Abstract
In the year 2016 the synthetic detergents complete 100 years and in this story they evolved as cleaners. They are already part of the routine of thousands of people worldwide. For a higher power of cleaning of the detergent, today, are added phosphates, the main responsible for environmental problems. After 100 years of synthetic detergents, the effect of the combination of various cleaners on the environment is a gap. Legislation and guidelines about the other components of the formula of cleaners still missing. Even the term biodegradable can be best placed on the diversity of products currently entitled biodegradable. A lot attitudes can still be taken to continuously improve the relationship between the parties involved, animals, plants, waters and men, so that in another 100 years continues to exist this interaction with the environment without destroying it. The marketing used by synthetic detergent companies evolved a lot over the years and showed maturity to deal with changes in theories and strategies for promotion and even with the constant social reform that its consumer lived, accompanying them intelligently to be able to capture their changing needs and desires, and so assemble the best way to connect to them. This paper focuses on the subject synthetic detergents as well as (i) types and applications, (ii) threats, (iii) sustainability, (iv) legislation, (v) packaging and (vi) marketing strategies.
Keywords: Synthetic detergents, Phosphates, Biodegradable, Environment, Marketing
1. Introduction
In the year 2016 the synthetic detergents complete 100 years and in this story they evolved as cleaners. Their uses and objectives have been expanded and today it is already part of the routine of thousands of people worldwide.
A detergent is any compound that can be used as a cleaning agent. Although soap is a detergent, this term is generally used to refer to synthetic substitutes of soap.
The soap, called anionic surfactant, of general formula RCO-ONa, is a salt of carboxylic acid of long chain containing 10–18 carbon atoms, wherein one hydrogen has been replaced by a cation. The long chain of hydrocarbon of the carboxylic acid salts is non-polar and capable of interacting with nonpolar species as fats and other impurities. The group ionized carboxylic acid, being polar, is able to interact with water molecules. This characteristic explains the interaction of soap with water and fats (Penteado et al., 2006, Cai and Hakkinen, 2014).
Generally, the common soap is a sodium salt, soluble in water. However, salts of Ca2+, Mg2+ or Fe3+ are insoluble in water. Thus, a soap cannot be used efficiently in a medium containing these ions, in case the hard water (Cai and Hakkinen, 2005).
Water hardness is usually measured as the amount of parts per million (ppm) of calcium carbonate (CaCO3). Water is considered hard if containing amounts of these cations above 150 mg L−1, water is considered soft if the contents are below 75 mg L−1 and if they are between 75 and 150 mg L−1 water is considered moderate.
The problems related to the use of common soaps in hard water led to the development of synthetic detergents.
Detergents have structural characteristics similar to soaps, polar region and a non-polar long chain and act basically the same way. However, chemical characteristics of the detergent are different and do not precipitate in hard water or acidic solutions, such as soaps.
Anionic synthetic detergents commonly used in cleaning contain alkyl benzene sulphonate of sodium, linear chain. On the market are found as a mixture of alkyl benzene sulfonates, wherein the main component of this mixture is dodecylbenzene sulphonate of sodium, established as standard of biodegradable anionic detergent.
2. Types and applications
Soaps and detergents belong to the same group of chemicals, the surfactants. There are four types of surfactants; anionic, cationic, nonionic and amphoteric (Kemmei et al., 2007).
Soaps and detergents belong to the group of anionic surfactants.
Non-ionic surfactants do not present radicals with electric charges and interact with water molecules by hydrogen bonds.
They are, together with the anionic surfactants, the most appropriate for the removal of dirt by washing, once in the water both surface of the fabric fibers and dirt particles are negatively charged.
The cationic surfactants are often used as bactericides, hair conditioners as well as fabric softener.
The amphoteric surfactants are used in shampoos and cosmetic creams.
3. Threat of synthetic detergents
Before the advent of synthetic detergents, there were problems with the effects of soaps on the ecosystem, once they have left an insoluble film on the surface of the water which, for example, decreased or even impeded the entry of oxygen. Therefore, always existed an environmental concern in relation to cleaning products, the observation of its effects on the ecosystem always existed.
3.1. Phosphates
The sequestering and chelating agents are added for greater cleaning power of the detergent (El-Gawad, 2014), such as phosphates, which remove calcium and magnesium ions that are present in water and can reduce detergent action.
Phosphates are non-toxic, increases the efficacy of the detergent and with them the cost of the final product is low. However, phosphates are largely responsible for problems to the environment (Ashforth and Calvin, 1973).
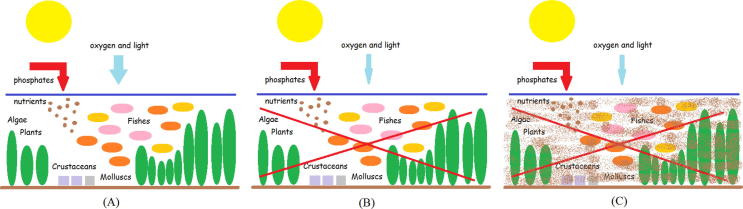
The phosphates act as nutrients to the environment. They favor the excessive growth of algae, which cause eutrophication of waters, as can be seen in Fig. 1. Thus, with respect to concern about the consequences caused by their indiscriminate use, brought the first laws restricting the addition of phosphates in detergents in various regions of the world (Cain, 1994, Osorio and Oliveira, 2001, Brazil, 2005, Warwick et al., 2013).
Fig. 1.
(A) Addition of phosphate in water causing proliferation of aquatic flora and consequently increase of aquatic fauna. (B) The increase of fauna and flora makes it difficult the entry of light and oxygen, which leads to the death of these species. (C) Water becomes dirty, smelly and improper for use.
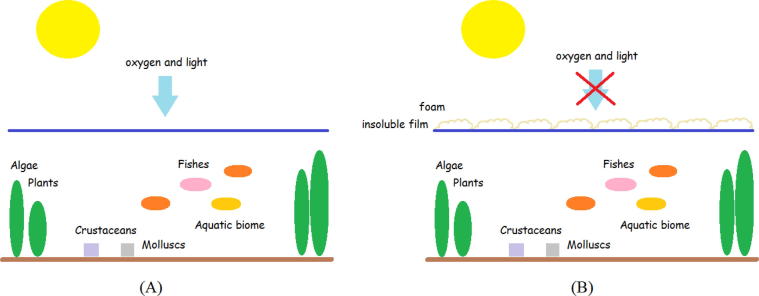
Phosphates are also responsible for the formation of white foam which act as a barrier to entry of oxygen and light in the water (Fig. 2).
Fig. 2.
(A) Normal condition. (B) Impedance of the entrance of light and oxygen by foams which affect the survival of aquatic species.
The union of the phosphate effects on waters as a nutrient and barrier to light and oxygen affect fauna and flora aquatic. The death of algae, plants, fish, mollusks and crustaceans are some examples of this effect. Water also becomes improper for consumption and future use by animals, plants and man. Many species are affected, as a cycle.
Another issue about the products with phosphates is the lack of knowledge of their degradation in the environment, since only isolated study of these substances is not enough. Degradation studies and simultaneous interaction of various cleaning products eliminated daily in the environment is a gap that drives the researchers.
This type of alert is not limited to phosphates used in synthetic detergents, but also to phosphate fertilizer, widely used in farming, which likewise have their final destinations to waters and watersheds (Warwick et al., 2013).
3.2. Biodegradable
The fact that a product is biodegradable does not make be non-polluting. The effect of the products entitled biodegradable in the ecosystem is unknown. A biodegradable product can be degraded by microorganisms, but this does not indicate that it does not cause damage to the ecosystem (Bailey et al., 1978).
Another point to be emphasized is the limit of biodegradability. Today, a product that is biodegradation in approximately 24 h receive the same titration that another product that is biodegraded in 28 days.
Currently one product with no residue after the biodegradation also receives the same titration biodegradable that another one which has residue of 10% after the biodegradation. Nowadays, there are no differences between products considered biodegradable in 24 h without waste or 28 days with up to 10% of waste.
It behooves us to continuously improve the relationship between the parties involved, animals, plants, waters and men, with the objective to exist this interaction with the environment without destroying it for another 100 years.
4. Sustainability
As synthetic detergents are already part of the routine of thousands of people worldwide and its use is already established, ideas emerged to overcome the threat of these products in environmentally friendly products.
The first action, in the relationship synthetic detergents and sustainability, was to change the precursor used in the manufacture of these products that originated surfactants with branched chains, which remained in the water for a long period of time, since the degradation by biological agent was more difficult. The permanence of these wastes caused the appearance of foams that spread by water sources and with the winds could carry contamination and dirt over long distances (Sutili et al., 2009).
Today are made surfactants of linear chain, which facilitate the biodegradation of the product.
An alternative method is the obtaining of non-ionic surfactant from the oil and bran of soy, a natural polymer, which when suffers acid hydrolysis produces amino acids which react with the oil to produce the surfactant. Brazil is a major producer of soy and, thus, a potential country in helping to decrease the environmental impact caused by the effects of synthetic detergents.
A possible replacement for phosphates, another impasse on the sustainability of synthetic detergents, are zeolites, aluminosilicate with high capacity of ion exchange and high porosity, which soften hard water (Ashforth and Calvin, 1973, Cai and Hakkinen, 2005, Warwick et al., 2013).
Today, unfortunately, the majority of detergent products on the market does not have the presence of zeolites among its components.
It is noteworthy also that alternative methods of cleaning using homemade products are, often, equally efficient. In this theme, the hot water, vinegar, sodium bicarbonate, lemon juice, salt, coffee powder, ascorbic acid, tocopherol and seed extract of grape fruit are examples.
In the year 2015 in Paris, actions were agreed by 195 nations in favor of the planet, “long live the planet, long live humanity, long live life itself”. The value of the knowledge of the effects of the use of synthetic detergents to the environment and the search for sustainable and alternative methods are valid and worthy of pride. This attitude comes to make us think in a multidimensional way (circular, horizontal, vertical and lateral). It comes to focus us the whole, the parts and especially the interaction among the parts of a system (Kogawa et al., 2014).
5. Legislation
5.1. Biodegradable synthetic detergents
In Europe and United States the prohibition of the use of synthetic detergents with branched chain started in 1965, since the problems caused by these detergents were related to the structures of their molecules. Products with the alkyl benzene sulphonate were strictly forbidden.
In Brazil, only in 1977, was presented a decree that banned the manufacture, sale or import of sanitizing of any kind, containing anionic surfactant, non-biodegradable (Brazil, 1977). In 1985, a law in which industries could only produce non-polluting detergents arises, and in it also contains a ban on imports of non-biodegradable detergents (Brazil, 1985).
5.2. Phosphates
European Union countries (Germany, Austria, Belgium, Ireland and Italy) have developed legislation with the support of environmentalists to control the addition of phosphates in detergents for clothes, in order to reduce water pollution (Cai and Hakkinen, 2005).
In Brazil, the Conselho Nacional do Meio Ambiente (CONAMA), established in 1981, presents in 2005 a resolution that stipulates a reduction by 1.5% of phosphorus concentration in powder detergents (Brazil, 2005).
5.3. Other components
The laws currently existing include standards for detergents and phosphates, however they do not provide guidance about the other components of the formula of an cleaner.
They can be 80% of the total components, non-biodegradable and toxic. These include antioxidants, antiseptics, fungicides, chlorine, chlorine amines, organochlorine and heavy metals such as mercury.
The urgency to legislation to control these other components is important and required by manufacturers.
5.4. Packaging labels
Regarding the labels on packets of synthetic detergents, they should contain information in case of accident, warning for not taking advantage of the empty package, recommendations for conservation, chemical group of the active components of the formula and their antidotes to use when there is therapeutic measures to be taken in case of accident and the highlight “KEEP OUT OF REACH OF CHILDREN AND PETS” (Brazil, 1977).
6. Packaging
The marketing of products stored in certain packaging has several objectives such as: facilitate handling, transporting and storing the product, ensure the quality and effect of it, contain the necessary information about the product, its origin and method of use, and as a tool to leverage sales of the product, that is, the packaging is also used in the marketing strategies of its producer.
In the case of synthetic detergents, with the passing the years and with the changing needs of consumers, the packaging of detergents were modified. The more elaborate packaging suggest a better quality product, that is, the client associates more durable packaging and visually attractive with a more effective product.
In addition to the relationship packing-effectiveness of the product, the packaging also suffer changes in their capacity to adapt to the social profile of your consumer. In Brazilian regions where there is a higher share of low-income population, the most marketed detergent containers are the ones with least capacity, while the regions with the highest income, the greater capacity of packaging lead sales.
6.1. Packaging and the environment
Another criterion commonly used by consumers when choosing their products are the environmental damage that their packaging may cause, being the less harmful preferred (Rokka and uusitalo, 2008). The packaging of detergents also changed with increasing environmental consciousness of the population, being produced with materials that allow greater reuse, reducing its impact on the environment, since the packages are produced and discarded on large scale.
In addition to environmental issues, the commercialization of illegal or pirated sanitizers has been highlighted by the increase in their consumption and risk. The black market exists because of lack of knowledge and producer's money to regularize its establishment and the existing demand of consumers who choose to purchase illegal products due to their attractive prices. Liquid detergents are among them, their commercialization is performed, in most cases, in PET bottles reused without identifying the origin, content and mode of use.
7. Marketing strategies
The marketing has emerged as a proposal to understand consumer needs and desires. For years, this form of interaction with the target audience has become indispensable for a product be able to enter profitably in market, and with the synthetic detergent is no different (Martins et al., 2011).
Firstly we must understand to whom they are directed to comprehend the strategies used for this type of product. The main target of detergents are women, housewives, because it is a product used in household cleaning. Other information is that the women, at the time of purchase, are interested in lower prices and higher quality. Thus, companies increasingly need to work on more refined strategies to achieve them (Sebastião, 2008).
The first approaches used were based on television advertisements showing the left side of the screen a T-shirt with a stain that an unknown brand of detergent could not take, and the right side of the same shirt and stain, but this time fully cleaned by detergent focus of advertising (Schmitt, 2002).
This format has been used for many years, however, it began to be replaced by other strategies. And this is due to two main reasons:
The first is the increasing appearance of new brands on the market with high competitive power and the appearance of new administration technologies, changes in the quality control and consumer demands (Martins et al., 2011).
In order to overcome these market requirements a strategy used by the leading brand at the time, which changed the focus of advertising, using as theories inspiration of experiential marketing, which differs from traditional marketing to focus on consumer experiences, generating sensory, emotional values, cognitive and identification (Schmitt, 2002, Lima, 2009).
This new vision uses cool and calm colors bringing to the consumer the promise to fresh and clean into their homes. This approach allowed the product to bind to its customers affectively taking the relationship beyond the product and its gross characteristics, establishing a level of fidelity that other brands have not been able to awaken (Schmitt, 2002).
The second reason for this change in strategy was the changing role of women in society. With the increasing integration of women in the market, since the 60s, the family structure has changed, and the relationships between parents and children and the own concept of “housewives”, mainly by changing the patriarchal system to a system equal within the homes, where all have equal voice. These kinds of changes made the leading brand reevaluate the way how communicate with your consumers, leaving aside the “washes whiter” to enhance the mother-child relationship (Limeira, 2006).
The theme used in the advertising, as well as other strategy presented, aims to make relations with consumers that go beyond price and quality, creating an intimate and sincere relationship, leaving behind potential competitors.
Therefore, the marketing used by detergent companies evolved a lot over the years and showed maturity to deal with changes in theories and strategies for promotion and even with the constant social reform that its consumer lived, accompanying them intelligently to be able to capture their changing needs and desires, and so assemble the best way to connect to them.
Declaration of interest
The authors report no declarations of interest.
Acknowledgments
The authors acknowledge CNPq (Brasília, Brazil), FAPESP (São Paulo, Brazil), CAPES (São Paulo, Brazil) and PADC/FCF/UNESP (Araraquara, Brazil).
Footnotes
Peer review under responsibility of King Saud University.
References
- Ashforth G.K., Calvin G. Safety evaluation of substitutes for phosphates in detergents. Water Res. 1973;7:309–320. [Google Scholar]
- Bailey R.A., Clark H.M., Ferris J.P., Krause S., Strong R.L. Soap and detergents. Chem. Environ. 1978;6:134–146. [Google Scholar]
- Brazil, Decreto n° 7.9095, de 05 de janeiro de, 1977. Diário Oficial da União. Brasília, 06 January. 1977.
- Brazil, Lei n° 7.365, de 13 de setembro de, 1985. Dispõe sobre a fabricação de detergentes não biodegradáveis. Diário Oficial da União. Brasília, 16 September 1985.
- Brazil, Conselho Nacional do Meio Ambiente (CONAMA). Resolução n° 357, de 17 de março de, 2005. Dispõe sobre a classificação dos corpos de água e diretrizes ambientais para o seu enquadramento, bem como estabelece as condições e padrões de lançamento de efluentes, e dá outras providências. Diário Oficial da União. Brasília, 18 mar. 2005.
- Cai, Z., Hakkinen, P.J., 2005. Detergent. Encyclopedia of Toxicology, second ed., pp. 739–742.
- Cai, Z., Hakkinen, P.J., 2014. Detergent reference module in biomedical sciences. Encyclopedia of Toxicology, third ed., pp. 10–13.
- Cain R.B. Biodegradation of detergents. Curr. Opin. Biotech. 1994;5:266–274. [Google Scholar]
- El-Gawad H.S.A. Aquatic environmental monitoring and removal efficiency of detergents. Water Sci. 2014;28:51–64. [Google Scholar]
- Kemmei T., Kodama S., Yamamoto A., Inoue Y., Hayakaw K. Determination of sequestering agents in cosmetics and synthetic detergents by high-performance liquid chromatography with ultraviolet detection. J. Chromatogr. A. 2007;1171:63–68. doi: 10.1016/j.chroma.2007.09.037. [DOI] [PubMed] [Google Scholar]
- Kogawa A.C., Aguiar F.A., Gaitani C.M., Salgado H.R.N. Validation of a stability indicating capillary electrophoresis method for the determination of darunavir in tablets and comparison with the of infrared absorption spectroscopic method. World J. Pharm. Pharm. Sci. 2014;3:283–297. [Google Scholar]
- Lima, M.C.L.O., 2009. Marketing Experimental. Brasília, Distrito Federal.
- Limeira T.M.V. FGV; São Paulo: 2006. Revolução Feminina. [Google Scholar]
- Martins C.A., Ribeiro P.H.A.C., Schvartzer A. FGV; Rio de Janeiro: 2011. Técnicas de Vendas. [Google Scholar]
- Osorio V.K.L., Oliveira W. Polifosfatos em detergentes em pó comerciais. Quím Nova. 2001;24:700–708. [Google Scholar]
- Penteado J.C.P., El Seoud A.O., Carvalho L.R.F. Alquilbenzeno sulfonato linear: uma abordagem ambiental e analítica. Quím Nova. 2006;29:1038–1046. [Google Scholar]
- Rokka J., Uusitalo L. Preference for green packaging in consumer product choices – do consumers care? Int. J. Consum. Stud. 2008;32:516–525. [Google Scholar]
- Schmitt B.H. Nobel; São Paulo: 2002. Marketing Experimental. [Google Scholar]
- Sebastião, M.K., 2008. Comportamento Organizacional: Fatores que Motivam a Compra. Taubaté, São Paulo.
- Sutili F.K., Miotto N., Rigoti E., Pergher S.B.C., Penha F.G. Aplicação de zeólitas sintéticas como coadjuvante em formulação detergente. Quím Nova. 2009;32:879–883. [Google Scholar]
- Warwick C., Guerreiro A., Soares A. Sensing and analysis of soluble phosphates in environmental samples: a review. Biosens. Bioelectron. 2013;41:1–11. doi: 10.1016/j.bios.2012.07.012. [DOI] [PubMed] [Google Scholar]