Abstract
Depression is a debilitating psychiatric condition that remains the second most common cause of disability worldwide. Currently, depression affects more than 4 per cent of the world’s population. Most of the drugs intended for clinical management of depression augment the availability of neurotransmitters at the synapse by inhibiting their neuronal reuptake. However, the therapeutic efficacy of antidepressants is often compromised as they are unable to reach brain by the conventional routes of administration. The purpose of the present study was to reconnoiter the potential of mucoadhesive PLGA-chitosan nanoparticles for the delivery of encapsulated Desvenlafaxine to the brain by nose to brain delivery route for superior pharmacokinetic and pharmacodynamic profile of Desvenlafaxine. Desvenlafaxine loaded PLGA-chitosan nanoparticles were prepared by solvent emulsion evaporation technique and optimized for various physiochemical characteristics. The antidepressant efficacy of optimized Desvenlafaxine was evaluated in various rodent depression models together with the biochemical estimation of monoamines in their brain. Further, the levels of Desvenlafaxine in brain and blood plasma were determined at various time intervals for calculation of different pharmacokinetic parameters. The optimized Desvenlafaxine loaded PLGA-chitosan nanoparticles (∼172 nm/+35 mV) on intranasal administration significantly reduced the symptoms of depression and enhanced the level of monoamines in the brain in comparison with orally administered Desvenlafaxine. Nose to brain delivery of Desvenlafaxine PLGA-chitosan nanoparticles also enhanced the pharmacokinetic profile of Desvenlafaxine in brain together with their brain/blood ratio at different time points. Thus, intranasal mucoadhesive Desvenlafaxine PLGA-chitosan nanoparticles could be potentially used for the treatment of depression.
Keywords: PLGA, Nanoparticles, Desvenlafaxine, Depression, Brain, Intranasal
Abbreviations: CNS, central nervous system; FST, forced swim test; BBB, blood brain barrier; BSF, blood-cerebrospinal fluid barrier; i.n., intranasal; 5 HT, 5-hydroxytryptamine; HCl, hydrochloric acid; PDI, poly dispersity index; PLGA, poly(lactic-co-glycolic acid); DVF, Desvenlafaxine; CN, chitosan; PVA, polyvinyl alcohol
1. Introduction
Depression is a debilitating mental disorder, characterized by low mood, hopelessness, sadness, low self-esteem, disturbed sleep and often with suicidal thoughts (Kircanski et al., 2012, Lenox and Frazer, 2010). As per current WHO report, depression is one of the leading causes of disability affecting more than 350 million people worldwide (Marcus et al., 2012). The World Mental Health Survey conducted in 17 countries reported that on average 1 in 20 people have suffered an episode of depression (WHO, 2015). Depression has also been held responsible for suicides which translate to 1 million lives per year (Marcus et al., 2012). The primary cause of depression is the disturbances in the monoaminergic i.e. norepinephrine, serotonin, dopamine transmission in brain due to complex interaction of several social, psychological and biological factors (Kircanski et al., 2012, Lenox and Frazer, 2010). Therefore, most of the drugs intended for treatment of depression enhance the availability of monoamines at the synapse by various mechanisms. Nevertheless, the therapeutic efficacy of these antidepressants is dependent on their continuous prolonged presence at the site of action in brain (Kircanski et al., 2012, Lenox and Frazer, 2010, Nutt, 2008). Currently antidepressants are mainly delivered by oral route due to chronic nature of treatment across several days. However, the efficacy of oral antidepressants is limited due to their inability to reach the brain effectively from systemic circulation as the blood brain barrier (BBB) and blood-cerebrospinal fluid (BSF) barrier restrict the transport of drugs from systemic circulation into the central nervous system (CNS) (Kilts, 2003). Also, the variation in plasma drug concentration following oral delivery leads to adverse effects, loss of efficacy and intolerability (Kilts, 2003). For many decades, different types of antidepressants such as tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRI), and serotonin and norepinephrine reuptake inhibitors (SNRI) have been used for treatment of depression but the remission rate after the first trial of an antidepressant is <30%, that continues to decline after a first antidepressant failure (Kircanski et al., 2012, http://mentalhealthdaily.com). Therefore, scientists and pharmaceutical companies have been competing to develop effective antidepressant drug capable of providing quick and prolonged remission. This is reflected by a series of new antidepressant drugs approved by FDA in the past 3 years such as vilazodone, levomilnacipran, vortioxetine and 20 more currently under clinical trials (http://mentalhealthdaily.com). These facts provide a strong rationale for the development of intranasal nanoformulation that can transport the antidepressant drug to the brain non-invasively and increase its brain concentration reducing the dosage, side effects and intolerability. In past few years intranasal (i.n.) nose to brain delivery has emerged as a novel technique for transporting therapeutic agents to the CNS (Kumar et al., 2008a, Kumar et al., 2008b). Nose to brain drug delivery is possible due to unique connection provided by the olfactory and/or trigeminal nerve system present between the olfactory epithelium and the CNS, bypassing the BBB (Kumar et al., 2008a, Kumar et al., 2008b). Recently, a large number of publications have reported the nose to brain delivery of many drugs due to its obvious benefits e.g. avoidance of BBB and hepatic first pass metabolism, non-invasiveness and ease of administration (Kumar et al., 2008a, Kumar et al., 2008b, Al-Ghananeem et al., 2010, Md et al., 2012).
In the present study Desvenlafaxine succinate (DVF), a second generation SNRI, was selected as the drug candidate for nose to brain delivery. Desvenlafaxine is an active metabolite of venlafaxine with an oral bioavailability of 80% and plasma half life of 11 h. Although Desvenlafaxine has better serotonin:norepinephrine ratio (10:1) than Venlafaxine (30:1), oral therapy is associated with a number of side effects such as increased blood pressure and heart rate, constipation, agitation, tremor, sweating, nausea, headache, and sleep disturbances (Mann, 2005, Perry and Cassagnol, 2009). For development of drug delivery system, biodegradable polymeric nanoparticles composed of polylactide-co-glycolide (PLGA) and chitosan (CN) were optimized. PLGA-CN nanoparticles are biodegradable, biocompatible and bioadhesive in nature to extend the residence time of drug in the nasal cavity and limit its nasal mucociliary clearance for better absorption across nasal epithelium including the olfactory regions for nose to brain delivery (Pawar et al., 2010).
The purpose of the present study was to optimize a nano-sized drug delivery system using PLGA and CN for intranasal nose to brain delivery of DVF and evaluate its antidepressant efficacy by pharmacodynamic and biochemical studies in rodents. The other objective of the present work was to evaluate the pharmacokinetics of DVF loaded PLGA-CN nanoparticles in brain and plasma, respectively for correlation with the outcomes of pharmacodynamic and biochemical studies.
2. Materials and methods
2.1. Materials
Poly (D,L-lactic-co-glycolic) (50:50, MW 9–12 KDa, carboxylic acid terminated) was purchased from Shandong Institute of Medical Instrument (Jinan, Shandong, China). DVF succinate was obtained from Chengdu-Kaijie Biopharm Co. Ltd (Chengdu, China). Polyvinyl alcohol (PVA, Av. MW 30–70 KDa), low MW Chitosan (CN, ∼75–85% degree of deacetylation, MW 50–190 KDa) and crude pig mucin (PM) were purchased from Sigma Chemical Co. All the other chemicals and reagents used were of the analytical grade.
2.2. Animals
Adult male Wistar rats (aged 6–8 weeks) weighing ∼250 g were selected for pharmacodynamic, biochemical and brain pharmacokinetic studies. The current study was approved by the Research review and ethics board (RREB), Tianjin Huanhu Hospital, P.R. China. All animal experiments were carried out as per the obligation of the national act for the use of experimental animals (People's Republic of China).
2.3. Preparation of PLGA-CN nanoparticles
PLGA-CN nanoparticles were prepared by emulsion solvent evaporation method (Wang et al., 2013). Briefly 100 mg of PLGA was dissolved in 2.5 mL of chloroform with or without DVF (30% w/w) and added to 10 ml aqueous phase (0.5% acetic acid solution with pH range of 4.6–4.8) containing 0.5% PVA and 0.5% CN. The primary emulsion was vortexed for 90 s and then sonicated by a probe sonicator at 50 W (Q700 Sonicator, CT USA) for 60 s on ice. The chloroform was removed by rotary evaporator under partial vacuum. The nanoparticles were obtained by ultracentrifugation (23,000g, 18 min at 4 °C, Beckmann coulter, USA). The concentrated nanoparticles were freeze-dried using mannitol as cryoprotectant (2.5% w/w).
2.4. Characterization of PLGA-CN
PLGA-CN nanoparticles were characterized for particle size and size distribution, zeta potential entrapment efficiency (EE) and drug loading (DL). The particle size, size distribution and zeta potential were measured by Zetasizer (model: Nano ZS, Malvern Instruments, UK). The particle size of the optimized formulation was also determined using transmission electron microscopy (TEM, Morgagni 268D, USA). The EE and DL were determined by the separation of free DVF from the DVF associated with PLGA-CN nanoparticles by ultracentrifugation at 45,000g for 30 min. The entrapment efficiency and drug loading were calculated using equations as given below:
The in vitro release of DVF from optimized formulation was determined by dialysis bag (MWCO 12 KDa; Sigma-Aldrich) dipped in a dissolution apparatus filled with phosphate buffer at pH 7.4 and pH 6.0 (at 37 °C ± 0.5 °C). At predetermined sampling time points, a 2 mL aliquot was withdrawn for analysis and replaced with equal amount of fresh phosphate buffer till 24 h. DVF was determined in the samples by reverse-phase HPLC with UV detection at 230 nm using mobile phase containing a mixture of buffer and acetonitrile in ratio of 70: 30. The buffer consists of 10 mM potassium dihydrogen phosphate and 2 mM 1-octane sulphonic acid sodium salt (pH 6.0) (Rao et al., 2014).
2.5. Mucoadhesive potency of nanoparticles
The mucoadhesive potency of nanoparticles was determined by their mucin binding efficacy as described by Yin and associates (Yin et al., 2006). Briefly, 2 ml of porcine mucin suspension (0.5 mg/ml) in phosphate buffer (pH7.4) was incubated with same volume of nanoparticle suspension at 37 °C for 60 min. After the incubation period, the samples were centrifuged at 65,000g for 20 min. The free mucin in the supernatant was measured by UV spectrometry (251 nm) and the mucin binding efficiency was determined by equation mentioned below.
2.6. Pharmacodynamic studies
2.6.1. Stress induced model
2.6.1.1. Forced swimming test
The forced swimming test was carried out to access and compare the antidepressant efficacy of intranasal DVF PLGA-CN NPs with that of oral/intranasal DVF (Porsolt et al., 1978, Kitada et al., 1981). In brief, rats were allowed to swim in a cylindrical glass vessel filled with water to 30 cm mark in order to force them to swim and/or float without support of tail and hindlimbs. Two swimming session were conducted, a 15 min pretest before the drug administration to acclimatize and train the rats for the test situation. The test session was conducted for 5 min on day 16 following an hour after the final dose. Test sessions were videotaped and behavioural swimming, climbing and immobility were recorded. The locomotor activity of all the four groups was recorded by digital photoactometer in the closed box (30 cm × 30 cm) equipped with infrared light sensitive photocells on 16th day 0.5 h after the test session. The activity was expressed as total counts for 5 min per rat. For FST the rats were sub-divided in four groups with six rats in each group. The doses were calculated by extrapolating the therapeutic dose of humans (50 mg/kg/day, oral bioavailability ∼80%) to rats on the basis of body surface area using dose conversion factor of 0.018 from human to rats (Paget and Barnes, 1964). Group 1 and group 2 rats were administered DVF PLGA-CN NPs (equivalent to 5 mg/kg/day DVF) and DVF solution (equivalent to 5 mg/kg/day) intranasally for 16 days, respectively. Group 3 was administered DVF solution (0.5 ml, equivalent to 5 mg/kg/day) by oral gavage for 16 days. Group 4 served as control and treated with normal saline solution (100 μl) intranasally for 16 days. For intranasal route, DVF/DVF PLGA-CN nanoparticles were dissolved/dispersed in 100 μl of normal saline and administered (50 μl/nostril) with the help of polyethylene tube (0.1 mm) positioned 5 mm deep in each nostril. The rats were anaesthetised before dosing and closely monitored till recovery. All the test groups were compared with control group.
2.6.2. Drug induced model
2.6.2.1. Reserpine reversal test (RRT)
Among the drug induced models of behavioural depression, the reversal of reserpine induced immobility, ptosis, hypothermia and sedation is used to measure the effectiveness of antidepressants (Costa et al., 1960). Similar to FST, the rats were divided into four groups with six rats in each group and administered with the same doses of each formulation. As per the method described by Costa et al., each group of rats was administered reserpine solution (2 mg/kg) intraperitoneally (Costa et al., 1960). Following an hour, the experimental groups received their respective treatments and the reduction in reserpine induced palpebral ptosis, diarrhoea, hypothermia and immobility was measured in individual rats of each group at 2, 4 and 8 h after the treatment, respectively. The diarrhoea and ptosis were measured by arbitrary scores and rated between 0, 1 and 2 with 0 for no symptoms, 2 for severe symptom and 1 for intermediate.
2.7. Biochemical estimation of serotonin, noradrenaline and dopamine
The rats were sacrificed after pharmacodynamic studies and the levels of three neurotransmitters i.e. serotonin, noradrenaline and dopamine were estimated in brain by a modified Schlumpf and coworkers method (Schlumpf et al., 1974). In the first step rat brain tissue extract was prepared. Briefly, the brain of rats was extracted and the subcortical parts of brain was separated from the cortex and weighed. The weighed tissue was homogenized using 4 ml of HCl-butanol (0.37:1) at 0 °C. The sample was centrifuged at 1150g at 0 °C for 10 min. An aliquot of supernatant (1 ml) was removed and added to heptane-HCl (1.5–0.3 ml, 0.1 M) at 0 °C. Following 10 min of vortexing, the tube was centrifuged at 700g rpm for 10 min at 0 °C. The aqueous layer (0.25 ml) was used for determination of the neurotransmitter as described below.
For estimation of serotonin (5 HT), O-phthaldialdehyde reagent (0.3 ml, strength-20 mg/100 ml conc. HCl) was added to aqueous phase (0.25 ml) as described in previous stage and heated at 100 °C for 10 min. All the samples were bought to room temperature and the absorbance was measured at 360–470 nm (excitation/emission) in the spectrofluorimeter (Spectrofluorimeter RF5301-PC, Shimadzu, Japan). The blank tissue sample was prepared by the same procedure but without the addition of O-phthaldialdehyde reagent.
For estimation of nor-adrenaline and dopamine, HCl (0.06 ml, 0.4 M) and EDTA sodium acetate buffer (0.1 ml, pH 6.9) were added to fresh aqueous phase (0.25 ml). Further, the samples were oxidized by adding 0.15 ml iodine solution (0.1 ml in ethanol). After 2 min, the reaction was blocked with 0.15 ml of Na2SO3 solution [Na2SO3 (0.5 g in 2 ml H2O) + NaOH (18 mL, 5 M)]. After 15 min, 0.1 ml of acetic acid (10 M) was added to the reaction mixture and heated at 100 °C for 6 min. The samples were bought to room temperature and absorbance was recorded at 395–485 nm for nor-adrenaline and 330–375 nm for dopamine. The blank samples were obtained by adding together all the reagents of the oxidation step in reversed order (Na2SO3 solution before iodine).
The internal standards were prepared by adding 250 μg of serotonin, dopamine and noradrenaline in 0.5 ml of distilled water: HCl-butanol (1:2). For internal reagent blank samples distilled water: HCl-butanol (1:2) was used.
2.8. Blood and brain pharmacokinetics
The blood and brain pharmacokinetics were performed as per experimental technique described previously (Al-Ghananeem et al., 2010, Md et al., 2012). Group 1 rats were administered DVF PLGA-CN NPs (equivalent to 5 mg/kg/day DVF) intravenously by tail vein. Groups 2 and 3 rats were administered DVF solution and DVF PLGA-CN nanoparticles (equivalent to 5 mg/kg/day DVF) intranasally. The pharmacokinetic studies were carried till 72 h to get the clear elimination phase for estimation of different pharmacokinetic parameters. Blood (100 μl) was collected by tail vein in precoated EDTA coated tubes and centrifuged at 3000 rpm for 5 min to obtain the plasma (50 μl). DVF was extracted from plasma by liquid extraction and citalopram was used as internal standard for its estimation by HPLC (Raut et al., 2003). For brain collection, the rats were sacrificed at schedule time intervals by cervical dislocation. Brain was dissected out from skull, washed with normal saline to remove blood stains and impurity, weighed and stored at −80 °C till further use. Brain was homogenized by a tissue homogeniser with 2 ml of normal saline. A small volume of brain tissue homogenate (200 μl) was used for estimation of DVF concentration in brain. The peak plasma concentration (Cmax) and the time required to reach the peak plasma (Tmax) were obtained from the plasma concentration-time profile. The drug targeting efficacy of the DVF PLGA-CN NPs after intranasal administration was obtained by the drug targeting efficiency (DTE) which is the ratio of the values of AUCbrain/AUCblood (intranasal): AUCbrain/AUCblood(intravenous) (Kumar et al., 2008a).
Nose to brain direct transport percentage (DTP%) was calculated as follows:
where Dz = (Di.v./Pi.v.) × Pi.n., Dz is the brain AUC fraction contributed by systemic circulation through the BBB following intranasal administration.
Di.v. is the AUC0–72h (brain) IV route.
Pi.v. is the AUC0–72h (blood) IV route.
Di.n. is the AUC0–72h (brain) intranasal route.
Pi.n. is the AUC0–72h (blood) intranasal route.
2.9. Statistical analysis
All the experiments are conducted at least in triplicate and results are expressed as mean ± standard error (SE) for all the in vivo data. Other results are expressed as mean ± standard deviation (SD). Statistical comparison of mean values was performed with analysis of variance (ANOVA) followed by Dunnett’s t-test. The P < 0.05 was considered significant and P < 0.01 was considered highly significant.
3. Results and discussion
3.1. Preparation and characterization of DVF loaded PLGA-CN nanoparticles
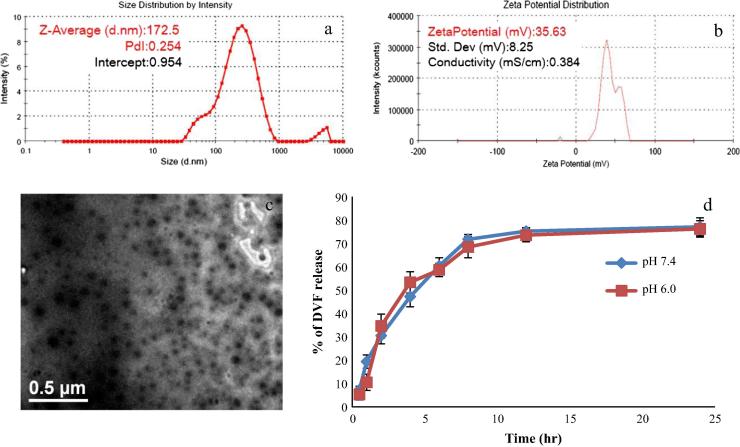
PLGA nanoparticles were prepared by emulsion solvent evaporation technique using CN as stabilizer. The CN was used as stabilizer as it provides mucoadhesive property to the nanoparticles as well as a positive surface charge. In the first step blank PLGA nanoparticles were synthesized and concentration of polymer and stabiliser was optimized. The particle size of the PLGA nanoparticles was found to be dependent on the ratio of polymer and stabilizer. Since CN alone is unable to stabilize the PLGA nanoparticles, therefore blend of CN and PVA was used for the preparation of NPs (Wang et al., 2013). The optimized placebo nanoparticles have PLGA: CN + PVA ratio 1:1 (CN:PVA = 1:1) with particle size of 151.5 ± 7.3 nm, PDI of 0.201 ± 0.11 and zeta potential of +42.97 ± 7.32 mV. In the next step DVF loaded nanoparticles were prepared by dissolving DVF in polymer phase and using the same condition/concentration as optimized for placebo nanoparticles. DVF concentration was optimized (DVF: PLGA 1:3) for particles size <200 nm (Table 1). Several studies have shown that nanoparticles smaller than 200 nm are easily transported through olfactory membrane transcellularly by olfactory neurones to the brain (Kumar et al., 2008a, Md et al., 2012). The mean particle size of DVF PLGA-CN nanoparticles (172.5 ± 10.2) (Fig. 1a) was found to be larger than the blank nanoparticles due to increase in the concentration of internal phase/polymer phase. TEM images of the DVF PLGA-CN nanoparticles also showed that size of these particles was <200 nm (Fig. 1c). The average zeta potential values were also found lower than the placebo nanoparticles due to DVF mediated increase in the net negative charge of the emulsion. Nevertheless, zeta potential value of 35.63 ± 8.25 mV (pH 7.4, Fig. 1b) represents a stable system. The %DL and %EE were found to be 30.8 ± 3.1 and 76.4 ± 4.2, respectively which shows that DVF can be entrapped within the PLGA-CN nanoparticles. The mucin binding efficacy for optimized PLGA-CN NPs and DVF PLGA NPs was found to be 64.0 ± 2.7% and 59.6 ± 1.5%, respectively. The decrease in mucoadhesive strength can be explained by the lower net positive charge of the DVF PLGA-CN nanoparticles in comparison with PLGA-CN nanoparticles. The in vitro release of DVF from optimized DVF PLGA-CN nanoparticles was carried out at pH 7.4 (physiological pH) and pH 6.0 (nasal mucosal pH) (Fig. 1d). It showed a biphasic release pattern with 30% (pH 7.4) and 34% (pH 6.0) drug release within 1 h followed by characteristic sustained release for more than 24 h. The cumulative percentage release of DVF from DVF PLGA-CN nanoparticles was 77.21 ± 3.87% (pH 7.4) and 76.32 ± 3.54% (pH 6.0) over a period of 24 h. The initial burst drug release was most likely due to the release of DVF loosely attached to the surface of the nanoparticles whereas the later slow release may be due to the DVF release from the core of PLGA-CN nanoparticles by the swelling and hydration of PLGA NPs matrix. These results showed that DVF was effectively entrapped within the PLGA-CN NPs matrix; however, there was no difference between their release profile at pH 7.4 and pH 6.0.
Table 1.
Concentration of ingredients and physiochemical parameter of the optimized Desvenlafaxine loaded PLGA-CN nanoparticles.
| Conc. of polymers | Conc. of stabiliser | Conc. of drug (mg/ml) | Mean particle size (nm ± SD) | Mean PDI (±SD) | Zeta potential (mV ± SD) | %EE (±SD) | %DL (±SD) |
|---|---|---|---|---|---|---|---|
| 1 mg/ml | 0.5 mg/ml (CN) 0.5 mg/ml (PVA) | 0.3 mg/ml | 172.5 ± 10.2 (pH 7.4) | 0.254 ± 0.02 (pH 7.4) | 35.63 ± 8.25 (pH 7.4) | 76.4 ± 4.2 | 30.8 ± 3.1 |
Figure 1.
(a) Particle size and PDI, (b) zeta potential of optimized Desvenlafaxine loaded PLGA-CN nanoparticles, (c) TEM image of the optimized DVF PLGA-CN nanoparticles and (d) the in vitro release of DVF PLGA-CN nanoparticles at pH 7.4 and pH 6.0 (n = 3 batches).
3.2. Pharmacodynamic studies
The FST is most widely used preclinical tests for assessing the antidepressant efficacy of experimental therapeutic moieties in rodents. Since its development in early 1970s, the FST has been continuously used for assessing the antidepressant activity of new compounds (Lucki, 1997). In forced swim models animals are compelled to swim in an inescapable environment where after an initial struggle the rodents embrace a motionless posture and float submissively with their head above water without any other movement. Posrsolt and associates suggested that this immobility was selectively reduced by variety of antidepressant drugs (Porsolt et al., 1978, Lucki, 1997). All the three parameters of the FST together with the locomotor activity were evaluated for their significance. The chronic antidepressant treatment regimen for 16 days was undertaken as it is known that the therapeutic effects of antidepressant takes several days or weeks to develop (Kitada et al., 1981, Berney, 2005). The 16-day drug administration was in line with the procedure reported earlier by Reneric et al. (2002). The control group i.e. the depressed rats displayed highest immobility and lowest swimming, climbing and locomotor count. The chronic treatment with DVF PLGA-CN NPs significantly reduced the immobility (P < 0.01) and enhanced the swimming time (P < 0.01), climbing time (P < 0.05) and locomotor count (P < 0.01) in comparison with the control group (Fig. 2). This shows that DVF PLGA-CN NPs provided sustained therapeutic levels of DVF in brain that reduced the symptoms of depression. A number of past studies have confirmed that an increase in swimming and climbing behaviours in the FST occurs only when the antidepressant treatment increases the levels of neurotransmitters i.e. serotonin, norepinephrine and dopamine levels in the nerve terminals (Rénéric and Lucki, 1978). On the other hand treatment with DVF solution decreased the immobility and improved the swimming time (P < 0.05) but didn’t enhance the climbing time and locomotor count at a significant level in comparison with the depressed control. This may be due to short residence time of DVF solution in nasal cavity as a result; sustained therapeutic levels of DVF were not maintained in brain for optimum antidepressant effect. Oral delivery of DVF produced inferior antidepressant effect than DVF (i.n.) and DVF PLGA-CN NPs. However DVF (oral) decreased the immobility of rodents (P < 0.05), but couldn’t enhance the swimming, climbing and locomotor count to a significant level. This may be due to combined effect of poor penetration of DVF to the brain, degradation in g.i.t, first pass metabolism and active efflux by P-glycoprotein and other efflux transporters present at the BBB. Multiple studies have shown that the brain uptake of drugs is low if it undergoes metabolism and/or is effluxed by transporters at the BBB (Hermann and Bassetti, 2007). These results are consistent with the earlier findings that have shown that oral delivery of drug leads to inferior antidepressant activity in comparison with intranasal nose to brain delivery (Alam et al., 2012, Haque et al., 2014).
Figure 2.
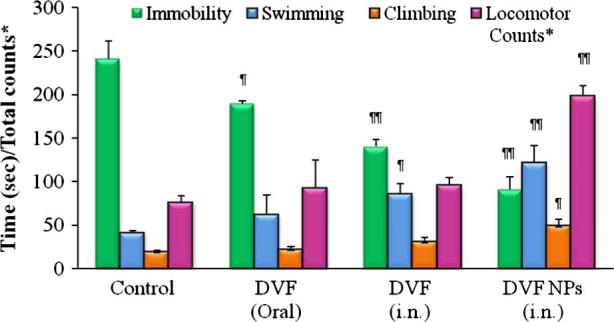
Evaluation of antidepressant activity of Desvenlafaxine loaded PLGA-CN nanoparticles in chronic depressed rats. (∗) means total locomotor count in 5 min. N = 6, Data represents Mean ± SEM; (¶) represents P < 0.05, (¶¶) represents P < 0.01.
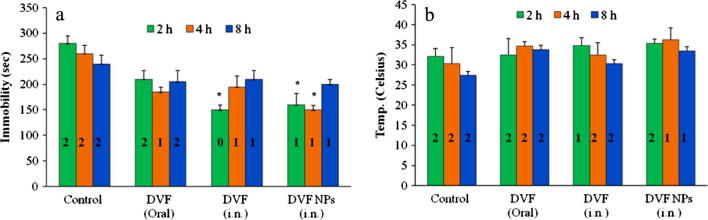
The RRT is also one of the most widely used drug induced depression model (Bourin et al., 1983). The reserpine has been known to irreversibly block the vesicular monoamine transporters (VMAT) and disrupts the monoaminergic transmission in brain (Henry and Scherman, 1989). The blockage of VMAT transporters prevents the vesicular uptake of monoamines and subsequent release in the synaptic cleft for post synaptic nerve excitation (Henry and Scherman, 1989). The depletion of monoamines at the nerve terminals leads to behavioural symptoms such hypothermia, ptosis, immobility, diarrhoea (Bourin et al., 1983, Henry and Scherman, 1989). All the three formulations reduced the reserpine induced immobility in rats but only DVF solution (i.n., 2 h) and DVF PLGA-CN NPs (i.n., 2 h, 4 h) reduced it to a significant extent (P < 0.05) (Fig. 3A). Similarly, all the three formulations reversed the reserpine induced hypothermia in rats but these differences were insignificant in comparison with control group (Fig. 3B). However, the ptosis and diarrhoea produced by reserpine were weakly antagonized only by DVF PLGA-CN NPs (Fig. 3A and B). Thus, these formulations showed better and more differentiating antidepressant activity in chronic FST models than drug induced depression model. Therefore, monoamine levels were determined in brain of rats that underwent FST.
Figure 3.
Effect of Desvenlafaxine loaded PLGA-CN nanoparticles on the reserpine induced (a) immobility and ptosis (b) hypothermia and diarrhoea in rats. N = 6, Data represents Mean ± SEM; (∗) represents P < 0.05, (∗∗) represents P < 0.01. [0, 1, 2 represents arbitrary score for reserpine induced ptosis and diarrhoea].
3.3. Biochemical studies
A large number of theories, hypothesis and mechanism have been proposed for depression. The most widely accepted theory for the pathogenesis of depression is the monoamine hypothesis, where functional deficiency of monoamines i.e. serotonin, noradrenaline and dopamine in the CNS has been linked to the symptoms of depression (Lenox and Frazer, 2010). Most of the currently used antidepressants increase the concentration of neurotransmitters in brain (Richelson, 1990). Therefore, we investigated the changes in the brain monoamine levels and their correlation with the antidepressant activity of different group of rats used in FST. Fig. 4 shows the concentration of serotonin, norepinephrine and dopamine in the brain post FST. The results showed that intranasal administration of DVF PLGA-CN nanoparticles enhanced both the serotonin (P < 0.01) and noradrenaline (P < 0.05) significantly in comparison with the depressed control, although noradrenaline levels were restored more effectively. Dopamine level was also found to be increased in the DVF PLGA-CN NPs treated rats but was statistically insignificant in comparison with the depressed control (p > 0.05). These results are in agreement with the published reports which suggest that monoamine especially serotonin and noradrenaline are diminished by stressful condition as observed in depressed rats (Arnsten, 2009). PLGA-CN nanoparticles facilitated the delivery of DVF to the brain and DVF prevented the reuptake of both serotonin and noradrenaline increasing their concentration in the nerve terminals. DVF PLGA-CN NPs effectively reversed the levels of monoamine neurotransmitters in chronic depressed rats and therefore they showed better swimming, climbing and locomotor activity. DVF (i.n.) treatment enhanced the levels of noradrenaline moderately, but failed to enhance the levels of serotonin and dopamine significantly. On the other hand DVF (oral) was unable to bring any significant change (p > 0.05) in the levels of three neurotransmitters which had good correlation with the results of pharmacodynamic studies. Based on these results, it could be inferred that chronic depression in rats was most affected by the changes in the levels of noradrenaline in brain and least affected by the changes in the dopamine levels. These results are in agreement with the previous studies which have shown that behavioural depression by uncontrollable shock is more sensitive towards changes in noradrenaline levels in the brain rather than serotonin or dopamine (Weiss et al., 1981). Further the levels of DVF in brain and systemic circulation were identified to strengthen these claims.
Figure 4.
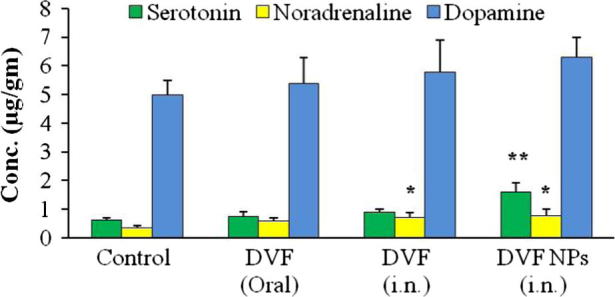
Effect of Desvenlafaxine loaded PLGA-CN nanoparticles on neurotransmitter level in brain of rats. N = 6, Data represents Mean ± SEM; (∗) represents P < 0.05, (∗∗) represents P < 0.01.
3.4. Blood and brain pharmacokinetics
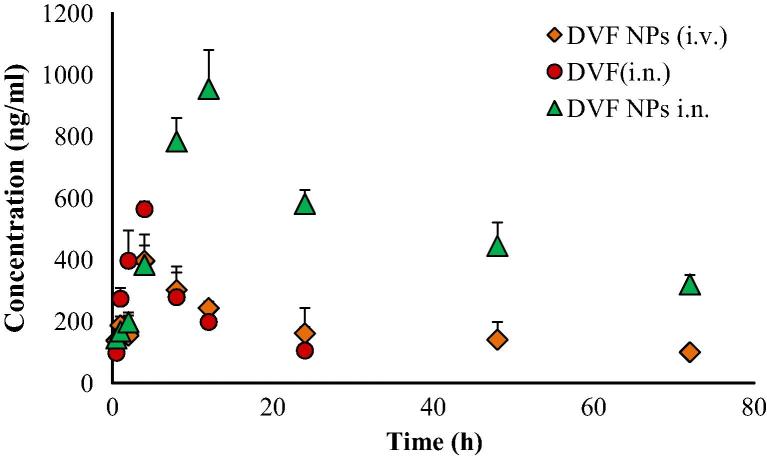
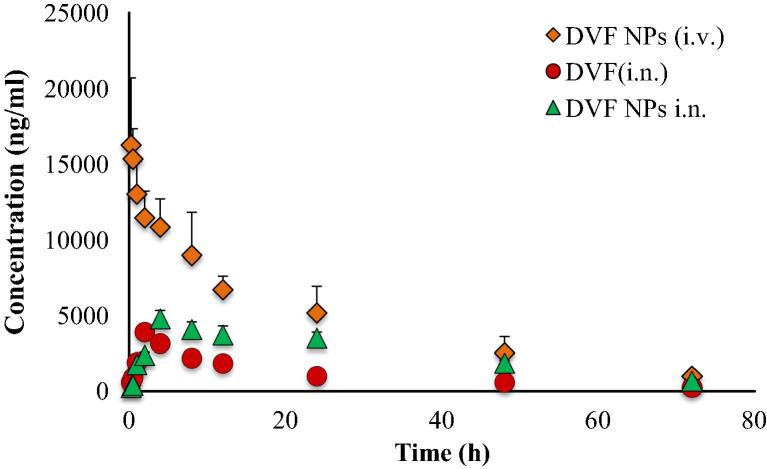
The concentrations of DVF in brain and blood plasma were analysed at different time points till 72 h following the dosing of DVF PLGA-CN nanoparticle (i.n.), DVF solution (i.n) and DVF PLGA-CN nanoparticle (i.v.). A higher DVF concentration was found in brain homogenate when DVF PLGA-CN nanoparticle was administered intranasally (954.56 ± 126.63 ng/ml) in comparison with the IV administration (396.91 ± 64.34 ng/ml) (Fig. 5). The other pharmacokinetic parameters such as half life, AUC, and AUMC were also found to be higher in brain for DVF PLGA-CN nanoparticle (i.n.) than DVF PLGA-CN nanoparticle (i.v.). Also, the rate of elimination of DVF PLGA-CN nanoparticle (i.n.) was found to be significantly lower (p < 0.05) in brain (Table 2). At initial time points the brain tissue concentration/time profile look similar for all the three formulations but they differ significantly at later time points. However DVF PLGA-CN nanoparticle (i.v.) was able to maintain DVF in brain for prolonged period of time but at much lower levels than DVF PLGA-CN nanoparticle (i.n.). These results prove that PLGA nanoparticles can transport the DVF to the brain by unique nose to brain transportation pathways and maintain its effective therapeutic concentration beyond 72 h in brain. Therefore, intranasal nose to brain delivery represents a better strategy for non-invasive delivery of DVF to the brain. The literature suggests that an intranasally administered therapeutic agent reaches the brain by a number of extracellular and intracellular mechanisms by olfactory and trigeminal nerve pathways (Illum, 2000, Thorne and Frey, 2001). A number of studies have comprehensively shown that mucoadhesive nanoparticles can transport the enclosed drugs to the brain by unique nose to brain transportation pathways and maintain its effective therapeutic concentration for prolonged time in brain (Kumar et al., 2008a, Kumar et al., 2008b, Al-Ghananeem et al., 2010, Md et al., 2012). Therefore, DVF was encapsulated in polymeric nanoparticles composed of PLGA and CN. The mucoadhesive nanoparticulate system of PLGA-CN increased the retention time of DVF in the nasal cavity to sustain their effective therapeutic concentration in brain. In addition, CN used in nose to brain formulations has been found to enhance the paracellular transport through epithelial tight junctions due to their specific interaction with either the protein kinase C pathway or the electrostatic interaction with negative charged sialic acid residues on mucosal epithelial cells (Artursson et al., 1994, Thorne et al., 2004). The DVF PLGA-CN nanoparticle (i.n.) improved the pharmacokinetic profile of DVF in brain which restored the level of monoaminergic neurotransmitters in brain and improved the pharmacodynamic response. Most surprising results were obtained following the administration of DVF (i.n.). Although its rate of absorption in the brain was faster than DVF PLGA-CN nanoparticle, it had a very short residence time in brain most likely due to mucociliary clearance of DVF from the nasal cavity. The rapid absorption can be explained on the basis of small molecular weight of DVF (399.48 Da) and log P value of 2.26 which facilitate its permeation through tight junctions of the epithelial cells and transportation by trigeminal and olfactory nerve pathways to the brain (Pund et al., 2013). In systemic circulation, DVF PLGA-CN nanoparticle attended a lower peak plasma concentration (4781.33 ± 561.25 ng/ml) than DVF PLGA-CN nanoparticle (i.v.) (16243.67 ± 4445.15 ng/ml) (Fig. 6). These results are in harmony with the earlier studies which have shown that drugs could be absorbed into systemic circulation by respiratory regions of the nasal cavity but at much lower levels than the intravenous route (Kumar et al., 2008a, Md et al., 2012). DVF (i.n.) was also detected beyond 24 h in plasma due to continuous absorption from respiratory regions, but based upon its plasma concentration it can be inferred that most of the dose was escalated out by the mucociliary clearance. The brain/blood ratio of DVF was found to be higher for DVF PLGA-CN nanoparticle (i.n.) on almost all the time points. Based on their plasma AUC values, the systemic bioavailability of DVF PLGA-CN nanoparticle (i.n.) and DVF (i.n.) was found to be 56.35% and 23.70%, respectively. The DTE shows the average partitioning of DVF between brain and blood with time whereas DTP stands for the percentage of DVF directly carried to the brain by nose to brain pathways. The DTE and DTP for DVF PLGA-CN nanoparticle (i.n.) were calculated to be 544.23 and 81.62, and for DVF (i.n.) were 202.41 and 50.59, respectively. The greater DTE and DTP values of DVF PLGA-CN nanoparticles showed that they have better brain targeting efficiency than other two formulations.
Figure 5.
Effect of Desvenlafaxine loaded PLGA-CN nanoparticles on brain concentration of DVF at various time points. N = 6, Data represents Mean ± SEM.
Table 2.
Blood and brain pharmacokinetic parameters of different formulations.
| Organ/tissue | Formulation | Cmax (ng/ml) | Tmax (h) | T1/2 (h) | Ke (h−1) | AUC 0-72 h (ng ml−1 h) | AUC 0-∞ (ng ml−1 h) | DTE% | DTP% |
|---|---|---|---|---|---|---|---|---|---|
| Brain | DVF NPs (i.n.) | 954.56 ± 126.63 | 12 | 130.23 ± 9.27 | 0.005 ± 0.000 | 37379.4 ± 1078.6 | 97589.99 ± 8751.6 | 544.23 | 81.62 |
| Blood | 4781.33 ± 561.25 | 4 | 197.56 ± 10.70 | 0.003 ± 0.000 | 181618.1 ± 31732.3 | 373597.1 ± 43241.5 | |||
| Brain | DVF (i.n.) | 564.5 ± 24.88 | 4 | 20.12 ± 4.92 | 0.034 ± 0.008 | 5858.4 ± 644.1 | 8931.3 ± 1236.3 | 202.41 | 50.59 |
| Blood | 3909.67 ± 149.09 | 2 | 27.01 ± 2.86 | 0.025 ± 0.001 | 76533.4 ± 3381.5 | 86450.1 ± 6431.8 | |||
| Brain | DVF NPs (i.v.) | 396.91 ± 64.34 | 4 | 19.21 ± 2.61 | 0.010 ± 0.002 | 12208.5 ± 2414.6 | 22079.5 ± 3526.7 | ||
| Blood | 16265.67 ± 4445.15 | - | 68.18 ± 5.46 | 0.036 ± 0.005 | 322833.4 ± 8999.8 | 350561.4 ± 15491.2 | |||
N = 3, Data represents Mean ± SEM
Figure 6.
Effect of Desvenlafaxine loaded PLGA-CN nanoparticles on the plasma concentration of DVF at various time points. N = 6, Data represents Mean ± SEM.
4. Conclusion
In the present study, a PLGA-CN nanosized drug delivery system was optimized for brain delivery of DVF. The optimized DVF PLGA-CN nanoparticles significantly improved the uptake of DVF to the brain. As evident from the pharmacodynamic and monoamine levels in brain, DVF PLGA-CN nanoparticles effectively reversed the symptoms of depression in rats. These results also indicate that nose to brain delivery is a viable non-invasive option for the therapy of depression and other brain disorders. However, these formulations require rigorous preclinical studies in higher animal models for predicting their efficacy in humans. A significant effort is also needed towards the development of delivery devices that could deposit the formulations exclusively to the olfactory region of the nose.
Conflict of interest
The authors report no conflict of interest associated with this work.
Contribution of authors
We declare that this work was done by the author named in this article and all liabilities pertaining to claims relating to the content of this article will be borne by the authors. Author “Gui-Feng Tong” wrote the first draft of the manuscript. Author “Gui-Feng Tong” designed the studies and authors “Gui-Feng Tong, Nan Qin and Li-Wei Sun performed the experiments. All authors contributed to and have approved the final manuscript.
Acknowledgement
The authors acknowledge the Tianjin Hospital for funding this research. The authors would like to thank the members of animal facility, Tianjin Hospital, for their help in carrying out animal work.
Footnotes
Peer review under responsibility of King Saud University.
References
- <http://mentalhealthdaily.com/2015/09/11/new-antidepressants-in-the-pipeline-2015-drugs-in-clinical-trials/> (accessed 30.3.2016).
- Alam M.I., Baboota S., Ahuja A., Ali M., Ali J., Sahni J.K. Intranasal administration of nanostructured lipid carriers containing CNS acting drug: pharmacodynamic studies and estimation in blood and brain. J. Psychiatr. Res. 2012;46:1133–1138. doi: 10.1016/j.jpsychires.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Al-Ghananeem A.M., Saeed H., Florence R., Yokel R.A., Malkawi A.H. Intranasal drug delivery of didanosine-loaded chitosan nanoparticles for brain targeting; an attractive route against infections caused by aids viruses. J. Drug Target. 2010;18:381–388. doi: 10.3109/10611860903483396. [DOI] [PubMed] [Google Scholar]
- Arnsten A.F.T. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artursson P., Lindmark T., Davis S.S., Illum L. Effect of chitosan on the permeability of monolayers of intestinal epithelial cells (Caco-2) Pharm. Res. 1994;11:1358–1361. doi: 10.1023/a:1018967116988. [DOI] [PubMed] [Google Scholar]
- Berney P. Dose-response relationship of recent antidepressants in the short-term treatment of depression. Dialogues Clin. Neurosci. 2005;7:249–262. doi: 10.31887/DCNS.2005.7.3/pberney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourin M., Poncelet M., Chermat R., Simon P. The value of the reserpine test in psychopharmacology. Arzneimittelforschung. 1983;33:1173–1176. [PubMed] [Google Scholar]
- Costa E., Garattini S., Valyelli L. Interactions between resperpine, chlorpromazine and imipramine. Experientia. 1960;16:461–463. doi: 10.1007/BF02171155. [DOI] [PubMed] [Google Scholar]
- Haque S., Md S., Sahni J.K., Ali J., Baboota S. Development and evaluation of brain targeted intranasal alginate nanoparticles for treatment of depression. J. Psychiatr. Res. 2014;48:1–12. doi: 10.1016/j.jpsychires.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Henry J., Scherman D. Radioligands of the vesicular monoamine transporter and their use as markers of monoamine storage vesicles. Biochem. Pharmacol. 1989;38:2395–2404. doi: 10.1016/0006-2952(89)90082-8. [DOI] [PubMed] [Google Scholar]
- Hermann D.M., Bassetti C.L. Implications of ATP-binding cassette transporters for brain pharmacotherapies. Trends Pharmacol. Sci. 2007;28:128–134. doi: 10.1016/j.tips.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Illum L. Transport of drugs from the nasal cavity to central nervous system. Eur. J. Pharm. Sci. 2000;11:1–18. doi: 10.1016/s0928-0987(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Kilts C.D. Potential new drug delivery system for antidepressants: an overview. J. Clin. Psychiatry. 2003;64:31–33. [PubMed] [Google Scholar]
- Kircanski K., Joormann J., Gotlib I.H. Cognitive aspects of depression. Wiley Interdiscip. Rev. Cogn. Sci. 2012;3:301–313. doi: 10.1002/wcs.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada Y., Miyauchi T., Satoh A., Satoh S. Effects of antidepressants in rat forced swimming test. Eur. J. Pharmacol. 1981;72:145–152. doi: 10.1016/0014-2999(81)90269-7. [DOI] [PubMed] [Google Scholar]
- Kumar M., Misra A., Babbar A.K., Mishra A.K., Mishra P., Pathak K. Intranasal nanoemulsion based brain targeting drug delivery system of risperidone. Int. J. Pharm. 2008;358:285–291. doi: 10.1016/j.ijpharm.2008.03.029. [DOI] [PubMed] [Google Scholar]
- Kumar M., Misra A., Mishra A.K., Mishra P., Pathak K. Mucoadhesive nanoemulsion-based intranasal drug delivery system of olanzapine for brain targeting. J. Drug Target. 2008;16:806–814. doi: 10.1080/10611860802476504. [DOI] [PubMed] [Google Scholar]
- Lenox R.H., Frazer A. Mechanism of action of antidepressants and mood stabilizers. In: Davis K.L., Charney D., Coyle J.T., Nemeroff C., editors. Neuropsychopharmacology: the fifth generation of progress. Lippincott Williams and Wilkins; Philadelphia: 2010. pp. 1139–1163. [Google Scholar]
- Lucki I. The forced swimming test as a model for core and component behavioural effects of antidepressant drugs. Behav. Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Mann J.J. The medical management of depression. N. Engl. J. Med. 2005;353:1819–1834. doi: 10.1056/NEJMra050730. [DOI] [PubMed] [Google Scholar]
- Marcus, M., Yasamy, M.T., Ommeren, M.V., Chishom, D., Saxena, S., Depression A global Public Health concern, 2012. <http://www.who.int/mental_health/management/depression/who_paper_depression_wfmh_2012.pdf/> (accessed 30.3.2016).
- Md S., Khan R.A., Mustafa G., Chuttani K., Baboota S., Sahni J.K., Ali J. Bromocriptine loaded chitosan nanoparticles intended for direct nose to brain delivery: pharmacodynamic, Pharmacokinetic and Scintigraphy study in mice model. Eur. J. Pharm. Sci. 2012;48:393–405. doi: 10.1016/j.ejps.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Nutt D.J. Relationship of neurotransmitters to the symptoms of major depressive disorder. J. Clin. Psychiatry. 2008;69:4–7. [PubMed] [Google Scholar]
- Pawar D., Goyal A.K., Mangal S., Mishra N., Vaidya B., Tiwari S., Jain A.K., Vyas S.P. Evaluation of mucoadhesive PLGA microparticles for nasal immunization. AAPS J. 2010;12:130–137. doi: 10.1208/s12248-009-9169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget G.E., Barnes J.M. Academic Press Inc., Ltd; London: 1964. pp. 135–166. [Google Scholar]
- Perry R., Cassagnol M. Desvenlafaxine: a new serotonin-norepinephrine reuptake inhibitor for the treatment of adults with major depressive disorder. Clin. Ther. 2009;31:1374–1404. doi: 10.1016/j.clinthera.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Porsolt R.D., Bertin A., Jalfre M. “Behavioral despair” in rats and mice: strain differences and the effect of imipramine. Eur. J. Pharmacol. 1978;51:291–294. doi: 10.1016/0014-2999(78)90414-4. [DOI] [PubMed] [Google Scholar]
- Pund S., Rasve G., Borade G. Ex vivo permeation characteristics of venlafaxine through sheep nasal mucosa. Eur. J. Pharm. Sci. 2013;48:195–201. doi: 10.1016/j.ejps.2012.10.029. [DOI] [PubMed] [Google Scholar]
- Rao K.V., Reddy K.P., Kumar Y.R. Stability indicating LC method for rapid determination of related substances of O-desmethyl venlafaxine in active pharmaceutical ingredients and pharmaceutical formulations. J. Chromatogr. Sci. 2014;52:1247–1254. doi: 10.1093/chromsci/bmt207. [DOI] [PubMed] [Google Scholar]
- Raut B.B., Kolte B.L., Deo A.A., Bagool M.A. A Rapid and sensitive HPLC method for the determination of venlafaxine and O-desmethylvenlafaxine in human plasma with UV detection. J. Liq. Chrom. Relat. Tech. 2003;26:1297–1313. [Google Scholar]
- Rénéric J.P., Lucki I. Antidepressant behavioral effects by dual inhibition of monoamine reuptake in the rat forced swimming test. Psychopharmacology. 1978;136:190–197. doi: 10.1007/s002130050555. [DOI] [PubMed] [Google Scholar]
- Rénéric J.P., Bouvard M., Stinus L. In the rat forced swimming test, chronic but not subacute administration of dual 5-HT/NA antidepressant treatments may produce greater effects than selective drugs. Behav. Brain Res. 2002;136:521–532. doi: 10.1016/s0166-4328(02)00203-6. [DOI] [PubMed] [Google Scholar]
- Richelson E. Antidepressants and brain neurochemistry. Mayo Clin. Proc. 1990;65:1227–1236. doi: 10.1016/s0025-6196(12)62747-5. [DOI] [PubMed] [Google Scholar]
- Schlumpf M., Lichtensteiger W., Langemann H., Waser P.G., Hefti F. A fluorometric micromethod for the simultaneous determination of serotonin, noradrenaline and dopamine in milligram amounts of brain tissue. Biochem. Pharmacol. 1974;23:2437–2446. doi: 10.1016/0006-2952(74)90235-4. [DOI] [PubMed] [Google Scholar]
- Thorne R.G., Frey W.H., II. Delivery of neurotrophic factors to the central nervous system: pharmacokinetic considerations. Clin. Pharmacokin. 2001;40:907–946. doi: 10.2165/00003088-200140120-00003. [DOI] [PubMed] [Google Scholar]
- Thorne R.G., Pronk G.J., Padmanabhan V., Frey W.H., 2nd Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127:481–496. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Wang Y., Li P., Kong L. Chitosan-modified PLGA nanoparticles with versatile surface for improved drug delivery. AAPS PharmSciTech. 2013;14:585–592. doi: 10.1208/s12249-013-9943-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J.M., Goodman P.A., Losito B.G., Corrigan S., Charry J.M., Bailey W.H. Behavioral depression produced by an uncontrollable stressor: Relationship to norepinephrine, dopamine, and serotonin levels in various regions of rat brain. Brain Res. Rev. 1981;3:167–205. [Google Scholar]
- World Health organization, 2015. Depression Fact sheet N°369. <http://www.who.int/mediacentre/factsheets/fs369/en/> (accessed 30.3.2016).
- Yin Y., Chen D., Qiao M., Lu Z., Hu H. Preparation and evaluation of lectin-conjugated PLGA nanoparticles for oral delivery of thymopentin. J. Control Release. 2006;116:337–345. doi: 10.1016/j.jconrel.2006.09.015. [DOI] [PubMed] [Google Scholar]