Abstract
Motor control is a ubiquitous aspect of human function, and from its earliest origins, abnormal motor control has been proposed as being central to schizophrenia. The neurobiological architecture of the motor system is well understood in primates and involves cortical and sub-cortical components including the primary motor cortex, supplementary motor area, dorsal anterior cingulate cortex, the prefrontal cortex, the basal ganglia, and cerebellum. Notably all of these regions are associated in some manner to the pathophysiology of schizophrenia. At the molecular scale, both dopamine and γ-Aminobutyric acid (GABA) abnormalities have been associated with working memory dysfunction, but particularly relating to the basal ganglia and the prefrontal cortex respectively. As evidence from multiple scales (behavioral, regional and molecular) converges, here we provide a synthesis of the bio-behavioral relevance of motor dysfunction in schizophrenia, and its consistency across scales. We believe that the selective compendium we provide can supplement calls arguing for renewed interest in studying the motor system in schizophrenia. We believe that in addition to being a highly relevant target for the study of schizophrenia related pathways in the brain, such focus provides tractable behavioral probes for in vivo imaging studies in the illness. Our assessment is that the motor system is a highly valuable research domain for the study of schizophrenia.
1. Introduction
Schizophrenia is the most disruptive of neuropsychiatric disorders, affecting approximately 1% of the world’s population (1). The disease is characterized by profound and lasting impairments particularly in language, memory and cognition, as well as in the structure and function of brain regions sub-serving these domains (2). Influential studies have rightfully advocated for the study of higher order cognitive impairments in schizophrenia, considering these impairments as a central pathway for understanding core elements of the illness’ underlying pathophysiology (3). Yet, a complimentary literature has come to emphasize that dysfunction in basic motor function and control represents a highly relevant physiological pathway in the illness (4). Dysfunction in motor behaviors has frequently been associated with prediction errors, for example, deficiencies that patients show in predicting the consequences of motor actions (5). However, motor dysfunction in schizophrenia is even more direct, involving impairments in basic motor processing and control. That this is the case is supported by evidence that brain regions such as the dorsal anterior cingulate cortex, the basal ganglia and the cerebellum, each of which are associated with higher order functions such as memory and executive control and implicated in schizophrenia (6-9) are also associated with basic motor function (10, 11).
Recent reviews have presciently acknowledged the singular and compelling role that motor abnormalities play in the illness. Hirjak and colleagues noted that these abnormalities cluster into at least three distinct classes: neurological soft signs, abnormal involuntary movements and catatonia (12), and argued for motivated imaging approaches to identify the correlates of these sub-types. Such approaches can parse apart the “motor endophenotype” in schizophrenia. In a separate review (13), Walther also focused on these classes of motor abnormalities, but emphasized focus on the cerebellar motor circuit, as a key neural pathway, impairments within which are relevant for many of the dimensional deficits in motor function in the illness. Here we provide a synthesis reaffirming the value of studying motor function and dysfunction in schizophrenia. Our approach summarizes some of the evidence indicating that motor dysfunction is at the heart of a core pathophysiological pathway in the illness (14), yet our synthesis adds complementary elements not previously marshalled in support of developing an integrative framework. Thus, we build our rationale as follows: We first briefly summarize the current and historical context for motivating the relevance of motor dysfunction in schizophrenia. We follow with a pivot to basic movement physiology, reviewing the architecture of the primate motor system, one that is well delineated by structural and electrophysiological studies, and confirmed with in vivo fMRI studies. Then we theoretically navigate the intersection between this organization of motor function, and schizophrenia-related pathophysiology that is observed in brain regions implicated in motor processing. We then transition to a discussion of the molecular correlates of schizophrenia, particularly in the context of the dopaminergic hypothesis that offers a manner of convergence with the motor system. Each of these principal sub-sections are summarized in accompanying schematic figures.
We reiterate that we provide more synthesis than review. Each of the subsections herein would demand more space for a comprehensive treatment (15-17), and as noted, separate reviews have effectively addressed some of these. Yet, in linking basic movement physiology, schizophrenia and neurotransmitters, we supplement previous advocacy for increased focus on the study of motor dysfunction and its pathophysiological bases in schizophrenia. The study of motor dysfunction in schizophrenia is in fact, not distinct from the study of higher order dysfunctions in the illness. Schizophrenia is a “thought disorder”, yet notably thinking has been conceptualized as an “active motor process” (18); structures such as the basal ganglia that are considered cognitive pattern generators, are also motor pattern generators (19), generating sequences of commands designed to sub serve future action. In this way motor function is a foundation for higher-level cognitive function. Indeed, impairments in extended domains of the motor system, including oculomotor deficits, have been directly linked to the core pathophysiology of psychosis itself (20). The motor system itself, provides a tractable framework for studying brain networks, as the simplest of tasks evoke sophisticated interactions between regions such as the anterior cingulate, supplementary motor area and primary motor cortex (21-23). Moreover, these tasks are not characterized by many of the patient-control performance differences that confound the interpretation of in vivo imaging studies of higher order cognitive function (24). A focus on motor function is also of significant clinical relevance. Schizophrenia patients with general motor abnormities have less favorable outcomes, and motor abnormalities in turn predict deficits in memory, executive functioning, and attention (25), supporting the notion of thinking as an active motor process. And, patients with motor dysfunction also develop more severe side effects of antipsychotic medications (26), and longitudinal studies indicate that neurological soft signs (NSS) predict elevations in negative symptoms of schizophrenia (27).
2. Motor dysfunction in schizophrenia: Background
Aberrant motor functioning in patients with schizophrenia has been noted since the earliest systematic clinical characterizations of the illness (28). General movement disorders in schizophrenia were described as early as 1919, in the pre-neuroleptic era with Kraepelin describing schizophrenia patients with dementia praecox and paraphrenia as having “…spasmodic phenomena in the musculature of the face and of speech”, “involuntary choreic movements”, and “atheoide ataxia” (29). More generally, studies have noted abnormal involuntary movements (dyskinesia), slowness in planning and execution of fine motor tasks (psychomotor slowing), problems with coordination and sensory-motor tasks (neurological soft signs; NSS), rigid posturing, grimacing, and mannerisms (catatonia), and Parkinsonism (30). With the advent of typical antipsychotics, motor dysfunction in schizophrenia has increasingly been associated with their extra-pyramidal side effects (31). However, in addition to the early characterization of motor dysfunction, evidence suggests that antipsychotic medications may in fact serve to exacerbate the emergence of spontaneous motor disorders, rather than being the single underlying cause (26, 32).
Estimates of the frequency of motor abnormalities in schizophrenia patients range between 50%-65% (as compared to ~5% in healthy controls) (25). Retrospective studies have suggested that motor abnormalities significantly predate the onset of psychosis, with delayed motor development in childhood seen as a risk factor for future schizophrenia spectrum disorders (33). These deficits also tend to aggregate in families of patients, suggesting that abnormal motor development is a vulnerability marker, and a fundamental correlate of psychosis (34). The underlying neurobiology of motor behavior, particularly associated with the hand effectors is reasonably well established both in primate (35) and neuroimaging studies (10). These detailed descriptions therefore provide a basis for the prospective assessment of the pathophysiology of brain networks in schizophrenia. In advocating this, we simply echo the “physiological approach” that has elucidated dysfunctional working memory in the illness (36, 37).
3. The architecture of the primate motor system: Motor regions and beyond
Studies of the primate motor system have provided some of the most carefully defined associations between a simple behavioral domain and mechanisms in the neural substrate. Motor behavior is sub served by highly coordinated brain sub-networks, principally involving the primary motor cortex (M1), the supplementary motor cortex (SMA), the pre-supplementary motor cortex (pre-SMA), the dorsal anterior cingulate (dACC), the inferior parietal cortex, the basal ganglia, and the anterior cerebellum (38, 39). Notably, dysfunction in several of these brain regions has also been implicated in schizophrenia and higher-level cognitive dysfunction associated with the disease.
The primary motor cortex (M1; Brodmann Area 4) is responsible for eliciting localized movements in various parts of the body, with its specific areas topographically organized. The extent of cortical surface area responsible for the control of a body part varies in approximate proportion to the precision of movement it demonstrates (40). BA 6, comprised of the premotor cortex and supplementary motor area (SMA), is another important region implicated in motor control. The SMA is associated with the planning, initiation, and anticipation of specific movements (41), and further divided into rostral pre-SMA and the caudal SMA that together form a dedicated subcortical-cortical temporal processing network (42). For example, the pre-SMA engages in the initiation and updating of non-automatized internally generated movements, as opposed to the SMA-proper, which plays a role in externally generated movements (43, 44).
The dACC also referred to as the mid cingulate cortex (45) lies outside the core motor circuit, yet is generally regarded as a critical interface between cognition and motor control (22, 46, 47). Located on the medial surface of the cerebral hemispheres, the dACC has extensive structural and functional connections to the lateral prefrontal cortex, limbic structures, SMA, and the striatum (48, 49). The structure’s regulatory role in a hierarchical model of motor control, is sub served through dense projections that transmit selective modulatory signals to the SMA and other structures (50, 51). Recent fMRI studies have confirmed such a modulatory influence that allows maintenance of timing expectancy (22), and in general, the dACC is highly active during tasks requiring inhibitory or excitatory motor control (52-54). fMRI studies in humans and single-unit electrophysiological studies of primates have demonstrated functionally heterogeneous regions of the dACC that respond distinctly and disproportionately to reward-based decisions of motor selection (55, 56). This willed control of motor behavior is consistent with the idea of dACC involvement in suppressing externally triggered motor routines, as seen in lesion studies of the structure in primates.
The selection of motor functions and higher-level cognitive decisions of voluntary movement are also influenced by the basal ganglia and its constituents including in particular the caudate nuclei and the putamen (57, 58). Furthermore, a series of parallel loops connect many cortical inputs to the basal ganglia and serve to control voluntary movements (59). These discrete loops also make reciprocal connections from the basal ganglia motor system that also includes the globus pallidus (GP), the substantia nigra and sub thalamic nuclei (STN) to cerebral cortical areas, including the dorsolateral prefrontal cortex, and the anterior cingulate (60). The basal ganglia’s components, together with the cerebral cortex and the ventrolateral nucleus of the thalamus, are implicated in movement control through a model (61, 62) in which the basal ganglia is controlled directly and indirectly by two discrete pathways. Both pathways are inhibitory, suppressing thalamic activity, and begin with cortical excitation of neurons located in the striatum. These motor pathways of the basal ganglia are of particular interest in schizophrenia because of their rich dopaminergic circuitry and the coinciding role of dopamine in reward, psychosis and movement (63). The implications for schizophrenia of this motor cortical – striatal motor pathway are self-evident both from the perspective of systems neuroscience, as well as dopaminergic models of the illness (64).
Dopamine produced and synthesized in dopaminergic neurons in the substantia nigra is released in the striatum, stimulating the direct pathway through D1 receptors. Dopamine also inhibits the indirect pathway by binding to D2 receptors of GABA neurons (65). This indirect pathway begins with cortical excitation of striatal neurons that excite populations of GABA neurons, distinct from those excited in the direct pathway. These project to neurons in the STN, which in turn project to the GP internal segment to inhibit motor related functions of the thalamus. The balance of the two conjugate pathways is thought to be key for normal motor function (66). The role of the basal ganglia in movement balances muscle activation, helps in sequencing activation for fast movements, and selectively inhibits competing movements that could interrupt voluntary action (67).
The cerebellum consists of two lateral hemispheres and a medial vermis (68), and these cerebellar regions have highly variegated and diverse roles in voluntary motor control, balance, coordination, and higher cognitive non-motor functions. The cerebellum can be further divided into ten mirrored lobules, which are somatotopically organized, in a series of sensory-motor “homunculi”, arranged dorso-ventrally (69). Cerebellar lobules IV, V, and VIII are robustly activated during motor tasks (70), and cognitively demanding tasks elicit activation of lobules VI and VII (71-73). Unsurprisingly, the structural architecture of the cerebellar lobules consists of motor and non-motor closed loop circuits to the thalamus other regions of the cerebral cortex (74). Connections to the cortico-ponto-cerebellar system make the cerebellum a key control region for voluntary motor control, enhancing the structures relevance for schizophrenia (75). The main output center of the cerebellum is the dentate nucleus, which links cortical structures such as premotor, prefrontal, posterior parietal, and primary motor cortex mainly via the thalamus. The cortex sends projections back to the cerebellum for processing via the pons. These cortico-cerebellar loops link function of the cerebellum to the function of the cerebral cortex and vice versa (76). The cerebellum is thought to act as a timer, updating and predicting body dynamics for fast movements based on sensory feedback (77), and this precision is assumed to have high temporal fidelity (78). This model provides the rationale for cerebellar involvement in coordination, precision, and timing of movement, as well as sequential processing of spatial and temporal information (79), and has been considered of fundamental importance in the context of schizophrenia (80, 81).
Figure 1 provides an overview of the general architecture of the motor system. It is notable that functional impairments and structural abnormalities in each of these regions have been associated with motor dysfunction in schizophrenia. In the subsequent section, we explore how and why the patterns of motor dysfunction in schizophrenia may emerge from the macroscopic network of brain regions depicted in Figure 1.
Figure 1.
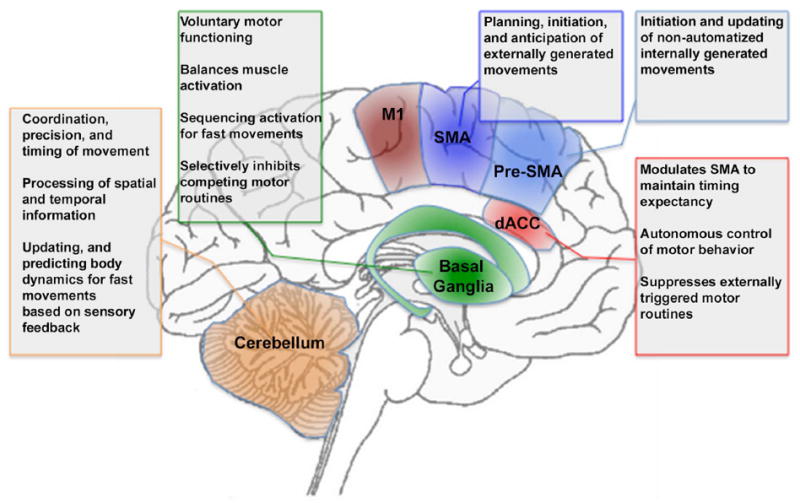
The figure provides a schematic depiction of motor, cingulate and cerebellar regions and their role in aspects of motor behavior. Highlighted are M1: Primary Motor Cortex, SMA: Supplementary Motor Area, Pre-SMA: Pre-Supplementary Motor Area, dACC: Dorsal Anterior Cingulate Cortex, Basal Ganglia, and Cerebellum. The corresponding text boxes summarize known aspects of the relative specialization of function of the regions.
4. Dysfunction in regions of the core and extended motor network in schizophrenia
Several different abnormalities in structure, function, and connectivity of brain regions of the motor system may be involved in the emergence of motor dysfunction in schizophrenia. Here we provide a succinct summary of several representative findings of regional and network dysfunction in schizophrenia, of motor-function related brain regions.
4.1 M1 and the Supplementary Motor Cortices
Aberrant activity in M1 and the pre-motor cortex has been observed in schizophrenia patients during finger sequence learning and relatively simple sensorimotor detection tasks, though the precise nature of the aberration appears task specific. Kodama and colleagues trained patients and controls on a simple finger sequence learning task, demonstrating that compared to healthy controls, patients hyper-activated pre-motor cortex following sequence learning (82). This pattern of hyper-activation is suggestive of impaired efficiency of motor control and learning mechanisms, and was conceptually replicated in a separate study by Minzenberg and colleagues (83). When patients were required a basic sensori-motor detection task they hyper-activated ipsi-lateral (to the response hand) M1 compared to healthy controls. However, aberrant hypo-activation of the motor cortices has also been documented using sequential and non-sequential finger tapping tasks that do not involve learning (84-86). As is the case with other classes of tasks, such as working memory, the direction of the aberrance in schizophrenia is most likely yoked to the specific tasks demands and performance metrics of patients (87, 88). Notably, impairments are observed not only in basic motor control when generating finger movements, but also during the application of motor force using the hand effectors. In a recent study, Martinelli and colleagues required participants to apply manual force to a lever that was commensurate with a visual cue signaling the degree of force that was to be applied (89). In healthy controls, the degree of applied force generated to this external cue was strongly correlated with fMRI estimated responses in the primary somatosensory cortex, indicative of normal force signaling in motor regions. This coupling was absent in patients, suggesting that schizophrenia may alter mechanisms that encode and predict sensorimotor responses (the notion of impaired predictive coding is re-visited below). What appears relevant is that basic motor behaviors evoke impaired responses in the motor network. These effects are also generally associated with aberrant metrics of morphology.
Schizophrenia patients who display aberrant motor behavior also show aberrant morphometric characterization of several of the motor structures depicted in Figure 1. In a study of first episode schizophrenia, reduced size of the pre-SMA was associated with impaired motor sequencing (90), an effect that has been replicated using the conjoint assessment of fMRI and voxel based morphometric studies (91). The changes in size have also been related to symptom dimensions and clinical scales. For example, volumetric reductions and fMRI measures of activation are negatively correlated with NSS scores in the SMA and M1 of recently diagnosed patients (92-94). Alterations in the size of the SMA are also thought to represent a compensatory cortico-cortical pathway that may offset insufficient basal ganglia output (95), and in catatonic patients with schizophrenia, increased severity of catatonia (based on the Bush Francis Catatonia Rating scale) predicts higher blood perfusion in the SMA (96). This latter effect is highly suggestive that schizophrenia is associated with dysregulation of the SMA in the resting state.
Diffusion tensor imaging (DTI) studies of white matter connections of motor cortices have demonstrated alterations in the cortico-basal and cortico-cortical pathways. Specifically, in patients with motor abnormalities, there is an associated increase in motor activity between the pre-SMA to SMA-proper connection with less motor activity in the right pre-SMA to GP connection (93). These findings are similar to compensatory motor activity of decreased basal gangia output proposed in Parkinson’s disease (97). This idea parallels DTI findings of abnormal white matter integrity found under the right SMA in schizophrenia patients with decreased resting state wrist movement (98).
As previously noted, cognitive and motor control is partly mediated by the dorsal anterior cingulate cortex (dACC). Cingulate cortical neurons project to multiple areas of the motor and premotor cortex, suggesting that numerous parallel pathways exist whereby cingulate neurons can modulate motor output systems of the brain. Structural MRI studies suggest that reductions in gray matter volume in the dorsal and rostral ACC, frequently predate the onset of psychosis in high-risk individuals (99, 100). In schizophrenia patients, decreased volume of the cingulate gyrus is significantly and selectively correlated with executive dysfunction (101). Also, reduced synaptic density, deficits in inhibitory interneurons, and aberrant synaptic pathology have been found in the cytoarchitecture of cingulate cortex in patients (102). These aberrations complement fMRI studies of finger tapping, which demonstrate failed motor task-related activation of the ACC and disrupted connectivity to medial frontal cortex (103), evidence of functional impairments in basic volition. Recent evidence sheds light on the structural correlations of avolition in the motor domain. Using estimates of non-Gaussian diffusion (based on diffusion kurtosis imaging, DKI), Docx and colleagues examined the relationship between volitional motor activity (assessed with actigraphy) and white matter structure. In general, patients were less active than healthy controls, and showed greater diffusivity and lower fractional anisotropy on longitudinal white matter tracts (104). Moreover, in patients, mean kurtosis (a measure that better distinguishes between hindered versus unhindered diffusivity), was strongly associated with actigraph estimates of activity in patients. These estimates of motor activity related to effects in the brain’s substrate also substantiate evidence of NSS deficits.
In schizophrenia, structural morphology of the basal ganglia, especially that of the caudate nucleus, putamen, and GP is atypical and associated with neurological soft signs (27), but the role that neuroleptics play appears to be quite heterogeneous. Grossly, the striatum is smaller than normal in antipsychotic-naive patients though upon antipsychotic treatment striatal and pallidial volumes enlarge (105), which is a possible explanation for increased basal ganglia volumes in medicated schizophrenia patients (106). In addition to size, alterations in shape of both the caudate and putamen have been found in schizophrenia patients as compared to healthy siblings (106). Furthermore, morphological abnormalities of the putamen are observed in regions where prominent connections to non-motor cortical areas exist. Striatal abnormalities are exacerbated in the context of deficit syndrome schizophrenia, a subclass of the diagnosis in which patients are characterized by pervasive negative symptoms, social withdrawal and increased anhedonia (107). Neuroleptic naïve deficit syndrome patients (relative to non-deficit syndrome patients) are characterized by increases in spontaneous movement disorders, consistent with the role of the basal ganglia in mediating both schizophrenia symptomatology and movement function (108). Thus, impaired activity in the basal ganglia with hyperactivity in the primary, premotor, and somatosensory cortices, is thought to lead to cortical motor overflow and subsequent motor disturbances (83).
Many of the alterations of motor symptoms described in schizophrenia, such as NSS, abnormal eye movements, and disequilibrium, indicate dysfunctions in the cerebellum. Abnormal cerebellar structure and connectivity with the motor cortex has been implicated in motor and timing dysfunction in the illness (109, 110). Transcranial magnetic stimulation directed at the cerebellum has shown increased excitability of the motor cortex in motor dysfunctions (111). These findings suggest a decrease in cortico-cerebellar inhibition of motor cortex, which is important in the coordination of motor sequencing. Investigations of schizophrenia patients with motor sequencing abnormalities revealed lower functional connectivity between the cerebellum and motor cortex during the resting state (112), a suggestive marker for structural dysconnectivity. Interestingly, schizophrenia patients with marked motor sequencing impairment had more psychotic episodes than those without movement sequencing disturbances (14), a finding that suggests that consecutive psychotic outbreaks play a role in progressive worsening of cortico-cerebellar connectivity. NSS, like impaired movement sequencing, seem to be a result of impaired coordination between these cortico-cerebellar areas involved in the motor system (14). From a neuropathological perspective, cerebellar purkinje cells, responsible for modulating input from the cerebellum to the cortex, have decreased size and linear density in schizophrenia (113). Purkinje cells and their extensive connectivity of climbing fibers in the inferior olive mediate cerebellar coordination of cognitive activity (114) and the many cortical and sensory inputs received permit the purkinje cells to detect error and selectively shuttle information back to the cerebral cortex through inhibitory output of deep nuclei. Thus, cerebellar organization influences the fidelity of perceptions, detects errors, and rapidly modulates coordination. Schizophrenia may impair the proper modulation and coordination of multiple signals causing a misinterpretation of erroneous sensory associations usually suppressed by the cerebellum, resulting in the frequently noted evidence of abnormal predictive coding in the illness (17).
The preceding discussion is summarized in Figure 2, wherein the text boxes that in Figure 1 represented the putative functions of each region, are replaced with a summary of regional deficits observed in schizophrenia. Figure 2 presents a systems level perspective on deficits in the motor system in schizophrenia, yet effects at this level can also be associated with evidence of functional alterations at the molecular scale in schizophrenia-relevant neurotransmitter systems. In the following section, we explore the synthesis between dopamine and dysfunctional motor control in schizophrenia.
Figure 2.
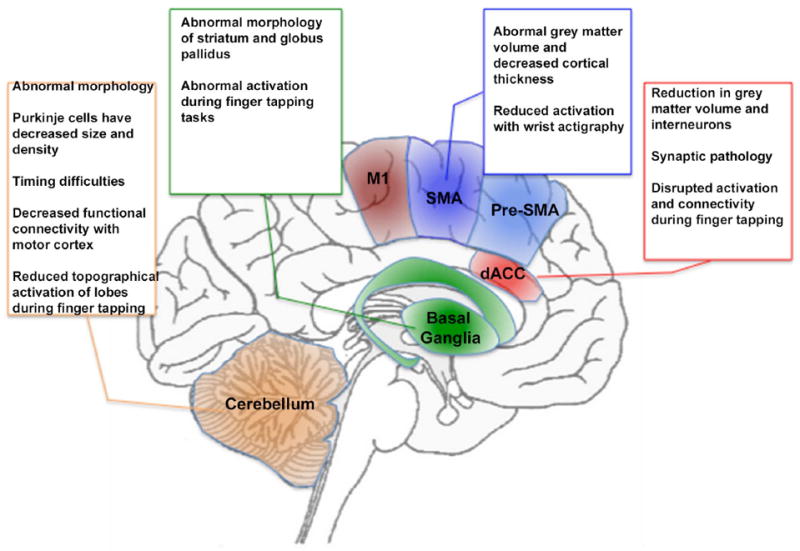
Figure 1 is adapted to summarize some of the known findings in schizophrenia. As seen in the text boxes (and more extensively discussed in the text), regions central to motor behavior also are characterized by widespread and multifaceted abnormalities in schizophrenia. These include abnormal structure (at multiple scales) and aberrant responses during motor tasks. The color and naming schemes are maintained from Figure 1.
5. Dopamine and motor dysfunction in schizophrenia
In 1966 van Rossum proposed an early version of the “dopamine hypothesis”, suggesting that the illness may be related to the pathophysiological overproduction of dopamine or overstimulation of dopamine receptors (115). Evidence in the 1950’s associating Parkinson’s disease with a deficiency of dopamine, laid the framework for altered dopamine transmission as the cause of extrapyramidal disturbances seen with antipsychotics, and later, the underlying pathology in schizophrenia itself (116). By 1975, the dopamine hypothesis was substantiated by direct binding assays of antipsychotics to dopamine receptors (117).
Dopaminergic projections most associated with schizophrenia involve the mesocortical system (118). This system arises in the ventral tegmental area (VTA) and projects to multiple cortical areas including the prefrontal cortex, anterior cingulate cortex, and areas of the temporal lobes. Alterations in dopamine signaling of this projection, as well as the mesolimbic and nigrostriatal projections are likely to be involved in schizophrenia. Higher dopamine levels are found in the substantia nigra and the striatum of the basal ganglia, with lower levels found in the cortex of schizophrenia patients (119). In neuroleptic-naive patients, an overactive striatal dopaminergic system is thought to result from changes in dopamine synthesis, storage, and release (63), and indeed, molecular imaging studies found increased pre-synaptic dopamine synthesis and storage in the striatum of schizophrenia patients (120). This elevated striatal dopamine function has been strongly associated with motor dysfunction and psychotic symptoms (121).
Dopamine receptor abnormalities, especially D1 and D2, likely underlie some of the behavioral and abnormal motor symptoms seen in schizophrenia (122). In the nigrostriatal projection, dopamine is produced and synthesized in the dopaminergic neurons in the substantia nigra, in turn stimulating movement via the direct pathway through D1 receptors. Dopamine inhibits the indirect pathway, suppressing movement, by binding to the D2 receptors that are on the indirect pathway GABA neurons. In the striatum, excessive stimulation of D2 receptors has been found in patients diagnosed early in the disease course (123). This hyper-stimulation is thought to result from highly sensitive D2 receptors, and it has been shown that schizophrenia patients who are exposed to dopamine-related drugs such amphetamine or methylphenidate often present with exacerbated psychotic symptoms (124).
In addition to hyper sensitivity, abnormal density of dopamine receptors may also impact motor areas implicated in schizophrenia. A decreased density of D2 receptors is found in the ACC, with higher density of D2 receptors in found the striatum (125, 126). In patients on antipsychotic treatment regimens, density of D2 receptors in the striatum is subsequently reduced, and is associated with an abnormal reduction in the speed of finger tapping (127). In a study where mice had up-regulated D2 receptors, increased inhibition of the indirect pathway was found, suggesting that the increased D2 receptor expression and increased striatal excitability could lead to reversible changes that would alter the way in which the direct pathway is activated in schizophrenia (123). This disruption of balance of the pallidal pathways, mediated by dopamine signaling, may play a role in the development of dysfunctional motor control. The link between putative pharmacologic mechanisms and treatment response, has led to the notion of dopamine (particularly striatal) sensitivity as a “final common pathway” in schizophrenia (64), though given the complex nature of dopamine signaling, it is unlikely that a single hypothesis is responsible for all the manifestations of the illness (118). Figure 3 (consistent with the format of previous figures), depicts the implicated dopamine pathways with a summary of pertinent findings in schizophrenia.
Figure 3.
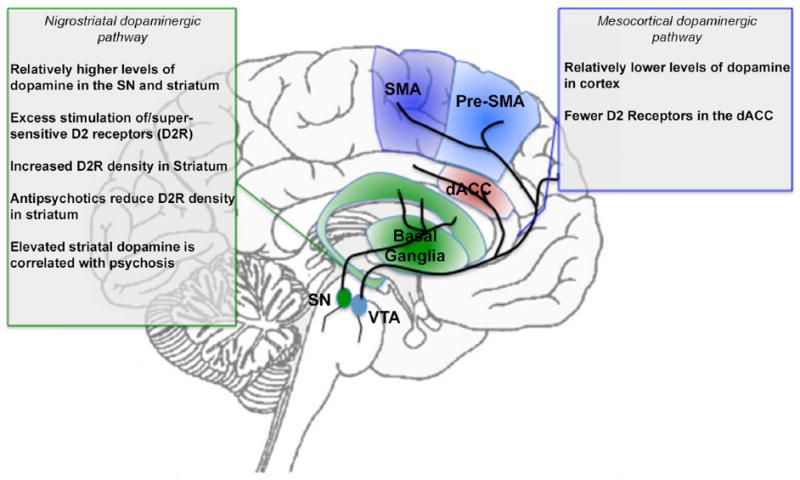
A summary of dopamine projections and implications for schizophrenia. The nigrostriatal dopaminergic projection system originates from the substantia nigra (SN), whereas the mesocortical dopaminergic projection system originates from the ventral tegmental area (VTA). These systems project to the basal ganglia, and the motor and cingulate regions respectively (see marked projections). The text boxes summarize evidence of dysfunction in schizophrenia. The color and naming schemes are consistent with previous figures.
6. Implications and Conclusions
Schizophrenia is a “multi-level” disorder with aberrations noted at micro-, meso- and macro-scopic scales in the neuronal substrate. Yet its origins are obscure and its pathophysiologic bases can only be reached through a process of “inference” from brain signals (128). The organization of motor function, certainly at the macroscopic scale is remarkably well understood (Figure 1), and as our synthesis suggests, aspects of motor function, intersect with questions of interest in the pathophysiology of schizophrenia (Figures 2-3).
Individuals with schizophrenia are characterized by a diverse set of motor symptoms, among them involuntary, uncoordinated, and inhibited movement. As noted, dyskinesia is frequently medication induced, but motor symptoms were noted long before the discovery of first-generation anti-psychotics, and frequently predate the onset of the illness. Given that motor symptoms are at the core of schizophrenia-related pathophysiology and provide a relatively well-understood mapping to brain systems, the motivation to study the neurobiological bases of motor dysfunction in schizophrenia is obvious. These motivations are not isolated. There is a vivid history of studying the oculomotor system in the context of schizophrenia and psychosis, in part because of the ready translational framework, and because motor control (or lack thereof) within the eye movement system can provide fundamental insights into the bases of psychosis (20). Thus, a framework involving motor dysfunction measured by neuroimaging of both neurotransmitter systems and brain activity may reconcile and delineate nonhomogeneous findings and help in categorizing endophenotypes of the disease. Moreover, the pathophysiology of brain regions known to be involved in motor function may help inform understanding of disturbed cognitive functions in schizophrenia, given that thinking may be an active motor process. With no single underlying etiology to connect these dysfunctions to clinical features, an exploratory approach involving task-underpinned neuroimaging will be essential. Basic motor control and behavior are linked with a rich set of cortical and sub-cortical brain regions (10), an aspect that is appropriately recognized in the Research Domain Criteria constructs matrix (129). Whether the explication of domain related effects will ultimately inform and transform the diagnostic concept of schizophrenia, is itself an open question (130, 131), yet one worthy of investigation.
Acknowledgments
Preparation of this work was supported by a Career Development Chair from Wayne State University, the Charles H. Gershenson Distinguished Faculty Fellowship from Wayne State University, the Lyckaki-Young Fund from the State of Michigan, the Prechter Family Bipolar Foundation, the Children’s Hospital of Michigan Foundation, the Children’s Research Center of Michigan, the Cohen Neuroscience Endowment, and the National Institute of Mental Health (MH 59299, MH111177).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saha S, Chant D, Welham J, McGrath J. A Systematic Review of the Prevalence of Schizophrenia. PLoS Medicine. 2005;2(5):e141. doi: 10.1371/journal.pmed.0020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keshavan MS, Tandon R, Boutros NN, Nasrallah HA. Schizophrenia, “just the facts”: what we know in 2008 Part 3: neurobiology. Schizophrenia Research. 2008;106(2-3):89–107. doi: 10.1016/j.schres.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Goldman-Rakic PS. The physiological approach: functional architecture of working memory and disordered cognition in schizophrenia. Biological Psychiatry. 1999;46(5):650–61. doi: 10.1016/s0006-3223(99)00130-4. [DOI] [PubMed] [Google Scholar]
- 4.Izawa J, Asai T, Imamizu H. Computational motor control as a window to understanding schizophrenia. Neurosci Res. 2016;104:44–51. doi: 10.1016/j.neures.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Synofzik M, Thier P, Leube DT, Schlotterbeck P, Lindner A. Misattributions of agency in schizophrenia are based on imprecise predictions about the sensory consequences of one’s actions. Brain. 2010;133(Pt 1):262–71. doi: 10.1093/brain/awp291. [DOI] [PubMed] [Google Scholar]
- 6.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Human Brain Mapping. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8(12):539–46. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266(5184):458–61. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- 9.Woodcock EA, Wadehra S, Diwadkar VA. Network profiles of the dorsal anterior cingulate and dorsal prefrontal cortex in schizophrenia during hippocampal-based associative memory. Front Systems Neurosci. 2016;10:32. doi: 10.3389/fnsys.2016.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witt ST, Laird AR, Meyerand ME. Functional neuroimaging correlates of finger-tapping task variations: an ALE meta-analysis. NeuroImage. 2008;42(1):343–56. doi: 10.1016/j.neuroimage.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31(2-3):236–50. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 12.Hirjak D, Thomann PA, Kubera KM, Wolf ND, Sambataro F, Wolf RC. Motor dysfunction within the schizophrenia-spectrum: A dimensional step towards an underappreciated domain. Schizophrenia Research. 2015;169(1-3):217–33. doi: 10.1016/j.schres.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 13.Walther S. Psychomotor symptoms of schizophrenia map on the cerebral motor circuit. Psychiatry Research. 2015;233(3):293–8. doi: 10.1016/j.pscychresns.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Kasparek T, Rehulova J, Kerkovsky M, Sprlakova A, Mechl M, Mikl M. Cortico-cerebellar functional connectivity and sequencing of movements in schizophrenia. BMC Psychiatry. 2012;12(1):17-. doi: 10.1186/1471-244X-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fredericks CM, Saladin LK. Pathophysiology of the motor systems: Principles and clinical presentations. Philadelphia, PA: F A Davis Company; 1996. [Google Scholar]
- 16.Howes OD, McCutcheon R, Owen MJ, Murray RM. The Role of Genes, Stress, and Dopamine in the Development of Schizophrenia. Biological Psychiatry. 2017;81(1):9–20. doi: 10.1016/j.biopsych.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friston K, Brown HR, Siemerkus J, Stephan KE. The dysconnection hypothesis (2016) Schizophrenia Research. 2016;176(2-3):83–94. doi: 10.1016/j.schres.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graybiel AM. The basal ganglia and cognitive pattern generators. Schizophrenia Bulletin. 1997;23:459–70. doi: 10.1093/schbul/23.3.459. [DOI] [PubMed] [Google Scholar]
- 19.Graybiel AM. The basal ganglia. Trends Neurosci. 1995;18(2):60–2. [PubMed] [Google Scholar]
- 20.Thakkar KN, Diwadkar VA, Rolfs M. Oculomotor Prediction: A Window into the Psychotic Mind. Trends in Cognitive Sciences. 2017 doi: 10.1016/j.tics.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grefkes C, Eickhoff SB, Nowak DA, Dafotakis M, Fink GR. Dynamic intra- and interhemispheric interactions during unilateral and bilateral hand movements assessed with fMRI and DCM. NeuroImage. 2008;41(4):1382–94. doi: 10.1016/j.neuroimage.2008.03.048. [DOI] [PubMed] [Google Scholar]
- 22.Asemi A, Ramaseshan K, Burgess A, Diwadkar VA, Bressler SL. Dorsal anterior cingulate cortex modulates supplementary motor area in coordinated unimanual motor behavior. Front Hum Neurosci. 2015;9:309. doi: 10.3389/fnhum.2015.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diwadkar VA, Asemi A, Burgess A, Chowdury A, Bressler SL. Potentiation of motor sub-networks for motor control but not working memory: Interaction of dACC and SMA revealed by resting-state directed functional connectivity. PLoS ONE. 2017;12(3):e0172531. doi: 10.1371/journal.pone.0172531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carter CS, Heckers S, Nichols T, Pine DS, Strother S. Optimizing the design and analysis of clinical functional magnetic resonance imaging research studies. Biological Psychiatry. 2008;64(10):842–9. doi: 10.1016/j.biopsych.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Bombin I, Arango C, Buchanan RW. Significance and meaning of neurological signs in schizophrenia: two decades later. Schizophrenia Bulletin. 2005;31(4):962–77. doi: 10.1093/schbul/sbi028. [DOI] [PubMed] [Google Scholar]
- 26.Peralta V, Cuesta MJ. Neuromotor abnormalities in neuroleptic-naive psychotic patients: antecedents, clinical correlates, and prediction of treatment response. Comprehensive Psychiatry. 2011;52(2):139–45. doi: 10.1016/j.comppsych.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Hirjak D, Wolf RC, Wilder-Smith EP, Kubera KM, Thomann PA. Motor Abnormalities and Basal Ganglia in Schizophrenia: Evidence from Structural Magnetic Resonance Imaging. Brain Topography. 2015;28(1):135–52. doi: 10.1007/s10548-014-0377-3. [DOI] [PubMed] [Google Scholar]
- 28.Walther S, Strik W. Motor Symptoms and Schizophrenia. Neuropsychobiology. 2012;66(2):77. doi: 10.1159/000339456. [DOI] [PubMed] [Google Scholar]
- 29.Kraepelin E. Dementia Praecox. In: Cutting J, Shepeherd M, editors. The Clinical Roots of Schizophrenia. Cambridge: Cambridge University Press; 1986. [Google Scholar]
- 30.Morrens M, Docx L, Walther S. Beyond boundaries: in search of an integrative view on motor symptoms in schizophrenia. Frontiers in Psychiatry. 2014;5:145. doi: 10.3389/fpsyt.2014.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitty PF, Owoeye O, Waddington JL. Neurological Signs and Involuntary Movements in Schizophrenia: Intrinsic To and Informative on Systems Pathobiology. Schizophrenia Bulletin. 2009;35(2):415–24. doi: 10.1093/schbul/sbn126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pappa S, Dazzan P. Spontaneous movement disorders in antipsychotic-naive patients with first-episode psychoses: a systematic review. Psychological Medicine. 2009;39(7):1065–76. doi: 10.1017/S0033291708004716. [DOI] [PubMed] [Google Scholar]
- 33.Murray GK, Jones PB, Moilanen K, Veijola J, Miettunen J, Cannon TD, et al. Infant motor development and adult cognitive functions in schizophrenia. Schizophrenia Research. 2006;81(1):65–74. doi: 10.1016/j.schres.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 34.Woods BT, Kinney DK, Yurgelun-Todd DA. Neurological “hard” signs and family history of psychosis in schizophrenia. Biological psychiatry. 1991;30(8):806–16. doi: 10.1016/0006-3223(91)90236-f. [DOI] [PubMed] [Google Scholar]
- 35.Armstrong E. A comparative review of the primate motor system. Journal of Motor Behavior. 1989;21(4):493. doi: 10.1080/00222895.1989.10735496. [DOI] [PubMed] [Google Scholar]
- 36.Goldman-Rakic PS. The physiological approach: functional architecture of working memory and disordered cognition in schizophrenia. Biological Psychiatry. 1999;46(5):650–61. doi: 10.1016/s0006-3223(99)00130-4. [DOI] [PubMed] [Google Scholar]
- 37.Lewis DA. Cortical circuit dysfunction and cognitive deficits in schizophrenia--implications for preemptive interventions. Eur J Neurosci. 2012;35(12):1871–8. doi: 10.1111/j.1460-9568.2012.08156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dum RP, Levinthal DJ, Strick PL. Motor, cognitive, and affective areas of the cerebral cortex influence the adrenal medulla. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(35):9922–7. doi: 10.1073/pnas.1605044113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dum RP, Strick PL. Motor areas in the frontal lobe of the primate. Physiology & Behavior. 2002;77(4-5):677–82. doi: 10.1016/s0031-9384(02)00929-0. [DOI] [PubMed] [Google Scholar]
- 40.Chouinard PA, Paus T. The primary motor and premotor areas of the human cerebral cortex. The Neuroscientist. 2006;12(2):143–52. doi: 10.1177/1073858405284255. [DOI] [PubMed] [Google Scholar]
- 41.Makoshi Z, Kroliczak G, van Donkelaar P. Human Supplementary Motor Area Contribution to Predictive Motor Planning. Journal of Motor Behavior. 2011;43(4):303–9. doi: 10.1080/00222895.2011.584085. [DOI] [PubMed] [Google Scholar]
- 42.Schwartze M, Rothermich K, Kotz SA. Functional dissociation of pre-SMA and SMA-proper in temporal processing. NeuroImage. 2012;60(1):290–8. doi: 10.1016/j.neuroimage.2011.11.089. [DOI] [PubMed] [Google Scholar]
- 43.Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nature Reviews Neuroscience. 2008;9(11):856–69. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- 44.Picard N, Strick PL. Activation of the supplementary motor area (SMA) during performance of visually guided movements. Cerebral Cortex. 2003;13(9):977–86. doi: 10.1093/cercor/13.9.977. [DOI] [PubMed] [Google Scholar]
- 45.Hoffstaedter F, Grefkes C, Caspers S, Roski C, Palomero-Gallagher N, Laird AR, et al. The role of anterior midcingulate cortex in cognitive motor control: evidence from functional connectivity analyses. Human Brain Mapping. 2014;35(6):2741–53. doi: 10.1002/hbm.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2(6):417–24. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- 47.Kronhaus DM, Willshaw DJ. The cingulate as a catalyst region for global dysfunction: a dynamical modelling paradigm. Cereb Cortex. 2006;16(8):1212–24. doi: 10.1093/cercor/bhj062. [DOI] [PubMed] [Google Scholar]
- 48.Hutchison RM, Womelsdorf T, Gati JS, Leung LS, Menon RS, Everling S. Resting-state connectivity identifies distinct functional networks in macaque cingulate cortex. Cereb Cortex. 2012;22(6):1294–308. doi: 10.1093/cercor/bhr181. [DOI] [PubMed] [Google Scholar]
- 49.Vogt BA, Pandya DN, Rosene DL. Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents. J Comp Neurol. 1987;262(2):256–70. doi: 10.1002/cne.902620207. [DOI] [PubMed] [Google Scholar]
- 50.Bates JF, Goldman-Rakic PS. Prefrontal connections of medial motor areas in the rhesus monkey. The Journal of Comparative Neurology. 1993;336(2):211–28. doi: 10.1002/cne.903360205. [DOI] [PubMed] [Google Scholar]
- 51.Luppino G, Matelli M, Camarda R, Rizzolatti G. Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. The Journal of Comparative Neurology. 1993;338(1):114–40. doi: 10.1002/cne.903380109. [DOI] [PubMed] [Google Scholar]
- 52.Friedman A, Burgess A, Ramaseshan K, Easter P, Khatib D, Chowdury A, et al. Brain network dysfunction in obsessive-compulsive disorder induced by simple uni-manual behavior: The role of the dorsal anterior cingulate cortex. Psychiatry Res Neuroimaging. 2017;260:6–15. doi: 10.1016/j.pscychresns.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108(3):624–52. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 54.Nakamura K, Roesch MR, Olson CR. Neuronal activity in macaque SEF and ACC during performance of tasks involving conflict. Journal of Neurophysiology. 2005;93(2):884–908. doi: 10.1152/jn.00305.2004. [DOI] [PubMed] [Google Scholar]
- 55.Shima K, Tanji J. Role for cingulate motor area cells in voluntary movement selection based on Reward. Science. 1998;282(5392):1335–8. doi: 10.1126/science.282.5392.1335. [DOI] [PubMed] [Google Scholar]
- 56.Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, et al. Dorsal anterior cingulate cortex: A role in reward-based decision making. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(1):523–8. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Avanzino L, Pelosin E, Vicario CM, Lagravinese G, Abbruzzese G, Martino D. Time processing and motor control in movement disorders. Front Hum Neurosci. 2016;10:631. doi: 10.3389/fnhum.2016.00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morris LS, Kundu P, Dowell N, Mechelmans DJ, Favre P, Irvine MA, et al. Fronto-striatal organization: Defining functional and microstructural substrates of behavioural flexibility. Cortex. 2016;74:118–33. doi: 10.1016/j.cortex.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nambu A. Seven problems on the basal ganglia. Current Opinion in Neurobiology. 2008;18(6):595–604. doi: 10.1016/j.conb.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 60.Leisman G, Braun-Benjamin O, Melillo R. Cognitive-motor interactions of the basal ganglia in development. Frontiers in Systems Neuroscience. 2014;8:16. doi: 10.3389/fnsys.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends in Neurosciences. 1989;12(10):366–75. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 62.Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Progress in Brain Research. 1990;85:119. [PubMed] [Google Scholar]
- 63.Perez-Costas E, Melendez-Ferro M, Roberts RC. Basal ganglia pathology in schizophrenia: dopamine connections and anomalies. Journal of Neurochemistry. 2010;113(2):287–302. doi: 10.1111/j.1471-4159.2010.06604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Howes OD, Murray RM. Schizophrenia: an integrated sociodevelopmental-cognitive model. Lancet. 2013 doi: 10.1016/S0140-6736(13)62036-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brittain J-S, Brown P. Oscillations and the basal ganglia: motor control and beyond. NeuroImage. 2014;85(Pt 2):637–47. doi: 10.1016/j.neuroimage.2013.05.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harrington D, Hening W, Poizner H. Basal Ganglia: Motor Functions. 2009:346–50. [Google Scholar]
- 67.Patel N, Jankovic J, Hallett M. Sensory aspects of movement disorders. The Lancet Neurology. 2014;13(1):100–12. doi: 10.1016/S1474-4422(13)70213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pollok B, Butz M, Gross J, Sudmeyer M, Timmermann L, Schnitzler A. Coupling between cerebellar hemispheres: Behavioural, anatomic, and functional data. The Cerebellum. 2006;5(3):212–9. doi: 10.1080/14734220600621294. [DOI] [PubMed] [Google Scholar]
- 69.Batson M, Petridou N, Klomp D, Frens M, Neggers S. Single session imaging of cerebellum at 7 tesla: Obtaining structure and function of multiple motor subsystems in individual subjects. PLoS ONE. 2015;10(8):e0134933. doi: 10.1371/journal.pone.0134933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Desmond JE, Gabrieli JD, Wagner AD, Ginier BL, Glover GH. Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional MRI. J Neurosci. 1997;17(24):9675–85. doi: 10.1523/JNEUROSCI.17-24-09675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. NeuroImage. 2012;59(2):1560–70. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stoodley CJ, Valera EM, Schmahmann JD. An fMRI study of intra-individual functional topography in the human cerebellum. Behav Neurol. 2010;23(1-2):65–79. doi: 10.3233/BEN-2010-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Diwadkar VA, Meintjes EM, Goradia D, Dodge NC, Warton C, Molteno CD, et al. Differences in cortico-striatal-cerebellar activation during working memory in syndromal and nonsyndromal children with prenatal alcohol exposure. Human Brain Mapping. 2013;34(8):1931–45. doi: 10.1002/hbm.22042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bernard JA, Seidler RD, Hassevoort KM, Benson BL, Welsh RC, Wiggins JL, et al. Resting state cortico-cerebellar functional connectivity networks: a comparison of anatomical and self-organizing map approaches. Front Neuroanat. 2012;6:31. doi: 10.3389/fnana.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Picazio S, Koch G. Is motor inhibition mediated by cerebello-cortical interactions? Cerebellum. 2015;14(1):47–9. doi: 10.1007/s12311-014-0609-9. [DOI] [PubMed] [Google Scholar]
- 76.Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Research Reviews. 2000;31(2):236–50. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 77.Salman MS, Tsai P. The role of the pediatric cerebellum in motor functions, cognition, and behavior. Neuroimaging Clinics of North America. 2016;26(3):317–29. doi: 10.1016/j.nic.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ivry RB, Spencer RM. The neural representation of time. Current Opinion in Neurobiology. 2004;14(2):225–32. doi: 10.1016/j.conb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 79.Molinari M, Chiricozzi FR, Clausi S, Tedesco AM, De Lisa M, Leggio MG. Cerebellum and detection of sequences, from perception to cognition. The Cerebellum. 2008;7(4):611–5. doi: 10.1007/s12311-008-0060-x. [DOI] [PubMed] [Google Scholar]
- 80.Andreasen NC. A unitary model of schizophrenia: Bleuler’s “fragmented phrene” as schizencephaly. Archives of General Psychiatry. 1999;56(9):781–7. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- 81.Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophrenia Bulletin. 1998;24(2):203–18. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- 82.Kodama S, Fukuzako H, Fukuzako T, Kiura T, Nozoe S, Hashiguchi T, et al. Aberrant brain activation following motor skill learning in schizophrenic patients as shown by functional magnetic resonance imaging. Psychological Medicine. 2001;31(6):1079–88. doi: 10.1017/s0033291701004196. [DOI] [PubMed] [Google Scholar]
- 83.Minzenberg MJ, Yoon JH, Soosman SK, Carter CS. Excessive contralateral motor overflow in schizophrenia measured by fMRI. Psychiatry Research: Neuroimaging. 2012;202(1):38. doi: 10.1016/j.pscychresns.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 84.Rogowska J, Gruber SA, Yurgelun-Todd DA. Functional magnetic resonance imaging in schizophrenia: cortical response to motor stimulation. Psychiatry Research. 2004;130(3):227–43. doi: 10.1016/j.pscychresns.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 85.Schroder J, Wenz F, Schad LR, Baudendistel K, Knopp MV. Sensorimotor cortex and supplementary motor area changes in schizophrenia. A study with functional magnetic resonance imaging. Br J Psychiatry. 1995;167(2):197–201. doi: 10.1192/bjp.167.2.197. [DOI] [PubMed] [Google Scholar]
- 86.Muller JL, Roder CH, Schuierer G, Klein H. Motor-induced brain activation in cortical, subcortical and cerebellar regions in schizophrenic inpatients. A whole brain fMRI fingertapping study. Progress in Neuro-psychopharmacology & Biological Psychiatry. 2002;26(3):421–6. doi: 10.1016/s0278-5846(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 87.Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophrenia Research. 2003;60:285–98. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- 88.Diwadkar VA, Pruitt P, Zhang A, Radwan J, Keshavan MS, Murphy E, et al. The neural correlates of performance in adolescents at risk for schizophrenia: Inefficiently increased cortico-striatal responses measured with fMRI. J Psychiatr Res. 2012;46:12–21. doi: 10.1016/j.jpsychires.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martinelli C, Rigoli F, Shergill SS. Aberrant Force Processing in Schizophrenia. Schizophrenia Bulletin. 2016 doi: 10.1093/schbul/sbw092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Exner C, Weniger G, Schmidt-Samoa C, Irle E. Reduced size of the pre-supplementary motor cortex and impaired motor sequence learning in first-episode schizophrenia. Schizophrenia Research. 2006;84(2–3):386–96. doi: 10.1016/j.schres.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 91.Singh S, Goyal S, Modi S, Kumar P, Singh N, Bhatia T, et al. Motor function deficits in schizophrenia: an fMRI and VBM study. Neuroradiology. 2014;56(5):413–22. doi: 10.1007/s00234-014-1325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hirjak D, Wolf RC, Stieltjes B, Hauser T, Seidl U, Schröder J, et al. Cortical signature of neurological soft signs in recent onset schizophrenia. Brain Topography. 2014;27(2):296–306. doi: 10.1007/s10548-013-0292-z. [DOI] [PubMed] [Google Scholar]
- 93.Bracht T, Schnell S, Federspiel A, Razavi N, Horn H, Strik W, et al. Altered cortico-basal ganglia motor pathways reflect reduced volitional motor activity in schizophrenia. Schizophrenia Research. 2013;143(2-3):269–76. doi: 10.1016/j.schres.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 94.Venkatasubramanian G, Jayakumar PN, Gangadhar BN, Keshavan MS. Neuroanatomical correlates of neurological soft signs in antipsychotic-naive schizophrenia. Psychiatry Research. 2008;164(3):215–22. doi: 10.1016/j.pscychresns.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 95.Stegmayer K, Horn H, Federspiel A, Razavi N, Bracht T, Laimbock K, et al. Supplementary motor area (SMA) volume is associated with psychotic aberrant motor behaviour of patients with schizophrenia. Psychiatry Research. 2014;223(1):49–51. doi: 10.1016/j.pscychresns.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 96.Walther S, Schappi L, Federspiel A, Bohlhalter S, Wiest R, Strik W, et al. Resting-State hyperperfusion of the supplementary motor area in catatonia. Schizophrenia Bulletin. 2016 doi: 10.1093/schbul/sbw140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grafton ST. Contributions of functional imaging to understanding parkinsonian symptoms. Current Opinion in Neurobiology. 2004;14(6):715–9. doi: 10.1016/j.conb.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 98.Walther S, Federspiel A, Horn H, Razavi N, Wiest R, Dierks T, et al. Alterations of white matter integrity related to motor activity in schizophrenia. Neurobiology of Disease. 2011;42(3):276–83. doi: 10.1016/j.nbd.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 99.Fornito A, Yücel M, Dean B, Wood SJ, Pantelis C. Anatomical abnormalities of the anterior cingulate cortex in schizophrenia: Bridging the gap between neuroimaging and neuropathology. Schizophrenia Bulletin. 2009;35(5):973–93. doi: 10.1093/schbul/sbn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Calabrese DR, Wang L, Harms MP, Ratnanather JT, Barch DM, Cloninger CR, et al. Cingulate gyrus neuroanatomy in schizophrenia subjects and their non-psychotic siblings. Schizophrenia Research. 2008;104(1):61–70. doi: 10.1016/j.schres.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Szeszko PR, Bilder RM, Lencz T, Ashtari M, Goldman RS, Reiter G, et al. Reduced anterior cingulate gyrus volume correlates with executive dysfunction in men with first-episode schizophrenia. Schizophrenia Research. 2000;43(2):97–108. doi: 10.1016/s0920-9964(99)00155-3. [DOI] [PubMed] [Google Scholar]
- 102.Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122(Pt 4):593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 103.Honey GD, Pomarol-Clotet E, Corlett PR, Honey RAE, McKenna PJ, Bullmore ET, et al. Functional dysconnectivity in schizophrenia associated with attentional modulation of motor function. Brain. 2005;128(Pt 11):2597–611. doi: 10.1093/brain/awh632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Docx L, Emsell L, Van Hecke W, De Bondt T, Parizel PM, Sabbe B, et al. White matter microstructure and volitional motor activity in schizophrenia: A diffusion kurtosis imaging study. Psychiatry Research. 2017;260:29–36. doi: 10.1016/j.pscychresns.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 105.Brandt GN, Bonelli RM. Structural neuroimaging of the basal ganglia in schizophrenic patients: a review. Wiener Medizinische Wochenschrift. 2008;158(3):84–90. doi: 10.1007/s10354-007-0478-7. [DOI] [PubMed] [Google Scholar]
- 106.Mamah D, Wang L, Barch D, de Erausquin GA, Gado M, Csernansky JG. Structural analysis of the basal ganglia in schizophrenia. Schizophrenia Research. 2007;89(1):59–71. doi: 10.1016/j.schres.2006.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Amador XF, Kirkpatrick B, Buchanan RW, Carpenter WT, Marcinko L, Yale SA. Stability of the diagnosis of deficit syndrome in schizophrenia. The American Journal of psychiatry. 1999;156(4):637–9. doi: 10.1176/ajp.156.4.637. [DOI] [PubMed] [Google Scholar]
- 108.Peralta V, Moreno-Izco L, Sanchez-Torres A, Garcia de Jalon E, Campos MS, Cuesta MJ. Characterization of the deficit syndrome in drug-naive schizophrenia patients: the role of spontaneous movement disorders and neurological soft signs. Schizophrenia Bulletin. 2014;40(1):214–24. doi: 10.1093/schbul/sbs152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biological Psychiatry. 2008;64(2):81–8. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang H, He H, Zhong J. Multimodal MRI characterisation of schizophrenia: a discriminative analysis. Lancet. 2016;388(Suppl 1):S36. [Google Scholar]
- 111.Fitzgerald PB, Brown TL, Daskalakis ZJ, Kulkarni J. A transcranial magnetic stimulation study of inhibitory deficits in the motor cortex in patients with schizophrenia. Psychiatry Research: Neuroimaging. 2002;114(1):11–22. doi: 10.1016/s0925-4927(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 112.Kasparek T, Prikryl R, Rehulova J, Marecek R, Mikl M, Prikrylova H, et al. Brain functional connectivity of male patients in remission after the first episode of schizophrenia. Hum Brain Mapp. 2013;34(3):726–37. doi: 10.1002/hbm.21469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tran KD, Smutzer GS, Doty RL, Arnold SE. Reduced Purkinje cell size in the cerebellar vermis of elderly patients with schizophrenia. The American Journal of Psychiatry. 1998;155(9):1288–90. doi: 10.1176/ajp.155.9.1288. [DOI] [PubMed] [Google Scholar]
- 114.Popa LS, Hewitt AL, Ebner TJ. The cerebellum for jocks and nerds alike. Front Syst Neurosci. 2014;8:113. doi: 10.3389/fnsys.2014.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Madras BK. History of the discovery of the antipsychotic dopamine D2 receptor: a basis for the dopamine hypothesis of schizophrenia. Journal of the History of the Neurosciences. 2013;22(1):62–78. doi: 10.1080/0964704X.2012.678199. [DOI] [PubMed] [Google Scholar]
- 116.Baumeister AA, Francis JL. Historical development of the dopamine hypothesis of Schizophrenia. Journal of the History of the Neurosciences. 2002;11(3):265–77. doi: 10.1076/jhin.11.3.265.10391. [DOI] [PubMed] [Google Scholar]
- 117.Seeman P, Lee T. Antipsychotic drugs: Direct correlation between clinical potency and presynaptic action on dopamine neurons. Science. 1975;188(4194):1217–9. doi: 10.1126/science.1145194. [DOI] [PubMed] [Google Scholar]
- 118.Fallon JH, Opole IO, Potkin SG. The neuroanatomy of schizophrenia: circuitry and neurotransmitter systems. Clinical Neuroscience Research. 2003;3(1):77–107. [Google Scholar]
- 119.Meador-Woodruff JH, Haroutunian V, Powchik P, Davidson M, Davis KL, Watson SJ. Dopamine receptor transcript expression in striatum and prefrontal and occipital cortex: Focal abnormalities in orbitofrontal cortex in schizophrenia. Archives of General Psychiatry. 1997;54(12):1089–95. doi: 10.1001/archpsyc.1997.01830240045007. [DOI] [PubMed] [Google Scholar]
- 120.McGowan S, Lawrence AD, Sales T, Quested D, Grasby P. Presynaptic dopaminergic dysfunction in schizophrenia: A Positron Emission Tomographic [18F]Fluorodopa study. Archives of General Psychiatry. 2004;61(2):134–42. doi: 10.1001/archpsyc.61.2.134. [DOI] [PubMed] [Google Scholar]
- 121.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: Version III-The Final common pathway. Schizophrenia Bulletin. 2009;35(3):549–62. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Laruelle M. Imaging dopamine transmission in schizophrenia: A review and meta-analysis. The Quarterly Journal of Nuclear Medicine. 1998;42(3):211. [PubMed] [Google Scholar]
- 123.Cazorla M, de Carvalho FD, Chohan MO, Shegda M, Chuhma N, Rayport S, et al. Dopamine D2 receptors regulate the anatomical and functional balance of basal ganglia circuitry. Neuron. 2014;81(1):153–64. doi: 10.1016/j.neuron.2013.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Seeman P, Seeman MV. Schizophrenia and the supersensitive synapse. Neuropsychiatry. 2011;1(3):233–42. [Google Scholar]
- 125.Tune LE, Wong DF, Pearlson G, Strauss M, Young T, Shaya EK, et al. Dopamine D2 receptor density estimates in schizophrenia: a positron emission tomography study with 11C-N-methylspiperone. Psychiatry Research. 1993;49(3):219. doi: 10.1016/0165-1781(93)90063-m. [DOI] [PubMed] [Google Scholar]
- 126.Suhara T, Okubo Y, Yasuno F, Sudo Y, Inoue M, Ichimiya T, et al. Decreased dopamine D2 receptor binding in the anterior cingulate cortex in schizophrenia. Archives of General Psychiatry. 2002;59(1):25–30. doi: 10.1001/archpsyc.59.1.25. [DOI] [PubMed] [Google Scholar]
- 127.Yang YK, Chiu NT, Chen M, Chen CC, Yeh TL, Lee IH. Correlation between fine motor activity and striatal dopamine D 2 receptor density in patients with schizophrenia and healthy controls. Psychiatry Research: Neuroimaging. 2003;123(3):191–7. doi: 10.1016/s0925-4927(03)00066-0. [DOI] [PubMed] [Google Scholar]
- 128.Silverstein B, Bressler S, Diwadkar VA. Inferring the dysconnection syndrome in schizophrenia: Interpretational considerations on methods for the network analyses of fMRI data. Frontiers in Psychiatry. 2016;7:132. doi: 10.3389/fpsyt.2016.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Morris SE, Cuthbert BN. Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues in Clinical Neuroscience. 2012;14(1):29–37. doi: 10.31887/DCNS.2012.14.1/smorris. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Morris SE, Vaidyanathan U, Cuthbert BN. Changing the diagnostic concept of schizophrenia: The NIMH Research Domain Criteria Initiative. Nebr Symp Motiv. 2016;63:225–52. doi: 10.1007/978-3-319-30596-7_8. [DOI] [PubMed] [Google Scholar]
- 131.Sprooten E, Rasgon A, Goodman M, Carlin A, Leibu E, Lee WH, et al. Addressing reverse inference in psychiatric neuroimaging: Meta-analyses of task-related brain activation in common mental disorders. Human Brain Mapping. 2017;38(4):1846–64. doi: 10.1002/hbm.23486. [DOI] [PMC free article] [PubMed] [Google Scholar]


