Abstract
Simulation is important in evaluating novel methods when input data is not easily obtainable or specific assumptions are needed. We present cophesim, a software to add the phenotype to generated genotype data prepared with a genetic simulator. The output of cophesim can be used as a direct input for different genome wide association study tools. cophesim is available from https://bitbucket.org/izhbannikov/cophesim.
Keywords: Phenotype simulation, GWAS
Introduction
Genome-wide association studies (GWAS) are routine in population research. New methods are being developed for better accessing complex associations between genotypes and phenotypes, uncovering genotype structures or testing evolutionary hypotheses. Testing the novel methods requires experimental data, which may not be easily obtainable. One solution is to use artificial data simulated with specific assumptions.
The best existing phenotype simulators, such as: GENOME 1, Plink 2, phenosim 3, CoaSim 4, Fregene 5, ForSim 6, QuantiNemo 7, GCTA 8, HapGen 9, SeqSimla 10, and SimRare 11 offer qualitative and dichotomous simulated phenotype. But the known phenotype simulation software tools have some limitations, which may prevent customers from using them: (i) the majority, if not all, of the phenotype simulation software tools do not offer simulation of survival traits/time-to-event outcome, making it impossible to test respective hypotheses of associations; (ii) some of the tools are not easy to use, due to wide range of parameters, which the user has to provide and control (rather than calculate them automatically), making them unnecessarily difficult to use and preventing the user from future use of the tool; (iii) phenotype simulation is often offered as an auxiliary part of the genetic simulation routine, and therefore the user first has to perform a time-consuming unavoidable genetic simulation in order to obtain the phenotype; (iv) in situations when the genetic data is already simulated from other tools, only phenosim and GCTA offer adding simulated phenotype to such data. Consequently, it is necessary to have a new, simple and flexible phenotype simulation tool with plain algorithmic assumptions.
Consequently, we present cophesim, a comprehensive phenotype simulation tool that was developed to add a phenotype to corresponding genotypes simulated by other simulation tool ( Table S1). cophesim offers simulation of continuous, dichotomous and survival traits, with different (user-provided) effect sizes of causal variants, with the ability to simulate epistatic interactions. It also can simulate phenotype within gene-environment interaction assumptions using up to 10 covariates.
Methods
Implementation
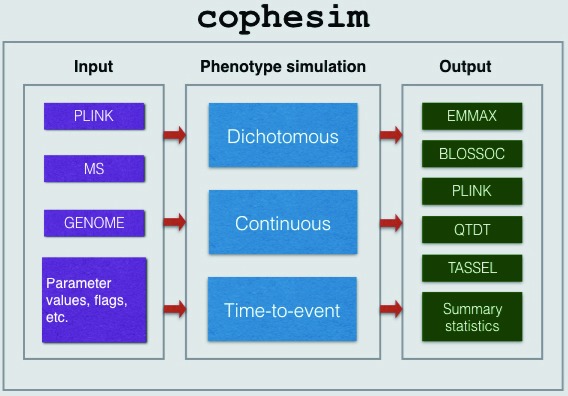
The workflow (see Figure 1) includes the following stages: (i) Input data pre-processing; (ii) phenotype simulation; (iii) generation of final output files.
Figure 1.
Workflow of cophesim has three stages: (1) Input stage, where the input data (can be provided in one of the three formats: Plink, MS and GENOME, see the user manual - Supplementary File 1) along with the other input parameters (such as causal variants with size effects, output format, etc.) is prepared for phenotype simulation; (2) Phenotype simulation stage, where different types of phenotypic traits are simulated: dichotomous, continuous and time-to-event (‘survival’); (3) Output stage – the final stage, where simulated phenotype data are packed to various formats in order to be directly usable by six GWAS tools: EMMAX, BLOSSOC, Plink, QTDT, TASSEL and GenABEL. Summary statistics are generated at the output stage as well.
Input data
Currently cophesim accepts the genotype output data from Plink, MS 12 and GENOME software applications. Phenotypes (dichotomous, continuous and survival) are then added according to the following simulation scenarios.
Dichotomous phenotype
Dichotomous phenotype for i th individual ( i = 1... N, where N is the total number of individuals in a dataset) is simulated according to the logistic model (if the user provided effect sizes for causal variants):
where p i is the probability of a particular outcome. In cophesim, it is a probability of a “case” (cases are marked by “1”, and “0” are controls in simulated dichotomous phenotype) for i th individual. If p i is greater than the some threshold p 0 (we use p 0 ~ U(0, 1)), then the phenotype for i th individual is set to “1” and to “0” otherwise. The variable z is determined with the following equation:
E j – effect size for j th variant, user-defined; g ij – value of j th genetic marker for i th individual; α j - effect size for j th covariate and X ij is a value of j th covariate for a i th individual (the term is added to represent gene-environment iterations); ϵ i – a standard normal residual, ϵ i N (0, 1), computed for i th individual, M is a total number of genetic variants and K is a total number of covariates used.
If the user did not provide the effect sizes for causal variants, the following strategy is then used:
Here w ij is a weight and computed as follows: (a standardization procedure, and the matrix W containing element w ij is called a standardized genotype matrix 8; MAF j – a minor allele frequency for j th genetic variant, and the other values are the same as described above. This strategy allows using defined genetic architecture in a simulated population.
Continuous phenotype
Qualitative (continuous) phenotype for i th individual is simulated according to the linear regression scenario according to the equations (2) or (3) (in case if effect sizes were not supplied).
Inverse Probability method
We model a survival phenotype from the proportional hazards model using the inverse probability method 13: if U is uniform in (0, 1) and if S(·| z) is the conditional survival function derived from the proportional hazards model: S( t| z) = e – H0( t) ez, then the random variable
has survival function S(·| z). In this equation, H 0( t) is a cumulative baseline hazard. By default, we use the Weibull cumulative baseline hazard: H 0( t) = λρt ρ–1; z is the same parameter that defined above, for each individual, and depends on whether the user provided effect sizes for causal variants or not. We also implemented exponential and Gompertz hazards.
Linkage Disequilibrium
The simplest way to simulate collinearity between two SNPs, g 1 and g 2, with effect sizes E 1 and E 2 is to replace some portion of g 2 with g 1 values according to provided coefficient, which reflects a correlation between two SNPs. We also consider applying other techniques, such as copulas, in order to simulate LD.
Epistatic interactions
These are modeled with the following equation for i th individual:
where the term E 12 g 1 i g 2 i is the interaction term in which E 12 is the epistatic effect size (user-defined, zero by default); α j is the effect size for j th covariate X.
Output files
Output files are in the formats as the direct inputs for the following tools: EMMAX 14, Blossoc 4, Plink (.ped file), QTDT 15, TASSEL 16, GenABEL 17 (see Table 1).
Table 1. Output file formats supported by phenotype simulator cophesim.
Applying one of the options shown below controls the output format. Each output format has a special suffix type, which defines the file format. These output formats are concordant to those used in phenosim.
| Application | Option | Commentary |
|---|---|---|
| EMMAX | -emmax | Suffices .emma_geno, .emma_pheno |
| BlOSSOC | -blossoc | Suffices .blossoc_pos, .blossoc_geno |
| PLINK | -plink | Used by default across all phenotypes,
except survival. Suffices .ped, .map, .pheno. |
| QTDT | -qtdt | Suffices .ped, .map, .dat |
| TASSEL | -tassel | Suffices .poly, .trait |
| GenABEL | - | This format is used in simulation of
survival phenotype. |
Operation
cophesim is freely available for download from the following link: https://bitbucket.org/izhbannikov/cophesim. Requirements: Python v2.7.10 and newer, plinkio v0.9.6, R v3.2.4 and newer, Plink v1.07, - in order to run the examples. The user manual is provided in a separate file “cophesim.pdf” located in the program directory and is also available as Supplementary File 1.
Use case
Below we present an example that shows simulation of genetic data and then simulation of three different phenotypic traits. Other examples and installation instructions are provided at the program website and also in the user manual. Refer to the user manual for description of input parameters.
#-------------------------------------------Example begins---------------------------------------#
#Step 1: genetic data simulation:
plink --simulate-ncases 5000 --simulate-ncontrols 5000 --simulate wgas.sim --out sim.plink --make-bed
#Step 2: Convert .bed to .ped:
plink --bfile sim.plink --recode --out sim.plink
#Step3: phenotype simulation from previously made genetic data:
python cophesim.py -i sim.plink -o testout -itype plink -otype plink -c -ce effects.txt -s -gomp
#-------------------------------------------End of example---------------------------------------#
In this example, we first (Step 1) simulate genetic data using Plink. We simulate N. cases = N. control = 5,000 cases and controls and 1,000 SNPs (defined in wgas.sim file, refer to the Plink website to see documentation for this type of file). Then (Step 2) we convert a binary sim.plink.bed file to sim.plink.ped (option --recode in Plink). This step is not required since cophesim can handle binary Plink files ( .bed, .bim, .fam), but we provide this step in order to show the ability of the program to deal with Plink PED format. Finally (Step 3), we simulate dichotomous (by default), continuous (option -c) and survival (option -s) traits from previously simulated data stored in files sim.plink.ped and sim.plink.map. Note that we simulate survival trait with Gompertz hazard function (option - gomp); effect sizes for causal variants are provided in file effects.txt (to include this file we use option -ce).
ROC curves
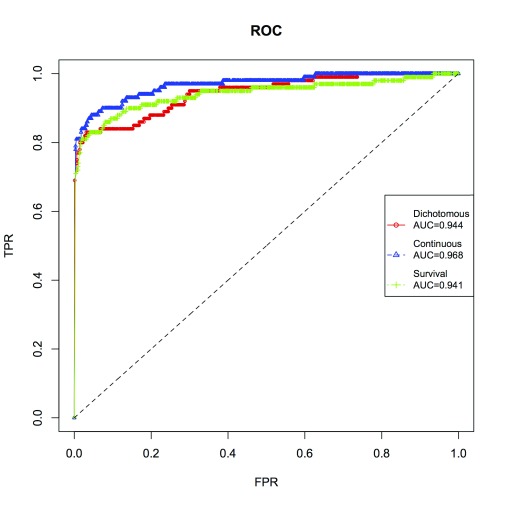
We provide Receiver-Operating Characteristic (ROC) curves ( Figure 2) constructed from association tests performed on a simulated dataset. Simulation and association testing were performed with Plink suite. The following parameters were used: N = 10,000 individuals, N. snp. c = 100 causal, with total N. snp = 1,000 variants. Causal variants were labeled with ‘1’ and the other (neutral) variants were labeled with ‘0’. These labels are then used later as true identifiers during calculation of TPR (true positive rate) and FPR (false positive rate). Dichotomous, continuous and survival phenotypic traits were simulated with cophesim. Then association tests were performed with Plink for dichotomous and continuous traits (using Plink flags –logistic and -regression, respectively). Association tests for survival trait were performed with the R package GenABEL. Then calculated p-values provided by association tests for each variant were compared to the significance threshold. Those variants passed the threshold were recognized as causal and associated with simulated phenotype. These classification results later were compared to the true identifiers (defined above) in order to obtain TPR and FPR. For all these tests, we varied the significance threshold from 0 to 1 with the increment of 0.001.
Figure 2. ROC curves constructed from results of association tests performed on a simulated dataset of N = 10,000 individuals, 100 causal and 1,000 of total SNP sites.
TPR: True Positive Ratio, FPR: False Positive Ratio. These results were calculated for dichotomous, continuous and survival traits. The dashed, 45 degrees line represents random guessing.
The R code to construct ROC curves is provided in the file “roc.R”. This file is attached to this computer note and also in the data repository: https://bitbucket.org/izhbannikov/cophesim_data/ROC/roc.R
Conclusion
In this work we presented the cophesim for phenotype simulation from genetic data obtained either from simulation or real data collecting. cophesim makes it possible to simulate various demographic models under user-defined scenarios.
Software and data availability
Tool and source code available from: https://bitbucket.org/izhbannikov/cophesim
Archived source code as at time of publication: doi: 10.5281/zenodo.810195 18
License: MIT
The example script and output files for the software are available at: https://doi.org/10.5281/zenodo.804090 19.
To test the cophesim we provided a repository “cophesim_data”: https://bitbucket.org/izhbannikov/cophesim_data. Download or clone this repository to be able to run tests.
Funding Statement
This work was supported by the National Institute on Aging of the National Institutes of Health (NIA/NIH) under award numbers P01AG043352, R01AG046860, and P30AG034424
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
Supplementary material
Table S1: Best available phenotype/genotype simulation software applications and their comparison to cophesim in terms of ability to simulate different types of phenotypic traits.
Supplementary File 1: User manual for cophesim.
References
- 1. Liang L, Zöllner S, Abecasis GR: Genome: a rapid coalescent-based whole genome simulator. Bioinformatics. 2007;23(12):1565–7. 10.1093/bioinformatics/btm138 [DOI] [PubMed] [Google Scholar]
- 2. Purcell S, Neale B, Todd-Brown K, et al. : Plink: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Günther T, Gawenda I, Schmid KJ: phenosim--A software to simulate phenotypes for testing in genome-wide association studies. BMC Bioinformatics. 2011;12(1):265. 10.1186/1471-2105-12-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mailund T, Schierup MH, Pedersen CN, et al. : Coasim: A flexible environment for simulating genetic data under coalescent models. BMC Bioinformatics. 2005;6(1):252. 10.1186/1471-2105-6-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoggart CJ, Chadeau-Hyam M, Clark TG, et al. : Sequence-level population simulations over large genomic regions. Genetics. 2007;177(3):1725–1731. 10.1534/genetics.106.069088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lambert BW, Terwilliger JD, Weiss KM: Forsim: a tool for exploring the genetic architecture of complex traits with controlled truth. Bioinformatics. 2008;24(16):1821–2. 10.1093/bioinformatics/btn317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Neuenschwander S, Hospital F, Guillaume F, et al. : quantinemo: an individual-based program to simulate quantitative traits with explicit genetic architecture in a dynamic metapopulation. Bioinformatics. 2008;24(13):1552–3. 10.1093/bioinformatics/btn219 [DOI] [PubMed] [Google Scholar]
- 8. Yang J, Lee SH, Goddard ME, et al. : Gcta: A tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88(1):76–82. 10.1016/j.ajhg.2010.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spencer CC, Su Z, Donnelly P, et al. : Designing genome-wide association studies: sample size, power, imputation, and the choice of genotyping chip. PLoS Genet. 2009;5(5):e1000477. 10.1371/journal.pgen.1000477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chung RH, Shih CC: SeqSIMLA: a sequence and phenotype simulation tool for complex disease studies. BMC Bioinformatics. 2013;14(1):199. 10.1186/1471-2105-14-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li B, Wang G, Leal SM: Simrare: a program to generate and analyze sequence-based data for association studies of quantitative and qualitative traits. Bioinformatics. 2012;28(20):2703–4. 10.1093/bioinformatics/bts499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ewing G, Hermisson J: MSMS: a coalescent simulation program including recombination, demographic structure and selection at a single locus. Bioinformatics. 2010;26(16):2064–5. 10.1093/bioinformatics/btq322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bender R, Augustin T, Blettner M: Generating survival times to simulate Cox proportional hazards models. Stat Med. 2005;24(11):1713–1723. 10.1002/sim.2059 [DOI] [PubMed] [Google Scholar]
- 14. Kang HM, Sul JH, Service SK, et al. : Variance component model to account for sample structure in genome-wide association studies. Nat Genet. 2010;42(4):348–54. 10.1038/ng.548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abecasis GR, Cardon LR, Cookson WO: A general test of association for quantitative traits in nuclear families. Am J Hum Genet. 2000;66(1):279–292. 10.1086/302698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bradbury PJ, Zhang Z, Kroon DE, et al. : TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics. 2007;23(19):2633–5. 10.1093/bioinformatics/btm308 [DOI] [PubMed] [Google Scholar]
- 17. Aulchenko YS, Ripke S, Isaacs A, et al. : GenABEL: an R library for genome-wide association analysis. Bioinformatics. 2007;23(10):1294–6. 10.1093/bioinformatics/btm108 [DOI] [PubMed] [Google Scholar]
- 18. Zhbannikov I: izhbannikov/release-1.4.1. Zenodo. 2017. Data Source [Google Scholar]
- 19. Zhbannikov I: izhbannikov/cophesim_data: First release. Zenodo. 2017. Data Source [Google Scholar]