Abstract
Objective
To determine differences in biomarker levels between radiographic phenotypes of facet joint osteoarthritis (FOA) only, spine OA only ((disc space narrowing (DSN) and vertebral osteophytes (OST)) or the combination of FOA and spine OA.
Design
A cross-sectional analysis of data from 555 participants in the Johnston County Osteoarthritis Project was performed. Lumbar spine levels were graded by severity (OST and DSN) and presence (FOA) of degeneration. Biomarkers included hyaluronan (HA) and type II collagen (CTX-II). Adjusted risk ratios (aRRR) were estimated using multinomial regression, with adjustment for age, race, sex, body mass index (BMI), and radiographic OA (knee, hip, hand). Interactions were tested between sex, race and low back symptoms.
Results
FOA only was present in 22.4%, 14.5% had spine OA only, and 34.6% had the combination of FOA and spine OA. Compared to the reference group of neither FOA or spine OA, a one unit higher ln HA level was associated with 31% higher relative risk ratio (RRR=1.31 ((95% 1.03, 1.67)) of having FOA only, while, a one unit higher ln uCTX-II level was associated with 84% higher relative risk ratio (RRR=1.84 ((95% CI 1.19, 2.84)) of having spine OA only. No significant interactions were identified.
Conclusion
Interestingly, OA affecting the synovial facet joint was associated with a marker of inflammation (HA). Spine OA, affecting intervertebral discs that contain collagen type II, was associated with a marker reflecting collagen type II degradation (CTX-II). These findings suggest that biomarkers may reflect the different pathophysiologic processes of lumbar spine OA phenotypes.
Chronic low back pain (cLBP) impacts over 31 million Americans at any given time1, has increased threefold in prevalence over a 10-year period2, and results in $100–$200 billion per year in total U.S expenditures.3 A large amount of these expenditures for interventions is attributed to two conditions, intervertebral disc (IVD) degeneration and facet joint osteoarthritis (FOA) because of their association with cLBP.4–10 Some of these interventions have high complication rates11 including potentially severe complications that lead to infection or death12, 13. A better understanding of the etiological process of spine degeneration may improve the delivery of interventions.
The radiographic features of spine degeneration including disc space narrowing [DSN], vertebral osteophytes [OST] and FOA), are commonly overlooked in discussions regarding osteoarthritis (OA). One reason is that characterization of IVD degeneration in the spine as an OA process has been debated14. This is in part due to anatomical differences that exist within the IVD that do not necessarily exhibit the same pathophysiology as OA in the knee, hip or hand7. Some consider the combination of at least mild radiographic DSN (analogous to joint space narrowing in an appendicular joint) and at least mild vertebral OST (at the same lumbar level) to be a definition of lumbar spine OA15, 16 based on a radiographic atlas definition (i.e., Kellgren-Lawrence)17. However, the facet or zygapophyseal joint, is the only structure in the spine that is classified as a synovial joint (i.e., contains articular cartilage, synovial lining and a joint capsule)18. When compared to lumbar spine DSN and vertebral OST, FOA has been strongly associated with OA of the knee and hand, suggesting a shared basis for degeneration7; this raises the intriguing question—whether the different features of spine OA reflect the same, or different pathophysiological process.
Biomarkers may be useful as indicators of specific subtypes of OA or of OA in specific joints19, 20. Previously, using data from the Johnston County Osteoarthritis Project (JoCo OA), we identified a significant association between a broad spectrum of biomarkers and lumbar spine DSN, but most were not independently associated with vertebral OST14. This suggests that these radiographic features differ to some extent in their pathophysiology and associated tissue metabolism14. We are unaware of previous analyses that have sought to differentiate between phenotypes of spine degeneration: those with FOA only, spine OA only, or the combination of FOA and spine OA, compared to those without FOA or spine OA. Therefore, the purposes of these analyses were 1) to determine whether biomarkers reflect differences in the phenotypes of spine degeneration, and 2) to determine whether these associations differ by sex, race and the presence of low back symptoms. We hypothesized that biomarkers would have a significant association with spine degeneration phenotypes and that these biomarkers would reflect differences in the tissues involved and their metabolism. Further, we expected that these associations between biomarkers and spine degeneration phenotypes would differ by sex, race and presence of low back symptoms.
Methods and Materials
Participants
Details of the sampling strategy and recruitment methods used for the JoCo OA are described elsewhere7, 21. Briefly, the JoCo OA is an ongoing cohort study set in 6 townships of Johnston County, North Carolina. The primary purpose of the JoCo OA is to determine the incidence, prevalence and progression of knee, hip, hand, and spine OA. Data for these cross-sectional analyses come from 1,015 participants enrolled in the JoCo OA during cohort enrichment (2003–2004). The cohort enrichment aimed to enrich the sample for African Americans (AAs) and younger participants. As such, participants enrolled during this time period were younger (mean age 59.3 vs. 65.8 years) and had a higher proportion of AAs (40% vs. 28%) than those at first follow-up of the original cohort; the 2 groups did not differ according to sex22.
Demographic data
Demographic data were collected by clinical interview and examination, including age and body mass index (BMI) at the time of interview (calculated from height measured without shoes and weight measured with a balance beam scale), race (Caucasian/AA), and sex. Low back symptoms were collected at clinical interview by asking participants to answer “yes” or “no” to “On most days do you have symptoms of pain, aching or stiffness in your lower back?”
Biomarkers
Details of the participants, collection and analyses of specimens of biomarkers have been described elsewhere14. Briefly, all participants had blood and urine collected at the clinic visit on the same day that radiographs were taken. Therefore, all samples were collected after completion of morning activity at a time (>1 hour after arising) when these serum markers have attained equilibrium23. Participants were sampled for biomarker analysis to represent a similar distribution of race and sex to the full cohort. A broad spectrum of serum and urine biomarkers was analyzed. Urinary type I collagen marker, N-terminal telopeptide (NTX-I, Osteomark, NJ), which reflects bone turnover or reabsorption24 and urinary type-II collagen (CTXII, IDS, Boldon, UK), the C-terminal cross-linked telopeptide of type II collagen measured by competitive ELISA and corrected for creatinine concentrations. The intra- and inter-assay precisions of NTX and CTXII are 5.86% and 9.97%, respectfully. Serum collagenase-generated neoepitope (C2C), which is a biomarker neoepitope of type II collagen degradation, was measured with a competitive ELISA (Ibex, Montreal, CA) with an intra- and inter-assay precision of 2.41% and 9.49%, respectfully. Serum C-propeptide of type II procollagen (CPII) which reflects cartilage synthesis24 was measured with a competitive ELISA (Ibex, Montreal, CA). The intra- and inter assay precisions are 3.68% and 9.08%, respectfully. Non-aggrecan and non-collagenous marker, serum cartilage oligomeric matrix protein (COMP), reflects cartilage degradation but may also be found in bone, ligaments, tendons and vascular smooth muscle25 was measured using an in-house sandwich ELISA. The reported precision is 5.8–6.6% intra-assay and between 8.7–9.7% inter-assay variability. Serum hyaluronic acid (HA) is a glycosaminoglycan indicative of OA and synovial inflammation24 was measured with the Hyaluronic Acid Test kit (Corgenix, Westminster, CO). The intra- and inter- assay precision is 3.6–4.7% and 5.7–7.0%, respectively.
Radiographic Spine Evaluation and Phenotypes
By protocol, women of reproductive age (<50 years of age) were excluded from having lumbar spine radiographs. Lateral lumbar spine films were taken with the participant lying on his/her left side with the central beam centered at the lumbar spine. All lateral lumbar spine radiographs were graded at each lumbar level by a single bone and joint radiologist (JBR) without regard to participants’ biomarker levels. The Burnett Atlas26 was used to grade lumbar spine radiographic features of FOA, DSN and OST. Facet joint OA was graded as absent or present at each lumbar level while DSN and OST were graded in a semi-quantitative fashion (0=none, 1=mild, 2=moderate and 3=severe). The grading for OST was done for each superior and inferior aspect of the anterior face of the lumbar vertebra. Spine OA was derived from the presence of at least a mild OST (either superior or inferior) and mild DSN at the same level of the lumbar spine for any level of the lumbar spine. FOA was coded as 1=present and 0=absent at any level of the lumbar spine. From these two different coding schemes we developed four spine degeneration phenotypes: 1) no FOA or spine OA (reference group), 2) FOA only, 3) spine OA only and 4) the combination of FOA and spine OA.
Spine Reliability Sub-Study
We conducted an intra-rater reliability analysis of the radiologist (JBR) for spine individual radiographic features of FOA, DSN and OST. A random sample of 50 participants from this cohort was selected for the reliability analysis. The radiologist completed the readings 2 weeks apart and was blinded to demographic, clinical characteristics, the reliability study and biomarker information of the participants. Data were analyzed using weighted kappa (wk) statistics for ordinal variables (DSN and OST) and kappa (k) statistics for the binary variable of FOA. Moderate-to-strong intra-rater reliability was found for FOA with a k=0.73, for DSN wk=0.89, and for OST wk=0.90.
Knee, Hip and Hand Osteoarthritis
Participants completed postero-anterior knee radiography of both knees in weight bearing with a Synaflexer™ (CCBR-Synarc, San Francisco, CA) positioning device and bilateral hip radiography with supine anteroposterior pelvis radiographs. The primary reason for a participant not having knee radiographs was presence of knee arthroplasty. The primary reason for missing hip radiographs was women of reproductive age (<50 years). Postero-anterior hand radiographs were obtained with the beam focused on the 3rd metacarpophalangeal joint for grading of 30 hand joints bilaterally (the distal interphalangeal [DIP], proximal interphalangeal [PIP], metacarpophalangeal [MCP], carpometacarpal [CMC] and thumb IP and MCP joints). All hip, knee and hand radiographs were read for Kellgren-Lawrence (K-L)27 score by a single bone and joint radiologist (JBR). Inter-rater and intra-rater reliability have been reported previously with a weighted kappa (wK) of 0.86 and 0.89 for the hip and knee, respectively28. Hip and knee OA, for these analyses, were defined as a K-L score of 2–4 in at least one extremity. Hand OA was defined, similar to a previous definition, as having at least one extremity with a K-L grade of 2–4 in one DIP and in at least 2 other similarly affected interphalangeal joints or CMC joints across both hands16.
Statistical Analysis
Descriptive statistics were generated for the total sample and each phenotype in the form of means and standard deviations or median and interquartile ranges for continuous covariates and counts and proportions for categorical covariates. Each biomarker demonstrated a right skewed distribution, so biomarkers were natural log (ln) transformed to meet model assumptions for analyses. Analysis of variance was used for continuous variables, and chi square tests were used for categorical variables to determine differences across FOA only, Spine OA only or the combination of FOA and spine OA. Post-hoc within group differences comparisons for continuous variables were conducted with Tukey Honestly Significant Difference.
We used multinomial regression to estimate unadjusted and adjusted associations between individual biomarkers and lumbar spine phenotypes. We chose to model each biomarker individually to determine their independent effects for the phenotypes of spine degeneration. The order of the categories is irrelevant as each category is independently compared to the referent group. Relative-risk ratios (RRR) and 95% confidence intervals were the measure of association. We report models adjusted for age, BMI, race and sex separately and in combination with adjustment for knee OA, hip OA, and hand OA to overcome confounding by appendicular OA as described in our previous work14. Associations between each spine phenotype and potential confounding factors of clinical, demographic and knee, hip or hand OA can be found in Appendix A. Pairwise interaction terms for sex, race and low back symptoms and each biomarker individually were used to assess effect measure modification while adjusting for all other covariates, however no significant (p<0.05) interaction effects were identified. All analyses were conducted in Stata 14 (Stata Corp, College Station, TX), and statistical significance was set at p<0.05.
Results
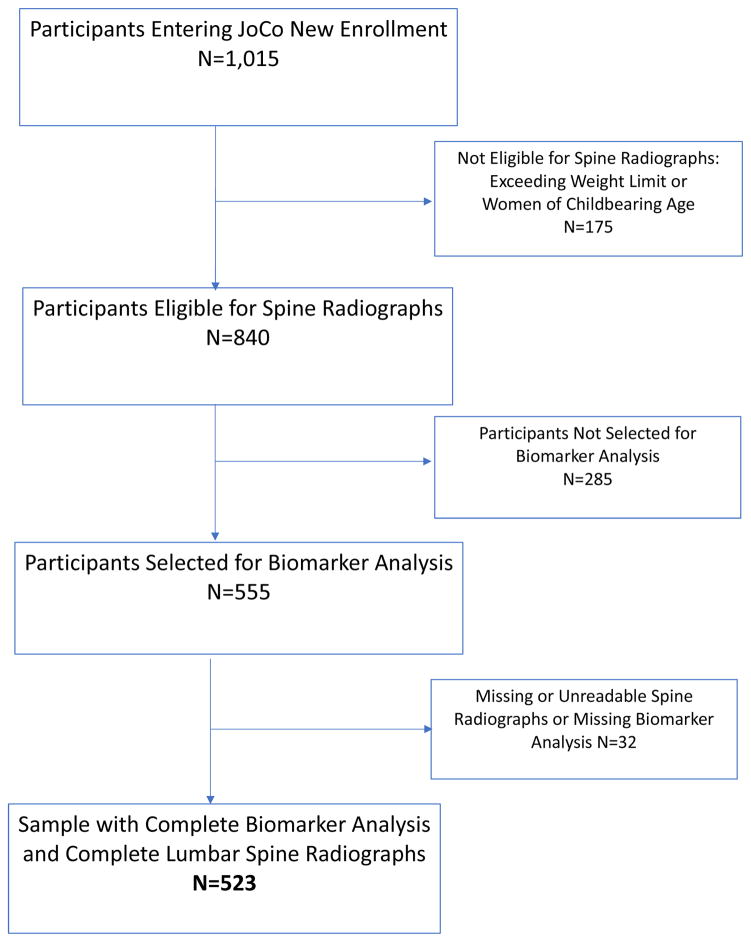
Figure 1 describes the flow of participants entering cohort enrichment for the JoCo OA Project (2003–2004). Of the 1,015 participants who underwent clinical examination, 175 were missing radiographs due to either exceeding the weight limit for the radiograph table or were women of child bearing age, leaving 840 participants with lumbar spine radiographs. A total of 555 participants had biomarker data. Some participants in the sample had unreadable (e.g., due to congenital defect or surgery) lumbar spine radiographs, leaving 523 participants with complete radiograph and biomarker data for the current study (Figure 1).
Figure 1.
Flow diagram of cohort enrichment participants 2003 – 2004 from the Johnston County Osteoarthritis (JoCo) Project with complete radiographic and biomarker data for these analyses.
Table 1 describes the demographic and clinical characteristics, frequency of peripheral joint OA and biomarker levels for included participants. On average the sample was older aged (mean 62.2 ((SD=9.9 years)) and classified as obese (BMI=30.2 ((SD=6.2)). Most participants were women (61.6%) and White (62.2%). There was a fairly equal distribution of spine degeneration phenotypes: 28.5% had no FOA or spine OA, 22.4% had only FOA, 14.5% had spine OA only, and 34.6% had the combination of FOA and spine OA. Low back symptoms were present in nearly half (49.0%) of the participants. Knee OA was present in 29.7%, 23.8% had hip OA and 28.0% had hand OA. Median biomarker levels for the whole cohort are reported in Table 1.
Table 1.
Distribution of demographic, clinical, radiographic and biomarker levels of participants for these analyses.
| Variable | Values |
|---|---|
| Age, years, mean (SD) | 62.2 (9.9) |
| BMI, kg/m2, mean (SD) | 30.2 (6.2) |
| Women, n (%) | 342 (61.6%) |
| Men, n (%) | 213 (38.4%) |
| African American, n (%) | 210 (37.8%) |
| White, n (%) | 345 (62.2%) |
| No FOA or spine OA, n (%) | 155 (28.5%) |
| Spine OA only, n (%) | 79 (14.5%) |
| FOA only, n (%) | 122 (22.4%) |
| FOA and spine OA, n (%) | 188 (34.6%) |
| Low back symptoms, n (%) | 271 (49.0%) |
| Knee OA, n (%) | 158 (29.7%) |
| Hip OA, n (%) | 130 (23.8%) |
| Hand OA, n (%) | 155 (28.0%) |
| uCTX-II (ng/mM Cr), median (IQR) | 0.207 (0.136, 0.332) |
| uNTX-I (nM BCE/mM Cr), median IQR | 60.2 (35.2, 107.9) |
| sCP-II (ng/ml), median IQR | 833.0 (614.1, 1043.9) |
| sC2C (ng/ml), median IQR | 167.5 (141.9, 195.3) |
| sCOMP (ng/ml) median IQR | 1561.4 (1206.5, 1989.8) |
| sHA (ng/ml) median IQR | 20.4 (9.5, 38.9) |
BMI=body mass index, OA=osteoarthritis, FOA=facet joint osteoarthritis. C-terminal crosslinked telopeptide (CTX-II) measured in nanogram per millimole, accounting for creatinine clearance. Cross-linked N-telopeptides (NTX-I) measured in nanomole of bone equivalents accounting for creatinine clearance. C-Propeptide (CP-II), Cleavage of type II collagen (C2C), Cartilage oligomeric matrix protein (COMP) and Hyaluronic Acid (HA) all measured in nanograms per milliliter. U=urine, s=serum
Table 2 describes the difference in demographic, clinical, peripheral joint OA and biomarker variables across phenotypes of spine degeneration. When compared to no FOA or spine OA, significant differences (p<0.001) in age were found for FOA only and the combination of FOA and spine OA. A greater proportion of Whites (68.0%), compared with African Americans, had FOA only and the combination of FOA and spine OA (74.5%). Those with FOA only and those with FOA and spine OA were more likely to have knee OA, 31.1% and 45.0% resepectively, compared to those without either FOA or spine OA. Similarly, those with FOA only and those with FOA and spine OA had a higher proportion of hand OA, 32.0% and 47.3%, respectively compared to those without FOA or spine OA. No significant differences were found for sex, BMI, hip OA or low back symptoms across spine phenotypes.
Table 2.
Distribution of participants with no spine osteoarthritis (OA) or facet joint OA (FOA) only, spine OA only, and the combination of FOA and spine OA across demographic, radiographic, clinical and biomarker variables.
| No FOA or spine OA | FOA only | Spine OA Only | FOA and spine OA | |
|---|---|---|---|---|
| Age, years mean (SD) | 57.0 (7.6) | 61.7 (9.0)* | 60.1 (8.7) | 67.8 (9.7) * |
| African American, n (%) | 78 (50.3%) | 39 (32.0%) | 42 (53.2) | 48 (25.5%) |
| White, n (%)* | 77 (49.7%) | 83 (68.0%) | 37 (46.8%) | 140 (74.5%) |
| Women, n (%) | 91 (58.7%) | 82 (67.2%) | 46 (58.2%) | 116 (61.7%) |
| Men, n (%) | 64 (41.2%) | 40 (32.8%) | 33 (41.8%) | 72 (38.3%) |
| BMI, kg/m2 mean (SD) | 29.3 (5.9) | 30.8 (6.4) | 30.0 (6.0) | 30.3 (6.3) |
| Knee OA, n (%)* | 25 (16.2%) | 37 (31.1%) | 13 (16.9%) | 77 (45.0%) |
| Hip OA, n (%) | 34 (22.2%) | 30 (24.8%) | 15 (19.2%) | 47 (25.5%) |
| Hand OA, n (%)* | 15 (9.7%) | 39 (32.0%) | 9 (11.7%) | 89 (47.3%) |
| Low Back Symptoms, n (%) | 66 (42.9%) | 54 (44.3%) | 43 (54.4%) | 99 (52.7%) |
| CTX-II (ng/mM Cr), median (IQR) | 0.183 (0.120, 0.257) | 0.198 (0.131, 0.284) | 0.221* (0.157, 0.368) | 0.247* (0.160, 0.383) |
| NTX-I (nM BCE/mM Cr), median IQR | 55.0 (33.9, 113.6) | 60.1 (34.1, 93.8) | 60.7 (34.0, 120.2) | 62.3 (39.2, 104.4) |
| CP-II (ng/ml), median IQR | 807.5 (609.6, 1047.9) | 896.3 (634.0, 11108.5) | 913.3 (683.5, 1088.0) | 825.4 (591.4, 1004.9) |
| C2C (ng/ml), median IQR | 167.3 (140.3, 200.0) | 170.2 (138.8, 190.0) | 174.7 (146.5, 206.9) | 166.1 (145.0, 192.5) |
| COMP (ng/ml) median IQR | 1401.0 (1080.0, 1854.6) | 1469.1 (1214.8, 1948.6) | 1646.9 (1296.9, 2064.5) | 1652.0 (1304.0, 2011.2) |
| HA (ng/ml) median IQR* | 15.9 (7.8, 27.8) | 20.3 (8.6, 37.2) | 17.3 (8.5, 36.2) | 25.3* (11.8, 55.4) |
OA=osteoarthritis, Spine OA=combination of disc space narrowing and osteophyte of the same level of the lumbar spine, FOA=facet joint OA, BMI=body mass index, SD=standard deviation. C-terminal crosslinked telopeptide (CTX-II) measured in nanograms per millimole, accounting for creatinine clearance. Cross-linked N-telopeptides (NTX-I) measured in nanomoles of bone equivalents accounting for creatinine clearance. C-Propeptide (CP-II), Cleavage neoepitope of type II collagen (C2C), Cartilage oligomeric matrix protein (COMP) and Hyaluronic Acid (HA) all measured in nanograms per milliliter.
p<0.001;
Table 3 describes the unadjusted and adjusted associations between each biomarker and the phenotypes of spine degeneration. For the FOA phenotype, a one unit higher lnHA level was associated with 31% higher relative risk ratio (RRR=1.31 ((95% 1.03, 1.67)) of having FOA only vs. having neither FOA nor spine OA; a similar association was found when adjusted for peripheral appendicular joint OA (RRR=1.30 ((95% CI 1.00, 1.69)). In contrast, for the spine OA phenotype, a one unit higher lnuCTX-II level was associated with 83% higher relative risk ratio (RRR=1.83 ((95% CI 1.21, 2.76) of having spine OA only vs having neither FOA nor FOA with spine OA; a similar association was found adjusting for demographic and clinical characteristics (RRR=1.72 ((1.14, 2.58)) and additionally adjusting for appendicular OA (RRR=1.84 ((95% CI 1.19, 2.84)). Among those with the combination of FOA and spine OA, significant associations were found between unadjusted lnCOMP and adjusted and unadjusted lnHA and lnuCTX-II. The association with COMP did not persist after adjustment for demographic and clinical characteristics. The associations with lnHA and FOA only were similar in strength to the combination of FOA and spine OA. In contrast, the association of lnuCTX-II and the combination of FOA and spine OA was somewhat attenuated (RRR=1.62 (95% CI 1.08, 2.42)) compared with the association of lnuCTXII and spine OA only (RRR=1.84 (95% CI 1.19, 2.84)).
Table 3.
Unadjusted and adjusted models for the relationship between biomarkers and facet joint OA (FOA) only, spine OA only, and the combination of FOA an spine OA compared to no FOA or spine OA.
| Unadjusted RRR (95% CI) |
Adjusted Model 1 RRR (95% CI) |
Adjusted Model 2 RRR (95% CI) |
|
|---|---|---|---|
| FOA Only | |||
| lnCTX-II (ng/mM Cr) | 1.03 (0.73, 1.47) | 1.06 (0.73, 1.54) | 1.07 (0.72, 1.59) |
| lnNTX-I (nM BCE/mM Cr) | 0.92 (0.69, 1.24) | 0.71 (0.49, 1.03) | 0.74 (0.51, 1.08) |
| lnCP-II (ng/ml) | 1.49 (0.80, 2.80) | 1.64 (0.85, 3.15) | 1.89 (0.95, 3.74) |
| lnC2C (ng/ml) | 0.60 (0.25, 1.46) | 0.69 (0.28, 1.73) | 0.65 (0.25, 1.64) |
| lnCOMP (ng/ml) | 1.23 (0.68, 2.34) | 1.03 (0.55, 1.91) | 0.95 (0.50, 1.81) |
| lnHA (ng/ml) | 1.20 (0.98, 1.47) | 1.31 (1.03, 1.67) | 1.30 (1.00, 1.69) |
| Spine OA Only | |||
| lnCTX-II (ng/mM Cr) | 1.83 (1.21, 2.76) | 1.72 (1.14, 2.58) | 1.84 (1.19, 2.84) |
| lnNTX-I (nM BCE/mM Cr) | 1.11 (0.80, 1.54) | 1.16 (0.81, 1.65) | 1.14 (0.80, 1.63) |
| lnCP-II (ng/ml) | 1.95 (0.95, 4.02) | 2.03 (0.97, 4.27) | 2.11 (0.98, 4.56) |
| lnC2C (ng/ml) | 1.56 (0.60, 4.08) | 1.48 (0.56, 3.93) | 1.40 (0.53, 3.67) |
| lnCOMP (ng/ml) | 1.91 (0.97, 3.81) | 1.67 (0.85, 3.27) | 1.65 (0.83, 3.27) |
| lnHA (ng/ml) | 1.11 (0.89, 1.40) | 1.10 (0.84, 1.43) | 1.10 (0.83, 1.46) |
| Combination of FOA and spine OA | |||
| lnCTX-II (ng/mM Cr) | 1.83 (1.32, 2.55) | 1.88 (1.29, 2.74) | 1.62 (1.08, 2.42) |
| lnNTX-I (nM BCE/mM Cr) | 1.03 (0.79, 1.34) | 0.92 (0.64, 1.32) | 0.95 (0.66, 1.37) |
| lnCP-II (ng/ml) | 0.95 (0.54, 1.65) | 1.12 (0.60, 2.10) | 1.20 (0.62, 2.32) |
| lnC2C (ng/ml) | 0.84 (0.38, 1.83) | 0.98 (0.41, 2.40) | 1.01 (0.41, 2.50) |
| lnCOMP (ng/ml) | 2.03 (1.18, 3.50) | 1.40 (0.75, 2.60) | 1.19 (0.62, 2.28) |
| lnHA (ng/ml) | 1.62 (1.29, 2.11) | 1.64 (1.29, 2.11) | 1.37 (1.05, 1.78) |
Spine OA=combination of disc space narrowing and osteophyte of the same level of the lumbar spine, RRR=relative-risk ratio; FOA=facet joint OA; C-terminal crosslinked telopeptide (CTX-II) measured in nanograms per millimole, accounting for creatinine clearance. Cross-linked N-telopeptides (NTX-I) measured in nanomoles of bone equivalents accounting for creatinine clearance. C-Propeptide (CP-II), Cleavage neoepitope of type II collagen (C2C), Cartilage oligomeric matrix protein (COMP) and Hyaluronic Acid (HA) all measured in nanograms per milliliter. Prior to natural logarithmic transformation, the biomarkers were expressed in the units of measure indicated. Referent is no FOA or spine OA for all analyses. Model 1 adjusted for age, body mass index, sex, and race. Model 2 adjusted for model 1 variables and knee, hip and hand osteoarthritis.
Discussion
Our intent was to determine if there was an independent relationship between a broad spectrum of biomarkers and phenotypes of spine degeneration and if those associations differed by sex, race or low back symptoms. Unlike previous studies, we grouped participants as having FOA only, spine OA only, and the combination of FOA and spine OA. Indeed, we identified biomarkers that may reflect structural differences between spine degeneration phenotypes. Although we anticipated significant differences across demographics and clinical characteristics, we did not identify any significant interactions with these factors and spine phenotypes. The biomarker differences with these phenotypes suggests that a unique etiological process of spine degeneration may underlie these phenotypes.
It has been reported that IVD degeneration precedes FOA because an increased load occurs on the facet joint from IVD degeneration29. However, in approximately 20% of individuals, FOA precedes IVD degeneration30. Our findings indicate that 22% of participants have FOA without the presence of spine OA. Although lower in prevalence, we also identified a proportion of participants with spine OA without FOA. This suggests that, in some individuals, these two degenerative processes in the lumbar spine may be independent of one another. We also identified a subgroup with the combination of FOA and spine OA and a similar proportion without any radiographic evidence of FOA or spine OA; the latter represents a unique group, considering the mean age of the sample, suggesting that age is an independent risk factor for spine degeneration. The roughly similar distribution of the different spine degeneration phenotypes is interesting and suggests that the etiological degenerative process may differ for these specific phenotypes.
We found that only one of these joint metabolism markers, HA, reflects OA of the lumbar spine facet joint. Our previous analyses between the individual radiographic features of DSN and OST found several significant associations between biomarkers, including HA and DSN8. In those analyses, we did not restrict our spine degeneration groups to DSN only and therefore did not account for FOA presence. The analyses reported here indicate that the presence of FOA only in the spine, but not spine OA, is significantly associated with HA. We did find that HA was associated with the combination of FOA and spine OA phenotype. The similar association between the FOA only phenotype and the combination of both FOA and spine OA suggests that FOA may be the driving factor behind this association. In prior reports, HA has been associated with OA in the knee, hip and hand reflecting a synovial inflammatory process7, 19, 32–36. The fact that HA is associated with FOA only and not spine OA only is consistent with biomarker results for appendicular joints and underscores the utility of this biomarker for synovial joints, which in the lumbar spine are found only in the facet joint.
Previous analyses have found the type II collagen marker, uCTX-II, to be significantly associated with DSN14, 37, vertebral OST14 and FOA38. Meulenbelt and colleagues, however, found uCTX-II to be significantly associated with FOA, but not DSN, in participants in the Genetics, Arthrosis and Progression (GARP) study38. Kraus and colleagues19 also found a relationship between uCTX-II and HA and lumbar spine radiographic OST or DSN. There are several reasons that our results may differ from these previous studies including anatomical location of FOA in the GARP study (i.e., cervical and lumbar sites combined), inclusion of samples from sibling pairs, and adjustment in multivariable models. Furthermore, the objective of both of these studies was to determine the association between biomarkers and total body burden of radiographic OA using the combined scores of OA grades from multiple joints. In the current analysis, we were interested in the association between biomarkers and distinct phenotypes of spine degeneration. In these adjusted analyses, there are clear differences in the associations of biomarkers and FOA only and spine OA only suggesting an independent association between uCTX-II and spine OA. In our previous analyses14, we identified a significant relationship between uCTX-II and both DSN and OST. We did not differentiate participants by distinct subset based on each radiographic feature so we are unable to determine the independent effect of uCTX-II on DSN from those results. In the current study, we identified a significant association between uCTX-II and the combination of FOA and spine OA. However, the weaker association found between uCTX-II and the combination of FOA and spine OA when compared to spine OA only suggests that spine OA only is the driving factor in the association. Although, we found moderate to strong associations with both CP-II and C2C and spine OA only, these associations were not statistically significant. One reason for the lack of statistical significance may be the large between-participant variability inherent to these biomarkers which could affect the statistical power.
There are several strengths and some limitations to our study. The primary limitation of this study is its cross-sectional design; thus, we could not address the temporal relationship between the onset of biomarker abnormalities and onset of spine degeneration. This can only be assessed using longitudinal analyses which we plan to conduct in future work. Lateral lumbar spine radiographs may not be the optimal image or view for FOA, which could lead to non-differential misclassification of FOA status. However, we observed similar prevalence estimates of FOA based on lateral spine radiography7 as previously reported using computed tomography scans39. We adjusted for hip, knee, and hand OA to take into account their potential contribution to biomarker levels; however, we did not control for every possible factor (such as medication use, liver function, and kidney function, diet/activity) that could affect serum levels of biomarkers. Per protocol, we excluded women of childbearing age and did not evaluate for factors that may influence biomarkers in the spine such as the presence of spondylolisthesis and dynamic instability, this may limit generalizability. Although we analyzed a broad spectrum of biomarkers, we did not include recently developed inflammatory biomarkers that may be more specific to IVD degeneration40, 41. Despite these limitations we found a difference in biomarker levels between distinct phenotypes of spine degeneration that potentially reflect a difference in pathophysiology of these important spine structures. The independent association of biomarkers with specific lumbar spine degeneration phenotypes could impact clinical practice by aiding more precise identification of the appropriate spinal structure to target with interventions.
Supplementary Material
Acknowledgments
Role of the funding source
APG receives funds from the NIH Loan Repayment Program, National Institute of Arthritis Musculoskeletal and Skin Diseases (1-L30-AR057661-01).
The Johnston County Osteoarthritis Project is supported in part by cooperative agreements S043, S1734, and S3486 from the Centers for Disease Control (CDC) and Prevention/Association of Schools of Public Health; the NIAMS Multipurpose Arthritis and Musculoskeletal Disease Center grant 5-P60-AR30701; the NIAMS Multidisciplinary Clinical Research Center grant 5 P60 AR49465-03; the NIA P30-AG-028716. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC, NIA or NIAMS.
Footnotes
Author contributions
Contributions: All authors of this work have made substantial contributions to the conception and design, acquisition of data, analysis, interpretation, drafting of manuscript and final approval of submission.
Conflict of interest
All authors disclose they have no financial or personal relationships with other people or organizations that could potentially and inappropriately bias their work and conclusions.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Adam P. Goode, Associate Professor, Department of Orthopedic Surgery, Duke Clinical Research Institute, Duke University School of Medicine.
Amanda E. Nelson, Assistant Professor, Thurston Arthritis Research Center, University of North Carolina, Chapel Hill.
Virginia B. Kraus, Professor, Duke Molecular Physiology Institute and Division of Rheumatology, Duke University School of Medicine, Durham, NC.
Jordan B. Renner, Professor, Thurston Arthritis Research Center, Department of Radiology, University of North Carolina, Chapel Hill.
Joanne M. Jordan, Professor, Thurston Arthritis Research Center, University of North Carolina, Chapel Hill.
References
- 1.Jensen MC, Brant-Zawadzki MN, Obuchowski N, Modic MT, Malkasian D, Ross JS. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331:69–73. doi: 10.1056/NEJM199407143310201. [DOI] [PubMed] [Google Scholar]
- 2.Freburger JK, Holmes GM, Agans RP, Jackman AM, Darter JD, Wallace AS, et al. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169:251–258. doi: 10.1001/archinternmed.2008.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(Suppl 2):21–24. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- 4.Raastad J, Reiman M, Coeytaux R, Ledbetter L, Goode AP. The association between lumbar spine radiographic features and low back pain: a systematic review and meta-analysis. Semin Arthritis Rheum. 2015;44:571–585. doi: 10.1016/j.semarthrit.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Goode AP, Carey TS, Jordan JM. Low back pain and lumbar spine osteoarthritis: how are they related? Curr Rheumatol Rep. 2013;15:305. doi: 10.1007/s11926-012-0305-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gellhorn AC, Katz JN, Suri P. Osteoarthritis of the spine: the facet joints. Nat Rev Rheumatol. 2013;9:216–224. doi: 10.1038/nrrheum.2012.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goode AP, Marshall SW, Renner JB, Carey TS, Kraus VB, Irwin DE, et al. Lumbar spine radiographic features and demographic, clinical, and radiographic knee, hip, and hand osteoarthritis. Arthritis Care Res (Hoboken) 2012;64:1536–1544. doi: 10.1002/acr.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Schepper EI, Damen J, van Meurs JB, Ginai AZ, Popham M, Hofman A, et al. The association between lumbar disc degeneration and low back pain: the influence of age, gender, and individual radiographic features. Spine (Phila Pa 1976) 2010;35:531–536. doi: 10.1097/BRS.0b013e3181aa5b33. [DOI] [PubMed] [Google Scholar]
- 9.Muraki S, Oka H, Akune T, Mabuchi A, En-Yo Y, Yoshida M, et al. Prevalence of radiographic lumbar spondylosis and its association with low back pain in elderly subjects of population-based cohorts: the ROAD study. Ann Rheum Dis. 2009;68:1401–1406. doi: 10.1136/ard.2007.087296. [DOI] [PubMed] [Google Scholar]
- 10.Pye SR, Reid DM, Smith R, Adams JE, Nelson K, Silman AJ, et al. Radiographic features of lumbar disc degeneration and self-reported back pain. J Rheumatol. 2004;31:753–758. [PubMed] [Google Scholar]
- 11.Nasser R, Yadla S, Maltenfort MG, Harrop JS, Anderson DG, Vaccaro AR, et al. Complications in spine surgery. J Neurosurg Spine. 2010;13:144–157. doi: 10.3171/2010.3.SPINE09369. [DOI] [PubMed] [Google Scholar]
- 12.Smith JS, Saulle D, Chen CJ, Lenke LG, Polly DW, Jr, Kasliwal MK, et al. Rates and causes of mortality associated with spine surgery based on 108,419 procedures: a review of the Scoliosis Research Society Morbidity and Mortality Database. Spine (Phila Pa 1976) 2012;37:1975–1982. doi: 10.1097/BRS.0b013e318257fada. [DOI] [PubMed] [Google Scholar]
- 13.Smith JS, Shaffrey CI, Sansur CA, Berven SH, Fu KM, Broadstone PA, et al. Rates of infection after spine surgery based on 108,419 procedures: a report from the Scoliosis Research Society Morbidity and Mortality Committee. Spine (Phila Pa 1976) 2011;36:556–563. doi: 10.1097/BRS.0b013e3181eadd41. [DOI] [PubMed] [Google Scholar]
- 14.Goode AP, Marshall SW, Kraus VB, Renner JB, Sturmer T, Carey TS, et al. Association between serum and urine biomarkers and lumbar spine individual radiographic features: the Johnston County Osteoarthritis Project. Osteoarthritis Cartilage. 2012;20:1286–1293. doi: 10.1016/j.joca.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson AE, Renner JB, Schwartz TA, Kraus VB, Helmick CG, Jordan JM. Differences in multijoint radiographic osteoarthritis phenotypes among African Americans and Caucasians: the Johnston County Osteoarthritis project. Arthritis Rheum. 2011;63:3843–3852. doi: 10.1002/art.30610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraus VB, Jordan JM, Doherty M, Wilson AG, Moskowitz R, Hochberg M, et al. The Genetics of Generalized Osteoarthritis (GOGO) study: study design and evaluation of osteoarthritis phenotypes. Osteoarthritis Cartilage. 2007;15:120–127. doi: 10.1016/j.joca.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalichman L, Hunter DJ. Lumbar facet joint osteoarthritis: a review. Semin Arthritis Rheum. 2007;37:69–80. doi: 10.1016/j.semarthrit.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Kraus VB, Kepler TB, Stabler T, Renner J, Jordan J. First qualification study of serum biomarkers as indicators of total body burden of osteoarthritis. PLoS One. 2010;5:e9739. doi: 10.1371/journal.pone.0009739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Catterall JB, Hsueh MF, Stabler TV, McCudden CR, Bolognesi M, Zura R, et al. Protein modification by deamidation indicates variations in joint extracellular matrix turnover. The Journal of biological chemistry. 2012;287:4640–4651. doi: 10.1074/jbc.M111.249649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2007;34:172–180. [PubMed] [Google Scholar]
- 22.Allen KD, Chen JC, Callahan LF, Golightly YM, Helmick CG, Renner JB, et al. Associations of occupational tasks with knee and hip osteoarthritis: the Johnston County Osteoarthritis Project. J Rheumatol. 37:842–850. doi: 10.3899/jrheum.090302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon CD, Stabler TV, Kraus VB. Variation in osteoarthritis biomarkers from activity not food consumption. Clin Chim Acta. 2008;398:21–26. doi: 10.1016/j.cca.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauer DC, Hunter DJ, Abramson SB, Attur M, Corr M, Felson D, et al. Classification of osteoarthritis biomarkers: a proposed approach. Osteoarthritis Cartilage. 2006;14:723–727. doi: 10.1016/j.joca.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Muller G, Michel A, Altenburg E. COMP (cartilage oligomeric matrix protein) is synthesized in ligament, tendon, meniscus, and articular cartilage. Connect Tissue Res. 1998;39:233–244. doi: 10.3109/03008209809021499. [DOI] [PubMed] [Google Scholar]
- 26.Burnett SJHD, Cooper C, Spector TD. A Radiographic Atlas of Osteoarthritis. London: SpringereVerlag; 1994. [Google Scholar]
- 27.Kellgren JH. The Epidemiology of Rheumatic Diseases. Annals of the rheumatic diseases. 1964;23:109–122. doi: 10.1136/ard.23.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jordan JM, Linder GF, Renner JB, Fryer JG. The impact of arthritis in rural populations. Arthritis care and research. 1995;8:242–250. doi: 10.1002/art.1790080407. [DOI] [PubMed] [Google Scholar]
- 29.Jinkins JR. Acquired degenerative changes of the intervertebral segments at and suprajacent to the lumbosacral junction. A radioanatomic analysis of the nondiscal structures of the spinal column and perispinal soft tissues. Eur J Radiol. 2004;50:134–158. doi: 10.1016/j.ejrad.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Videman T, Battie MC, Gill K, Manninen H, Gibbons LE, Fisher LD. Magnetic resonance imaging findings and their relationships in the thoracic and lumbar spine. Insights into the etiopathogenesis of spinal degeneration. Spine (Phila Pa 1976) 1995;20:928–935. doi: 10.1097/00007632-199504150-00009. [DOI] [PubMed] [Google Scholar]
- 31.Kalichman L, Guermazi A, Li L, Hunter DJ. Association between age, sex, BMI and CT-evaluated spinal degeneration features. J Back Musculoskelet Rehabil. 2009;22:189–195. doi: 10.3233/BMR-2009-0232. [DOI] [PubMed] [Google Scholar]
- 32.Elliott AL, Kraus VB, Luta G, Stabler T, Renner JB, Woodard J, et al. Serum hyaluronan levels and radiographic knee and hip osteoarthritis in African Americans and Caucasians in the Johnston County Osteoarthritis Project. Arthritis Rheum. 2005;52:105–111. doi: 10.1002/art.20724. [DOI] [PubMed] [Google Scholar]
- 33.Filkova M, Senolt L, Braun M, Hulejova H, Pavelkova A, Sleglova O, et al. Serum hyaluronic acid as a potential marker with a predictive value for further radiographic progression of hand osteoarthritis. Osteoarthritis Cartilage. 2009;17:1615–1619. doi: 10.1016/j.joca.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Davis CR, Karl J, Granell R, Kirwan JR, Fasham J, Johansen J, et al. Can biochemical markers serve as surrogates for imaging in knee osteoarthritis? Arthritis Rheum. 2007;56:4038–4047. doi: 10.1002/art.23129. [DOI] [PubMed] [Google Scholar]
- 35.Garnero P, Piperno M, Gineyts E, Christgau S, Delmas PD, Vignon E. Cross sectional evaluation of biochemical markers of bone, cartilage, and synovial tissue metabolism in patients with knee osteoarthritis: relations with disease activity and joint damage. Ann Rheum Dis. 2001;60:619–626. doi: 10.1136/ard.60.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharif M, George E, Shepstone L, Knudson W, Thonar EJ, Cushnaghan J, et al. Serum hyaluronic acid level as a predictor of disease progression in osteoarthritis of the knee. Arthritis Rheum. 1995;38:760–767. doi: 10.1002/art.1780380608. [DOI] [PubMed] [Google Scholar]
- 37.Garnero P, Sornay-Rendu E, Arlot M, Christiansen C, Delmas PD. Association between spine disc degeneration and type II collagen degradation in postmenopausal women: the OFELY study. Arthritis Rheum. 2004;50:3137–3144. doi: 10.1002/art.20493. [DOI] [PubMed] [Google Scholar]
- 38.Meulenbelt I, Kloppenburg M, Kroon HM, Houwing-Duistermaat JJ, Garnero P, Hellio Le Graverand MP, et al. Urinary CTX-II levels are associated with radiographic subtypes of osteoarthritis in hip, knee, hand, and facet joints in subject with familial osteoarthritis at multiple sites: the GARP study. Ann Rheum Dis. 2006;65:360–365. doi: 10.1136/ard.2005.040642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalichman L, Li L, Kim DH, Guermazi A, Berkin V, O’Donnell CJ, et al. Facet joint osteoarthritis and low back pain in the community-based population. Spine. 2008;33:2560–2565. doi: 10.1097/BRS.0b013e318184ef95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weber KT, Alipui DO, Sison CP, Bloom O, Quraishi S, Overby MC, et al. Serum levels of the proinflammatory cytokine interleukin-6 vary based on diagnoses in individuals with lumbar intervertebral disc diseases. Arthritis Res Ther. 2016;18:3. doi: 10.1186/s13075-015-0887-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weber KT, Satoh S, Alipui DO, Virojanapa J, Levine M, Sison C, et al. Exploratory study for identifying systemic biomarkers that correlate with pain response in patients with intervertebral disc disorders. Immunol Res. 2015;63:170–180. doi: 10.1007/s12026-015-8709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.