;56:1320–1325. doi: 10.1128/iai.56.5.1320-1325.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magee DM, Williams DM, Smith JG, Bleicker CA, Grubbs BG, Schachter J, Rank RG. Role of CD8 T cells in primary Chlamydia infection. Infect Immun. 1995;63:516–521. doi: 10.1128/iai.63.2.516-521.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrison SG, Morrison RP. A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. J Immunol. 2005;175:7536–7542. doi: 10.4049/jimmunol.175.11.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su H, Feilzer K, Caldwell HD, Morrison RP. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infection and immunity. 1997;65:1993–1999. doi: 10.1128/iai.65.6.1993-1999.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darville T, Andrews CW, Jr, Laffoon KK, Shymasani W, Kishen LR, Rank RG. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect Immun. 1997;65:3065–3073. doi: 10.1128/iai.65.8.3065-3073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darville T, O'Neill JM, Andrews CW, Jr, Nagarajan UM, Stahl L, Ojcius DM. Toll-like receptor-2, but not Toll-like receptor-4, is essential for development of oviduct pathology in chlamydial genital tract infection. J Immunol. 2003;171:6187–6197. doi: 10.4049/jimmunol.171.11.6187. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Zhou Z, Chen J, Wu G, Yang Z, Zhou Z, Baseman J, Zhang J, Reddick RL, Zhong G. Lack of long-lasting hydrosalpinx in A/J mice correlates with rapid but transient chlamydial ascension and neutrophil recruitment in the oviduct following intravaginal inoculation with Chlamydia muridarum. Infect Immun. 2014;82:2688–2696. doi: 10.1128/IAI.00055-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrison SG, Morrison RP. In situ analysis of the evolution of the primary immune response in murine Chlamydia trachomatis genital tract infection. Infect Immun. 2000;68:2870–2879. doi: 10.1128/iai.68.5.2870-2879.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nature reviews. Immunology. 2005;5:149–161. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- 22.Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nature reviews. Immunology. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 23.Moschovakis GL, Forster R. Multifaceted activities of CCR7 regulate T-cell homeostasis in health and disease. European journal of immunology. 2012;42:1949–1955. doi: 10.1002/eji.201242614. [DOI] [PubMed] [Google Scholar]
- 24.Platt AM, Randolph GJ. Dendritic cell migration through the lymphatic vasculature to lymph nodes. Advances in immunology. 2013;120:51–68. doi: 10.1016/B978-0-12-417028-5.00002-8. [DOI] [PubMed] [Google Scholar]
- 25.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 26.Hopken UE, Wengner AM, Loddenkemper C, Stein H, Heimesaat MM, Rehm A, Lipp M. CCR7 deficiency causes ectopic lymphoid neogenesis and disturbed mucosal tissue integrity. Blood. 2007;109:886–895. doi: 10.1182/blood-2006-03-013532. [DOI] [PubMed] [Google Scholar]
- 27.Junt T, Scandella E, Forster R, Krebs P, Krautwald S, Lipp M, Hengartner H, Ludewig B. Impact of CCR7 on priming and distribution of antiviral effector and memory CTL. J Immunol. 2004;173:6684–6693. doi: 10.4049/jimmunol.173.11.6684. [DOI] [PubMed] [Google Scholar]
- 28.Kursar M, Hopken UE, Koch M, Kohler A, Lipp M, Kaufmann SH, Mittrucker HW. Differential requirements for the chemokine receptor CCR7 in T cell activation during Listeria monocytogenes infection. J Exp Med. 2005;201:1447–1457. doi: 10.1084/jem.20041204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scandella E, Fink K, Junt T, Senn BM, Lattmann E, Forster R, Hengartner H, Ludewig B. Dendritic cell-independent B cell activation during acute virus infection: a role for early CCR7-driven B-T helper cell collaboration. J Immunol. 2007;178:1468–1476. doi: 10.4049/jimmunol.178.3.1468. [DOI] [PubMed] [Google Scholar]
- 30.Kahnert A, Hopken UE, Stein M, Bandermann S, Lipp M, Kaufmann SH. Mycobacterium tuberculosis triggers formation of lymphoid structure in murine lungs. The Journal of infectious diseases. 2007;195:46–54. doi: 10.1086/508894. [DOI] [PubMed] [Google Scholar]
- 31.Voedisch S, Koenecke C, David S, Herbrand H, Forster R, Rhen M, Pabst O. Mesenteric lymph nodes confine dendritic cell-mediated dissemination of Salmonella enterica serovar Typhimurium and limit systemic disease in mice. Infect Immun. 2009;77:3170–3180. doi: 10.1128/IAI.00272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stary G, Olive A, Radovic-Moreno AF, Gondek D, Alvarez D, Basto PA, Perro M, Vrbanac VD, Tager AM, Shi J, Yethon JA, Farokhzad OC, Langer R, Starnbach MN, von Andrian UH. VACCINES. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science. 2015;348:aaa8205. doi: 10.1126/science.aaa8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scidmore MA. Cultivation and Laboratory Maintenance of Chlamydia trachomatis. Current protocols in microbiology. 2005;Chapter 11(Unit 11A):11. doi: 10.1002/9780471729259.mc11a01s00. [DOI] [PubMed] [Google Scholar]
- 34.Cotter TW, Meng Q, Shen ZL, Zhang YX, Su H, Caldwell HD. Protective efficacy of major outer membrane protein-specific immunoglobulin A (IgA) and IgG monoclonal antibodies in a murine model of Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4704–4714. doi: 10.1128/iai.63.12.4704-4714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moon JJ, Chu HH, Hataye J, Pagan AJ, Pepper M, McLachlan JB, Zell T, Jenkins MK. Tracking epitope-specific T cells. Nat Protoc. 2009;4:565–581. doi: 10.1038/nprot.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol. 2013;14:509–513. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scimone ML, Felbinger TW, Mazo IB, Stein JV, Von Andrian UH, Weninger W. CXCL12 mediates CCR7-independent homing of central memory cells, but not naive T cells, in peripheral lymph nodes. J Exp Med. 2004;199:1113–1120. doi: 10.1084/jem.20031645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winter S, Rehm A, Wichner K, Scheel T, Batra A, Siegmund B, Berek C, Lipp M, Hopken UE. Manifestation of spontaneous and early autoimmune gastritis in CCR7-deficient mice. The American journal of pathology. 2011;179:754–765. doi: 10.1016/j.ajpath.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeruva L, Spencer N, Bowlin AK, Wang Y, Rank RG. Chlamydial infection of the gastrointestinal tract: a reservoir for persistent infection. Pathogens and disease. 2013;68:88–95. doi: 10.1111/2049-632X.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Q, Huang Y, Gong S, Yang Z, Sun X, Schenken R, Zhong G. In Vivo and Ex Vivo Imaging Reveals a Long-Lasting Chlamydial Infection in the Mouse Gastrointestinal Tract following Genital Tract Inoculation. Infect Immun. 2015;83:3568–3577. doi: 10.1128/IAI.00673-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moon JJ, McSorley SJ. Tracking the dynamics of salmonella specific T cell responses. Curr Top Microbiol Immunol. 2009;334:179–198. doi: 10.1007/978-3-540-93864-4_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Obar JJ, Lefrancois L. Memory CD8+ T cell differentiation. Ann N Y Acad Sci. 2010;1183:251–266. doi: 10.1111/j.1749-6632.2009.05126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 45.Schneider MA, Meingassner JG, Lipp M, Moore HD, Rot A. CCR7 is required for the in vivo function of CD4+ CD25+ regulatory T cells. J Exp Med. 2007;204:735–745. doi: 10.1084/jem.20061405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iijima N, Iwasaki A. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science. 2014;346:93–98. doi: 10.1126/science.1257530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol. 2005;6:895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 48.Farris CM, Morrison RP. Vaccination against Chlamydia genital infection utilizing the murine C. muridarum model. Infection and immunity. 2011;79:986–996. doi: 10.1128/IAI.00881-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moschovakis GL, Bubke A, Dittrich-Breiholz O, Braun A, Prinz I, Kremmer E, Forster R. Deficient CCR7 signaling promotes TH2 polarization and B-cell activation in vivo. European journal of immunology. 2012;42:48–57. doi: 10.1002/eji.201141753. [DOI] [PubMed] [Google Scholar]
- 50.Landers DV, Erlich K, Sung M, Schachter J. Role of L3T4-bearing T-cell populations in experimental murine chlamydial salpingitis. Infect Immun. 1991;59:3774–3777. doi: 10.1128/iai.59.10.3774-3777.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morrison RP, Feilzer K, Tumas DB. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4661–4668. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morrison SG, Morrison RP. Resolution of secondary Chlamydia trachomatis genital tract infection in immune mice with depletion of both CD4+ and CD8+ T cells. Infect Immun. 2001;69:2643–2649. doi: 10.1128/IAI.69.4.2643-2649.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cain TK, Rank RG. Local Th1-like responses are induced by intravaginal infection of mice with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect Immun. 1995;63:1784–1789. doi: 10.1128/iai.63.5.1784-1789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barral R, Desai R, Zheng X, Frazer LC, Sucato GS, Haggerty CL, O'Connell CM, Zurenski MA, Darville T. Frequency of Chlamydia trachomatis-specific T cell interferon-gamma and interleukin-17 responses in CD4-enriched peripheral blood mononuclear cells of sexually active adolescent females. Journal of reproductive immunology. 2014;103:29–37. doi: 10.1016/j.jri.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bai H, Cheng J, Gao X, Joyee AG, Fan Y, Wang S, Jiao L, Yao Z, Yang X. IL-17/Th17 promotes type 1 T cell immunity against pulmonary intracellular bacterial infection through modulating dendritic cell function. J Immunol. 2009;183:5886–5895. doi: 10.4049/jimmunol.0901584. [DOI] [PubMed] [Google Scholar]
- 56.Scurlock AM, Frazer LC, Andrews CW, Jr, O'Connell CM, Foote IP, Bailey SL, Chandra-Kuntal K, Kolls JK, Darville T. Interleukin-17 contributes to generation of Th1 immunity and neutrophil recruitment during Chlamydia muridarum genital tract infection but is not required for macrophage influx or normal resolution of infection. Infection and Immunity. 2011;79:1349–1362. doi: 10.1128/IAI.00984-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang X, Gao L, Lei L, Zhong Y, Dube P, Berton MT, Arulanandam B, Zhang J, Zhong G. A MyD88-dependent early IL-17 production protects mice against airway infection with the obligate intracellular pathogen Chlamydia muridarum. J Immunol. 2009;183:1291–1300. doi: 10.4049/jimmunol.0803075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu H, Jiang X, Shen C, Karunakaran KP, Jiang J, Rosin NL, Brunham RC. Chlamydia muridarum T-cell antigens formulated with the adjuvant DDA/TDB induce immunity against infection that correlates with a high frequency of gamma interferon (IFN-gamma)/tumor necrosis factor alpha and IFN-gamma/interleukin-17 double-positive CD4+ T cells. Infection and Immunity. 2010;78:2272–2282. doi: 10.1128/IAI.01374-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moniz RJ, Chan AM, Gordon LK, Braun J, Arditi M, Kelly KA. Plasmacytoid dendritic cells modulate nonprotective T-cell responses to genital infection by Chlamydia muridarum. FEMS Immunol. Med. Microbiol. 2010;58:397–404. doi: 10.1111/j.1574-695X.2010.00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang J, Kelly KA. Phenotype and function of regulatory T cells in the genital tract. Curr. Trends Immunol. 2011;12:89–94. [PMC free article] [PubMed] [Google Scholar]
- 61.Moore-Connors JM, Fraser R, Halperin SA, Wang J. CD4(+)CD25(+)Foxp3(+) regulatory T cells promote Th17 responses and genital tract inflammation upon intracellular Chlamydia muridarum infection. J Immunol. 2013;191:3430–3439. doi: 10.4049/jimmunol.1301136. [DOI] [PubMed] [Google Scholar]