Abstract
IMPORTANCE
The natriuretic peptides are biochemical markers of heart failure (HF) severity and predictors of adverse outcomes. Smaller studies have evaluated adjusting HF therapy based on natriuretic peptide levels (“guided therapy”) with inconsistent results.
OBJECTIVE
To determine whether an amino-terminal pro–B-type natriuretic peptide (NT-proBNP)-guided treatment strategy improves clinical outcomes compared to usual care in high-risk patients with HF and reduced ejection fraction (HFrEF).
DESIGN, SETTINGS, AND PARTICIPANTS
The GUIDing Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure (GUIDE-IT) study was a randomized multi-center clinical trial conducted between January 16, 2013 and September 20, 2016 at 45 clinical sites in the United States and Canada. This study planned to randomize 1,100 patients with HFrEF (ejection fraction ≤40%), elevated natriuretic peptide levels within the prior 30 days, and a history of a prior HF event (HF hospitalization or equivalent) to either an NT-proBNP-guided strategy or usual care.
INTERVENTION
Patients were randomized to either an NT-proBNP-guided strategy or usual care. Patients randomized to the guided strategy (n = 446) had HF therapy titrated with the goal of achieving a target NT-proBNP <1,000 pg/mL. Patients randomized to usual care (n = 448) had heart failure care in accordance with published guidelines, with emphasis on titration of proven neurohormonal therapies for heart failure. Serial measurement of NT-proBNP testing was discouraged in the usual care group.
MAIN OUTCOMES AND MEASURES
The primary endpoint was the composite of time-to-first HF hospitalization or cardiovascular mortality. Prespecified secondary endpoints included all-cause mortality, total hospitalizations for HF, days alive and not hospitalized for cardiovascular reasons, the individual components on the primary endpoint, and adverse events.
RESULTS
The Data and Safety Monitoring Board recommended stopping the study for futility when 894 (median age, 63; 286 (32%) women) of the planned 1,100 patients had been enrolled and followed for a median of 15 months. The primary endpoint occurred in 164 patients (37%) in the biomarker-guided group and 164 patients (37%) in the usual care group (adjusted hazard ratio=0.98; 95% confidence interval 0.79–1.22; p=0.88). Cardiovascular mortality was 12% in the biomarker guided group and 13% in the usual care group (hazard ratio=0.94 (95% confidence interval 0.65–1.37, p = 0.75). Neither other secondary endpoints nor achieved decreases in NT-proBNP levels were significantly different between the groups.
CONCLUSIONS AND RELEVANCE
In high-risk patients with HFrEF, a strategy of NT-proBNP-guided therapy was not more effective than a usual care strategy in improving outcomes.
TRIAL REGISTRATION
Clinicaltrials.gov identifier: NCT01685840
Keywords: amino-terminal pro–B-type natriuretic peptide, heart failure with reduced ejection fraction, patient outcomes
Evidence-based therapies such as targeting neurohormonal activation significantly improve outcomes in patients with heart failure (HF). Nevertheless, available data suggest that many patients in clinical practice are either not treated with these agents or are treated with lower than recommended doses.1,2 The natriuretic peptides, specifically B-type natriuretic peptide (BNP) and amino-terminal pro–B-type natriuretic peptide (NT-proBNP), are biomarkers that reflect HF severity and are significantly associated with adverse outcomes in HF.3,4 These markers decline in response to the use of guideline-recommended HF therapies, and rising levels portend a poor prognosis.5 These observational data have led to the hypothesis that serial measurements of natriuretic peptides may be used to guide titration of chronic medical therapy in HF.
Previous clinical trials of varying size and design have tested this hypothesis over the last two decades, with mixed results.6–11 These studies have generally been limited by their small size and also by significant heterogeneity between studies. Several meta-analyses have suggested substantial benefits with this approach, but no individual study has been of sufficient power to be definitive.12,13 In light of this uncertainly, current guidelines do not recommend the use of serial measurements of natriuretic peptides to guide titration of HF therapy.14,15 The Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure (GUIDE-IT) multicenter randomized clinical trial was designed to evaluate the efficacy of a NT-proBNP-guided HF treatment strategy compared to optimal medical therapy alone in high-risk patients with heart failure and reduced ejection fraction (HFrEF).
Methods
Study Design
The details of the rationale and design for this study have been published previously.16 The study protocol, including the statistical analysis plan, is provided in the Supplemental Material. The study was approved by the institutional review board at each study site, and all participants provided written informed consent. An independent Data and Safety Monitoring Board (DSMB) appointed by the National Heart Lung and Blood Institute monitored study conduct and patient safety. In order to maximize adherence to the study protocol, an adherence committee reviewed episodes in which HF therapy was not titrated despite NT-proBNP values being above the target and provided general feedback to the Executive Committee and the study sites (including study site score cards indicating cumulative site performance with regard to protocol adherence) on a regular basis. The adherence committee had a stepped approached for sites with consistently poor performance, including contact from the coordinating center and escalation to the Executive Committee to reinforce study goals and site training.
Study Participants
Patients were eligible for enrollment if they had chronic HFrEF with an ejection fraction of 40% or less, a history of a prior HF event (hospitalization for HF, emergency department visit for HF, or outpatient treatment with intravenous diuretics for HF) within the prior 12 months, and an NT-proBNP >2,000 pg/mL or BNP >400 pg/mL within the prior 30 days. Patients were excluded if they had an acute coronary syndrome or revascularization procedure within the prior 30 days, cardiac resynchronization therapy within the prior 3 months, end-stage renal disease, or anticipated heart transplantation or mechanical cardiac support within the next 12 months. In accordance with NIH policy, patient reported race and ethnicity information was collected using fixed categories.
Randomization and Treatment Assignments
Enrolled patients were randomized in a 1:1 fashion using computer generated random numbers using a simple randomization design with no restrictions to either the NT-proBNP-guided therapy strategy or usual care. Given the nature of the study intervention, treatment assignment was not blinded. For patients randomized to the NT-proBNP-guided strategy, clinicians were instructed to titrate HF therapy to target an NT-proBNP level <1,000 pg/mL. Specific adjustments of therapy for individual patients were at the discretion of the treating physician, but sites were encouraged to prioritize titration of neurohormonal antagonists over diuretics unless there was clinical evidence of congestion or volume overload. Patients randomized to the NT-proBNP-guided group used local laboratory NT-proBNP measurements to make decisions about titration of HF therapy. All patients in either group also had blinded NT-proBNP concentrations measured in a core laboratory at each study visit. For patients in either group, investigators were provided with the most recent American Heart Association (AHA)/American College of Cardiology (ACC) practice guidelines for the management of HF and specific information on target doses of proven medical therapies. After an initial visit at 2 and 6 weeks, visits occurred every 3 months throughout the remainder of the study. After therapy adjustment for HF (whether driven by NT-proBNP levels or clinical reasons), patients had a 2-week follow-up visit for reassessment.
Study Outcomes
The primary outcome was a composite of time-to-first HF hospitalization or death from cardiovascular causes. Prespecified secondary endpoints included all-cause mortality, total hospitalizations for HF, days alive and not hospitalized for cardiovascular reasons, the individual components on the primary endpoint, health related quality of life, resource utilization, costs, cost effectiveness, and safety. Results of the economic and quality of life analyses are not reported in this article. Adjudication of all deaths and hospitalizations was carried out by a blinded clinical endpoint committee according to pre-specified criteria. We predefined four adverse events of interest that might be anticipated to occur more frequently with more aggressive HF treatment: symptomatic hypotension, symptomatic bradycardia, hyperkalemia, and worsening renal function.
Statistical Analysis
A total sample size of 1,100 patients (550 per group) was expected to provide approximately 90% power to detect a difference in the primary endpoint with an assumed type I error rate of 0.05 two-sided. We estimated that the annual event rate for the composite endpoint would be 40% in the usual care group. We targeted a 20% decrease in the primary endpoint at 12 months for the biomarker-guided group in the sample size calculation, based on the recognition that this treatment effect would be consistent with other effective heart failure therapies that have been incorporated into clinical practice.17 According to protocol, all patients were to be followed for between 12 and 24 months after randomization (the last patient enrolled to be followed for 12 months). For the analysis of the primary endpoint, the adjusted hazard ratio would be adjusted for 5 prespecified baseline covariates—age, sex, ejection fraction, NT-proBNP level, and the presence of diabetes mellitus—within the Cox regression model. For missing baseline categorical variables, we imputed the most common value. For missing baseline NT-proBNP values, we utilized the NT-proBNP value from screening. For missing baseline ejection fraction values, we imputed the population median. We also performed the primary endpoint analysis with site as a random effect as a sensitivity analysis. We tested for heterogeneity of effect on the primary endpoint by testing for interactions within a number of subgroups defined by demographics and baseline characteristics (see Online Supplement). A subgroup analysis based on age (≥ 75 years vs. < 75 years) was prespecified based on prior data suggesting biomarker guided therapy was more effective in younger patients.9 For secondary analyses, inverse probability weighting was used to estimate mean days alive out of the hospital using the Bang-Tsiatis partitioned estimator.18 The total number of recurrent HF hospitalizations by treatment group was modelled using the Andersen-Gill intensity model.19 All analyses were based on the principle of intention to treat. All analyses were performed using SAS 9.4. The threshold for statistical significance was 2 sided with a Type 1 error rate of 0.05. There was no adjustment performed for multiple comparisons and thus secondary outcomes were considered exploratory.
Results
Study Patients
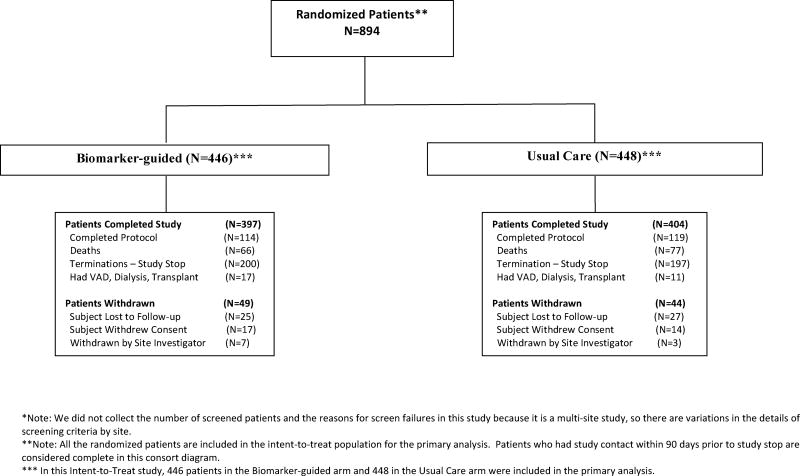
A total of 894 patients were enrolled at 45 sites in the United States and Canada between January 2013 and July 2016 (Figure 1). The groups were generally well balanced with respect to baseline characteristics (Table 1). The study enrolled patients with high-risk HF, as characterized by a low ejection fraction (median 25%), significantly elevated NT-proBNP (median 2,653 pg/mL), and a history of prior HF hospitalization (or equivalent) in the past year. Most patients were receiving recommended pharmacologic therapy for chronic heart failure at baseline. The median-follow up time for all patients was 15 months. Missing data for the 5 prespecified adjustment covariates was rare (none for age or sex, 1 for diabetes mellitus, 14 for baseline NT-proBNP, and 12 for ejection fraction).
Figure 1. Flowchart of Patient Accountability.
This figure displays a flowchart of patient accountability, from the initial randomized patients through the number of patients who completed the study or withdrew from both the biomarker-guided and usual care groups. Data on the number of patients screened for eligibility are not available.
Abbreviations: VAD, ventricular assist device
Table 1.
Baseline Characteristics
| Characteristic | NT-proBNP guided (n=446) |
Usual care (n=448) |
|---|---|---|
| Age (years) median [25th–75th] | 62 [51,70] | 64 [54,72] |
| Sex, No (% female) | 139 (31%) | 147 (33%) |
| Race, No (%) | ||
| White | 230 (54%) | 260 (59%) |
| Black | 168 (39%) | 156 (35%) |
| Other | 35 (7%) | 26 (6%) |
| Ethnicity, No. (% Hispanic) | 30 (7%) | 28 (6%) |
| Duration of HF (months) median [25th–75th] | 12 [1, 65] | 16 [1, 61] |
| Ejection fraction (%) median [25th–75th] | 24 [19,30] | 25 [20,30] |
| NYHA class at enrollment, No (%) | 36 (8%) | 23 (5%) |
| I | 218 (50%) | 229 (52%) |
| II | 176 (40%) | 182 (41%) |
| III | 8 (2%) | 9 (2%) |
| IV | ||
| History of: No,(%) | ||
| Ischemic heart disease | 203 (46%) | 244 (55%) |
| Diabetes mellitus | 198 (44%) | 212 (47%) |
| Atrial fibrillation | 162 (36%) | 196 (44%) |
| Chronic kidney disease | 161 (36%) | 169 (38%) |
| Systolic BP(mmHg) median [25th–75th] | 114 [102,128] | 114 [101,128] |
| Heart rate (beats/min) median [25th–75th] | 77 [68,87] | 76 [67,86] |
| NT-proBNP (pg/mL) median [25th–75th] | 2632 [1462–5235] | 2668 [1481–5604] |
| Creatinine (mg/dL) median [25th–75th] | 1.3 [1.1,1.7] | 1.3 [1.1,1.7] |
| Beta-blocker, No. (%) | 415 (93%) | 416 (93%) |
| Angiotensin Converting Enzyme inhibitor, Angiotensin receptor blocker, or Angiotensin receptor blocker neprilysin inhibitor, No. (%) | 345 (77%) | 339 (76%) |
| Mineralocorticoid antagonist (%) No. (%) | 223 (50%) | 217 (48%) |
| Implantable cardioverter defibrillator (%) No (%) | 182 (41%) | 178 (40%) |
| Cardiac resynchronization therapy No (%) | 87 (20%) | 76 (17%) |
At the regularly scheduled DSMB meeting on July 8, 2016, at which time about 50% of planned primary endpoint events had occurred, the study met prespecified inefficacy criteria and the DSMB made a recommendation to the NHLBI to discontinue the study due to lack of efficacy evidence for the biomarker-guided treatment group compared to usual care. The NHLBI accepted this recommendation and enrollment was discontinued after 894 patients had been enrolled (81% of planned enrollment). Final study visits for all patients still actively participating in the trial were completed prior to database lock.
Medical Treatment by Strategy and Follow-up
Patients randomized to the biomarker-guided strategy had a greater number of study clinic visits (median 12 vs. 10, Wilcoxon p=0.002) and more adjustments to HF therapy (median 6 vs. 4, Wilcoxon p<0.001) compared to patients randomized to usual care. Over the course of the study, there was modest intensification of HF therapy in both groups, without statistically significant differences between those randomized to NT-proBNP-guided therapy or usual care (Table 2).
Table 2.
Differences in Medical Therapy Over Time Between Treatment Groups
| NT-proBNP- guided (n=446) |
Usual Care (n=448) |
p- value* |
|||
|---|---|---|---|---|---|
| Baseline | 12 mo. | Baseline | 12 mo. | ||
| Beta-Blocker | |||||
| % taking beta-blocker | 93% | 91% | 93% | 91% | 0.86 |
| Mean dose achieved (% of target dose) | 33% | 48% | 35% | 45% | 0.60 |
| Proportion at 50% of target dose | 37% | 60% | 33% | 57% | 0.97 |
| Proportion at 100% of target dose | 7% | 15% | 6% | 11% | 0.31 |
| ACE/ARB | |||||
| % taking ACE/ARB | 77% | 75% | 74% | 71% | 0.63 |
| Mean dose achieved (% of target dose) | 41% | 55% | 43% | 53% | 0.35 |
| Proportion at 50% of target dose | 41% | 51% | 41% | 49% | 0.74 |
| Proportion at 100% of target dose | 17% | 31% | 20% | 27% | 0.11 |
| MRA | |||||
| % taking MRA | 50% | 54% | 48% | 52% | 1.00 |
| Mean dose achieved (% of target dose) | 98% | 115% | 94% | 103% | 0.29 |
| Proportion at 50% of target dose | 98% | 99% | 100% | 99% | 0.42 |
| Proportion at 100% of target dose | 76% | 85% | 75% | 75% | 0.06 |
| Loop Diuretics | |||||
| Mean dose (mg furosemide equivalents)† | 77 | 86 | 76 | 77 | 0.26 |
p-value is for comparison of change over time in NT-proBNP guided group compared to change over time in usual care group
Abbreviations: ACE/ARB, angiotensin-converting enzyme/angiotensin receptor blocker; BB, beta-blocker; MRA, mineralocorticoid receptor antagonist; NT-proBNP-guided, amino-terminal pro–B-type natriuretic peptide
Study Outcomes
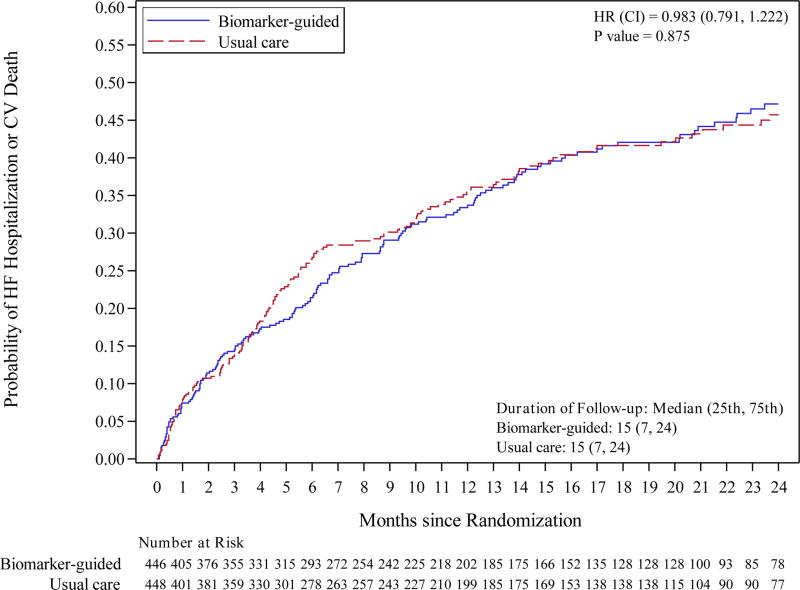
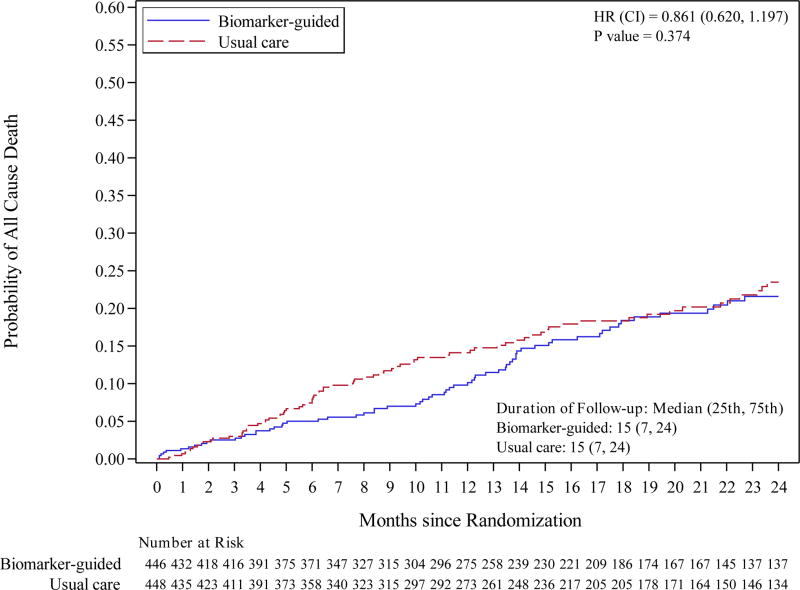
The composite endpoint of first hospitalization for HF or death from a cardiovascular cause occurred in 164 patients (37%) in the biomarker-guided group and 164 patients (37%) in the usual care group with 12-month Kaplan-Meier event rates of 33.8% and 36.0%, respectively and a treatment difference of −2.2% (95% confidence interval [CI] −9.1% to 4.6%). After adjustment for prespecified covariates, the adjusted hazard ratio for the primary endpoint was 0.98 (95% CI 0.79 – 1.22, p=0.88) (Figure 2). These results were not significantly changed by including site as a random effect (HR=0.99, 95% CI 0.79 – 1.23, p=0.92). Data for all-cause mortality, cardiovascular mortality, HF hospitalization, and all-cause hospitalization were also not significantly different between treatment groups (Table 3). Death occurred in 66 patients (15%) in the biomarker-guided group and 77 (17%) in the usual care group. The 12-month Kaplan-Meier event rates for all-cause mortality were 9.8% for biomarker-guided and 14.1% in the usual-care group for a treatment difference of −4.3% (95% CI −8.9% to 0.3%). After adjustment for the pre-specified covariates, the adjusted hazard ratio for all-cause mortality was 0.86 (95% CI 0.62 – 1.20; p=0.37).
Figure 2. Primary Endpoint (Heart failure hospitalization or CV mortality) and All-Cause Mortality.
Kaplan-Meier curves for: A) primary endpoint (heart failure hospitalization or CV mortality); and B) all-cause mortality.
Abbreviations: CV, cardiovascular; HF, heart failure
Table 3.
Secondary Outcomes
| NT-proBNP- guided |
Usual Care | Effect (95% CI) | p-value | |
|---|---|---|---|---|
| Mortality, No. (%) | 66 (15%) | 77 (17%) | 0.86 (0.62–1.20) | 0.37 |
| CV mortality | 53 (12%) | 57 (13%) | 0.94 (0.65–1.37) | 0.75 |
| Non-CV mortality | 13 (3%) | 20 (5%) | 0.66 (0.33–1.32) | 0.24 |
| First HF hospitalization, No (%) | 147 (33%) | 141 (32%) | 1.04 (0.82–1.31) | 0.76 |
| Total HF hospitalizations (No.) | 350 | 277 | 1.29 (0.97–1.72) | 0.083* |
| Days alive and not hospitalized for CV reasons, mean (SD) | 581 days (14.4) | 562 days (15.1) | 19.26 (−21.58–60.10) | 0.36# |
Abbreviations: CI, confidence interval; CV, cardiovascular;
All other abbreviations can be found in Table 1.
Based on Andersen-Gill Intensity model.
Based on Bang-Tsiatis portioned estimator
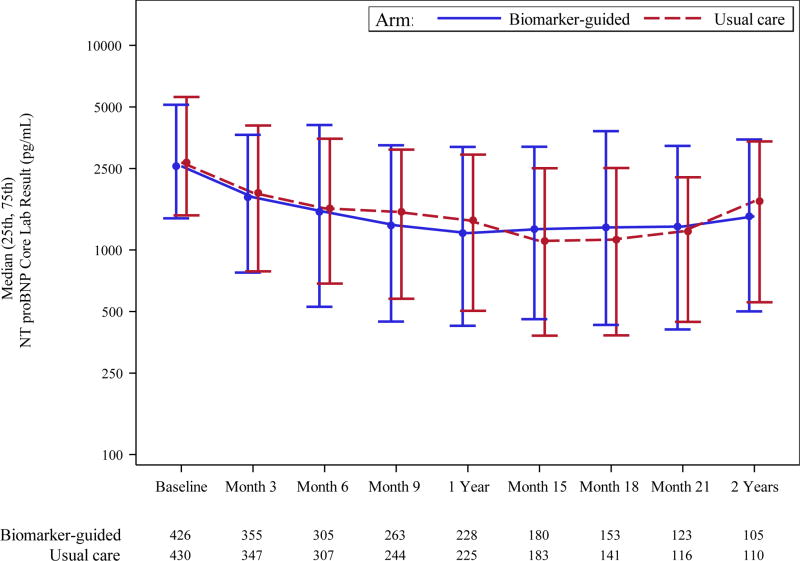
There was generally no evidence of heterogeneity of treatment effect in a number of prespecified and post-hoc subgroups (eFigure 1). Changes in the concentrations of NT-proBNP (based on blinded central core lab data) decreased over time in both groups and were not significantly different between groups; at 12 months, median NT-proBNP had decreased from a median of 2,568 to 1,209 pg/mL (53% decrease) in the biomarker-guided group, and from a median of 2,678 to 1,397 pg/mL (48% decrease) in the usual care group (Figure 3). The proportion of patients in both groups achieving the target value of NT-proBNP <1,000 pg/mL at 12 months was 46% for biomarker-guided group vs. 40% for usual care group (p=0.21).
Figure 3. Change in NT-proBNP Levels.
NT-proBNP levels between the groups over time.
Abbreviations: NT-proBNP, amino-terminal pro–B-type natriuretic peptide. B-guided, Biomarker guided therapy
Adverse Events
The rates of the predefined adverse events of interest (i.e., symptomatic hypotension, symptomatic bradycardia, hyperkalemia, and worsening renal function) were generally low and similar between the groups (eTable 1).
Discussion
The primary finding of this study is that in high-risk patients with HFrEF, a strategy of guiding therapy based on concentrations of NT-proBNP was not more effective than a usual care strategy in reducing the composite endpoint of time-to-first HF hospitalization or cardiovascular death. Similarly not significantly different results were seen in other clinical endpoints. Although there were more adjustments to therapy in the biomarker-guided group, neither doses of guideline-directed medical therapy, the achieved NT-proBNP concentrations, nor clinical outcomes were significantly different between the treatment groups.
These results differ from other data, including a recent comprehensive patient-level meta-analysis of data from 2,431 patients from 11 trials which showed a reduction in all-cause mortality with natriuretic peptide-guided therapy compared to usual care (hazard ratio = 0.62).13 A consistent feature of other studies in which natriuretic peptide-guided therapy was shown to be effective was the differential utilization of neurohormonal therapies as well as a separation of achieved natriuretic peptide concentrations between the two study groups. The up-titration of medical therapy in the NT-proBNP group in this study was substantially less than that seen in some smaller studies of biomarker-guided therapy. For example, a randomized study of 278 patients in eight Austrian hospitals achieved 100% of angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) target doses and 77% of beta-blocker target doses in patients randomized to biomarker-guided therapy, which was accompanied by a substantial reduction in HF events.10 Although it is challenging to compare across studies, the achieved dosing of these classes of drugs in the NT-proBNP-guided group was substantially less in this study (55% for ACE/ARB and 48% for beta-blockers at 12 months, Table 2). Whether the lack of up-titration of medical therapy observed in this study was related to patient characteristics (e.g., inability to up-titrate due to azotemia or hypotension) or physician behavior (e.g., unwillingness to up-titrate due to concern over adverse effects) in not clear from these data. This study enrolled patients with high-risk features (elevated natriuretic peptide levels within the prior 30 days and an HF event within the prior 12 months) and allowed a broad range of renal function, resulting in a study population with relatively advanced HF compared to most other clinical trials in ambulatory patients with HFrEF. By way of comparison, the median baseline NT-proBNP value in this study (2,607 pg/mL) was 1.6 fold that of patients enrolled in the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) study of sacubitril/valsartan17 and 3.2 fold that of patients enrolled in the Heart Failure and a Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) study.20 Patients with more severe HF such as those in this study may have more limitations to intensification of HF therapy, in particular hypotension and azotemia, which may have limited this ability to aggressively up-titrate medical therapy in the guided therapy group in response to above target NT-proBNP levels.
Another potential difference between this study and other data may relate to difference in the control group. In the single center ProBNP Outpatient Tailored Chronic HF Therapy (PROTECT) study, patients randomized to biomarker-guided therapy achieved a 44% decrease in the NT-proBNP level over time (compared to a 5% decrease in the usual care group), which was associated with a significant improvement in clinical outcomes for those patients randomized to the NT-proBNP-guided strategy.8 By contrast, in the current trial, both the decrease in NT-proBNP concentrations (Figure 3) and the proportion of patients in each group who reached the target NT-proBNP value of <1,000 pg/mL (46% vs. 40%) were not significantly different between the groups. This suggests that a key difference between this study and PROTECT may be in the usual care group rather than the NT-proBNP-guided treatment group. Patients enrolled in the usual care group of the this study had relatively frequent study-related clinic visits (median 10 visits over 15 months of follow-up) and adjustments to HF therapy (median of 4 adjustments), which represents a greater intensity of care (more akin to a disease management program) than would typically occur in routine clinical practice. Whether this frequency of clinical contact affected outcomes through mechanisms other than medication titration (e.g., by earlier detection and intervention on heart failure decompensation) is unknown. Although this study included both academic and community sites, the majority of this study sites had substantial focus and expertise in HF care which may have tended to lessen differences in the optimization of evidenced-based HF therapies between the study groups.
Limitations
This study has several important limitations. First, given the nature of the study intervention, the study was unblinded, which could be a potential source of bias. The design was based on an objective primary endpoint (cardiovascular death and heart failure hospitalization) that was adjudicated by a clinical events committee blinded to the treatment assignment in order to mitigate this bias. Second, although the study protocol discouraged measurement of NT-proBNP in patients in the usual care group, some patients may have had NT-proBNP levels assessed at non-study sites or by non-study clinicians, which may have served to diminish the difference between study groups. Finally, patients in both groups had more frequent clinical encounters than would typically occur in clinical practice, which may have influenced the results.
Conclusions
In high-risk patients with HFrEF, a strategy of NT-proBNP-guided therapy was not more effective than a usual care strategy in improving outcomes.
Supplementary Material
Key Points.
Question
Does a strategy of titrating therapy to a specific NT-proBNP target improve clinical outcomes in high-risk patients with heart failure and reduced ejection fraction?
Findings
In this randomized clinical trial including 894 adults, a strategy of NT-proBNP-guided therapy compared with usual care did not significantly improve time to first hospitalization or cardiovascular mortality (hazard ratio, 0.98).
Meaning
These findings do not support NT-proBNP-guided therapy for management of heart failure with reduced ejection fraction.
Acknowledgments
We would like to acknowledge the contributions of the GUIDE-IT investigators, study coordinators, and the participating patients to the GUIDE-IT study. A listing of the GUIDE-IT investigators and study coordinators is shown in the Supplemental Material. The sponsor (the NHLBI) had no role in the design of the study but NHLBI staff did participate in study conduct, collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication as members of the Steering Committee (Drs. Cooper, Leifer, and Desvigne-Nickens). Drs. Felker and Anstrom had full access to the all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Sources of Funding
The GUIDE-IT study was funded by National Institutes of Health grants HL105448, HL105451, and HL105457. Roche Diagnostics provided support for NT-proBNP testing. The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; National Institutes of Health; or the United States Department of Health and Human Services.
Footnotes
Author Contributions
All authors have been involved in the study design, analysis, and manuscript revision. All authors read and approved the final manuscript.
GM Felker: Dr. Felker contributed to the conception and design of the study, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
KJ Anstrom: Dr Anstrom contributed to the conception and design of the study, the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
KF Adams: Dr. Adams contributed to the conception and design of the study, the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
JA Ezekowitz: Dr. Ezekowitz contributed to the conception and design of the study, the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
M Fiuzat: Dr. Fiuzat contributed to the conception and design of the study, the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
N Houston-Miller: Ms. Houston-Miller contributed to the conception and design of the study, the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
JL Januzzi: Dr. Januzzi contributed to the conception and design of the study, the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
DB Mark: Dr. Mark contributed to the conception and design of the study, the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
IL Piña: Dr. Piña contributed to the conception and design of the study, the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
G Passmore: Ms. Passmore contributed to the conception and design of the study, the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
DJ Whellan: Dr. Whellan contributed to the conception and design of the study, the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
H Yang: Dr. Yang contributed to the conception and design of the study, the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
LS Cooper: Dr. Cooper contributed to the conception and design of the study, the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
ES Leifer: Dr. Leifer contributed to the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
P Desvigne-Nickens: Dr. Desvigne-Nickens contributed to the conception and design of the study, the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
CM O’Connor: Dr. O’Connor contributed to the conception and design of the study, the supervision, data acquisition and interpretation, the manuscript drafting, and the critical revision of the manuscript.
Conflict of Interest Disclosures
GM Felker: Dr. Felker reports research support from NIH, AHA, Roche Diagnostics, Amgen, Novartis, Merck; consulting for Novartis, Amgen, Medtronic, GSK, BMS, Myokardia, Stealth
KJ Anstrom: Dr. Anstrom reports no relevant disclosures.
KF Adams: Dr. Adams reports research funding for Roche Diagnostics; consulting for Roche Diagnostics.
JA Ezekowitz: Dr. Ezekowitz reports grants or honoraria from Novartis, Servier, Bayer, Merck, Trevena, Amgen, Canadian Institutes of Health Research, National Institutes of Health, and the Heart and Stroke Foundation of Canada.
M Fiuzat: Dr. Fiuzat reports research funding from Roche Diagnostics.
N Houston-Miller: Ms. Houston-Miller reports no relevant disclosures.
JL Januzzi: Dr. Januzzi reports received grant support from Siemens, Singulex and Prevencio, consulting income from Roche Diagnostics, Critical Diagnostics, Sphingotec, Phillips, and Novartis, and participates in clinical endpoint committees/data safety monitoring boards for Abbvie, Pfizer, Novartis, Amgen, Janssen, and Boehringer Ingelheim. Dr. Januzzi is supported in part by the Hutter Family Professorship in Cardiology.
DB Mark: Dr. Mark reports consulting for Medtronic, CardioDx, and St. Jude Medical; research grants from Eli Lilly, Medtronic, Bristol Myers Squibb, AstraZeneca, Merck & Company, Oxygen Therapeutics, and Gilead.
IL Piña: Dr. Piña reports no relevant disclosures.
G Passmore: Ms. Passmore reports no relevant disclosures.
DJ Whellan: Dr. Whellan reports no relevant disclosures.
H Yang: Dr. Yang reports no relevant disclosures.
LS Cooper: Dr. Cooper reports no relevant disclosures.
ES Liefer: Dr. Liefer reports no relevant disclosures.
P Desvigne-Nickens: Dr. Desvigne-Nickens reports no relevant disclosures.
CM O’Connor: Dr. O’Connor reports grant funding and consulting for Roche Diagnostics.
References
- 1.Lee DS, Tu JV, Juurlink DN, et al. Risk-treatment mismatch in the pharmacotherapy of heart failure. JAMA. 2005;294(10):1240–1247. doi: 10.1001/jama.294.10.1240. [DOI] [PubMed] [Google Scholar]
- 2.Fonarow GC, Albert NM, Curtis AB, et al. Improving evidence-based care for heart failure in outpatient cardiology practices: primary results of the Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF) Circulation. 2010;122(6):585–596. doi: 10.1161/CIRCULATIONAHA.109.934471. [DOI] [PubMed] [Google Scholar]
- 3.Januzzi JL, Jr, Sakhuja R, O'Donoghue M, et al. Utility of amino-terminal pro-brain natriuretic peptide testing for prediction of 1-year mortality in patients with dyspnea treated in the emergency department. Arch Intern Med. 2006;166(3):315–320. doi: 10.1001/archinte.166.3.315. [DOI] [PubMed] [Google Scholar]
- 4.Anand IS, Fisher LD, Chiang YT, et al. Changes in Brain Natriuretic Peptide and Norepinephrine Over Time and Mortality and Morbidity in the Valsartan Heart Failure Trial (Val-HeFT) Circulation. 2003;107(9):1278–1283. doi: 10.1161/01.cir.0000054164.99881.00. [DOI] [PubMed] [Google Scholar]
- 5.Masson S, Latini R, Anand IS, et al. Prognostic Value of Changes in N-Terminal Pro-Brain Natriuretic Peptide in Val-HeFT (Valsartan Heart Failure Trial) J Am Coll Cardiol. 2008;52(12):997–1003. doi: 10.1016/j.jacc.2008.04.069. [DOI] [PubMed] [Google Scholar]
- 6.Eurlings LWM, van Pol PEJ, Kok WE, et al. Management of Chronic Heart Failure Guided by Individual N-Terminal Pro-B-Type Natriuretic Peptide Targets: Results of the PRIMA (Can PRo-brain-natriuretic peptide guided therapy of chronic heart failure IMprove heart fAilure morbidity and mortality?) Study. Journal of the American College of Cardiology. 2010;56(25):2090–2100. doi: 10.1016/j.jacc.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 7.Troughton RW, Frampton CW, Yandle TG, Espiner EA, Nicholls MG, Richards AM. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet. 2000;355:1126–1130. doi: 10.1016/s0140-6736(00)02060-2. [DOI] [PubMed] [Google Scholar]
- 8.Januzzi JL, Jr, Rehman SU, Mohammed AA, et al. Use of amino-terminal pro-B-type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J Am Coll Cardiol. 2011;58(18):1881–1889. doi: 10.1016/j.jacc.2011.03.072. [DOI] [PubMed] [Google Scholar]
- 9.Pfisterer M, Buser P, Rickli H, et al. BNP-guided vs symptom-guided heart failure therapy: the Trial of Intensified vs Standard Medical Therapy in Elderly Patients With Congestive Heart Failure (TIME-CHF) randomized trial. Jama. 2009;301(4):383–392. doi: 10.1001/jama.2009.2. [DOI] [PubMed] [Google Scholar]
- 10.Berger R, Moertl D, Peter S, et al. N-Terminal Pro-B-Type Natriuretic Peptide-Guided, Intensive Patient Management in Addition to Multidisciplinary Care in Chronic Heart Failure: A 3-Arm, Prospective, Randomized Pilot Study. J Am Coll Cardiol. 2010;55(7):645–653. doi: 10.1016/j.jacc.2009.08.078. [DOI] [PubMed] [Google Scholar]
- 11.Lainchbury JG, Troughton RW, Strangman KM, et al. N-Terminal Pro-B-Type Natriuretic Peptide-Guided Treatment for Chronic Heart Failure: Results From the BATTLESCARRED (NT-proBNP-Assisted Treatment To Lessen Serial Cardiac Readmissions and Death) Trial. J Am Coll Cardiol. 2009;55(1):53–60. doi: 10.1016/j.jacc.2009.02.095. [DOI] [PubMed] [Google Scholar]
- 12.Felker GM, Hasselblad V, Hernandez AF, O'Connor CM. Biomarker-guided therapy in chronic heart failure: A meta-analysis of randomized controlled trials. American Heart Journal. 2009;158(3):422–430. doi: 10.1016/j.ahj.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 13.Troughton RW, Frampton CM, Brunner-La Rocca HP, et al. Effect of B-type natriuretic peptide-guided treatment of chronic heart failure on total mortality and hospitalization: an individual patient meta-analysis. Eur Heart J. 2014;35(23):1559–1567. doi: 10.1093/eurheartj/ehu090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128(16):e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 15.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016 doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 16.Felker GM, Ahmad T, Anstrom KJ, et al. Rationale and design of the GUIDE-IT study: Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure. JACC Heart failure. 2014;2(5):457–465. doi: 10.1016/j.jchf.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 18.Bang H, Tsiatis A. Estimating medical costs with censored data. Biometrika. 2000;87:329–343. [Google Scholar]
- 19.Andersen PK, Gill RD. Cox's Regression Model for Counting Processes: A Large Sample Study. Annals of Statistics. 1982;10(4):1100–1120. [Google Scholar]
- 20.Felker GM, Whellan D, Kraus WE, et al. N-terminal pro-brain natriuretic peptide and exercise capacity in chronic heart failure: data from the Heart Failure and a Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) study. American Heart Journal. 2009;158(4 Suppl):S37–44. doi: 10.1016/j.ahj.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.