Abstract
The aim of the present study was to determine the effect of different cryoprotectants and their concentration on the physicochemical characteristics of chitosan nanoparticles (CS-NPs). The effect of coating of CS-NPs with hyaluronic acid (HA) and alginic acid (ALG) before and after lyophilization was also evaluated. The ionic gelation method was used for the preparation of NPs and six different types of cryoprotectants (sucrose, glucose, trehalose, mannitol, polyethylene glycol-2000, and polyethylene glycol-10,000) were investigated at 5%, 10%, 20%, and 50% concentration levels. Coating of CS-NPs with HA and their protection with high amount of cryoprotectants indicated better particle size stability. Samples that were lyophilized without cryoprotectants resulted in an increase in average size due to high agglomeration. All cryoprotectants with varying amount provided some sort of size stability for the NPs except for the PEG-10,000 which had no protective effect at higher concentrations. Sucrose and trehalose sugars were found to have the highest protective effect with HA coated and uncoated CS-NPs. In conclusion, using cryoprotectants along with surface coating, the CS-NPs could achieve the desired physicochemical characteristics for a prolonged duration.
Keywords: Chitosan, Cryoprotectant, Hyaluronic-acid, Alginic-acid, Lyophilization, Nanoparticle
1. Introduction
Due to exceptional unique physical and biological characteristics, the polysaccharides have got prime attention as a carrier in drug delivery systems (Liu et al., 2008). Chitosan (CS) is a cationic polysaccharide derived from chitin which is a biopolymer, isolated from the exoskeleton of crustaceans, and some fungi (Sagheer et al., 2009). CS has drawn great attention in industrial areas such as paper technology, food, water filtration, agriculture, pharmaceuticals and biomedical industries (Jain et al., 2014). Use of CS for NPs preparation has been broadly employed for delivery of different kinds of payloads including drugs, proteins, peptides, genes, DNA, because of its numerous attractive features such as less immunogenic and relatively low toxic, excellent biocompatibility and biodegradability (Almalik et al., 2013a). The CS-NPs have found to have larger activated surface area than other physical forms of materials which support drug loading capacity, long shelf life, good permeability to epithelia and convenience of transporting into the body owing to its unique structure (Gokce et al., 2014). CS-NPs could be synthesizing by different methods such as ionic gelation, synthesizing with carboxymethyl cellulose using glutaraldehyde as cross-linker, with alginic acid or hyaluronic acid, coacervation, reverse micellar and polymerization with poly (hydroxyethyl methacrylate) techniques (Fabregas et al., 2013, Jain et al., 2014). Among these methods ionic gelation technique is widely preferred for the preparation of CS-NPs (de Pinho Neves et al., 2014). The formation of particles using ionic gelation process is somewhat mild and avoids demanding organic solvents and high temperatures allowing effective encompassing of delicate molecules without any damage and loss (Fan et al., 2012). This method principally depends up on ionic interactions between cationic chains of CS and anionic charged polyanions e.g. tripolyphosphate (TPP) as cross linker (Deng et al., 2014).
Besides encapsulation and drug loading CS-NP‘s could also be coated with certain materials to enhance their efficacy, better targeting and to make them more specific as a carrier (Almalik et al., 2013a). Polyionic nano-complexes (PICMs) composed of polycations and opposite polyanions, have been found to have a great potential in biomedical and nano-biotechnological applications including controlled drug release and gene transfection (Deng et al., 2014). Among numerous polyionic nano-complexes, hyaluronic acid based chitosan nanoparticles (HA-CS-NPs) and alginic acid based chitosan nanoparticles (ALG-CS-NPs) have been investigated fundamentally in recent years (Azevedo et al., 2014, Gokce et al., 2014). The coating of CS-NPs causes the conversion of surface charge of the NPs. HA and sodium-ALG have been found to provide negative charges on the surface of NPs as HA and ALG molecules are mainly present in the outer shell of the NPs (Borges et al., 2006). However positively charged nanocarriers enhance membrane association, internalization and endosomal escape, while those bearing a negative zeta potential has shown more specific and efficient uptake, especially when coated with targeting ligands (Almalik et al., 2013b).
The ionic gelation method causes the aggregation and fusion of NPs after synthesis when stored for long time which might be due to the restricted physicochemical stability of NP-suspensions (Gokce et al., 2014). Physical instability frequently occurs when NPs were stored for long term in aqueous medium which restricted their use (Rampino et al., 2013). Freeze drying of NP-suspension prevents the aggregation and fusion of particles also maintains the size-stability during long-term storage of NPs. However, if the NPs are lyophilized without any cryoprotectant the formation of aggregates can greatly hinder the re-dispersion of NPs, and thus affect their sizes (Fabregas et al., 2013). Similarly, CS-NPs form aggregation after freeze-drying due to inter-and intramolecular hydrogen bonding unless the cryoprotectant is used (Abdelwahed et al., 2006). Hence, cryoprotectants must be used during the freeze-drying of CS-NPs to remove excess moisture and increase long-term storage and to protect the CS-NPs size stability. The use of trehalose, mannitol, sorbitol or glycerol as cryoprotectant is an efficient way during freeze-drying of NPs to maintain their physical properties (Gokce et al., 2014). The purpose of this study was first to develop coated and uncoated CS-NPs by ionic gelation method. The desired sized coated CS-NPs were developed with the ideal formulation conditions. After their synthesis, the CS-NPs were mixed with different cryoprotectants at different ratios and lyophilized to get the freeze-dried product of CS-NPs. The CS-NPs were further subjected to physicochemical characterization by using zeta-sizer to measure particle size, their population, and size distribution before and after lyophilization to determine the best suitable cryoprotectants and their optimum combination or mixture to get the best sized and stable CS-NPs.
2. Materials and methods
2.1. Materials
Chitosan (deacetylation degree over 60% mol, from white mushrooms; Sigma, UK), 1 M hydrochloric acid (HCl), 1 M sodium hydroxide (NaOH) and sodium triphosphate pentabasic (TPP) were obtained from Sigma–Aldrich (Gillingham, UK); hyaluronic acid (HA) 200 kDa was obtained from Medipol SA (Lausanne, Switzerland) and Sodium Alginate (400 kDa) was obtained purchased from Sigma, UK. Glacial acetic acid and sodium acetate were purchased from VWR, BDH Chemicals (Poole, UK). Trehalose dihydrate was obtained from Merck (Darmstadt, Germany). Sucrose, glucose, mannitol, polyethylene glycols as PEG 2,000 and PEG 10,000 were obtained from Sigma-Aldrich Co. (St. Louis, Missouri). The RC-dialysis membrane of MWCO 10 kDa was obtained from (Spectra Por, Spectrum Laboratories Inc., Rancho Dominguez CA, USA). The other chemicals and reagents were of AR grade and were used as received.
2.2. Formulation of uncoated CS-NPs
The CS-NPs were prepared by ionic gelation method using tripolyphosphate (TPP) as crosslinking agent. Accurately 0.069%w CS solution was prepared by dissolving purified CS in 4.6 mM HCl and the pH of this solution was adjusted to 5 by the addition of appropriate volumes of 0.1 M NaOH. The obtained solution was kept on magnetic stirring overnight prior to use. A 0.1%w tripolyphosphate (TPP) was dissolved in deionized water and the pH of the solution was maintained to 5 with 0.1 M HCl. Both the solutions were filtered through a 0.22 μm size Millipore® filter. Exactly 215 μL of the TPP solution was added to the CS-solution to get a final volume of 3 mL leading to 9:1 CS: TPP mass ratio and the actual concentrations of CS and TPP in terms of percentage weight (%w), reached to 0.064 and 0.0071 respectively (Almalik et al., 2013a).
2.3. Development of HA and ALG-coated NPs
The prepared CS-NPs were subjected for coating with HA and ALG. Briefly, chitosan-TPP NPs were dispersed at a concentration of 0.025 weight percent (wt%) in 0.1 M acetic acid/acetate buffer at pH 5 and mixed by using magnetic stirring (500 rpm for 15 min). The dispersions were then slowly added under the continuous and vigorous stirring at 1200 rpm for 30 min to an equal amount and an equal strength of acetate buffer, containing HA (200 KDa) and ALG separately at a concentration of 1.5 mg mL−1 (Almalik et al., 2013b). The obtained dispersions were then dialyzed against deionized water by using dialysis membrane (MWCO = 10 KDa). Then, equal volumes of HA-coated and uncoated CS-NPs in deionized water were lyophilized and reweighted, and the actual dry weight for HA-coated CS-NPs was roughly double than that of the uncoated CS-NPs, suggesting a 0.5 HA weight fraction (Almalik et al., 2013a).
2.4. Particle size, polydispersity and zeta potential measurement
Hydrodynamic diameter (Z-average), polydispersity (PDI) and zeta potential measurements were always measured on three independent samples of the CS-NPs at 25 °C temperature using a Malvern Zetasizer Nano-ZS (ZEN3600, Malvern Instruments Ltd, UK) equipped with a solid state HeNe laser (633 nm λmax) at 173 degree scattering angle. The hydrodynamic diameter and particle size distribution were determined by Dynamic Light Scattering (DLS) also known as Photon Correlation Spectroscopy (PCS) or Quasi-Elastic Light Scattering (QELS) which is the most popular light scattering technique as it permits particle sizing even down to a diameter of 1 nm. Dynamic light scattering technique determines the diffusivity of the particles in the suspension based on the time-dependent fluctuations in the intensity of scattered light resulting from the Brownian motion. Size of the particles is therefore calculated from the Stokes-Einstein equation.
2.5. Lyophilization of CS-NPs and their recovery
The freshly prepared CS-NPs were filtered through 0.22 μm hydrophilic Millipore® syringe filters (to remove the lumps formed during ionic gelation of CS and TPP) and subjected to the process of lyophilization by using FreeZone Triad Cascade Benchtop Freeze Dry System (Labconco, Missouri, USA). About 1 mL of each ALG and HA coated CS-NPs suspensions, was freeze-dried with and without using cryoprotectants (glucose, sucrose, trehalose, mannitol, PEG 2000, or PEG 10,000) at 5%, 10%, 20%, and 50%, w/v concentration levels. All the samples were freeze-dried in triplicate. The uncoated CS-NPs and HA and ALG coated CS-NPs were stored at room temperature for 3 months to perform further characterization by DLS measurement at specific time points (initial, 1st month and at 3rd month). The lyophilized NPs were then re-dispersed in 1 mL deionized water and dialyzed against deionized water through dialysis membrane (MWCO = 10 KDa) in order to remove the excess amount of cryoprotectant and subjected to DLS measurement for the determination of particle size (Z-average), polydispersity and size distributions and zeta potentials. All the measurements were done in triplicate and the results were expressed in terms of ratio of Z-average before and after the process of lyophilization.
3. Results
3.1. Formulation of CS-NPs, HA- and ALG-coated CS-NPs
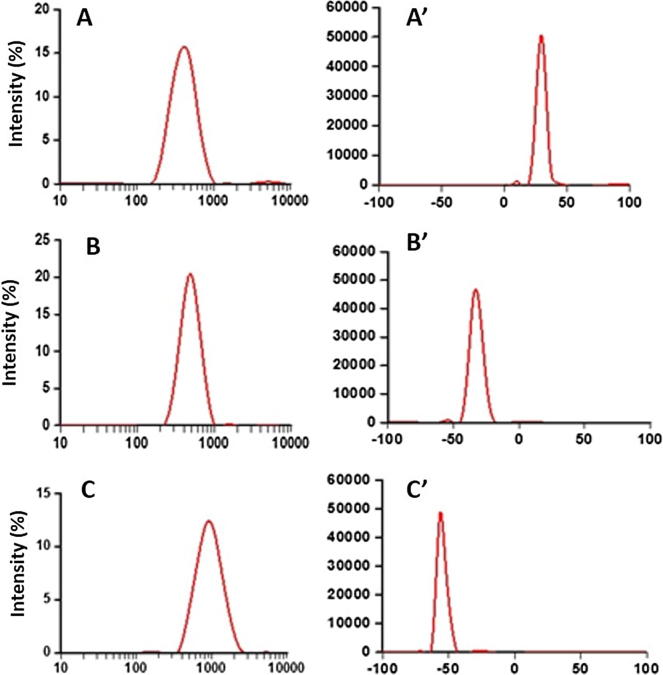
The ionic gelation method for the preparation of CS-NPs as well as the HA and ALG coated CS-NPs was found to be an effective technique which was confirmed by the DLS measurements for particle size distribution (Fig. 1A–C), zeta potential measurements and estimation of polydispersity index of the prepared NPs. Zeta potentials were recorded for the three different types of the NPs directly after synthesis and conversion of surface charge of NPs was found apparent which can be seen in the zeta potential curves where the positive zeta potential value of CS-NPs (uncoated) changed to negative values when CS-NPs were coated with HA and ALG (Fig. 1A’, 1B’ and 1C’).
Figure 1.
Particle size (nm) distributions for uncoated CS-NPs (A), HA-coated CS-NPs (B) and ALG-coated CS-NPs (C); zeta potential (mV) distributions for uncoated CS-NPs (A’), HA-coated CS-NPs (B’) and ALG-coated CS-NPs (C’).
The average size of CS-NPs (uncoated) was found around (298.11 ± 20.15) nm, while coating of these CS-NPs with HA increased an average particle size around 100 nm (390.84 ± 27.28 nm) and shifting of zeta potential value from positive (+34.3 ± 5.2) to negative (−36.1 ± 5.4) was found, which was due to the polyanionic nature of the HA molecules. Similarly, for the ALG coated NPs the particle size was increased by more than double (843.42 ± 70.54 nm) that of the HA coated NPs and around three times than that of the CS-NPs. Moreover, a great shifting of zeta potential value from a positive (+34.3 ± 5.2) to a high negative (−64.2 ± 8.2) was found (Table 1). These apparent changes in the zeta potential values were expected, because primarily, ALG has high negative charge density (approximately 2-fold than that of HA), secondly, ALG could be adsorbed rapidly and efficiently on the highly cationic surfaces of CS-NPs and hence, resulted in a significantly higher negative surface charge density on the coated NPs which was the main cause of the significant increase in the size of the coated NPs (Table 1) (Almalik et al., 2013c). The redispersion of coated, lyophilized CS-NPs in deionized water was found to have variable effects even at the same concentration levels of the same cryoprotectant, which was due to the electrostatic interaction effects between the surface of CS-NPs and the coating materials used i.e. hyaluronic and alginic acids to alter their surface properties. NPs that were lyophilized without using the cryoprotectant showed significant aggregations indicated by the increase in the values of their polydispersity before and after lyophilization as seen in Table 2. Moreover, this can be evidenced by the sufficient aggregation of NPs (observed by optical microscopy) after freeze-drying of coated and uncoated CS-NPs. The aggregation was observed in all the three NPs (Table 2), the minimum aggregation was found in case of HA-coated CS-NPs, and moderately higher aggregation was observed in case of ALG-coated CS-NPs when compared with the uncoated CS-NPs where the highest magnitude of aggregation was observed (Table 2). The lowest value of aggregation in HA-coated CS-NPs was also clearly evidenced by the lowest value of polydispersity among the three NPs (Kalam, 2016). The effect of cryoprotection was found even better in case HA-coated NPs as compared to the uncoated one, as it has resulted lowest aggregation with minimum values of polydispersity index in HA-coated F2. The higher aggregation was observed in uncoated F1 after freeze drying (Table 2), which might be due to the expansion of CS-NPs and fracture of CS-matrix that caused the inflow of aqueous phase into CS-NPs by the mechanism of imbibition and osmosis due to the presence of hydrophilic CS and TPP as excipients in the CS-NPs.
Table 1.
Physical characteristics of uncoated and coated CS-NPs measured by Zetasizer (mean ± SD, n = 3).
| Optimized formulations | Particle size (nm) ± SD | Polydispersity (PDI) ± SD | Zeta potential (mV) ± SD |
|---|---|---|---|
| Uncoated CS-NPs | 298.11 ± 20.15 | 0.356 ± 0.034 | 34.3 ± 5.2 |
| CS-NPs coated with HA | 390.84 ± 27.28 | 0.123 ± 0.041 | −36.1 ± 5.4 |
| CS-NPs coated with ALG | 843.42 ± 70.54 | 0.566 ± 0.054 | −64.2 ± 8.2 |
Table 2.
Physical characteristics of uncoated and coated CS-NPs before and after lyophilization without using cryoprotectant (mean ± SD, n = 3).
| Parameters | Before lyophilization |
After lyophilization |
||||
|---|---|---|---|---|---|---|
| Uncoated CS-NPs | HA-coated CS-NPs | ALG-coated CS-NPs | Uncoated CS-NPs (F1) | HA-coated CS-NPs (F2) | ALG-coated CS-NPs (F3) | |
| Polydispersity index | 0.356 ± 0.055 | 0.123 ± 0.015 | 0.566 ± 0.038 | 0.503 ± 0.056 | 0.351 ± 0.134 | 0.769 ± 0.162 |
| Particle size (nm) | 298.11 ± 20.15 | 390.84 ± 27.28 | 843.42 ± 51.74 | 346.33 ± 13.16 | 398.62 ± 13.98 | 925.36 ± 57.89 |
| Zeta potential (mV) | +34.3 ± 5.2 | −36.1 ± 5.4 | −64.2 ± 8.2 | +35.5 ± 4.5 | −34.7 ± 3.9 | −62.3 ± 5.8 |
| Aggregation* | – | – | – | +++ | + | ++ |
Aggregation: “+” = minimum, “++” = medium and “+++” = maximum.
3.2. Effect of cryoprotectant on CS-NPs, HA- and ALG-coated CS-NPs
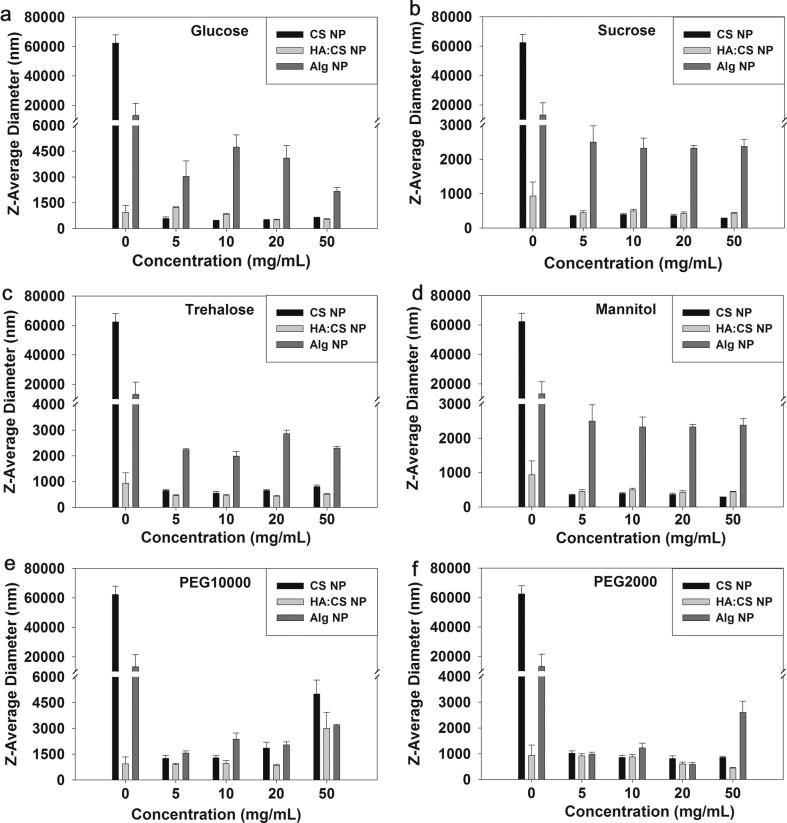
The effects of varying concentration of different sugars and glycols as cryoprotectant were investigated well on the three types of developed CS-NPs. The best protection of particle size was found when sucrose as cryoprotectant (at 5–50%, w/v concentrations) was used in case of uncoated CS-NPs, as there was no particle size increase due to the fact that aggregation was not observed due to the use of cryoprotectant which could be represented well in Fig. 2, that is clearly evidenced by the polydispersity index measurements of the aqueous dispersion of CS-NPs at varying cryoprotectant concentrations (Table 3A). It was observed that a poor quality of protection was observed in case of uncoated and ALG-coated CS-NPs when mannitol and PEG-10,000 were used as cryoprotectants. The other cryoprotectants used with CS-NPs showed little protection as there was a distinct increase in average particle size observed after re-dispersion in deionized water which was attributed to sufficient aggregation of the NPs (Fig. 2a–f).
Figure 2.
Variations in the average particle sizes of uncoated CS-NPs, HA-coated CS-NPs and ALG-coated CS-NPs, due to the effect of varying concentrations of different cryoprotectants.
Table 3.
Polydispersity index of coated and uncoated CS-NPs with different concentrations of different cryoprotectants (mean ± SD, n = 3).
| Concentration of cryoprotectant (w/v) | Polydispersity index at different levels of different cryoprotectants |
|||||
|---|---|---|---|---|---|---|
| Glucose | Sucrose | Trehalose | PEG 10,000 | PEG 2,000 | Mannitol | |
| ||||||
| 5% | 0.485 ± 0.014 | 0.432 ± 0.041 | 0.617 ± 0.181 | 0.986 ± 0.025 | 0.996 ± 0.007 | 0.996 ± 0.007 |
| 10% | 0.478 ± 0.058 | 0.405 ± 0.066 | 0.515 ± 0.072 | 0.635 ± 0.158 | 0.561 ± 0.059 | 0.995 ± 0.008 |
| 20% | 0.497 ± 0.004 | 0.425 ± 0.084 | 0.724 ± 0.159 | 0.814 ± 0.151 | 0.611 ± 0.105 | 0.996 ± 0.006 |
| 50% | 0.615 ± 0.129 | 0.408 ± 0.081 | 0.816 ± 0.232 | 0.674 ± 0.454 | 0.598 ± 0.106 | 0.996 ± 0.006 |
| ||||||
| 5% | 0.597 ± 0.181 | 0.392 ± 0.037 | 0.287 ± 0.022 | 0.378 ± 0.062 | 0.516 ± 0.022 | 0.587 ± 0.081 |
| 10% | 0.591 ± 0.207 | 0.386 ± 0.094 | 0.351 ± 0.045 | 0.310 ± 0.034 | 0.402 ± 0.057 | 0.503 ± 0.063 |
| 20% | 0.383 ± 0.050 | 0.323 ± 0.075 | 0.309 ± 0.084 | 0.347 ± 0.069 | 0.324 ± 0.017 | 0.462 ± 0.089 |
| 50% | 0.368 ± 0.031 | 0.361 ± 0.043 | 0.403 ± 0.088 | 0.878 ± 0.211 | 0.321 ± 0.023 | 0.464 ± 0.051 |
| ||||||
| 5% | 0.959 ± 0.069 | 0.987 ± 0.023 | 0.996 ± 0.006 | 0.919 ± 0.139 | 0.719 ± 0.243 | 0.996 ± 0.006 |
| 10% | 0.995 ± 0.007 | 0.966 ± 0.059 | 0.851 ± 0.258 | 0.899 ± 0.123 | 0.885 ± 0.198 | 0.965 ± 0.059 |
| 20% | 0.945 ± 0.079 | 0.858 ± 0.122 | 0.789 ± 0.032 | 0.709 ± 0.222 | 0.738 ± 0.099 | 0.993 ± 0.013 |
| 50% | 0.844 ± 0.271 | 0.982 ± 0.031 | 0.805 ± 0.179 | 0.742 ± 0.446 | 0.996 ± 0.007 | 0.965 ± 0.061 |
All the used cryoprotectants that could not protect well the HA-coated different NPs, a 20%, w/v, concentration of all the used cryoprotectant showed supreme protection with HA-coated CS-NPs as evidenced in Fig. 2a–f, indicating that the coating of CS-NPs with HA can function along with cryoprotectants in the shielding of the HA-coated NPs during lyophilization to conserve their sizes. The effects of the cryoprotectants such as glucose, sucrose, trehalose and mannitol on particle sizes of all the three types of NPs are represented well in Fig. 2a–d, respectively. At 50%, w/v concentration levels of cryoprotectants (except PEG 10,000) a sufficient increase in particle sizes were observed especially in case of ALG-coated CS-NPs. Moreover, at the other concentration level of cryoprotectants, the HA-coated CS-NPs were found to have comparatively closest sizes when relating to freshly prepared NPs except for the PEG-10,000 at 50%, w/v, concentration level (Fig. 2e), indicating sufficient aggregation of HA-coated CS-NPs that can also be supported by the increased value of polydispersity index of HA-coated CS-NPs with 50%, w/v, concentration level of PEG-10,000 as cryoprotectant (Table 3B).
Unpredictably, the ALG-coated chitosan-NPs, PEG-2000 showed the best results in their protection at 5–20%, w/v, concentration levels of the used cryoprotectant (Fig. 2f), to have less effect on particle size disturbance, while the polydispersity index was highly increased (Table 3C). After all the investigating effects for the utilization of sucrose, glucose, trehalose, mannitol, PEG-2000 and PEG-10,000 as cryoprotectants in the process of lyophilization, in general the best results were obtained with sucrose (Fig. 2b) and trehalose (Fig. 2c), especially when they were used to protect the HA-coated CS-NPs. The two sugars namely sucrose and trehalose provided the size conservation of the developed HA-coated CS-NPs and protected well at all the investigated concentration levels in the present study as well as also resulted in the lowest polydispersity values for HA-coated CS-NPs with all of the other used cryoprotectants.
3.3. Physical stability of coated and uncoated CS-NPs
The physical stability characterization parameters by DLS measurement of freeze-dried samples of NPs i.e. the uncoated CS-NPs and HA and ALG coated CS-NPs for a period of 3 months were analyzed at 1 month and at 3 month storage period at 25 °C temperature. After 1 month and 3 months of storage, there were only small changes in the different physical parameters were found in the case of coated-CS-NPs; however, a noticeable change in particle size was found in case of uncoated CS-NPs (Table 4). The mean particle sizes and polydispersity were found to increase while the magnitude of zeta-potentials was deceasing in the case of all CS-NPs, but the variation in particle size was not so much obvious in case of HA and ALG-coated CS-NPs, while it was significantly high in CS-NPs.
Table 4.
Effect of storage on particle size, zeta potential and polydispersity of coated and uncoated CS-NPs with 10% glucose for 3 months at 25 °C (mean ± SD, n = 3).
| For 3 months storage period at 25 °C | Particle size (nm) | Zeta-potential (mV) | Polydispersity index |
|---|---|---|---|
| At initial time | |||
| Uncoated CS-NPs | 302.18 ± 12.24 | +34.3 ± 5.2 | 0.356 ± 0.034 |
| HA-coated CS-NPs | 397.49 ± 17.58 | −36.1 ± 5.4 | 0.123 ± 0.041 |
| ALG-coated CS-NPs | 845.37 ± 21.28 | −64.2 ± 8.2 | 0.566 ± 0.054 |
| After 1 month | |||
| Uncoated CS-NPs | 331.54 ± 12.56 | +32.3 ± 4.3 | 0.378 ± 0.041 |
| HA-coated CS-NPs | 402.75 ± 18.24 | −30.6 ± 3.9 | 0.184 ± 0.063 |
| ALG-coated CS-NPs | 851.53 ± 23.16 | −61.2 ± 6.5 | 0.635 ± 0.071 |
| After 3 months | |||
| Uncoated CS-NPs | 345.66 ± 13.24 | +30.5 ± 3.6 | 0.392 ± 0.046 |
| HA-coated CS-NPs | 409.71 ± 17.54 | −28.5 ± 4.1 | 0.205 ± 0.034 |
| ALG-coated CS-NPs | 858.44 ± 22.35 | −58.6 ± 7.2 | 0.694 ± 0.085 |
4. Discussion
4.1. Formulation of CS-NPs, HA-coated, ALG-coated-NPs and their cryoprotection
In this study we prepared and characterized CS-NPs, HA-coated and ALG-coated CS-NPs, by evaluating their size stability before and after lyophilization with different cryoprotectants and at varying concentrations. HA is a natural, non-toxic and biodegradable polysaccharide widely distributed in the connective tissues and has beneficial effects throughout the body (Oyarzun-Ampuero et al., 2009). ALG is of algal origin and widely used in pharmaceuticals and biomedical applications, including drug delivery as a carrier due to its inherent biodegradable, biocompatible and mucoadhesive properties (Azevedo et al., 2014, Motwani et al., 2007). Thus, using HA and ALG as coating material for CS-NPs would be safely accepted.
The results of DLS measurement analysis indicated the particles with an anticipated size distribution that could be very reproducible under the correct conditions. However, the NPs are unstable and the particles will agglomerate after long-term storage without lyophilization with cryoprotectants, so lyophilization is important for continuing preservation of particle size for long-term storage. To prevent the significant agglomeration, degradation of NPs, and contents leakage from the loaded NPs use of cryoprotectant during lyophilization is needed (Abdelwahed et al., 2006, Gokce et al., 2014, Rampino et al., 2013).
The NPs-suspensions to be lyophilized are frozen that allow the ice crystal formation of water so samples become concentrated. In case of NPs further crystallization of water takes place in the samples (Gokce et al., 2014), that could have some negative impacts such as freezing, mechanical and dehydration stresses on the stability of NPs, and such stresses may cause the NPs destabilization (Abdelwahed et al., 2006).
4.2. Cryoprotection and its mechanism
The stabilization and prevention from the degradation of a molecule during freeze-drying and storage are known as cryoprotection (Townsend and DeLuca, 1988). The CS-NPs prepared and lyophilized without using cryoprotectant were found to have difficulty in their re-dispersion and showed sufficient aggregation. Hence, a protective agent must be added in lyophilizing samples to prevent the aggregation of NPs. For long-term storage of the NPs, freeze-drying with cryoprotectants and coating is recommended due to their fruitful effects on NPs size and distribution stability during the lyophilization.
For the purpose of cryoprotection and cryopreservation of lyophilized samples different sugars are commonly used (Abdelwahed et al., 2006). The protection mechanism by sugars are due to their ability to remain in amorphous forms when are lyophilized, and preserve the NPs in a “pseudo-hydrated” form when dehydrated due to the interactions of cryoprotectants with the NPs via hydrogen bonding (Rampino et al., 2013). This capability of sugars provides a shield from ice-crystals that would damage the NPs during lyophilization and in later stages during their dispersion. After lyophilization, most of the uncoated-NPs with different concentrations of sucrose, glucose or trehalose as cryoprotectant exhibited almost common behavior and those were more similar to newly synthesized NPs. A significant increase in particle size was observed in coated NPs and their reconstitution in water needed a long time when the mannitol, PEG-2000 and PEG-10,000 were used as cryoprotectants.
It was found that the excess amounts of cryoprotectant may cause agglomeration; hence, their type and quantity must be considered during the lyophilization of NPs. This effect is the explanation for the PEG-10,000, when it was used as cryoprotectant, it resulted an obvious particle size increase when measured without the same cryoprotectant.
The best results were obtained when trehalose and sucrose were used as cryoprotectants in case of HA coated NPs, which could be due to the low hygroscopicity of these sugars. The hygroscopicity of such sugars enables them to attract and hold water molecules from the surrounding environment. Moreover, a scientist Crowe in 1996 has described that these sugars do not have internal hydrogen bonding (Crowe et al., 1996), so, they facilitate an increase in hydrogen bonds formation with the NPs. Low chemical reactivity is the other feature of these cryoprotectants. Finally, sugars are recognized to vitrify and change into glass or a glass-like substance typically by exposure to the specific higher glass transition temperature (Abdelwahed et al., 2006, Gokce et al., 2014, Rampino et al., 2013).
HA is also has water retention property that is defined in terms of intrinsic viscosity (the volume of water held together by unit weight of the material of interest), which is a measure of hydrodynamic volume. HA has a very large hydrodynamic volume, which is made possible by its unique structural characteristics and high molecular weight. The viscosity of even very dilute HA solutions was extreme higher than that of the solvent used, due to the large differences in size between the HA and water molecules. The factors such as high intrinsic viscosity and larger hydrodynamic volume of HA, as well as the hygroscopicity of sucrose and trehalose would probably contributed to an effective cryoprotection which was observed after lyophilization of HA-coated CS-NPs (Shimada and Matsumura, 1975).
4.3. Physical stability of CS-NPs
The increase in particle size in case of uncoated CS-NPs was assumed due to the swelling and aggregation characteristics of CS in aqueous environment. When the CS-NP was stored in aqueous suspension form, there would be fast diffusion of aqueous phase into the CS-NPs, and such diffusion was much high in case of uncoated CS-NPs as compared to coated ones, and the mechanism of protonation of CS-molecules reorganizes the constituent molecule, which was attributed to the significant increase in uncoated CS-NPs size during storage (Gan et al., 2005). The physical stability results suggested that the rate of changes of uncoated particle size was significantly greater and faster when it was compared with HA and ALG-coated CS-NPs, which indicated the involvement of aggregates and cluster formation of uncoated CS-NPs and caused the higher particle size enhancement during storage. The mechanism of such aggregation was though because of the collision, which in turn causes adhesion of CS-NPs during long-term storage at room temperature (Kalam, 2016). In another postulation, the water absorption nature and swelling behavior of CS and tripolyphosphate were assumed the prime reasons for the enlargement and expansion of CS-NPs (Jain et al., 2014, Kalam, 2016, Shimada and Matsumura, 1975).
5. Conclusion
The ionotropic gelation technique was found perfect to formulate the CS-NPs. An obvious change in the mean particle size was observed after lyophilization of the coated/ uncoated CS-NPs, but the application of cryoprotectants provided a significant reduction in the particle size after lyophilization and prevented aggregation among the NPs. Lyophilization of the coated CS-NPs in the presence of a suitable cryoprotectant at an optimum concentration level results in a significantly effective treatment to control the particle size distribution and maintain the size, shape and integrity of the developed coated as well as uncoated CS-NPs.
Acknowledgment
This project was funded by the Research Groups Program (Research Group number RG-1436-027), Deanship of Scientific Research, King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdelwahed W., Degobert G., Stainmesse S., Fessi H. Freeze-drying of nanoparticles: formulation, process and storage considerations. Adv. Drug Deliv. Rev. 2006;58:1688–1713. doi: 10.1016/j.addr.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Almalik A., Day P.J., Tirelli N. HA-coated chitosan nanoparticles for CD44-mediated nucleic acid delivery. Macromol. Biosci. 2013;13:1671–1680. doi: 10.1002/mabi.201300302. [DOI] [PubMed] [Google Scholar]
- Almalik A., Donno R., Cadman C.J., Cellesi F., Day P.J., Tirelli N. Hyaluronic acid-coated chitosan nanoparticles: molecular weight-dependent effects on morphology and hyaluronic acid presentation. J. Control. Release. 2013;172:1142–1150. doi: 10.1016/j.jconrel.2013.09.032. [DOI] [PubMed] [Google Scholar]
- Almalik A., Karimi S., Ouasti S., Donno R., Wandrey C., Day P.J., Tirelli N. Hyaluronic acid (HA) presentation as a tool to modulate and control the receptor-mediated uptake of HA-coated nanoparticles. Biomaterials. 2013;34:5369–5380. doi: 10.1016/j.biomaterials.2013.03.065. [DOI] [PubMed] [Google Scholar]
- Azevedo M.A., Bourbon A.I., Vicente A.n.A., Cerqueira M.A. Alginate/chitosan nanoparticles for encapsulation and controlled release of vitamin B2. Int. J. Biol. Macromol. 2014;71:141–146. doi: 10.1016/j.ijbiomac.2014.05.036. [DOI] [PubMed] [Google Scholar]
- Borges O., Cordeiro-da-Silva A., Romeijn S.G., Amidi M., de Sousa A., Borchard G., Junginger H.E. Uptake studies in rat Peyer's patches, cytotoxicity and release studies of alginate coated chitosan nanoparticles for mucosal vaccination. J. Control. Release. 2006;114:348–358. doi: 10.1016/j.jconrel.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Crowe L.M., Reid D.S., Crowe J.H. Is trehalose special for preserving dry biomaterials? Biophys J. 1996;71:2087–2093. doi: 10.1016/S0006-3495(96)79407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pinho Neves A.L., Milioli C.C., Muller L., Riella H.G., Kuhnen N.C., Stulzer H.K. Factorial design as tool in chitosan nanoparticles development by ionic gelation technique. Colloids Surf., A. 2014;445:34–39. [Google Scholar]
- Deng X., Cao M., Zhang J., Hu K., Yin Z., Zhou Z., Xiao X., Yang Y., Sheng W., Wu Y., Zeng Y. Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials. 2014;35:4333–4344. doi: 10.1016/j.biomaterials.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Fabregas A., Minarro M., Garcia-Montoya E., Perez-Lozano P., Carrillo C., Sarrate R., Sanchez N., Tico J.R., Sune-Negre J.M. Impact of physical parameters on particle size and reaction yield when using the ionic gelation method to obtain cationic polymeric chitosan-tripolyphosphate nanoparticles. Int. J. Pharm. 2013;446:199–204. doi: 10.1016/j.ijpharm.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Fan W., Yan W., Xu Z., Ni H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf., B. 2012;90:21–27. doi: 10.1016/j.colsurfb.2011.09.042. [DOI] [PubMed] [Google Scholar]
- Gan Q., Wang T., Cochrane C., McCarron P. Modulation of surface charge, particle size and morphological properties of chitosan-TPP nanoparticles intended for gene delivery. Colloids Surf. B Biointerf. 2005;44:65–73. doi: 10.1016/j.colsurfb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Gokce Y., Cengiz B., Yildiz N., Calimli A., Aktas Z. Ultrasonication of chitosan nanoparticle suspension: influence on particle size. Colloids Surf., A. 2014;462:75–81. [Google Scholar]
- Jain A., Thakur K., Kush P., Jain U.K. Docetaxel loaded chitosan nanoparticles: formulation, characterization and cytotoxicity studies. Int. J. Biol. Macromol. 2014;69:546–553. doi: 10.1016/j.ijbiomac.2014.06.029. [DOI] [PubMed] [Google Scholar]
- Kalam M.A. Development of chitosan nanoparticles coated with hyaluronic acid for topical ocular delivery of dexamethasone. Int. J. Biol. Macromol. 2016;89:127–136. doi: 10.1016/j.ijbiomac.2016.04.070. [DOI] [PubMed] [Google Scholar]
- Liu Z., Jiao Y., Wang Y., Zhou C., Zhang Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008;60:1650–1662. doi: 10.1016/j.addr.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Motwani S., Chopra S., Talegaonkar S., Kohli K., Ahmad F., Khar R. Chitosan-sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: formulation, optimisation and in vitro characterisation. Eur. J. Pharm. Biopharm. 2007;68:513–525. doi: 10.1016/j.ejpb.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Oyarzun-Ampuero F.A., Brea J., Loza M.I., Torres D., Alonso M.J. Chitosan-hyaluronic acid nanoparticles loaded with heparin for the treatment of asthma. Int. J. Pharm. 2009;381:122–129. doi: 10.1016/j.ijpharm.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Rampino A., Borgogna M., Blasi P., Bellich B., Cesaro A. Chitosan nanoparticles: preparation, size evolution and stability. Int. J. Pharm. 2013;455:219–228. doi: 10.1016/j.ijpharm.2013.07.034. [DOI] [PubMed] [Google Scholar]
- Sagheer F.A.A., Al-Sughayer M.A., Muslim S., Elsabee M.Z. Extraction and characterization of chitin and chitosan from marine sources in Arabian Gulf. Carbohydr. Polym. 2009;77:410–419. [Google Scholar]
- Shimada E., Matsumura G. Viscosity and molecular weight of hyaluronic acids. J. Biochem. 1975;78:513–517. doi: 10.1093/oxfordjournals.jbchem.a130935. [DOI] [PubMed] [Google Scholar]
- Townsend M.W., DeLuca P.P. Use of lyoprotectants in the freeze-drying of a model protein, ribonuclease A. J. Parenter Sci. Technol. 1988;42:190–199. [PubMed] [Google Scholar]