Abstract
Choline is essential to the development and function of the central nervous system and supplemental choline during development is neuroprotective against a variety of insults, including neurotoxins like dizocilpine (MK-801). MK-801 is an NMDA receptor antagonist that is frequently used in rodent models of psychological disorders, particularly schizophrenia. At low doses, it causes cognitive impairments, and at higher doses it induces motor deficits, anhedonia, and neuronal degeneration. The primary goals of the present study were to investigate whether prenatal choline supplementation protects against the cognitive impairments, motor deficits, and neuropathologies that are precipitated by MK-801 administration in adulthood. Adult male Sprague Dawley rats were fed a standard or supplemented choline diet prenatally. Using the novelty preference test of object recognition, we found that only prenatal standard-fed rats displayed memory consolidation deficits induced by low-dose MK-801 administered immediately following study of sample objects; all other groups, including prenatal choline supplemented rats given MK-801, showed intact memory. Following high-dose MK-801, prenatal choline supplementation significantly alleviated rats’ motor response to MK-801, particularly ataxia. Using doublecortin and Ki67 to mark neurogenesis and cell division, respectively, in the hippocampus, we found that prenatal choline supplementation, in the face of MK-801 toxicity, protected against reduced hippocampal plasticity. Taken together, the current findings suggest that prenatal choline supplementation protects against a variety of behavioral and neural pathologies induced by the neurotoxin, MK-801. This research contributes to the growing body of evidence supporting the robust neuroprotective capacity of choline.
Keywords: nutrition, neuroprotection, novelty preference, adult neurogenesis, doublecortin, Ki67
Introduction
Choline is an essential nutrient crucial to the development and function of the central nervous system due to its roles in cell signaling, cell membrane structure, neurotransmission, and gene expression. The availability of choline during development modulates adult behavior and brain function; perinatal choline supplementation in rats enhances cognitive functioning throughout adulthood (reviewed in Meck and Williams, 2003; Meck et al., 2008) and protects against a diverse range of cognition-impairing neural insults (Blusztajn and Mellott, 2013). Within this larger ongoing investigation of the neuroprotective mechanisms of choline, the present study aimed to elucidate the role of prenatal choline supplementation in preventing the cognitive impairments, motor deficits, and hippocampal-based neuropathologies induced by the neurotoxin, MK-801.
The capacity of early life choline supplementation to modify behavior, brain function, and be neuroprotective may arise from its pervasive importance to the developing nervous system (Blusztajn et al., 1998; Zeisel, 2006): it promotes cell structure as a component of the phospholipid cell membrane, giving rise to both phosphatidylcholine and sphingomyelin (Blusztajn et al., 1987); supports cell signaling, particularly in neurons, through its conversion to the neurotransmitter, acetylcholine (Blusztajn and Wurtman, 1983); and regulates gene expression as a methyl group donor through its metabolite, S-adenosylmethionine (Niculescu and Zeisel, 2002). This epigenetic function of choline is of particular interest for research on choline’s protective capacity because its broad neuroprotective effects are likely mediated by a fundamental role in the expression of various genes. All of these processes to which choline makes essential contributions are often at risk of malfunctioning, as many people do not acquire a sufficient amount of choline (Zeisel, 2011). Choline is synthesized in the body, but the amount produced is inadequate for proper biological functioning (Zeisel and Blusztajn, 1994). Therefore, individuals must obtain this vitamin from foods such as eggs, beef, chicken, fish, broccoli, and cauliflower (Zeisel et al., 2003). Typically, men and women require an intake of 550 or 425 mg of choline per day, respectively (Zeisel and Caudill, 2010); however, less than 10% of people consistently meet this standard (Jensen et al., 2007). Adequate choline intake is crucial throughout the lifespan, but particularly in development as this is an especially sensitive period for the brain (Zeisel, 2006). For example, pregnant women with low choline intake have a higher risk of having a child with a neural tube defect or cleft palate (Shaw et al., 2004; 2006).
While choline deficiency is detrimental, choline supplementation, as mentioned, is protective in a variety of ways. Though dietary choline supplementation is protective throughout the lifespan (Zeisel, 2006), a greater protective capacity is exhibited when the supplementation occurs in early development as opposed to in adolescence or in adulthood (Meck et al., 2008; Wong-Goodrich et al., 2008a; Glenn et al., 2012). Early life choline supplementation in rats, either prenatally, neonatally, or post-weaning, exerts neuroprotection in a wide array of disease models, including epilepsy (Wong-Goodrich et al., 2008b; 2011), Down syndrome (Strupp et al., 2016), Rett syndrome (Nag and Berger-Sweeney et al., 2007; Nag et al., 2009), fetal alcohol syndrome (Thomas et al., 2003; 2007; Schneider and Thomas, 2016), depression (Glenn et al., 2012; McCall et al., 2015: Schulz et al., 2014), and schizophrenia (Corriveau and Glenn, 2012; Stevens et al., 2008; 2014). In addition to these disease models, early life choline supplementation also attenuates the effects of exposure to the neurotoxin, MK-801 (Guo-Ross et al., 2002; 2003). MK-801 is a noncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist (Wong et al., 1986) that blocks the activity of the excitatory neurotransmitter, glutamate, preventing it from activating the NMDA receptor. Because glutamate is the primary excitatory neurotransmitter in the nervous system, the disruption of its activity by MK-801 causes a variety of problems. In rats, chronic or large acute doses of MK-801 induce motor deficits (Andiné et al., 1999), anhedonia (Vardigan et al., 2010), and neuronal degeneration (Guo-Ross et al., 2002; 2003), while low acute doses induce cognitive impairment in both spatial memory (Åhlander et al., 1999) and object recognition memory (de Lima et al., 2005). These are symptoms frequently observed in psychological disorders for which pathology is associated with reduced excitatory neurotransmission, such as schizophrenia.
We previously reported that periadolescent choline supplementation, beginning post-weaning and continuing until just before adulthood (postnatal days 25–50), prevented cognitive impairment in a two-hit model of schizophrenia in which male rats were prenatally stressed and then administered an acute, large dose of MK-801 (5 mg/kg) as adults (Corriveau and Glenn, 2012). Because the cognitive deficits reported in that study only occurred in rats sustaining both hits, the prenatal stress and the adult MK-801 exposure, it was not possible to determine the extent to which choline supplementation reversed or slowed the damaging effects of prenatal stress or buffered rats against the damaging effects of adult MK-801. Thus, in the present study, we aimed to build on previous research showing that prenatal choline supplementation protected against MK-801’s neurotoxic properties (Guo-Ross et al., 2002; 2003) by investigating whether prenatal choline supplementation would also protect against MK-801’s adverse effects on memory. The specific focus was on whether prenatal choline supplementation would prevent memory consolidation deficits with low dose MK-801 (0.2 mg/kg) administered immediately after a study phase of the novelty preference test of object memory and 3 hours before memory retrieval of the studied objects. This small acute dose of MK-801 is used to isolate the cognitive response and prevent induction of motor deficits that would obscure the mnemonic test results (de Lima et al., 2005). We also examined whether prenatal choline supplementation would prevent locomotor and neurological deficits with high dose MK-801 (3 mg/kg), which is well known to induce declines in locomotion and increases in ataxia and stereotypy (e.g. Tricklebank et al., 1989) damage neurons (Horváth et al., 1997) and impact adult hippocampal neurogenesis and neuron survival (Ikonomidou et al., 1999; Mochizuki et al., 2007; Petrus et al., 2009; and see Nacher and McEwen, 2006). We previously saw only modest evidence that adolescent choline supplementation mildly attenuated motor symptoms using a 5 mg/kg dose of MK-801 (Corriveau and Glenn, 2012). However, in that case, it is probable that the dose was large enough to produce a ceiling effect in the rats. Also, rats were only observed during the first 30 minutes following exposure and it is possible that attenuated responses would be more evident as the effects of MK-801 on locomotor behaviors were dissipating. Thus, in the present study, rats were assessed on the same three scales of motor behavior we previously used—locomotion, stereotypy, and ataxia—but with the lower dose, as described above, and the time frame of behavioral observation was expanded to 3 hours to more comprehensively characterize the progression and resolution of motor effects. Three days following this dose, adult hippocampal neurogenesis and cell proliferation was assessed. Prenatal choline supplementation increases adult hippocampal neurogenesis (Glenn et al., 2007; McCall et al., 2015) and cell proliferation (Glenn et al., 2007; 2008), but there is not a clear understanding of the role of MK-801 in this process: mixed results indicate that MK-801 both increases (Nacher et al., 2003) and decreases neurogenesis (Arvidsson et al., 2001). Therefore, the present research seeks to clarify this inconsistency as well as determine the interactive effects of MK-801 and prenatal choline diet. It is hypothesized that declines in hippocampal plasticity, marked here by numbers of new-born neurons, would couple with behavioral deficits and both indices (behavior and hippocampal plasticity) would be ameliorated in prenatal choline supplemented rats.
Experimental Procedures
Colony Conditions
All rats were housed in clear polycarbonate cages (30.5 × 30.5 × 18.5 cm), which were individually ventilated (Thoren Caging Systems, Inc., Hazleton, PA). Pregnant dams and dams with litters were housed individually; after weaning, pups were housed in same-sex cage pairs. All cages contained a thin layer of corncob bedding. The colony room was maintained at 20–23°C with 10–55% humidity and was kept on a 12-hour light/12-hour dark cycle, with the lights on at 0800 h daily; every procedure was conducted in the light phase of the cycle. All rats had ad libitum access to food and water throughout the experiment. Food and water intake were recorded every other day throughout the experiment, and body weights were noted once a week. All testing procedures were approved by the Colby College Institutional Animal Care and Use Committee and performed in accordance with federal standards.
Prenatal Choline Manipulation, Cross-Fostering, and Experimental Groups
Twelve timed-pregnant female Sprague Dawley rats arrived at the lab from Charles River Laboratories (Wilmington, MA) on gestational day (GD) 8. Pregnant dams, housed as described above, were fed commercially available rat chow (Harlan Teklad, Madison, WI) until GD 10. On GD 10, pregnant dams were switched to a synthetic choline diet prepared based on formulations by the American Institute of Nutrition (AIN76A; Dyets Inc., Bethlehem, PA). Half of the pregnant dams (n = 6) were placed on a standard choline diet (STD; AIN76A with 1.1 g/kg choline chloride substituted for choline bitartrate), while the remaining half (n = 6) were placed on a choline-supplemented diet (SUP; AIN76A with 5 g/kg choline chloride). Following birth, on postnatal day (PD) 2, male and female pups from standard-fed and choline-supplemented mothers were gathered, toe-clipped to indicated diet condition, and cross-fostered among all dams so that each litter contained 10–12 pups with a mix of males and females, both standard-fed and choline-supplemented. Cross-fostering of pups ensures minimal influence of litter effects as all conditions are represented in each of the balanced litters. Also on PD 2, all dams were switched to the standard choline diet (1.1 g/kg). On PD 23, rat pups were weaned into same-sex groups of 4–6 littermates and switched to the regular, commercially available rat chow. Rats were separated into same-sex cage pairs on PD 30.
The male offspring were the subjects of the current study; female offspring were used in a different study. At present, there is great interest in, and indeed a national imperative, to use sex as a biological imperative in experiments. However, at the time that this study was conducted, we were building on two lines of research: one in males (e.g. Corriveau and Glenn, 2012) and another in females (Glenn et al., 2012). In future studies, we aim to include females and males in all of our experiments whenever possible. Of the male subjects of the present study (n = 40), 20 were on the standard choline diet prenatally (STD), while 20 were fed supplemental choline prenatally (SUP). Within each diet condition, half were randomly selected to receive MK-801 injections (MK), and the remaining half was selected to receive 0.9% saline (SAL). Therefore, there were four experimental groups: SUP-MK (n = 10), SUP-SAL (n = 10), STD-MK (n = 10), and STD-SAL (n = 9; one rat in the STD-SAL condition died of unknown causes prior to behavioral testing on PD 65).
Drug Administration
MK-801 (5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate) exists in two enantiomers. (−)-MK-801, as opposed to (+)-MK-801, is the less active enantiomer, binding to NMDA receptors with a slightly decreased affinity (Wong et al., 1986). The present study utilized (−)-MK-801 because the primary goal was to investigate cognitive deficits in adult rats as a function of prenatal choline levels. A lower dose (de Lima et al., 2005; van der Staay et al., 2011) of the less active form of MK-801 may aid in generating cognitive impairment without causing the other deficits induced by larger doses of MK-801 or by the (+)-MK-801 enantiomer with higher activity. This allows for the isolation of the cognitive deficits in the current rat model.
The (−) enantiomer of MK-801 hydrogen maleate (MK-801) was used in all behavioral tests (Sigma-Aldrich, St. Louis, MO). Testing began when rats reached adulthood (PD 80) and continued until sacrifice (PD 125). As described in the following sections, MK-801 was administered immediately following the study phase of the object recognition test to assess its effects on memory consolidation of the previously studied objects. The low dose of MK-801 used for this part of the study was 0.2 mg/kg i.p. in a volume of 1 mL/kg. This dosage was chosen because it was large enough to induce cognitive deficits in rats without causing changes in motor behaviors (de Lima et al., 2005; van der Staay et al., 2011). The large dose of MK-801 was 3 mg/kg i.p. in a volume of 1 mL/kg; this dose was used for the motor response test that followed cognitive assessments and just preceded sacrifice. This dose was chosen because it was large enough to induce motor deficits and neuronal dysfunction in rats, the two additional focuses of the current study (Andiné et al., 1999; Guo-Ross et al., 2002; 2003).
Object Recognition Memory Following Low-Dose MK-801 Administration
The novelty preference test of object recognition was used to assess cognition in response to low-dose MK-801 administration and prenatal choline diet. This test operates on the premise that rats explore novel objects in a familiar environment: when presented with a novel object and a previously studied object, a typical rat will spend more time investigating the novel object relative to the studied object (e.g. Dix and Aggleton, 1999), indicative of intact memory for the previously encountered study object. The testing apparatus was an open field constructed of wood with dimensions 70 × 70 × 60 cm. It was lined on the bottom with approximately 2 cm of corncob bedding. In each corner of the field, under the bedding, a jar lid was screwed to the wood, upside down, so that objects could be glued to glass jars and firmly secured to the apparatus by attaching the jars to their lids. This allowed rats to explore the objects without knocking any over, since a suddenly fallen object is an inherently more interesting object and skews the outcome of the test. All objects used were ceramic figurines of different colors and shapes, and all were approximately 3–6 inches tall. The objects were cleaned with OdoBan® thoroughly between each trial. A video camera (Logitech HD Pro Webcam C920, Fremont, CA), placed directly above the arena, was used to record each study and test phase. Each rat’s object exploration was tracked through the use of key presses that were collected and summarized with ANY-maze software (Stoelting Co., Wood Dale, IL).
Before the object recognition tests, rats were habituated to the testing apparatus over 3 consecutive days. During habituation sessions, the apparatus contained no objects, only the jar lids and corncob bedding, as described above. On the first day of habituation, rats spent 10 minutes in the field with their cage mate. On days 2 and 3 of habituation, each rat spent 5 minutes in the field alone. On the day following the last habituation day, the novelty preference test—consisting of a study phase, retention delay, and test phase—was conducted. For the study phase, each rat spent 5 minutes alone in the field and could investigate two identical objects placed in opposite corners. Immediately following the study phase, rats were injected with either MK-801 or saline, as described above and based on previous research (de Lima et al., 2005; van der Staay et al., 2011). As the rats had already studied the objects, but had yet to process them, MK-801 given at this time could influence consolidation of memories for the objects just investigated while leaving encoding and retrieval processes intact. The retention delay between the study phase and test phase was 3 h in duration. This time frame was chosen so that the test phase occurred at a time when the drug’s non-specific effects had subsided (de Lima et al., 2005). For the test phase, each rat was returned to the field for 3 min with two objects: one from the study phase and one novel object. The object from the pair that served as study or novel and the position of the objects in the apparatus during the test phase were counterbalanced within and between conditions.
Object investigation was operationally defined as time rats spent within 2 cm of an object with its head oriented toward it. The primary dependent measure from the test phase was an exploration ratio that was calculated for each rat by dividing the total time spent investigating the novel object with the total time spent exploring both the novel and study objects. Values of this ratio that were close to 0.5 indicated that the rat spent equivalent amounts of time investigating both objects; this lack of a bias for the novel object was taken as evidence of poor memory for the study object. Values of this ratio that were closer to 1.0, significantly greater than 0.5 (see section 4.7.), indicated that rats spent more time investigating the novel object than the study object; this bias for the novel object was taken as evidence of intact memory for the study object.
Motor Responses Following High-Dose MK-801 Administration
Ten days after the object recognition test, the motor reactions of rats following a high dose of MK-801 were observed. Rats were given a single injection of either MK-801 (3 mg/kg i.p. in a volume of 1 mL/kg) or saline. Directly following injection, rats were placed in a clear polycarbonate cage lined with corncob bedding and covered with a metal cage top in a quiet room. Their behavior was recorded via a camera connected to a computer in an adjacent room for a total of 3 h. Following the 3-h observation period, rats were returned to their home cages in the colony rooms. The video footage was analyzed by an experimenter blind to the experimental conditions in 5-min increments (including t = 0 and t = 180 min) for a total of 37 assessments per rat. Each rat’s behavior was coded for locomotion, stereotypy, and ataxia at each time point based on published scales specifically designed to assess motor effects of drugs like MK-801 (Andiné et al., 1999; Manahan-Vaughan et al., 2008; Corriveau and Glenn, 2012).
Behavioral ratings on the locomotor scale were used to gauge overall activity levels in the rats. The scale is designed to take into account a wide range of locomotor features, including the use of the entire enclosed area, the frequency of movements, and the full use of limbs in the movement. Scores on this scale ranged from 0–5: 0 = stationary, 1 = movements within a localized area with forelimbs only, 2 = intermittent movements within half of the area of the cage, 3 = continuous movement within half of the cage, 4 = intermittent movements within the whole area of the cage, and 5 = continuous movement within the whole area of the cage. Behavioral ratings on the stereotypy scale were used to detect expression of stereotyped movement in the rats. This behavioral feature is indicated by the repetitive engagement in motor behavior, in this case sniffing behavior. Scores on this scale ranged from 0–2: 0 = no sniffing, 1 = discontinuous sniffing (‘normal’ sniffing), and 2 = continuous sniffing. Behavioral ratings on the ataxia scale were used to detect the presence of ataxia: the ability of rats to maintain sufficient postural control to remain upright. Scores on this scale ranged from 0–3: 0 = normal body control, 1 = falling tendency upon movement, 2 = falling upon movement, and 3 = almost unable to move. Note that for this scale, rats that are given a saline injection will display normal body control thereby consistently scoring a 0. Accordingly, the analyses included are only on drug treated rats (see Statistical Analyses).
Markers of Adult Hippocampal Plasticity: Doublecortin and Ki67 Immunohistochemistry
Two markers of hippocampal plasticity were used in the present study: doublecortin to mark immature neurons and Ki67 to mark dividing cells. To assay these markers, rats were sacrificed 3 days after the high-dose MK-801 administration and motor assessments (approximately PD 125). This 3-day delay between MK-801 administration and sacrifice was chosen to allow MK-801 sufficient time to induce neural effects (e.g. Guo-Ross et al., 2003). The first marker, doublecortin (DCX), is a microtubule protein used to mark newly-born neurons as it is transiently expressed during differentiation and migration; this marker was of interest to determine whether the single high dose of MK-801 would affect the overall rate of addition of new neurons to the functional circuitry. However, DCX is expressed in new, immature neurons for approximately 10–14 days and thus marks a large pool of neurons in different stages of development. For this reason, we were also interested in a more momentary marker of cell division and used Ki67, a cellular protein expressed during cell division, to mark the numbers of cells that were newly proliferated 3 days after the acute high dose of MK-801.
To collect brain tissue for the neural assays, each rat was deeply anesthetized with isoflurane in 1.5% O2 prior to decapitation. The brains were extracted and post-fixed in 4% paraformaldehyde in 1M phosphate buffer (PB) at 4°C for approximately 4 weeks before vibratome sectioning. Two series of 60-µm coronal sections through the rostral hippocampus were collected and stored in 0.1% sodium azide in 1M PB at 4°C until immunohistochemistry to mark new neurons (doublecortin) and recently divided cells (Ki67) was performed. Issues with deriving sectioned tissue or processing resulted in non-usable tissue sets: 1 STD-SAL rat had only one viable set and 1 STD-SAL and 1 SUP-SAL had none.
DCX immunohistochemistry was based on methods as described previously (Rao and Shetty, 2004; Glenn et al., 2007). One set of the hippocampal tissue sections, free-floating, were rinsed in tris-buffered saline (TBS), incubated in 0.6% hydrogen peroxide for 30 min at room temperature, rinsed again in TBS, incubated in a 3% normal horse serum (Vector Laboratories) and 0.1% triton-X-100 (TTX) solution for 30 min at room temperature, and then incubated with the primary DCX antibody (1:200; Santa Cruz Biotechnology, Inc.). The sections remained in this primary antibody solution overnight on a shaker kept at 4°C. The following day, the tissue was rinsed with TBS and then incubated with the secondary antibody (biotinylated horse anti-goat, 1:200; Vector Laboratories) for 1 h at room temperature. After the incubation, the sections were rinsed in TBS and transferred to an avidin-biotin complex (ABC; Vector Laboratories) for 1 h, also at room temperature. The tissue was rinsed in TBS, then SG peroxidase was used to visualize the stained neurons (Vector-SG, Vector Laboratories). Following a final rinse in TBS, tissue was mounted on 1% gelatin-coated slides, counterstained using methyl green, and cover-slipped.
The other set of free-floating hippocampal sections was used for Ki67 immunohistochemistry and the procedures were the same as for DCX with different antibodies. Briefly, all rinses between steps were done with TBS; quenching and blocking were 30 min each at room temperature. Sections were then incubated overnight in the primary antibody (anti-Ki67 rabbit monoclonal, 1:200; Vector Laboratories) on a shaker at 4°C. Following this, the sections were incubated in the secondary antibody (biotinylated horse anti-rabbit IgG, 1:200; Vector Laboratories), then ABC, each for 1 h at room temperature. SG peroxidase was used for visualization and, after sections were mounted, methyl green was used for counterstaining prior to cover-slipping.
Unbiased Stereological Methods to Quantify Numbers of DCX+ and Ki67+ Hippocampal Cells
DCX- and Ki67-labeled cells in the dentate gyrus of the hippocampus were estimated using unbiased stereological techniques (Mouton et al., 2002; West, 1999); these methods are also described in detail in Glenn et al., 2007. Five sections from each rat with representation through the rostral-caudal extent of the dorsal hippocampus were used for quantification. Using StereoInvestigator software (Microbrightfield Inc., Williston, VT), 30–40 100 × 100-µm counting frames were systematically and randomly selected within a contour drawn to bound the dorsal and ventral blades of dentate gyrus resulting in 150–200 frames per rat. Cells were identified at 400× magnification using an optical dissector height of 20 µm with 2-µm guard zones and estimated by the software using the optical fractionator method (West, 1993; 1999).
Statistical Analyses
Means and standard errors of the means, displayed in the figures, were calculated for all behavioral and histological results including the object recognition test, the motor response test, neurogenesis and cell proliferation. As previously described, exploration ratios were calculated from object recognition test data to test for biases toward the novel object, indicative of memory for the study object. Two-way analyses of variance (ANOVAs) comparing between-subject factors of Diet (STD and SUP) and Drug (SAL and MK) were conducted on the exploration ratios. Additionally, one-sample t-tests were conducted comparing the mean exploration ratio of each group to the no preference value of 0.5 to validate the object recognition task as a test of memory. For both locomotion and stereotypy behaviors of the motor response test, 3-way ANOVAs were conducted comparing the effects of Diet, Drug, and Time on the motor behavior score. For stereotypy, average scores were collapsed over 3 time points, respectively, to elucidate patterns over time and an additional 3-way ANOVA was conducted to analyze the data in this way. For ataxia, a 2-way ANOVA was conducted comparing the effects of Diet and Time on the average ataxia score; the drug component was eliminated from statistical analysis because only rats given MK-801 exhibited ataxia. For DCX and Ki67 analyses, 2-way ANOVAs of between subject factors Diet and Drug were conducted on estimates of numbers of DCX and Ki-67-labeled cells. Levine’s test for equality of error variances and Mauchly’s test of sphericity used to test for homogeneity of variance and, when ε < 0.75, a Huynh-Feldt correction was applied to the analysis. This was necessary for the locomotion analysis and one comparison in the stereotypy analysis. Of note, homogeneity tests on the ataxia data revealed equal variance for the first half of the observations and significant lack of homogeneity of variance in the final portions of the observation period—a time when scores of all rats were close to normal. Thus, ataxia results for the first half of the observation (observations 1–18) only were analyzed. For all analyses, planned, directional, or post hoc pairwise t-tests were conducted as appropriate. The significance level was 0.05 for all tests.
Results
Object Recognition Memory Following Low-Dose MK-801 Administration
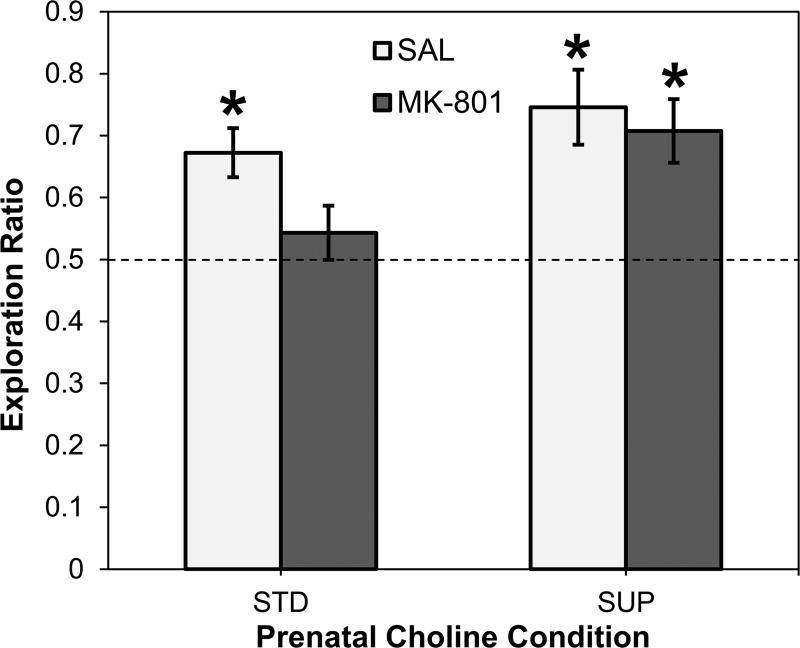
Fig. 1 shows the mean novelty preference exploration ratios calculated over the 3-min test phase and evaluated across prenatal choline diet and adult drug conditions. A 2×2 ANOVA revealed a main effect of prenatal choline diet on the exploration ratios (F (1, 34) = 5.789, p = 0.022): Overall, SUP rats had higher exploration ratios than STD rats. The main effect of adult drug administration was not statistically significant (F (1, 34) = 2.879, p > 0.05), nor was there a significant interaction between diet and drug (F < 1). To confirm that STD-SAL rats exhibited novelty preference and to pursue the hypothesis that prenatal choline supplementation protects memory consolidation against adult MK-801 toxicity, one-sample t-tests of each of the four groups were performed comparing the mean novelty preference ratios to the no-preference value of 0.5. STD-SAL rats displayed a significant preference for the novel object (t (8) = 4.350, p = 0.002), and STD-MK rats did not (t (9) = 0.988, p > 0.05). The SUP-SAL rats also exhibited a significant preference for the novel object (t (8) = 4.062, p = 0.004) and, unlike the STD-MK rats, the SUP-MK rats also displayed significant novelty preference (t (9) = 4.041, = 0.003).
Figure 1.
Object recognition memory using the novelty preference task as a function of prenatal dietary condition and low dose MK-801 or saline administration. Average exploration ratios for each of the four groups are shown as reflection of the extent to which rats displayed biases toward the novel object during the test phase of the task. The dashed line reflects no bias for either object. *p < 0.05 versus no bias value of 0.50.
Motor Responses Following High-Dose MK-801 Administration
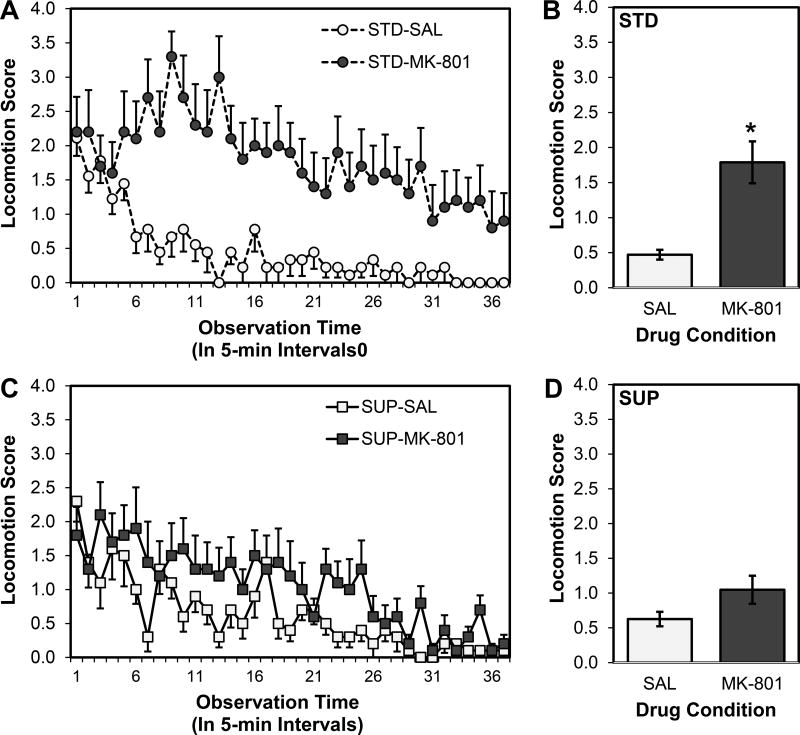
A 2×2×37 ANOVA on locomotion scores obtained at 37 5-min intervals over the observation period revealed main effects of both Time (F (9.788, 1260) = 9.258, p < 0.001) and Drug (F (1, 35) = 19.797, p < 0.001), but no main effect of Diet (F (1, 35) = 2.239, p > 0.05). There was neither a significant interaction between Time and Diet (F < 1) nor a three-way interaction among Time, Diet, and Drug (F < 1); however, there were significant interactions between Time and Drug (F (9.788, 1260) = 1.920, p = 0.043; see Fig. 2A and 2C) and Diet and Drug (F (1, 35) = 5.255, p = 0.028; see Fig. 2B and 2D). To pursue the significant Diet by Drug interaction, pairwise comparisons were conducted first comparing SAL and MK rats within the STD group, and then comparing SAL and MK rats within the SUP group. STD-MK rats had significantly higher mean locomotion scores compared with STD-SAL rats (t (17) = 4.086, p = 0.001; see Fig. 2B). SUP-MK and SUP-SAL rats did not have significantly different locomotion scores (t (18) = 1.858, p > 0.05; see Fig. 2D). Additionally, STD-MK rats had significantly higher locomotion scores than SUP-MK rats (t (18) = 2.055, p = 0.028).
Figure 2.
Locomotion scores as a function of prenatal dietary condition and high dose MK-801 or saline administration. Scores of standard-fed rats are shown in the top panel (A and B) and scores of prenatal choline-supplemented rats are shown in the bottom panel (C and D). The left panels (A and C) show scores of saline- and MK-801-treated rats collected at each of the 37 time points (one every 5 min for 3 hr) and the right panels (B and D) show the mean scores over time. Overall MK-801 significantly increased locomotion, particularly in early portions of the observation period for both diet conditions; this reduction was significantly attenuated in rats treated with prenatal choline supplementation. *p < 0.05 STD-MK-801 versus STD-SAL and SUP-MK-801
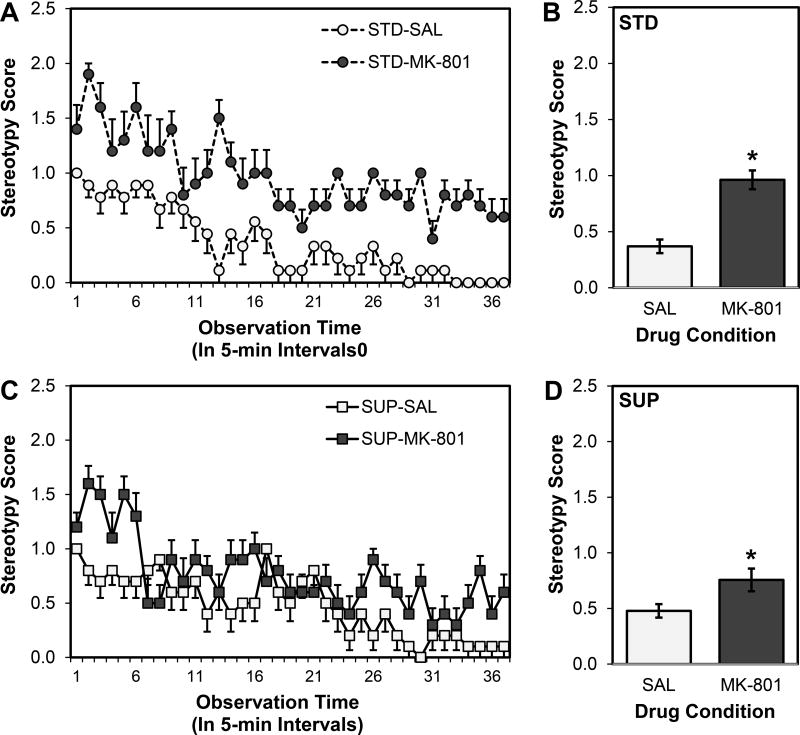
A 2×2×37 ANOVA revealed no main effect of Diet on mean stereotypy score (F < 1), but there were main effects of Drug (F (1, 35) = 29.898, p < 0.001) and Time (F (36, 1260) = 16.132, p < 0.001). There was no three-way interaction among Time, Diet, and Drug (F (36, 1260) = 1.281, p > 0.05). There was, however, a significant interaction between Time and Drug (F (36, 1260) = 2.524, p < 0.001; see Fig. 3A and 3C), and the interactions between Diet and Drug (F (1, 35) = 3.894, p = 0.056; see Fig. 3B and 3C) and Time and Diet (F (36, 1260) = 1.331, p = 0.092) approached statistical significance. Though the MK-801 treated rats displayed higher rates of stereotypy than SAL rats for both STD (t (9.981) = 4.297, p = 0.002; equal variances not assumed) and SUP (t (18) = 2.348, p = 0.031) rats, appears evident from Fig. 3 that the magnitude of the deficits greater in STD than SUP rats; this difference was not statistically significant (t (18) = 1.557, p = 0.068).
Figure 3.
Stereotypy scores as a function of prenatal dietary condition and high dose MK-801 or saline administration. Scores of standard-fed rats are shown in the top panel (A and B) and scores of prenatal choline-supplemented rats are shown in the bottom panel (C and D). The left panels (A and C) show scores of saline- and MK-801-treated rats collected at each of the 37 time points (one every 5 min for 3 hr) and the right panels (B and D) show the mean scores over time. Overall, MK-801 significantly increased stereotypy in standard- and prenatal choline-supplemented rats. *p < 0.05 MK-801 group versus saline-treated group for each diet condition
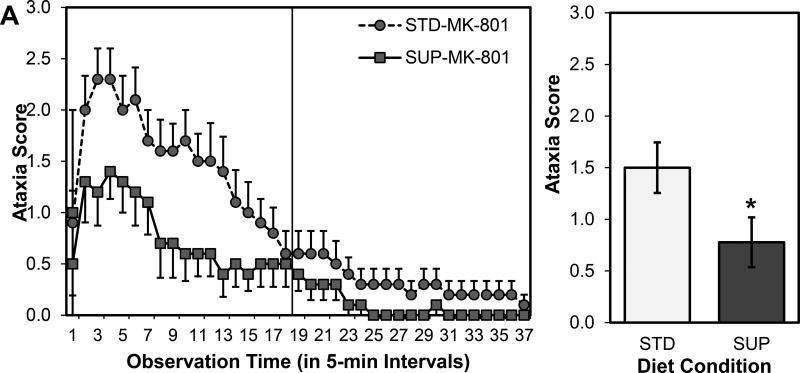
For the ataxia scores only the MK-801 treated rats were included in the analysis because at no time did a saline-injected rat register a score on the ataxia scale. Also, as outlined in the statistical plan, the first 18 observations were analyzed so as not to violate assumptions about homogeneity of variance that occurred in the last 19 observations; by the 18th observation, scores, overall, were close to normal values as the effects of MK-801 were dissipating (see Fig. 4). A 2×18 ANOVA on ataxia scores from the first part of the observation revealed main effects of Time (F (17, 306) = 13.807, p < 0.001) and Diet (F (1, 18) = 4.419, p = 0.050), but not a significant interaction between Time and Diet (F (17, 306) = 1.535, p = 0.081; see Fig. 4A). Both MK-801 treated groups showed ataxia over the observation period, but the SUP-MK rats had significantly lower ataxia scores (see Fig. 4B).
Figure 4.
Ataxia scores over a 3-hr observation period (A) and collapsed over time (B) as a function of prenatal dietary condition in rats given a high dose MK-801. The analysis focused on the scores in the left panel of A; scores in the right panel, the second half the observation period, were all close to normal as the drug effects dissipated. Overall, MK-801significnatly increased ataxia in the first half of the observation period and this increase was significantly attenuated in rats treated with prenatal choline supplementation. *p < 0.05 STD-MK-801 versus SUP-MK-801
Quantification of Adult Hippocampal Plasticity
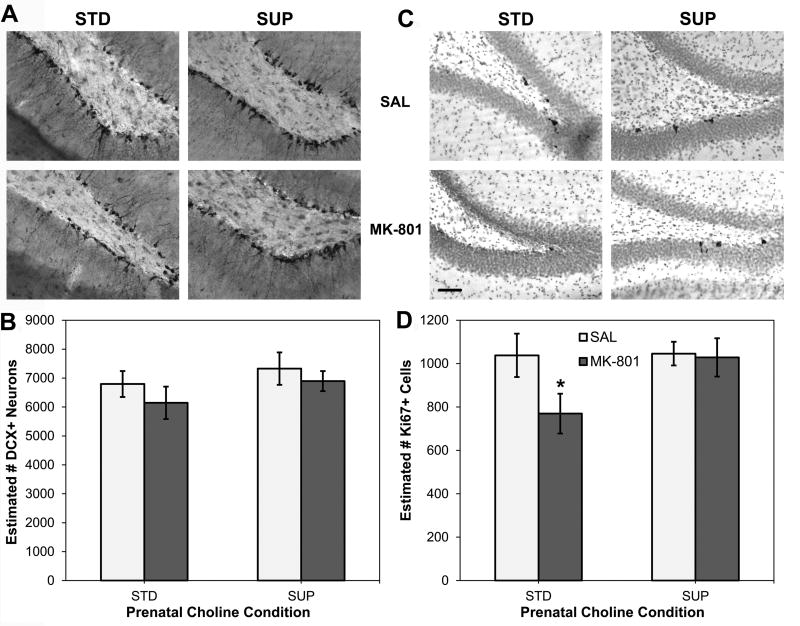
Fig. 5A shows the mean estimates of DCX-labeled neurons in the dentate gyrus calculated to quantify adult hippocampal neurogenesis. A 2×2 ANOVA revealed no significant main effects of Diet (F (1, 33) = 1.706, p > 0.05) or Drug (F (1, 33) = 1.212, p > 0.05), nor was there a significant interaction between Diet and Drug (F < 1). A planned comparison to test the prediction that MK-801 would interrupt normal neurogenic processes failed to detect a statistically significant difference between STD-SAL and STD-MK rats (p > 0.05), accordingly, there was not a discernable event from which to seek protection in the SUP rats. The pattern of Ki67 labeling shown in Fig. 5B aligns with that of DCX labeling shown in Fig. 5A. Here too, neither the main effects of Diet (F (1, 33) = 2.424, p > 0.05), Drug (F (1, 33) = 2.782, p > 0.05), nor their interaction (F (1, 33) = 2.142, p > 0.05) were statistically significant. However, unlike the DCX results, planned comparisons revealed that STD-MK-801 rats had fewer marked cells than STD-SAL rats (t (16) = 1.979, p = 0.032), a difference not detected between SUP-MK-801 and SUP-Sal rats (t (17) = 0.164, p > 0.05). Furthermore, STD-MK-801 rats also had fewer marked cells than SUP-MK-801 rats (t (17) = 2.034, p = 0.029).
Figure 5.
Adult hippocampal neurogenesis and cell proliferation as a function of prenatal dietary condition and high dose MK-801 or saline administration. Photomicrographs of doublecortin (DCX) immunohistochemistry to mark new, immature neurons from representative rats from each condition are shown in (A). There were no significant changes to numbers DCX+ neurons in the dentate gyrus of the hippocampus with either MK-801 administration or prenatal choline supplementation (B). Photomicrographs of Ki67 immunohistochemistry to mark dividing cells from representative rats from each condition are shown in (C). MK-801 administration significantly reduced the numbers of Ki67+ cells in the dentate gyrus of the hippocampus and this effect was prevented by prenatal choline supplementation (D). Photomicrographs are 200× magnification and bar in STD-MK-801 image in (C) represents 25 µm and applies to all images. *p<0.05 versus STD-SAL and SUP-MK-801.
Discussion
The present research sought to investigate the neuroprotective capacity of prenatal choline supplementation against adult MK-801 toxicity. Prenatal choline supplementation was hypothesized to protect against cognitive impairments, motor deficits, and disruptions to hippocampal neurogenesis and cell proliferation in adult male rats. Behaviorally, the early life choline treatment protected the memory consolidation process against an acute low dose of MK-801 as measured by the novelty preference test of object recognition. Additionally, in response to a high dose of MK-801, prenatal choline supplementation prevented locomotor deficits, most evidently in ataxia and general locomotion, and reductions in hippocampal cell proliferation. Taken together, these findings indicate that prenatal choline is neuroprotective against adult MK-801 toxicity. This research helps elucidate discrepancies regarding cognitive and neural outcomes in past studies and also contributes to the growing body of evidence supporting the neuroprotective capacity of choline.
Prenatal choline supplementation prevented MK-801-induced memory deficits
MK-801 administration in rats disrupts memory consolidation (de Lima et al., 2005; van der Staay et al., 2011), and it exerted this effect in the present study as well: when standard-fed rats were given a low dose of MK-801 immediately following a study phase in the novelty preference task, during which they could freely investigate two identical objects, they failed to show memory for those objects in a retention test conducted 3 hours later. It is possible that these effects are the result of MK-801’s adverse actions on the hippocampus, marked in the present study by declines in cell proliferation. However, as object recognition is critically supported by structures outside the hippocampus, in particular, the perirhinal cortex, and it is likely that the effects of MK-801 extend outside the hippocampus. Choline supplementation may also have widespread effects and support for the hypothesis that rats treated with prenatal choline supplementation would be protected from the amnestic properties of MK-801 was obtained: supplemented rats given MK-801 did not have impaired memory for the previously studied objects. This result fits with previous research showing that memory was protected in a rat model of schizophrenia in which rats were prenatally stressed, supplemented with choline in adolescence, and given an MK-801 insult as adults (Corriveau and Glenn, 2012). However, in that study, no conclusion could be drawn regarding whether the choline protected memory deficits induced by prenatal stress, the MK-801 insult, or a combination of the two. In the current study, the effect of MK-801 on memory was isolated and thus at least one way that choline was neuroprotective in the previous study is by buffering rats from MK-801’s toxic effects. That said, the possibility remains that supplemental choline, either administered prenatally, as in the present study, or during adolescence, as in Corriveau and Glenn (2012), is also neuroprotective against the adverse impacts of prenatal stress (e.g. Schulz et al., 2014).
Prenatal choline supplementation attenuated MK-801-induced motor deficits
Chronic or large acute doses of MK-801 cause motor deficits in rats (Andiné et al., 1999). This was undoubtedly supported in the current results: MK-801-treated rats exhibited increased locomotion, stereotypy, and ataxia compared with saline-treated rats. As there were was some evidence that adolescent choline supplementation was neuroprotective against general locomotion impairments in a previous study (Corriveau and Glenn, 2012), prenatal choline supplementation in the present study was hypothesized to prevent these locomotion deficits caused by MK-801. As expected, MK-801 treatment in both diet groups caused increased locomotion compared to the saline-treated groups, and activity levels decreased over time in all groups. In agreement with the hypothesis, standard-fed rats given MK-801 exhibited significantly higher locomotion compared to those treated with saline rats, while both saline- and MK-801-treated supplemented rats demonstrated levels of locomotion that were not significantly different. Therefore, as hypothesized, the prenatal choline supplementation prevented MK-801-induced increases in locomotion. It was also hypothesized that stereotypy would similarly be affected by the diet treatment, however, when stereotypy scores were gathered every 5 minutes over the 3-hour observation period, there was only modest, non-significant attenuation of it in the choline-supplemented rats. To search for a clearer pattern in the data, the average of stereotypy scores gathered from each of the 3 hours were analyzed but only revealed a somewhat more noticeable benefit of the diet. Thus, on this measure, the choline supplementation was not as effective and this may reflect the potency of this outcome of MK-801 toxicity. It is also possible that the stereotypy scale used was not sensitive enough to reveal choline’s effects. While 0 indicated no sniffing and 2 indicated pathological stereotypy, a 1 indicated normal sniffing behavior. Noting normal sniffing behavior appeared to be a distracting measure from the true MK-801-induced behavior of interest. In the future, this scale could be changed to mark either presence or absence of head-weaving (stereotypy) behavior and more gradations of it. This may uncover a clearer result; in the present study we aimed to stay aligned with the scale as it is widely used in the field.
Unlike the modest protections described above, the effect of prenatal choline supplementation on MK-801-induced ataxia was notable. In the rat model of schizophrenia in which rats were prenatally stressed, supplemented with choline in adolescence, and given MK-801 in adulthood, choline-supplemented rats were most protected from the MK-801-induced ataxia observed in the standard-fed rats (Corriveau and Glenn, 2012). Accordingly, choline was expected to prevent MK-801-induced ataxia in the current study as well, especially because the supplementation in the present study occurred prenatally when choline seems to exert greater neuroprotection (Meck et al., 2008; Wong-Goodrich et al., 2008b; Glenn et al., 2012). Also, over the 3-hour observation period in the current study, ataxia was significantly attenuated in the choline-supplemented rats in comparison to those that were standard-fed. This effect was quite robust with marked reductions in ataxia in the early parts of the observation period when the drugs’ effects are particularly potent. As the drugs’ effects dissipated over the 3-hour period, ataxia declined overall, and with it the difference between standard-fed and choline supplemented rats. When considered in the larger context of all the motor behaviors analyzed, the results of the 3-hour observation revealed compelling support for the notion that prenatal choline supplementation leads to organizational changes in neural structure that prevents the toxicity to their function brought on by exposure to a large MK-801 dose in adulthood. In light of MK-801’s properties as an antagonist of NMDA receptors, choline may be exerting changes to their sensitivity, either directly by altering their metabolic properties (Alkondon et al., 1999) or indirectly via acetylcholine neurotransmission. Choline is a selective agonist for the α-7 nicotinic acetylcholine receptor and the abundance of choline during early development may alter the availability of this receptor subtype in adult animals. It is also well documented that prenatal choline supplementation alters the adult properties of cholinergic neurons—leading to larger cell bodies (Williams et al., 1998), along with greater choline stores and increased acetylcholine production and release (Cermak et al., 1998; Blusztajn, 1998). Increases in acetylcholine function is likely to have widespread effects on hippocampal circuitry, including GABAergic inhibitory interneurons (Griguoli and Cherubini, 2012). These hippocampal interneurons are innervated by cholinergic neurons originating in the septum and contain nicotinic and muscarinic receptors. By altering their overall activity and tone, the introduction of the potent dose of an NMDA antagonist may be, at least partially, counteracted. NMDA receptor blockade induces a number of effects that lead to excitotoxicity and enhance GABAergic tone in this system through enhanced cholinergic activity may be a potential mechanism through which early life choline is protective against neurotoxins later in life. These hypotheses are fruitful targets for future study.
Prenatal choline supplementation prevented MK-801-induced declines hippocampal plasticity
A potential neural correlate of the behavioral indices used in the present study was the extent to which MK-801 may affect features of adult hippocampal neurogenesis (Arvidsson et al., 2001; but see Nacher et al., 2003). In the present study, hippocampal neurogenesis and, more generally, cell proliferation were viewed as markers of neural plasticity, a process that supports learning and memory. Accordingly, MK-801 was hypothesized to reduce neurogenesis in the hippocampus to match the hypothesized MK-801-induced memory deficits. However, contrary to our expectations, there was no significant difference in the number of new neurons, as assessed by DCX, between the saline- and MK-801-treated rats. However, the findings from the cell proliferation marker, Ki67 did support our hypothesis: among standard-fed rats, fewer dividing cells were detected in those given MK-801 compared to those given saline injections. This effect may be linked to the motor deficits observed by indirectly affecting neural function in associated areas, such as the striatum. Hippocampal-striatal interactions are integral in a number of motor output patterns and long-term alterations to the hippocampal circuitry through enhanced addition of new, excitable neurons could affect their interplay. It should be noted that not all newly proliferated cells will survive and give rise to new functional neurons, and further work would help clarify the extent to which MK-801-induced decreases in the pool of newly divided cells affects the numbers of surviving neurons. The modest increase in the sensitivity to MK-801 of Ki67, compared to DCX, is not surprising giving the drug-assay window. DCX is marking immature neurons with a window of approximately 2 weeks (Brown et al., 2003) and rats in the present study were sacrificed 3 days after MK-801 administration. Thus, even if MK-801 affected neuron birth and differentiation it might be hard to detect with DCX’s broad window. Ki67, by contrast, is a momentary indicator of cell division at the time of death and thus better captures the effects of MK-801 in the 3-day window of interest. In fact, it is possible that the effects of the large dose of MK-801 on cell proliferation, marked by Ki67, may have been larger closer to the time of injection and future studies could address this. Another key way to further study these effects of MK-801 would be through the use of the exogenous cell division marker, BrdU; the timing of this marker is under the experimenter’s control and can provide more information about the timing cell birth and survival.
Past research indicates that dietary supplementation with choline increases cell proliferation and hippocampal neurogenesis (Glenn et al., 2007; 2008; McCall et al., 2015) and accordingly the prenatal choline supplementation of the current study was expected to combat reductions in these markers caused by MK-801 in adult rats. In accordance with this hypothesis, the current investigation revealed that choline-supplemented rats given MK-801 had significantly more newly-divided cells in the dentate gyrus compared to standard-fed rats given MK-801. These results support the hypothesis that augmenting hippocampal plasticity is one mechanism by which supplemental choline may act to enhance memory under normal conditions and protect memory under disordered conditions. Interestingly, though choline-supplemented saline-treated rats tended to exhibit more marked neurons and cells than standard-fed saline-treated rats, these differences were not statistically significant and are in contrast to previously reported findings (Glenn et al., 2007; 2008; McCall et al., 2015). Several differences between past work and the current study may have contributed to this result. The rats in the present study were approximately 4 months old at the time of sacrifice, while rats in previous studies were quite a bit older: over 7 months old at the time of sacrifice in Glenn et al., 2007, and over 24 months old in Glenn et al., 2008. Both rates of cell proliferation and neurogenesis in the hippocampus decline with age (e.g. Kuhn et al., 1996) and it may be that choline works to maintain levels at a higher rate through this downward slope. Accordingly, aged rats (24 months old) that were treated prenatally with choline supplementation have enhanced cell proliferation compared to standard-fed rats of the same age (Glenn et al., 2008). Given the already high rates of neurogenesis in young rats then, choline may do little earlier in life to induce further increases, which could, in fact, be detrimental to normal hippocampal function. However, once rates and overall numbers decline, the evidence of choline’s effects is apparent (Glenn et al., 2007; 2008). It should be noted that the present lack of an effect of choline on neurogenesis does stand in contrast to the report of increased neurogenesis by McCall et al., 2015. In that study, rats were of a similar age, however choline was administered differently—in water, and from weaning through to the end of the study. Additionally, rats in that study were assessed for emotional behaviors and underwent a battery of anxiety-provoking tests. These stressful experiences may have reduced overall levels of neurogenesis in control groups, allowing the beneficial effects of choline on this measure to be detected. Also, the overall magnitude of the effect in McCall et al., 2015 was generally more modest than previous reports (Glenn et al., 2007), which, again, may be due to differences in the age of the rats. Experiments designed to specifically target these discrepancies is clearly warranted but, in general, they do not detract from the neuroprotective findings of the present report.
Conclusion
The findings of the current investigation reveal that prenatal choline supplementation is protective against a variety of behavioral and neural changes induced following adult MK-801 toxicity. Regarding behavior, adult rats that were prenatally choline supplemented, unlike adult rats that were prenatally standard fed, did not show any adverse effects of MK-801 on memory consolidation and motor function, especially in locomotion and ataxia. Within the brain, developmental choline supplementation protected against reductions in hippocampal cell proliferation. These results contribute to the growing evidence in support of the broad neuroprotective capacity of choline and help elucidate discrepancies regarding neural outcomes from previous work. Given the present focus on the adult MK-801 toxicity component in rodent schizophrenia models (Corriveau and Glenn, 2012) as a viable target of choline neuroprotection, future research investigating long-term exposure and withdrawal models is warranted. This will provide further insights into the ways early life choline’s organizational impact on the developing brain intersects with glutamate-based hypothesis of schizophrenia. This investigation and other research exploring the protective ability of choline demonstrate that a mere modification of diet may prove a viable, safer alternative to drug treatment in the face of behavioral impairments and neural pathologies observed in numerous psychological disorders.
Research Highlights.
Low dose MK-801 impaired memory consolidation for objects in adult male rats
Adult MK-801 memory deficits were prevented by prenatal choline supplementation
High dose MK-801 impaired motor function and hippocampal cell proliferation
Adult MK-801 motor deficits were prevented by prenatal choline supplementation
Adult MK-801 hippocampal effects were prevented by prenatal choline supplementation
Acknowledgments
The authors would like to thank Amanda Kimball for her technical assistance and Sarah Steimel for her editorial assistance. This project was supported by a grant to MJG from the National Center for Research Resources (5P20RR016463-12) and the National Institute of General Medical Sciences (8P20GM103423-12) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Åhlander M, Misane I, Schött PA, Ögren SO. A behavioral analysis of the spatial learning deficit induced by the NMDA receptor antagonist MK-801 (dizocilpine) in the rat. Neuropsychopharmacology. 1999;21:414–426. doi: 10.1016/S0893-133X(98)00116-X. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Choline and selective antagonists identify two subtypes of nicotinic acetylcholine receptors that modulate GABA release from CA1 interneurons in rat hippocampal slices. J Neurosci. 1999;19:2693–2705. doi: 10.1523/JNEUROSCI.19-07-02693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andiné P, Widermark N, Axelsson R, Nyberg G, Olofsson U, Mårtensson E, Sandberg M. Characterization of MK-801-induced behavior as a putative rat model of psychosis. J Pharmacol Exp Ther. 1999;290:1393–1408. [PubMed] [Google Scholar]
- Arvidsson A, Kokaia Z, Lindvall O. N-methyl-D-aspartate receptor-mediated increase of neurogenesis in adult rat dentate gyrus following stroke. Eur J Neurosci. 2001;14:10–18. doi: 10.1046/j.0953-816x.2001.01611.x. [DOI] [PubMed] [Google Scholar]
- Blusztajn JK. Choline, a vital amine. Science. 1998;281:794–795. doi: 10.1126/science.281.5378.794. [DOI] [PubMed] [Google Scholar]
- Blusztajin JK, Mellott TJ. Neuroprotective actions of perinatal choline nutrition. Clin Chem Lab Med. 2013;51:591–599. doi: 10.1515/cclm-2012-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blusztajn JK, Wurtman RJ. Choline and cholinergic neurons. Science. 1983;221:614–620. doi: 10.1126/science.6867732. [DOI] [PubMed] [Google Scholar]
- Blusztajn JK, Cermak JM, Holler T, Jackson DA. Imprinting of hippocampal metabolism of choline by its availability during gestation: Implications for cholinergic neurotransmission. J Physiol. 1998;92:199–203. doi: 10.1016/s0928-4257(98)80010-7. [DOI] [PubMed] [Google Scholar]
- Blusztajn JK, Liscovitch M, Richardson UI. Synthesis of acetylcholine from choline derived from phosphatidylcholine in a human neuronal cell line. Proc Natl Acad Sci USA. 1987;84:5474–5477. doi: 10.1073/pnas.84.15.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JP, Couillard-Després S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Cermak JM, Holler T, Jackson DA, Blusztajn JK. Prenatal availability of choline modifies development of the hippocampal cholinergic system. FASEB J. 1998;12:349–357. doi: 10.1096/fasebj.12.3.349. [DOI] [PubMed] [Google Scholar]
- Corriveau JA, Glenn MJ. Postnatal choline levels mediate cognitive deficits in a rat model of schizophrenia. Pharmacol Biochem Behav. 2012;103:60–68. doi: 10.1016/j.pbb.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima MNM, Laranja DC, Bromberg E, Roesler R, Schröder N. Pre- or post-training administration of the NMDA receptor blocker MK-801 impairs object recognition memory in rats. Behav Brain Res. 2005;156:139–143. doi: 10.1016/j.bbr.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Dix SL, Aggleton JP. Extending the spontaneous preference test of recognition: Evidence of object-location and object-context recognition. Behav Brain Res. 1999;99:191–200. doi: 10.1016/s0166-4328(98)00079-5. [DOI] [PubMed] [Google Scholar]
- Glenn MJ, Adams RS, McClurg M. Supplemental dietary choline during development exerts antidepressant-like effects in adult female rats. Brain Res. 2012;1443:52–63. doi: 10.1016/j.brainres.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn MJ, Gibson EM, Kirby ED, Mellott TJ, Blusztajn JK, Williams CL. Prenatal choline availability modulates hippocampal neurogenesis and neurogenic responses to enriching experiences in adult female rats. Eur J Neurosci. 2007;25:2473–2482. doi: 10.1111/j.1460-9568.2007.05505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn MJ, Kirby ED, Gibson EM, Wong-Goodrich SJ, Mellott TJ, Blusztajn JK, Williams CL. Age-related declines in exploratory behavior and markers of hippocampal plasticity are attenuated by prenatal choline supplementation in rats. Brain Res. 2008;1237:110–123. doi: 10.1016/j.brainres.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griguoli M, Cherubini E. Regulation of hippocampal inhibitory circuits by nicotinic acetylcholine receptors. J Physiol. 2012;590:655–666. doi: 10.1113/jphysiol.2011.220095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo-Ross SX, Clark S, Montoya DAC, Jones KH, Obernier J, Shetty AK, White AM, Blusztajn JK, Wilson WA, Swartzwelder HS. Prenatal choline supplementation protects against postnatal neurotoxicity. J Neurosci. 2002;22:1–6. doi: 10.1523/JNEUROSCI.22-01-j0005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo-Ross SX, Jones KH, Shetty AK, Wilson WA, Swartzwelder HS. Prenatal dietary choline availability alters postnatal neurotoxic vulnerability in the adult rat. Neurosci Lett. 2003;341:161–163. doi: 10.1016/s0304-3940(03)00119-8. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Bechmann I, Naftolin F, Kalra SP, Leranth C. Heterogeneity in the neuropeptide Y-containing neurons of the rat arcuate nucleus: GABAergic and non-GABAergic subpopulations. Brain Res. 1997;756:283–286. doi: 10.1016/s0006-8993(97)00184-4. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vöckler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- Jensen HH, Batres-Marquez SP, Carriquiry A, Schalinske KL. Choline in the diets of the US population: NHANES, 2003–2004. FASEB J. 2007;21:219. [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D, von Haebler D, Winter C, Juckel G, Heinemann U. A single application of MK-801 causes symptoms of acute psychosis, deficits in spatial memory, and impairment of synaptic plasticity in rats. Hippocampus. 2008;18:125–134. doi: 10.1002/hipo.20367. [DOI] [PubMed] [Google Scholar]
- McCall N, Mahadevia D, Corriveau JA, Glenn MJ. Adult emotionality and neural plasticity as a function of adolescent nutrient supplementation in male rats. Pharmacol Biochem Behav. 2015;132:125–135. doi: 10.1016/j.pbb.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: Implications for memory and attentional processing across the lifespan. Neurosci Biobehav Rev. 2003;27:385–399. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL, Cermak JM, Blusztajn JK. Developmental periods of choline sensitivity provide an ontogenetic mechanism for regulating memory capacity and age-related dementia. Front Integr Neurosci. 2008;1:1–11. doi: 10.3389/neuro.07.007.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki N, Takagi N, Kurokawa K, Kawai T, Besshoh S, Tanonaka K, Takeo S. Effect of NMDA receptor antagonist on proliferation of neurospheres from embryonic brain. Neurosci Lett. 2007;417:143–148. doi: 10.1016/j.neulet.2007.02.066. [DOI] [PubMed] [Google Scholar]
- Mouton PR, Long JM, Lei DL, Howard V, Jucker M, Calhoun ME, Ingram DK. Age and gender effects on microglia and astrocyte numbers in brains of mice. Brain Res. 2002;956:30–35. doi: 10.1016/s0006-8993(02)03475-3. [DOI] [PubMed] [Google Scholar]
- Nacher J, McEwan BS. The role of N-methyl-D-aspartate receptors in neurogenesis. Hippocampus. 2006;16:267–270. doi: 10.1002/hipo.20160. [DOI] [PubMed] [Google Scholar]
- Nacher J, Alonso-Llosa G, Rosell DR, McEwen BS. NMDA receptor antagonist treatment increases the production of new neurons in the aged rat hippocampus. Neurobiol Aging. 2003;24:273–284. doi: 10.1016/s0197-4580(02)00096-9. [DOI] [PubMed] [Google Scholar]
- Nag N, Berger-Sweeney JE. Postnatal dietary choline supplementation alters behavior in a mouse model of Rett syndrome. Neurobiol of Dis. 2007;26:473–480. doi: 10.1016/j.nbd.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Nag N, Moriuchi JM, Peitzman CG, Ward BC, Kolodny NH, Berger-Sweeney JE. Environmental enrichment alters locomotor behaviour and ventricular volume in Mecp2 1lox mice. Behav Brain Res. 2009;196:44–48. doi: 10.1016/j.bbr.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Niculescu MD, Zeisel SH. Diet, methyl donors and DNA methylation: interactions between dietary folate, methionine and choline. Journal Nutr. 2002;132:2333S–2335S. doi: 10.1093/jn/132.8.2333S. [DOI] [PubMed] [Google Scholar]
- Petrus DS, Fabel K, Kronenberg G, Winter C, Steiner B, Kempermann G. NMDA and benzodiazepine receptors have synergistic and antagonistic effects on precursor cells in adult hippocampal neurogenesis. Eur J Neurosci. 2009;29:244–252. doi: 10.1111/j.1460-9568.2008.06579.x. [DOI] [PubMed] [Google Scholar]
- Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19:234–246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- Schneider RD, Thomas JD. Adolescent choline supplementation attenuates working memory deficits in rats exposed to alcohol during the third trimester equivalent. Alcohol Clin Exp Res. 2016;40:897–905. doi: 10.1111/acer.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Pearson JN, Gasparrini ME, Brooks KF, Drake-Frazier C, Zajkowski ME, Kreisler AD, Adams CE, Leonard S, Stevens KE. Dietary choline supplementation to dams during pregnancy and lactation mitigates the effects of in utero stress exposure on adult anxiety-related behaviors. Behav Brain Res. 2014;268:104–110. doi: 10.1016/j.bbr.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw GM, Carmichael SL, Laurent C, Rasmussen SA. Maternal nutrient intakes and risk of orofacial clefts. Epidemiol. 2006;17:285–291. doi: 10.1097/01.ede.0000208348.30012.35. [DOI] [PubMed] [Google Scholar]
- Shaw GM, Carmichael SL, Yang W, Selvin S, Schaffer DM. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am Journal Epidemiol. 2004;160:102–109. doi: 10.1093/aje/kwh187. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Adams CE, Yonchek J, Hickel C, Danielson J, Kisley MA. Permanent improvement in deficient sensory inhibition in DBA/2 mice with increased perinatal choline. Psychopharmacology. 2008;198:413–420. doi: 10.1007/s00213-008-1170-3. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Choo KS, Stitzel JA, Marks MJ, Adams CE. Long-term improvements in sensory inhibition with gestational choline supplementation linked to α7 nicotinic receptors through studies in Chrna7 null mutation mice. Brain Res. 2014;1552:26–33. doi: 10.1016/j.brainres.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strupp BJ, Powers BE, Velazquez R, Ash JA, Kelley CM, Alldred MJ, Strawderman M, Caudill MA, Mufson EJ, Ginsberg SD. Maternal choline supplementation: A potential prenatal treatment for Down Syndrome and Alzheimer’s Disease. Curr Alzheimers Res. 2016;13:97–106. doi: 10.2174/1567205012666150921100311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, Biane JS, O’Bryan KA, O’Neill TM, Dominguez HD. Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behav Neurosci. 2007;121:120–130. doi: 10.1037/0735-7044.121.1.120. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Leany BD, Riley EP. Differential vulnerability to motor deficits in second replicate HAS and LAS rats following neonatal alcohol exposure. Pharmacol Biochem Behav. 2003;75:17–24. doi: 10.1016/s0091-3057(03)00031-5. [DOI] [PubMed] [Google Scholar]
- Tricklebank MD, Singh L, Oles RJ, Preston C, Iversen SD. The behavioural effects of MK-801: A comparison with antagonists acting non-competitively and competitively at the NMDA receptor. Eur J Pharmacol. 1989;167:127–135. doi: 10.1016/0014-2999(89)90754-1. [DOI] [PubMed] [Google Scholar]
- van der Staay FJ, Rutten K, Erb C, Blokland A. Effects of the cognition impairer MK-801 on learning and memory in mice and rats. Behav Brain Res. 2011;220:215–229. doi: 10.1016/j.bbr.2011.01.052. [DOI] [PubMed] [Google Scholar]
- Vardigan JD, Huszar SL, McNaughton CH, Hutson PH, Uslaner JM. MK-801 produces a deficit in sucrose preference that is reversed by clozapine, D-serine, and the metabotropic glutamate 5 receptor positive allosteric modulator CDPPB: Relevance to negative symptoms associated with schizophrenia? Pharmacol Biochem Behav. 2010;95:223–229. doi: 10.1016/j.pbb.2010.01.010. [DOI] [PubMed] [Google Scholar]
- West MJ. New stereological methods for counting neurons. Neurobiol Aging. 1993;14:275–285. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- West MJ. Stereological methods for estimating the total number of neurons and synapses: Issues of precision and bias. TINS. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- Williams CL, Meck WH, Heyer DD, Loy R. Hypertrophy of basal forebrain neurons and enhanced visuospatial memory in perinatally choline-supplemented rats. Brain Res. 1998;794:225–238. doi: 10.1016/s0006-8993(98)00229-7. [DOI] [PubMed] [Google Scholar]
- Wong EHF, Kemp JA, Priestley T, Knight AR, Woodruff GN, Iversen LL. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. PNAS. 1986;83:7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Goodrich SJE, Mellott TJ, Glenn MJ, Blusztajn JK, Williams CL. Prenatal choline supplementation attenuates neuropathological response to status epilepticus in the adult rat hippocampus. Neurobiol Dis. 2008a;30:255–369. doi: 10.1016/j.nbd.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Goodrich SJE, Glenn MJ, Mellot TJ, Blusztajn JK, Meck WH, Williams CL. Spatial memory and hippocampal plasticity are differentially sensitive to the availability of choline in adulthood as a function of choline supply in utero. Brain Res. 2008b;1237:153–166. doi: 10.1016/j.brainres.2008.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH. Choline: Critical role during fetal development and dietary requirements in adults. Annu Rev Nutr. 2006;26:229–250. doi: 10.1146/annurev.nutr.26.061505.111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH. Nutritional genomics: Defining the dietary requirement and effects of choline. J Nutr. 2011;141:531–534. doi: 10.3945/jn.110.130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH, Blusztajn JK. Choline and human nutrition. Annu Rev Nutr. 1994;14:269–296. doi: 10.1146/annurev.nu.14.070194.001413. [DOI] [PubMed] [Google Scholar]
- Zeisel SH, Caudill MA. Choline. Adv Nutr. 2010;1:46–48. doi: 10.1093/advances/nmx004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH, Mar MH, Hower JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J Nutr. 2003;133:1302–1307. doi: 10.1093/jn/133.5.1302. [DOI] [PubMed] [Google Scholar]