Abstract
Purpose
Pathologic complete response to neoadjuvant chemoradiation therapy (CRT) is associated with improved outcomes for patients with locally advanced rectal cancer (LARC). Increased response rates have been reported with higher radiation doses, but these studies often lack long-term outcome and/or toxicity data. We conducted a case-control analysis of patients with LARC who underwent definitive CRT to determine the efficacy and safety of intensified treatment with a concomitant boost (CB) approach.
Methods and materials
From 1995 to 2003, a phase 2 protocol examined CRT with 5-fluorouracil and CB radiation therapy (52.5 Gy in 5 weeks) for patients with LARC. Seventy-six protocol patients were matched (case-control approach) for surgery type, tumor (T) stage, and clinical nodal (N) stage with patients who received standard dose (SD) CRT (5-fluorouracil, 45 Gy). A chart review was performed. McNemar's test and Kaplan-Meier analyses were used for statistical analysis.
Results
The SD and CB groups did not differ in tumor circumferential involvement and length, but the tumors of CB patients were closer to the anal verge (4.7 vs 5.7 cm; P = .02). Although tumor downstaging was higher in the CB cohort (76% vs 51%; P < .01), pathologic complete response rates did not differ (CB, 17.1% vs SD, 15.8%, P = 1.00). The incidence of grade ≥3 radiation-related toxicities was low and similar in both groups (CB, 10% vs SD, 3%, P = .22). Postoperative (anastomotic leak, wound complications/abscess, bleeding) and late (small bowel obstruction, stricture) complication rates did not differ between the groups (P > .05). The median follow-up was 11.9 years. The 5-year local control rates were higher for CB (100.0%) compared with SD (90.0%) patients (P = .01). CB patients had higher rates of 10-year progression-free survival (71.9% vs 57.6%, P < .01) and overall survival (71.6% vs 62.4%, P = .01) compared with SD patients.
Conclusions
CRT dose escalation for patients with LARC is safe and effective. The improved T-downstaging and local control observed in CB patients should encourage further dose escalation studies.
Summary.
Patients with locally advanced rectal cancer (LARC) who achieve complete pathologic response after neoadjuvant chemoradiation experience improved outcomes. Radiation dose escalation has been shown to improve the rates of tumor response. We conducted a case-control study of patients with LARC who received standard dose or concomitant boost chemoradiation prior to surgical resection. Boost patients had improved tumor downstaging and local control without increases in early or late toxicity, including surgical complications.
Introduction
Standard-of-care treatment for locally advanced rectal cancer (LARC) includes neoadjuvant chemoradiation therapy (CRT) followed by total mesorectal excision. This approach is based on randomized prospective data that show both local control and sphincter preservation benefits.1 However, even with this validated treatment regimen, only approximately 15% of patients benefit from a pathologic complete response (pCR) at the time of surgery, and a significant proportion of lesions fail to be downstaged by treatment.2, 3 Because achievement of a pCR has been shown to confer a survival benefit in patients with LARC, there is obvious interest in improving the outcomes of preoperative therapy.3
Recently, investigations in rectal cancer treatment have focused on organ preservation strategies because surgical resection can be accompanied by significant morbidity for the patient. Several studies have reported patient outcomes for CRT followed by either local excision only or observation with careful follow-up.4, 5, 6 Although randomized data are limited, the results of these studies are encouraging, and local control rates for tumors with excellent response to CRT were comparable to surgical outcomes (0%-23%).5 These regimens have the potential to improve the quality of life of patients, but they must also provide safe oncologic outcomes. Patients who are recommended for these alternative regimens must demonstrate an excellent clinical response to neoadjuvant CRT.
To achieve this, other researchers have investigated radiation therapy dose escalation using both external beam radiation therapy (EBRT) and brachytherapy techniques.7, 8, 9, 10, 11, 12, 13, 14, 15 A dose-response relationship for rectal cancer has been confirmed, and patients receiving boost doses have demonstrated increased rates of tumor response with acceptable rates of early toxicity (gastrointestinal complaints, dermatitis, leukopenia/neutropenia, pain).16 Escalated radiation doses leading to the possibility of less morbid surgical procedures must also result in reasonable late toxicities because these complications also have the potential to significantly decrease a patient's quality of life. However, long-term reports of toxicity associated with high-dose radiation therapy are not often available.
We sought to explore the potential relationship between escalated dose regimens during neoadjuvant CRT and increased rates of tumor response while carefully considering any increase in radiation-related toxicity or surgical complications. To accomplish this, we conducted a case-control analysis of patients with LARC treated with neoadjuvant CRT, with and without a concomitant boost (CB). We performed long-term follow-up of these patients to evaluate outcomes and identify any possible late toxicities associated with boost treatment.
Methods and materials
Patient selection
From 1995 to 2003, 78 patients were treated with neoadjuvant CRT with continuous infusion 5-fluorouracil (5-FU) 300 mg/m2 on the days of radiation and 52.5 Gy radiation in 5 weeks given as 45 Gy at 1.8 Gy per fraction to the pelvis and an additional 1.5 Gy per fraction given as a second daily dose to the tumor with 2 to 3 cm margins in the fifth week of treatment (ie, CB). Forty-five of these patients were enrolled on an institutional review board–approved phase 2 institutional protocol that examined the feasibility of adding a CB to neoadjuvant CRT.17 In the same time period, 137 patients were treated off-protocol with neoadjuvant CRT without a CB (standard dose [SD], 45 Gy in 5 weeks). Each patient in the CB group was matched for type of surgery performed, tumor stage (T-stage), and clinical nodal metastases (N-stage) with a patient from the SD group using a computerized shuffling algorithm with a random number generator. Seventy-six pairs of patients were generated in this manner. For all 152 patients, a chart review was performed to determine clinicopathologic parameters and treatment outcomes.
Treatment
All patients received neoadjuvant CRT followed by surgical resection. Protocol patients in the CB group received a total dose of 52.5 Gy over 5 weeks. They were administered 45 Gy in 25 fractions to the pelvis, typically with 18 mV photons at 1.8 Gy per fraction daily with a 3-field belly board technique. The treatment fields were typically weighted 2:1:1 for the posterior, right lateral, and left lateral fields, respectively, with 45-degree wedges on the lateral fields. During the last week of radiation therapy, patients were given a CB to the tumor with a 2 to 3 cm margin. This boost dose totaled 7.5 Gy over 5 fractions at 1.5 Gy/d and was delivered as a second daily fraction with a 6-hour interfraction interval. The matched pair patients (SD) were treated to a total dose of 45 Gy in 25 fractions, delivered at 1.8 Gy/d over 5 weeks. These patients did not receive a boost. All other therapy details were identical to the protocol patients. All patients received continuous venous infusion 5-FU at 300 mg/m2 on the days of radiation. Surgical resection was performed approximately 6 weeks after completion of CRT. The type of operative procedures included low anterior resection, abdominoperineal resection, and proctectomy with coloanal anastomosis.
Follow-up
Follow-up evaluations included a physical examination with carcinoembryonic antigen measurement every 3 to 6 months with proctoscopy and cross-sectional imaging every 6 to 12 months for 5 years. Recurrence was defined as local (within the colon near the anastomosis), regional (within the pelvis), or distant (outside of the pelvis).
Study endpoints
The primary endpoint of our study was efficacy of the CB approach, including both early (tumor downstaging and pCR rates) and late efficacy (local control rates). Secondary endpoints included toxicity and complication rates.
Statistical analysis
The clinicopathologic parameters of the 2 groups were compared using the Student t or χ2 test, as appropriate. Categorical data were summarized by frequency, and McNemar's test was used to determine differences in clinicopathologic factors and toxicities between matched pairs. Time-to-event analyses (time to local, regional, and distant recurrence, progression-free survival [PFS], and overall survival [OS]), calculated from the date of diagnosis to time of recurrence or death, were assessed by the Kaplan-Meier method. Patients who were alive at last follow-up and those lost to follow-up were censored. The log-rank test was used to compare groups. Effects were considered statistically significant at P < .05. All statistical analyses were performed using statistical software (JMP Pro, Version 11, SAS Institute, Cary, NC).
Toxicity analysis
Toxicities and complications were assessed in a subset of pairs with complete toxicity data available. A radiation oncologist (J.R.G.) abstracted radiation toxicity information from the medical record and used the Common Terminology Criteria for Adverse Events, Version 4.0 for grading. Two surgical oncologists (K.V.K. and I.J.P.) abstracted all surgical complication information that was reported in the medical record. Toxicities that were clearly secondary to disease progression were not reported (eg, bowel obstruction secondary to carcinomatosis).
Results
Clinical and pathologic characteristics
Clinical characteristics of the CB and SD cohorts were well matched by type of surgery performed, T-stage, and N-stage (P = 1.0 for all 3 variables; Table 1). In addition to the selection matching criteria, there were no significant differences between patients who received SD treatment versus those who received a CB in terms of age (57.1 vs 55.9 years, P = .54), male sex (64.5% vs 65.8%, P = .86), or race/ethnicity (P = .17). Neither percentage of circumferential involvement (54.4% vs 58.4%, P = .41) nor tumor length (5.1 vs 5.1 cm, P = .97) differed between the groups (information available only for a subset of patients). However, the CB patient tumors were located closer to the anal verge (CB, 4.7 cm vs SD, 5.7 cm, P = .02). The time interval between radiation and surgery (P = .67) and the duration of CRT (P = .80) did not differ between the groups.
Table 1.
Clinical characteristics of matched patient pairs
| Variable | Standard Dose (n = 76) | Concomitant Boost (n = 76) | P-value |
|---|---|---|---|
| Age (y) | 57.1±13.0 | 55.9±11.7 | .54 |
| Sex (male) | 49 (64.5%) | 50 (65.8%) | .86 |
| Ethnicity/Race (Caucasian) | 62 (81.6%) | 68 (89.5%) | .17 |
| T Stage | Matched | ||
| T2 | 2 (2.6%) | 2 (2.6%) | |
| T3 | 71 (93.4%) | 71 (93.4%) | |
| T4 | 3 (4.0%) | 3 (4.0%) | |
| N Stage | Matched | ||
| N0 | 42 (55.3%) | 42 (55.3%) | |
| N1 | 34 (44.7%) | 34 (44.7%) | |
| AJCC Stage | Matched | ||
| I (T1-T2, N0, M0) | 1 (1.3%) | 1 (1.3%) | |
| IIA (T3, N0, M0) | 39 (51.3%) | 39 (51.3%) | |
| IIB (T4, N0, M0) | 2 (2.6%) | 2 (2.6%) | |
| IIIA (T1-T2, N1, M0) | 1 (1.3%) | 1 (1.3%) | |
| IIIB (T3-T4, N1, M0) | 33 (43.4%) | 33 (43.4%) | |
| Operative procedure | |||
| Low anterior resection | 19 (25.0%) | 19 (25.0%) | |
| Abdominoperineal resection | 21 (27.6%) | 21 (27.6%) | 1 |
| Proctectomy with coloanal anastomosis | 36 (47.4%) | 36 (47.4%) | |
| Distance from anal verge (cm) | 5.7 ± 3.1 | 4.7 ± 2.5 | .02 |
| Circumferential involvement (%) | 54.4 ± 20.5 (n = 41) | 58.4 ± 22.2 (n = 36) | .41 |
| Tumor length (cm) | 5.1 ± 2.6 (n = 45) | 5.1 ± 2.0 (n = 54) | .97 |
| Time from radiation therapy to surgery | 48 (24-83) | 42 (24-256) | .67 |
| Duration of chemoradiation therapy (d), (range) | 35 (22-42) | 35 (31-42) | .80 |
AJCC, American Joint Committee on Cancer.
Pathologic outcomes
The rate of tumor downstaging was substantially improved in the CB cohort, with 76% of patients found to have a reduction in T-stage versus 51% of patients in the SD arm (odds ratio, 2.9; confidence interval, 1.4-6.7; P < .01; Table 2). However, the rates of pCR did not differ significantly (CB, 17.1% vs SD, 15.8%; P = 1.00). Other pathologic variables, including lymphovascular space invasion (P = 1.00), perineural invasion (P = .22), and radial resection margin (P = .22) did not differ between the cases and controls. The number of pathologically evaluated and pathologically positive lymph nodes did not differ between the CB and SD arms (P = .13 and P =.74, respectively).
Table 2.
Pathologic factors of matched pairs
| Parameter (n = 76 pairs, else specified) | CB/Yes SD/Noa |
CB/No SD/Yes |
CB/Yes SD/Yes |
CB/No SD/No |
Odds Ratio (CI) | P-value |
|---|---|---|---|---|---|---|
| Tumor downstaging | 29 | 10 | 29 | 8 | 2.9 (1.4-6.7) | .004 |
| pCR | 6 | 5 | 7 | 58 | 1.2 (0.3-5.0) | 1 |
| LVSI | 4 | 3 | 1 | 68 | 1.3 (0.2-9.1) | 1.00 |
| PNI | 1 | 5 | 1 | 69 | 0.2 (0.0-1.8) | .22 |
| Radial resection margin | 12 | 20 | 3 | 26 | 0.6 (0.3-1.3) | .22 |
CB, concomitant boost; CI, confidence interval; LVSI, lymphovascular space invasion; pCR, pathologic complete response; PNI, perineural invasion; SD, standard dose.
Yes means patient experienced an outcome; no means patient did not experience an outcome.
Survival outcomes
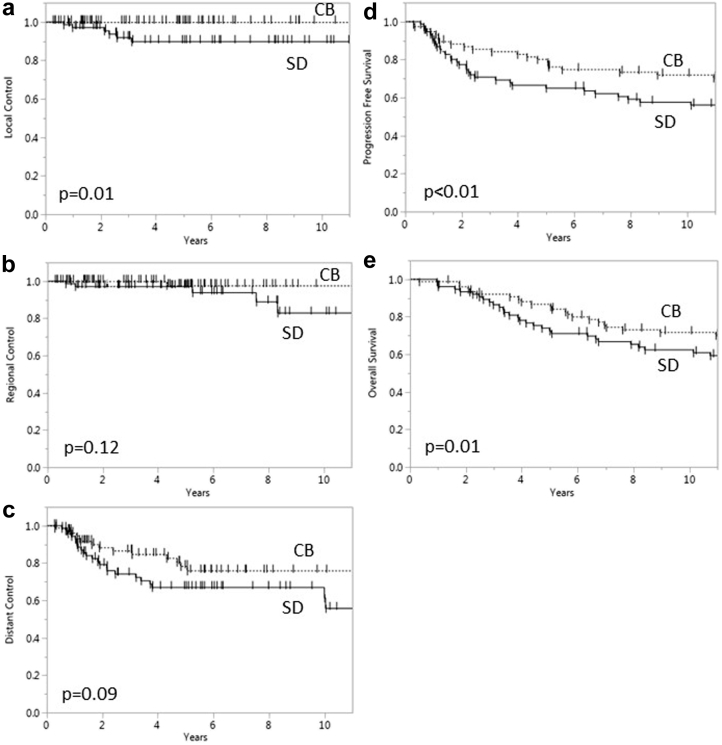
The median clinical follow-up for the entire cohort was 11.9 years (cases, 13.5 years; controls, 10.8 years), and radiographic follow-up was 5.0 years (cases, 5.2 years; controls, 5.0 years). Figure 1 depicts the local, regional, and distant control rates and the PFS and OS rates for the CB and SD patients. The 5-year local control rates were significantly higher for CB patients (100.0%) compared with SD patients (90.0%; P = .01). CB patients also showed a trend toward higher 5 and 10 year regional control rates compared with SD patients (97.7% and 97.7% vs 97.1% and 82.9%, respectively; P = .12 for both) at 5 and 10 years, respectively. Similarly, there was a trend toward improved 5-year distant control rates (78.2% vs 67%; P = .09) for the CB patients compared with SD patients. CB patients had significantly higher 10-year PFS and OS rates compared with SD patients (71.9% and 71.6% vs 57.6% and 62.4%, respectively; P < .01 and P = .01). Notably, all patients received 5-FU as adjuvant therapy except for one patient who received capecitabine and one who received 5-FU and cisplatin.
Figure 1.
Local control (a), regional control (b), distant control (c), progression-free survival (d), and overall survival (e) of patients treated with concomitant boost and standard dose radiation therapy.
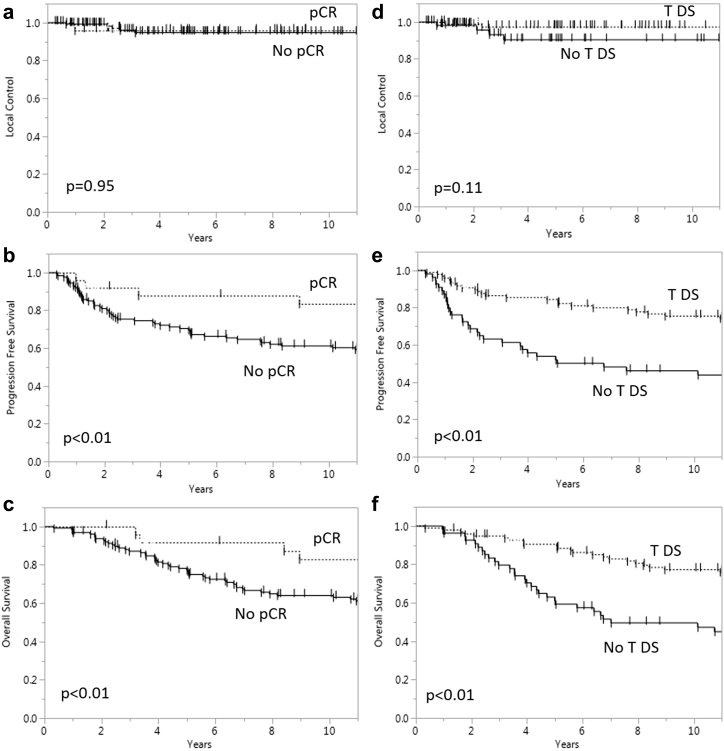
Figure 2 compares the local control, PFS, and OS of patients with and without pCR and with or without T-downstaging. Patients with pCR had significantly higher rates of PFS (P < .01) and OS (P < .01) compared with those without pCR. Similarly, patients with T-downstaging had higher PFS (P < .01) and OS rates (P < .01) compared with patients without T-downstaging.
Figure 2.
Local control (a), progression-free survival (b), and overall survival (c) for patients with and without pathologic complete response. Local control (d), progression-free survival (e), and overall survival (f) for patients with and without tumor downstaging.
Radiation outcomes and toxicities
Radiation-related toxicities were similar for both groups (Table 3). The groups had no significant differences in radiation therapy interruptions (P = .37) or neoadjuvant chemotherapy reductions (P = .22). In general, the cohorts did not differ in terms of overall toxicities noted (grades 1-4, and any grade; P > .05). Additional toxicities, including skin reaction (grade ≥2), fatigue, nausea, mucositis, diarrhea, pain, and acute urinary symptoms, did not differ between the cohorts (P > .05).
Table 3.
Radiation toxicity of matched pairs
| Parameter (n = 76 pairs, unless specified) | CB/Yes SD/Noa |
CB/No SD/Yes |
CB/Yes SD/Yes |
CB/No SD/No |
Odds ratio (CI) | P-value |
|---|---|---|---|---|---|---|
| Interruption in radiation | 4 | 1 | 0 | 58 | 4.0 (0.5-197.0) | .37 |
| Neoadjuvant chemotherapy reduction | 5 | 1 | 0 | 56 | 5.0 (0.6-236.5) | .22 |
| Radiotoxicity (n = 63 pairs) | ||||||
| Grade I | 9 | 7 | 45 | 2 | 1.3 (0.4-4.1) | .8 |
| Grade II | 17 | 12 | 9 | 25 | 1.4 (0.6-3.3) | .46 |
| Grade III | 4 | 1 | 1 | 57 | 4.0 (0.4-196.9) | .37 |
| Grade IV | 1 | 0 | 0 | 62 | NA | 1 |
| Any Grade | 6 | 5 | 52 | 0 | 1.2 (0.3-4.9) | 1 |
| Fatigue | 4 | 3 | 0 | 56 | 1.3 (0.2-9.1) | 1 |
| Nausea | 12 | 4 | 9 | 38 | 3.0 (0.9-12.8) | .08 |
| Mucositis | 10 | 3 | 2 | 48 | 3.3 (0.9-18.9) | .1 |
| Diarrhea | 17 | 16 | 25 | 5 | 1.1 (0.5-2.3) | 1.0 |
| Hand/foot syndrome | 2 | 2 | 0 | 59 | 1.0 (0.1-13.8) | .62 |
| Skin reactionb (52 pairs) | 12 | 22 | 11 | 7 | 1.8 (0.9-4.1) | .12 |
| Pain | 12 | 5 | 3 | 43 | 2.4 (0.8-8.7) | .15 |
| Acute urinary symptoms | 4 | 7 | 1 | 51 | 0.6 (0.1-2.3) | .55 |
CB, concomitant boost; CI, confidence interval; SD, standard dose.
Yes means patient experienced an outcome; no means patient did not experience an outcome.
Grade 1 dermatitis treated as unaffected.
Surgical outcomes and toxicities
Surgical wound complications that required treatment did not differ between the groups (P > .05). Urinary side effects, including early urinary retention (P = .04) and urinary tract infection (P = .004), were higher in the SD group compared with the CB group. Bowel complications, including anastomotic leak, bleeding, wound complication, and abscess, were not different between the groups (P > .05). Late toxicities, including small bowel obstruction and stricture, also did not differ significantly (P > .05). Surgical outcomes, including sphincter preservation (P-value not calculable), temporary ileostomy (P = 1.00), and permanent colostomy (P = .62), also did not differ between the groups. Full details are presented in Table 4.
Table 4.
Surgical outcomes and toxicities of matched pairs
| Parameter (n = 76 pairs, else specified) | CB/Yes SD/Noa |
CB/No SD/Yes |
CB/Yes SD/Yes |
CB/No SD/No |
Odds ratio (CI) | P-value |
|---|---|---|---|---|---|---|
| Temporary ileostomy | 5 | 4 | 45 | 22 | 1.3 (0.3-6.3) | 1.00 |
| Permanent colostomy | 1 | 3 | 19 | 53 | 0.3 (0.0-4.2) | .62 |
| Urinary | ||||||
| Early urinary retention | 0 | 6 | 1 | 69 | b | .04 |
| Urinary tract infection | 0 | 10 | 0 | 66 | b | .004 |
| Bowel | ||||||
| Mucositis (63 pairs) | 10 | 3 | 2 | 48 | 3.3 (0.9-18.9) | .1 |
| Diarrhea (63 pairs) | 17 | 16 | 25 | 5 | 1.1 (0.5-2.3) | 1.0 |
| Bowel obstruction | ||||||
| Early small bowel | 0 | 3 | 0 | 73 | b | .25 |
| Late small bowel | 8 | 5 | 0 | 63 | 1.6 (0.5-6.2) | .58 |
| Anastomotic Leak | ||||||
| Radiologic | 5 | 4 | 0 | 67 | 1.3 (0.3-6.3) | 1.0 |
| Clinical | 3 | 3 | 0 | 70 | 1.0 (0.1-7.5) | .68 |
| Wound complications | ||||||
| Abdominal wound complication | 12 | 7 | 1 | 56 | 1.7 (0.6-5.1) | .36 |
| Perineal wound complication | 4 | 3 | 2 | 67 | 1.3 (0.2-9.1) | 1.00 |
| Pelvic abscess | ||||||
| Abdominal/pelvic abscess | 8 | 4 | 1 | 63 | 2.0 (0.5-9.1) | .39 |
| Abdominal/pelvic abscess needing percutaneous drainage | 5 | 2 | 1 | 68 | 2.5 (0.4-26.3) | .45 |
| Abdominal/pelvic abscess needing operative drainage | 2 | 2 | 0 | 72 | 1.0 (0.1-13.8) | .62 |
| Postoperative bleeding | 1 | 1 | 0 | 74 | 1.0 (0.01-78.5) | .48 |
| Perineal hernia | 3 | 1 | 0 | 72 | 3.0 (0.2-157.5) | .61 |
| Anastomotic stricture | 7 | 4 | 1 | 64 | 1.75 (0.5-8.2) | .55 |
CB, concomitant boost; CI, confidence interval; SD, standard dose.
Yes means patient experienced an outcome; no means patient did not experience an outcome.
odds ratio not calculable due to value of zero.
Discussion
Our results suggest that dose escalation in patients with LARC receiving neoadjuvant CRT leads to improved outcomes without a significant increase in radiation toxicity or surgical complication rates. The increased T-downstaging seen in the CB group supports the dose-response relationship confirmed by other studies and lends support to the argument for moderate dose escalation to improve complete response rates.7
Other researchers have reported a highly significant dose-response relationship for patients with LARC after neoadjuvant CRT for dose levels in the range of 50.4 to 70 Gy, notably higher than a conventional dose.7 Additionally, the efficacy and safety of dose escalation delivered using both EBRT and/or brachytherapy in LARC has been reported by several groups.4, 9, 10, 11, 12, 13 Jakobsen et al reported a phase 3 randomized trial that compared 50.4 Gy in 28 fractions of EBRT and 50.4 Gy with an endorectal brachytherapy boost of 10 Gy in 2 fractions.10 The authors concluded that a higher dose increased the rate of major response by 50% for T3 tumors and that an endorectal boost is feasible with no increased toxicity or surgical complications. Passoni et al described the use of adaptive radiation therapy plans using a CB technique to give an additional dose to the residual tumor near the end of treatment.11 In this study, 25 patients with LARC received concurrent CRT that consisted of 41.4 Gy in 18 fractions to the full field with a CB to the residual tumor over the last 6 fractions to give 45.6 Gy in 18 fractions (equivalent dose in 2 Gy fractions = 54 Gy). They concluded that this adaptive strategy is feasible with acceptable toxicity and tumor response rates, and they encouraged further dose escalation in these patients. Given the significant interest in this area, Burbach et al compiled a meta-analysis of patients with LARC treated with dose escalation above 60 Gy and concluded that these doses resulted in high pCR rates (estimated pCR, 20.4%) and acceptable acute grade ≥3 toxicity (estimate, 10.3%).8 Resectability was estimated at 89.5%. Interestingly, the total equivalent dose in 2 Gy fractions did not correlate with acute toxicity, resectability, or pCR rates in this study. Due to the inherent limitations of meta-analysis, the authors recommended additional larger randomized phase 3 studies of dose escalation in this population. These same authors also designed a randomized controlled trial to compare 50 Gy standard CRT with a 15 Gy EBRT boost plus 50 Gy CRT. This trial is currently ongoing.16
However, these studies are limited by a lack of control patients, nonstandard chemotherapy protocols, or nonuniform pathologic response assessment. Without SD patients for comparison, interpretation of the boost dose contribution to patient toxicity is impossible. Additionally, several studies included patients who eventually declined surgical resection; therefore, the pathologic response cannot be known.8 In this report, we used a matched case-control approach to ensure that the patients in the CB and SD cohorts were similar in terms of T-stage, N-stage, and type of surgery performed. Chemotherapy regimen and standard pathologic assessment were also identical for both cohorts. This careful study design increases the likelihood that the differences noted between the patient groups are attributable to the effects of the boost radiation dose rather than the measured patient or tumor factors.
Our study is also unique in the significant length of follow-up for the included patients. Nearly all other studies reported only on the acute toxicities of radiation treatment and surgical complication rates. As data on late toxicity were scarce, this information was not discussed in the recent meta-analysis.8, 18 Potential long-term complications include fistula, anastomotic stricture, or recurrent bowel obstruction, all of which affect a patient's quality of life. Interestingly, many prior studies used higher radiation doses with larger fraction sizes or brachytherapy, which resulted in biologically equivalent doses that were much higher than both the prescribed dose and the standard CRT. Accordingly, with these dose schedules, there may be greater potential for late toxicities, which is only notable with long-term follow-up. We report both the immediate and long-term complication rates for CB and SD patient groups with no significant differences between the measured outcomes. These data may be considered during treatment planning in anticipation of subsequent successful resection.
The LARC treatment paradigm leads to excellent outcomes, but the morbidity associated with treatment can be significant. Thus, there is considerable interest in alternative treatment strategies that deintensify or exclude one of the treatment modalities to decrease toxicity and morbidity. Appelt et al recently reported a series of patients with distal rectal cancer in a prospective observational trial who received 60 Gy in 30 fractions to the tumor with a 5 Gy endorectal brachytherapy boost. Forty of 51 patients had a complete clinical response, and local regrowth for those who were observed (no surgical resection) was 15.5% at 1 year.4 The Preoperative Radiation Or Selective Preoperative Radiation and Evaluation before Chemotherapy and Total Mesorectal Excision (PROSPECT) trial (currently recruiting patients) will compare receipt of chemotherapy alone versus CRT followed by surgical resection for patients with LARC. However, these alternative treatment regimens need a longer follow-up to ensure good oncologic outcomes.
There is considerable interest in the identification of clinical, molecular, and imaging-based predictors of response to treatment either prior to or early during the course of therapy, which would allow for individualized treatment strategies.19 Patients who are predicted to be poor responders could undergo treatment intensification (eg, high dose radiation boost) or alternative therapies with the potential for improved outcomes. Those predicted to have excellent response to therapy could benefit from treatment de-escalation with associated decreased morbidity.
Our study provides evidence for the efficacy and safety of moderate radiation dose escalation. We show that a boost dose is associated with increased tumor downstaging, with potential implications for tailoring subsequent treatments. Practically, this CB approach could be used in patients who are predicted upfront (prior to treatment initiation) to be poor responders, particularly given the emergence of new information with regard to rectal cancer subtypes, with implications for treatment sensitivity. Alternatively, if a midtreatment assessment (via imaging or examination) predicts a poor overall response to preoperative therapy, the CB could be planned during the first few weeks of treatment and safely delivered at the end of the radiation therapy course. This boost dose delivery schedule is therefore advantageous compared with treatments that deliver a dose throughout the entire treatment course.
It is now well established that tumor regression (measured by regression grade or pathologic response) is associated with improved long-term outcomes for patients who are treated with neoadjuvant CRT.2, 20, 21, 22, 23 Several studies report an association between improved pathologic response and improved local control and disease-free survival.2 We confirmed that patients who achieved a pCR or T-downstaging had improved PFS and OS rates. In the present study, we did not observe a significant increase in pCR for the CB patients, but we did observe increased rates of tumor downstaging after receipt of a CB. In this study, surgery was performed at a mean of 6 to 7 weeks after completion of CRT. However, a longer interval to surgical resection can also increase the rates of pCR, and it is likely that those patients with more significant downstaging at 6 weeks would have ultimately achieved a complete response if given more time.
There are several limitations of our study. The study enrollment period was over a decade ago, which allows for a long period of actual follow-up for both the SD and CB groups, especially for documenting late toxicities, but also makes comparisons to current standard-of-care treatments more challenging. The overall treatment strategy with regard to concurrent chemotherapy agents and the use of adjuvant chemotherapy has not fundamentally changed during this time. However, the use of 45 Gy was relatively standard at the time that these patients were treated; we currently typically use a sequential boost to 50.4 Gy after treating the pelvis to 45Gy. Therefore, the impact of an additional radiation boost beyond the current standard of care of 50.4 Gy on tumor downstaging and/or treatment toxicity would need to be addressed separately.
The patients in this study were treated with 3-dimensional conformal radiation therapy; however, recent investigations have shown acute toxicity benefits24, 25, 26, 27 with the use of intensity modulated radiation therapy for LARC CRT treatment when compared with 3-dimensional techniques, and many have adopted the routine use of intensity modulated radiation therapy in this setting. Additionally, there is the possibility of higher staging inaccuracies with the endorectal ultrasound staging that was employed for these patients (albeit for patients on both arms being compared). Currently, we routinely use magnetic resonance imaging for staging. Furthermore, to compare the outcomes after SD and CB radiation treatment, matching was based on clinical stage information, which is subject to risk for stage inaccuracy. Clinical stage information is less predictive of survival than pathologic stage, but any inaccuracies of clinical staging would have been equally likely to affect both groups.
Finally, despite matching on the known factors, it is possible that other residual confounding factors could account for the survival differences. However, it is notable that the CB was associated with greater treatment response despite initial tumor characteristics, such as circumferential extent and size, that did not differ between the groups. The improvement in PFS and OS seems intuitively incongruous with merely an incremental increase in radiation dose via a CB. Given that some patients on the CB arm were treated on protocol, there may have been an inherent but intangible bias in selection by the treating team (although eligibility criteria were similar to those for off-protocol patients) and/or with patient willingness to participate in the protocol that eventually translated to a PFS or OS benefit. This is unlikely to be due to more intensive systemic therapy administered to patients in the CB group because there was no difference in adjuvant chemotherapy between the groups.
These data demonstrate that dose escalation in LARC treatment is feasible, safe, and associated with increased tumor response. Dose escalation may have important implications for novel treatment strategies for rectal cancer that rely on pCR, such as nonoperative management. Therefore, strategies to enhance radiation therapy response through dose optimization should be further pursued.
Acknowledgments
The authors wish to thank Dr. Chirag Patel for assistance with the creation of matched pairs for data analysis.
Footnotes
Sources of support: This work was partially supported by the MD Anderson Cancer Center Support Grant P30-CA16672 and the John E. and Dorothy J. Harris Endowed Professorship to SK.
Conflicts of interest: None.
References
- 1.Sauer R., Becker H., Hogenberger W. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 2.Park I.J., You Y.N., Agarwal A. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol. 2012;30:1770–1776. doi: 10.1200/JCO.2011.39.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee Y.C., Hsieh C.C., Chuang J.P. Prognostic significance of partial tumor regression after preoperative chemoradiotherapy for rectal cancer: A meta-analysis. Dis Colon Rectum. 2013;56:1093–1101. doi: 10.1097/DCR.0b013e318298e36b. [DOI] [PubMed] [Google Scholar]
- 4.Appelt A.L., Ploen J., Harling H. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol. 2015;16:919–927. doi: 10.1016/S1470-2045(15)00120-5. [DOI] [PubMed] [Google Scholar]
- 5.Smith F.M., Waldron D., Winter D.C. Rectum-conserving surgery in the era of chemoradiotherapy. Br J Surg. 2010;97:1752–1764. doi: 10.1002/bjs.7251. [DOI] [PubMed] [Google Scholar]
- 6.Habr-Gama A., Sabbaga J., Gama-Rodrigues J. Watch and wait approach following extended neoadjuvant chemoradiation for distal rectal cancer: Are we getting closer to anal cancer management? Dis Colon Rectum. 2013;56:1109–1117. doi: 10.1097/DCR.0b013e3182a25c4e. [DOI] [PubMed] [Google Scholar]
- 7.Appelt A.L., Ploen J., Vogelius I.R., Bentzen S.M., Jakobsen A. Radiation dose-response model for locally advanced rectal cancer after preoperative chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2013;85:74–80. doi: 10.1016/j.ijrobp.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burbach J.P., den Harder A.M., Intven M., van Vulpen M., Verkooijen H.M., Reerink O. Impact of radiotherapy boost on pathological complete response in patients with locally advanced rectal cancer: A systematic review and meta-analysis. Radiother Oncol. 2014;113:1–9. doi: 10.1016/j.radonc.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 9.Caravatta L., Picardi V., Tambaro R. Neoadjuvant accelerated concomitant boost radiotherapy and multidrug chemotherapy in locally advanced rectal cancer: A dose-escalation study. Am J Clin Oncol. 2012;35:424–431. doi: 10.1097/COC.0b013e31821a5844. [DOI] [PubMed] [Google Scholar]
- 10.Jakobsen A., Ploen J., Vuong T., Appelt A., Lindebjerg J., Rafaelsen S.R. Dose-effect relationship in chemoradiotherapy for locally advanced rectal cancer: A randomized trial comparing two radiation doses. Int J Radiat Oncol Biol Phys. 2012;84:949–954. doi: 10.1016/j.ijrobp.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Passoni P., Fiorino C., Slim N. Feasibility of an adaptive strategy in preoperative radiochemotherapy for rectal cancer with image-guided tomotherapy: Boosting the dose to the shrinking tumor. Int J Radiat Oncol Biol Phys. 2013;87:67–72. doi: 10.1016/j.ijrobp.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Radu C., Norrlid O., Braendengen M., Hansson K., Isacsson U., Glimelius B. Integrated peripheral boost in preoperative radiotherapy for the locally most advanced non-resectable rectal cancer patients. Acta Oncol. 2013;52:528–537. doi: 10.3109/0284186X.2012.737022. [DOI] [PubMed] [Google Scholar]
- 13.Vestermark L.W., Jensen H.A., Pfeiffer P. High-dose radiotherapy (60 Gy) with oral UFT/folinic acid and escalating doses of oxaliplatin in patients with non-resectable locally advanced rectal cancer (LARC): A phase I trial. Acta Oncol. 2012;51:311–317. doi: 10.3109/0284186X.2011.652740. [DOI] [PubMed] [Google Scholar]
- 14.Mohiuddin M., Paulus R., Mitchell E. Neoadjuvant chemoradiation for distal rectal cancer: 5-year updated results of a randomized phase 2 study of neoadjuvant combined modality chemoradiation for distal rectal cancer. Int J Radiat Oncol Biol Phys. 2013;86:523–528. doi: 10.1016/j.ijrobp.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiltshire K.L., Ward I.G., Swallow C. Preoperative radiation with concurrent chemotherapy for resectable rectal cancer: Effect of dose escalation on pathologic complete response, local recurrence-free survival, disease-free survival, and overall survival. Int J Radiat Oncol Biol Phys. 2006;64:709–716. doi: 10.1016/j.ijrobp.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Burbach J.P., Verkooijen H.M., Intven M. Randomized controlled trial for pre-operative dose-escalation BOOST in locally advanced rectal cancer (RECTAL BOOST study): Study protocol for a randomized controlled trial. Trials. 2015;16:58. doi: 10.1186/s13063-015-0586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krishnan S., Janjan N.A., Skibber J.M. Phase II study of capecitabine (Xeloda) and concomitant boost radiotherapy in patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2006;66:762–771. doi: 10.1016/j.ijrobp.2006.05.063. [DOI] [PubMed] [Google Scholar]
- 18.Engels B., Platteaux N., Van den Begin R. Preoperative intensity-modulated and image-guided radiotherapy with a simultaneous integrated boost in locally advanced rectal cancer: Report on late toxicity and outcome. Radiother Oncol. 2014;110:155–159. doi: 10.1016/j.radonc.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Kim N.K., Hur H. New perspectives on predictive biomarkers of tumor response and their clinical application in preoperative chemoradiation therapy for rectal cancer. Yonsei Med J. 2015;56:1461–1477. doi: 10.3349/ymj.2015.56.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodel C., Martus P., Papadoupolos T. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688–8696. doi: 10.1200/JCO.2005.02.1329. [DOI] [PubMed] [Google Scholar]
- 21.Maas M., Nelemans P.J., Valentini V. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–844. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 22.McCoy MJ, Hemmings C, Hillery S, et al. Neoadjuvant chemoradiotherapy for rectal cancer: How important is tumour regression [e-pub ahead of print]? ANZ J Surg.http://dx.doi.org/10.1111/ans.13394, accessed March 5, 2017. [DOI] [PubMed]
- 23.Agarwal A., Chang G.J., Hu C.Y. Quantified pathologic response assessed as residual tumor burden is a predictor of recurrence-free survival in patients with rectal cancer who undergo resection after neoadjuvant chemoradiotherapy. Cancer. 2013;119:4231–4241. doi: 10.1002/cncr.28331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jabbour S.K., Patel S., Herman J.M. Intensity-modulated radiation therapy for rectal carcinoma can reduce treatment breaks and emergency department visits. Int J Surg Oncol. 2012;2012:891067. doi: 10.1155/2012/891067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parekh A., Truong M.T., Pashtan I. Acute gastrointestinal toxicity and tumor response with preoperative intensity modulated radiation therapy for rectal cancer. Gastrointest Cancer Res. 2013;6:137–143. [PMC free article] [PubMed] [Google Scholar]
- 26.Samuelian J.M., Callister M.D., Ashman J.B., Young-Fadok T.M., Borad M.J., Gunderson L.L. Reduced acute bowel toxicity in patients treated with intensity-modulated radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2012;82:1981–1987. doi: 10.1016/j.ijrobp.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 27.Ng S.Y., Colborn K.L., Cambridge L. Acute toxicity with intensity modulated radiotherapy versus 3-dimensional conformal radiotherapy during preoperative chemoradiation for locally advanced rectal cancer. Radiother Oncol. 2016;121:252–257. doi: 10.1016/j.radonc.2016.09.010. [DOI] [PubMed] [Google Scholar]