Abstract
Background
Pancreatic exocrine insufficiency (PEI) affects patients with chronic pancreatitis (CP) and cystic fibrosis (CF) who produce insufficient digestive pancreatic enzymes. Common symptoms include steatorrhoea, diarrhea, and abdominal pain.
Objective
The objective of the study was to develop and test the content validity of a patient-reported outcome (PRO) instrument assessing PEI symptoms and their impact on health-related quality of life.
Methods
Instrument development was supported by a literature review, expert physician interviews (n = 10: Germany 4, UK 3, France 3), and exploratory, qualitative, concept-elicitation interviews with patients with CF and CP with PEI (n = 61: UK 29, Germany 18, France 14) and expert physicians (n = 10). Cognitive debriefing of the draft instrument was then performed with patients with PEI (n = 37: UK 24, Germany 8, France 5), and feasibility was assessed with physicians (n = 3). For all interviews, verbatim transcripts were qualitatively analysed using thematic analysis methods and Atlas.ti computerized qualitative software. All themes were data driven rather than a priori.
Results
Patient interviews elicited symptoms and impacts not reported in the literature. Six symptom concepts emerged: pain, bloating, bowel symptoms, nausea/vomiting, eating problems, and tiredness/fatigue. Six impact domains were also identified. A 45-item instrument was developed in English, French, and German for testing in cognitive debriefing patient interviews. Following cognitive debriefing, 18 items were deleted.
Conclusion
Rigorous qualitative patient research and expert clinical input supported development of a PEI-specific PRO with the potential to aid management and monitoring of unmet needs among patients with PEI. The next step is to perform psychometric evaluation of the resulting instrument.
Key Points for Decision Makers
| A pancreatic exocrine insufficiency (PEI)-specific patient-reported outcome instrument has been developed on the basis of a rigorous process that included a review of the literature, patient interviews in three countries, and interviews with expert physicians. |
| The instrument includes assessment of symptoms (primarily gastrointestinal symptoms) and domains of physical, emotional, and social functioning. |
| The qualitative research was conducted in three different European countries, which should help ensure the cross-cultural validity of the instrument and ease of translation into other languages. |
| The instrument is being developed for use in research studies and clinical practice to aid treatment decisions and evaluation of disease severity. |
Introduction
Pancreatic exocrine insufficiency (PEI) is the reduction in the synthesis and secretion of pancreatic digestive enzymes into the duodenum [1]. The most common causes of PEI are chronic pancreatitis (CP) in adults and cystic fibrosis (CF) in children/adolescents. Other causes include pancreatic tumors, acute pancreatitis, gastrointestinal surgery, and the partial or complete removal of the pancreas [2–4]. Patients with PEI resulting from any of these conditions have an increased risk of malnutrition, which is associated with increased complications, higher mortality [5], and poor survival in advanced pancreatic cancer [6].
PEI has a long subclinical course and is underdiagnosed in patients with conditions such as CP [7]. In children with CF, PEI is usually present from infancy but can develop in later life. If untreated, PEI results in reduced nutrient absorption, leading to malnutrition-related complications such as poor growth and development and impaired immune response to infections. In patients with CF, malnutrition is associated with respiratory morbidity and shortened survival [8, 9].
Steatorrhoea (fatty stools) is the defining PEI symptom. However, patients may also experience diarrhea, weight loss, vitamin deficiency symptoms, abdominal distension, and flatulence [5], which can adversely impact patients’ health-related quality of life (HRQoL) and survival [10, 11]. In Europe and the USA, patients diagnosed with PEI are treated with pancreatic enzyme-replacement therapy (PERT), but many are undiagnosed or under-treated [12, 13]. To avoid malnutrition-related morbidity and mortality in patients with CP, PERT should commence as soon as PEI is diagnosed [14]. Lack of diagnosis and under-treatment intensify the symptom experience and may increase the impact on HRQoL [10, 11].
Given the subjective nature of PEI symptoms (e.g., abdominal pain and diarrhea), a patient-reported outcome (PRO) instrument that collects information directly from the patient about symptom severity and impacts could be of value in clinical practice to inform treatment decisions and aid tracking of disease severity [15–17]. The development of any PRO should start with rigorous qualitative research in the target patient population combined with expert physician input to ensure that all concepts that are important and relevant to patients are included [15]. It is then crucial that items, response scales, and recall periods are worded simply enough that they are interpreted and understood consistently and as intended. A PRO measure for use in PEI (PEI-Q) could provide physicians with valuable information about patients’ symptoms and impacts on their HRQoL that may not emerge organically during all clinical interviews. For example, insomnia and fatigue are reported by most patients with CP [10, 11] but are rarely discussed in clinical interviews.
The objective of this study was to follow best practice methods for PRO instrument development and validation to develop a PRO instrument to assess all PEI symptoms and the associated impact on HRQoL for use in clinical practice [16, 18]. Such a PRO instrument may also be useful when assessing symptoms in studies and randomized trials of PEI treatments. Specific objectives were to identify key symptoms and impacts reported by patients with PEI and CP or CF, physicians, and published literature and to use concepts elicited by patients to develop a PRO instrument to assess PEI symptoms and impacts.
Materials and Methods
The PEI instrument was developed in six key stages (Fig. 1).
Fig. 1.
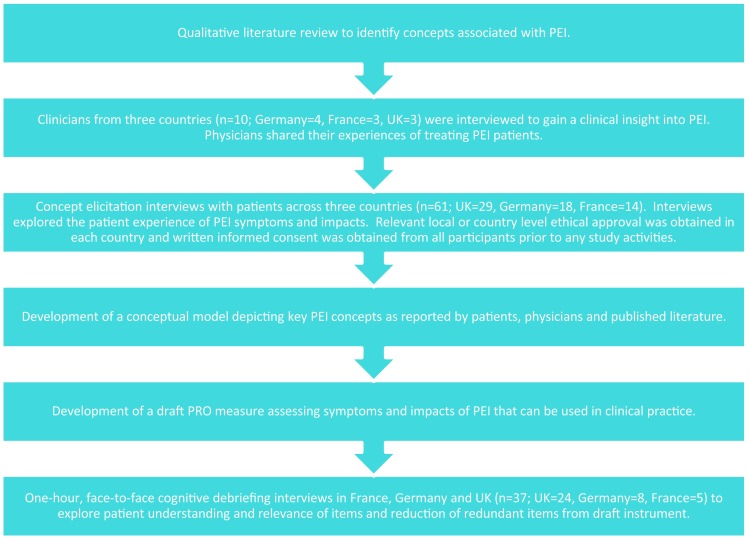
Overview of pancreatic exocrine insufficiency-specific patient-reported outcome development process. PEI pancreatic exocrine insufficiency, PRO patient-reported outcome
Literature and Patient-Reported Outcomes Instrument Review
A targeted peer-reviewed literature search was conducted in the PubMed, PsycINFO, and EMBASE databases to identify qualitative research articles outlining patient-reported PEI symptom and impact concepts. Searches were conducted using disease and qualitative research search terms (Table 1) yielding 100 abstracts, six of which met inclusion criteria and were reviewed in full. Symptom and impact concepts identified from the articles informed the development of a preliminary PEI conceptual model.
Table 1.
Qualitative literature review search strategy
| Search type | Search terms |
|---|---|
| Disease-related terms | Pancreatitis, cystic fibrosis AND pancreas OR pancreatic insufficiency, pancreatic cancer, pancreas AND disease, pancreatic exocrine insufficiency OR PEI OR exocrine pancreatic insufficiency OR EPI, pancreas AND surgery OR pancreatectomy OR gastrectomy |
| Qualitative research terms | Qualitative OR phenomenology OR grounded theory OR thematic analysis OR narrative OR focus group OR interview OR subjective experience OR patient experience OR lived experience |
EPI exocrine pancreatic insufficiency, PEI pancreatic exocrine insufficiency
A review of 1520 abstracts identified six existing PRO instruments that have been used in PEI for full review: the European Organisation for Research and Treatment of Cancer core questionnaire [19], the European Organisation for Research and Treatment of Cancer pancreatic cancer questionnaire [20], the Digestive Diseases Questionnaire [21], the Functional Assessment of Cancer Therapy Hepatobiliary Symptom Index [22], the Gastrointestinal Symptom Rating Scale [23], and the Gastrointestinal Quality of Life Index [24]. However, none of the instruments reviewed had adequate evidence of content validity and psychometric validity in PEI as none had been specifically developed or validated in a PEI population.
Physician Interviews
Open-ended qualitative telephone interviews were conducted with ten CF and CP expert physicians in Germany (n = 4), France (n = 3) and the UK (n = 3) to provide insight into the most important and relevant PEI concepts from a clinical perspective. Findings informed revisions to the conceptual model.
Concept-Elicitation Patient Interviews
Semi-structured qualitative concept-elicitation interviews of 1-h in duration (n = 61: UK 29, Germany 18, France 14) were conducted with adult patients with PEI (aged ≥18 years and diagnosed with either CP or CF) in France, Germany, and the UK and adolescent patients with CF (aged 12–17 years) in the UK. In the UK, all interviews were conducted face-to-face either at the participant’s home or at the clinical site. In France and Germany, all interviews were conducted remotely via telephone. Participants were mostly interviewed on their own, but if a family member was present they were asked not to partake in or contribute to the interview. The qualitative sample size was determined based on an aim to include a sample sufficient to be likely to achieve ‘conceptual saturation’ (the point when no new concepts emerge from patient interviews) [25, 26]. Others have suggested that, in a relatively homogenous population, a sample of 12 patients can be sufficient to achieve conceptual saturation [25, 26]. With that in mind, a minimum sample of at least 12 in each country was targeted, such that the total sample (and also the total CF and CP samples) would be considerably larger. Conceptual saturation was assessed for the whole sample and by condition and country; patients were ordered according to date of interview and split into three groups. The concepts elicited in each group were compared. Conceptual saturation was continually evaluated in parallel with interviews, and interviews were conducted until saturation was achieved; saturation was considered to be achieved if no new concepts were elicited in the third group of patients. A purposive approach to sampling with the inclusion of recruitment quotas was also used to ensure the concept-elicitation sample included patients with a range of demographic characteristics, including age, sex, education level, and disease condition.
Eligible patients had to have a physician-confirmed diagnosis of PEI and either CP or CF. Patients with CP had to have been treated for ≤10 years, and both patients with CP and those with CF had to have experienced PEI symptoms in the year prior to recruitment. All patients were required to be literate in French, German, or English and willing to participate in a 1-h interview. The following patients were excluded: those with ileus/bowel obstruction, acute abdomen, malignancy involving the digestive system, coeliac disease, or Crohn’s disease; those who had undergone solid organ transplant or surgery affecting the large or small bowel or for isolated gastrectomy; and patients with CF with a history of fibrosing colonopathy.
Participants were identified by their physician using a purposive sampling methodology whereby participants who met the inclusion criteria and had an upcoming clinical appointment were invited to participate. In all countries, participants were invited to participate in the study by their physician during a routine face-to-face appointment. They were provided with a study letter providing information about the study aims and goals, and written informed consent was obtained prior to commencement of study-related activities. Participants had no known pre-existing relationship with or knowledge about the interviewers. Prior to each interview, the interviewer informed participants about the purpose of doing the interviews and the interviewer’s involvement in the research. No record of refusal to participate was recorded for the study, and no participants withdrew from the study after agreeing to participate.
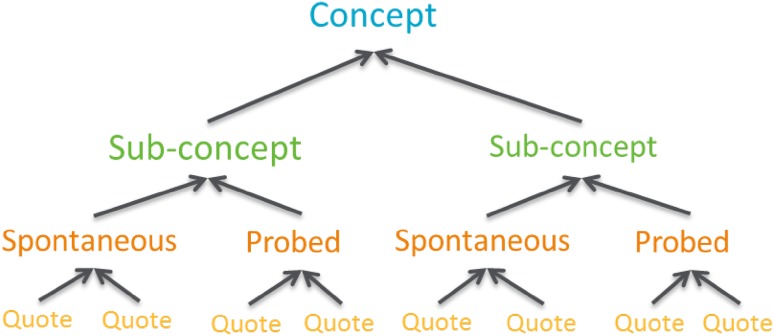
A semi-structured interview guide was developed with expert clinical input and included concepts identified from the literature review to ensure questions and prompts were appropriate to participants. Interviews were conducted by a team of experienced qualitative interviewers (N. Williamson, C. Tolley, and L. Maguire in the UK; Caroline Jonquet in France; Sabine Bielfeldt in Germany) who represented different age groups to minimize interviewer bias. Additionally, interviewers had limited knowledge of the disease area, maximizing spontaneous elicitation of information of importance to the participant without potential bias. Interviews started with open-ended questions and were followed with more direct questions to allow for spontaneous discussion concerning PEI symptoms, impact, treatments, and coping strategies while ensuring all important topics were covered if they did not emerge spontaneously. Field notes were taken during the interviews to ensure all topics were covered and to document any notable non-verbal behaviors of participants that should be considered when interpreting the results. All interviews were audio-recorded, transcribed verbatim, and translated into English where relevant. Transcripts were quality checked by the interviewer who conducted the interview to ensure consistency and accuracy in transcription prior to analysis; however, transcripts were not returned to participants for comment because of project time restraints. Qualitative analysis was performed using Atlas.ti software, which involved grouping of quotes into themes using thematic analysis methods [27]. All themes identified were driven by data rather than a priori assumptions. A coding tree was developed prior to analysis of any interviews to structure how concepts and sub-concepts would be coded. The coding tree was used consistently to guide coding of all the interviews (Fig. 2). A team of three experienced qualitative data coders (N. Williamson, L. Maguire, and C. Tolley) coded the transcripts in parallel using a constant comparative method, and all coding was overseen by the project lead (N. Bonner) to ensure codes were consistently applied.
Fig. 2.
Example coding tree
Ethical approval was obtained in the UK (National Research Ethics Service reference: 12/YH/0500) and Germany (Universitätsmedizin Greifswald Ethikkommision reference: BB 123/12). A waiver was granted in France (reference: AV128200A).
PRO Instrument Development
Items were simultaneously developed in British English, French, and German with expert linguistic and clinical input. They were developed using verbatim content from the interviews and worded using patient language to be comprehensible to all education levels while considering conceptual relevance across languages/cultures. The linguistic expert helped ensure linguistic and cultural equivalence of the formulations across the language versions and that the items developed would be relatively easy to translate into other languages in the future.
Cognitive Debriefing Patient Interviews
Content validity of the PRO was evaluated through cognitive debriefing interviews with 37 adult patients with CF or CP [28] in the UK (n = 16), Germany (n = 8), and France (n = 5) as well as adolescents in the UK (n = 8). Patients were identified and recruited through their physician using the same recruitment process as for the concept-elicitation interviews. Although no formal sample size was calculated, recruitment quotas were adopted to ensure the sample effectively represented different demographic characteristics, including age, sex, education, and disease condition. This ensured that any heterogeneity in the population and likely responses was captured, and the PEI-Q was tested in patients who were representative of the target population, including participants who may have difficulty interpreting or completing the PRO [29, 30]. Inclusion and exclusion criteria were the same as for the concept-elicitation interviews except that participation in the concept-elicitation stage was an exclusion criterion. As in concept elicitation, participants were approached by their physician and invited to participate, and written informed consent form was obtained prior to commencement of study-related activities. Interviews in both stages were therefore one-off interviews scheduled with individual patients. Patients completed the 45-item questionnaire using a ‘think-aloud’ method and were asked detailed debriefing questions to evaluate their understanding and the relevance of questions to their PEI experience [28]. Qualitative analysis of verbatim transcripts was performed and results used to inform modification or deletion of items, although the earlier concept-elicitation findings and expert clinical input were also taken into account.
Results
Literature Review
The literature search identified key symptoms (including painful gastrointestinal sensations, other gastrointestinal sensations, trapped wind, changes in appearance of stools, changes in bowel movements, eating-related symptoms) and impact concepts (including psychological, family, occupational, eating-related, tiredness/fatigue, loss of physical strength, and PEI treatment impacts). Additionally, coping concepts (e.g., altering administration of enzymes, denial, socializing with people who know about the condition, relying on others for support, balancing benefits and risk, modifying diet, and performing upright gentle activities) and triggers (e.g., eating-related) associated with patients’ PEI experience were also elicited (Table 2).
Table 2.
Pancreatic exocrine insufficiency concepts elicited from qualitative literature review
| Concept | Sub-concepts identified |
|---|---|
| Symptoms | Painful gastrointestinal sensations, other gastrointestinal sensations, trapped wind, changes in appearance of stools, changes in bowel movements, symptoms related to eating |
| Impacts | Psychological, family, occupational, and eating-related impacts; fatigue/tiredness/lack of energy; loss of physical strength; and PEI treatment-related impacts |
| Coping strategies | Altering administration of enzymes, denial, socializing with people who know about the condition, relying on others for support, balancing benefits and risk, modifying diet, and performing upright gentle activities |
| Triggers | Eating-related |
PEI pancreatic exocrine insufficiency
Physician Interviews
All physician interviews were conducted between July and October 2012. The PEI symptoms most commonly reported by CF and CP physicians were weight loss (considered highly important by the physicians interviewed, but not identified in the literature review), abdominal pain, diarrhea, and fatty stools (all reported by six physicians each). The physicians suggested that diet (n = 10) and, to a lesser extent, social functioning (n = 3) were the HRQoL domains most impacted by PEI. Physicians specializing in CF reported fewer PEI symptom and impact concepts than those specializing in CP. Additionally, the physicians mentioned problems with treatment adherence (n = 4), particularly among children and adolescents, who may miss medication to avoid taking treatment in front of peers, and among adult patients with CP who continue to consume alcohol.
Concept-Elicitation Patient Interviews
A total of 61 concept-elicitation interviews were conducted with patients with CF or CP between October 2012 and September 2013. Table 3 summarizes the numbers of patients interviewed in each diagnosis group in each country.
Table 3.
Diagnosis of patients interviewed at the concept-elicitation stage by country
| Country | Adult CF | Adolescent CF | Adult CP | Total |
|---|---|---|---|---|
| France | 6 | 0 | 8 | 14 |
| Germany | 9 | 0 | 9 | 18 |
| UK | 12 | 9 | 8 | 29 |
| Total | 27 | 9 | 25 | 61 |
CF cystic fibrosis, CP chronic pancreatitis
Demographic and Clinical Characteristics
The sample was evenly distributed in terms of sex (32 males, 29 females), and the mean age was 36 years (range 12–81). Steatorrhoea was the most common symptom leading to diagnosis of PEI across all conditions; however, most patients were diagnosed using multiple methods (Table 4). Unsurprisingly, adult patients with CF had a longer mean time since diagnosis (26 years) and more years receiving medication (25 years) than adults with CP (5 and 4 years, respectively) and adolescents with CF (11 and 13 years, respectively).
Table 4.
Demographic and clinical characteristics of the concept-elicitation sample (N tot = 61)
| Adult CV (N = 27) | Adult CP (N = 25) | Adolescent CF (N = 9) | Total (N = 61) | |
|---|---|---|---|---|
| Patient-reported | ||||
| Sex | ||||
| Male | 11 (41) | 16 (64) | 5 (56) | 32 (52) |
| Female | 16 (59) | 9 (36) | 4 (44) | 29 (48) |
| Age | ||||
| Mean | 30 | 52 | 14 | 36 |
| Minimum, maximum | 19, 64 | 20, 81 | 12, 16 | 12, 81 |
| Missing data | 0 | 1 (4) | 0 | 1 (2) |
| Ethnicity | N = 21a | N = 17a | N = 47 | |
| White/Caucasian | 18 (86) | 16 (94) | 9 (100) | 43 (91) |
| African, Caribbean, or Black | 0 | 0 | 0 | 0 |
| Asian | 2 (10) | 1 (6) | 0 | 3 (6) |
| Mixed race | 1 (5) | 0 | 0 | 1 (2) |
| Adults only—education | N = 52 | |||
| Some high school, but no diploma or GED | 3 (11) | 5 (20) | NA | 8 (15) |
| High school diploma or equivalent | 4 (15) | 5 (20) | 9 (17) | |
| Some college or associate’s degree/bachelor’s degree | 10 (37) | 6 (24) | 16 (31) | |
| Some graduate work | 4 (15) | 2 (8) | 6 (12) | |
| Post-graduate degree | 3 (11) | 5 (20) | 8 (15) | |
| GCSE | 1 (4) | 0 | 1 (2) | |
| GCE A level | 1 (4) | 0 | 1 (2) | |
| CIMA (accountant) | 0 | 1 (4) | 1 (2) | |
| PhD | 1 (4) | 0 | 1 (2) | |
| National Craftsman Certificate | 0 | 1 (4) | 1 (2) | |
| Physician-reported | ||||
| How PEI was diagnosed | ||||
| Weight loss | 0 | 10 (40) | 2 (22) | 12 (20) |
| Steatorrhea | 19 (70) | 17 (68) | 5 (56) | 41 (67) |
| Diarrhea | 4 (15) | 15 (60) | 4 (44) | 23 (38) |
| Bloating | 5 (19) | 12 (48) | 2 (22) | 19 (31) |
| Abdominal discomfort | 7 (26) | 16 (64) | 4 (44) | 27 (44) |
| Fecal elastase test | 7 (26) | 12 (48) | 9 (100) | 28 (46) |
| Recurrent rectal prolapse | 1 (4) | 0 | 0 | 1 (2) |
| Meconium level | 1 (4) | 0 | 0 | 1 (2) |
| Neonatal screening | 2 (7) | 0 | 0 | 2 (3) |
| Failure to thrive | 1 (4) | 0 | 0 | 1 (2) |
| Missing data | 2 (7) | 0 | 0 | 2 (3) |
| Years since diagnosis | ||||
| Mean | 26 | 5 | 11 | 16 |
| Minimum, maximum | 13, 48 | 0, 23 | 3, 16 | 0, 48 |
| Missing data | 2 (7) | 3 (12) | 0 | 5 (8) |
| Years receiving PEI medication | ||||
| Mean | 25 | 4 | 13 | 15 |
| Minimum, maximum | 3, 47 | 0, 9 | 11, 16 | 0, 47 |
| Missing data | 1 (4) | 0 | 0 | 1 |
Data are presented as n (%) unless otherwise indicated
CF cystic fibrosis, CIMA Chartered Institute of Management Accountants, CP chronic pancreatitis, GED General Educational Development, GCE General Certificate of Education, GCSE General Certificate of Secondary Education, NA not applicable, N tot number in total sample, PEI pancreatic exocrine insufficiency
aData protection rules precluded patient ethnicity being collected in France
Symptoms
Six primary symptom concepts were elicited from the patient interviews: pain, bloating symptoms, bowel movements, nausea/vomiting, eating-related symptoms, and tiredness/fatigue. Table 5 presents the sub-concepts and example quotes, and details of findings are provided in the following sections.
Table 5.
Pancreatic exocrine insufficiency conceptual framework
| Concepts | Sub-concepts | Example quote from qualitative patient research [condition, sex, age (years)] |
|---|---|---|
| PEI symptoms | ||
| Pain | Abdominal pain | “Well, it’s like having a knife being rammed in” (CF, female, 24) |
| Non-abdominal pain | “The only way I can describe that is like having toothache in your back on both sides …, which goes, vertically up your back” (CP, male, 60) | |
| Bloating symptoms | Bloating | “It’s a strange feeling, as if I’ve ate too much and I haven’t had anything to eat at all” (CP, male, 55) |
| Distension | “It hurts and it also doesn’t look very attractive because it makes you a bit fat” (CF, female, 22) | |
| Bowel noises | “It sounded like a washing machine, it would start to grumble like crazy” (CF, female, 22) | |
| Flatulence | “Sometimes, I was like sitting on the toilet, and it would just be wind that would be coming out” (CF, female, 40) | |
| Trapped wind | “You can feel that it’s filling up a … a gas … in your stomach and your guts” (CP, male, 55) | |
| Bowel movement/stool symptoms | Constipation | “I would no longer go to have bowel movements … it just didn’t come.” (CP, male, 68) |
| Frequency of bowel movements | “If it’s really bad, four or five times a day.” (CP, female, 29) | |
| Diarrhea | “You’d be working in someone’s garden, and you’d have diarrhea, and you can’t really use their toilet …” (CF, male, 47) | |
| Pain in the bottom | “I would have thought it’s more like – say like if you’re having trouble going to the toilet, more like straining and trying to like go, rather than it just going normally.” (CF, female, 25) | |
| Stool appearance | “Often it is very varied … the diarrhea is actually always orange … There are brownish to black spots in it.” (CP, male, 54) | |
| Bowel urgency | “It happens quite often, so that I have to plan that I know if I am outside my apartment, there is a toilet close by.” (CF, female, 51) | |
| Stool color | “It varies depending on what I eat … it is usually a weird yellowish brown. But oddly, when I have fatty stools, then it’s as if oil is coming out with it and then it is usually always red.” (CF, female, 21) | |
| Stool smell | “It just really smells. Just it’s … uh, for me, it smells like baby poo” (CF, female, 34) | |
| Fatty stools | “This oily, fatty feces always sticks to the toilet …” (CP, male, 55) | |
| Nausea/vomiting | Nausea | “Really it’s just, you know, feeling like you need to be sick or … but not.” (CF, male 32) |
| Vomiting | “One of the major blockages that I had, I was actually … I was actually vomiting with that …” (CF, female, 34) | |
| Eating | Loss of appetite | “When digestion still hasn’t happened, you get the feeling … No, no, you really get the feeling that you have been eating for three weeks straight, and then you’re really not hungry any longer” (CF, male, 28) |
| Weight loss | “I lost about two stone. I went down to nine stone and I looked terrible” (CP, male, 55) | |
| Tiredness | Tiredness | “Well I can go to work. Yeah, but I get tired and exhausted.” (CP, female, 29) |
| PEI impacts | ||
| Impact on daily activities | Daily activities | “When I do have a bad day then it has an extreme effect, where I just can’t do anything or I am limited in what I can do or I simply cannot do anything else.” (CF, male, 24) |
| Physical activities | “If I’ve got a stomachache, I find, um, like running around a bit more difficult, but nothing hugely.” (CF, male, 12) | |
| Concentration | “I can’t concentrate on things. I can keep my mind off things if I’m doing something that doesn’t take any concentration. Which is like watching the telly or something.” (CF, male, 16) | |
| Proximity to toilet | “If I wasn’t taking my tablets, I would be like on the toilet 24/7.” (CF, female, 15) | |
| Impact on emotional wellbeing | Embarrassment | “I clasp my hands together over my head and just have to let it out, because that also causes the cramps, then it’s quite embarrassing, when I have to go to the toilet there.” (CF, female, 21) |
| Frustration | “Sometimes I couldn’t even get to my check-up appointments here or to my doctor’s office consultations in the morning because I just never came down from the toilet and that was always the most frustrating thing to me.” (CF, female, 22) | |
| Worry/anxiety/stress | “I am always worried that something is going to happen, that the ducts become obstructed again.” (CP, female, 75) | |
| Sadness | “Sometimes if I want to do something and can’t because my stomach hurts, then I’ll be sad.” (CF, female, 12) | |
| Impact on diet | Diet awareness | “I mean the thing … I’m eating … I’ve been doing this whole diet … the whole [inaudible] dietary management thing since I was like six, so you sort of know in your head, you’re supposed to go for the high-calorie stuff.” (CF, male, 22) |
| Managing medication | “If you don’t [take] the right amount of Creon, or if you forget the Creon, it’s kind of embarrassing, but you’re just like on the toilet after that.”(CF, female, 26) | |
| Avoiding fatty food | “Also mindful about eating fatty food and cutting fat off and that sort of stuff.” (CP, male, 60) | |
| Impact on social functioning | Social activities | “You have to postpone what you’ve planned because you don’t know how it’s going to continue and how the day or next few hours will go, depending on how bad it is.” (CP, male, 24) |
| Relationship with friends | “When I was younger, I had different friends. Perhaps we’d run around at breaks. Obviously, if I had a stomachache, I perhaps wouldn’t run around as much.” (CF, male, 12) | |
| Staying at home | “The best thing to do is stay home and somewhere that’s comfortable, where there’s a toilet, and you can sit there in peace and wait as long as it takes.” (CF, male, 24) | |
| Family | “My parents had to cope with always running after me, giving me tablets and nagging me when I didn’t take them, and despite that, they were there for me when I hadn’t taken them and I was suffering.” (CF, female, 22) | |
| Intimate relations | “It is always a bit strained anyway. Look, first he has to look first if I … we can’t say yet, “Now, tomorrow we’re going to get up at 5”, and I always have to get up earlier, because I need at least an hour to deal with the sugar, inhalation, physio and I have to do a little bit.” (CF, female, 24) | |
| Impact on work or study | Time missed at work/study | “I was absent, missing work hours because I was constantly tired.” (CP, male, 60) |
| Performance at work/study | “Physically while working, it still shows somehow that this here … at least I imagine it, that there is something that inhibits me a little. When I work in a bent over position or something like that. I want to say I’m not as efficient.” (CP, male, 62) | |
| Sleep | Sleep | “If digestion goes badly, then you sleep poorly afterward.” (CF, female, 32) |
CF cystic fibrosis, CP chronic pancreatitis, PEI pancreatic exocrine insufficiency, pt patient
Pain
In total, 49 (80%) patients reported experiencing pain; 51 (84%) reported experiencing abdominal pain, 49 (96%) of them spontaneously. Of those, 40 (78%) described abdominal pain occurring mostly in their stomach; patients with CF most often reported pain in the upper and lower abdominal quadrants, whereas patients with CP more often reported upper-right abdominal pain. Both patients with CF and those with CP also reported non-abdominal pain, but whether that pain should be attributed to PEI or comorbid conditions was unclear; no adolescents with CF reported non-abdominal pain.
Bloating Symptoms
Bloating or other gas-related abdominal symptoms were reported by 39 (64%) patients; 50 (82%) patients reported stomach noises, 20 (33%) reported high levels of flatulence, and nine (15%) reported “trapped wind”.
Bowel Movement/Stool Symptoms
Patients described several bowel movement-related symptoms; 29 (48%) experienced constipation (although it is recognized that may be due to treatment-related side effects), 11 (18%) increased frequency of bowel movements, and 20 (33%) bowel urgency. In total, 46 (75%) patients experienced diarrhea, 30 (49%) passed fatty stools, a key PEI symptom, and 31 (51%) described a change in stool color associated with PEI, e.g., passing more ‘light colored’ (n = 15 [48%]), ‘yellow/brown’ (n = 15 [48%]), and ‘orange’ (n = 10 [32%]) stools; 13 (42%) described stools having an unusual or strong odor.
Nausea and Vomiting
Nausea and vomiting symptoms were reported by 39 (64%) patients, with 17 (44%) reporting experiencing nausea only, eight (21%) vomiting only, and eight (21%) both nausea and vomiting.
Eating-Related Symptoms
Patients had experienced weight loss (n = 41 [67%]) and loss of appetite (n = 20 [33%]). More adults with CP reported weight loss (n = 22 [54%]) and lack of appetite (n = 10 [24%]) than adults with CF (n = 6 [15%]) and adolescent patients (n = 4 [10%]).
Tiredness
In total, 25 (41%) patients reported experiencing tiredness but did not specifically associate this with PEI.
Impact
More HRQoL impact concepts were reported in the patient interviews than in the literature or from physicians. The six reported HRQoL impact concepts were grouped into the domains daily activities, emotional, dietary, social, family and relationships, work, and school. The most common daily activities impacted were ‘housework,’ ‘holidays,’ and ‘travelling’ (all n = 6 [10%]). In terms of emotional impacts, patients commonly reported being embarrassed by their symptoms (particularly bowel symptoms; n = 22 [36%]). A large proportion of patients described having to avoid fatty foods (n = 23 [38%]), or other dietary restrictions. Finally, a large proportion of patients described avoiding going out (n = 19 [31%]) and their relationships with friends being impacted by their PEI (n = 17 [28%]). Over half of the sample (n = 33 [54%]) described their work and/or schoolwork being impacted by their PEI.
Patients also discussed symptom trigger sub-concepts, most commonly eating fatty foods (n = 25 [41%]), eating the wrong food (n = 20 [33%]), and stress related to eating (n = 11 [18%]). Patients also described coping strategies such as lying down (n = 13 [21%]), avoiding eating (n = 11 [18%]), and applying heat to the abdomen (n = 9 [15%]).
Conceptual saturation was achieved within all samples analysed, with no new symptom or impact concepts emerging from the last group of interviews analysed.
Management of Pancreatic Exocrine Insufficiency Medication
Patients described difficulties estimating PEI medication dosage for food consumed, and commonly reported difficulties with medication adherence, including forgetting to take or taking too much medication, resulting in diarrhea, lighter/orange stools (n = 6 [10%] for each), constipation (n = 5 [8%]), fatty/oily stools (n = 3 [5%]), abdominal discomfort, gas (n = 2 [3%] for each), weight loss, changes in stool color, needing to be close to a toilet, and impact on relationships (n = 1 [2%] for each).
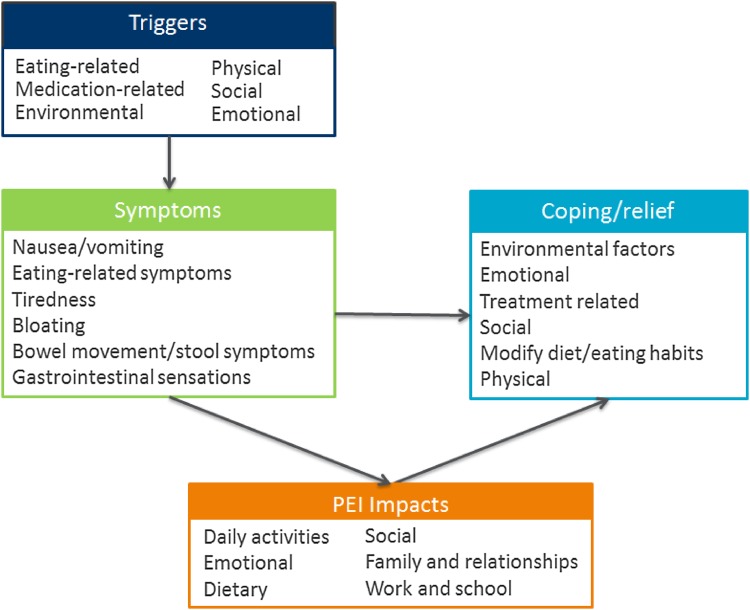
Conceptual Model
Figure 3 presents the conceptual model for all concepts that were derived from the literature review and physicians and the patient concept-elicitation interviews. Following analysis of all interviews, the developed conceptual model was shared with expert CF and CP physicians practicing in the UK (n = 3), France (n = 2), and Germany (n = 2), who verified the relevance of concepts. These clinical experts noted that evidence from the patient interviews was consistent with their experience but also extended their understanding of the patient experience and impact of PEI on HRQoL. The sub-concepts that assess different aspects of these relatively broad concepts are detailed in the conceptual framework in Table 5. For example, symptom is a concept and abdominal pain is a specific type (sub-concept) of symptom.
Fig. 3.
Pancreatic exocrine insufficiency (PEI) conceptual model
PRO Instrument Development
A draft 45-item PEI-specific PRO instrument, the PEI-Q, was developed based on the above conceptual model, patient interview findings, and clinical input. Example symptom and impact items included in the PEI-Q are shown in Fig. 4. Separate symptom and impact conceptual frameworks for the instrument are presented in Table 5.
Fig. 4.
Sample questions from the pancreatic exocrine insufficiency-specific patient-reported outcomes measure (PEI-Q)
Cognitive Debriefing Patient Interviews
The relevance and patient understanding and interpretation of the items included in the draft instrument was evaluated through cognitive debriefing interviews (n = 37) conducted in the UK (n = 24), Germany (n = 8), and France (n = 5) between March and September 2014. Target samples were achieved in Germany and the UK; however, recruitment was below target in France. Physician input was sought on proposed deletions to ensure key PEI symptoms and impacts were retained. Discussions between the researchers, study sponsor, and expert physicians were held at each stage of the decision-making process until consensus was reached. Items were removed that were either not strongly relevant to patients’ PEI experience or overlapped with other concepts. Specifically, ten items were deleted as they were not considered specific to PEI or were relevant to a small proportion of participants: constipation; vomiting; pain in bottom; tiredness; stomach noises; feeling down; and impact on family life, relationship with partner, work/school, and performance at work. Eight items were also considered to overlap conceptually with other items and thus were deleted: stomach looking big and round (overlapped with bloating), trapped wind (overlapped with bloating), needing to go to the toilet (overlapped with average bowel movements), impact on sports/exercise (overlapped with daily activities), and relationship with friends (overlapped with social activities). The instrument was reduced to 27 items.
Discussion
This paper describes the development of the first known PEI-specific PRO instrument, developed on the basis of in-depth and rigorous qualitative research. The instrument has been developed with the goal of providing patients and physicians with more comprehensive and systematically collected information about PEI patients’ symptoms and HRQoL functioning to aid patient–physician communication and management of PEI. The qualitative interviews with both patients with CP and those with CF and with physicians identified many patient-reported concepts relevant to capturing the full range of PEI symptoms and impacts on patients’ daily lives [15, 31]. Many of these concepts have not previously been documented in the literature, highlighting the value of collecting data directly from patients to fully understand their experience of symptoms and impacts. During the qualitative interviews, physicians specializing in CF reported fewer PEI symptom and impact concepts than those specializing in CP. This may reflect that patients with CF have usually been receiving treatment for longer than those with CP and have usually experienced symptoms since childhood, reducing the impact. It may also be the case that certain symptoms are features of CP and not of CF. Set against that possibility, the results from the qualitative interviews indicate that, overall, the PEI symptom experience is similar in both patient groups. This possibility will be further explored during quantitative validation of the PEI-Q, at which point it is expected that some items may be deleted if they do not appear equally relevant to patients with CP and those with CF. Findings also identified that patients may have difficulty estimating correct enzyme doses, which could result in poor treatment adherence. The PEI-Q has the potential to help physicians identify the need to adjust treatment regimens, address dosing issues, and maintain treatment adherence, thus contributing to better outcomes for patients. The instrument may have particular value in PEI management following diagnosis or alongside existing diagnostic tests, providing supplementary information to physicians.
Our findings provide evidence that, in addition to direct effects on bowel function and digestive function, PEI symptoms also distress patients and interfere with daily activities. They can also result in patients avoiding foods that are difficult to digest rather than correctly adjusting doses of enzyme replacement. This may lead to malnutrition [8, 9, 14, 32] and worsening of malnutrition-related symptoms, which is associated with early mortality in patients with CF [8, 9]. Achieving adequate enzyme replacement is important for all of these conditions [14]. We believe the PEI-Q will assist physicians to assess the adequacy of PERT and should help improve the management of PEI symptoms.
Guidelines for PEI diagnosis and management in CP do not include PROs [33–35] but are reliant on stool elastase measurements, the only widely available test for PEI. Guidelines suggest that a trial period of PERT can clarify whether patients’ symptoms are due to PEI [33, 34], but many physicians understand that responses to PERT vary widely. Additional symptoms found in our qualitative patient interviews highlight the benefit of a PRO to monitor PEI symptoms and the full patient experience. This will improve assessment of treatment response and will enable adjustment of PERT doses after consideration of patient-reported severity of symptoms using a standardized measure.
It is well established that PROs can provide important endpoints for the evaluation of treatment benefits and adverse effects in clinical trials [16, 18]. In addition, Velikova et al. [36] provided evidence that use of a quality-of-life questionnaire in clinical practice can improve patient–doctor communication and can highlight symptoms previously untreated or discounted by the doctor and patient. The PEI-Q could help identify untreated symptoms, adjust enzyme-replacement doses to effective levels, and maintain good nutritional and general health status.
A strength of the study is that we included patients with CF and CP, the two primary causes of PEI; thus, the findings provide evidence of content validity in different sub-groups. Inclusion of both patients with CF and those with CP maximized the likelihood of identifying symptoms and impacts that result from PEI rather than these comorbid conditions. It is still possible that some of the symptoms identified are due to CP or CF, for example, epigastric pain. This will be further explored in a quantitative validation study that is already underway, and it is possible that some symptom concepts currently included may be deleted based on the findings of that study. Another strength of the study is that patients were recruited from three countries, ensuring findings have cross-cultural relevance.
However, the study has some limitations. Data were collected in France, Germany, and the UK only; further study in other countries and outside Europe would provide further confidence in the cross-cultural validity of the PEI-Q. That said, major differences are considered unlikely given that no differences were found across the countries studied. Another study limitation is that some patients’ PEI was diagnosed by symptoms reported rather than laboratory-based tests, allowing the possibility that patients may not have been correctly diagnosed with PEI. This method was chosen to ensure feasibility and practicality of the study and also reflects clinical practice. Moreover, most participants were diagnosed using clinical tests such as the fecal elastase test, and no differences were identified in symptoms reported between patients diagnosed via different methods. However, we do recommend that future studies collect data from patients diagnosed using laboratory-based tests to cross-validate these findings.
This paper describes the first phase of development of the PEI-Q using qualitative interviews, ensuring the full patient experience is captured. Work thus far has been qualitative, leading to a list of symptom and impact concepts to document the comprehensive PEI symptom experience and the associated impact on patients’ well-being. Symptoms and impacts identified have been integrated into the PEI-Q instrument. Quantitative testing of the psychometric validity and reliability of the instrument is necessary before it can be used in clinical practice [37, 38]; the psychometric properties of the PEI-Q will be evaluated in a planned validation study.
Conclusions
This paper describes the development of a PEI-specific PRO instrument based on in-depth qualitative research of a diverse PEI patient sample. Data provide useful insights to improve understanding of the disease experience of patients with PEI and to better inform clinical management.
Acknowledgements
Mathias Schifflers was an employee at Abbott at the time this work was conducted and was involved in all aspects of the research. Chloe Tolley and Laura Maguire were employees at Adelphi Values and were involved in the conduct of interviews and qualitative analysis of the interview data. Caroline Jonquet was employed by Zeste Research and conducted the interviews in France. Sabine Bielfeldt, as an employee of Medical Market Research, conducted the patient interviews in Germany.
Author contributions
Professors Johnson, Levy, Staab, and Lerch and Dr. Connett provided input into the study design, reviewed and provided interpretation of the literature review findings, and reviewed and provided clinical input into the qualitative study documents. They also provided review and interpretation of the qualitative results. Professor Dominguez-Munoz was involved in development of the PEI-Q, review of study documents, and providing clinical input and interpretation of the qualitative results. Mr. Arbuckle and Ms. Bonner and Williamson are employees of Adelphi Values, a health outcomes research company that was paid by Abbott to conduct the research. They were involved in all of the research activities, leading the study design, development of all study documents, and the analysis and write up of the data. All authors contributed to the writing of the manuscript and reviewed and approved the final manuscript.
Compliance with Ethical Standards
Funding
This study was funded by Abbott.
Conflict of interest
Mr. Arbuckle, Ms. Bonner, and Ms. Williamson were contracted by Abbott as consultants to perform the research. Professors Lerch, Levy, Johnson, and Dominguez-Munoz and Drs. Connett and Staab were engaged as expert scientific advisors by Adelphi Values on behalf of Abbott and received honoraria for their participation. The authors declare there are no other competing interests.
References
- 1.Toouli J, et al. Management of pancreatic exocrine insufficiency: Australasian Pancreatic Club recommendations. Med J Aust. 2010;193(8):461–467. doi: 10.5694/j.1326-5377.2010.tb04000.x. [DOI] [PubMed] [Google Scholar]
- 2.Messner JKV. Pancreatic enzyme therapy. Dtsch Arztebl Int. 2011;108:578–582. doi: 10.3238/arztebl.2011.0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahl S, et al. The effect of oral pancreatic enzyme supplementation on the course and outcome of acute pancreatitis: a randomized, double-blind parallel-group study. JOP. 2014;15(2):165–174. doi: 10.6092/1590-8577/797. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez CA, Lerch MM. Sphincter stenosis and gallstone migration through the biliary tract. Lancet. 1993;341(8857):1371–1373. doi: 10.1016/0140-6736(93)90942-A. [DOI] [PubMed] [Google Scholar]
- 5.Keller J, Layer P. Human pancreatic exocrine response to nutrients in health and disease. Gut. 2005;54(Suppl 6):vi1–28. doi: 10.1136/gut.2005.065946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Partelli S, et al. Faecal elastase-1 is an independent predictor of survival in advanced pancreatic cancer. Dig Liver Dis. 2012;44(11):945–951. doi: 10.1016/j.dld.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Garg PK, Tandon RK. Survey on chronic pancreatitis in the Asia-Pacific region. J Gastroenterol Hepatol. 2004;19(9):998–1004. doi: 10.1111/j.1440-1746.2004.03426.x. [DOI] [PubMed] [Google Scholar]
- 8.Gaskin KJ. Nutritional care in children with cystic fibrosis: are our patients becoming better? Eur J Clin Nutr. 2013;67(5):558–564. doi: 10.1038/ejcn.2013.20. [DOI] [PubMed] [Google Scholar]
- 9.Sinaasappel M, et al. Nutrition in patients with cystic fibrosis: a European Consensus. J Cyst Fibros. 2002;1(2):51–75. doi: 10.1016/S1569-1993(02)00032-2. [DOI] [PubMed] [Google Scholar]
- 10.Fitzsimmons D, et al. Symptoms and quality of life in chronic pancreatitis assessed by structured interview and the EORTC QLQ-C30 and QLQ-PAN26. Am J Gastroenterol. 2005;100(4):918–926. doi: 10.1111/j.1572-0241.2005.40859.x. [DOI] [PubMed] [Google Scholar]
- 11.Mokrowiecka A, et al. Clinical, emotional and social factors associated with quality of life in chronic pancreatitis. Pancreatology. 2010;10(1):39–46. doi: 10.1159/000225920. [DOI] [PubMed] [Google Scholar]
- 12.Dominguez-Munoz JE, Iglesias-Garcia J. Oral pancreatic enzyme substitution therapy in chronic pancreatitis: is clinical response an appropriate marker for evaluation of therapeutic efficacy? JOP. 2010;11(2):158–162. [PubMed] [Google Scholar]
- 13.Sikkens EC, Cahen DL, van Eijck C, Kuipers EJ, Bruno MJ. Patients with exocrine insufficiency due to chronic pancreatitis are undertreated: a Dutch national survey. Pancreatology. 2012;12(1):71–73. doi: 10.1016/j.pan.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Sikkens EC, et al. Pancreatic enzyme replacement therapy in chronic pancreatitis. Best Pract Res Clin Gastroenterol. 2010;24(3):337–347. doi: 10.1016/j.bpg.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Lasch KE, et al. PRO development: rigorous qualitative research as the crucial foundation. Qual Life Res. 2010;19(8):1087–1096. doi: 10.1007/s11136-010-9677-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Food and Drug Administration . Guidance for industry: patient reported outcome measures: use in medicinal product development to support labeling claims. Silver Spring, MD: US FDA; 2012. [Google Scholar]
- 17.Patrick DL, et al. Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health. 2007;10(Suppl 2):S125–S137. doi: 10.1111/j.1524-4733.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 18.European Medicines Agency . Reflection paper on the regulatory guidance for the use of health-related quality of life (HRQL) measures in the evaluation of medicinal products. London: EMA; 2009. [Google Scholar]
- 19.Fayers P, Bottomley AO. Quality of life research within the EORTC—the EORTC QLQ-C30. Eur J Cancer. 2002;38:125–133. doi: 10.1016/S0959-8049(01)00448-8. [DOI] [PubMed] [Google Scholar]
- 20.Fitzsimmons D, et al. Development of a disease specific quality of life (QoL) questionnaire module to supplement the EORTC core cancer QoL questionnaire, the QLQ-C30 in patients with pancreatic cancer. EORTC Study Group on Quality of Life. Eur J Cancer. 1999;35(6):939–941. doi: 10.1016/S0959-8049(99)00047-7. [DOI] [PubMed] [Google Scholar]
- 21.Palesch YY, et al. Digestive disease quality of life instrument (DDQ-15): a validation study. Gastroenterology. 1998;114(Supple 1):A32–A33. doi: 10.1016/S0016-5085(98)80133-9. [DOI] [Google Scholar]
- 22.Yount S, et al. Assessment of patient-reported clinical outcome in pancreatic and other hepatobiliary cancers: the FACT Hepatobiliary Symptom Index. J Pain Symptom Manage. 2002;24(1):32–44. doi: 10.1016/S0885-3924(02)00422-0. [DOI] [PubMed] [Google Scholar]
- 23.Dimenas E, et al. Well-being and gastrointestinal symptoms among patients referred to endoscopy owing to suspected duodenal ulcer. Scand J Gastroenterol. 1995;30(11):1046–1052. doi: 10.3109/00365529509101605. [DOI] [PubMed] [Google Scholar]
- 24.Eypasch E, et al. Gastrointestinal quality of life index: development, validation and application of a new instrument. Br J Surg. 1995;82(2):216–222. doi: 10.1002/bjs.1800820229. [DOI] [PubMed] [Google Scholar]
- 25.Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods. 2006;18:59–82. doi: 10.1177/1525822X05279903. [DOI] [Google Scholar]
- 26.Glaser B, Strauss A. The constant comparative methods of qualitative analysis. In: Glaser BG, Strauss AL, editors. The discovery of grounded theory: strategies for qualitative research. New York: Aldine de Gruyter; 2010. pp. 101–116. [Google Scholar]
- 27.Atlas.ti software. ATLAS.ti Scientific Software Development GmbH. Berlin:Atlas;2013.
- 28.Willis GB. Cognitive interviewing: a tool for improving questionnaire design. Thousand Oaks: Sage Publications; 2004. [Google Scholar]
- 29.Perneger TV, et al. Sample size for pre-tests of questionnaires. Qual Life Res. 2015;24(1):147–151. doi: 10.1007/s11136-014-0752-2. [DOI] [PubMed] [Google Scholar]
- 30.Patrick DL, et al. Content validity–establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO Good Research Practices Task Force report: part 2—assessing respondent understanding. Value Health. 2011;14(8):978–988. doi: 10.1016/j.jval.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Abbott J, et al. Ways of coping with cystic fibrosis: implications for treatment adherence. Disabil Rehabil. 2001;23(8):315–324. doi: 10.1080/09638280010004171. [DOI] [PubMed] [Google Scholar]
- 32.Tan CS, et al. The relationship between nutritional status, inflammatory markers and survival in patients with advanced cancer: a prospective cohort study. Support Care Cancer. 2015;23(2):385–391. doi: 10.1007/s00520-014-2385-y. [DOI] [PubMed] [Google Scholar]
- 33.Chronic Pancreatitis German Society of Digestive and metabolic diseases (DGVS), Hoffmeister A, Mayerle J, et al. S3-Consensus guidelines on definition, etiology, diagnosis and medical, endoscopic and surgical management of chronic pancreatitis German Society of Digestive and Metabolic Diseases (DGVS) [in German]. Z Gastroenterol. 2012;50(11):1176–224. [DOI] [PubMed]
- 34.Mayerle J, et al. Chronic pancreatitis–definition, etiology, investigation and treatment. Dtsch Arztebl Int. 2013;110(22):387–393. doi: 10.3238/arztebl.2013.0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellis I, Lerch MM, Whitcomb DC. Genetic testing for hereditary pancreatitis: guidelines for indications, counselling, consent and privacy issues. Pancreatology. 2001;1(5):405–415. doi: 10.1159/000055840. [DOI] [PubMed] [Google Scholar]
- 36.Velikova G, et al. Measuring Quality of Life in Routine Oncology Practice Improves Communication and Patient Well-Being: A Randomized Controlled Trial. J Clin Oncol. 2004;22(4):714–724. doi: 10.1200/JCO.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 37.Hays R, Anderson R, Reyiki DA. Assessing reliability and validity of measurement in clinical trials. In: Staquet MJ, Hays RD, Fayers PM, editors. Quality of life assessment in clinical trials. New York: Oxford University Press; 1998. [Google Scholar]
- 38.Aaronson N, et al. Assessing health status and quality-of-life instruments: attributes and review criteria. Qual Life Res. 2002;11(3):193–205. doi: 10.1023/A:1015291021312. [DOI] [PubMed] [Google Scholar]