Abstract
The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA)- Outcome Measures in Rheumatology (OMERACT) PsA Core Set working group recently published the updated 2016 psoriatic arthritis (PsA) core domain set, a set of disease features that should be measured in all clinical trials. At the GRAPPA annual meeting in July 2016, the PsA working group presented the updated PsA core domain set endorsed by 90% of participants at OMERACT in May 2016 and held a working group meeting where the working group drafted a roadmap for the development of the PsA core outcome measurement set. In this manuscript, we review the development process of the PsA core domain set and the ongoing and proposed workstreams for development of a PsA core measurement set.
Key Indexing Terms: psoriatic arthritis, psoriasis, outcome measures, GRAPPA, OMERACT
Outcome measure development has rapidly progressed over the past 10 years and so too has our knowledge of psoriatic arthritis (PsA) pathophysiology and the multiple facets of life impacted by this disease.(1, 2) The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA)-Outcome Measures in Rheumatology (OMERACT) PsA working group developed a Core Domain Set to be measured in randomized controlled trials (RCTs) and longitudinal observational studies (LOS), and is working to develop a Core Outcome Measurement Set. At the GRAPPA annual meeting in July 2016, the working group presented results and next steps.
PsA Updated Core Domain Set
The PsA working group includes an international group of rheumatologists, dermatologists, patient research partners (PRPs), methodologists, and two fellows. The working group developed a plan to update the 2006 PsA Core Domain Set (3) following the OMERACT meeting in May 2014.(4) At that meeting, OMERACT participants voted that the PsA core domain set should be revised given updated knowledge about PsA (including the importance of individual disease manifestations), the need to incorporate patient input, and new OMERACT “Filter 2.0” methodology (a set of guidelines proposed by OMERACT for development of core domain sets).(5) The group developed a plan to address these issues through individual workstreams that culminated in a series of consensus activities. PRPs were included in all phases including one PRP on the steering committee, and at least one PRP was involved in the planning and interpretation of each of the workstreams.(6, 7)
Using the OMERACT Filter 2.0 framework, the working group performed an update (2010–2015) of the systematic literature review of domains measured in RCTs and LOS.(8) Next, patient domains were identified through international focus groups (130 patients in 7 countries). A total of 39 domains were identified from focus groups and literature review. In a phone conference, the working group discussed how to organize and describe these 39 domains in patients’ words. Patient and provider Delphi surveys were then sent to 75 patients and 125 physicians (rheumatologists and dermatologists) to rate the importance of the items for measurement in RCTs and LOS. These data formed the basis for a group discussion at a Nominal Group Technique meeting held in Jersey City, NJ, in March 2016. Twelve PRPs and twelve physicians came to consensus on a preliminary core domain set through a moderated discussion. The preliminary core domain set was then proposed at the OMERACT meeting in Whistler, British Columbia, in May 2016. The final core domain set (Figure 1) includes three parts: an inner circle (should be measured in all RCTs and LOS), a middle circle (important but not mandatory), and the outer circle that represents the research agenda. The inner circle includes musculoskeletal (MSK) disease activity (peripheral arthritis, enthesitis, dactylitis, and spine symptoms), skin disease activity (skin psoriasis and nail dystrophy), pain, patient global, physical function, health related quality of life, fatigue, and systemic inflammation. The middle circle includes economic cost, emotional well-being, participation, and structural damage. Finally, stiffness, independence, treatment burden, and sleep are in the research agenda. Some domains included in the 2016 core domain set were also present in the 2006 and 2014 Core Domain Sets, some moved from the outer circle, and others were added (Figure 1 shows these changes).
Figure 1. 2006 and 2016 GRAPPA-OMERACT PsA Core Domain Sets.
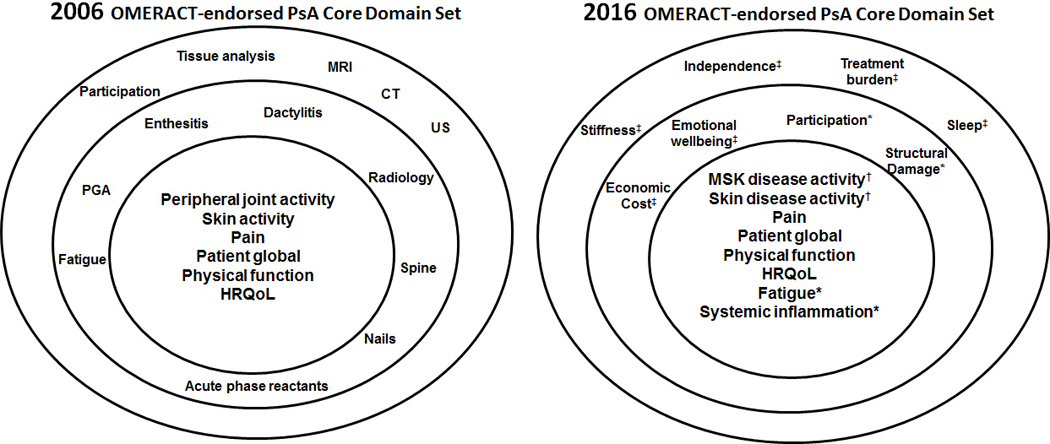
In the 2016 core domain set (right), domains that moved from one of the outer circles to either the middle or inner circle are noted with an asterisk. MSK disease activity (now includes peripheral arthritis, enthesitis, dactylitis, and spine symptoms) and skin disease activity (psoriasis and nail dystrophy) were redefined (marked with “†”). Finally, items marked with “‡” are new additions to the set. This figure was modified from Figure 3 in Orbai et al. Ann Rheum Dis 2016 with permission.
Having achieved ratification of the core domain set, the next step for the PsA working group is to select outcome measurement instruments. One of the issues raised at OMERACT and again at the GRAPPA meeting was the number of domains included within the core domain set. The feasibility of capturing the full set even within the inner circle is a daunting task. It is important to recognize that each domain will not necessarily need to be measured by an individual instrument. Most recent RCTs are already measuring these domains.(8) In fact, among the eight most recently conducted RCTs, most of these domains were captured although fatigue, participation, and spine symptoms are less commonly measured (Table 1).
Table 1.
Core Domains Measured and Reported in Recent Randomized Controlled Trials
| Domain | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Peripheral arthritis | √ | √ | √ | √ | √ | √ | √ | √ |
| Skin Disease | √ | √ | √ | √ | √ | √ | √ | √ |
| Enthesitis | √ | √ | √ | √ | √ | √ | √ | √ |
| Dactylitis | √ | √ | √ | √ | √ | √ | √ | √ |
| Health-related Quality of Life | √ | √ | √ | √ | √ | √ | √ | √ |
| Physical function | √ | √ | √ | √ | √ | √ | √ | √ |
| Systemic Inflammation | √ | √ | √ | √ | √ | √ | √ | |
| Structural Damage | √ | √ | √ | √ | ||||
| Nail Disease | √ | √ | √ | √ | ||||
| Spine symptoms | √ | √ | √ | |||||
| Fatigue | √ | √ | √ | |||||
| Participation | √ |
PsA Core Set working group meeting
During this meeting, the working group discussed and reviewed organization of workstreams to tackle development of the PsA Core Outcome Measurement Set (Figure 2). Each workstream includes at least two PRPs. A series of systematic literature reviews are ongoing to assess measurement properties of candidate instruments including patient-reported outcomes assessing skin and joint disease activity, fatigue, pain, patient global, health-related quality of life, participation, and emotional well-being; physical examination methods for assessing MSK and skin disease activity; systemic inflammation; imaging modalities to assess structural damage and disease activity; and composite disease activity measures. A qualitative assessment of the feasibility and the content validity of available instruments from the perspective of patients and physicians has been launched. Simultaneously, instrument assessments are being performed within RCTs and LOS. These three efforts will produce data about the measurement performance of each instrument. These data will be presented and discussed in a workshop and breakout groups at the next GRAPPA annual meeting, after which the group may realize that additional data are needed. The working group will then refine the data obtained and structure the patient and physician Delphi surveys using similar methods to those used in the development of the core domain set. The patient and physician Delphis will be used to achieve consensus on the optimal instruments to address some, if not all, of the core domains. If needed, an in-person consensus meeting will be held following the Delphi. The working group aims to present a preliminary core outcome measurement set for discussion and voting at the next OMERACT meeting in May 2018.
Figure 2. Roadmap for developing the Psoriatic Arthritis (PsA) Core Outcome Measurement Set.
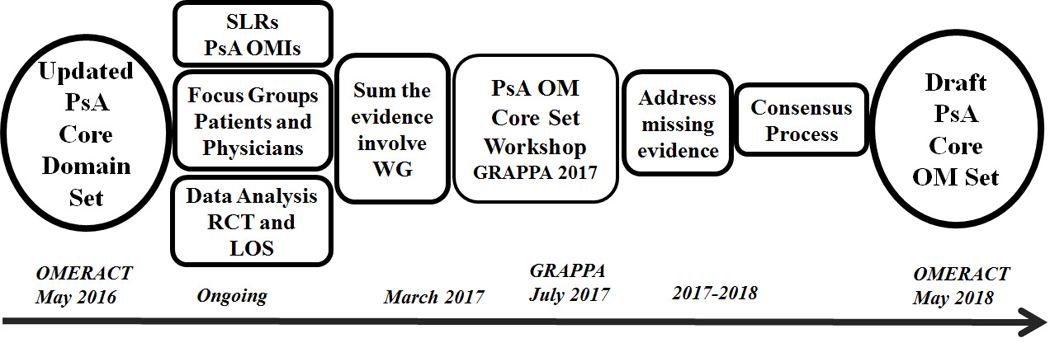
Workstreams are listed in chronological order.
GRAPPA = Group for Research and Assessment of Psoriasis and Psoriatic Arthritis;
OMERACT = Outcome Measures in Rheumatology; WG = working group;
LOS = longitudinal observational studies; OMIs = outcome measurement instruments;
RCT = randomized controlled trials; SLR: systematic literature review.
Conclusion
As the number of new therapies for psoriasis and PsA increases, the need to understand how to best measure disease activity, impact and long-term patient outcomes has become more important. Additionally, standardizing outcome measurement among RCTs and LOS will assist patients, clinicians, and other stakeholders in better evaluating the evidence available for a particular treatment. To this end, patients, rheumatologists, dermatologists, and methodologists are working together through GRAPPA, OMERACT, and the International Dermatology Outcome Measurers (IDEOM) to develop and standardize core outcome measurement sets for psoriatic disease.
Acknowledgments
We would like to acknowledge all members of the GRAPPA-OMERACT PsA working group, which includes the authors along with Laure Gossec, Pil Hoejgaard, Neil McHugh, Robin Christensen, and Katy Leung who were unable to attend the GRAPPA meeting, as well as Rodrigo Firmino.
Funding: AO was supported by NIH K23 AR063764 and the Rheumatology Research Foundation. A-MO was supported in part by a Scientist Development Award from the Rheumatology Research Foundation and the Johns Hopkins Arthritis Center Discovery Fund.
Contributor Information
Alexis Ogdie, Assistant Professor of Medicine and Epidemiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Maarten de Wit, Patient Research Partner, VU Medical Centre, Amsterdam, The Netherlands.
Kristina Callis Duffin, University of Utah, Salt Lake City, UT, USA.
Willemina Campbell, Patient Research Partner, Toronto Western Hospital, Toronto, Ontario, Canada.
Jeffrey Chau, Patient Research Partner, Hong Kong Psoriatic Arthritis Association, Hong Kong, China.
Laura C Coates, UK National Institute for Health Research Clinical Lecturer, Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Leeds, UK.
Lihi Eder, Assistant Professor of Medicine, Women’s College Research Institute, Women’s College Hospital. University of Toronto, Toronto, ON, Canada.
Musaab Elmamoun, Fellow, University of Toronto, Toronto Western Hospital, Toronto, Ontario, Canada.
Oliver FitzGerald, Newman Clinical Research Professor, St Vincent’s University Hospital and University College Dublin, Ireland.
Dafna D Gladman, Professor of Medicine, University of Toronto, Senior Scientist, Krembil Research Institute, Director, Psoriatic Disease Program, Toronto Western Hospital, Toronto, Ontario, Canada.
Niti Goel, Patient Research Partner, Vice President, Advisory Services, QuintilesIMS, Adjunct Assistant Professor, Duke University School of Medicine, Durham, North Carolina, USA.
Jana James, Patient Research Partner, Royal United Hospital, Bath, UK.
Umut Kalyoncu, Assistant Professor of Internal Medicine, Division of Rheumatology, Hacettepe University Faculty of Medicine, Ankara, Turkey.
John Latella, Patient Research Partner; Board Member, International Dermatology Outcome Measurers, West Granby, CT, USA.
Chris Lindsay, Patient Research Partner, Thousand Oaks, CA, USA.
Philip J Mease, Rheumatology Research, Swedish Medical Center, Clinical Professor, University of Washington School of Medicine, Seattle, WA, USA.
Denis O’Sullivan, Patient Research Partner, Our Lady’s Hospice & Care Services, Dublin, Ireland.
Ingrid Steinkoenig, Patient Research Partner, Cleveland Clinic, Cleveland, Ohio, USA.
Vibeke Strand, Division of Immunology/Rheumatology, Stanford University School of Medicine, Palo Alto, CA, USA.
William Tillett, Consultant Rheumatologist, Senior Lecturer, Royal United Hospital, Bath; Pharmacy and Pharmacology, University of Bath, UK.
Ana-Maria Orbai, Assistant Professor of Medicine, Johns Hopkins University School of Medicine, Director Psoriatic Arthritis Program, Johns Hopkins Arthritis Center, Baltimore, Maryland, USA.
References
- 1.Gladman DD. Clinical Features and Diagnostic Considerations in Psoriatic Arthritis. Rheum Dis Clin North Am. 2015;41:569–79. doi: 10.1016/j.rdc.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Gossec L, de Wit M, Kiltz U, Braun J, Kalyoncu U, Scrivo R, et al. A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: elaboration and preliminary validation of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire, a 13-country EULAR initiative. Ann Rheum Dis. 2014;73:1012–9. doi: 10.1136/annrheumdis-2014-205207. [DOI] [PubMed] [Google Scholar]
- 3.Gladman DD, Mease PJ, Strand V, Healy P, Helliwell PS, Fitzgerald O, et al. Consensus on a core set of domains for psoriatic arthritis. J Rheumatol. 2007;34:1167–70. [PubMed] [Google Scholar]
- 4.Tillett W, Eder L, Goel N, De Wit M, Gladman DD, FitzGerald O, et al. Enhanced Patient Involvement and the Need to Revise the Core Set - Report from the Psoriatic Arthritis Working Group at OMERACT 2014. J Rheumatol. 2015;42:2198–203. doi: 10.3899/jrheum.141156. [DOI] [PubMed] [Google Scholar]
- 5.Boers M, Kirwan JR, Wells G, Beaton D, Gossec L, d’Agostino MA, et al. Developing core outcome measurement sets for clinical trials: OMERACT filter 2.0. J Clin Epidemiol. 2014;67:745–53. doi: 10.1016/j.jclinepi.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Orbai AM, Mease PJ, de Wit M, Kalyoncu U, Campbell W, Tillett W, et al. Report of the GRAPPA-OMERACT Psoriatic Arthritis Working Group from the GRAPPA 2015 Annual Meeting. J Rheumatol. 2016;43:965–9. doi: 10.3899/jrheum.160116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orbai AM, de Wit M, Mease P, Shea JA, Gossec L, Leung YY, et al. International patient and physician consensus on a psoriatic arthritis core outcome set for clinical trials. Ann Rheum Dis. 2016 doi: 10.1136/annrheumdis-2016-210242. [Epub ahead of print; September 9, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalyoncu U, Ogdie A, Campbell W, Bingham CO, 3rd, de Wit M, Gladman DD, et al. Systematic literature review of domains assessed in psoriatic arthritis to inform the update of the psoriatic arthritis core domain set. RMD Open. 2016;2:e000217. doi: 10.1136/rmdopen-2015-000217. [DOI] [PMC free article] [PubMed] [Google Scholar]


