Abstract
The influence of an inorganic support – halloysite nanotubes – on the release rate and biological activity of the antibiotic encapsulated in alginate-based dressings was studied. The halloysite samples were loaded with approx. 10 wt.% of the antibiotic and then encapsulated in Alginate and Gelatin/Alginate gels. The material functionalized with aliphatic amine significantly extended the release of vancomycin from alginate-based gels as compared to that achieved when silica was used. After 24 h, the released amounts of the antibiotic immobilized at silica reached 70%, while for the drug immobilized at halloysite the released amount of vancomycin reached 44% for Alginate discs. The addition of gelatin resulted in even more prolonged sustained release of the drug. The antibiotic was released from the system with a double barrier with Higuchi kinetic model and Fickian diffusion mechanism. Only the immobilized drug encapsulated in Alginate gel demonstrated very good antimicrobial activity against various bacteria. The inhibition zones were greater than those of the standard discs for the staphylococci and enterococci bacteria tested. The addition of gelatin adversely affected the biological activity of the system. The inhibition zones were smaller than those of the reference samples. A reduction in the drug dose by half had no significant effect on changing the release rate and microbiological activity. The in vivo toxicity studies of the material with immobilized drug were carried out with Acutodesmus acuminatus and Daphnia magna. The material studied had no effect on the living organisms used in the bioassays. The proposed system with a double barrier demonstrated high storage stability.
Keywords: Halloysite nanotubes, Vancomycin, Alginate, Wound dressings
1. Introduction
Halloysite nanotubes (HNTs) are naturally occurring clays composed of aluminosilicate sheets. The wall contains 10–15 bilayers of aluminium and silicon oxide. Unlike kaolinite, HNTs have an additional monolayer of water between adjacent layers. HNTs are characterized by the presence of siloxane groups at the exterior surfaces and aluminol groups at the interior surfaces. As alumina layer is at the inner surface and silica layer at the outer surface, thus the tube surfaces are oppositely charged in water. Different literature sources provide slightly divergent values for the basic dimensions of HNTs, but it can be assumed that the internal diameter (lumen), outer diameter and length of nanotubes are in the range 1–30 nm, 30–100 nm and 0.1–2.0 μm, respectively (Joussein et al., 2005, Pasbakhsh and Churchman, 2015).
HNTs are a low cost, natural mineral nanotubes with a wide range of potential applications in different fields. They have been investigated for thermal resistance, corrosion prevention, in polymerisation reaction, for remediation or as carrier systems (Kamble et al., 2012, Du et al., 2010, Rawtani and Agrawal, 2012, Lvov et al., 2008) and delivery systems (Massaro et al., 2015b, Massaro et al., 2016, Massaro et al., 2016). They have many possible applications in the area of medical science (Lvov et al., 2016a, Lvov et al., 2016b, Lvov and Abdullayev, 2013, Massaro et al., 2015a, Massaro et al., 2014, Vergaro et al., 2010, Vergaro et al., 2012), which is not only a consequence of the nanotubes structure (Levis and Deasy, 2002), but also of biocompatibility of the material (Abdullayev and Lvov, 2013). Release of different bioactive compounds, like 5-aminosalicylic acid (Aguazzi et al., 2013), tetracycline HCl (Price et al., 2001) or ofloxacin (Wang et al., 2014) from HNTs has been studied. Moreover, surface modification of HNTs can result in improvement in the material properties, e.g. modification with dopamine allows enzyme immobilization (Chao et al., 2013), while surface functionalization with 3-Aminopropyltrimethoxysilane increases loading with ibuprofen (Tan et al., 2013). The inorganic HNTs can be also combined with biopolymer materials in order to obtain, e.g. scaffolds for tissue engineering (Naumenko et al., 2016) or electrospun fiber membranes for clinical application (Xue et al., 2015).
Halloysite is one of many inorganic carriers of biologically active substances. In our previous work we used modified silica as a carrier of the antibiotic vancomycin (Kurczewska et al., 2015a). Vancomycin is a glycopeptide water-soluble drug, used for the treatment of infection caused by Gram-positive bacteria. Literature provides information on a number of different carriers of this antibiotic. Silica-based mesoporous material SBA-15 has been found to be effective in local delivery of vancomycin (Molina-Manso et al., 2012), while functionalized mesoporous silica participated in the reaction with the antibiotic in order to obtain vancomycin-modified silica nanoparticles for killing pathogenic bacteria (Qi et al., 2013). Also microporous hydroxyapatite fibres have been tested as carriers of the drug (Ravelingien et al., 2010).
A large group of systems, used as drug carriers are hydrogels (Peppas et al., 2000, Hamidi et al., 2008). Vancomycin can be encapsulated within hydrogel beads (Lin et al., 2010) or it can be covalently bonded in order to form antibacterial hydrogel (Lakes et al., 2014). Hydrogels with vancomycin can be applied as wound healing dressings (Zhang et al., 2008, Zhao et al., 2014). Modern dressings should not only increase patients’ comfort, but mostly they are designed to create appropriate environment around a wound that facilitates and participates in its healing (Boateng et al., 2008, Ovington, 2007). Alginate is relatively common drug carrier in modern dressings. It is a natural polysaccharide polymer composed of alternating residues of 1–4 α-L-guluronic (G-blocks) and β-D-mannuronic acid (M-blocks). Gel formation of alginate is carried out under mild environment, and it is possible thanks to the reaction with divalent cations (Ca, Ba, Sr). Calcium alginate wound dressing with impregnated vancomycin has been investigated as a system for treatment of surgical infections (Lin et al., 1999). However, more sophisticated systems,- ensuring a slow-release of an active substance, are continuously searched for. One of the proposed solutions is the use of bilayer films, in which one layer contains the active drug and the other provides an additional barrier that must be overcome by this drug to reach the treatment site (Thu et al., 2012, Thu and Ng, 2013). In our previous studies we investigated the properties of Vancomycin immobilized at the functionalized silica surfaces, encapsulated in alginate-based gel (Kurczewska et al., 2015b). The system proposed was investigated as a potential modern wound dressing with a slowly-released antibiotic.
There are also some other materials that are composed of halloysite and alginate. Bionanocomposite beads of halloysite/alginate have been characterized and studied as a new drug carriers (Chiew et al., 2014, Karnik et al., 2015). A hybrid nanocomposite containing halloysite nanotubes, chitosan and sodium alginate has been used for a sustained release of an analgesic (Li et al., 2016).
In this work, encouraged by a high binding capacity of different drugs by halloysite, we modified the system with a double barrier. The main aim of the present study was to investigate halloysite nanotubes as carriers of vancomycin, to demonstrate the influence of surface functionalization with (3-Aminopropyl)-trimethoxysilane and finally to investigate the effect of inorganic carrier on release rate and biological activity of vancomycin in potential wound dressings, in which halloysite with ionically-bonded drug is encapsulated in alginate-based matrix.
2. Materials and methods
2.1. Materials
Halloysite nanotubes (HNTs), (3-Aminopropyl)-trimethoxysilane (APTS), alginic acid sodium salt, gelatin powder and glycerol were commercial products of Aldrich and were used as received. Vancomycin hydrochloride was used as a commercial product of Xelia Pharmaceuticals ApS (Denmark). Hydrochloric acid, ethanol and toluene were purchased from POCH (Poland). All the chemicals were of analytical grade. Demineralized water was used for aqueous solutions preparation.
2.2. Synthesis of APTS-modified halloysite
HNTs were first purified with 10% (v/v) hydrochloric acid solution for 24 h at room temperature. Then purified nanotubes were suspended in dry toluene with (3-Aminopropyl)-trimethoxysilane. The mixture was refluxed under constant stirring for 12 h. A calcium chloride drying tube was used to maintain a dry environment. Then the product was filtered off and washed several times with toluene and ethanol to remove the excess of organosilane. Finally APTS-modified halloysite (HNTs_APTS) was dried overnight at 120 °C.
2.3. Characterization of unmodified and modified halloysite
Transmission electron microscope (TEM) images were recorded on a Hitachi HT7700 microscope, operating at accelerating voltage of 100 kV. The infrared spectra were taken on an IFS 66v/s Fourier transform infrared (FTIR) spectrophotometer from Bruker, equipped with an MCT detector (125 scans, resolution 2 cm−1). The spectra were recorded in the 400–4000 cm−1 range for KBr pellets. The thermogravimetric studies were carried out in a Setsys 1200 apparatus (Setaram) at a heating rate of 10 °C/min under helium atmosphere. Elemental analysis was carried out on a Vario ELIII (Elementar, USA) analyzer.
2.4. Drug loading and fabrication of alginate-based dressings
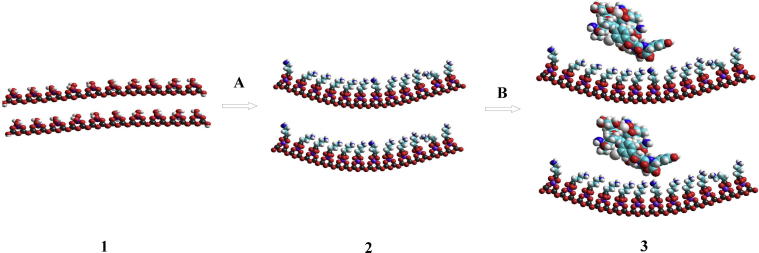
Three different HNT samples were used for vancomycin immobilization: commercial product (HNTs), halloysite activated with hydrochloric acid (HNTs_HCl) and halloysite modified with (3-Aminopropyl)-trimethoxysilane (HNTs_APTS). The procedure of drug loading was analogous to that presented for the silicas (Kurczewska et al., 2015b). A schematic presentation of the preparation of vancomycin immobilized at HNTs_APTS is shown in Fig 1.
Fig. 1.
A schematic presentation of the drug loading at HNTs_APTS, where A is APTS, B – Vancomycin, 1 – HNTs_HCl, 2 – HNTs_APTS, 3 – Vancomycin immobilized at HNTs_APTS.
Alginate-based dressings were prepared only for the samples with vancomycin immobilized at the HNTs_APTS according to the procedure previously described (Kurczewska et al., 2015b). In brief, for Alginate dressing 1.50 ± 0.02 g of sodium alginate (6%, w/v) was dissolved in water. Then 1.5 mL of a plasticizer (glycerol) was added and finally 0.25 or 0.50 ± 0.05 g of the HNTs_APTS was added under rapid stirring of the sample. For making Gelatin/Alginate dressing, at first 1.50 ± 0.04 g of gelatin powder was dissolved in hot water. The following steps were the same as for Alginate gel (alginate was dissolved in the gelatin solution). Alginate or Gelatin/Alginate gels were poured into petri dishes (diameter 7.5 cm) and dried for 48 h. Then the gels were weighted and stored in conditions that ensured a constant weight.
The drug loading at halloysite surfaces was determined by the standard procedure, i.e. by subtracting the unreacted vancomycin in the supernatant from the total amount added. A calibration curve of vancomycin was plotted using standard solutions. The drug concentration in the solution was determined with UV–Vis spectrophotometry and the amount of the drug immobilized at the halloysite was calculated with the following equation:
| (1) |
where C0 and Ci are the initial and the final drug concentration [mg/mL], V the drug solution volume [mL] and W the halloysite dose added [g].
For the alginate-based dressings with the antibiotic immobilized at HNTs_APTS surface, the drug loading ability was considered to be 100%.
In order to determine the cumulative release efficiency, the samples were immersed in phosphate buffered saline (PBS, pH 7.4), heated and stirred at 37 °C for 6 days. The samples of the dried alginate-based films were prepared by trimming with scissors several 1 cm × 1 cm area pieces. Than the absorbance of the solution relative to the blank PBS solution was used for determination of the antibiotic concentration released from the materials studied (mg of vancomycin per g of halloysite powder or dried alginate-based material). The process for the samples of the dried alginate-based films was carried out as long as the structure of the alginate gel was broken and the whole drug released corresponded to the amount released from HNTs_APTS carrier. The results for each sample were averaged and expressed as mean ± standard deviation.
2.5. Release tests
For in vitro release studies, the procedure previously described was used (Kurczewska et al., 2015b). The weighted samples containing vancomycin, were immersed in PBS solution and stirred at 37 °C. At a specified time intervals, -aliquots (5 mL) were withdrawn from the release medium and replaced with fresh PBS in order to keep constant the volume of the release medium. The samples were analyzed on a UV–Vis Agilent 8453 Spectrophotometer at 280 nm to determine the amount of released vancomycin using the Lambert–Beer law. The in vitro tests were made in triplicate.
Total amounts of the drug released (Ft) were calculated as follows:
| (2) |
where Vm and Ct are volume and concentration of the drug at time t, Va is the volume of the sample withdrawn and Ci is the drug concentration at time i (i < t).
2.6. Microbiological tests
The study was performed for four gel samples with Vancomycin-immobilized at HNTs_APTS (0.50 or 0.25 ± 0.02 g) in Alginate or Gelatin/Alginate. As a standard, a disc containing 30 μg of Vancomycin was applied. The following cultures of reference strains, for the study of antibacterial activity of antibiotic, were applied: Staphylococcus epidermidis ATCC 12228; Staphylococcus aureus ATCC 25923; Staphylococcus haemolyticus ATCC 29970; Streptococcus pneumoniae ATCC 49619; Streptococcus pyogenes ATCC 19615; Enterococcus faecalis ATTC 29212.
The study was performed according to the methodology recommended by the EUCAST (The European Committee on Antimicrobial Susceptibility Testing), using disc diffusion method with Mueller–Hinton Agar (MHA) and Mueller–Hinton Agar with 5% horse blood (MHS), respectively, in accordance to the conditions required for the particular bacteria growth. The inhibition zone was determined by the appropriate diameter measurement (in mm).
At first, the gel discs were excised, in sterile conditions, using appropriate template with 8 mm diameter. Then, 0.1 mL suspension of a density of 1.5 × 108 CFU/mL of the tested microorganisms was applied on the MHA medium (in case of Staphylococcus haemolyticus ATCC 29970, Staphylococcus aureus ATCC 25923, Staphylococcus epidermidis ATCC 12228, Enterococcus faecalis ATTC 29212) or MHS (in case of Streptococcus pyogenes ATCC 19615, Streptococcus pneumoniae ATCC 49619) and spread using a sterile glass rod on the surface of these media. The earlier prepared gel discs were placed on the so-prepared plates, under sterile conditions. On a single 10 cm diameter plate five discs were placed simultaneously (all 5 together), including the standard disc. All plates were incubated for 16–18 h at 36 ± 1 °C in accordance with the EUCAST recommendations. After incubation, the impact of the tested products on the growth of microorganisms was observed and measured by the diameter of inhibition zones. The obtained data were analyzed using non-parametric method – Kruskal–Wallis test. The results were considered significant at p value <0.05.
2.7. Toxicity of the materials studied
For bioassays pure cultures of Acutodesmus acuminatus (syn. Scenedesmus acuminatus) from the algal bank of Department of Hydrobiology, Faculty of Biology, Adam Mickiewicz University in Poznan were used. The culture of Acutodesmus acuminatus was grown in sterile conditions at 23–25 °C under artificial lighting with white light (4300 lux). The bioassays were carried out in flasks after inserting the culture medium (100 mL) with Acutodesmus acuminatus cells (stabilized phase of growth). Moreover, the toxicity bioassays were conducted with cladocerans Daphnia magna, which were cultivated in accordance with International Standard (ISO) recommendations. Daphnia bioassays were carried out in Petri dishes in water (100 mL) at temperature 20–21 °C under low exposure of light. Reference and bioassay samples contained 50 individuals of Daphnia magna.
The cultures of Acutodesmus acuminatus and Daphnia magna were treated with Vancomycin immobilized at HNTs_APTS (powder) and at HNTs_APTS encapsulated in alginate matrix (alginate discs). The amount of Acutodesmus acuminatus cells and Daphnia magna individuals was counted in the range of 0 to 72 h. A number of Acutodesmus acuminatus cells was counted under a light microscope (chamber of 0.2 mm × 0.0625 mm2), while a number of Daphnia magna individuals – under a stereoscopic microscope. The reference and bioassay tests were repeated three times. The results were averaged and expressed as mean ± SD.
3. Results and discussion
3.1. Characterization
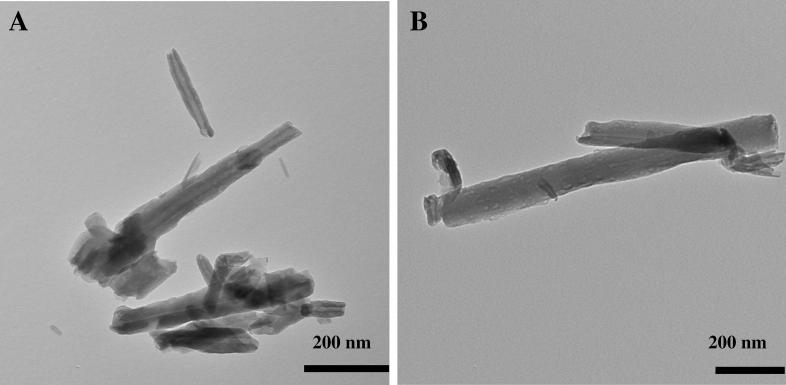
Halloysite material before and after functionalization with aminopropyl unit was characterized by several physicochemical methods. TEM imaging was used to observe morphological structure of the material studied. The exemplary TEM images of commercial HNTs and modified HNTs_APTS are shown in Fig. 2. The particles of the commercial inorganic support are characterized by cylindrical shape with a transparent central area, which indicates that the particles are hollow and open-ended. The halloysite tube (HNT) is approximately 600–1000 nm long (but some shorter tubes with a length of 200 nm are also present) and has an external diameter of 25–50 nm and internal diameter of 5–15 nm. The dimensions were determined based on statistical analysis of several TEM images by analyzing measurements for 100 nanotubes. HCl-treatment of commercial HNTs was carried out at room temperature. Sulphuric acid treatment of halloysite at higher temperatures can result in enlargement of halloysite lumen by etching of alumina (Abdullayev et al., 2012). However, for our purposes, the process was conducted under mild conditions in order to eliminate mineral impurities and disaggregate halloysite particles. After acid activation and organic functionalization, the tubular structure is preserved. However, the external diameter for HNTs_APTS tubes is wider than that for HNTs as a consequence of organic functionalization. The average outer diameter of HNTs_APTS tubes increased (40–80 nm) compared with pristine HNTs, while the length remained unchanged.
Fig. 2.
Transmission electron microscopy images (200 nm) of original halloysite HNTs (a) and modified HNTs_APTS (b).
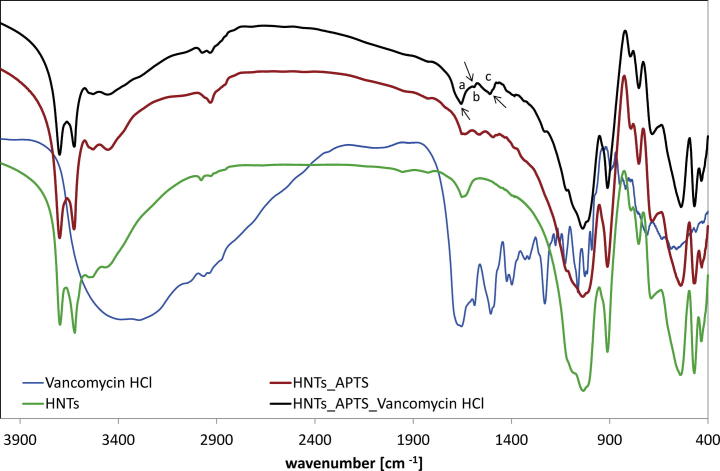
The presence of organic groups was confirmed by FTIR spectroscopy (Fig 3). Most of the signals are derived from the inorganic support. The bands at 3701 and 3624 cm−1 were assigned to the stretching vibrations of Al—OH. The signals at 3540 and 3451 cm−1 come from stretching and bending vibrations of water molecules. The bands corresponding to the stretching mode of Si—O appear at 1049, 779, 760 and 701 cm−1. The signal at 915 cm−1 originates from O—H deformation (Al—OH). The signals at 546 and 474 cm−1 correspond to the bending vibrations of Al—O—Si and Si—O—Si respectively. The signal at 436 cm−1 was assigned to the Si—O deformation mode. The band at 2940 cm−1, observed for the functionalized halloysite, was attributed to the C—H stretching vibrations of alkyl groups. The presence of primary amine in HNTs_APTS was manifested by the signal at 1553 cm−1 (bending vibrations of NH2). The presence of vancomycin was confirmed in the region below 1700 cm−1. The bands at 1607 and 1517 cm−1 could be attributed to aromatic adsorption. The band corresponding to the stretching mode of carbonyl in amide appears at 1663 cm−1.
Fig. 3.
FTIR spectra of halloysite before and after immobilization of Vancomycin, where bands at (a) 1663; (b) 1607; (c) 1517 cm−1 in HNTs_APTS_Vancomycin HCl were indicated.
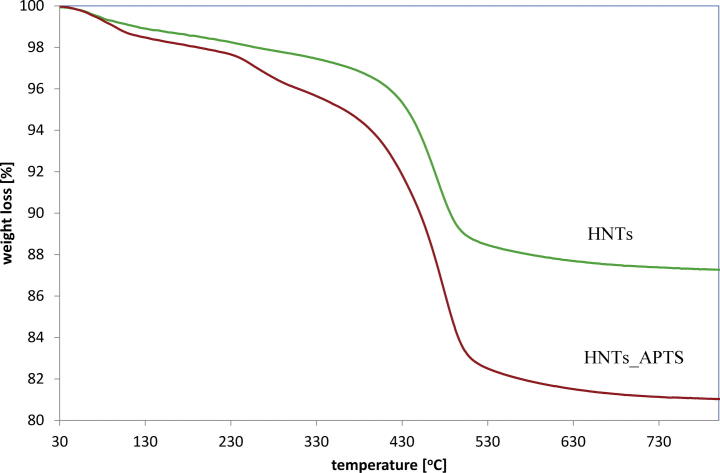
The thermogravimetric analysis also showed the differences between original and modified halloysite. The original HNTs show mass loss due to dehydration of physically adsorbed water and interlayer water, as well as dehydroxylation of aluminol groups. In the studied range (30–800 °C), the weight percentage of HNTs and HNTs_APTS decreased by about 11% and 18% respectively. The TG curves obtained by measuring the weight loss of the materials studied are shown in Fig 4. The amount of grafted APTS was calculated on the basis of elemental analysis (%C 3.467; %N 1.163; %H 2.456) and it amounted 0.83 mmol g−1. The molar ratio of C/N was 3, suggesting that complete hydrolysis of APTS occurred.
Fig. 4.
TGA curves of HNTs and HNTs_APTS.
3.2. Release analysis
Vancomycin was immobilized at three solid materials: HNTs, HNTs_HCl and HNTs_APTS. The amount of the antibiotic per unit weight of the carrier was chosen to be able to compare the results with those obtained for the silica (Kurczewska et al., 2015b). The antibiotic content in the solids was very similar for all samples and it amounted to approximately 100.0 mg per g of the inorganic support. The amount of drug loaded for each carrier was 102.0 ± 5.0 mg/g for HNTs, 95.0 ± 4.0 mg/g for HNTs_HCl and 97.0 ± 6.0 mg/g for HNTs_APTS (average ± standard deviation). The use of (3-Aminopropyl)-trimethoxysilane for the functionalization of halloysite surface was prompted by several factors. Firstly, it has been demonstrated (Peixoto et al., 2016) that on grafting of several organosilanes into HNTs, the APTS-functionalized halloysite showed the highest silylation efficiency. Secondly, the antibiotic molecule contains amino, carboxyl and phenolic groups. Therefore, in our previous studies we applied the silica surfaces functionalized with amine, diol and carboxyl groups for immobilization of the drug (Kurczewska et al., 2015b). However, only the silica with amine groups (Silica_APTS) demonstrated gradual instead of very rapid release of vancomycin. It was explained by different strength of bindings between vancomycin and the functional groups at the inorganic surface. Moreover, we assumed that as with other drugs having carboxyl groups (Tan et al., 2013), the presence of aminopropyl groups should ensure much stronger anchoring of the antibiotic (electrostatic interactions) as compared to the unmodified halloysite (hydrogen bonding).
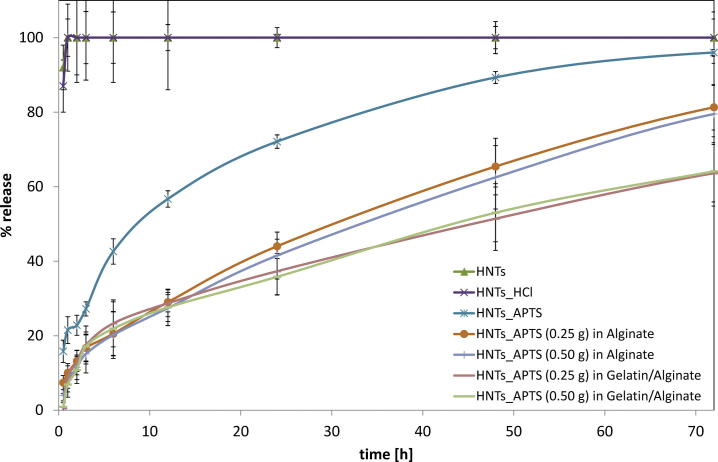
The total amount of the drug released within 6 days of the study coincides with the amount of the antibiotic immobilized at the inorganic carriers (HNTs, HNTs_HCl and HNTs_APTS). Therefore the amount of drug loaded in the unit weight of the support was the reference value for the study of the drug release versus time. At first in vitro release studies were carried out in order to determine which of the halloysite forms applied was the most conducive to the sustained release of the antibiotic (Fig 5). In systems with HNTs and HNTs_HCl a burst release of the drug was observed, in which about 90% of the drug was released in 30 min. When using APTS-functionalized halloysite, the release rate was different. After 30 min only 15% of the drug was released, while after 24 h, the amount of the antibiotic released from the carrier increased to 70%. This behaviour is in accordance with the assumption about a formation of strong interactions between amine groups in the carrier with the carboxyl groups in the antibiotic. For this reason, only vancomycin immobilized at HNTs_APTS was introduced into alginate-based matrices.
Fig. 5.
The release profiles of Vancomycin from HNTs_APTS in Alginate or Gelatin/Alginate matrix.
In order to prepare alginate-based dressings, either 0.25 g or 0.50 g of HNTs_APTS with immobilized vancomycin were used. The total amounts of drug loaded in dressings with double barrier (halloysite-alginate) were as follows: 17.6 ± 3.2 mg or 37.1 ± 4.5 mg (which corresponds to 5.0 mg of Vancomycin per g of dried material or 9.8 mg/g) in Alginate discs and 16.2 ± 4.2 mg or 39.8 ± 3.9 mg (3.2 mg/g or 7.5 mg/g) in Gelatin/Alginate discs. The different concentrations of the antibiotic per g of the dried discs are related to the absence or the presence of gelatin.
The materials studied with immobilized Vancomycin were stored at 4 °C. Due to the presence of the antibiotic in the complex formula, it is very important to determine the stability of the drug used on storage. For this purpose both the samples of HNTs_APTS and the alginate-based dressings were tested in order to determine the total amount of the drug released immediately after a sample preparation, and also after 10 and 30 days of storage. The values obtained were compared with the amount of loaded drug. It was found that after 10 days, Vancomycin released from each sample tested retained entirely original activity. However, after 30 days only for the antibiotic immobilized at HNTs_APTS, the amount of drug released remained unchanged. For alginate discs, a decrease in the amount of drug released was observed, but still about 90% of the drug remained active.
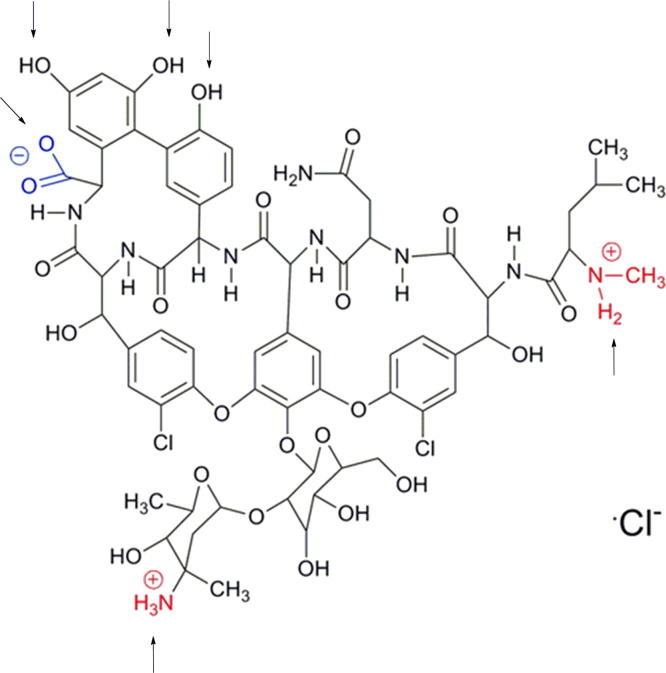
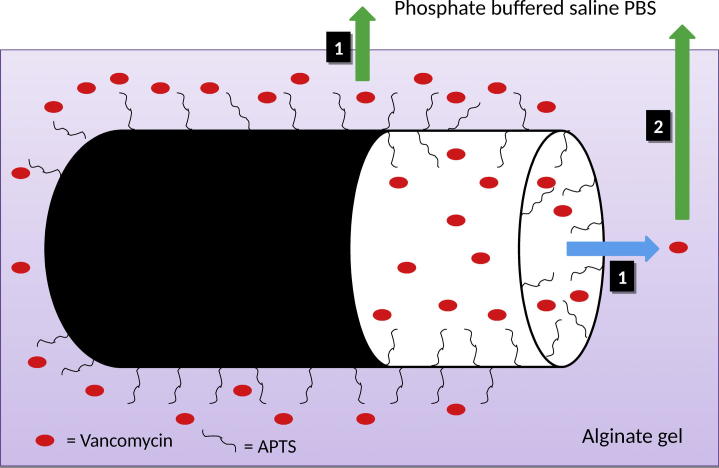
Further analysis concerned the character of the drug release rate form alginate-based dressings. On the basis of the results of vancomycin release (Fig 5), it can be observed that the difference in the dose of the drug in the alginate-based carriers had no effect on the transport rate of the antibiotic into the PBS medium. On the other hand, the systems with a smaller content of halloysite (0.25 g) were characterized by a lower stability when compared to those containing 0.50 g of the carrier in the alginate discs. The release profile of vancomycin from the systems studied with halloysite differs from that for the system with APTS-functionalized silica carrier. The release of the antibiotic immobilized at Silica_APTS encapsulated in alginate matrices was much slower when compared to that of the drug immobilized at APTS-functionalized silica without a gel carrier or encapsulated in free form in alginate matrices. Moreover, the addition of gelatin had an beneficial effect on slowing down the release of the drug from the complexed matrix. As expected, a similar behaviour was observed after replacing silica with halloysite with analogous functional groups (Kurczewska et al., 2015b). However, the amounts of the drug released in a time unit are much different for the systems with silica and with halloysite. After 24 h, the released amounts of vancomycin immobilized at Silica_APTS reached 70% or 60% for Alginate or Gelatin/Alginate discs, while for vancomycin immobilized at HNTs_APTS the released amount of the drug reached 44% or 37% for Alginate or Gelatin/Alginate discs. Furthermore, the shape of the curve of drug release for the systems examined in this work is different than that recorded for the systems based on silica. It appears that in the initial period (up to 6 h) the process of transport of drug through the double barrier occurs quite rapidly. Then the process is considerably slowed down and the character of the drug release is linear. One probable factor responsible for the observed differences is the structure of halloysite. As it is possible that functionalization of both the outer surface and the inner lumen can take place (Lun et al., 2014), we may assume that the antibiotic is immobilized not only on the outer surface but it also penetrates (at least partially) inside the carrier. Vancomycin has six pKa values, two basic and four acidic (Fig 6). It is amphotheric molecule that can react with acids and bases (Johnson and Yalkowsky, 2006). As a consequence of the silanization with APTS, the halloysite surface is positively charged, which should enhance adsorption. However, the loading efficiency for HNTs and HNTs_APTS was comparable. During the hydrolysis of APTS, some molecules of the silane can condense with each other and form oligomers. Therefore, it could contribute to less space in the lumen available for vancomycin loading. On the other hand, the introduction of APTS on halloysite surface influenced the release of the antibiotic. Vancomycin molecules are larger than other antibiotics (1.77 nm) but small enough to penetrate inside the lumen. On this basis we can propose the probable mechanism of the drug release (Fig 7). In the first phase, i.e. up to 6 h, the antibiotic derived primarily from the outer surface of halloysite is transported through the double barrier (indicated in the scheme as 1 and green arrow). Under this assumption and on the basis of release data, we can estimate that only about 20% of the drug is immobilized on the outer surface. The drug inside the lumen is initially released into the alginate gel (1 and blue arrow) and later it is transported into the medium PBS (2 and green arrow). A two-stage release of the antibiotic from the inner surface causes a significant reduction in the release rate of the active agent.
Fig. 6.
Structure of Vanocomycin; arrows represent functional groups responsible for six pKa values.
Fig. 7.
A schematic presentation of the mechanism proposed of Vancomycin release from HNTs_APTS encapsulated in alginate matrix.
In order to further study the release behaviour of the antibiotic, the in vitro release data were fitted to several models to determine the kinetics of drug release for the systems studied. The data were analysed using zero-order (3), first order (4), simplified Higuchi (5) and Korsmeyer-Peppas (6) models represented by the following equations (Dash et al., 2010, Costa and Lobo, 2001):
| (3) |
| (4) |
| (5) |
| (6) |
where Ft is the fraction of the drug released at time t in minutes, k – the release constant of the respective equation, n – the diffusion exponent.
The parameters of the release kinetics are presented in Table 1. Calculated correlation coefficients (R2) suggested that the release of Vancomycin from HNT_APTS followed first-order kinetics. This model is characteristic of water-soluble drugs in porous matrices. However, for the system with a double barrier, Higuchi equation showed very good correlation coefficients. This model finds application in polymeric matrix system, modified release pharmaceutical dosage forms and some transdermal systems. It is important that Higuchi and Korsmeyer-Peppas models can be applied only for amount of drug released < 60%. However, the application of Higuchi equation to the overall data again resulted in R2 above 0.980. This model describes drug release as a diffusion process. It was also confirmed by the diffusion exponent (n) value in Korsmeyer-Peppas model, which amounted n ≤ 0.5 (Fickian diffusion mechanism). Additionally, the observed Higuchi constants indicate that Vancomycin release from HNTs_APTS in Gelatin/Alginate is slightly slower (k = 0.9479 min−1/2) than that from HNTs_APTS in Alginate (k = 1.1074 min−1/2).
Table 1.
The kinetic parameters for the vancomycin release from the systems studied.
| Sample | Zero-order |
First-order |
Higuchi |
Korsmeyer-Peppas |
||||
|---|---|---|---|---|---|---|---|---|
| k | R2 | k | R2 | k | R2 | k | R2 | |
| HNT_APTS | 0.008 ± 0.006 | 0.853 | 0.001 ± 0.001 | 0.997 | 1.943 ± 0.225a | 0.979a | 3.885 ± 0.563a | 0.956a |
| 0.971 ± 0.530 | 0.866 | 5.054 ± 0.512 | 0.936 | |||||
| HNT_APTS in Alginate | 0.021 ± 0.004 | 0.848 | 0.001 ± 0.001 | 0.960 | 1.107 ± 0.116a | 0.995a | 1.435 ± 0.268a | 0.980a |
| 1.193 ± 0.124 | 0.997 | 1.206 ± 0.095 | 0.987 | |||||
| HNT_APTS in Gelatin/Alginate | 0.015 ± 0.009 | 0.763 | >0.001 ± 0.001 | 0.940 | 0.948 ± 0.207a | 0.986a | 1.058 ± 0.280a | 0.969a |
| 0.950 ± 0.238 | 0.995 | 1.224 ± 0.345 | 0.974 | |||||
Values obtained considering only drug released <60%.
3.3. Biological activity analysis
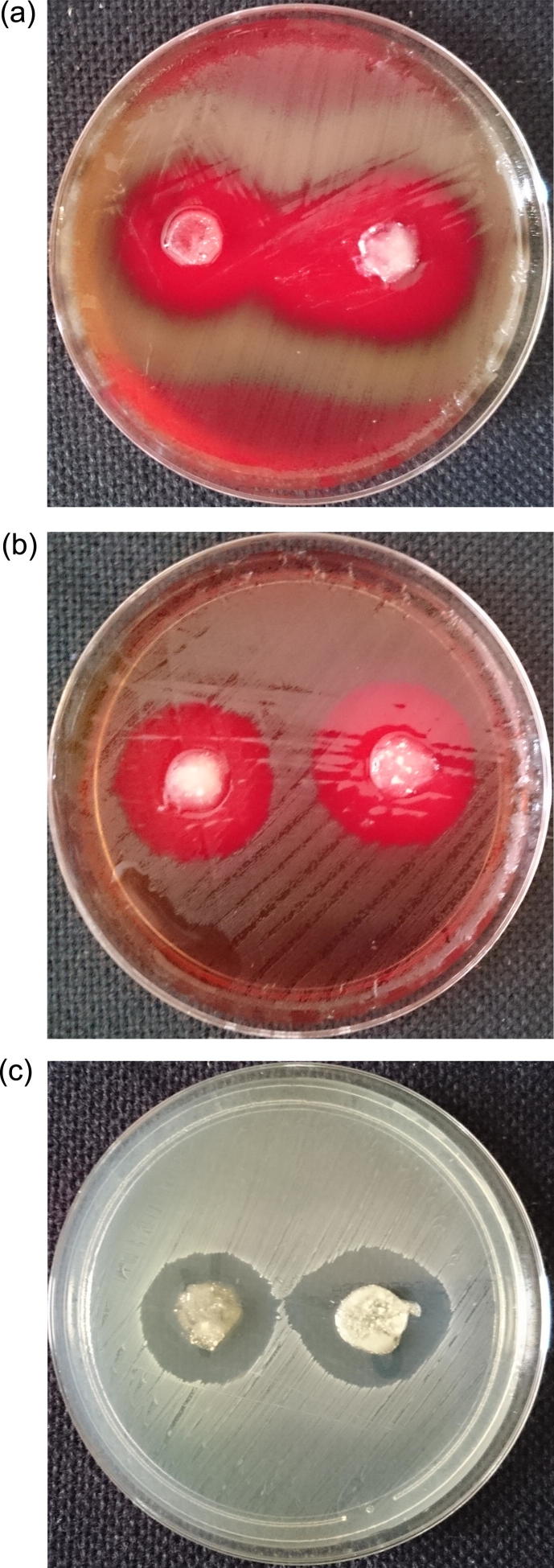
For microbiological studies gram positive bacteria were only evaluated. Vancomycin is dedicated to infections of cocci etiology. The newest EUCAST recommendation does not include vancomycin treatment of infections caused by gram-negative bacilli. The discs for the microbiological activity study have diameters of 10–13 mm. The sizes of the inhibition zones for vancomycin immobilized at HNTs_APTS in alginate-based matrices are presented in Table 2. The exemplary images showing anti-microbial activity of the material studied are shown in Fig 8.
Table 2.
The sizes of the inhibition zones in the samples with Vancomycin immobilized at the halloysite nanotubes.
| Sample | Inhibition zones ± standard deviation [mm] for bacteria tested |
||||||
|---|---|---|---|---|---|---|---|
| Staphylococcus epidermidis | Staphylococcus aureus | Staphylococcus haemolyticus | Streptococcus pneumoniae | Streptococcus pyogenes | Enterococcus faecalis | ||
| ATCC 12228 | ATCC 25923 | ATCC 29970 | ATCC 49619 | ATCC 19615 | ATTC 29,212 | ||
| Alginate | HNT-NH2 | 23.0 ± 2.5 | 21.0 ± 0.6 | 20.0 ± 0.6 | 28.0 ± 0.6 | 21.0 ± 0.6 | 19.0 ± 0.6 |
| HNT-NH2a | 21.0 ± 1.5 | 18.0 ± 0.6 | 20.0 ± 1.5 | 26.0 ± 1.5 | 21.0 ± 1.2 | 18.0 ± 1.0 | |
| Gelatin/Alginate | HNT-NH2 | 15.0 ± 1.5 | 17.0 ± 1.5 | 14.0 ± 2.0 | 20.0 ± 1.2 | 17.0 ± 1.0 | 14.0 ± 2.9 |
| HNT-NH2a | 13.0 ± 2.6 | 13.0 ± 1.5 | 14.0 ± 1.5 | 22.0 ± 1.0 | 16.0 ± 2.1 | 11.0 ± 3.2 | |
| Reference sample | 18.0 ± 0.6 | 18.0 ± 0.6 | 18.0 ± 1.2 | 26.0 ± 0.6 | 20.0 ± 0.6 | 17.0 ± 0.6 | |
The samples with twice lower weighted amount of halloysite with immobilized Vancomycin.
Fig. 8.
Images of inhibition zones in the samples with Vancomycin immobilized at HNTs_APTS (left side – 0.25 g; right side – 0.50 g) in Alginate for bacteria: (a) S. pneumoniae; (b) S. pyogenes; (c) E. faecalis.
All the gels tested showed antimicrobial activity. However, Alginate gels demonstrated greater inhibition zones in the bacteria tested than Gelatin/Alginate materials. For the staphylococci and enterococci bacteria, the inhibition zones of Alginate discs are generally greater than those of the Vancomycin standard disc. The only exception is the sample with 0.25 g of HNTs_APTS tested on S. aureas ATCC strain, for which the inhibition zones are the same as for the standard discs of the antibiotic studied. For S. pyogenes ATCC 19615 and S. pneumoniae ATCC 49619 strains the results are comparable to those for the reference standard discs. For the samples in Gelatin/Alginate gels, the inhibitions zones were smaller than those of the reference samples for all bacterial strains investigated. The differences in microbial activity between Alginate and Gelatin/Alginate gels are related to different release rates from these matrices. The samples were incubated for 16–18 h. Therefore, the amount of vancomycin released from Alginate gels on the first day is satisfactory. After addition of gelatin, the observed slowing down of the drug release can lead to the loss of the desired therapeutic effect. Furthermore, for the same sample type, but with different content of the active substance (antibiotic), no significant differences in biological activity were noted.
Statistical analysis was performed to evaluate the antibacterial activity of the examined gels. The six tested cocci strains were evaluated separately. The differences between the inhibition zone diameter and the disc diameter were calculated and statistically evaluated. Statistically significant activity of all tested gels was confirmed and determined at p value in range of 0.0218–0.0348.
3.4. Toxicity analysis
Toxicity of halloysite nanotubes can be investigated in vitro using human cell cultures ad microbial cells or in vivo using living organisms. The in vivo toxicity studies of HNTs can be carried out using different model organisms, like Caenorhabditis elegans nematode (Fakhrullina et al., 2015) or Paramecium caudatum (a model aqueous organism), (Kryuchkova et al., 2016).
In order to study toxicity of the materials presented in this paper, bioassays with Acutodesmus acuminatus and Daphnia magna were used. Daphnia magma are very often used in bioassays studies as they are very sensitive to any changes in the environment chemistry. Moreover, they provide important information about a potential impact of substances on human beings. Besides, Daphnia are transparent, thus it is possible not only to observe their death, but also other symptoms informing of their stress.
The bioassays were carried out for Vancomycin immobilized at HNTs_APTS (powder) and at HNTs_APTS encapsulated in alginate matrix (alginate discs). The samples studied did not affected the organisms tested. The average values of subsequent repetitions of each bioassay show a similar trend that was observed for a reference sample. A number of Acutodesmus acuminatus cells (×106/mL) and Daphnia magna individuals in 100 mL are presented in Table S1 in Supplementary Information (SI). It evidences no effect of the materials studied on the living organisms applied in the bioassays. The only difference observed when compared to the reference sample is a decreased mobility of Daphnia in samples with alginate discs due to increased density of the environment.
4. Conclusions
The model studied of the potential modern wound dressing, based on a double barrier with the antibiotic vancomycin as an antimicrobial agent, which was proposed as improvement to the earlier described one, was presented. After replacement of previously studied silica with halloysite nanotubes, the release rate of the drug decreased. However, an essential factor in preventing the burst process of releasing the immobilized drug was the presence of amine groups on the inorganic surface. The halloysite structure allows functionalization of exterior and interior surfaces with organic ligands. Thus, the immobilized drug is released at different rates, depending on where it is located in the carrier. This process takes longer than that of release of the drug deposited only on the outer surface. The modification of gel composition by the addition of gelatin had a favourable impact on the slowing down of the drug release from the dressing proposed. However, this change had a negative effect on the antimicrobial properties of the system studied. Although all the systems investigated, i.e. both Alginate and Gelatin/Alginate gels, showed antimicrobial properties, only the former gave satisfactory results. The reduction in the release rate of vancomycin from HNTs_APTS in Gelatin/Alginate gel was so large, that the antimicrobial activity results after 24 h were much worse than for the reference sample. Therefore, only vancomycin immobilized at HNTs_APTS and encapsulated in Alginate gel can be considered as a potential dressing material for treatment of wounds. The proposed new dressing exhibits high stability and neutral character in relation to the living organisms applied in the bioassays.
The results obtained indicate that the designed potential wound dressing with optimized parameters can be effective strategy in long-term treatment of wounds. The system with a double barrier can be further examined to become more versatile by encapsulation of various drugs applied in a wound healing process.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors declare that they have no personal financial or non-financial conflicts of interest.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jsps.2017.02.007.
Appendix A. Supplementary material
References
- Abdullayev E., Joshi A., Wei W., Zhao Y., Lvov Y. Enlargement of halloysite clay nanotube lumen by selective etching of aluminum oxide. ACS Nano. 2012;6(8):7216–7226. doi: 10.1021/nn302328x. [DOI] [PubMed] [Google Scholar]
- Abdullayev E., Lvov Y.M. Halloysite clay nanotubes as a ceramic “skeleton” for functional biopolymer composites with sustained drug release. J. Mater. Chem. B. 2013;1:2894–2903. doi: 10.1039/c3tb20059k. [DOI] [PubMed] [Google Scholar]
- Aguazzi C., Viseras C., Cerezo P., Salcedo I., Sanchez-Espejo R., Valenzuela C. Release kinetics of 5-aminosalicylic acid from halloysite. Coll. Surf. B Biointerf. 2013;105:75–80. doi: 10.1016/j.colsurfb.2012.12.041. [DOI] [PubMed] [Google Scholar]
- Boateng J.B., Matthews K.H., Stevens H.N.E., Eccleston G.M. Wound healing dressings and drug delivery systems. J. Pharm. Sci. 2008;97(8):2892–2922. doi: 10.1002/jps.21210. [DOI] [PubMed] [Google Scholar]
- Chao C., Liu J., Wang J., Zhang Y., Zhang B., Zhang Y., Xiang X., Chen R. Surface modification of halloysite nanotubes with dopamine for emzyme immobilization. ACS Appl. Mater. Interf. 2013;5:10559–10564. doi: 10.1021/am4022973. [DOI] [PubMed] [Google Scholar]
- Chiew C.S.C., Poh P.E., Pasbakhsh P., Tey B.T., Yeoh H.K., Chan E.S. Physicochemical characterization of halloysite/alginate bionanocomposite hydrogel. Appl. Clay Sci. 2014;101:444–454. [Google Scholar]
- Costa P., Lobo J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001;13:123–133. doi: 10.1016/s0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]
- Dash S., Murthy P.N., Nath L., Chowdhury P. Kinetic modeling drug delivery release from controlled drug delivery systems. Acta Polon. Pharm. Drug. Res. 2010;67:217–223. [PubMed] [Google Scholar]
- Du M., Guo B., Jia D. Newly emerging applications of halloysite nanotubes: a review. Polym. Inter. 2010;59:574–582. [Google Scholar]
- Fakhrullina G.I., Akhatova F.S., Lvov Y.M., Fakhrullin R. Toxicity of halloysite clay nanotubes in vivo: a Caenorhabditis elegans study. Environ. Sci. Nano. 2015;2:54–59. [Google Scholar]
- Hamidi M., Azadi A., Rafiei P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008;60:1638–1649. doi: 10.1016/j.addr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Johnson J.L.H., Yalkowsky S.H. Reformulation of a new vancomycin analog: an example of the importance of buffer species and strength. AAPS Pharm. Sci. Tech. 2006;7(1):E33–E37. doi: 10.1208/pt070105. [DOI] [PubMed] [Google Scholar]
- Joussein E., Petit S., Churchman J., Theng B., Righi D., Delvaux B. Halloysite clay minerals – a review. Clay Miner. 2005;40:383–426. [Google Scholar]
- Kamble R., Ghag M., Gaikawad S., Panda B.K. Halloysite nanotubes and applications: a review. J. Adv. Sci. Res. 2012;3(2):25–29. [Google Scholar]
- Karnik S., Hines K., Mills D.K. Nanoenhanced hydrogel system with sustained release capabilities. J. Biomed. Mater. Res. 2015;103(7):2416–2426. doi: 10.1002/jbm.a.35376. [DOI] [PubMed] [Google Scholar]
- Kryuchkova M., Danilushkina A., Lvov Y., Fakhrullin R. Evaluation of toxicity of nanocalys and grapheme oxide in vivo: a Paramecium caudatum study. Environ. Sci. Nano. 2016;3:442–452. [Google Scholar]
- Kurczewska J., Sawicka P., Ratajczak M., Gajęcka M., Schroeder G. Vancomycin-modified silica: synthesis, controlled release and biological activity of the drug. Int. J. Pharm. 2015;486:226–231. doi: 10.1016/j.ijpharm.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Kurczewska J., Sawicka P., Ratajczak M., Gajęcka M., Schroeder G. Will the use of double barrier result in sustained release of vancomycin? Optimization of parameters for preparation of a new antibacterial alginate-based modern dressing. Int. J. Pharm. 2015;496:526–533. doi: 10.1016/j.ijpharm.2015.10.075. [DOI] [PubMed] [Google Scholar]
- Lakes A.L., Peyyala R., Ebersole J.L., Puleo D.A., Zach Hilt J., Dziubla T.D. Synthesis and characterization of an antibacterial hydrogel containing covalently bound vancomycin. Biomacromolecules. 2014;15:3009–3018. doi: 10.1021/bm5006323. [DOI] [PubMed] [Google Scholar]
- Levis S.R., Deasy P.B. Characterisation of halloysite for use as a microtubular drug delivery system. Int. J. Pharm. 2002;243:125–134. doi: 10.1016/s0378-5173(02)00274-0. [DOI] [PubMed] [Google Scholar]
- Li H., Zhu X., Xu X., Peng W., Zhong S., Wang Y. The combination of adsorption by functionalized halloysite nanotubes and encapsulation by polyelectrolyte coatings for sustained drug delivery. RCS Adv. 2016;6:54463–54470. [Google Scholar]
- Lin H.R., Ou L.H., Lin Y.J., Ling M.H. Hollow, pH-sensitive calcium-alginate/poly(acrylic acid) hydrogel beads as drug carriers for vancomycin release. J. Appl. Polym. Sci. 2010;118:1878–1886. [Google Scholar]
- Lin S.S., Uang S.W.N., Lee S.S., Chan E.C., Chen K.T., Yang C.Y., Chen C.Y., Chan Y.S. In vitro elution of antibiotic from antibiotic-impregnated biodegradable calcium alginate wound dressing. J. Trauma Inj. Inf. Crit. Care. 1999;47:136–141. doi: 10.1097/00005373-199907000-00027. [DOI] [PubMed] [Google Scholar]
- Lun H., Ouyang J., Yang H. Natural halloysite nanotubes modified as an aspirin carrier. RCS Adv. 2014;4(83):44197–44202. [Google Scholar]
- Lvov Y.M., Abdullayev E. Functional polymer–clay nanotube composites with sustained release of chemical agents. Prog. Polym. Sci. 2013;38(10–11):1690–1719. [Google Scholar]
- Lvov Y.M., DeVilliers M.M., Fakhrullin R.F. The application of halloysite tubule nanoclay in drug delivery. Expert Opin. Drug Deliv. 2016;13(7):977–986. doi: 10.1517/17425247.2016.1169271. [DOI] [PubMed] [Google Scholar]
- Lvov Y.M., Shchukin D.G., Mohwald H., Price R.R. Halloysite clay nanotubes for controlled release of protective agents. ACS Nano. 2008;2(5):814–820. doi: 10.1021/nn800259q. [DOI] [PubMed] [Google Scholar]
- Lvov Y.M., Wang W., Zhang L., Fakhrullin R. Halloysite clay nanotubes for loading and sustained release of functional compounds. Adv. Mater. 2016;28(6):1227–1250. doi: 10.1002/adma.201502341. [DOI] [PubMed] [Google Scholar]
- Massaro M., Amorati R., Cavallaro G., Guernelli S., Lazzara G., Milioto S., Noto R., Poma P., Riela S. Direct chemical grafted curcumin on halloysite nanotubes as dual-responsive prodrug for pharmacological applications. Colloids Surf. B. 2016;140:505–513. doi: 10.1016/j.colsurfb.2016.01.025. [DOI] [PubMed] [Google Scholar]
- Massaro M., Colletti C.G., Noto R., Riela S., Poma P., Guernelli S., Parisi F., Milioto S., Lazzara G. Pharmaceutical properties of supramolecular assembly of co-loaded cardanol/triazole-halloysite systems. Int. J. Pharm. 2015;478(2):476–485. doi: 10.1016/j.ijpharm.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Massaro M., Piana S., Colletti C.G., Noto R., Riela S., Baiamonte C., Giordano C., Pizzolanti G., Cavallaro G., Milioto S., Lazzara G. Multicavity halloysite–amphiphilic cyclodextrin hybrids for co-delivery of natural drugs into thyroid cancer cells. J. Mater. Chem. B. 2015;3:4074–4081. doi: 10.1039/c5tb00564g. [DOI] [PubMed] [Google Scholar]
- Massaro M., Riela S., Baiamonte C., Blanco J.L.J., Giordano C., Lo Meo P., Milioto S., Noto R., Parisi F., Pizzolanti G., Lazzara G. Dual drug-loaded halloysite hybrid-based glycocluster for sustained release of hydrophobic molecules. RSC Adv. 2016;6:87935–87944. [Google Scholar]
- Massaro M., Riela S., Lo Meo P., Noto R., Cavallaro G., Milioto S., Lazzara G. Functionalized halloysite multivalent glycocluster as a new drug delivery system. J. Mater. Chem. B. 2014;2:7732–7738. doi: 10.1039/c4tb01272k. [DOI] [PubMed] [Google Scholar]
- Molina-Manso D., Manzano M., Doadrio J.C., Del Prado G., Ortiz-Perez A., Vallet-Regi M., Gomez-Barrena E., Esteban J. Usefulness of SBA-15 mesoporous ceramics as a delivery system for vancomycin, rifampicin and linezolid: a preliminary report. Int. J. Antimicrob. Ag. 2012;40:252–256. doi: 10.1016/j.ijantimicag.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Naumenko E.A., Guryanov I.D., Yendluri R., Lvov Y.M., Fakhrullin R.F. Clay nanotube-biopolymer composite scaffolds for tissue engineering. Nanoscale. 2016;8:7257–7271. doi: 10.1039/c6nr00641h. [DOI] [PubMed] [Google Scholar]
- Ovington L.G. Advances in wound dressings. Clin. Dermatol. 2007;25:33–38. doi: 10.1016/j.clindermatol.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Pasbakhsh P., Churchman G.J., editors. Natural Mineral Nanotubes: Properties and Applications. first ed. CRC Press (Taylor&Francis Group), Boca Raton and Apple Academic Press; Oakwille: 2015. [Google Scholar]
- Peixoto A.F., Fernandes A.C., Peraira C., Pires J., Freire C. Physicochemical characterization of organosilylated halloysite clay nanotubes. Micropor. Mesopor. Mater. 2016;219:145–154. [Google Scholar]
- Peppas N.A., Bures P., Leobandung W., Ichikawa H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000;50:27–46. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- Price R.R., Gaber B.P., Lvov Y.M. In-vitro release characteristics of tetracycline HCl, khellin and nicotinamide adenine dineculeotide from halloysite; a cylindrical mineral. J. Microencapsul. 2001;18(6):713–722. doi: 10.1080/02652040010019532. [DOI] [PubMed] [Google Scholar]
- Qi G., Li L., Yu F., Wang H. Vancomycin-modified mesoporous silica nanoparticles for selective recognition and killing of pathogenic gram-positive bacteria over macrophage-like cells. ACS Appl. Mater. Interf. 2013;5:10874–10881. doi: 10.1021/am403940d. [DOI] [PubMed] [Google Scholar]
- Ravelingien M., Mullens S., Luyten J., D’Hondt M., Boonen J., De Spiegeleer B., Coenye T., Vervaet C., Remon J.P. Vancomycin release from poly(d,l-lactic acid) spray-coated hydroxyapatite fibres. Eur. J. Pharm. Biopharm. 2010;76:366–370. doi: 10.1016/j.ejpb.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Rawtani D., Agrawal Y.K. Multifarious applications of halloysite nanotubes: a review. Rev. Adv. Mater. Sci. 2012;30:282–295. [Google Scholar]
- Tan D., Yuan P., Annabi-Bergaya F., Yu H., Liu D., Liu H., He H. Natural halloysite nanotubes as mesoporous carriers for the loading of ibuprofen. Micropor. Mesopor. Mater. 2013;179:89–98. [Google Scholar]
- Thu H.E., Ng S.F. Gelatine enhances drug dispersion in alginate bilayer film via the formation of crystalline microaggregates. Int. J. Pharm. 2013;454:99–106. doi: 10.1016/j.ijpharm.2013.06.082. [DOI] [PubMed] [Google Scholar]
- Thu H.E., Zulfakar M.H., Ng S.F. Alginate based bilayer hydrocolloidal films as potential slow-release modern wound dressing. Int. J. Pharm. 2012;434:375–383. doi: 10.1016/j.ijpharm.2012.05.044. [DOI] [PubMed] [Google Scholar]
- Vergaro V., Abdullayev E., Lvov Y.M., Zeitoun A., Cingolani R., Rinaldi R., Leporatti S. Cytocompatibility and uptake of halloysite clay nanotubes. Biomacromolecules. 2010;11(3):820–826. doi: 10.1021/bm9014446. [DOI] [PubMed] [Google Scholar]
- Vergaro V., Lvov Y.M., Leporatti S. Halloysite clay nanotubes for resveratrol delivery to cancer cells. Macromol. Biosci. 2012;12(9):1265–1271. doi: 10.1002/mabi.201200121. [DOI] [PubMed] [Google Scholar]
- Wang Q., Zhang J., Zheng Y., Wang A. Adsorption and release of ofloxacin from acid- and heat- treated halloysite. Coll. Surf. B Biointerf. 2014;113:51–58. doi: 10.1016/j.colsurfb.2013.08.036. [DOI] [PubMed] [Google Scholar]
- Xue J., Niu Y., Gong M., Shi R., Chen D., Zhang L., Lvov Y. Electrospun microfiber membranes embedded with drug-loaded clay nanotubes for sustained antimicrobial protection. ACS Nano. 2015;9(2):1600–1612. doi: 10.1021/nn506255e. [DOI] [PubMed] [Google Scholar]
- Zhang L.F., Yang D.J., Chen H.C., Sun R., Xu L., Xiong Z.C., Govender T., Xiong C.D. An ionically crosslinked hydrogel containing vancomycin coating on a porous scaffold for drug delivery and cell culture. Int. J. Pharm. 2008;353:74–87. doi: 10.1016/j.ijpharm.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zhang X., Wang Y., Wu Z., An J., Lu Z., Mei L., Li C. In situ cross-linked polysaccharide hydrogel as extracellular matrix mimics for antibiotics delivery. Carbohydr. Polym. 2014;105:63–69. doi: 10.1016/j.carbpol.2014.01.068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.