Abstract
To realize photothermal therapy (PTT) of cancer/tumor both the photothermal conversion and temperature detection are required. Usually, the temperature detection in PTT needs complicated instruments, and the therapy process is out of temperature control in the present investigations. In this work, we attempt to develop a novel material for achieving both the photothermal conversion and temperature sensing and control at the same time. To this end, a core-shell structure with NaYF4:Er3+/Yb3+ core for temperature detection and NaYF4:Tm3+/Yb3+ shell for photothermal conversion was designed and prepared. The crystal structure and morphology of the samples were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Furthermore, the temperature sensing properties for the NaYF4:Er3+/Yb3+ and core-shell NaYF4:Er3+/Yb3+@NaYF4:Tm3+/Yb3+ nanoparticles were studied. It was found that the temperature sensing performance of the core-shell nanoparticles did not become worse due to coating of NaYF4:Tm3+/Yb3+ shell. The photothermal conversion behaviors were examined in cyclohexane solution based on the temperature response, the NaYF4:Er3+/Yb3+@NaYF4:Tm3+/Yb3+ core-shell nanoparticles exhibited more effective photothermal conversion than that of NaYF4:Er3+/Yb3+ nanoparticles, and a net temperature increment of about 7 °C was achieved by using the core-shell nanoparticles.
Introduction
In the clinical research and practice, hyperthermia alone or its combination with other therapies including surgery, radiation therapy, chemotherapy and anticancer drug, have been applied in the control and treatment for tumors and cancers1–5. Due to the fact that the tumor cells and tissues are sensitive to the temperature above normal6, thus hyperthermia works based on increasing the temperature of lesion location for a period of time and killing cancerous cells with less damage on normal cells and tissues. The successful cases of treatments on breast, head and neck tumors have indicated that hyperthermia as adjuvant therapy effectively controls tumors and reduces recurrence7.
As an interesting alternative for hyperthermia, PTT has attracted much attention due to its advantages of less side effects, minimal invasiveness, high specificity and short treatment period8–11. The mechanism for PTT is based on the photothermal conversion agents that convert their absorbed light energy into heat to thermally ablate targeted cancer cells at a certain temperature8. Thus, external excitation light sources, temperature measurements and photothermal conversion materials are imperative conditions for the whole process of PTT. Firstly, for the excitation source, the light lying in the biological windows (first window: 700–980 nm, second window: 1000–1400 nm) is commonly used as excitation source12,13. In fact, though the excitation wavelength is located at the biological windows, the penetration depth of the light is finite. However, the tumors located at superficial tissues such as galactophore cancer and cutaneum carcinoma ask for short penetration distance of excitation light, and the tumors in deep tissues can also be treated in assistance of medical endoscope technique14. When exposed in the spectral windows, the photothermal agents can selectively work in the lesions where the agents are incorporated into, and extremely avoid unnecessary injury to the surrounding normal tissues. This is to say, the tumors, which are targeted by the photothermal agents, can reach the expected temperature and be cured before the healthy issue is injured. Moreover, the excitation lights, whose wavelengths fall into the biological windows, can penetrate biotic tissues with a few centimeters depth, which makes deep-tissue hyperthermia treatments possible. Secondly, for the temperature sensing, precisely measuring the temperature is crucial to control heat release and treatment time maintained in the location of tumor cells. Currently, a variety of temperature-measuring methods, such as infrared thermal imaging15,16, magnetic resonance imaging17, photoacoustic technique18,19 and ultrasound20, have been extensively used. The non-contact thermometry still suffers from many applied limitations, although these methods can be used to detect the average temperature or surface temperature of the photothermal heaters at different heating time, excitation power and thermal dosage. For instance, real-time thermal measurement of tumors located in surface can be achieved by infrared thermal imaging, but the temperature in deep tissue fails to be detected. Photoacoustic measurement gives high resolution images, but the sensitivity is lower. The resolution and sensitivity of magnetic resonance imaging measurement are higher, whereas the temperature feedback time is long and the equipment is heavy and costly. Considering these disadvantages of temperature monitoring at present, it is vitally important to obtain real-time thermal feedback of photothermal heaters in deep tumors for controlling temperature and thermal dosage injection in PTT process. Lastly, the photothermal conversion materials are placed in lesions to enhance the light absorption and heat conversion in the tumors. The rapid development of biotechnology and nanotechnology provides several kinds of photothermal conversion materials including metallic nanoparticles21,22, magnetic nanoparticles23, semiconductor quantum dots (CdSe, CdTe)24,25, carbon-based nanomaterials (graphene, carbon nanotubes)26,27, organic nanoparticles (dyes, polymer, biomacromolecule)28–30 for PTT. Despite the effective conversion from light energy to heat, these photothermal conversion materials still exhibit some limitations such as weak biological compatibility and long-term toxicity in clinical applications31. The body may suffer from the acute inflammation and cell apoptosis which are caused by poor-metabolized metallic nanoparticles32,33. Carbon nanotubes not only suppress the immune system but also cause pulmonary inflammation and granuloma after bronchial instillation34,35. Graphene produces serious oxidative stress and irritates the skin, eye and respiratory system after intravenous injection26,36. Therefore, novel photothermal agents with low systemic toxicity and high conversion efficiency require to be developed for meeting clinical demands.
Rare earth (RE) doped upconversion nanoparticles (UCNPs) with many advantages of unique anti-Stokes fluorescence, large penetration depth of infrared (IR) excitation light into tissues, low background noise, high chemical stability and low toxicity, have been extensively investigated in biodetection, drug delivery, fluorescence labeling and imaging and optical temperature sensors37–42. Typically, Yb3+ is widely used as photo-sensitizer due to its larger absorption cross section at 980 nm, and then it could transfer the absorbed energy to the quenchers (for example Sm3+) which can accept the energy and produce heat via nonradiative relaxation43. Under 808 nm excitation, Nd3+ is commonly selected as sensitizer to absorb the light energy effectively. The photothermal conversion effect induced by 808 nm laser irradation in Sm3+/Nd3+ co-doped NaY(WO4)2 microstructures was investigated44. The advantage of adopting Nd3+ as both the photothermal conversion and temperature sensing is that both the excitation emission wavelengths are in the biological window and the deep tissue treatment can be achieved45,46. These investigations revealed that the RE doped nanoparticles can be photothermal conversion candidates, and can also achieve effective temperature detection at the exact position where the RE doped nanoparticles located at. Compared with the traditional temperature measurements, the kind of non-contact optical temperature sensor can monitor and feed back the real-time temperature of tumors in deep tissues. From what mentioned above, combining the photothermal conversion and temperature sensing in one nanoplatform would be beneficial to the practical application in advanced PTT47. Although scientists have made significant progresses in the vivo photothermal therapy treatments by using optical temperature sensing nanoparticles to read temperature48,49, developing novel nanoparticles possessing both the photothermal conversion and temperature sensing is still a challenge50–52.
According to the above analysis, in this work, we intend to develop a nano-system in which the functions of photothermal conversion and temperature sensing are expected to be integrated. Thereby, a core-shell nanostructure composed of the NaYF4:Er3+/Yb3+ core and NaYF4:Tm3+/Yb3+ shell is designed. The reason of choosing this nanostructure is that Er3+/Yb3+ couple can realize temperature sensing53–56, and Tm3+/Yb3+ couple exhibited strong photo-heat conversion57, meanwhile the NaYF4 core-shell structured nanoparticles can be easily prepared via thermal decomposition route. Therefore, in this work, the NaYF4:Er3+/Yb3+@NaYF4:Tm3+/Yb3+ core-shell nanoparticles were prepared, and their crystal structure and morphology were characterized by means of XRD, SEM and TEM. Furthermore, the spectroscopic study indicated that the core-shell nanoparticles presented excellent temperature sensing and photothermal conversion performance. It should be emphasized that the heat converted from the core-shell structure comes not only from the NaYF4:Tm3+/Yb3+ shell but also from the NaYF4:Er3+/Yb3+ core, and the former contributes more heat than that of the latter. This is because of the fact that the full upconversion luminescence process of the NaYF4:Er3+/Yb3+ core is accompanied by the nonradiative transitions from all the populated energy levels, and these nonradiative transitions will produce heat.
Results and Discussion
Structure and morphology of NaYF4 core and core-shell structure
The XRD patterns for the pulverized NaYF4: Er3+/Yb3+ core and NaYF4: Er3+/Yb3+@NaYF4: Tm3+/Yb3+ core-shell are shown in Fig. 1. All the diffraction peaks can be well indexed in accordance with diffraction pattern of hexagonal NaYF4 powder (in JCPDS card no. 28-1192), which indicates that the samples prepared are free of impurities, neither the introduction of RE3+ into the host nor the shell coating has effect on the crystal phase. The diffraction peaks of NaYF4:Er3+/Yb3+@NaYF4:Tm3+/Yb3+ core-shell nanoparticles are slightly narrower than that of the core. From the diffraction peaks at 2θ angle of 17°, the crystallite sizes of the samples are estimated based on the Debye-Scherrer formula () to be approximately 21.2 nm for the core and 26.2 nm for the core-shell nanoparticles, respectively. This means that the expected NaYF4:Er3+/Yb3+@NaYF4:Tm3+/Yb3+ core-shell structure might be received via the synthesis approach stated in Experimental section.
Figure 1.
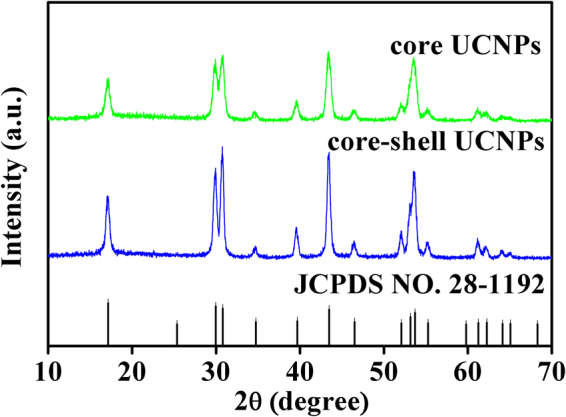
XRD patterns of the NaYF4:Er3+/Yb3+ core and NaYF4: Er3+/Yb3+@NaYF4: Tm3+/Yb3+ core-shell UCNPs, the standard card of β-NaYF4 (JCPDS: no. 28-1192) is shown as reference.
To observe the microscopic morphology for the NaYF4:Er3+/Yb3+ core and NaYF4:Er3+/Yb3+@NaYF4:Tm3+/Yb3+ core-shell structure, SEM images were taken and are shown in Fig. 2a,b, respectively. It can be seen from the images that both core and core-shell samples are composed of uniform and regular sphere-like particles. For more clearly observing histological morphology of the samples, the TEM images of the synthesized core and core-shell samples were measured and are displayed in Fig. 2c,d, respectively. It is found that particles in the naked core sample in Fig. 2c are monodispersed and sphere-shaped particles, but fewer particles showed hexagonal shape. Figure 2d for the core-shell sample displays that the particles remain monodispersed, but the morphology of all particles is of hexagonal shape. To estimate the particle sizes of the core and core-shell samples, the sizes of 70 particles for each sample were measured from the TEM images by using noncommercial software. The particle size histograms of core and core-shell samples are shown in Fig. 2f,g, respectively. It is found from Fig. 2f,g that the particle size distribution is narrow, and the average diameter of core sample is determined to be 19.2 nm, and the mean particle size of core-shell sample increases up to 23.2 nm. The results are nearly consistent with the calculation results derived from XRD patterns. These statistical results for particle sizes imply that NaYF4:Er3+/Yb3+ nanoparticles were successfully coated with about 2.0 nm thickness NaYF4:Tm3+/Yb3+ shell. Figure 2e exhibits the HRTEM images of core-shell UCNPs, the two adjacent lattice fringes with a lattice spacing of 0.52 nm matches well with that of the (100) plane in hexagonal NaYF4. The obvious lattice fringes in the HRTEM images further confirm the core-shell structure possesses high crystallinity.
Figure 2.
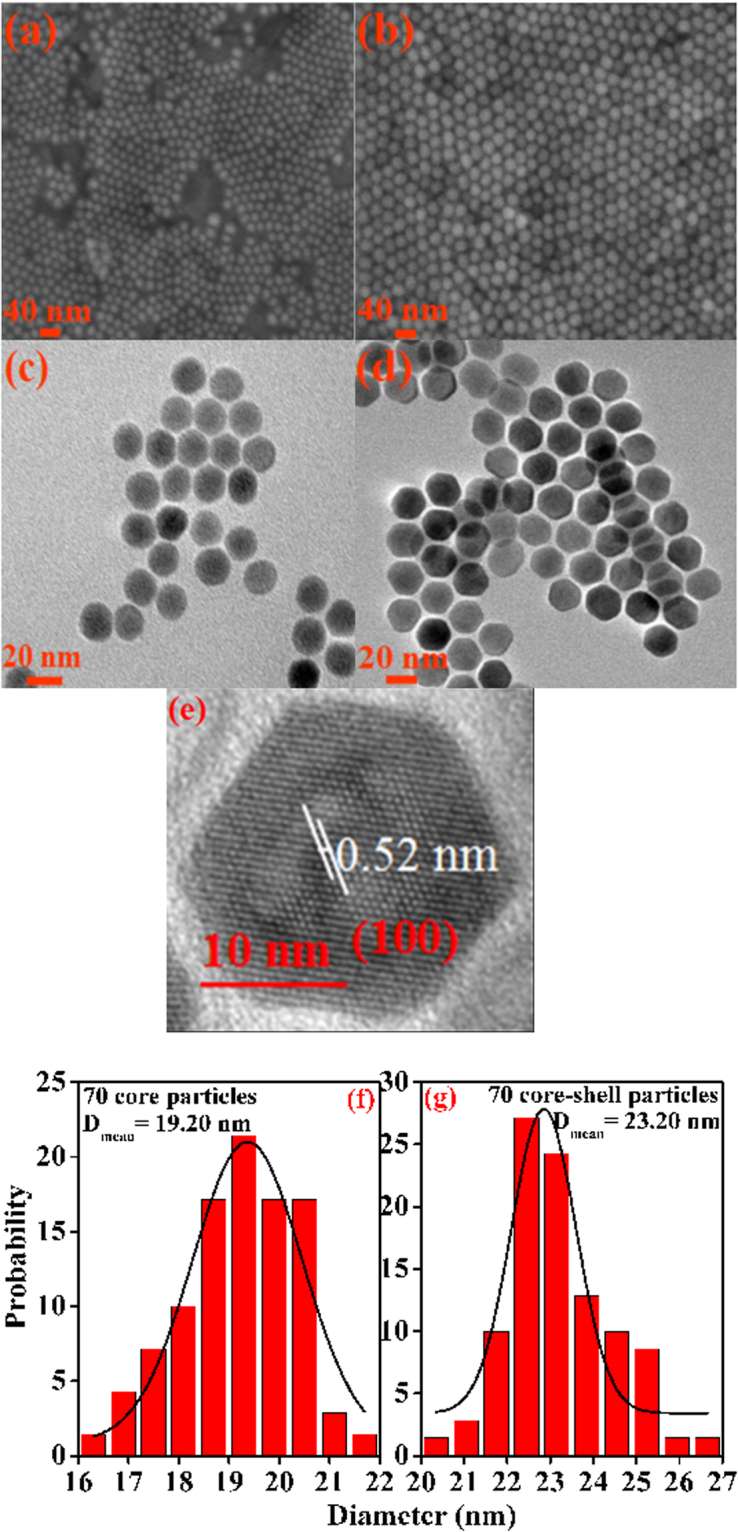
(a) and (b) SEM images of core and core-shell UCNPs, (c) and (d) TEM images of core and core-shell UCNPs, (e) HRTEM images of the core-shell sample, (f) and (g) Size distribution histograms of the core and core-shell nanoparticles derived from the TEM images.
Temperature sensing properties of NaYF4:Er3+/Yb3+ and NaYF4:Er3+/Yb3+@NaYF4: Tm3+/Yb3+ core-shell particles
Usually, the temperature at tissue surface is relatively easy to be detected with non-contact thermal camera, but the PTT process of tumor located in deep tissue would require real time temperature reading. Therefore, it is necessary to develop a non-contact thermometer. The present studied NaYF4:Er3+/Yb3+@NaYF4:Tm3+/Yb3+ core-shell in which Er3+ has two adjacent levels, namely 4S3/2 and 2H11/2 in thermal equilibrium, may be a good temperature sensing material. Because the emission intensity ratio of 2H11/2 → 4I15/2 to 4S3/2 → 4I15/2 is equal to the population ratio of 2H11/2 to 4S3/2 which is only dependent on the sample temperature for a certain system. These relations can be mathematically expressed as below.
| 1 |
In above equation, R stands for the fluorescence intensity ratio; I H and I S represents the integrated emission intensities of 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2, respectively; C is a constant depending on the doped RE3+ and host materials; ΔE is the energy gap between 2H11/2 and 4S3/2 states, k is the Boltzmann constant, and T is the absolute temperature. Therefore, the sample temperature can be readily derived by taking the measured fluorescence intensity ratio into equation (1) as long as ΔE and C are confirmed.
In order to obtain the parameters C and ΔE for the NaYF4:Er3+/Yb3+ and NaYF4:Er3+/Yb3+@NaYF4:Tm3+/Yb3+ core-shell particles, the temperature calibration experiments were carried out. In doing so, the cyclohexane solution containing OA-capped NaYF4:Er3+/Yb3+ particles or OA-capped NaYF4:Er3+/Yb3+@NaYF4:Tm3+/Yb3+ core-shell particles were prepared. The reason for using cyclohexane is that the OA-capped UCNPs could be well dispersed in cyclohexane to form stable colloidal solution directly without further surface modification, and the colloidal solution can maintain transparent and homogeneous for several days. The inset of Fig. 3 shows the images of the solution under irradiation of daylight and 980 nm fiber laser in dark background. The solution in both cases is homogeneous and ready for the spectral measurements. In the course of temperature calibration, 2 mL solution (4 Wt% of the nanoparticles) in cuvette was firstly heated via water bath to 373 K, and then moved to the sample chamber of the spectrometer for spectral measurement. The UC emission spectra at various temperatures were recorded when the solution was naturally cooled down until room temperature, and the solution temperatures were recoded via a K-type thermocouple connected to a proportional-integral-differential (PID) controller. It should be pointed out that when studying the temperature sensing property, the excitation power should be set as low as possible in order to avoid the laser-induced thermal effect39,40,43,57. To check this effect, the green UC emission spectra for the core-shell solution at room temperature were measured at different time under 980 nm fiber laser working at electric current of 1.0 A, and are shown in Fig. 3. It can be seen that all the spectral lines are accurately overlapped, thus implying that under the laser irradiation the solution temperature does not change obviously with time, that is to say, the thermal effect induced by constant laser irradiation can be omitted under these conditions. Therefore, these conditions except for the solution temperature were kept for the full processes of spectral measurements. It should be mentioned that the excitation power densities in this work were about 0.24 × 102 W/cm2 and 1.29 × 102 W/cm2 when the laser working currents were 1.0 and 2.0 A.
Figure 3.
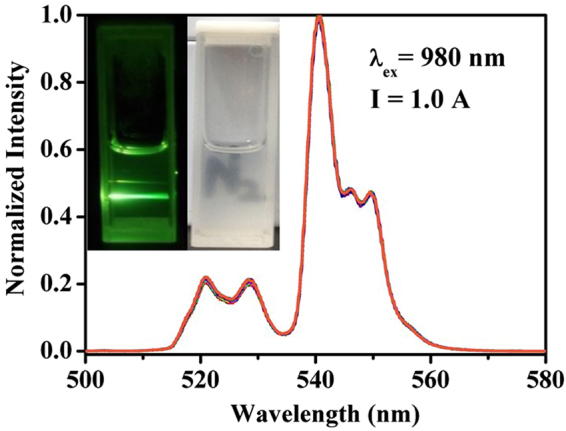
Normalized green UC emission spectra of NaYF4:Er3+/Yb3+@NaYF4:Tm3+/Yb3+ core-shell sample measured at different moments within 70 min when continuously excited by 980 nm fiber laser working at current of 1 A. The inset shows digital photos of core-shell particles in cyclohexane solution under daylight (right side) and under 980 nm irradiation in dark background (left side).
The insert in Fig. 4a shows the UC spectra for solution with core-shell sample at various temperatures ranged from 300 to 336 K. It can be seen that the emission intensities of 4S3/2 → 4I15/2 and 2H11/2 → 4I15/2 both decrease with increasing solution temperature, which is caused by the higher nonradiative transition rate of 4S3/2 level58. The integrated intensities at different temperatures for 4S3/2 → 4I15/2 and 2H11/2 → 4I15/2 transitions were calculated, and then the fluorescence intensity ratios were derived. The solid circles in Fig. 4a represent the dependence of integrated fluorescence intensity ratio on the solution temperature, and the solid line shows the fitting curve by using equation (1). The free parameters C and ΔE/k were confirmed to be 6.53 and 936.55 K from the fitting processes. In view of the above, the temperature response curve for the core-shell nanoparticles can be received by taking the parameters C and ΔE/k into equation (1). When the temperature response curve is known, the sensitivity (S) for temperature detection can be defined as derivative of fluorescence intensity ratio (R) with respect to temperature (T), and written as follows,
| 2 |
Figure 4.
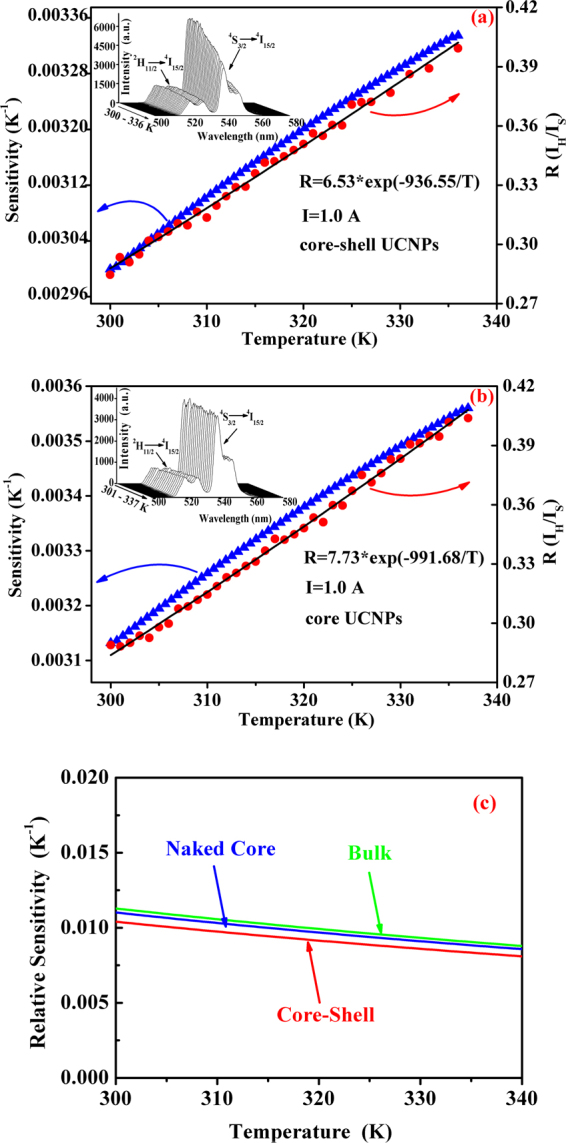
Dependence of green emission intensity ratio (IR/IS) (•) and sensitivity (▴) on sample temperature, the solid curves represent the fitting curves of experimental data to equation (1) (a) for NaYF4:Er3+/Yb3+@ NaYF4:Tm3+/Yb3+ core-shell particles and (b) for NaYF4:Er3+/Yb3+ particles. The inserts show the upconversion spectra for both the samples measured at different temperatures. (c) Shows the dependence of relative sensitivities for the naked core, core-shell structure and the bulk on the temperature.
On the basis of equation (2), the temperature sensing sensitivity curve (solid triangle dots) was obtained and is shown in Fig. 4a. It is confirmed that the sensitivity ranges from 0.0030 to 0.0033 K−1 in the temperature range of 300–336 K.
It should be mentioned that in above-studied core-shell structure the temperature sensing component, namely the NaYF4:Er3+/Yb3+ core, is covered by NaYF4:Tm3+/Yb3+ shell which is expected to play the part of calorifiers. Therefore, it is necessary to clarify if the temperature sensing performance of the core-shell particles becomes worse. For this purpose, the temperature sensing properties for the NaYF4:Er3+/Yb3+ particles were studied in a analogical way as done for the core-shell sample. The obtained results are shown in Fig. 4b where the insert depicts the UC spectra measured at various temperatures, and the solid circles present the dependence of fluorescence intensity ratio on the sample temperature, the solid up-triangles gives the temperature-dependent sensitivity. The parameters C and ΔE/k in equation (1) were confirmed to be 7.73 and 991.68 K, and the sensitivity increases from 0.003 to 0.0035 K−1 as temperature increases from 301 to 337 K. In comparison, the temperature sensing properties of the naked core and the core-shell are almost the same, thus indicating that the temperature sensing performance of NaYF4:Er3+/Yb3+ component in core-shell structure does not abate after coating with NaYF4:Tm3+/Yb3+ shell. It should be mentioned that the UC luminescence intensity of NaYF4:Er3+/Yb3+@ NaYF4:Tm3+/Yb3+ core-shell is 1.6 times higher than that of the naked NaYF4:Er3+/Yb3+ core under the same experimental conditions. The mechanism of luminescence enhancement in the core-shell structure can not be easily deduced from the present spectral data, nonetheless, the intense UC emissions would be beneficial to the temperature measurement in the PTT application, since intense enough fluorescence signal would lower the requirement for high performance sensor in the spectral measurements, and meanwhile make the extraction of UC emissions from tissue easier. Here, we should emphasize that both the green emissions of Er3+ are in the outside of the biological windows, therefore the core-shell nanoparticles can not be used for the tumor treatment in deep tissue, but they may be useful for treatment of superficial tissue.
In fact, the temperature sensing of lanthanide ions has been widely studied45,59–61. To further evaluate the temperature sensing performance of the designed core-shell structure, the relative sensitivities for the naked core, core-shell structure and the bulk NaYF4:Er3+/Yb3+ were calculated in the temperature region from 300 to 340 K by using the data obtained in this work and our previous work60. The relative sensitivity is defined as where R and S are determined by Eqs (1) and (2). The calculated results are shown in Fig. 4c. From Fig. 4c it can be seen that all the relative sensitivities for these three particles decrease with increasing temperature, and the relative sensitivity for NaYF4:Er3+/Yb3+@ NaYF4:Tm3+/Yb3+ core-shell structure does not change obviously in comparison with the naked NaYF4:Er3+/Yb3+ cores and the bulk NaYF4:Er3+/Yb3+ produced by our group. To compare the relative sensitivity of our designed core-shell structure with those in references, the ΔE/k for NaYF4:Er3+/Yb3+ particles under different excitation wavelengths and in different forms including core-shell structures and different crystal phases are collected and listed in Table 1 62–71. The 1st column of Table 1 presents the size, morphology or structure; the phase and the excitation wavelength for each NaYF4:Er3+/Yb3+ particles are listed in 2nd and 3rd columns; the 4th column contains the ΔE/k values for all the NaYF4:Er3+/Yb3+ samples; the last column gives references. From Table 1 it can be found that the parameter ΔE/k determining relative sensitivity of NaYF4:Er3+/Yb3+ particles changes greatly from sample to sample, and our samples exhibit moderate ΔE/k values amongst all the results from different research groups. In this study, the temperature sensing properties of the naked core and core-shell structure were derived under the condition that the nanoparticles were immersed in cyclohexane liquid. Here we should point out that the most recent investigation proves that the temperature sensing properties of rare earth doped nanoparticles depend also on the immersion liquid environment72, thus the obtained results can be different when they are used in in-vivo systems.
Table 1.
Δ E/k for NaYF4:Er3+/Yb3+ with different sizes, morphologies and structures.
| Status of NaYF4:Er3+/Yb3+ | Crystal Phase | Excitation Length (nm) | Δ E/k (k−1) | Refs. |
|---|---|---|---|---|
| core-shell NaYF4:Er3+/Yb3+@NaYF4:Nd3+/Yb3+ | beta- | 800 | 993.2±18.8 | 62 |
| silane modified NaYF4:Er3+/Yb3+ | beta- | 980 | 1065.9 | 63 |
| 75 nm NaYF4:Er3+/Yb3+ | alpha- | 980 | 1025.8 | 60 |
| rods, 200 nm in diameter, 400 nm in length, NaYF4:Er3+/Yb3+ | beta- | 980 | 1085.3 | 60 |
| 15 nm, NaGdF4:Er3+/Yb3+ | beta- | 980 | 1013±28 | 64 |
| 15 nm core, 5 nm shell NaGdF4:Er3+/Yb3+@NaYF4 | 1060±55 | |||
| 15 nm core and 10 nm shell NaGdF4:Er3+/Yb3+@NaYF4 | 1035±44 | |||
| 15 nm core and 15 nm shell NaGdF4:Er3+/Yb3+@NaYF4 | 1027±32 | |||
| 27 nm, NaYF4:Er3+/Yb3+@SiO2 | beta- | 980 | 969 | 65 |
| bulk NaYF4:Er3+/Yb3+/Li+ | beta- | 980 | 1198.2±41.18 | 66 |
| polydimethylsiloxane stabled NaYF4:Er3+/Yb3+ | beta- | 980 | 1044.16 | 67 |
| 370 nm in diameter, 419 nm in length NaYF4:Er3+/Yb3+ | beta- | 980 | 748.9 | 68 |
| nanosized NaYF4:Er3+/Yb3+ | beta- | 980 | 1028.5 | 69 |
| nanosized NaYF4:Er3+/Yb3+@SiO2 | 1031.5 | |||
| bulk NaYF4:Er3+/Yb3+ | beta- | 980 | 1306.72±12.57 | 70 |
| nanowire NaYF4:Er3+/Yb3+ | 1317.39±4.81 | |||
| nanorods NaYF4:Er3+/Yb3+ | 1343.14±29.35 | |||
| nanoplates NaYF4:Er3+/Yb3+ | 1397.41±9.39 | |||
| hexagonal microprisms 6 μm in length and 2μm in diameter NaYF4:Er3+/Yb3+ | beta- | 980 | 1082.1 | 71 |
| 19 nm, NaYF4:Er3+/Yb3+ | beta- | 980 | 936.55 | This work |
| 19 nm core, 4 nm shell NaYF4:Er3+/Yb3+@ NaYF4:Tm3+/Yb3+ | 991.68 |
Photothermal conversion effect of NaYF4 core-shell structure as nano-calorifier
In above section, the NaYF4:Er3+/Yb3+@NaYF4:Tm3+/Yb3+ core-shell particles were qualified for temperature sensing as good as NaYF4:Er3+/Yb3+ particles. The aim of this work is to develop bifunctional nanomaterials having both temperature sensing and photothermal conversion for PTT, therefore, in this section we will discuss about the photothermal conversion properties of the NaYF4:Er3+/Yb3+@NaYF4:Tm3+/Yb3+ core-shell particles.
To examine the photothermal conversion effect of the core-shell nanoparticles, the cyclohexane solutions with NaYF4:Er3+/Yb3+@NaYF4:Tm3+/Yb3+ core-shell particles and NaYF4:Er3+/Yb3+ nanoparticles were again used, and 980 nm fiber laser was adopted as excitation source. The procedure for photothermal conversion measurements is designed as follows. The solution containing nanoparticles is continuously irradiated by 980 nm laser, and then the UC spectra are measured at different time when the laser works at the current of 2.0 A. For the sake of studying the influence of core-shell particles’ content in solution on the heat generation, three solutions containing 5 Wt% naked NaYF4:Er3+/Yb3+ nanoparticles (named as solution I), 6 Wt% (solution II) and 13 Wt% (solution III) NaYF4:Er3+/Yb3+@NaYF4:Tm3+/Yb3+ core-shell particles in 7 mL cyclohxane were prepared, and 2 mL of each solution was used for the spectral measurements.
To confirm that the heat generation of the system was caused by the nanoparticles but not cyclohexane via absorbing 980 nm irradiation, the absorption spectra of the pure cyclohexane and solution III were measured and are shown in Fig. 5. The absorption peak at about 925 nm can be seen in both solutions, which originates from the solvent cyclohexane. It should be noted that the absorption of pure cyclohexane at 980 nm is very weak, and the absorbed light may convert to other lights with different wavelengths or transform to heat energy. To examine the emissions of pure cyclohexane under 980 nm excitation, its emission spectra in the ranges from 200–900 nm on F-4600 (Hitachi) and from 900–2250 nm on NIRQuest-256 (Ocean Optics) were measured, and no any emissions were observed, thus implying that the absorbed light energy by cyclohexane can completely converted into heat energy. This means that the heat generation by cyclohexane can not be neglected. The absorption peak at about 976 nm corresponding to the 2F7/2 → 2F5/2 transition of Yb3+ is observed in solution III. The intense absorption centered at 976 nm in solution III containing core-shell nanoparticles is prerequisite for effective photothermal conversion, but this does not mean that the photothermal conversion can be achieved. Therefore, the photothermal conversion behavior should be further experimentally checked.
Figure 5.
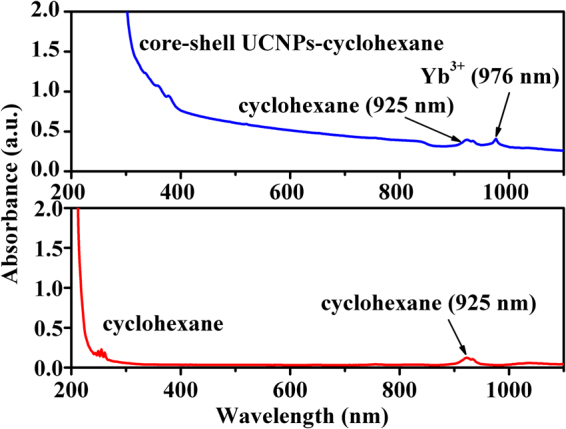
Absorption spectra of pure cyclohexane (control) and solution III.
As an example, Fig. 6 shows the UC spectra for solution III measured at different moments within 70 min under irradiation of 980 nm laser working at current of 2.0 A. To find the change of solution temperature with time, the UC emission intensity ratios R (I H /I S) were calculated, and then the solution temperatures at different moments were derived based on the obtained temperature response curve (Fig. 4a). Hereby, the relations between the temperature of all the solutions and laser irradiation time are shown in Fig. 7. For comparison, the time-dependent temperature for the pure cyclohexane was also given in Fig. 7. The temperature for the pure cyclohexane was measured with a thermocouple under the same experimental condition. From Fig. 7 it can be seen that the temperature of pure cyclohexane grows steadily with the increase of irradiation time within 30 min, and then keeps unchanged after reaching around 304 K, thus indicating that a thermal equilibrium is achieved in the studied system. This means that though the absorption of pure cyclohexane is weak at 980 nm, the photothermal conversion can not be ignored. This observed result for pure cyclohexane coincides with the deduction we made based on Fig. 5. In fact, the absorption of body fluid is also existent in the practical applications of PTT. Therefore, the investigation for photothermal conversion in solution environment may have more practical significance than that in solid powders. It can be found from Fig. 7 that the other solutions with nanoparticles display a similar variation trend of temperature toward irradiation time as the pure cyclohexane does, but they are really different from each others. The temperature increasing rate for solution I at initial moments is larger than that of pure cyclohexane, and the temperature at the final thermal equilibrium is higher than that of cyclohexane. These results indicate that NaYF4:Er3+/Yb3+ nanoparticles have contribution to the photothermal conversion. In comparison with solution I, the solution II and III exhibit more effective photothermal conversion since the final thermal equilibrium temperatures are higher, and the temperature increasing rates in initial stage of laser irradiation are larger. It should also be noted that solution III, which contains more amounts of core-shell particles than that of solution II, displays more effective photothermal conversion behavior. Additionally, though solution I and solution II contain approximately equal amount of nanoparticles (naked NaYF4:Er3+/Yb3+ core in solution I; NaYF4:Er3+/Yb3+@NaYF4:Tm3+/Yb3+ core-shell in solution II), the final equilibrium temperature of solution II is much higher than that of solution I, thus indicating that the NaYF4:Tm3+/Yb3+ shell plays important role in the photothermal conversion. It should be emphasized that a net temperature incensement of about 15 K from room temperature 295 K to final equilibrium temperature around 310 K can be obtained in solution III. The net temperature increment is around 7 K (see Fig. 7) when the contribution of heat generation by the solvent (cyclohexane) was deducted. It should be mentioned that less than one fifth of volume for the solution but not the full volume were irradiated by the laser beam. Meanwhile, it should also be noted that this 7 K increment is accomplished in the solution with low amount of the studied core-shell nanoparticles (photothermal agent), and the solution in a thermally open environment, thus it can be expected that a 6 K temperature increment from 37 (normal body temperature) to 43 °C (effective tumor treatment temperature) can be obtained by increasing amount of the core-shell nanoparticles deposited on tumor tissues which are surrounded by other tissues other than the open environment, since the temperature increment is proportional to the absorbed heat quality.
Figure 6.
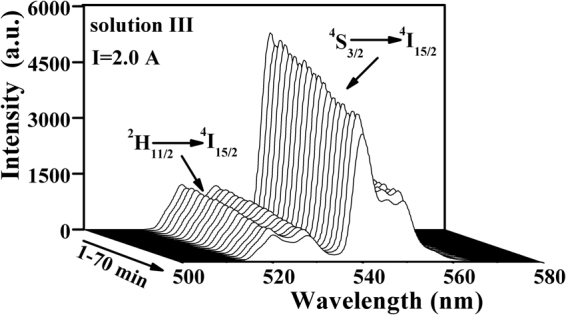
UC emission spectra of core-shell particles functioned as nano-calorifiers in solution III excited by 980 nm laser working at 2 A for 70 min.
Figure 7.
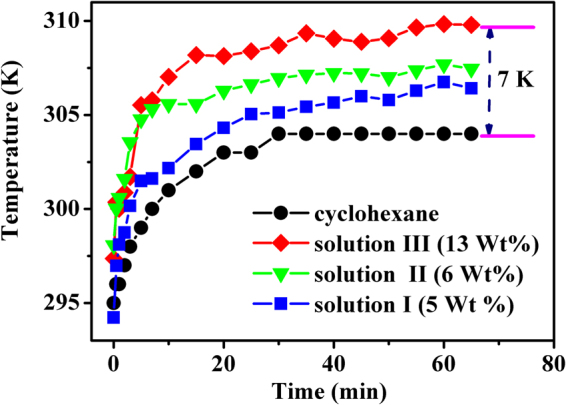
Relationship between the temperature of cyclohexane, solutions (I, II and III) and the laser irradiation time. Solid dots show the experimental data, solid curves indicate the variation trend.
Conclusions
The core-shell structured NaYF4:Er3+/Yb3+@NaYF4:Tm3+/Yb3+ nanoparticles were successfully synthesized via a thermolysis reaction, and characterized by means of XRD, SEM, TEM/HRTEM, from which it was found that the average core diameter was 19.2 nm and shell thickness was about 2.0 nm. In assistance of water bath heating the optical temperature sensing for the NaYF4:Er3+/Yb3+@NaYF4:Tm3+/Yb3+ core-shell nanoparticles was studied and compared with naked NaYF4:Er3+/Yb3+ core nanoparticles. It was found that the coating of photothermal converting NaYF4:Tm3+/Yb3+ shell did not obviously injure the temperature sensing performance of the core-shell particles, but improved the luminescence intensities of the thermally coupled 2H11/2 and 4S3/2 levels, that further made the optical signal measurements easy. The photothermal conversion behavior for the core-shell nanoparticles were studied, and it was found that the cyclohexane solution with NaYF4:Er3+/Yb3+@NaYF4:Tm3+/Yb3+ core-shell nanoparticles exhibited more effective photothermal conversion than that of the NaYF4:Er3+/Yb3+ nanoparticles, and a net temperature increment of 7 K was achieved.
Experimental section
Materials
Yttrium oxide (Y2O3, 99.99%), ytterbium oxide (Yb2O3, 99.99%), erbium oxide (Er2O3, 99.99%) and thulium oxide (Tm2O3, 99.99%) were purchased from Shanghai Second Chemical Reagent Factory (China). Other chemicals including cyclohexane, absolute ethanol, methanol, sodium hydroxide and ammonium fluoride were analytical grade reagents which were purchased from Tianjin Reagent Chemicals Co., Ltd (China). Oleic acid (OA, 90%) and 1-octadecene (ODE, 90%) were purchased from Sigma-Aldrich and Aladdin, respectively.
Synthesis of lanthanide chloride with crystal water
A certain amount of RE2O3 (RE = Y, Er, Yb, Tm), water and hydrochloric acid (the volume ratio of hydrochloric acid to water is 1: 1) were added into a 250 mL beaker, then the mixture was heated under vigorous stirring until all the oxides were dissolved. Next, the transparent solution was re-crystallized five times with adding distilled water repeatedly. Lastly, the RECl3·6H2O (RE = Y, Er, Yb, Tm) samples were obtained when their corresponding crystallized products were well dried in air at 80°C for 12 h.
Synthesis of NaYF4: Er3+/Yb3+ core UCNPs
NaYF4: Er3+/Yb3+ core particles were synthesized by a thermal decomposition route. As an example, a typical procedure is presented below. A total amount of 2 mmol RECl3·6H2O (RE:78 mol% Y3+, 20 mol% Yb3+ and 2 mol% Er3+), 12 mL OA and 30 ml ODE were added into a 100 ml three-necked flask which was then vacuumized for 30 min to remove oxygen under magnetic stirring at room temperature. Next, the flask containing the mixture was heated to 150 °C to dissolve lanthanide chloride, and a yellow transparent solution under vacuum with magnetic stirring was formed. When the solution cooled down to room temperature naturally, 10 mL methanol solution with 5 mmol NaOH and 8 mmol NH4F was dropped into the flask slowly. After stirring and reacting at room temperature, the mixed solution was then heated to 100 °C in N2 atmosphere and kept for 1 h to remove residual methanol, oxygen and water. Subsequently, the solution was heated to 310 °C, vigorously stirred and hold at this temperature for 1 h in N2 atmosphere. After cooling down to room temperature, the final products were collected with absolute ethanol and centrifugated at 9000 rpm for 10 min. The white precipitate was washed three times with absolute ethanol/cyclohexane (3:1 v/v). Finally, the NPs were dispersed in 5 mL of cyclohexane for further characterizations.
Synthesis of NaYF4: Er/Yb@NaYF4: Tm/Yb core-shell structure
Following the same synthesis route above, the shell precursor solution containing 2 mmol RE2O3·6H2O (RE:79.5% mol Y3+, 20%mol Yb3+, 0.5%mol Tm3+), 12 mL OA and 30 ml ODE were prepared in the reaction vessel, after cooling down to room temperature, 2 mmol core product was added into the reaction vessel before adding methanol solution. After that the temperature was again increased up to 100 °C in N2 atmosphere and kept for 1 h to remove cyclohexane. After removing cyclohexane, the following synthesis procedure is the same as that of core particles presented above.
Characterization
The crystalline structure of the samples was characterized by XRD (X-ray diffractometer using Cu-Kα1 radiation source, λ = 0.15406 nm, SHIMADZU, Japan). The XRD data in 2θ ranging from 10° to 80° were collected with a scanning step size of 0.02°. Morphologies and microscopic structures were observed by a field emission SEM (FE-SEM, SUPRA 55 SAPPHIRE, RIGAKU, Janpan) and a high resolution TEM (HR-TEM, JEM-2100F, JOEL, Japan). The UC emission spectra were recorded by using F-4600 spectrophotometer (HITACHI, Japan) under excitation of an externally introduced 980 nm fiber laser. It should be mentioned that if without specific statement, the excitation power density was around 0.24 × 102 and 1.29 × 102 W/cm2 when the working currents of 980 nm laser were 1.0 and 2.0 A, respectively. The reason why the low power was used for the temperature calibration experiments is to avoid the laser-irradiation-induced heating effect. The temperature controlling experiments were carried out with quartz cuvette (10 m/m) heated by water bath, and a thermocouple was used to monitor the solution temperature.
Acknowledgements
This work was partially supported by NSFC (National Natural Science Foundation of China, Grant No. 11774042), Fundamental Research Funds for the Central Universities (Grant No. 3132016333), China Postdoctoral Science Foundation (Grant No. 2016M591420), the Natural Science Foundation of Zhejiang Province (Grant No. LZ17E020001), the Open Fund of the State Key Laboratory on Integrated Optoelectronics (IOSKL2015KF27).
Author Contributions
Yanqiu Zhang prepared the samples and wrote the first edition of the manuscript. Baojiu Chen made the research proposal and provided the financial support for this work, and also took the responsibility of all correspondence. Sai Xu revised the manuscript, made the final check. Xiangping Li, Jinsu Zhang, Jiashi Sun and Hui Zheng established the sample temperature controlling setup. Lili Tong and Hua Zhong edited a program for the temperature data collection, calibrated the temperatures. Guozhu Sui measured all the spectra. Haiping Xia provided the supports of measurements of SEM/TEM. Ruinian Hua made the XRD measurements and gave instructive suggestions for the spectral analyses; meanwhile he also took the responsibility of correspondence.
Competing Interests
All the authors have read the final version of the manuscript, agreed with the whole contents included in the manuscript and were aware of the submission to Scientific Reports.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Baojiu Chen, Email: chenmbj@sohu.com.
Ruinian Hua, Email: rnhua@dlnu.edu.cn.
References
- 1.Ebrahimi M. On the temperature control in self-controlling hyperthermia therapy. J. Magn. Magn. Mater. 2016;416:134–140. doi: 10.1016/j.jmmm.2016.04.095. [DOI] [Google Scholar]
- 2.DeNardo GL, DeNardo SJ. Turning the heat on cancer. Cancer. Biother. Radio. 2009;23:671–679. doi: 10.1089/cbr.2008.0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chicheł A, Skowronek J, Kubaszewska M, Kanikowski M. Hyperthermia-description of a method and a review of clinical applications. Rep. Pract. Oncol. Radiother. 2007;12:267–275. doi: 10.1016/S1507-1367(10)60065-X. [DOI] [Google Scholar]
- 4.Takahashi I, et al. Clinical application of hyperthermia combined with anticancer drugs for the treatment of solid tumors. Surgery. 2002;131:78–84. doi: 10.1067/msy.2002.119308. [DOI] [PubMed] [Google Scholar]
- 5.Franckena M, et al. Hyperthermia dose-effect relationship in 420 patients with cervical cancer treated with combined radiotherapy and hyperthermia. Eur. J. Cancer. 2009;45:1969–1978. doi: 10.1016/j.ejca.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Issels RD. Hyperthermia adds to chemotherapy. Eur. J. Cancer. 2008;44:2546–2554. doi: 10.1016/j.ejca.2008.07.038. [DOI] [PubMed] [Google Scholar]
- 7.Beik J, et al. Nanotechnology in hyperthermia cancer therapy: from fundamental principles to advanced applications. J. Control. Release. 2016;235:205–221. doi: 10.1016/j.jconrel.2016.05.062. [DOI] [PubMed] [Google Scholar]
- 8.Chen Q, Liu XD, Zeng JF, Cheng ZP, Liu Z. Albumin-NIR dye self-assembled nanoparticles for photoacoustic pH imaging and pH-responsive photothermal therapy effective for large tumors. Biomaterials. 2016;98:23–30. doi: 10.1016/j.biomaterials.2016.04.041. [DOI] [PubMed] [Google Scholar]
- 9.O’Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004;209:171–176. doi: 10.1016/j.canlet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Chen QW, et al. Recent advances in different modal imaging-guided photothermal therapy. Biomaterials. 2016;106:144–166. doi: 10.1016/j.biomaterials.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Jiang XJ, Li Z, Yao JR, Shao ZZ, Chen X. One-step synthesis of soy protein/graphene nanocomposites and their application in photothermal therapy. Mat. Sci. Eng C. 2016;68:798–804. doi: 10.1016/j.msec.2016.07.034. [DOI] [PubMed] [Google Scholar]
- 12.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 2003;7:626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Idris NM, Jayakumar MKG, Bansal A, Zhang Y. Upconversion nanoparticles as versatile light nanotransducers for photoactivation applications. Chem. Soc. Rev. 2015;44:1449–1478. doi: 10.1039/C4CS00158C. [DOI] [PubMed] [Google Scholar]
- 14.Liang C, et al. Magnetic field-enhanced photothermal ablation of tumor sentinel lymph nodes to inhibit cancer metastasis. Small. 2015;11:4856–4863. doi: 10.1002/smll.201501197. [DOI] [PubMed] [Google Scholar]
- 15.Arora N, et al. Effectiveness of a noninvasive digital infrared thermal imaging system in the detection of breast cancer. Am. J. Surg. 2008;196:523–526. doi: 10.1016/j.amjsurg.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Kontos M, Wilson R, Fentiman I. Digital infrared thermal imaging (DITI) of breast lesions: sensitivity and specificity of detection of primary breast cancers. Clin. Radiol. 2011;66:536–539. doi: 10.1016/j.crad.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Steiner P, et al. Radio-frequency-induced thermoablation: monitoring with T-weighted and proton-frequency-shift MR imaging in a interventional 0.5 T environment. Radiol. 1998;206:803–810. doi: 10.1148/radiology.206.3.9494505. [DOI] [PubMed] [Google Scholar]
- 18.Vasallo OD, Marin E. The application of the photoacoustic technique to the measurement of the thermal effusivity of liquids. J. Phys. D: Appl. Phys. 1999;32:593–597. doi: 10.1088/0022-3727/32/5/014. [DOI] [Google Scholar]
- 19.Raveendranath K, Ravi J, Jayalekshmi S, Rasheed TMA, Nair KPR. Thermal diffusivity measurement on LiMn2O4 and its de-lithiated form (λ-MnO2) using photoacoustic technique. Mater. Sci. Eng.,B. 2006;131:210–215. doi: 10.1016/j.mseb.2006.04.012. [DOI] [Google Scholar]
- 20.Seip R, VanBaren P, Cain CA, Ebbini ES. Noninvasive real-time multipoint temperature control for ultrasound phased array treatments. IEEE. T. Ultrason. Ferr. 1996;43:1063–1073. doi: 10.1109/58.542050. [DOI] [Google Scholar]
- 21.Dickerson EB, et al. Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer Lett. 2008;269:57–66. doi: 10.1016/j.canlet.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang XH, El-Sayed MA. Gold nanoparticles: optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010;1:13–28. doi: 10.1016/j.jare.2010.02.002. [DOI] [Google Scholar]
- 23.Kumar CSSR, Mohammad F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug. Deliver. Rev. 2011;63:789–808. doi: 10.1016/j.addr.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu MQ, et al. The therapeutic efficacy of CdTe and CdSe quantum dots for photothermal cancer therapy. Biomaterials. 2012;33:7071–7083. doi: 10.1016/j.biomaterials.2012.06.062. [DOI] [PubMed] [Google Scholar]
- 25.Jiang TT, et al. A Au nanoflower@SiO2@CdTe/CdS/ZnS quantum dot multi-functional nanoprobe forphotothermal treatment and cellular imaging. RSC Adv. 2014;4:23630–23636. doi: 10.1039/C4RA02965H. [DOI] [Google Scholar]
- 26.Kim SH, et al. In vitro and vio tumor targeted photothermal cancer therapy using functionalized graphene nanoparticles. Biomacromolecules. 2015;16:3519–3529. doi: 10.1021/acs.biomac.5b00944. [DOI] [PubMed] [Google Scholar]
- 27.Chou HT, Wang TP, Lee CY, Tai NH, Chang HY. Photothermal effects of multi-walled carbon nanotubes on the viability of BT-474 cancer cells. Mat. Sci. Eng C. 2013;33:989–995. doi: 10.1016/j.msec.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 28.Yang K, et al. In vitro and in vivo near-infrared photothermal therapy of cancer using polypyrrole organic nanoparticles. Adv. Mater. 2012;24:5586–5592. doi: 10.1002/adma.201202625. [DOI] [PubMed] [Google Scholar]
- 29.Cheng L, Yang K, Chen Q, Liu Z. Organic stealth nanoparticles for highly effective in vivo near-infrared photothermal therapy of cancer. Acs. Nano. 2012;6:5605–5613. doi: 10.1021/nn301539m. [DOI] [PubMed] [Google Scholar]
- 30.Zha ZB, Yue XL, Ren QS, Dai ZF. Uniform polypyrrole nanoparticles with high photothermal conversion efficiency for photothermal ablation of cancer cells. Adv. Mater. 2013;25:777–782. doi: 10.1002/adma.201202211. [DOI] [PubMed] [Google Scholar]
- 31.Jaque D, et al. Nanoparticles for photothermal therapies. Nanoscale. 2014;6:9494–9530. doi: 10.1039/C4NR00708E. [DOI] [PubMed] [Google Scholar]
- 32.Bayazitoglu Y, Kheradmand S, Tullius K. An overview of nanoparticles assisted laser therapy. Int. J. Heat Mass Transfer. 2013;67:469–486. doi: 10.1016/j.ijheatmasstransfer.2013.08.018. [DOI] [Google Scholar]
- 33.Pan Y, et al. Size-dependent cytotoxicity of gold nanoparticles. Small. 2007;3:1941–1949. doi: 10.1002/smll.200700378. [DOI] [PubMed] [Google Scholar]
- 34.Popov AM, Lozovik YE, Fiorito S, Yahia L. Biocompatibility and applications of carbon nanotubes in medical nano robots. Int. J. Nanomedicine. 2007;2:361–372. [PMC free article] [PubMed] [Google Scholar]
- 35.Chiu-wing L, John TJ, Richard M, Sivaram A, Robert LH. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit. Rev. Toxicol. 2006;36:189–217. doi: 10.1080/10408440600570233. [DOI] [PubMed] [Google Scholar]
- 36.Vallabani NVS, et al. Toxicity of graphene in normal human lung cells (BEAS-2B) J. Biomed. Nanotechnol. 2011;7:106–107. doi: 10.1166/jbn.2011.1224. [DOI] [PubMed] [Google Scholar]
- 37.Xu S, et al. NaYF4:Yb,Tm nanocrystals and TiO2 inverse opal composite films: a novel device for upconversion enhancement and solid-based sensing of avidin. Nanoscale. 2014;6:5859–5870. doi: 10.1039/C4NR00224E. [DOI] [PubMed] [Google Scholar]
- 38.Xu S, et al. A novel upconversion, fluorescence resonance energy transfer biosensor (FRET) for sensitive detection of lead ions in human serum†. Nanoscale. 2014;6:12573–12579. doi: 10.1039/C4NR03092C. [DOI] [PubMed] [Google Scholar]
- 39.Zheng H, et al. Microwave-assisted hydrothermal synthesis and temperature sensing application of Er3+/Yb3+ doped NaY(WO4)2 microstructures. J. Colloid. Interf. Sci. 2014;420:27–34. doi: 10.1016/j.jcis.2013.12.059. [DOI] [PubMed] [Google Scholar]
- 40.Zheng H, et al. Temperature sensing and optical heating in Er3+ single-doped and Er3+/Yb3+ codoped NaY(WO4)2 particles†. RSC Adv. 2014;4:47556–47563. doi: 10.1039/C4RA05492J. [DOI] [Google Scholar]
- 41.Tian Y, et al. Optical transition, electron-phonon coupling and fluorescent quenching of La2(MoO4)3:Eu3+ phosphor. J. Appl. Phys. 2011;109:053511. doi: 10.1063/1.3551584. [DOI] [Google Scholar]
- 42.Tian BN, et al. Excitation pathway and temperature dependent luminescence in color tunable Ba5Gd8Zn4O21:Eu3+ phosphors. J. Mater. Chem. C. 2013;1:2338–2344. doi: 10.1039/c3tc00915g. [DOI] [Google Scholar]
- 43.Zheng H, et al. Rod-shaped NaY(MoO4)2:Sm3+/Yb3+ nanoheaters for photothermal conversion: influence of doping concentration and excitation power density. Sensor. Actuat. B-Chem. 2016;234:286–293. doi: 10.1016/j.snb.2016.04.162. [DOI] [Google Scholar]
- 44.Xu S, et al. 808 nm laser induced photothermal effect on Sm3+/Nd3+ doped NaY(WO4)2 microstructures. Sensor. Actuat. B-Chem. 2017;240:386–391. doi: 10.1016/j.snb.2016.08.176. [DOI] [Google Scholar]
- 45.Xu W, Qi HY, Zheng LJ, Zhang ZG, Cao WW. Multifunctional nanoparticles based on the Nd3+/Yb3+ codoped NaYF4. Opt. Lett. 2015;40:5678–5681. doi: 10.1364/OL.40.005678. [DOI] [PubMed] [Google Scholar]
- 46.Rocha U, Kumar KU, Jacinto C, Ramiro J, Caamano A. Nd3+ doped LaF3 nanoparticles as self-monitored photothermal agents. Appl. Phys. Lett. 2014;104:053703. doi: 10.1063/1.4862968. [DOI] [Google Scholar]
- 47.Debasu ML, et al. All-in-one optical heater-thermometer nanoplatform operative from 300 to 2000 K based on Er3+ emission and blackbody radiation. Adv. Mater. 2013;25:4868–4874. doi: 10.1002/adma.201300892. [DOI] [PubMed] [Google Scholar]
- 48.Carrasco E, et al. Intratumoral thermalreading during photo-thermal therapy by multifunctional fluorescent nanoparticles. Adv. Funct. Mater. 2015;25:615–626. doi: 10.1002/adfm.201403653. [DOI] [Google Scholar]
- 49.Zhu XJ, et al. Temperature-feedback upconversion nanocomposite for accurate photothermal therapy at facile temperature. Nat. Comm. 2016;7:10437. doi: 10.1038/ncomms10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosal B, et al. Infrared-emitting QDs for thermal therapy with real-time subcutaneous temperature feedback. Adv. Funct. Mater. 2016;26:6060–6068. doi: 10.1002/adfm.201601953. [DOI] [Google Scholar]
- 51.Rosal B, Ximendes E, Rocha U, Jaque D. In Vivo luminescence nanothermometry: from materials to applications. Adv. Opt. Mater. 2017;5:1600508. doi: 10.1002/adom.201600508. [DOI] [Google Scholar]
- 52.Rosal B, Rocha U, Ximendes EC, Martin Rodriguez E, Jaque D. Nd3+ ions in nanomedicine: Perspectives and applications. Opt. Mater. 2017;63:185–196. doi: 10.1016/j.optmat.2016.06.004. [DOI] [Google Scholar]
- 53.Huang F, Gao Y, Zhou JC, Xua J, Wang YS. Yb3+/Er3+ co-doped CaMoO4: a promising green upconversion phosphor for optical temperature sensing. J. Alloy. Compd. 2015;639:325–329. doi: 10.1016/j.jallcom.2015.02.228. [DOI] [Google Scholar]
- 54.Du P, Luo LH, Yu JS. Upconversion emission, cathodoluminescence and temperature sensing behaviors of Yb3+ ions sensitized NaY(WO4)2:Er3+ phosphors. Ceram. Int. 2016;42:5635–5641. doi: 10.1016/j.ceramint.2015.12.083. [DOI] [Google Scholar]
- 55.Sinha S, Mahata MK, Kumar K, Tiwari SP, Rai VK. Dualistic temperature sensing in Er3+/Yb3+ doped CaMoO4 upconversion phosphor. Spectrochim. Acta A. 2017;173:369–375. doi: 10.1016/j.saa.2016.09.039. [DOI] [PubMed] [Google Scholar]
- 56.Siaï A, González PH, Naifer KH, Férid M. La2O3: Tm, Yb, Er upconverting nano-oxides for sub-tissue lifetime thermal sensing. Sensor. Actuat. B-Chem. 2016;234:541–548. doi: 10.1016/j.snb.2016.05.019. [DOI] [Google Scholar]
- 57.Xiang SY, et al. Microwave-assisted hydrothermal synthesis and laser-induced optical heating effect of NaY(WO4)2:Tm3+/Yb3+ microstructures. Opt. Mater. Express. 2014;4:1966–1980. doi: 10.1364/OME.4.001966. [DOI] [Google Scholar]
- 58.Zhou TM, Zhang YQ, Wu ZL, Chen BJ. Concentration effect and temperature quenching of upconversion luminescence in BaGd2ZnO5:Er3+/Yb3+ phosphor. J. Rare. Earth. 2015;33:686–692. doi: 10.1016/S1002-0721(14)60471-3. [DOI] [Google Scholar]
- 59.Xu X, et al. α-NaYb(Mn)F4:Er3+/Tm3+@NaYF4 UCNPs as band-shape luminescent nanothermometers over a wide temperature range. ACS Appl. Mater. Interfaces. 2015;7:20813–20819. doi: 10.1021/acsami.5b05876. [DOI] [PubMed] [Google Scholar]
- 60.Tong LL, et al. Microwave-assisted hydrothermal synthesis, temperature quenching and laser-induced heating effect of hexagonal microplate β-NaYF4:Er3+/Yb3+ microcrystals under 1550 nm laser irradiation. Sensor. Actuat. B-Chem. 2017;246:175–180. doi: 10.1016/j.snb.2017.02.030. [DOI] [Google Scholar]
- 61.Lu HY, et al. Optical temperature sensing in beta-NaLuF4:Yb3+/Er3+/Tm3+ based on thermal, quasi-thermal and non-thermal coupling levels. RSC Adv. 2016;6:55307–55311. doi: 10.1039/C6RA08311K. [DOI] [Google Scholar]
- 62.Jiang GC, et al. 794 nm excited core-shell upconversion nanoparticles for optical temperature sensing. RSC Adv. 2016;6:11795–11801. doi: 10.1039/C5RA27203C. [DOI] [Google Scholar]
- 63.Chen BT, et al. Amphiphilic silane modified NaYF4:Yb, Er loaded with Eu(TTA)3(TPPO)2 nanoparticles and their multi-functions:dual mode temperature sensing and cell imaging. Nanoscale. 2013;5:8541–8549. doi: 10.1039/c3nr02670a. [DOI] [PubMed] [Google Scholar]
- 64.Chen DQ, Xu M, Huang P. Core-shell upconverting nanoarchitectures for luminescenct sensing of temperature. Sens. Actuat. B-Chem. 2016;231:576–883. doi: 10.1016/j.snb.2016.03.070. [DOI] [Google Scholar]
- 65.Huo LL, et al. Dual-functional β-NaYF4:Er3+, Yb3+ nanoparticles for bioimaging and temperature sensing. Opt. Mater. Express. 2016;6:1056–1064. doi: 10.1364/OME.6.001056. [DOI] [Google Scholar]
- 66.Dubey A, Soni AK, Kumari A, Dey R, Rai VK. Enhanced green upconversion emission in NaYF4:Er3+/Yb3+/Li+ phosphors for optical thermometry. J. Alloys Compd. 2017;693:194–200. doi: 10.1016/j.jallcom.2016.09.154. [DOI] [Google Scholar]
- 67.Wang Y, Cao WB, Li SB, Wen WJ. Facile and high spatial resolution ratio-metric luminescence thermal mapping in microfluidics by near infrared excited upconversion nanoparticles. Appl. Phys. Lett. 2016;108:051902. doi: 10.1063/1.4940746. [DOI] [Google Scholar]
- 68.Du P, Luo LH, Yu JS. Facile synthesis of Er3+/Yb3+-codoped NaYF4 Nanoparticles: A promising multifunctional upconverting luminescenct materials for versatile applications. RSC Adv. 2016;6:94539–94546. doi: 10.1039/C6RA22349D. [DOI] [Google Scholar]
- 69.Geitenbeek RG, et al. NaYF4:Er3+,Yb3+/SiO2 core/shell upconverting nanocrystals for luminescence thermometry up to 900 K. J. Phys. Chem. C. 2017;121:3503–3510. doi: 10.1021/acs.jpcc.6b10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li DD, Shao QY, Dong Y, Jiang JQ. Thermal sensitivity and stability of NaYF4:Er3+,Yb3+ upconversion nanowires, nanorods and nanoplates. Mater. Lett. 2013;110:233–236. doi: 10.1016/j.matlet.2013.08.047. [DOI] [Google Scholar]
- 71.Zhou SS, et al. Upconversion luminescence of NaYF4:Er3+,Yb3+ for temperature sensing. Opt. Comm. 2013;291:138–142. doi: 10.1016/j.optcom.2012.11.005. [DOI] [Google Scholar]
- 72.Skripka A, et al. Double rare-earth nanothermometer in aqueous media: opening the third optical transparency window to temperature sensing. Nanoscale. 2017;9:3079–3085. doi: 10.1039/C6NR08472A. [DOI] [PubMed] [Google Scholar]


