Abstract
Objectives
To assess the tolerability and efficacy of bevacizumab with carboplatin and weekly paclitaxel as first-line adjuvant therapy for advanced stage ovarian cancer.
Methods
After IRB approval, this single-institution, phase II study enrolled patients with stage III or IV epithelial ovarian cancer after primary cytoreductive surgery to treatment with carboplatin (AUC 5), weekly paclitaxel (80 mg/m2), and bevacizumab (15 mg/kg) every 3 weeks for at least 6 cycles. The primary endpoint was tolerability of at least 4 cycles of therapy, with a target treatment success rate of >60%. Secondary endpoints included progression-free survival (PFS) and response rate. Plasma biomarkers were analyzed by the multiplex ELISA assays.
Results
Thirty-three patients were enrolled with 30 evaluable patients receiving at least one cycle of combination treatment. Twenty-three patients (77%) were able to complete at least 4 cycles of therapy per protocol, and the posterior probability that the treatment success rate is > 60% is 0.77. Twenty-one patients (70%) were able to complete ≥ 6 cycles of therapy. Median PFS was 22.4 months for patients with optimal (R0) compared to 16.9 months for optimal ≤1cm (HR 1.71, 95% CI 0.58-4.98, p=0.33), and 16.9 months for suboptimal > 1cm (HR 3.75, 95% CI 1.05-13.34, p=0.04) disease. Increases in mean Flt-3L was significantly higher in responders versus non- responders (83.4 vs. 28 pg/mL, p=0.05).
Conclusions
Adjuvant bevacizumab with dose-dense chemotherapy is associated with acceptable toxicity and a high likelihood of completing 4 cycles of therapy. Dynamic changes in Flt-3L may represent a predictive marker to treatment response.
Introduction
In 2017, a projected 22,440 cases of ovarian cancer will be diagnosed in the United States, leading to 14,080 deaths. [1] Due to a paucity of effective screening modalities, most patients are diagnosed at advanced stages. The most widely adopted standard treatment regimen for advanced ovarian cancer includes cytoreductive surgery and tri-weekly carboplatin and paclitaxel chemotherapy. [2] With this treatment regimen, over 50% of patients achieve an initial complete clinical response, however, the majority develop relapsed disease within the first 2 years, with only 10–30% achieving long-term survival. [3] Newer first-line treatment regimens through phase III randomized controlled trials have shown significantly improved initial response rates and prolonged the time to recurrence. These include combination intraperitoneal (IP) and intravenous (IV) cisplatin and paclitaxel [4], dose-dense weekly IV paclitaxel and carboplatin [5–6], and combination therapy with the anti-angiogenic monoclonal antibody, bevacizumab. [7–8]
Alternative dosing schedules of chemotherapy with weekly paclitaxel and carboplatin have been evaluated as first-line and salvage chemotherapy in patients with ovarian cancer. [9–13] A phase III trial from the Japanese Gynecologic Oncology Group (JGOG 3016) showed that dose-dense paclitaxel and carboplatin significantly improved progression-free survival (PFS) (28.2 vs. 17.5 months; HR 0.76, 95% CI 0.62-0.91; p=0.004) compared to the conventional regimen given every 21 days. Median overall survival (OS) was also significantly improved (100.5 vs. 62.2 months; HR 0.79, 95% CI 0.63-0.99; p=0.04) in the dose-dense treatment group. Rates of adverse events were similar in each treatment group, except grade 3 and 4 anemia was higher in the dose-dense group (69% vs. 44%, p<0.0001). Hematologic toxicity led to discontinuation of therapy in 60% of the dose-dense group compared to 43% on conventional regimen (p=0.02). Moreover, at least one treatment cycle was delayed in more patients on dose-dense treatment (76% vs. 67%, p=0.02). [5–6]
Bevacizumab has been studied in many phase I, II, and III clinical trials in primary and recurrent ovarian cancer, due to the significant role that angiogenesis and neovascularization plays in tumor growth, invasion, and metastases. [14–15] The early use of bevacizumab in Gynecologic Oncology Group (GOG)-170D was as a single agent 15mg/kg every 21 days which led to 40% of patients surviving progression-free for at least 6 months. [16] The combination of bevacizumab with other cytotoxic therapies stems from preclinical models suggesting possible synergistic interaction. [17] It is hypothesized that increased sensitization to apoptosis or reversal of chemotherapeutic drug resistance occurs when VEGF pathway is interrupted. [18] Two large randomized phase III trials, GOG-218 [7] and International Collaboration on Ovarian Neoplasms (ICON)-7 [8], evaluated bevacizumab and tri-weekly carboplatin and paclitaxel followed by single-agent maintenance bevacizumab as front-line treatment for ovarian cancer. Both trials demonstrated a significantly improved PFS with the addition of bevacizumab to front-line therapy followed by maintenance bevacizumab. Similarly, the favorable results with the use of bevacizumab in the recurrent setting have broadened use of this agent in platinum-resistant ovarian cancer. [19]
Recent phase II data has supported the use of bevacizumab (7.5 mg/kg) with dose-dense paclitaxel (80 mg/m2) and carboplatin (AUC 6) in stage I-IV ovarian cancer with median PFS of 24 months. With the interest in the use of bevacizumab in the front-line setting and concern for excess hematologic toxicity with weekly paclitaxel, there have been limited reports on the safety and tolerability of using a combination of bevacizumab (15 mg/kg) with dose-dense paclitaxel and carboplatin. We sought to assess the tolerability, efficacy, and response rate of bevacizumab with weekly paclitaxel and carboplatin.
Methods
Patients
After MD Anderson Institutional Review Board approval, this single-institution, single-arm, non-randomized phase II study enrolled patients from April 2010 until October 2013 with stage III or IV epithelial ovarian, primary peritoneal, or fallopian tube carcinoma. All patients must have undergone primary tumor reductive surgery with a maximal effort at debulking. Patients with no gross residual disease (R0) or residual disease of 1cm or less after primary surgery were defined as “optimal” and greater than 1cm defined as “suboptimal”. Patients undergoing neoadjuvant chemotherapy were excluded. Patients were included if they were enrolled no later than 12 weeks after initial surgery; had measurable or non-measurable disease; Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1, or 2; normal organ and marrow function as defined per the protocol. All patients signed approved institutional informed consent.
Treatment
Enrolled patients received intravenous carboplatin (AUC 5) on day 1 and paclitaxel (80mg/m2) on days 1, 8, and 15 of each cycle. Bevacizumab (15mg/kg) was given on day 1 of cycles 2 through 6, thus bevacizumab was not given with the first cycle after primary cytoreductive surgery. One cycle of therapy was given every 21 days. Patients were treated for a total of 6 cycles. The dose of carboplatin at an AUC 5 was chosen given the previous hematologic toxicity reported in JGOG 3016. [5] Changes to the chemotherapy regimen in patients found to have persistent disease after 6 cycles of protocol-directed therapy or maintenance chemotherapy was made at the discretion of the treating physician.
Toxicity
Toxicity was monitored before each treatment cycle, with adverse events defined and graded according to Common Terminology Criteria for Adverse Events (version-4). Treatment with bevacizumab, carboplatin, and paclitaxel on day 1 of each cycle was not given unless the absolute neutrophil count (ANC) was ≥1500 cells/mm3 and the platelet count was ≥100,000/µl. Treatment with weekly paclitaxel on day 8 and 15 of each cycle was not given unless the ANC was ≥1000 cells/mm3 and the platelet count is ≥75,000/ µl. Therapy was delayed for a maximum of 3 weeks until these values were achieved. Patients who failed to recover adequate counts within a 3 week delay were removed from the study. Patients did not receive prophylactic growth factors (filgrastim [GCSF], sargramostim [GM-CSF]) unless they experienced recurrent neutropenic complications after specified treatment modifications. Patients were not eligible to receive PEG-filgrastim on study due to the weekly dosing schedule of paclitaxel. If required, growth factor support was initiated the day after the last dose of chemotherapy and continued until the ANC is sustained 1000 cells/mm3. For the first occurrence of febrile neutropenia and/or documented grade 4 neutropenia persisting ≥ 7 days, growth factors (G-CSF) were added at a dose of 5µg/kg/day (or equivalent dose of sargramostim) given subcutaneously starting the day after the last dose of chemotherapy and continuing through hematopoietic recovery. Dose modifications for the management of hematologic toxicity were applicable to carboplatin and were allowed for paclitaxel non-hematologic toxicities per protocol. No dose reductions were allowed for bevacizumab dosing. If adverse events occurred that required holding the bevacizumab, then the dose remained the same once treatment resumed. If unmanageable toxicity occurred at any time during the study, treatment with bevacizumab was discontinued.
Evaluation criteria
A CT scan or MRI of at least the abdomen and pelvis was required to establish post-surgical baseline for the extent of residual disease within 4 weeks of start of chemotherapy. Patients considered “suboptimal” received radiographic disease assessment after completion of 3 cycles of study therapy and within 4 weeks after completion of protocol directed chemotherapy. Patients considered “optimal” received radiographic disease assessment after completion of protocol directed chemotherapy. All patients could receive radiographic disease assessment as clinically indicated for suspicion of progressive disease. All patients that had measurable or non- measurable disease were evaluated for clinical efficacy using Response Evaluation Criteria in Solid Tumors (RECIST) guidelines version 1.1. [20] Target lesions were to be ≥ 1cm in longest diameter by computed tomography or magnetic resonance imaging, ≥ 2cm by chest X-ray, or ≥ 1cm by physical exam using calipers, except lymph nodes, which were to be ≥ 1.5cm on short axis. [21] All measurable lesions were documented at baseline as being “target” or “non-target”. Response rates were determined based on these measurements and documented as one of the following: complete response (CR), partial response (PR), increasing disease, symptomatic deterioration, stable disease (SD), or inevaluable (IE) for response. Progression could also be based on serum CA-125 elevation of greater than or equal to two times the nadir CA-125 value on two occasions at least one week apart.
Assessment of circulating cytokines
Cytokine/chemokine evaluation analyses were performed by the Multiplex ELISA assays. Plasma was collected from 12 of the 33 total patients at the following time points: pre-cycle 1 (i.e., prior to starting therapy), pre-cycle 2, pre-cycle 6 for responders, or at the time the patient went-off treatment before cycle 6. Multiple cytokine/chemokine levels were measured using the human cytokine/chemokine magnetic bead panel - Immunology Multiplex Assay (HCYTOMAG-60K, EMD Millipore Corporation) based on the manufacturer’s instructions. All plasma samples were run in duplicate. Positive controls were used for the assay quality control.
Statistical methods
Patients were considered evaluable if they received at least 1 complete cycle of chemotherapy on protocol. The primary outcome of treatment success was defined as a patient completing at least 4 cycles of combination therapy regardless of dose delay or dose modification. The target sample size was 30 patients and treatment success rate was estimated at 60%. A Bayesian monitoring rule was used following the example of Thall and Simon. [22] Stopping rules were predetermined and a uniform prior distribution for the treatment success rate was assumed. The treatment success rate was estimated with a 90% credible interval. The posterior probability that the treatment success rate is 60% or more was reported. Progression-free survival (PFS) was estimated with the Kaplan-Meier product-limit estimator stratified by debulking status. [23] Cox proportional hazards regression model [24] was used to assess the association of gene expression and cytokines with PFS as described below. The frequency of objective response and duration of objective response was estimated with a 95% confidence interval separately for optimally and suboptimally debulked patients. The frequency and severity of observed adverse effects was reported. All statistical analyses were performed using SAS 9.3 for Windows (SAS Institute Inc., Cary, NC).
Results
Thirty-three patients were enrolled to the study, and 30 evaluable patients received at least one cycle of combination treatment. One patient was considered inevaluable after enrollment onto the protocol, but before receiving any chemotherapy due to a post-surgical complication. The other 2 patients were considered inevaluable after receiving only 1 week of cycle 1 treatment on protocol due to surgical complications deemed not related to chemotherapy. Median age was 57 years (range 31–76) and median follow-up was 20.5 months (range 7.8–51.5). Most patients enrolled were Caucasian (n=22, 67%), had stage IIIC (n=26, 87%) high-grade (n=26, 87%) disease, and of serous histology (n=21, 70%, Table 1.) Four patients (13%) had low-grade serous carcinoma. Eleven patients (33%) had a prior history of hypertension. Following surgical cytoreduction, 16 patients (53%) had no gross residual disease (R0), 9 (30%) had ≤1cm residual, and 5 (17%) had > 1cm residual disease. Five patients (17%) had a bowel resection during the primary surgical cytoreduction.
Table 1.
Demographics and Clinical Characteristics
| Number of patients (%) | |
|---|---|
| Age, median (range) | 57 (31–76) |
| Race | |
| Caucasian | 22 (67%) |
| Black | 1 (3%) |
| Asian | 4 (12%) |
| Other | 3 (10%) |
| Body mass index, median (range) | 24.8 (17.2–41.2) |
| History of hypertension | |
| Yes | 11 (33%) |
| No | 19 (67%) |
| Stage | |
| IIIB | 3 (10%) |
| IIIC | 26 (87%) |
| IV | 1 (3%) |
| Grade | |
| Low-grade | 4 (13%) |
| High-grade | 26 (87%) |
| Histology | |
| Serous | 21 (70%) |
| Mixed endometrioid and serous | 3 (10%) |
| Clear cell | 4 (13%) |
| Poorly differentiated adenocarcinoma | 2 (7%) |
| Surgical cytoreduction | |
| Optimal (R0) | 16 (53%) |
| Optimal (<1cm) | 9 (30%) |
| Suboptimal (≥1cm) | 5 (17%) |
| BRCA positive | |
| Yes | 4 (13%) |
| No | 26 (87%) |
| Cycles completed on Protocol | |
| <4 cycles | 7 (23%) |
| ≥4 cycles | 23 (77%) |
| Treatment delay | |
| Yes | 22 (73%) |
| No | 8 (27%) |
| Number cycles growth factor support | |
| <2 cycles | 20 (67%) |
| ≥2 cycles | 10 (33%) |
| Maintenance chemotherapy | |
| Yes | 3 (10%) |
| No | 27 (90%) |
Twenty-three patients (77%) completed at least 4 cycles of therapy per protocol, and the posterior probability that the treatment success rate is > 60% was 0.77. Twenty-one (70%) of patients were able to complete ≥ 6 cycles of therapy. Twenty-two patients (73%) experienced at least one treatment delay. The most common reasons for treatment delay were neutropenia (n=17, 77%), thrombocytopenia (n=5, 23%), chemotherapy reaction (n=1, 5%), febrile illness (n=1, 5%), and other causes (n=7, 32%). Sixty percent of patients required no growth factor support. Ten patients (33%) used G-CSF and 2 patients (7%) used GM-CSF. Of the 12 patients that required growth factor support, the median number of cycles requiring growth factor support was 3.5 cycles (range 1-5). Adverse events are listed in Table 2. The most common grade 3 (n=19) and grade 4 (n=5) adverse event was neutropenia. Grade 1 (n=22) and grade 2 (n=3) neuropathy was common, however, no grade 3 or 4 neuropathy was reported. Similarly, grade 1 (n=21) and grade 2 (n=17) anemia was seen, however, only 2 reported events of grade 3 anemia was reported, and no grade 4 anemia events. Only 2 hypertension adverse events were seen, one was grade 1 and the other grade 4 requiring the patient to come off of bevacizumab. One patient also experienced cardiomyopathy after cycle 3 on protocol requiring discontinuing protocol therapy. No gastrointestinal perforation events occurred in this cohort. Two patients (7%) of patients experienced poor healing of the vaginal cuff requiring a repeat surgical procedure, one occurred after cycle 2 and the other after cycle 4.
Table 2.
Adverse Events
| Adverse Events | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total |
|---|---|---|---|---|---|
| Alopecia | 11 | 22 | 0 | 0 | 33 |
| Constipation | 20 | 10 | 1 | 0 | 31 |
| Dermatologic | 14 | 1 | 1 | 0 | 16 |
| Diarrhea | 16 | 1 | 0 | 0 | 17 |
| Fatigue | 27 | 15 | 4 | 0 | 46 |
| Anemia | 21 | 17 | 2 | 0 | 40 |
| Hypertension | 1 | 0 | 0 | 1 | 2 |
| Electrolyte | 12 | 0 | 0 | 0 | 12 |
| Mucositis | 13 | 7 | 0 | 0 | 20 |
| Nausea/vomiting | 24 | 6 | 0 | 0 | 30 |
| GI perforation | 0 | 0 | 0 | 0 | 0 |
| Neurology | 22 | 3 | 0 | 0 | 25 |
| Neutropenia | 5 | 21 | 19 | 5 | 50 |
| Pain | 28 | 3 | 2 | 0 | 33 |
| Thrombocytopenia | 19 | 6 | 2 | 0 | 26 |
| Wound healing | 0 | 0 | 2 | 0 | 2 |
| Other | 44 | 16 | 0 | 0 | 60 |
| Total | 277 | 128 | 33 | 6 | 444 |
Four patients (13%) of patients had measurable disease by RECIST criteria after primary cytoreduction, and 25 patients (83%) had non-measurable disease (Table 3). One patient did not have imaging after primary cytoreductive surgery. Of those patients with measurable disease after primary cytoreduction, disease response assessment after 6 cycles of chemotherapy demonstrated a complete response (n=1), partial response (n=1), stable disease (n=1), and one patient was inevaluable. Of those patients with non-measurable disease after primary cytoreduction, disease response assessment after 6 cycles of chemotherapy resulted in a complete response in 12 patients (48%), stable disease in 6 patients (24%), progressive disease in 2 patients (8%), and 5 patients were inevaluable.
Table 3.
Disease Response by RECIST
| Disease Status after primary cytoreduction |
CR | PR | SD | PD | IE |
|---|---|---|---|---|---|
| Measurable disease (n=4) | 1 | 1 | 1 | --- | 1 |
| Non-measurable disease (n=25) | 12 | --- | 6 | 2 | 5 |
CR=Complete response, PR=Partial response, SD=Stable disease, PD=Progressive disease, IE=Inevaluable
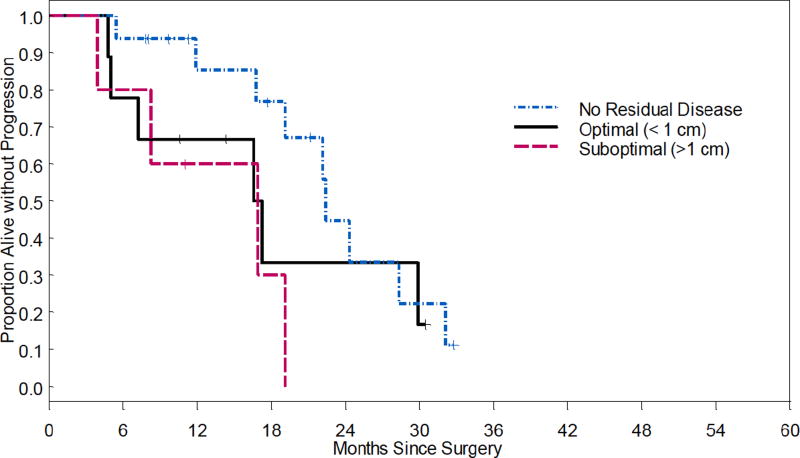
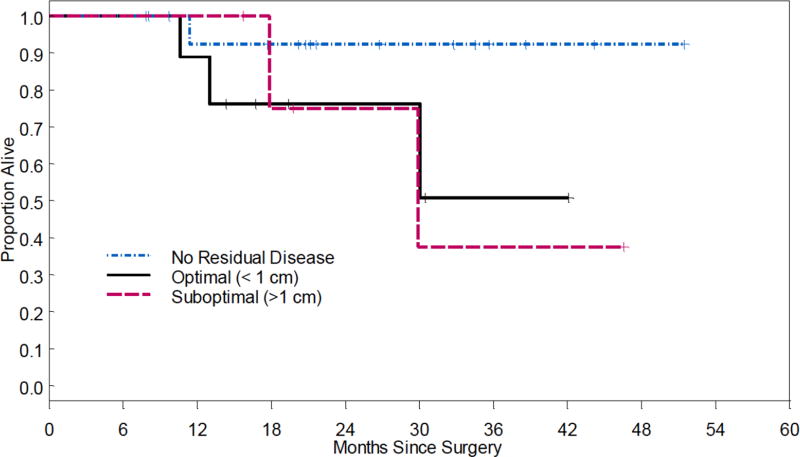
Fifteen patients (50%) developed recurrence, and 4 patients (13%) had progressive disease. Median PFS was 22.4 months in optimal (R0) compared to 16.9 months for optimal <1cm (HR 1.71, 95% CI 0.58-4.98, p=0.33), and 16.9 months for suboptimal ≥1cm (HR 3.75, 95% CI 1.05-13.34, p=0.04, Figure 1). Median OS was not reached for optimal (R0) and optimal <1cm. The OS for suboptimal ≥1cm was 29.9 months (HR 6.02, 95% CI 0.54-67.11, p=0.14, Figure 2).
Figure 1.
Progression-free survival
Figure 2.
Overall survival
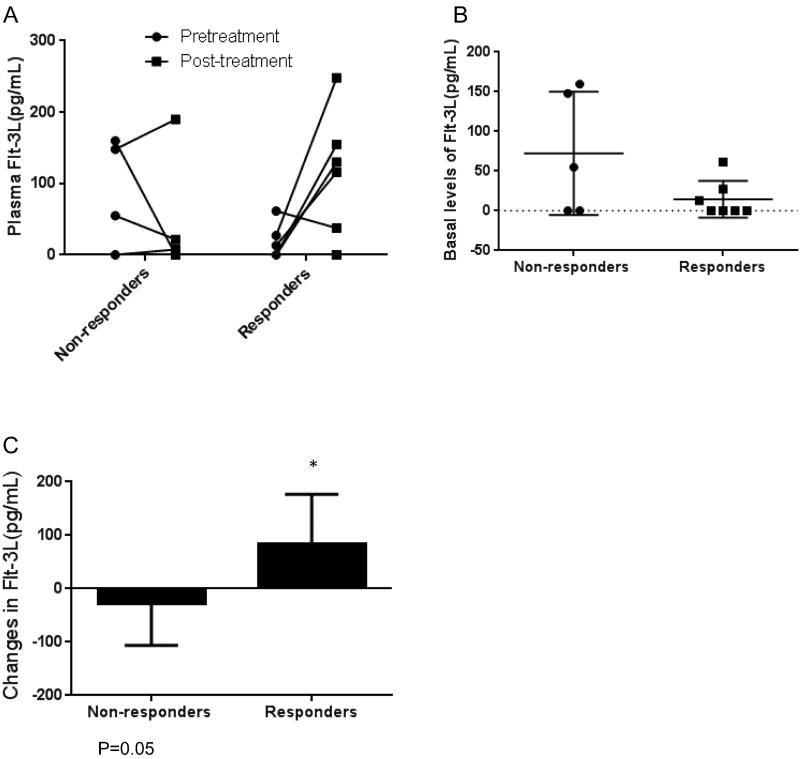
Dynamic changes in Flt-3L levels correlates with the treatment response
To investigate whether the paired pre and post-treatment plasma markers could potentially predict the response to the treatment, we measured the plasma biomarkers related to inflammation or immune response with the MILLIPLEX Immunology Multiplex Assay in 12 available patients. Among the 40 human cytokines/chemokines tested, there was a trend between baseline levels of Flt-3L and treatment response (Figure 3A and 3B). Furthermore, analyzing the dynamic changes in Flt-3L levels in relation to the treatment response revealed that mean Flt-3L increase during the treatment was higher in the responders (83.43±34.99 pg/mL) than that in the non-responders (−28±34.93 pg/mL, p=0.05), suggesting that dynamic changes in Flt-3L may represent potential predictive markers to the treatment response.
Figure 3.
Discussion
Majority of patients receiving dose dense paclitaxel, carboplatin, and bevacizumab chemotherapy were able to complete at least 4 cycles of therapy per protocol, leading to an acceptable treatment success rate as defined by the protocol. Although 73% of patients experienced at least one treatment delay, only 40% required growth factor support. There were few grade 3 and 4 adverse events, leading to an acceptable toxicity profile. One grade 4 hypertension adverse event did occur in a patient with pre-existing hypertension. No gastrointestinal perforation occurred. Two patients experienced poor healing of the vaginal cuff requiring a second surgical procedure. It is unclear the direct relationship between this complication and the combination regimen given the small sample size, but warrants close monitoring in patients on this therapy in the future. Progression-free survival was significantly better in patients that underwent optimal primary cytoreductive surgery (R0) on protocol therapy (22.4 months) compared to those that underwent either optimal (<1cm) or suboptimal (≥1cm) (16.9 months, respectively, p=0.04). This adds to the growing body of literature supporting complete R0 surgical cytoreduction in ovarian cancer surgery. Our plasma biomarker analysis revealed that Flt-3L may represent a marker for response to dose dense paclitaxel, carboplatin and bevacizumab regimen.
The JGOG 3016 study was the first randomized phase III trial showing a significant improvement in PFS and OS with weekly paclitaxel and carboplatin compared to the conventional regimen given every 21 days in advanced stage ovarian cancer. However, there was a high incidence of hematologic toxicity in the dose-dense group, leading to treatment delays and discontinuing therapy. [5–6] With the interest in the use of bevacizumab in the front- line setting, there have been limited reports on the safety and tolerability of using a combination of bevacizumab (15 mg/kg) with weekly paclitaxel and carboplatin.
Bevacizumab has been studied in many phase I, II, and III clinical trials in primary and recurrent ovarian cancer, due to the significant role that angiogenesis and neovascularization plays in tumor growth, invasion, and metastases. [14–15] Promising results from the AURELIA trial showing significantly improved PFS and objective response rate lead to the FDA approval of the use of bevacizumab in platinum-resistant recurrent ovarian cancer. [19] There have been 2 large randomized phase III trials using bevacizumab in the front-line setting, GOG-218 [7] and International Collaboration on Ovarian Neoplasms (ICON)-7. [8] These trials evaluated bevacizumab and tri-weekly carboplatin and paclitaxel followed by single-agent bevacizumab maintenance as adjuvant treatment for advanced ovarian cancer after primary cytoreductive surgery. Both trials demonstrated a significantly improved PFS with the addition of bevacizumab to front-line therapy followed by maintenance bevacizumab therapy.
Gonzalez-Martin et al. reported a single-arm phase II study evaluating weekly paclitaxel, carboplatin, and bevacizumab (OCTAVIA) in patients with stage I-IV ovarian cancer. Eligible patients were similar to those in ICON-7. Patients received 6 to 8 cycles of intravenous bevacizumab (7.5mg/kg, day 1 every 21 days), weekly paclitaxel (80mg/m2, days 1, 8, 15 every 21 days), and carboplatin (AUC 6, day 1 every 21 days). Bevacizumab 7.5 mg/kg was continued as a single agent every 3 weeks for up to 17 cycles. This multi-institution trial enrolled 189 patients in 9 countries. Over 90% of patients in this trial received ≥ 6 cycles of protocol therapy and 89% of patients received bevacizumab maintenance therapy. Thirty-seven percent of patients received G-CSF, 26% blood transfusions, and 21% paclitaxel dose reductions. Eleven percent of patients had bevacizumab discontinued due to adverse events, including vascular thrombosis, hypertensive crisis, and gastrointestinal disorders, including one case of gastrointestinal perforation. Median PFS was 28.8 months (95% CI 26.7 to NA months) in R0 group compared to 24.4 months (17.5 to NA months) in <1cm and 18.1 months (16 to 21.7 months) in ≥1cm resection group. RECIST response rate was 84.6%. [13] Our data showed a similar rate of growth factor support (33% G-CSF and 7% GM-CSF) and grade 3 or 4 anemia (7%) leading to 77% of patients completing at least 4 cycles and 70% completing 6 or more cycles of combination therapy. Our PFS estimates in the R0 group (22.4 months) compared to < 1cm (16.9 months) or ≥1cm (16.9 months) cannot be directly compared to the OCTAVIA data given that was not our primary endpoint, small patient sample, and that our data included only patients with stage III-IV disease. But altogether, both studies show an acceptable toxicity profile and majority of patients completing intended therapy.
Data was recently published from GOG-262, a phase III randomized trial of patients with stage II to IV epithelial ovarian cancer after optimal or suboptimal primary cytoreduction to either dose-dense paclitaxel (80 mg/m2) and carboplatin (AUC 6) or conventionally-dosed paclitaxel (175 mg/m2) and carboplatin (AUC 6). Bevacizumab 15mg/kg was optional in both arms, however, was administered to over 80% of patients. Most patients had advanced stage disease (stage III/IV, 97%) and had gross residual disease (63%) at enrollment. Overall, there was no difference in PFS between the 2 arms (HR 0.89, 95% CI 0.74-1.06). When stratified by those that did not receive bevacizumab (n=112), dose-dense paclitaxel led to an improved PFS (10 vs. 14 months, HR 0.62, 95% CI 0.4-0.95, p=0.03). However, in those patients that did receive bevacizumab (n=580), there was no difference in PFS between the 2 arms (15 months in both arms, HR 0.99, 95% CI 0.83-1.20). Based on this data, the authors suggested that bevacizumab may reduce any benefit to dose-dense therapy. While data on adverse events and toxicity were available, data regarding dose delays, growth factor support, and number of patients completing intended dose-dense therapy was not available in the manuscript. [27
Interestingly, we found that there was a suggestive trend between the dynamic changes in Flt-3L level and treatment response to this treatment. Our data is limited given that our translational endpoints could only be tested on 12 of 33 patient samples due to incomplete blood samples available. However, our limited analysis showed that patients with increased Flt-3L during the treatment might be more sensitive to this combination therapy than those with low-level changes in Flt-3L. This suggests that inducible Flt-3L might be an useful molecular determinant to use bevacizumab with dose-dense paclitaxel and carboplatin as first-line treatment in advanced ovarian cancer. It has been reported that the mechanism of Flt-3L may be a dendritic cell stimulator and Flt-3L has showed promise as a novel antitumor agent in animal models. [28–29] Whether inducible Flt-3L and dynamic changes in Flt-3L over treatment time could be used as a predictive biomarker to this treatment will require larger studies.
While our current trial is limited by the small sample size, the data regarding tolerability to therapy, dose delays, growth factor support, and adverse events adds to the current body of literature regarding feasibility of administering dose-dense paclitaxel, carboplatin and bevacizumab after primary cytoreductive surgery and further biomarker development.
Highlights.
Bevacizumab with dose-dense paclitaxel and carboplatin has acceptable toxicity.
Dose-delay is expected but majority complete therapy without growth factor support.
Changes in Flt-3L may represent a biomarker to response to therapy.
Acknowledgments
Funding statement: This research is supported, in part, by the National Institutes of Health (CA016672, P50 CA083639, UH3 TR000943), the Judy Rees Fund, the V-Foundation, the American Cancer Society Research Professor Award, the Ovarian Cancer Research Fund Alliance, the Blanton Davis Ovarian Cancer Research Program, the Frank McGraw Memorial Chair in Cancer Research, and the Ann Rife Cox Chair in Gynecology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: The authors declare no potential conflicts of interest.
References
- 1.American Cancer Society. Cancer Facts & Figures. 2017. [Google Scholar]
- 2.Du Bois A, Luck HJ, Meier W, et al. A randomized trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003;95:1320–9. doi: 10.1093/jnci/djg036. [DOI] [PubMed] [Google Scholar]
- 3.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–29. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 5.Katsumata N, Yasuda M, Takahashi F, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomized controlled trial. Lancet. 2009;374:1331–8. doi: 10.1016/S0140-6736(09)61157-0. [DOI] [PubMed] [Google Scholar]
- 6.Katsumata N, Yasuda M, Isonishi S, et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomized, controlled, open-label trial. Lancet Oncol. 2013;14:1020–6. doi: 10.1016/S1470-2045(13)70363-2. [DOI] [PubMed] [Google Scholar]
- 7.Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–83. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 8.Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2482–96. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 9.Wu CH, Yang CH, Lee JN, Hsu SC, Tsai EM. Weekly and monthly regimens of paclitaxel and carboplatin in the management of advanced ovarian cancer. A preliminary report on side effects. Int J Gynecol Cancer. 2001;11:295–9. doi: 10.1046/j.1525-1438.2001.011004295.x. [DOI] [PubMed] [Google Scholar]
- 10.Sehouli J, Stengel D, Mustea A, et al. Weekly paclitaxel and carboplatin (PC-W) for patients with primary advanced ovarian cancer: results of a multicenter phase II study of the NOGGO. Cancer Chemother Pharmacol. 2008;61:243–50. doi: 10.1007/s00280-007-0466-z. [DOI] [PubMed] [Google Scholar]
- 11.Pignata S, Breda E, Scambia G, et al. A phase II study of weekly carboplatin and paclitaxel as first-line treatment of elderly patients with advanced ovarian cancer. A Multicentre Italian Trial in Ovarian Cancer (MITO-5) study. Crit Rev Oncol Hematol. 2008;66:229–36. doi: 10.1016/j.critrevonc.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Havrilesky LJ, Alvarez AA, Sayer RA, et al. Weekly low-dose carboplatin and paclitaxel in the treatment of recurrent ovarian and peritoneal cancer. Gynecol Oncol. 2003;88:51–7. doi: 10.1006/gyno.2002.6859. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Martin A, Gladieff L, Tholander B, et al. Efficacy and safety results from OCTAVIA, a single-arm phase II study evaluating front-line bevacizumab, carboplatin and weekly paclitaxel for ovarian cancer. Eur J Cancer. 2013;49:3831–38. doi: 10.1016/j.ejca.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Folkman J. Angiogenesis in cancer, vascular, rheumatoid, and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 15.Fidler IF, Ellis LM. The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell. 1994;79:185–8. doi: 10.1016/0092-8674(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 16.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:5165–71. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 17.Sweeney CJ, Miller KD, Sissons SE, et al. The antiangiogenic property of docetaxel is synergistic with a recombinant humanized monoclonal antibody against vascular endothelial growth factor or 2-methoxyestradiol but antagonized by endothelial growth factors. Cancer Res. 2001;61:3369–72. [PubMed] [Google Scholar]
- 18.Wildiers H, Guetens G, De Boeck G, et al. Effect of antivascular endothelial growth factor treatment on the intratumoral uptake of CPT-11. Br J Cancer. 2003;88:1979–86. doi: 10.1038/sj.bjc.6601005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label phase III trial. J Clin Oncol. 2014;32:1302–8. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Chen TT, Ng TH. Optimal flexible designs in phase II clinical trials. Stat Med. 1998;17:2301–12. doi: 10.1002/(sici)1097-0258(19981030)17:20<2301::aid-sim927>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 22.Thall PF, Simon R. Practical Bayesian guidelines for phase IIB clinical trials. Biometrics. 1994;50:337–49. [PubMed] [Google Scholar]
- 23.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–81. [Google Scholar]
- 24.Cox DR. Regression models and life tables. Journal of the Royal Statistical Society. 1972;34:187–220. [Google Scholar]
- 25.Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–64. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 26.Pounds S, Morris SW. Estimating the occurrence of false positives and false negatives in microarray studies by approximating and partitioning the empirical distribution of pvalues. Bioinformatics. 2003;19:1236–42. doi: 10.1093/bioinformatics/btg148. [DOI] [PubMed] [Google Scholar]
- 27.Chan JK, Brady MF, Penson RT, et al. Weekly vs. Every-3-week paclitaxel and carboplatin for ovarian cancer. N Engl J Med. 2016;374:738–48. doi: 10.1056/NEJMoa1505067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong J, Bohinski RJ, Van Waes C, et al. Antitumor effect of secreted Flt3-ligand can act at distant tumor sites in a murine model of head and neck cancer. Cancer Gene Ther. 2003;10:96–104. doi: 10.1038/sj.cgt.7700534. [DOI] [PubMed] [Google Scholar]
- 29.Dong J, McPherson CM, Stambrook PJ. Flt-3 ligand: a potent dendritic cell stimulator and novel antitumor agent. Cancer Biol Ther. 2002;1:486–9. doi: 10.4161/cbt.1.5.161. [DOI] [PubMed] [Google Scholar]