Abstract
The kidney vasculature is extremely complex, yet, despite recent progress, our understanding of how the renal vascular system develops is limited. By using advanced tissue engineering techniques and in vivo and in vitro depletion of specific populations of endothelial cell precursors, Halt et al. have identified a CD146-expressing precursor as an important player in the development of the renal vasculature.
The kidney is a highly vascularized organ that receives ~20% of the cardiac output. The proper spatial and timely assembly of the renal vessels is a crucial developmental process required for formation of the functional postnatal kidney. Nearly all kidney diseases affect the renal vasculature. A pressing challenge in the field of tissue engineering is to achieve efficient vascularization of organs; this is particularly true for the renal vascular system, which is extremely complex.
Blood vessels may develop via angiogenesis (budding from preexisting vessels) or vasculogenesis (de novo formation from precursors that differentiate into vascular cells) (extensively reviewed in Sequeira-Lopez1). The early embryonic kidney initially generates its vasculature by vasculogenesis and then by both mechanisms.1,2 Hemovasculogenesis (concomitant formation of blood precursors and vessels) is an additional mechanism for the development of renal blood vessels.1,2 During both normal development and pathological processes, all these mechanisms may coexist.
All blood and lymphatic vessels in the kidney contain an inner endothelial cell layer surrounded by mural cells. In arterioles, mural cells include smooth muscle cells, or pericytes, renin cells, and fibroblasts. In the glomerulus, mesangial cells surround and support the glomerular capillaries. Peritubular capillaries are also surrounded by interstitial pericytes, fibroblasts, and other cell types including erythropoietin-synthesizing cells. The renal vasculature develops in parallel with the nephrons, formed through inductive interactions between the ureteric bud (UB) (an outgrowth of the Wolffian duct) and committed renal progenitor cells within the metanephric mesenchyme (MM) (a mass of loosely associated mesenchymal cells surrounding the Wolffian duct) (reviewed in Little and McMahon3). Cells within the MM expressing the Wilm tumor 1 gene (WT1) respond to signals from the UB, start expressing Cited1 and Six2 transcription factors, and undergo a mesenchyme-to-epithelium transition to form the various segments of the developing nephron; each emerging nephron fuses to the collecting system derived from the arborized UB. WT1− cells within the MM express either the Forkhead transcription factor (Foxd1)4 and give rise to all the above-mentioned mural cells5 or the helix-loop-helix transcription factor stem cell leukemia (SCL/Tal1) and give rise to endothelium and hematopoietic precursors2 (Figure 1a). Foxd1+-derived stromal cells are critical for vascular patterning, nephrogenesis, and branching of the UB.4,5 In recent years, the mechanisms that control UB branching and nephron induction and maturation have been substantially advanced.3 However, our understanding of the origin and development of the renal vasculature is more limited. To some extent, this is due to the lack of reliable markers and the cell and tissue culture systems that allow fate mapping of cell lineages that form the vasculature.
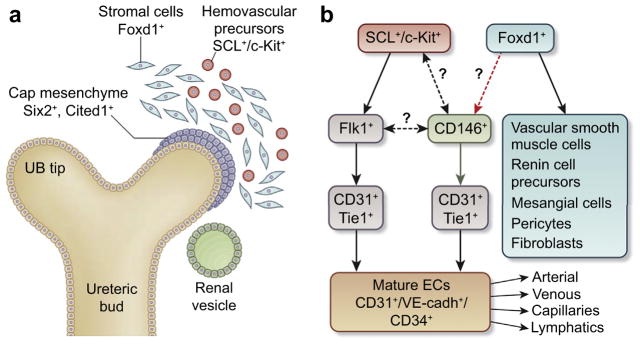
Figure 1. Early stages of renal vasculature development.
(a) Cellular compartments in the early metanephric kidney (modified from Sequeira-Lopez et al.5). (b) Provisional place of CD146+ precursors within the lineage hierarchy of the renal vasculature. Black arrows indicate current evidence, green arrow indicates a novel discovery by Holt et al.,7 dashed black arrows indicate lineage relationships that remain to be studied. Red dashed arrow indicates a suggested link that requires further experimentation. Specific factors characteristic of arterial, venous, capillary, and lymphatic ECs are not depicted for clarity. cadherin, calcium-dependent adhesion; CD, cluster of differentiation; c-Kit, tyrosine kinase receptor; EC, endothelial cell; Flk1, fetal liver kinase; Foxd1, Forkhead transcription factor; SCL, stem cell leukemia transcription factor; Six2, sine oculis-related homeobox gene 2; Tie1, tyrosine kinase with Ig-like and EGF-like domains 1; VE, vascular endothelial; UB, ureteric bud.
Results of grafting experiments have revealed that the prevascular embryonic mouse kidneys contain progenitors of all cell types required for development of the vascular system, including endothelial cell (EC) progenitors, precursors of the smooth muscle cells, pericytes, juxtaglomerular renin+ cells, and mesangial cells.1,5 In 2006, Schmidt-Ott et al.6 showed that, dispersed within the cortical Foxd1+ stromal compartment, there is a population of Foxd1−/c-Kit+ angioblasts. C-Kit, a tyrosine kinase receptor, is among the earliest markers of hematopoietic cells and angioblasts.6 Embryologically, c-Kit+ cells align with aorta-gonad-mesonephric cells adjacent ventrally and laterally to the dorsal aorta rather than with Foxd1+ cells, indicating their distinct embryonic origin.6 The relation of c-Kit+ cells to aorta-gonad-mesonephric cells suggests that EC precursors may have migrated to the presumptive MM very early. The population of c-Kit+ cells is mixed: some of the c-Kit+ cells express the early EC marker, kinase insert domain receptor/vascular endothelial growth factor receptor 2/fetal liver kinase 1 (Kdr/VEGFR2/Flk1), whereas others are Flk1-negative. Overall, the population of renal EC precursors at any given time during development is highly heterogeneous: in addition to Flk1-expressing cells, there are EC precursors expressing tyrosine kinase with Ig-like and EGF-like domains 1 (TIE/Tie1), a population of cells expressing the platelet/endothelial cell adhesion molecule-1/cluster of differentiation 31 (PECAM-1/CD31), and cells expressing podocalyxin. The relationship among these many subtypes of renal ECs is not well understood. It is possible that cells expressing various surface markers represent different developmental and maturation stages of EC precursors initially derived from the same early lineage. In 2015, a thorough lineage and fate study2 identified a progenitor within the early embryonic kidney that expresses SCL and gives rise to endothelium and hematopoietic precursors by hemovasculogenesis. However, the lineage relationship of the various potential pools of precursor cells and their differentiation pathways have not been resolved.
The study by Halt et al.7 (2016) published in this issue of Kidney International identifies a potential new player involved in the early development of renal endothelial cells. The investigators used a clever combination of well-established mouse transgenic models with an innovative ex vivo dissociation/reaggregation assay and live-imaging and immunofluorescence-mediated sorting approaches to address the following questions: (i) Which subtypes of EC precursors are crucial for kidney vascularization? (ii) Is there continuity between various populations of EC subtypes? (iii) Are there specific factors which are critical for EC development and survival? The study provides an elegant analysis of important players in renal vascular development and raises additional fundamental questions.
First, Halt et al.7 identified CD146+ cells in the early mouse embryonic (E) kidney. CD146 encodes an Flk1 coreceptor that decorates cells not previously known to be present in kidney. The investigators established the presence of Flk1+/CD31+, CD146+/ CD31+, and CD146+/CD31− cells in the E11.5–E12.5 mouse kidney (Figure 1b). This raises crucial questions for future studies such as: (i) Do all Flk1+ precursors express the CD146+ coreceptor and give rise to all combinations of the above-mentioned markers? Or (ii) if there are different nonoverlapping Flk1+ and CD146+ precursors, do both lineages coexpress SCL? In a subsequent experiment, the investigators used a Tie1 promoter-driven Cre recombinase to activate expression of the diphtheria toxin receptor to render Tie1-expressing cells sensitive to diphtheria toxin in the culture medium. Although, Tie1+ cells were eliminated from early kidney explants, the CD31+ vascular tree was still detected. These results suggest that there are Tie1-negative (Diphtheria toxin–insensitive) alternative EC precursors in the embryonic kidney. To hunt for the elusive CD31+ precursors, the investigators employed an elegant cell reaggregation approach similar to that used by other groups to analyze renal progenitors, embryonic stem cells, or inducible pluripotent cells for engineering a mammalian “kidney in a dish.”8,9 Halt et al. dissociated prevascular kidney tissues, depleted the resultant cell suspension of either CD31+ or CD146+ cells by immunomagnetic positive selection or fluorescence-activated cell sorter–mediated isolation and allowed the remaining cells to reaggregate. Surprisingly, after depletion of CD31+ cells, there was recovery of a CD31+ vasculature, whereas eradication of CD146+ cells completely disrupted the CD31+ vascular system, indicating that CD146+ cells might have given rise to CD31+ cells. By using chimeric reaggregation cultures in which only CD146+/CD31− cells were labelled with enhanced green fluorescent protein, the investigators confirmed that CD31+ cells originated from CD146+ precursors. As addressed by Halt et al.,7 the generation of a CD146-Cre mouse will allow further characterization of the differentiation pathways of the CD146 precursors and ascertain whether they are also hemogenic, as recently was shown for the SCL+ precursors.2 In fact, it would be interesting to determine whether CD146+ and SCL+ cells are expressed by the same or different precursor(s). Next, by examining fluorescent cells from Fox-D1EGFPCre;TdTomato embryonic kidneys, the investigators found that a subset of TdTomato-tagged cells was positive for either CD146+ or CD31+ and suggested that both CD146+ and CD31+ cells might have originated from Foxd1+ precursors. However, this point remains uncertain because after depletion of CD146+ cells, Foxd1+ precursors should have been able to contribute at least to a subset of ECs and this was not the case (Figure 1b). Finally, using reaggregation assays, they showed the importance of VEGFR signaling in vascular system survival, yet found no role for the hypoxia-activated pathway in vascular tree development and maintenance.
In summary, the study by Halt et al.7 demonstrates the utility of the ex vivo reaggregation assay in unraveling cell lineages that give rise to the renal vasculature. By using advanced tissue engineering techniques coupled with in vivo and in vitro depletion of specific populations of EC precursors, the investigators gather new evidence in support of a vasculogenic mechanism for renal vascular development. Furthermore, the study shows that MM cells expressing CD146+ play an important role in the development of the renal vasculature.
Footnotes
see basic research on page 311
DISCLOSURES
All the authors declared no competing interests.
References
- 1.Sequeira-Lopez ML. The origin and regulation of renal vasculature. In: Little MH, editor. Kidney Development, Disease, Repair and Regeneration. New York, NY: Academic Press; 2015. pp. 147–163. [Google Scholar]
- 2.Hu Y, Li M, Göthert JR, et al. Hemovascular progenitors in the kidney require sphingosine-1-phosphate receptor 1 for vascular development [e-pub ahead of print] [accessed November 3, 2015];J Am Soc Nephrol. doi: 10.1681/ASN.2015060610. http://dx.doi.org/10.1681/ASN.2015060610. [DOI] [PMC free article] [PubMed]
- 3.Little MH, McMahon AP. Mammalian kidney development: principles, progress, and projections. Cold Spring Harb Perspect Biol. 2012:4. doi: 10.1101/cshperspect.a008300. http://dx.doi.org/10.1101/cshperspect.a008300. [DOI] [PMC free article] [PubMed]
- 4.Levinson R, Mendelsohn C. Stromal progenitors are important for patterning epithelial and mesenchymal cell types in the embryonic kidney. Semin Cell Dev Biol. 2003;14:225–231. doi: 10.1016/s1084-9521(03)00025-9. [DOI] [PubMed] [Google Scholar]
- 5.Sequeira-Lopez ML, Lin EE, Li M, et al. The earliest metanephric arteriolar progenitors and their role in kidney vascular development. Am J Physiol Regul Integr Comp Physiol. 2015;308:R138–R149. doi: 10.1152/ajpregu.00428.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt-Ott KM, Chen X, Paragas N, et al. C-kit delineates a distinct domain of progenitors in the developing kidney. Dev Biol. 2006;299:238–249. doi: 10.1016/j.ydbio.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 7.Halt KJ, Pärssinen HE, Junttila SM, et al. CD146+ cells are essential for kidney vasculature development. Kidney Int. 2016;90:311–324. doi: 10.1016/j.kint.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 8.Junttila S, Saarela U, Halt K, et al. Functional genetic targeting of embryonic kidney progenitor cells ex vivo. J Am Soc Nephrol. 2015;26:1126–1137. doi: 10.1681/ASN.2013060584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takasato M, Er PX, Chiu HS, et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2015;526:564–568. doi: 10.1038/nature15695. [DOI] [PubMed] [Google Scholar]