Abstract
Here we interrogated, using three separate but complementary experimental approaches, the impact of vitamin B12 availability and methotrexate exposure on Daphnia magna, which we hypothesised should have an opposite effect on One carbon metabolism (OCM). OCM is a vital biological process supporting a variety of physiological processes, including DNA methylation. Contrary to mammalian models, this process remains largely unexplored in invertebrates. The purpose of this study was to elucidate the impact of OCM short-term alteration on the fitness and epigenome of the keystone species, Daphnia. We used maternal age at reproduction, brood size and survival rates in combination with DNA methylation sensitive comet assay to determine the effects of vitamin B12 or MTX on fitness and the epigenome. Vitamin B12 had a positive influence on Daphnia fitness and we provide evidence demonstrating that this may be associated with an increased level of genome-wide DNA methylation. Conversely, exposing D. magna to MTX negatively influenced the fitness of the animals and was associated with loss of global DNA methylation, translating in decreased fitness. These results highlight the potential importance of OCM in invertebrates, providing novel evidence supporting a potential role for epigenetic modifications to the genome in D. magna environmental adaptability.
Introduction
In recent years the importance of the water flea, Daphnia, as a model organism for studying toxicological genomics, ecology and evolution has been highlighted by a number of groups1–4. This freshwater planktonic microcrustacean has also been proposed as a model organism for studying developmental programming and epigenetics, as clonal lines contain genetically identical but phenotypically divergent individuals5. Although Daphnia has been identified as an important species for epigenetics research and DNA methylation exists in its genome6, there are still very few studies in the literature7,8 connecting directly the DNA methylation status with its ecological function.
Daphnia species are cyclic parthenogens that are capable of reproducing sexually or asexually, depending on their environment, under favourable environmental conditions they reproduce asexually, developing apomictically from diploid oocytes9. Sexual reproduction is favoured under more challenging or restrictive environments experiencing variations in photoperiod, decrease in temperature, increased population density and/or food shortages9,10. Daphnia spp. have also been shown to exhibit phenotypic plasticity in response to other environmental cues, such as the formation of helmets and neckteeth when exposed to predators and tolerance adaptation to increased levels of salinity5,11,12. Recently, Daphnia has been proposed as a model organism to study Environmental Epigenetics13.
DNA methylation, a vital epigenetic modification involved with gene regulation, has been extensively studied in mammals for nearly two decades14. It is widely regarded that DNA methylation in mammals is susceptible to external environmental cues and that these changes may result in phenotypic alterations15–17. Conversely, the genomic levels and role of DNA methylation has only recently begun to be unraveled in invertebrate species18. Most recently, analysis of gene body methylation in two species of Daphnia revealed both highly methylated and unmethylated gene bodies; with the level of methylation being associated to gene family size7. Furthermore, differential gene body methylation (specifically exonic methylation) has been shown to occur in Daphnia exposed to the toxic cyanobacterium Microcystis aeruginosa 8.
DNA methylation reactions are catalyzed by DNA methyltransferases (DNMTs), which utilize methyl groups supplied by OCM19. Folate and methionine metabolism constitute the OCM pathway20. S-adenosylmethionine (SAM), the universal methyl donor for DNA, RNA and histone methylation reactions, is generated through OCM21. Intracellular availability of SAM is dependent upon the availability of several key nutrients and vitamins such as methionine, vitamin B6, vitamin B12 and folate20. Diets with suboptimal and/or supraphysiological levels of these key players can impair methyl group availability, resulting in aberrant DNA methylation patterns22. While evidence linking nutritional deficit and epigenome alterations (aberrant DNA methylation) is abundant in mammals22–27 these studies are currently lagging behind in aquatic invertebrate species. Although there is evidence in Daphnia spp. demonstrating that reproductive performance is linked to dietary vitamin B12 28. In addition, links between nutrition, DNA methylation and caste determination have been identified in social insects such as honeybees and ants29,30. In the invertebrate, Caenorhabditis elegans, B12 is essentially required for growth and its genome contains a full complement of homologs of mammalian B12-related metabolic pathways31; suggesting a possible role for B12 in OCM in other invertebrate species. To increase our understanding of invertebrates, with respect to vitamin B12 regulation and OCM, using D. magna as a model organism, we raised three main questions to test our hypothesis that environmentally induced phenotypic changes, observed in Daphnia spp., are associated with underlying epigenetic alterations to the genome: (i) Do Daphnia respond to quick dietary shifts (acute exposure) in vitamin B12 concentration, being a key component of one-carbon metabolism and DNA methylation? (ii) How do animals respond when food conditions are not changed, but OCM is perturbed using methotrexate (MTX)?, a known antagonist of one-carbon metabolism in mammals and invertebrates32–34 (iii) Do vitamin B12 supplementation or MTX exposure differentially impact DNA methylation of daphnids?
To address these questions, we first subjected D. magna to culture under concentration gradients of vitamin B12 or methotrexate (MTX) and recorded their fitness. We then assessed global DNA methylation levels using an easily accessible, medium-resolution DNA methylation comet assay developed for this purpose35.
Materials and Methods
Sample preparation
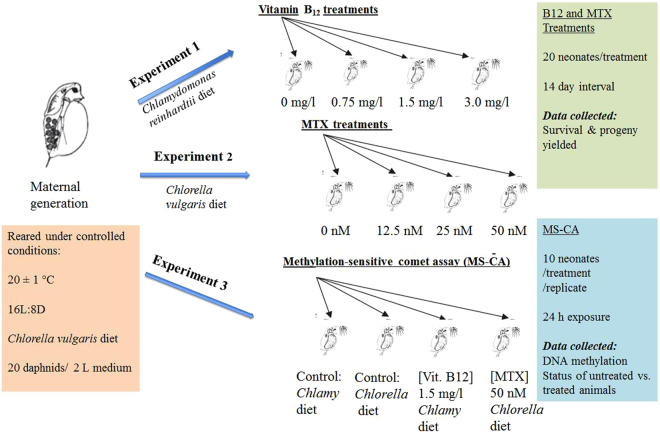
Daphnia magna clones (Straus, Bham2 clone housed in Birmingham, UK) were cultured for several generations prior to the experiment under standardized conditions according to the US Environmental Protection Agency guidelines (EPA-821-R-02–012) to minimize maternal effects. Unless stated, all reagents were supplied by Sigma–Aldrich, Dorset, UK. During initial culture and acclimation 20 daphnid neonates (≤24 h) were maintained in 20 °C ± 1 °C charcoal and UV filtered and aerated borehole water and fed daily, ad libitum, with 2.7 × 104 Chlorella vulgaris cells per daphnid for 7 days and 5 × 105 cells/daphnid, thereafter. Culture media was replaced weekly and culture vessels were kept under a summer photoperiod (16:8 h – light:dark). In the first experiment, investigating the acute effects of vitamin B12 on D. magna, Chlamydomonas reinhardtii algae (vitamin B12 deficient strain) were used as a food source as they are able to grow in vitamin B12 deficient media36,37. Chlamydomonas (+) 15–2040 (Carolina Biological Supply Company, USA) was prepared by inoculation in modified Bolds Basic Medium (Bold 1949, Bischoff and Bold 1963) for 5 days at 25 °C with permanent light. The Chlamydomonas was harvested by centrifugation at 3000 × g for 15 min and resuspended in borehole water. In the second experiment investigating the impact of MTX on D. magna, Daphnids were fed another chlorophyte, Chlorella vulgaris (grown in the same medium, light and temperature conditions as Chlamydomonas). The overall experimental design is outlined in Fig. 1.
Figure 1.
Experimental Design. A scheme showing overview of three experiments including chronic exposure— vitamin B12 and methotrexate (MTX) treatments and methylation sensitive comet assay (MS-CA) in Daphnia.
Experimental design
Experiment 1 – evaluating the effect of vitamin B12 availability on overall D. magna fitness
Neonates (≤24 h) from stock cultures were switched to Chlamydomonas, a B12 deficient diet and exposed to four vitamin B12 culture conditions over 14 days; high (3.0 mg L−1), medium (1.5 mg L−1), low (0.75 mg L−1) and control-deficient (0 mg L−1 vitamin B12 deficient). By increasing media concentrations of B12, on a background diet deficient in B12, we hypothesised that any improvement of fitness observed, would be due to increasing availability of dietary B12 – required for OCM. The selection of vitamin B12 concentrations was based on a preliminary acute experiment (48 h) indicating that animals survived and displayed no immobilization at concentrations of B12 ranging from 0 to 6.0 mg L−1 (data not shown). For chronic exposure treatments, twenty D. magna neonates (from separate mothers) (≤24 h), from the second brood, were cultured individually in 80 ml of test solution per treatment. During the first 6 days of culture Daphnids were fed 3 × 104 Chlamydomonas cells per day and on days 7–14 the concentration was increased to 6 × 106, to fulfil their increased metabolic requirements. The culture media was renewed every second day, during which time organismal survival and reproductive capacity (number of progeny produced) was recorded.
Experiment 2 – evaluating the effect of methotrexate on D. magna fitness
An initial test of different methotrexate (MTX) concentrations (50 nM, 25 nM, 12.5 nM, 6.25 nM, and control (lacking MTX)) on D. magna was performed for 48 h. All of the Daphnids survived for 48 h and no immobility was determined by stereomicroscopic examination. Based on the observation that low-dose MTX had no impact on survival or mobility, treatment groups containing 50 nM, 25 nM and 12.5 nM and a control group lacking MTX were investigated. These low-dose concentrations were selected based on previous findings by Wang et al.33. For each group, 20 Daphnids were cultured individually in 80 ml media over a 14 day exposure period. Daphnia were each fed 2.7 × 104 Chlorella vulgaris cells for 6 days, and 5.5 × 104 cells of algae per Daphnia for days 7–14. Every second day the media was renewed, survival, the number of live progeny and the time from the release of the first brood and second brood by each daphnid was recorded. Neonates were removed during this time to prevent their exposure to MTX and consumption of algae intended for the adult Daphnia.
Experiment 3 - Methylation sensitive comet assay (MS-CA)
Sensitivity of Daphnia spp. to environmental insults has been reported previously38 and, typically, phenotypic changes are observed rapidly39–41. We hypothesised that rapid eco-response of Daphnia may be mediated through alterations to their epigenome. To address this, DNA methylation, in the context of CCGG sequences throughout the Daphnia genome, was assessed over a short period of time (24 h) following dietary changes (i.e. vitamin B12 supplementation) or in the presence of xenobiotics (MTX). Global DNA methylation patterns were determined using comet assay combined with methylation sensitive DNA digestion35, adapted by our group for use in Daphnia (MS-CA). This method is cost effective, allows for processing a large number of samples simultaneously and does not require advanced bioinformatics; thus offering a rapid method for screening DNA methylation alterations. However, the method has a disadvantage in terms of resolution – identification of loci specific alterations to DNA methylation is not possible. For the MS-CA experiment, 10 second brood D. magna juveniles (≤24 h) were exposed to (1) 1.5 mg L−1 vitamin B12 or (2) 50 nM MTX for 24 h and respective controls. Animals in the vitamin B12 group were fed 3 × 104 Chlamydomonas cells per daphnid and animals in the MTX group fed 2.7 × 104 Chlorella vulgaris cells per daphnid. Experiments were performed in duplicate alongside control conditions lacking either vitamin B12 or MTX. After 24 h, 10 whole juvenile daphnids from each treatment and control group were sampled to eliminate tissue selection bias of the Daphnia and/or the presence of eggs in the brood pouch that could lead to potential variations in DNA methylation. Animals were placed in 1 ml phosphate buffered saline (PBS) containing 20 mM EDTA and 10% DMSO, as described previously42. Samples were homogenised by continuous pipetting for 2 min at 4 °C then centrifuged at 5000 rpm for 5 min and the supernatant removed. Homogenised pellets were resuspended in a 100 µl solution of 0.7% low melting point agarose (Promega, Southampton, UK) and spread onto microscope slides pre-coated with 1% standard agarose and the agarose allowed to set by cooling slides on a cold metal block at 4 °C. Next, slides were submerged in ice cold lysis buffer containing 10 mM Tris, 100 mM EDTA, 2.5 M NaCl, 10% DMSO and 1% Triton X-100 for 1 h to prepare nucleoids. Exposed nuclei were digested for 30 min at 37 °C in 50 µl reactions containing 1 x CutSmart buffer and 10 U HpaII, MspI (New England Biolabs, Hertfordshire, UK) or no enzyme, respectively. Prior to enzymatic digestion, slides were saturated in endonuclease buffer (10 mM Tris–HCl, 10 mM NaCl, 1 mM β-mercaptoethanol and 2 mM EDTA) for 10 min to create optimal conditions for enzyme catalytic activity. Digested nuclei were exposed to alkali conditions (1 mM Na2EDTA and 300 mM NaOH) for 20 min at 4 °C, followed by electrophoresis at 300 mA, 0.8 V/cm at 4 °C in the dark for 20 min. After electrophoresis, the slides were neutralised with 0.4 M Tris-HCl buffer, pH 7.5 for 15 min. DNA was fluorescently labelled with a 1:10,000 dilution of SYBR gold for 1 hour (Life Technologies ltd, Paisley, UK). Comet slides were analysed using a fluorescence microscope (Zeiss, Axiovert) equipped with a 515 to 560 nm excitation filter and a barrier filter of 590 nm. at 400 X magnification using a 40 X oil immersion lens and Komet IV image analysis software (Perceptive Instruments, Bury St Edmunds, UK). Measurements of percent (%) tail DNA of 50 random comets per slide were taken and the median value used as the unit for statistical analysis as recommended by Duez et al.43. DNA digestion was quantified by measuring the percentage of DNA migrating from the comet head (% tail intensity). For all samples, genome methylation was calculated using the formula [(100–HpaII\MspI × 100) – control], where HpaII\MspI represent are the average percentage tail DNA resulting from HpaII- and MspI-digested nucleoids and35. Control (slide no enzyme) was subtracted to account for the basal DNA damage prior to enzymatic digestion. As an internal control, the skin cancer cell line (HaCaT) with known methylation level, was also included.
Statistical Analysis
Malthusian parameter (r) was calculated as a metric of performance following supplementation with vitamin B12 or exposure to MTX according to the formula 1 = (ert1lt1mt1) + (ert2 lt2mt2), where t1 = time to first brood, t2 = time to second brood, m = brood size and l = survivorship that was set to 1 as mortality was not observed during exposures. Small r was iterated with left part of the equation set to 1 for comparability. A higher value of this parameter suggests a fitter organism. The environment for statistical computing “R” version 3.1 (R development Core Team, 2008) was used for statistical analysis. The effect of vitamin B12 and MTX on fitness was analysed separately by generalized linear model (glm) with the treatment concentration as the explanatory variable. The models did not show any significant sign of violation of the assumption for the test (data not shown). The effects of different treatments (food regimes) were compared using generalized linear model. MS-CA was also compared by glm, with treatment as the explanatory variable. Two independent experiments were performed so that we were able to test if the experiment had an effect.
Results
Vitamin B12 supplementation is associated with improved D. magna fitness
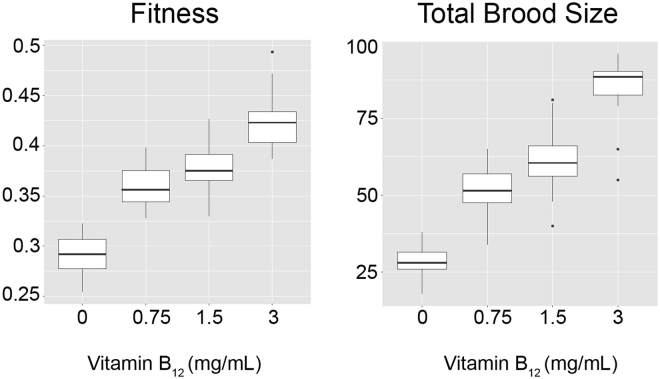
Life history traits for 80 D. magna exposed to different concentrations of vitamin B12 (control – no supplemental vitamin B12, low – 0.75 mg/L, medium– 1.5 mg/L and high – 3.0 mg/L) were recorded over a 14 day period to determine the relationship between vitamin B12 availability and D. magna fitness. These traits enable population growth rate calculations, which translate into fitness. Age at first reproduction, age at second reproduction, size of first brood, size of second brood and survival rate were recorded. Generalized linear model analysis revealed that for every 1 mg L−1 increase of vitamin B12, D. magna fitness significantly increased by 0.039 (P ≤ 0.001) (Supplementary Table 1). Average fitness for each treatment group was as follows: 0.26 ± 0.09 (0 mg L−1), 0.36 ± 0.02 (0.75 mg L−1), 0.37 ± 0.03 (1.5 mg L−1) and 0.42 ± 0.02 (3.0 mg L−1) (Fig. 2). Progeny yielded by D. magna was also proportional to the concentration of vitamin B12 that they were exposed to in the media. In the 0 mg L−1 group there were a total of 33 juveniles, increasing by 17 for every mg of vitamin B12 added per litre of media (Supplementary Table 2 and Fig. 2). Data related to the impact of vitamin B12 concentration on the size of the first and second broods is outlined in Supplementary Figure 1.
Figure 2.
Impact of vitamin B12 availability on Daphnia magna fitness and reproductive performance. Left panel – Box-plot of fitness versus vitamin B12 concentration. Right panel – total brood size relative to vitamin B12 concentration. First and third quartiles presented with a vertical line inside to indicate the mean value respectevely for all figures.
Reduction of Daphnia magna fitness following exposure to methotrexate
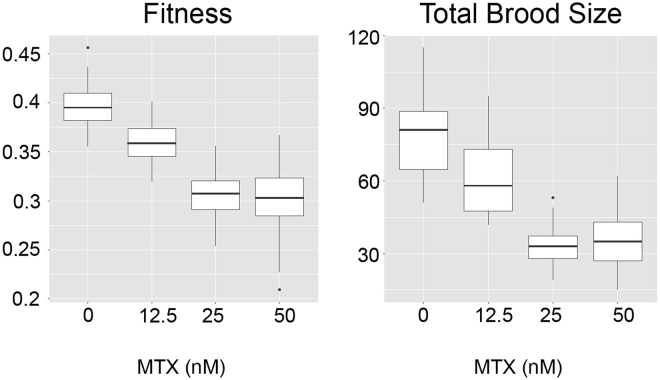
A similar experimental strategy as above was employed over a 14-day period to examine the effects of MTX exposure on D. magna fitness. Fitness was compared across four experimental groups maintained under the following media conditions; control animals (no MTX), 12.5 nM, 25 nM and 50 nM MTX using the generalized linear model. It was observed that for every 1 nM increase in MTX concentration D. magna fitness decreased by 0.002 and it was statistically significant compared to the animals that were not exposed to MTX (P ≤ 0.001) (Supplementary Table 3). D. magna mothers that were not exposed to MTX had the highest fitness and increasing levels of MTX was proportional to decreased fitness; average fitness for each treatment group was as follows: 0.39 ± 0.03 (0 nM), 0.36 ± 0.02 (12.5 nM), 0.30 ± 0.02 (25 nM) and 0.25 ± 0.12 (50 nM) (Fig. 3). A marked decline in reproductive performance was observed along the MTX concentration gradient – total number of offspring (first and second brood combined) in the unexposed group was equal to 72 and decreased by 8.9 for every 10 nM increase of MTX in the media (Supplementary Table 4 and Fig. 3). Data related to the impact of increasing concentrations of MTX on the size of the first and second broods are outlined in Supplementary Figure 2.
Figure 3.
Impact of MTX exposure on Daphnia magna fitness and reproductive performance. Left panel – Box-plot of fitness versus MTX concentration. Right panel – total brood size relative to MTX concentration.
Vitamin B12 availability and methotrexate (MTX) exposure impact global DNA methylation levels
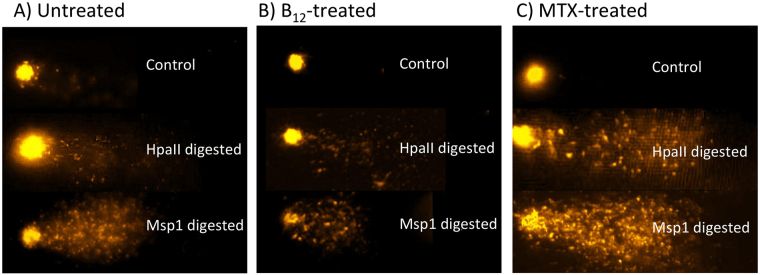
We next used methylation sensitive comet assays (MS-CA) to evaluate the effect of vitamin B12 supplementation and MTX exposure on genome wide methylation levels (Fig. 4). Animals were exposed to media supplemented with either 1.5 mg/L vitamin B12 or 50 nM MTX for 24 h alongside daphnids maintained in non-supplemented media. The average level of methylation for B12-treated animals was 9.09 ± 2.4%, which was significantly larger than that observed for the control animals; Chlamydomonas, 5.34 ± 2.4% and Chlorella 5.8 ± 1.9% (Table 1 and Fig. 5). MS-CA analysis comparing untreated control animals to MTX-exposed animals revealed that a 24 h exposure of D. magna to media supplemented with 50 nM MTX significantly reduced global DNA methylation, average methylation in MTX-treated animals was 1.81 ± 1.2% (Table 1 and Fig. 5). Summary statistics of vitamin B12 vs control and MTX are outlined in Table 2. The average level of methylation in Human keratinocytes (HaCaT), with a documented degree of DNA methylation44, was observed to be 48.7 ± 1.2% for two independent experiments (Supplementary file 3). This level of methylation is in agreement with data previously published using HaCaT cells44; thus serving as an internal control validating the MS-CA method.
Figure 4.
Methylation sensitive comet assay (MS-CA). The observed comet tails represent double-strand DNA breaks, introduced by the isoschizomeric enzymes MspI and HpaII; in the presence of an electric field, the HpaII- and MspI-digested DNA migrates faster, compared to the significantly slower migration of the undigested DNA. Digestion of the control (Chlamy) and treated (vitamin B12 and MTX) samples resulted in a tail DNA increase for MTX and decrease for vitamin B12, relative to the intact DNA in the comet head of the untreated controls – indicative of decreased DNA methylation levels in MTX-treated animals and increased DNA methylation in vitamin B12-treated animals.
Table 1.
Global DNA methylation levels in treated and untreated Daphnia magna
| Treatment group | Mean (%) | Standard Deviation |
|---|---|---|
| Vitamin B12 | 9.09 | 2.4 |
| Methotrexate | 1.81 | 1.2 |
| Chlamy-Control | 5.34 | 2.4 |
| Chlorella-Control | 5.78 | 1.9 |
| Skin (HaCaT) | 48.7 | 1.2 |
Data presented in this table shows the mean global methylation level (±standard deviation (SD)) of treated and untreated D. magna in two independent experiments, in duplicates. Mammalian skin cell line (HaCaT) with known DNA methylation levels, served as an internal control for MS-CA and DNA methylation calculations.
Figure 5.
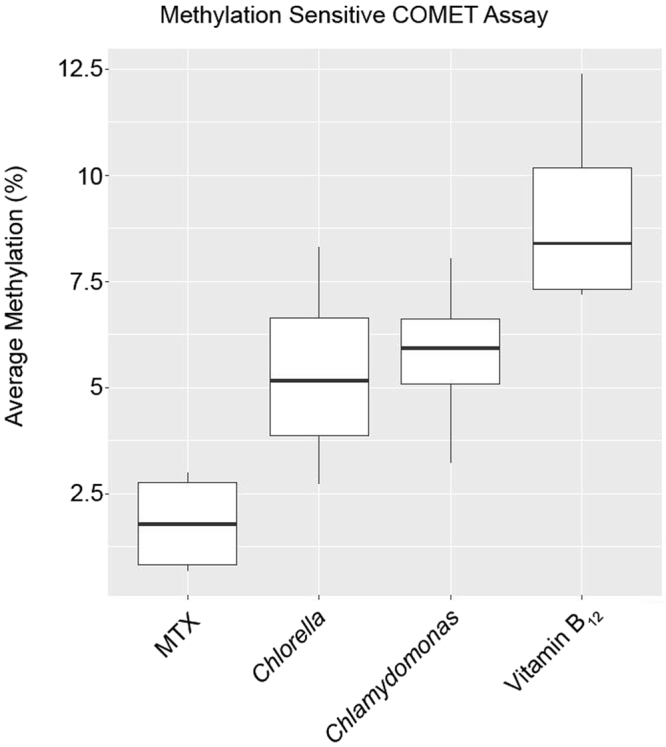
Global methylation profiles of Daphnia magna supplemented with vitamin B12 or exposed to MTX. The y-axis represents percentage DNA methylation levels following methylation-sensitive comet assay. The x-axis denotes the Daphnia sample groups; vitamin B12-treated, MTX-exposed and untreated controls (Chlamydomonas and Chlorella). A box and whiskers are present for each sample.
Table 2.
Summary statistics of MS-CA performed by generalized linear model (Vitamin B12 vs. control and MTX). Null deviance was 157 on 15 degrees of freedom and AIC was 73.8.
| Value | Standard error | t-value | p-value | |
|---|---|---|---|---|
| Intercept | 10.96 | 1.82 | 6.03 | <<0.001 |
| Methotrexate | −7.28 | 1.42 | −5.11 | <<0.001 |
| Chlamy-control | −3.75 | 1.42 | −2.63 | 0.023 |
| Chlorella-control | −3.31 | 1.42 | −2.32 | 0.040 |
| Experiment | −1.24 | 1.01 | −1.23 | 0.24 |
glm revealed that MTX exposed animals display a 7.28% decrease in global DNA methylation compared to Daphnia supplemented with 1.5 mg/L vitamin B12. A similar trend was observed in control animals fed with Chlamydomonas (Chlamy) and Chlorella (with decreases of 3.78% and 3.31% in DNA methylation, respectively, relative to vitamin B12).
Discussion
To date, the ecological stoichiometry field has largely focused on other limitations such as nitrogen, phosphorous, zinc or iron45–47. However, the potential role of epigenetic mechanisms in ecological stoichiometry has yet to be evaluated. To investigate epigenetically driven ecological stoichiometry we interrogated genomic methylation and fitness in D. magna using two separate, but complementary, approaches. This involved supplementation of growth media vitamin B12 and MTX, molecules with known effects on OCM.
D. magna fitness and global methylation levels differed according to vitamin B12 availability and exposure to MTX. Vitamin B12 had a positive effect on fitness over a two week period and was associated with increased genomic methylation after only 24 h; while MTX was detrimental to fitness and exposure resulted in loss of DNA methylation. Vitamin B12 had a similar positive influence on fitness in D. magna to that observed previously in Daphnia pulex 28, however the underlying molecular mechanisms in this early investigation were unexplored and the effect of the vitamin has not been addressed subsequently. Here, we provide evidence that supports a role for epigenetic mechanisms in D. magna’s ability to adapt to their surroundings/dietary composition and convey environmental cues to measurable changes in phenotype. It is indeed plausible that the observed variations of fitness in response to dietary manipulation may be mediated through DNA methylation-dependent changes in gene expression. It is possible that the observed effects are purely correlational and acting via alternative pathways than OCM; however this is unlikely due to the bi-directionality of the effect of manipulation. Therefore, further high resolution profiling of DNA methylation and gene expression in vitamin B12 supplemented and MTX-exposed animals is required to reveal which genes and pathways that are directly involved in the observed effects.
Vitamin B12, an important factor in the one-carbon metabolic network, is interlinked with methionine synthase and is involved in the donation of one-carbon units from the folate cycle into the methionine cycle through methylation of homocysteine to methionine32. Therefore, given that methionine is the precursor to the universal methyl donor S-adenosylmethionine (SAM), required for methylation of DNA, it is plausible to interpret that disruptions to this cycle of events can lead to alterations to genomic methylation levels, as exemplified by the increased levels of DNA methylation observed between vitamin B12 supplemented and control animals in the current study. Indeed, this relationship between vitamin B12 availability and DNA methylation has been reported previously in vertebrates48, but not with dramatic changes to fitness. Additionally, we observed that global DNA methylation was reduced following exposure of D. magna to a low-dose concentration of MTX. Interpretation of this finding is that the antifolate, MTX (a dihydrofolate reductase inhibitor49), inhibits SAM production through reduction of methionine adenyltransferase (MAT) activity33, thus limiting the availability of methyl groups that are required for establishing and maintaining genomic methylation. This is consistent with previous findings showing that low-dose MTX induced DNA demethylation in human osteosarcoma cells50. Furthermore, a recent study by Blatch et al. in Drosophila showed that several metabolites, associated with OCM (cystathionine, methylgycine, and methylmalonic acid), were disrupted in larvae consuming MTX51; providing evidence that MTX may affect OCM in invertebrates.
Our results demonstrate that potential perturbations of one-carbon metabolism, elicited through vitamin B12 deficiency or MTX exposure, affect not only the fitness of the animals but also impact their epigenome. These findings may have a wider context in the natural environment and the growth rate hypothesis (GRH). The GRH states that higher growth rate (μ) is related to higher phosphorus concentration and lower carbon∶phosphorus and nitrogen∶phosphorous ratios52 but it implies that any limitation might have an effect on an organisms growth. For instance, a diet deficient in vitamin B12 resulted in non-viable eggs in Blattella germanica, suggesting that it may affect the growth of some insects (Gordon, 1959). If vitamin availability can influence global DNA methylation and this can drive organism growth, as observed in the current study, then this phenomenon sheds new light on undiscovered mechanisms of growth regulation in these invertebrates; and calls for urgent verification of these findings within the natural habitat. Our work may also be applied to investigating the possible molecular mechanisms involved in the contentious area of transgenerational epigenetic inheritance research53. Broadly defined, transgenerational epigenetic inheritance is the transmission of heritable non-DNA-based information (i.e. DNA methylation, histone marks and non-coding RNA molecules) from one generation to the next, through multiple generations54. Indeed, intergenerational inheritance has been previously demonstrated in a zinc exposure study in D. magna, associated with DNA hypomethylation in non-exposed generations that were linked with changes in gene transcription47. In another study, Daphnia mothers fed a low phosphorous diet produced smaller neonates with lower body P content compared to control (P-rich) mothers55. However, the underlying molecular mechanisms were not interrogated and epigenetic mechanisms represent an attractive possibility to potentially explain some of the observed variation. The use of Daphnia to investigate transgenerational epigenetic inheritance is enticing – given their clonal nature and genetic identicalness, thus permitting the separation of genetic and epigenetic influences on phenotype5.
It has recently been reasoned that invertebrates (specifically insects) are suitable organisms for studying human diseases with an epigenetic component, due to conservation of underlying molecular mechanisms56. Additionally, despite being vital for development in non-mammalian species the mechanisms of vitamin function are poorly understood in invertebrates51. The observations outlined in our investigation, showing a link between vitamin B12 availability or MTX exposure and levels of DNA methylation, provide novel information on nutritional mechanisms in a non-mammalian species. Our results provide supporting evidence for the use of invertebrate species, particularly D. magna, as models for studying the molecular mechanisms involved in environmental epigenetics and transgenerational epigenetic inheritance. These findings also suggest that one carbon metabolism may be limited by vitamins in the natural environment; and we propose that future experiments in natural environments are performed to test this hypothesis. Further experiments are also required to uncover whether B12 supplementation or MTX exposure directly affect OCM in invertebrates. Finally, the results of our study show promise for determining whether chronic outcomes of environmental insults are associated with perturbed DNA methylation; using a reliable, cost-effective and rapid assay developed for this purpose.
Availability of data and material
Should the manuscript be accepted, the data supporting the results will be archived in an appropriate public repository such as Dryad or Figshare and the data DOI will be included at the end of the article.
Electronic supplementary material
Acknowledgements
MW is grateful to European Union for the Intra-European Fellowships (IEF), Independent Research Fellowship, Marie Skłodowska Curie (FP7-PEOPLE-2013-IEF) with the project, Ghosts in parthenogenetic daughters - epigenetic effects on clonal organisms can reveal the degree of phenotypic plasticity due to biotic cues (dGHOST, project no 629892)) and also “TOPP finansiering” (IS-TOPP projekt 238820) from Norwegian Research Council to MW. The work has been done in Prof. John K. Colbourne laboratory. We are greatful to him and his Technician Mr. Ian G. Sewell.
Author Contributions
M.W. conceived the experiment as part of a Marie Skłodowska-Curie, dGhost project, in University of Birmingham. F.K. and M.W. designed the experiments. F.K. conducted the experiments under her master thesis in Birmingham. F.K. learned the comet assay technique under NHs guidance. M.W. and F.K. developed the methylation sensitive assay (MS-CA) for Daphnia. A.O.D. drafted the manuscript and contributed in developing the idea of using restriction enzymes and was instrumental in the analysis and interpretation of the data together with the co-authors. M.W. performed the final statistics and arranged the figures in discussion with A.O.D. and F.K. All co-authors commented on the drafted manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Fitore Kusari and Alan M. O’Doherty contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-12148-2.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shaw JR, et al. Daphnia as an emerging model for toxicological genomics. Advances in Experimental Biology-Comparative Toxicogenomics. 2008;2:165–219. doi: 10.1016/S1872-2423(08)00005-7. [DOI] [Google Scholar]
- 2.Heckmann LH, et al. Systems biology meets stress ecology: linking molecular and organismal stress responses in Daphnia magna. Genome Biol. 2008;9:R40. doi: 10.1186/gb-2008-9-2-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stollewerk A. The water flea Daphnia–a ‘new’ model system for ecology and evolution? Journal of biology. 2010;9:21. doi: 10.1186/jbiol212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colbourne JK, et al. The ecoresponsive genome of Daphnia pulex. Science. 2011;331:555–561. doi: 10.1126/science.1197761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris KD, Bartlett NJ, Lloyd VK. Daphnia as an emerging epigenetic model organism. Genet Res Int. 2012;2012:147892. doi: 10.1155/2012/147892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vandegehuchte MB, et al. Occurrence of DNA methylation in Daphnia magna and influence of multigeneration Cd exposure. Environment international. 2009;35:700–706. doi: 10.1016/j.envint.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Asselman J, De Coninck DI, Pfrender ME, De Schamphelaere KA. Gene Body Methylation Patterns in Daphnia Are Associated with Gene Family Size. Genome biology and evolution. 2016;8:1185–1196. doi: 10.1093/gbe/evw069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asselman J, et al. Bisulfite Sequencing with Daphnia Highlights a Role for Epigenetics in Regulating Stress Response to Microcystis through Preferential Differential Methylation of Serine and Threonine Amino Acids. Environ Sci Technol. 2017;51:924–931. doi: 10.1021/acs.est.6b03870. [DOI] [PubMed] [Google Scholar]
- 9.Xu S, Innes DJ, Lynch M, Cristescu ME. The role of hybridization in the origin and spread of asexuality in Daphnia. Molecular ecology. 2013;22:4549–4561. doi: 10.1111/mec.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleiven OT, Larsson P, Hobaek A. Sexual Reproduction in Daphnia-Magna Requires 3 Stimuli. Oikos. 1992;65:197–206. doi: 10.2307/3545010. [DOI] [Google Scholar]
- 11.Vandegehuchte MB, Janssen CR. Epigenetics and its implications for ecotoxicology. Ecotoxicology. 2011;20:607–624. doi: 10.1007/s10646-011-0634-0. [DOI] [PubMed] [Google Scholar]
- 12.Coldsnow, K. D., Mattes, B. M., Hintz, W. D. & Relyea, R. A. Rapid evolution of tolerance to road salt in zooplankton. Environmental pollution, doi:10.1016/j.envpol.2016.12.024 (2016). [DOI] [PubMed]
- 13.Wojewodzic, M.W. & Beaton, M. The Future of Environmental Epigenetics: Insights Using the Clonal Water Flea Model. In Heleen Verlinden, editor: Insect Epigenetics, Vol 53, AIIP, UK: Academic Press (2017).
- 14.Li E, Zhang Y. DNA methylation in mammals. Cold Spring Harbor perspectives in biology. 2014;6:a019133. doi: 10.1101/cshperspect.a019133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burris HH, Baccarelli AA. Environmental epigenetics: from novelty to scientific discipline. Journal of applied toxicology: JAT. 2014;34:113–116. doi: 10.1002/jat.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pacchierotti F, Spano M. Environmental Impact on DNA Methylation in the Germline: State of the Art and Gaps of Knowledge. BioMed research international. 2015;2015:123484. doi: 10.1155/2015/123484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruiz-Hernandez A, et al. Environmental chemicals and DNA methylation in adults: a systematic review of the epidemiologic evidence. Clinical epigenetics. 2015;7:55. doi: 10.1186/s13148-015-0055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts SB, Gavery MR. Is There a Relationship between DNA Methylation and Phenotypic Plasticity in Invertebrates? Frontiers in physiology. 2012;2:116. doi: 10.3389/fphys.2011.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson OS, Sant KE, Dolinoy DC. Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. The Journal of nutritional biochemistry. 2012;23:853–859. doi: 10.1016/j.jnutbio.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ducker, G. S. & Rabinowitz, J. D. One-Carbon Metabolism in Health and Disease. Cell metabolism, 10.1016/j.cmet.2016.08.009 (2016). [DOI] [PMC free article] [PubMed]
- 21.Mentch SJ, Locasale JW. One-carbon metabolism and epigenetics: understanding the specificity. Annals of the New York Academy of Sciences. 2016;1363:91–98. doi: 10.1111/nyas.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandovici I, et al. Maternal diet and aging alter the epigenetic control of a promoter-enhancer interaction at the Hnf4a gene in rat pancreatic islets. Proc Natl Acad Sci USA. 2011;108:5449–5454. doi: 10.1073/pnas.1019007108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci USA. 2007;104:13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolff GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. 1998;12:949–957. [PubMed] [Google Scholar]
- 25.Lim U, Song MA. Dietary and lifestyle factors of DNA methylation. Methods Mol Biol. 2012;863:359–376. doi: 10.1007/978-1-61779-612-8_23. [DOI] [PubMed] [Google Scholar]
- 26.Soubry, A. et al. Newborns of obese parents have altered DNA methylation patterns at imprinted genes. International journal of obesity, doi:10.1038/ijo.2013.193 (2013). [DOI] [PMC free article] [PubMed]
- 27.Dominguez-Salas P, et al. Maternal nutrition at conception modulates DNA methylation of human metastable epialleles. Nature communications. 2014;5:3746. doi: 10.1038/ncomms4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keating KI. The Influence of Vitamin-B12 Deficiency on the Reproduction of Daphnia-Pulex Leydig (Cladocera) J Crustacean Biol. 1985;5:130–136. doi: 10.2307/1548225. [DOI] [Google Scholar]
- 29.Kucharski R, Maleszka J, Foret S, Maleszka R. Nutritional control of reproductive status in honeybees via DNA methylation. Science. 2008;319:1827–1830. doi: 10.1126/science.1153069. [DOI] [PubMed] [Google Scholar]
- 30.Chittka A, Wurm Y, Chittka L. Epigenetics: the making of ant castes. Curr Biol. 2012;22:R835–838. doi: 10.1016/j.cub.2012.07.045. [DOI] [PubMed] [Google Scholar]
- 31.Bito T, Watanabe F. Biochemistry, function, and deficiency of vitamin B12 in Caenorhabditis elegans. Experimental biology and medicine. 2016;241:1663–1668. doi: 10.1177/1535370216662713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer. 2013;13:572–583. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang YC, Chiang EP. Low-dose methotrexate inhibits methionine S-adenosyltransferase in vitro and in vivo. Molecular medicine. 2012;18:423–432. doi: 10.2119/molmed.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Affleck JG, Neumann K, Wong L, Walker VK. The effects of methotrexate on Drosophila development, female fecundity, and gene expression. Toxicological sciences: an official journal of the Society of Toxicology. 2006;89:495–503. doi: 10.1093/toxsci/kfj036. [DOI] [PubMed] [Google Scholar]
- 35.Lewies A, Van Dyk E, Wentzel JF, Pretorius PJ. Using a medium-throughput comet assay to evaluate the global DNA methylation status of single cells. Frontiers in genetics. 2014;5:215. doi: 10.3389/fgene.2014.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Croft MT, Lawrence AD, Raux-Deery E, Warren MJ, Smith AG. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature. 2005;438:90–93. doi: 10.1038/nature04056. [DOI] [PubMed] [Google Scholar]
- 37.Kazamia E, et al. Mutualistic interactions between vitamin B12 -dependent algae and heterotrophic bacteria exhibit regulation. Environmental microbiology. 2012;14:1466–1476. doi: 10.1111/j.1462-2920.2012.02733.x. [DOI] [PubMed] [Google Scholar]
- 38.Schindler, D. W. Detecting Ecosystem Responses to Anthropogenic Stress. Canadian Journal of Fisheries and Aquatic Sciences, doi:10.1139/f87-276 (1987).
- 39.Tautz D. Not just another genome. BMC biology. 2011;9:8. doi: 10.1186/1741-7007-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyakawa H, et al. Gene up-regulation in response to predator kairomones in the water flea, Daphnia pulex. BMC Dev Biol. 2010;10:45. doi: 10.1186/1471-213X-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyakawa H, Gotoh H, Sugimoto N, Miura T. Effect of juvenoids on predator-induced polyphenism in the water flea, Daphnia pulex. Journal of experimental zoology. Part A, Ecological genetics and physiology. 2013;319:440–450. doi: 10.1002/jez.1807. [DOI] [PubMed] [Google Scholar]
- 42.Park SY, Choi J. Genotoxic effects of nonylphenol and bisphenol A exposure in aquatic biomonitoring species: freshwater crustacean, Daphnia magna, and aquatic midge, Chironomus riparius. Bulletin of environmental contamination and toxicology. 2009;83:463–468. doi: 10.1007/s00128-009-9745-1. [DOI] [PubMed] [Google Scholar]
- 43.Duez P, Dehon G, Kumps A, Dubois J. Statistics of the Comet assay: a key to discriminate between genotoxic effects. Mutagenesis. 2003;18:159–166. doi: 10.1093/mutage/18.2.159. [DOI] [PubMed] [Google Scholar]
- 44.Florea AM. DNA methylation pyrosequencing assay is applicable for the assessment of epigenetic active environmental or clinical relevant chemicals. BioMed research international. 2013;2013:486072. doi: 10.1155/2013/486072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karasov, W. H. & Martínez del Rio, C. Physiological ecology: how animals process energy, nutrients, and toxins. (Princeton University Press, 2007).
- 46.Sterner, R. W. & Elser, J. J. Ecological stoichiometry: the biology of elements from molecules to the biosphere. (Princeton University Press, 2002).
- 47.Vandegehuchte MB, Lemiere F, Janssen CR. Quantitative DNA-methylation in Daphnia magna and effects of multigeneration Zn exposure. Comparative biochemistry and physiology. Toxicology & pharmacology: CBP. 2009;150:343–348. doi: 10.1016/j.cbpc.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 48.Fernandez-Roig S, Lai SC, Murphy MM, Fernandez-Ballart J, Quadros EV. Vitamin B12 deficiency in the brain leads to DNA hypomethylation in the TCblR/CD320 knockout mouse. Nutrition & metabolism. 2012;9:41. doi: 10.1186/1743-7075-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schweitzer BI, Dicker AP, Bertino JR. Dihydrofolate reductase as a therapeutic target. FASEB J. 1990;4:2441–2452. doi: 10.1096/fasebj.4.8.2185970. [DOI] [PubMed] [Google Scholar]
- 50.Sramek M, Neradil J, Sterba J, Veselska R. Non-DHFR-mediated effects of methotrexate in osteosarcoma cell lines: epigenetic alterations and enhanced cell differentiation. Cancer cell international. 2016;16:14. doi: 10.1186/s12935-016-0289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blatch SA, Stabler SP, Harrison JF. The effects of folate intake on DNA and single-carbon pathway metabolism in the fruit fly Drosophila melanogaster compared to mammals. Comparative biochemistry and physiology. Part B, Biochemistry & molecular biology. 2015;189:34–39. doi: 10.1016/j.cbpb.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 52.Gounand, I. et al. Size evolution in microorganisms masks trade-offs predicted by the growth rate hypothesis. Proceedings. Biological sciences283, doi:10.1098/rspb.2016.2272 (2016). [DOI] [PMC free article] [PubMed]
- 53.Heard E, Martienssen RA. Transgenerational epigenetic inheritance: myths and mechanisms. Cell. 2014;157:95–109. doi: 10.1016/j.cell.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Doherty, A. M. & McGettigan, P. A. Epigenetic processes in the male germline. Reprod Fertil Dev, doi:10.1071/RD14167 (2014). [DOI] [PubMed]
- 55.Frost PC, et al. Transgenerational effects of poor elemental food quality on Daphnia magna. Oecologia. 2010;162:865–872. doi: 10.1007/s00442-009-1517-4. [DOI] [PubMed] [Google Scholar]
- 56.Mukherjee K, Twyman RM, Vilcinskas A. Insects as models to study the epigenetic basis of disease. Progress in biophysics and molecular biology. 2015;118:69–78. doi: 10.1016/j.pbiomolbio.2015.02.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.