Abstract
Objective
The aim of the study was to compare costs associated with excision of routine cavity shave margins (CSM) versus standard partial mastectomy (PM) in patients with breast cancer.
Background
Excision of CSM reduces re-excision rates by more than 50%. The economic implications of this is, however, unclear.
Methods
Between October 21, 2011 and November 25, 2013, 235 women undergoing PM for Stage 0–III breast cancer were randomized to undergo either standard PM (“no shave”, n = 116) or have additional CSM taken (“shave”, n = 119). Costs from both a payer and a hospital perspective were measured for index surgery and breast cancer surgery–related care through subsequent 90 days.
Results
The 2 groups were well-matched in terms of baseline characteristics. Those in the “shave” group had a longer operative time at the initial surgery (median 76 vs 66 min, P < 0.01), but a lower re-excision rate for positive margins (13/119 = 10.9% vs 32/116 = 27.6%, P < 0.01). Actual direct hospital costs associated with operating room time ($1315 vs. $1137, P = 0.03) and pathology costs ($1195 vs $795, P < 0.01) were greater for the initial surgery in patients in the “shave” group. Taking into account the index surgery and the subsequent 90 days, there was no significant difference in cost from either the payer ($10,476 vs $11,219, P = 0.40) or hospital perspective ($5090 vs $5116, P = 0.37) between the “shave” and “no shave” groups.
Conclusions
Overall costs were not significantly different between the “shave” and “no shave” groups due to significantly fewer reoperative surgeries in the former.
Keywords: breast cancer, cavity shave margins, costs, partial mastectomy, randomized controlled trial
Many breast cancer patients opt for breast conserving surgery for treatment of their disease. Critical to minimizing the risk of local recurrence after this procedure is the need to obtain clear margins, defined as no invasive cancer cells touching the edge of the resected specimen.1 Failure to achieve an adequate resection initially often mandates further surgery to minimize this risk.
Although a number of studies have evaluated techniques to reduce positive margin and re-excision rates, few have carefully considered the cost implications of these approaches. As nearly 300,000 patients with invasive and in situ breast cancer are diagnosed annually in the United States,2 many of whom will opt for breast conserving surgery, understanding the cost implications of alternative strategies for both payers and providers is essential. We recently reported the results of a randomized controlled trial in which we found that routine resection of cavity shave margins (ie, additional tissue circumferentially around the initial resection cavity at the time of the original surgery) resulted in a halving of the positive margin and re-excision rate beyond standard partial mastectomy.3 Although the reduction in re-excisions may reduce overall costs, the added operative time and costs associated with histopathologic examination of additional shave margins may result in increased costs. We therefore sought to objectively determine, in this prospective randomized controlled trial, the economic impact of taking routine cavity shave margins versus standard partial mastectomy.
METHODS
Study Design and Oversight
A randomized controlled trial was conducted to determine the impact of taking routine cavity shave margins in patients undergoing standard partial mastectomy. This was an investigator-initiated, single-center study conducted at an academic medical center in the United States. The trial involved 235 patients with stage 0–3 breast cancer. A consort diagram is shown in Figure 1. Prespecified outcome measures included positive margin and re-excision rates, volume of tissue excised, cosmetic outcomes, locoregional recurrence rates, intraoperative time, and cost. Here, we report the economic evaluation of this procedure (ie, the latter 2 outcome measures). Follow-up will continue for 5 years to assess long-term recurrence rates, cosmesis, and quality of life.
FIGURE 1.
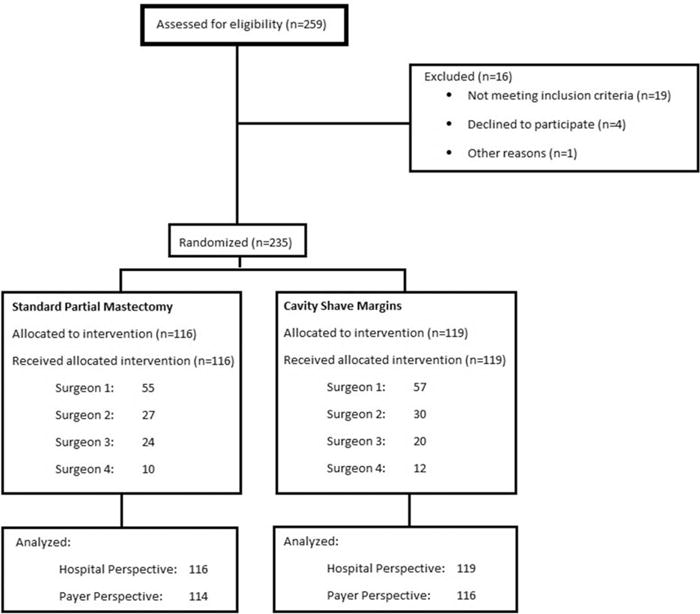
Consort diagram. Outline of randomized controlled trial.
This study was approved by the Yale University Human Investigations Committee and overseen by the Data Safety and Monitoring Committee of the Yale Cancer Center, with internal audits by the Yale Center for Clinical Investigation.
Patients
Patients older than 18 years who presented with stage 0–3 breast cancer diagnosed on core needle biopsy who were scheduled to undergo a partial mastectomy were eligible to participate. Patients who previously had an excisional biopsy or attempt at partial mastectomy were excluded. Preoperative needle localization for nonpalpable tumors was left to the discretion of the surgeon. Patients were stratified into 1 or 2 groups based on clinical stage (stage 0–2 vs stage 3).
Study Randomization and Treatment
After informed consent, patients in each of the 2 strata were randomized 1:1 to have, at the time of partial mastectomy, either additional circumferential cavity shave margins taken (“shave”) or not (“no shave”). Randomization lists were generated by the Yale Center for Analytical Sciences. Surgeons, initially blinded to the randomization assignment, were instructed to perform their standard partial mastectomy, taking additional selective margins as they deemed necessary based on their own gross evaluation of the specimen and/or intraoperative imaging. Neither the main specimen nor the additional margins were sent for frozen section, and no other technology for margin assessment was used. Once the surgeon was satisfied with the procedure he/she had performed, before closing, a sealed randomization envelope was opened intraoperatively and the surgeon was instructed to either take further tissue circumferentially around the cavity (“shave”) or close (“no shave”). Shave margins were to include the superior, medial, lateral, and inferior margins; anterior and posterior margins were also required if the original resection did not extend to skin or pectoralis fascia, respectively. The shave margins were not sent for frozen section, but were evaluated based on permanent histopathological analysis.
Positive margins were defined as tumor cells touching the edge of the resected specimen for invasive tumors,1 and tumor within 1 mm of the edge of the resected specimen for ductal carcinoma in situ.4 Pathologists were blinded to the randomization group of patients, and in fact, to whether the patient was participating in the trial. Patients with positive margins were recommended to return to the operating room for re-excision at the discretion of the surgeon.
Outcome Measures
Intraoperative time was measured from the time of incision to dressing. These data were collected prospectively by the circulating nurse in the operating room, and verified in the medical record.
In terms of costs, 2 perspectives were considered. From a payer perspective, amounts reimbursed for facility and provider fees for all breast surgery–related care from the index case through the subsequent 90 days were obtained from the hospital and the medical group, respectively. From a hospital perspective, direct costs incurred by the facility related to breast surgery, including (but not limited to) operating room time and pathology services were calculated by hospital personnel from the hospital’s cost accounting system and provided to the investigators. Costs included all breast surgery–related care from the index case through the subsequent 90 days. This included additional operative interventions that may have been done for indications aside from margin clearance (eg, lymph node evaluation). Hospital personnel were blinded as to patients’ randomization group. It should be noted that these costs do not include indirect costs that would also be required for provision of these services.
Statistical Analysis
Sample size for the trial had been calculated on the basis of the primary endpoint of margin positivity. A sample size of 250 was calculated to provide 80% power to detect a difference between the anticipated 30% positive margin rate in the partial mastectomy group and a 15% positive margin rate in the shaved group with a 1-sided significance level of 0.025 using the Inequality Tests for Two Proportions module in PASS 2008 (Kaysville, UT), and the normal approximation.
For the current analysis, patients’ characteristics, including sociodemographic and clinicopathologic factors and payer type, were compared between randomized groups using Fisher exact tests or χ2 tests for categorical variables, and Mann-Whitney U tests for continuous variables as appropriate. Although direct hospital costs and transaction amounts incurred by payers related to facility fees were obtained for all 235 patients, data related to payments for professional fees were missing for 5 patients. Costs from a payer perspective were therefore analyzed on the basis of the 230 patients for whom complete data were available. To assess the impact of excluding these 5 patients, multiple imputation was conducted to generate 10 imputed “filled-in” datasets, with assumption of data missing at random. Each dataset was analyzed separately. These results had little change compared to the primary analysis of 230 patients; therefore, only the latter are presented. Multiple imputation analyses were performed using SAS 9.3 (SAS Institute, Cary, NC); the remainder of the statistical analyses was performed using IBM SPSS Statistics (Version 21.0; Armonk, NY).
RESULTS
Patient Characteristics
Between October 21, 2011 and November 25, 2013, 235 patients were enrolled in this trial; 119 were randomized to the “shave” arm and 116 to the “no shave” arm. The 2 groups were well-matched in terms of baseline characteristics (P > 0.05 for all comparisons, Table 1). Four surgeons participated in the trial; each had roughly the same number of cases in each of the 2 arms of the study (P = 0.87). Of note, the payer mix was also similar between the 2 groups (Table 1, P =.71), as was the reoperative rate for indication aside from margins (eg, for lymph node assessment alone), P = 1.00.
TABLE 1.
Patient Characteristics
| Characteristic | Shave (N = 119) | No Shave (N = 116) |
|---|---|---|
| Age, median (range), yr | 62 (35–88) | 61 (33–94) |
| Race/ethnicity, no. (%)* | ||
| • White | 93 (78.2) | 90 (77.6) |
| • Black | 15 (12.6) | 15 (12.9) |
| • Asian | 2 (1.7) | 2 (1.7) |
| • Other | 9 (7.6) | 9 (7.8) |
| Hispanic ethnicity, no. (%)*,† | 3/96 (3.1) | 3/96 (3.1) |
| Palpable tumor, no. (%) | 26 (21.8) | 26 (22.4) |
| Pathological stage, no. (%) | ||
| • 0 | 24 (20.2) | 32 (27.6) |
| • 1 | 69 (58.0) | 53 (45.7) |
| • 2 | 25 (21.0) | 29 (25.0) |
| • 3 | 1 (0.8) | 2 (1.7) |
| Invasive tumor size, median (range), cm | 1.0 (0.0–6.0) | 1.1 (0.0–6.5) |
| Invasive histologic subtype, no. (%)‡ | ||
| • Ductal | 80/95 (84.2) | 73/84 (86.9) |
| • Lobular | 10/95 (10.5) | 6/84 (7.1) |
| • Other | 5/95 (5.3) | 5/84 (6.0) |
| DCIS component, no. (%) | 83 (69.7) | 87 (75.0) |
| DCIS size, median (range), cm | 1.0 (0.0–9.3) | 1.0 (0.0–8.1) |
| Neoadjuvant chemotherapy, no. (%) | 4 (3.4) | 3 (2.6) |
| No residual disease, no. (%) | 4 (3.4) | 7 (6.0) |
| Complex closure, no. (%) | 20 (16.8) | 27 (23.3) |
| Initial lymph node procedure, no. (%) | ||
| • None | 22 (18.5) | 25 (21.6) |
| • SLNB alone | 86 (72.3) | 82 (70.7) |
| • SLNB and completion ALND | 7 (5.9) | 7 (6.0) |
| • ALND alone | 4 (3.4) | 2 (1.7) |
| Insurance | ||
| • Self-pay | 5 (4.2) | 2 (1.7) |
| • Medicare | 37 (31.1) | 31 (26.7) |
| • Medicaid | 7 (5.9) | 6 (5.2) |
| • Medicare managed care | 10 (8.4) | 12 (10.3) |
| • Nongovernmental managed care | 33 (27.7) | 31 (26.7) |
| • Commercial/private insurance | 27 (22.7) | 34 (29.3) |
| Reoperative surgery for lymph node evaluation alone | 2 (1.7) | 1 (0.9) |
Race and ethnicity were self-reported by patients.
Ethnicity not specified in 43 (18.3%) of patients.
A total of 179 patients had invasive disease, with or without concomitant DCIS; the remaining 45 has DCIS alone.
ALND indicates axillary lymph node dissection; DCIS, ductal carcinoma in situ; SLNB, sentinel lymph node biopsy.
Intraoperative Time Associated With Routine Cavity Shave Margins
The median time from incision to dressing was 66 minutes (range 24–265) in the “no shave” group and 76 minutes (range 34–196) in the “shave” group, P < 0.01. Although the range for operative time varied widely in both arms due to additional procedures performed at the initial procedure, it should be noted that the proportion of patients who had complex closure of the resected cavity (P = 0.22) and additional procedures for lymph node evaluation such as sentinel lymph node biopsy and/or axillary dissection at the initial surgical procedure (P = 0.82) were similar between the 2 arms (Table 1). Hence the additional 10 minutes of operative time was likely due solely to the resection of cavity shave margins. We further analyzed operative time in the “shave” and “no shave” group according to type of lymph node evaluation and type of closure. These data are shown in Table 2.
TABLE 2.
Operative Times According to Lymph Node Evaluation and Closure Type in the “Shave” and “No Shave” Groups
| Median Operative Time (Incision to Dressing, min)
|
|||
|---|---|---|---|
| Shave | No Shave | P | |
| Type of lymph node evaluation: | |||
| None | 58.5 (n = 22) | 48 (n = 25) | 0.10 |
| SLNB alone | 79 (n = 86) | 62 (n = 82) | 0.01 |
| SLNB and ALND | 107 (n = 7) | 97 (n = 7) | 1.00 |
| ALND alone | 125 (n = 4) | 154 (n = 2) | 1.00 |
| Complex closure: | |||
| Yes | 82 (n = 20) | 69 (n = 27) | 0.11 |
| No | 75 (n = 99) | 66 (n = 89) | 0.02 |
ALND indicates axillary lymph node dissection; SLNB, sentinel lymph node biopsy.
In addition, we had asked the circulating nurse in the operating room to record the time at which the envelope was opened. This, by definition in this trial, was the time at which the surgeon was ready to close. Therefore the time between when the envelope was opened and dressing would be the time for closure, with or without resection of additional cavity shave margins based on randomization group. The median time from envelope opening to dressing was 41 minutes in the “shave” group, and 31 minutes in the “no shave” group, P < 0.01. This confirms our hypothesis that the difference of 10 minutes in total operative time was likely due to resection of cavity shave margins.
Cost Analysis From a Hospital Perspective
Direct hospital costs were higher at the initial procedure in the “shave” group (Table 3). In particular, direct costs associated with increased operating room time were higher in the “shave” group at the initial procedure (mean $1315 vs $1138, P = 0.03). The median number of shave margins removed at the initial procedure in patients randomized to the “shave” group was 4 (range 3–6). Given the additional specimens produced, the actual direct hospital-related costs associated with pathologic evaluation of specimens from the initial procedure were also higher in the “shave” group (mean $1195 vs $795, P < 0.01).
TABLE 3.
Actual Direct Costs Incurred by the Hospital
| Mean (95% CI) Costs Per Patient | “Shave” (n = 119) | “No Shave” (n = 116) | P |
|---|---|---|---|
| Index surgery: | |||
| OR costs | $1315 ($1179–$1451) | $1138 ($1034–$1,241) | 0.03 |
| Pathology costs | $1195 ($1111–$1280) | $795 ($701–$890) | <0.01 |
| Total costs | $4758 ($4515–$5001) | $4133 ($3897–$4370) | <0.01 |
| Additional breast surgery within 90 days: | |||
| OR costs | $94 ($46–$141) | $247 ($160–$334) | <0.01 |
| Pathology costs | $51 ($15–$87) | $112 ($70–$154) | <0.01 |
| Total costs | $332 ($159–$506) | $983 ($609–$1356) | <0.01 |
| Total 90-day breast surgery–related costs: | |||
| OR costs | $1409 ($1258–$1559) | $1385 ($1258–$1511) | 0.86 |
| Pathology costs | $1247 ($1150–$1343) | $909 ($806–$1012) | <0.01 |
| Total costs | $5090 ($4761–$5420) | $5116 ($4692–$5540) | 0.37 |
CI indicates confidence interval; OR, operating room.
A total of 48 patients required further surgery: 3 for sentinel node biopsy for a previously undiagnosed invasive breast cancer (2 in the “shave” group and 1 in the “no shave” group, P = 0.58), and 45 for re-excision of positive margins. Patients randomized to the “shave” group were significantly less likely to have a positive margin at the conclusion of the initial procedure (23/119 = 19.3% vs 39/116 = 33.6%, P = 0.01), and to require subsequent re-excision for margin clearance (13/119 = 10.9% vs 32/116 = 27.6%, P < 0.01) than those in the “no shave” group. Six patients required more than 1 re-excision for margin clearance (5 in the “no shave” group vs 1 in the “shave” group, P = 0.09). Ultimately, 7 patients opted for a total mastectomy (5 in the “no shave” group vs 2 in the “shave” group, P = 0.24).
The mean cost to the hospital for additional surgeries for those who required them was not significantly different between the “shave” and “no shave” groups [$2422 (95% CI: $1809–$3035) vs $3461 (95% CI: $2562–$4360), P = 0.23]. Given the significantly lower rate of reoperative surgery in the “shave” group, direct hospital costs were, however, significantly lower for subsequent surgeries in this group (mean $332 vs $983, P < 0.01). Taking into account all surgeries (including the index case and any additional surgeries within 90 days), there was no significant difference in cost (from a hospital perspective) between the 2 groups.
Of note, patients in the “shave” group had a slightly higher rate of having more aggressive lymph node evaluation on their initial surgery (Table 1). Given that this may skew costs, we therefore analyzed total costs both from a hospital and a payer perspective based on type of lymph node evaluation at the initial surgery. These data are shown in Table 4.
TABLE 4.
Mean Total Costs Incurred by Hospitals and Payers Based on Initial Lymph Node Evaluation Procedure in the “Shave” and “No Shave” Groups
| Median Total Costs ($) Inclusive of Index Surgery and Breast Surgery-related Care Within 90 Days
|
|||
|---|---|---|---|
| Shave | No Shave | P | |
| Direct costs incurred by hospital | |||
| Type of lymph node evaluation: | |||
| None | 3681 (n = 22) | 4078 (n = 25) | 0.75 |
| SLNB alone | 5356 (n = 86) | 5387 (n = 82) | 0.17 |
| SLNB and ALND | 6344 (n = 7) | 5942 (n = 7) | 0.90 |
| ALND alone | 4935 (n = 4) | 4076 (n = 2) | 0.27 |
| Total payments incurred by payers | |||
| Type of lymph node evaluation: | |||
| None | 7075 (n = 22) | 9870 (n = 25) | 0.04 |
| SLNB alone | 11,237 (n = 83) | 11,561 (n = 80) | 1.00 |
| SLNB and ALND | 10,982 (n = 7) | 11,886 (n = 7) | 0.90 |
| ALND alone | 12,504 (n = 4) | 12,014 (n = 2) | 1.00 |
ALND indicates axillary lymph node dissection; SLNB, sentinel lymph node biopsy.
Cost Analysis From a Payer Perspective
Considering the initial procedure and any breast surgery–related care during the subsequent 90 days, the total amount paid for facility and provider fees was not significantly different between the 2 groups (mean $10,476 vs $11,219 for “shave” vs “no shave” groups, respectively, P = 0.40). The breakdown of these costs incurred by payers for facility versus provider fees is shown in Table 5. Of note, although the overall costs incurred by payers were not different between the “shave” and “no shave” groups, the professional fees paid for pathology services were higher in the “shave” group (mean $1052 vs $811, P < 0.01).
TABLE 5.
Total Payments Incurred by Payers*
| Mean Total Payments (95% CI) | “Shave” (n = 116) | “No Shave” (n = 114) | P |
|---|---|---|---|
| Facility fees | $6546 ($5690–$7403) | $7287 ($6385–$8189) | 0.17 |
| Provider fees | |||
| Anesthesia | $801 ($674–$928) | $918 ($779–$1057) | 0.38 |
| Surgery | $1810 ($1566–$2053) | $1856 ($1588–$2124) | 0.93 |
| Radiology | $239 ($199–$280) | $262 ($216–$309) | 0.47 |
| Pathology | $1052 ($910–$1194) | $811 ($685–$938) | <0.01 |
| Total provider fees | $3929 ($3433–$4426) | $3932 ($3411–$4,452) | 0.87 |
| Total payments | $10,476 ($9406–$11,545) | $11,219 ($10,065–$12,372) | 0.40 |
Includes index surgery and breast surgery–related payments in the ensuing 90 days.
CI indicates confidence interval.
Factors correlating with total costs from a payer perspective are shown in Table 6. Although not significantly different based on the randomization arm (“shave” vs “no shave”), total payments were lower in patients who had negative margins at the conclusion of the initial surgery, who required fewer re-excisions, and who were less likely to require mastectomy.
TABLE 6.
Factors Associated With Total Costs From a Payer Perspective*
| Factor | Mean Total Cost (95% CI) | P |
|---|---|---|
| Randomization arm:† | 0.40 | |
| • No shave (n = 116) | $11,219 ($10,065–$12,372) | |
| • Shave (n = 114) | $10,476 ($9406–$11,545) | |
| Margin status at index case: | <0.01 | |
| • Positive (n = 61) | $14,045 ($11,870–$16,219) | |
| • Negative (n = 169) | $9688 ($9034–$10,342) | |
| Subsequent surgery: | <0.01 | |
| • Yes (n = 47) | $16,014 ($13,621–$18,408) | |
| • No (n = 183) | $9516 ($8862–$10,170) | |
| Mastectomy: | <0.01 | |
| • Yes (n = 7) | $21,642 ($12,782–$30,503) | |
| • No (n = 223) | $10,505 ($9769–$11,241) | |
| Pathological tumor stage: | 0.44 | |
| • 0 (n = 56) | $10,687 ($8811–$12,563) | |
| • 1 (n = 119) | $10,725 ($9769–$11,680) | |
| • 2 (n = 52) | $11,066 ($9234–$12,899) | |
| • 3 (n = 3) | $14,642 ($747–$28,537) |
Actual transaction amounts incurred by payers, inclusive of facility and provider fees, for index case and any other breast surgery–related care in the ensuing 90 days.
Based on complete data available in 230 patients.
CI indicates confidence interval.
DISCUSSION
In this prospective randomized controlled trial, we found that the excision of cavity shave margins not only results in significantly fewer subsequent surgeries for margin clearance, but also does not increase payer or hospital costs. Although the initial procedure adds an average of 10 minutes to initial operative time and results in additional pathology costs, these are offset by the reduction in re-excisions. Assessing the cost implications from both the hospital and payer perspective is important, as both are increasingly focused on increasing value and reducing wasteful spending. We note that, from both perspectives, there was no difference in cost between the 2 arms. When considered in the context of the lower re-excision rate in the “shave” group, an outcome that has important implications for patients, these findings suggest that the adoption of routine excision of cavity shave margins may provide a superior patient experience without increasing costs.
Although a number of retrospective studies of cavity shave margins have found a halving of positive margin and re-excision rates similar to our study,5–10 none have objectively reported the associated costs. One study by Rizzo et al examined use of cavity shave margins, but reported primarily on use of pathology services and did not report on costs. Similar to our findings, they found that patients who had additional margins taken at the initial surgery were less likely to have positive margins at the conclusion of the initial procedure (10.8% vs 24.8%, P < 0.01), but had more histology slides reviewed than patients who had standard breast conserving surgery (mean 20.3 vs 14.2, P < 0.01).11 The Rizzo study also found that fewer slides were submitted for histopathologic review at the time of re-excision in patients who had cavity shave margins excised at the initial surgery (mean 6.4 vs 16.0, P < 0.01). This mirrors the trend toward lower direct pathology-related costs incurred by the hospital at the time of re-excision for the “shave” relative to the “no shave” arms (mean $257 vs $386, respectively, P = 0.10) in our trial.
From a payer perspective, mean payments for facility and individual provider groups were similar in the “shave” versus “no shave” group with the exception of higher costs associated with pathology provider fees in the “shave” arm. This correlates with the higher total direct costs from a hospital perspective related to pathology services. In the context of this trial, examination of a minimum of 2 sections perpendicular to each margin of the partial mastectomy specimen and the additional margins was mandated, such that quantitative margin distances on each could be specified. Given that cavity shave margins are by definition circumferential, it may only be necessary to analyze these rather than the margins on the primary specimen thereby reducing pathology-related costs. The financial impact of doing so, however, could not be assessed in this study. Future studies, however, could assess this issue.
Of note, we did not use frozen section for intraoperative margin analysis. Several studies have advocated intraoperative frozen section for margin analysis to reduce positive margin rates.12,13 This technique is associated with an additional 53 minutes of operative time,14 as opposed to 10 minutes with the routine cavity shave technique. Although a decision analysis suggested that intra-operative frozen section could be associated with cost-savings if the initial rate of re-excision was greater than 26% and could be reduced to 3%, actual costs in these studies have not been evaluated.14 Sabel et al15 reported that their re-excision rate fell from 26% to 9% using frozen section, similar to our reduction in re-excision rates from 27.6% to 10.9% using routine cavity shave margins. In a model based on their clinical experience, Sabel et al estimated a cost savings of between $400 and $800 (based on charges) per patient using frozen section for both lymph nodes and margins. In Sabel’s study, however, it is unclear what fraction of these savings is related to use of frozen section for lymph node evaluation versus margins. Although we did not perform frozen sections on margins, we did use this technique for sentinel node evaluation in both arms of the trial. Furthermore, without data regarding the payer mix or payment-to-charge ratio in their patient population, it is difficult to compare Sabel’s $400 to $800 estimate of savings (based on charges) to our results.
Others have used gross assessment by the pathologist, with frozen section only if deemed necessary. In a study of 2 hospitals that differed in their use of intraoperative assessment, Uecker et al16 found that the re-excision rate was significantly higher when no intraoperative assessment was used (60% vs 24%, P < 0.05). Although costs of the index surgery were higher when intraoperative assessment was used ($14,562 vs $11,786, P < 0.05), the total surgical costs inclusive of re-excisions were lower ($15,341 vs $22,013, P < 0.05). It is difficult to compare Uecker’s data to ours, however, as they do not specify whether the “costs” listed in their study pertain to actual payments, direct costs, or charges. Of note, the “costs” in the Uecker study were significantly higher than either the direct hospital costs (from a hospital perspective) or the actual payments received (from a payer perspective) in our study. Furthermore, although our data are homogeneous, in that all cases were done in 1 hospital setting with 1 practice plan for providers, and were randomly distributed between the 2 arms, theirs involved 2 different facilities with presumably different providers that were unevenly distributed between the 2 groups in their study. This may have contributed to the cost difference between the 2 groups in their study.
More recently, novel technology has been touted to aid surgeons in identifying and resecting potentially positive margins intraoperatively. Devices such as MarginProbe have been found to reduce positive margin rates by 23% to 56%, but costs associated with intraoperative time, pathology evaluation, and capital equipment costs have not been reported.17,18 Similarly, Ueo et al19 recently reported a novel fluorescent probe for intraoperative evaluation of margins during breast conserving surgery; again, costs have not been elucidated.
Despite the strengths of our study, we note several limitations. In particular, our study perspective is limited to that of the hospital and the payer, but we were unable to incorporate the patient perspective.20 Re-excision procedures are associated with financial costs to the patient associated with increased time off work, childcare, transportation, and parking. In addition, there is the emotional burden patients endure with a second operative procedure. These costs would be expected to be higher in the “no shave” group due to higher re-excision rates. Taking these factors into account would only add to the value of the “shave” technique. Although we cannot assess actual financial costs associated with this technique from a patient’s perspective, we plan on assessing patient satisfaction with the procedure (from an emotional, financial, and cosmetic standpoint) at 5 years, including an assessment of quality of life.
Although its impact on local recurrence remains to be evaluated based on long-term follow-up in this trial, we have demonstrated that excising routine cavity shave margins requires no additional capital cost, adds only 10 minutes to operative time, and is associated with a lower positive margin and re-excision rate. Although the technique is associated with a higher cost at the initial procedure, this is offset by the significant reduction in re-excision rates it provides.
Acknowledgments
This study was supported by the Yale Cancer Center.
This work was funded by Yale Cancer Center.
Footnotes
To be presented at the San Antonio Breast Cancer Symposium on December 10, 2015, in San Antonio, TX.
Trial Registration: ClinicalTrials.gov: NCT01452399
The authors report no conflicts of interest.
References
- 1.Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Ann Surg Oncol. 2014;21:704–716. doi: 10.1245/s10434-014-3481-4. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts & Figures 2016. Atlanta: American Cancer Society; 2016. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. [Google Scholar]
- 3.Chagpar AB, Killelea BK, Tsangaris TN, et al. A randomized, controlled trial of cavity shave margins in breast cancer. N Engl J Med. 2015;373:503–510. doi: 10.1056/NEJMoa1504473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. Breast Cancer (Version 2.2016) https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed May 10, 2016.
- 5.Kobbermann A, Unzeitig A, Xie XJ, et al. Impact of routine cavity shave margins on breast cancer re-excision rates. Ann Surg Oncol. 2011;18:1349–1355. doi: 10.1245/s10434-010-1420-6. [DOI] [PubMed] [Google Scholar]
- 6.Unzeitig A, Kobbermann A, Xie XJ, et al. Influence of surgical technique on mastectomy and reexcision rates in breast-conserving therapy for cancer. Int J Surg Oncol. 2012;2012:725121. doi: 10.1155/2012/725121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marudanayagam R, Singhal R, Tanchel B, et al. Effect of cavity shaving on reoperation rate following breast-conserving surgery. Breast J. 2008;14:570–573. doi: 10.1111/j.1524-4741.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 8.Cao D, Lin C, Woo SH, et al. Separate cavity margin sampling at the time of initial breast lumpectomy significantly reduces the need for reexcisions. Am J Surg Pathol. 2005;29:1625–1632. doi: 10.1097/01.pas.0000180448.08203.70. [DOI] [PubMed] [Google Scholar]
- 9.Tengher-Barna I, Hequet D, Reboul-Marty J, et al. Prevalence and predictive factors for the detection of carcinoma in cavity margin performed at the time of breast lumpectomy. Mod Pathol. 2009;22:299–305. doi: 10.1038/modpathol.2008.186. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson AF, Asad J, Boolbol SK, et al. Do additional shaved margins at the time of lumpectomy eliminate the need for re-excision? Am J Surg. 2008;196:556–558. doi: 10.1016/j.amjsurg.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Rizzo M, Iyengar R, Gabram SG, et al. The effects of additional tumor cavity sampling at the time of breast-conserving surgery on final margin status, volume of resection, and pathologist workload. Ann Surg Oncol. 2010;17:228–234. doi: 10.1245/s10434-009-0643-x. [DOI] [PubMed] [Google Scholar]
- 12.Tan MP, Sitoh NY, Sim AS. The value of intraoperative frozen section analysis for margin status in breast conservation surgery in a nontertiary institution. Int J Breast Cancer. 2014;2014:715404. doi: 10.1155/2014/715404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boughey JC, Hieken TJ, Jakub JW, et al. Impact of analysis of frozen-section margin on reoperation rates in women undergoing lumpectomy for breast cancer: evaluation of the National Surgical Quality Improvement Program data. Surgery. 2014;156:190–197. doi: 10.1016/j.surg.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 14.Osborn JB, Keeney GL, Jakub JW, et al. Cost-effectiveness analysis of routine frozen-section analysis of breast margins compared with reoperation for positive margins. Ann Surg Oncol. 2011;18:3204–3209. doi: 10.1245/s10434-011-1956-0. [DOI] [PubMed] [Google Scholar]
- 15.Sabel MS, Jorns JM, Wu A, et al. Development of an intraoperative pathology consultation service at a free-standing ambulatory surgical center: clinical and economic impact for patients undergoing breast cancer surgery. Am J Surg. 2012;204:66–77. doi: 10.1016/j.amjsurg.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 16.Uecker JM, Bui EH, Foulkrod KH, et al. Intraoperative assessment of breast cancer specimens decreases cost and number of reoperations. Am Surg. 2011;77:342–344. [PubMed] [Google Scholar]
- 17.Schnabel F, Boolbol SK, Gittleman M, et al. A randomized prospective study of lumpectomy margin assessment with use of MarginProbe in patients with nonpalpable breast malignancies. Ann Surg Oncol. 2014;21:1589–1595. doi: 10.1245/s10434-014-3602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allweis TM, Kaufman Z, Lelcuk S, et al. A prospective, randomized, controlled, multicenter study of a real-time, intraoperative probe for positive margin detection in breast-conserving surgery. Am J Surg. 2008;196:483–489. doi: 10.1016/j.amjsurg.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 19.Ueo H, Shinden Y, Tobo T, et al. Rapid intraoperative visualization of breast lesions with gamma-glutamyl hydroxymethyl rhodamine green. Sci Rep. 2015;5:12080. doi: 10.1038/srep12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim P. Cost of cancer care: the patient perspective. J Clin Oncol. 2007;25:228–232. doi: 10.1200/JCO.2006.07.9111. [DOI] [PubMed] [Google Scholar]


