Abstract
The putative tumor suppressor gene WW domain containing oxidoreductase (WWOX) spans a common fragile site (CFS) on chromosome 16q23.3. CFSs are regions of profound genomic instability and sites for genomic deletions in cancer cells. Therefore, WWOX is structurally altered in diverse nonhematological cancer types. However, the function of WWOX in hematological tumor types, including multiple myeloma (MM) and monoclonal gammopathy of undetermined significance (MGUS) remains unclear. WWOX expression and methylation in patients with MM, MGUS, or noninvasive lymphoma (control) were analyzed using reverse transcription- and methylation specific-polymerase chain reaction analysis. Variant WWOX transcripts were detected in 65 and 50% of patients with MM and MGUS, respectively, compared with 10% of controls. WWOX expression was higher in patients with MM, and WWOX promoter methylation was detected in 35% of patients with MM compared with 5% of patients with MGUS and 4% of controls. WWOX promoter methylation was significantly associated with shorter overall survival time of patients, in particular those with MM who were never treated with novel agents. Genomic alterations, including deletions and promoter methylation that affect WWOX expression occur early and may be involved in the pathogenesis, progression, and prognosis of MM.
Keywords: multiple myeloma, monoclonal gammopathy of undetermined significance, WW domain containing oxidoreductase gene, common fragile site, methylation
Introduction
The underlying molecular mechanism of multiple myeloma (MM) is associated with oncogene activation caused by the translocation of the immunoglobulin heavy chain gene (1,2). Mutations or deletions of certain genes are also involved (3,4). Although MM is incurable, the median survival time is currently 5–7 years compared with 3 years before 2000 due to the development of novel therapies (5). The prognosis of patients with MM is associated with genetic abnormalities (3), and the identification of molecular genetic markers for predicting prognosis is therefore essential. The application of high-throughput next-generation sequencing techniques has contributed to efforts to understanding the pathogenesis of MM (3,4); however, full coverage of the genomic alterations involved remains unclear.
Chromosomal instability is a feature of certain cancer types and is associated with the presence of chromosomal fragile sites (CFSs) (6). Common fragile sites are present in normal chromosomes, and are prone to forming chromosomal gaps and breaks under conditions that partially inhibit DNA synthesis (7). Furthermore, CFSs are regions of profound genomic instability and are frequent sites for deletions and other alterations in cancer cells (8,9). Fragile site aphidicolin type common fragile site (16) (q23.2) (FRA16D) on chromosome 16q23.2 is the second most frequently expressed CFS region (10) and has been identified as deleted in multiple types of cancer (11–13).
The WW domain containing oxidoreductase (WWOX) gene was identified as a putative tumor suppressor gene (14–17) and spans FRA16D (16,18,19). Genomic alterations, including homozygous and hemizygous deletions that affect the WWOX locus, occur in certain types of cancer (20–22). Deletion of all or part of chromosome 16q and loss of heterozygosity occurs in patients with MM (23–25). In addition, relatively low expression levels of WWOX and the cylindromatosis gene, which reside on chromosome 16q, have been associated with worse prognosis (24).
WWOX is the target of recurrent deletions of chromosome 16q (15–17,19) that disrupt one WWOX allele by removing exons 6–8 that encode the oxidoreductase domain, leading to the production of variant transcripts. WWOX variant transcripts are frequently identified in breast, lung, esophageal and hematological malignancies (17,26–30).
There is evidence to indicate that the loss of full length WWOX expression is due to the localization of WWOX at one of the most active human CFSs (15–17,19); however, other studies have demonstrated that the methylation of the WWOX promoter leads to decreased expression (31–33). Furthermore, epigenetic processes, particularly DNA methylation, are involved in carcinogenesis, and multiple studies have reported an association between the methylation of tumor suppressor genes and poor prognosis of patients with MM (34–40).
Genes with CFSs are often methylated in various types of malignancy (41,42), but little information is available regarding hematologic malignancy types (43,44). In addition, the methylation of the WWOX promoter has been associated with worse prognosis of patients with different types of cancer (31,45–47). To the best of our knowledge, no previous studies have reported an association between alterations of WWOX and progression of MM. The present study aimed to elucidate the function of WWOX in the pathogenesis of MM.
Materials and methods
Cell lines
The human myeloma cell line RPMI8226 was obtained from the American Type Culture Collection (Manassas, VA, USA), and KMS11, KMS12PE, KMM1, KMS18, and KMS26 human myeloma cell lines were provided by Dr Takemi Otsuki (Kawasaki Medical School, Okayama, Japan). The cell lines were cultured in 10 ml RPMI-1640 medium (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in an atmosphere containing 5% CO2.
Patients
Subjects included 165 patients with MM (82 male and 83 female) and an average age of 67 (range 34–87), 33 patients (16 male and 17 female) with monoclonal gammopathy of undetermined significance (MGUS) with an average age of 67 (range 44–81), diagnosed according to International Myeloma Working Group (IMWG) Criteria for the Diagnosis of Multiple Myeloma (48) and 25 patients with lymphoma lacking infiltration of the bone marrow as a control. All patients were treated at Gunma University Hospital between January 2004 and September 2011. The present study was approved by the Institutional Review Board of Gunma University Hospital (IRB no. 810). Written informed consent was obtained from all patients prior to 3 ml of bone marrow aspirate collection.
Plasma cell purification
The plasma cells were purified from BM mononuclear cells from 30 MM patients using anti-CD138 antibody conjugated with PE (Beckman-Coulter, Brea, CA) and Easy Sep PE positive selection containing anti-PE antibody conjugated with micro-magnetic beads kit (STEMCELL Technologies, Vancouver, BC, Canada). The purity of the CD138 positive plasma cells was analyzed using a flow cytometer (FACSCanto II, Becton Dickinson, San Jose, CA, USA).
Isolation of nucleic acids
DNA was extracted from 88 patients with MM using QIAamp DNA Blood Midi kit (Qiagen, Inc., Valencia, CA, USA), according to the manufacturer's protocol. DNA and RNA were extracted from 77 patients with MM and 33 patients with MGUS and 25 patients with lymphoma lacking infiltration of the bone marrow, respectively, using an All-Prep mini-kit (Qiagen, Inc.) according to the manufacturer's protocol.
Nested reverse transcription-polymerase chain reaction (RT-PCR) analysis of WWOX transcripts
cDNA was synthesized from 10 ng total RNA obtained from 77 patients with MM, 33 patients with MGUS and 25 control patients, and cell lines KMM1, KMS11, KMS12PE, KMS18, KMS26 and RPMI8226 using a PrimeScript RT-PCR kit with gDNA Eraser (Takara Bio, Inc., Otsu, Japan). The first and second PCR amplifications were performed using the nested primers as follows: First forward, 5′-AGTTCCTGAGCGAGTGGACC-3′ and reverse, 5′-TTACTTTCAAACAGGCCACCAC-3′ and second forward, 5′-AGGTGCCTCCACAGTC-3′ and reverse, 5′-GTGTGTGCCCATCCGCTCT-3′ (29,30). Each reaction (50 µl each) contained 0.2 µmol of each primer, 2.0 mM MgCl2, 0.2 mM dNTP mix, 1X PCR buffer and 1.25 units of Takara ExTaq Hot Start Version (Takara Bio, Inc.). The thermocycling conditions maintained were as follows: 95°C for 8 min; 35 cycles at 94°C for 30 sec, 57°C for 30 sec, and 72°C for 1 min; and an extension step at 72°C for 5 min. A total of 1 µl amplification product from the first reaction was used for the second reaction. The amplicons were electrophoresed through a 2% agarose gel and visualized using ethidium bromide.
Methylation-specific PCR (MSP)
The CpG island of the WWOX gene is located 406 bp upstream of the transcription start site and is considered the promoter region. MSP was used to detect the methylation levels of this region. DNA obtained from 165 patients with MM, 33 patients with MGUS and 25 control patients, and cell lines KMM1, KMS11, KMS12PE, KMS18, KMS26 and RPMI8226 were used for the MSP analysis. Each 0.5 µg sample of genomic DNA was treated with sodium bisulfite using the MethylEasy Xceed Rapid DNA Bisulfite Modification kit (Takara Bio, Inc.) following manufacturer's protocol, and the converted DNA was subjected to PCR. MSP was performed using specific primers designed to amplify methylated or unmethylated sequences of the WWOX promoter. The primer sequences for methylated or unmethylated DNA are as follows: Methylated forward, 5′-TATGGGCGTCGTTTTTTTAGTT-3′ and reverse, 5′-CAATCTCCGCAATATCGCGACA-3′; unmethylated forward, 5′-TATGGGTGTTGTTTTTTTAGTT-3′ and reverse, 5′-CAATCTCCACAATATCACAACA-3′ (31). Each reaction (20 µl each) contained 0.2 µmol of each primer, 2.0 mM MgCl2, 0.2 mM dNTP mix, 1X PCR buffer and 1.25 units of Takara ExTaq Hot Start Version (Takara Bio, Inc.). The thermocycling conditions maintained were as follows: 95°C for 8 min; 35 cycles at 94°C for 30 sec, 58°C for 30 sec, and 72°C for 1 min; and an extension step of 72°C for 5 min. The amplicons were electrophoresed through a 2% agarose gel and visualized using ethidium bromide.
Statistical analysis
IBM SPSS software (version 22.0; IBM SPSS, Armonk, NY, USA) was used for statistical analysis. Frequencies were compared using the χ2 test, and mean values were compared using the Student's t-test or the Mann-Whitney U test. Overall survival (OS) and statistical significance were calculated using the Kaplan-Meier estimator method, log-rank test, and generalized Wilcoxon test.
Results
Analysis of WWOX mRNAs
Nested PCR assays (Fig. 1A) detected the full-length wild-type WWOX mRNA and a short variant WWOX mRNA lacking exons 6–8. The variant type was expressed at low levels by KMM1 and KMS18 cells, and at higher levels by KMS11 and RPMI8226 cells. Full-length wild-type WWOX was detected in KMS12PE and KMS26 cells, and RPMI8226 cells expressed the wild-type and variant transcripts.
Figure 1.
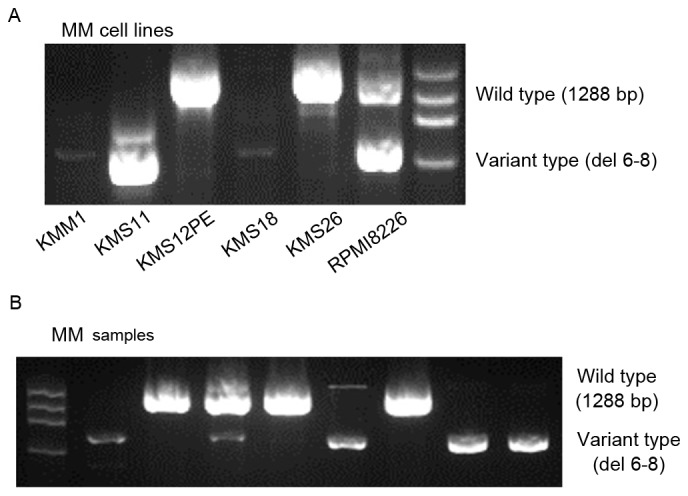
Detection of wild-type and variant WWOX mRNAs using nested reverse transcription-polymerase chain reaction. (A) The wild-type WWOX and variant amplicons migrated as 1,288 and 600 bp bands, respectively. The variant WWOX amplicon was detected in the KMM1, KMS11, KMS18 and RPMI8226 cell lines, and RPMI8226 cells expressed both forms. A weak band was detected in KMM1 and KMS18 cells, in which the WWOX promoter was methylated. (B) Detection of wild-type and variant WWOX amplicons in the plasma cells of patients with MM. WWOX, WW domain containing oxidoreductase; bp, base pairs; MM, multiple myeloma.
WWOX transcripts were undetectable in 9 patients with MM, 7 patients with MGUS and 4 patients with lymphoma (data not shown), and those patients were excluded from the following analysis. The variant WWOX mRNA was detected in 44/68 (65%) patients with MM (Fig. 1B), 13/26 (50%) cases of patients with MGUS and in 2/21 (10%) patients with lymphoma (Table I). This indicated that MM and MGUS bone marrow cells expressed the variant WWOX at a similar frequency (P=0.16), and at a significantly higher frequency compared with lymphoma bone marrow cells (P<0.001 and P=0.004, respectively; Table I). The similar high frequencies of detection of variant WWOX mRNA in patients with MM and MGUS suggested that WWOX alteration occurred during the premalignant stage of MM.
Table I.
Frequencies of variant WWOX mRNA, WWOX promoter methylation, and RASSF1A promoter methylation in patients with MM or MGUS and control subjects.
| No. patients (%) | ||||
|---|---|---|---|---|
| Genomic alteration | Lymphoma | MGUS | MM | P-value |
| Variant WWOX | 2/21 (10) | 13/26 (50) | 44/68 (65) | 0.1620a |
| 0.0001b | ||||
| 0.0030c | ||||
| WWOX methylation | 1/25 (4) | 2/33 (6) | 58/165 (35) | 0.0012a |
| 0.0020b | ||||
| 0.7300c | ||||
| RASSF1A methylation | 3/25 (12) | 14/33 (42) | 63/165 (38) | 0.7120a |
| 0.0120b | ||||
| 0.0190c | ||||
MM vs. MGUS;
MM vs. lymphoma;
MGUS vs. lymphoma. WWOX, WW domain containing oxidoreductase; MM, multiple myeloma; MGUS, monoclonal gammopathy of undetermined significance; RASSF1A, Ras association domain family member 1 isoform A.
CD138-positive plasma cells and CD138-negative bone marrow cells obtained from the same patients with MM were purified and analyzed to determine whether variant WWOX was expressed by plasma cells. The variant WWOX mRNA was detected in the CD138-positive plasma cells of 17/30 patients (57%), which was equivalent to the results of the analysis of whole-marrow mononuclear cells of patients with MM described above (P=0.45; data not shown). This was significantly higher compared with the detection in 6/30 (24%) of the CD138-negative cell samples (P=0.01). These results indicated that the variant form of WWOX mRNA was a genuine abnormality of MM cells and not a characteristic of all hematopoietic cells.
Methylation of the WWOX promoter in MM cell lines and tumor cells
MSP analysis detected WWOX promoter methylation in 2/6 (KMM1 and KMS18) cell lines (Fig. 2A) and in 3/4 patients with MM (Fig. 2B). Methylation of the WWOX promoter was detected in samples from 58/165 (35%) patients with MM, 2/33 (6%) patients with MGUS, and in 1/25 patients with lymphoma (4%). The frequency of WWOX promoter methylation in patients with MM was significantly higher compared with those with MGUS (P=0.001) or lymphoma (P=0.002), but the difference was not significant between patients with MGUS or lymphoma (P=0.73; Table I). This indicated that WWOX was preferentially methylated in MM cells.
Figure 2.
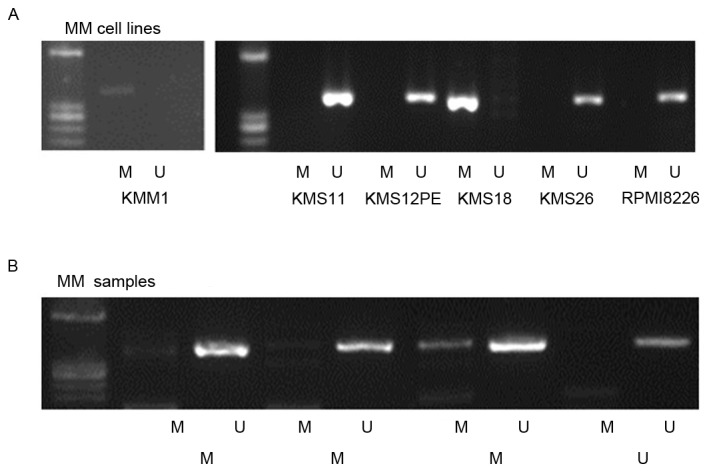
Methylation-specific polymerase chain reaction analysis of the WWOX promoter. (A) Lane M, methylated WWOX promoter; Lane U, unmethylated WWOX promoter. WWOX promoter methylation was detected in the DNA of the KMM1 and KMS18 cell lines. (B) Methylated and unmethylated WWOX promoter DNA of patients with MM was detected. WWOX, WW domain containing oxidoreductase; MM, multiple myeloma.
To determine whether methylation was specific for WWOX or reflected the methylation status of tumor suppressor genes in MM cells, Ras association domain family member 1 isoform A (RASSF1A) promoter methylation was analyzed. RASSF1A promoter methylation was detected in 63/165 patients with MM (38%), 14/33 patients with MGUS (42%), and in 3/25 patients with lymphoma (12%; Table I). No significant difference in the frequency between the number of patients with MM and MGUS was identified (P=0.70). In patients with MM, the frequency of RASSF1A promoter methylation was equivalent to that of WWOX hypermethylation. However, the RASSF1A promoter was more frequently methylated compared with WWOX in patients with MGUS (P=0.04; data not shown), suggesting that specific methylation of WWOX was associated with the progression of MM.
Association between the prognosis of patients with MM and WWOX promoter methylation
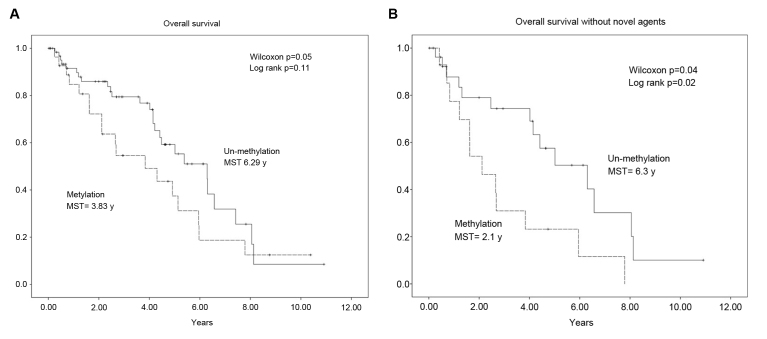
The association between WWOX promoter methylation and the OS of patients with MM was determined using the Kaplan-Meier estimator method, log-rank, and the generalized Wilcoxon test. The median OS time was shorter in patients with methylated WWOX sequences compared with those without methylation (3.83 vs. 6.29 years; Fig. 3A). This difference was significant according to the results of the generalized Wilcoxon test (P=0.02) but not those of the log-rank test (P=0.11). As novel agents, namely bortezomib, thalidomide and lenalidomide, are effective for treating MM, the patients were stratified according to those treated with or without novel agents and the data were analyzed again. The median OS time of patients with WWOX promoter methylation was shorter compared with those without methylation (2.1 vs. 6.3 years). The difference was significant according to the results of the generalized Wilcoxon test (P=0.04) and the log-rank test (P=0.02; Fig. 3B). In contrast, no significant difference between the median OS times of each class of patients treated with novel agents were identified (methylation, 5.1 years; undetectable methylation, 5.4 years; P=0.73; data not shown).
Figure 3.
Analyses of OS and WWOX promoter methylation. The solid and dashed lines represent the OS of patients with or without WWOX promoter methylation, respectively. (A) Kaplan-Meier analysis of patients with and without WWOX promoter methylation revealed that the median survival times were 3.83 and 6.29 years, respectively. (B) Stratification of patients according to the type of chemotherapy. The median survival times of the patients with and without WWOX promoter methylation were 2.1, and 6.3 years, respectively. OS, overall survival; WWOX, WW domain containing oxidoreductase.
Analysis of WWOX promoter methylation, β2-microglobulin levels, and International Staging System (ISS) classification
The higher incidence of WWOX promoter methylation in patients with MM compared with those with MGUS, and the association between methylation and shorter OS time, indicated that WWOX promoter methylation was associated with disease progression. To support this hypothesis, the association between WWOX promoter methylation, serum β2-microglobulin levels and MM stage was analyzed according to the ISS (49). The mean β2-microglobulin level was significantly higher in patients with WWOX promoter methylation compared with those without (6.80 vs. 4.68 mg/l; P=0.02; data not shown). The frequency of patients at ISS stage 3 with WWOX promoter methylation was significantly higher compared with those without methylation (P=0.02; data not shown).
Discussion
In the present study, recurrent expression of a short form of WWOX mRNA was demonstrated in patients with MM or MGUS. Furthermore, it was revealed that the WWOX promoter was frequently methylated in patients with MM, and that this increased during the progression of disease from MGUS to advanced MM. In addition, WWOX promoter methylation was identified to be associated with a shorter median OS time.
The wild-type transcript is ubiquitously expressed and shorter variants also occur (15,17). For example, homozygous deletion of chromosome 16q23.2 in various cancer cell lines includes deletions of WWOX exons (17) that generate shorter WWOX mRNA variants. In a previous study, numerous truncated WWOX variants lacking exons 5–8 were identified in clinical samples obtained from patients with breast cancer (27). Reduced expression of the full-length WWOX transcript by cancer cells and the detection of high levels of variant WWOX transcripts that occur specifically in tumors indicates that WWOX may be involved in oncogenesis (27).
In the present study, variant WWOX mRNAs were detected in myeloma cell lines and plasma cells of patients with MM or MGUS, but at a significantly lower frequency in the cells of control patients. These results indicated that WWOX alteration was associated with aberrant plasma cells.
The instability of CFSs correlates with genomic instability in precancerous lesions (50) and the early stages of oncogenesis are associated with the DNA damage response (50,51). Genomic instability and abnormalities are hallmarks of MM, and aberrant DNA repair pathways are involved in disease onset and progression (52). WWOX deficiency reduces the levels of ATM serine/threonine kinase and impairs DNA repair, which may drive genomic instability (51). Therefore, WWOX alterations may also cause genomic instability. The results of the present study on variant WWOX mRNA expression in patients with MGUS suggested that the alteration of a CFS indicates genomic instability at an early stage of the disease.
Studies of WWOX protein knockout and hypomorphic mice have demonstrated that a functional defect of WWOX leads to the induction of various types of tumor, including lymphoma and plasmacytoma (53–55). In vitro, WWOX inhibits β-catenin (56) and suppresses the transcriptional activity of the nuclear factor-κB (NF-κB)-RELA proto-oncogene, NF-κB subunit complex (57), which are involved in the pathogenesis of MM. Variant WWOX serves as a dominant-negative factor in vitro to inhibit the tumor suppressor function of wild-type WWOX (26). These findings, taken together with those of the present study, support the hypothesis that the loss of WWOX function serves a causative role in the pathogenesis of MM.
The frequent detection of WWOX promoter methylation in the cells of patients with MM, in contrast to patients with MGUS and the control group, indicates that WWOX promoter methylation is associated with MM progression. This is consistent with findings that WWOX methylation correlates with poor prognosis of patients with ovarian cancer (47), head and neck cancer (58), and chorangiocarcinoma (59).
No significant correlation was identified between WWOX methylation and OS. This result may be due to improved treatment outcomes using novel agents. Therefore, patients were stratified according to the types of therapy they received and prognosis was identified as being worse for patients with WWOX methylation if they had not received treatment with a novel agent, suggesting that WWOX methylation may be associated with resistance to conventional cytotoxic drugs.
In conclusion, the present study demonstrated that WWOX promoter methylation is associated with β2-microglobulin levels and ISS, indicating that WWOX methylation contributes to MM progression. Unlike WWOX, the rate of methylated RASSF1A was similar between patients with MGUS and MM. Therefore, the association of WWOX methylation with a more progressive and worse phenotype may not reflect the methylation of tumor suppressor genes.
Genomic instability is a hallmark of the majority of types of cancer, and is potentially involved in oncogenesis and the response to therapy. As WWOX is a putative human tumor suppressor gene, it appears possible that the selection for loss of function driven by fragile site instability is involved in MM progression. Further mechanistic studies are required to determine the role of WWOX and other genes within other CFSs in the pathogenesis of MM.
Acknowledgements
The present study was supported by the Ministry of Education, Science and Culture, Japan (grant no. 20590556).
References
- 1.Bergsagel PL, Kuehl WM. Chromosome translocations in multiple myeloma. Oncogene. 2001;20:5611–5622. doi: 10.1038/sj.onc.1204641. [DOI] [PubMed] [Google Scholar]
- 2.Chesi M, Bergsagel PL. Molecular pathogenesis of multiple myeloma: Basic and clinical updates. Int J Hematol. 2013;97:313–323. doi: 10.1007/s12185-013-1291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan GJ, Walker BA, Davies FE. The genetic architecture of multiple myeloma. Nat Rev Cancer. 2012;12:335–348. doi: 10.1038/nrc3257. [DOI] [PubMed] [Google Scholar]
- 4.Egan JB, Shi CX, Tembe W, Christoforides A, Kurdoglu A, Sinari S, Middha S, Asmann Y, Schmidt J, Braggio E, et al. Whole-genome sequencing of multiple myeloma from diagnosis to plasma cell leukemia reveals genomic initiating events, evolution, and clonal tides. Blood. 2012;120:1060–1066. doi: 10.1182/blood-2012-01-405977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vincent Rajkumar S. Multiple myeloma: 2014 Update on diagnosis, risk-stratification and management. Am J Hematol. 2014;89:999–1009. doi: 10.1002/ajh.23810. [DOI] [PubMed] [Google Scholar]
- 6.Dillon LW, Burrow AA, Wang YH. DNA instability at chromosomal fragile sites in cancer. Curr Genomics. 2010;11:326–337. doi: 10.2174/138920210791616699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glover TW. Common fragile sites. Cancer Lett. 2006;232:4–12. doi: 10.1016/j.canlet.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 8.Gao G, Smith DI. Very large common fragile site genes and their potential role in cancer development. Cell Mol Life Sci. 2014;71:4601–4615. doi: 10.1007/s00018-014-1753-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debatisse M, Le Tallec B, Letessier A, Dutrillaux B, Brison O. Common fragile sites: Mechanisms of instability revisited. Trends Genet. 2012;28:22–32. doi: 10.1016/j.tig.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Sutherland GR, Richards RI. The molecular basis of fragile sites in human chromosomes. Curr Opin Genet Dev. 1995;5:323–327. doi: 10.1016/0959-437X(95)80046-8. [DOI] [PubMed] [Google Scholar]
- 11.Balsara BR, Pei J, De Rienzo A, Simon D, Tosolini A, Lu YY, Shen FM, Fan X, Lin WY, Buetow KH, et al. Human hepatocellular carcinoma is characterized by a highly consistent pattern of genomic imbalances, including frequent loss of 16q23.1-24.1. Genes Chromosomes Cancer. 2001;30:245–253. doi: 10.1002/1098-2264(2000)9999:9999<::AID-GCC1083>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 12.Paris PL, Witte JS, Kupelian PA, Levin H, Klein EA, Catalona WJ, Casey G. Identification and fine mapping of a region showing a high frequency of allelic imbalance on chromosome 16q23.2 that corresponds to a prostate cancer susceptibility locus. Cancer Res. 2000;60:3645–3649. [PubMed] [Google Scholar]
- 13.Hansen LL, Yilmaz M, Overgaard J, Andersen J, Kruse TA. Allelic loss of 16q23.2-24.2 is an independent marker of good prognosis in primary breast cancer. Cancer Res. 1998;58:2166–2169. [PubMed] [Google Scholar]
- 14.O'Keefe LV, Richards RI. Common chromosomal fragile sites and cancer: Focus on FRA16D. Cancer Lett. 2006;232:37–47. doi: 10.1016/j.canlet.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 15.Ried K, Finnis M, Hobson L, Mangelsdorf M, Dayan S, Nancarrow JK, Woollatt E, Kremmidiotis G, Gardner A, Venter D, et al. Common chromosomal fragile site FRA16D sequence: Identification of the FOR gene spanning FRA16D and homozygous deletions and translocation breakpoints in cancer cells. Hum Mol Genet. 2000;9:1651–1663. doi: 10.1093/hmg/9.11.1651. [DOI] [PubMed] [Google Scholar]
- 16.Bednarek AK, Laflin KJ, Daniel RL, Liao Q, Hawkins KA, Aldaz CM. WWOX, a novel WW domain-containing protein mapping to human chromosome 16q23.3-24.1, a region frequently affected in breast cancer. Cancer Res. 2000;60:2140–2145. [PubMed] [Google Scholar]
- 17.Paige AJ, Taylor KJ, Taylor C, Hillier SG, Farrington S, Scott D, Porteous DJ, Smyth JF, Gabr H, Watson JE. WWOX: A candidate tumor suppressor gene involved in multiple tumor types. Proc Natl Acad Sci USA. 2001;98:11417–11422. doi: 10.1073/pnas.191175898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ludes-Meyers JH, Bednarek AK, Popescu NC, Bedford M, Aldaz CM. WWOX, the common chromosomal fragile site, FRA16D, cancer gene. Cytogenet Genome Res. 2003;100:101–110. doi: 10.1159/000072844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paige AJ, Taylor KJ, Stewart A, Sgouros JG, Gabra H, Sellar GC, Smyth JF, Porteous DJ, Watson JE. A 700-kb physical map of a region of 16q23.2 homozygously deleted in multiple cancers and spanning the common fragile site FRA16D. Cancer Res. 2000;60:1690–1697. [PubMed] [Google Scholar]
- 20.Alsop AE, Taylor K, Zhang J, Gabra H, Paige AJ, Edwards PA. Homozygous deletions may be markers of nearby heterozygous mutations: The complex deletion at FRA16D in the HCT116 colon cancer cell line removes exons of WWOX. Genes Chromosomes Cancer. 2008;47:437–447. doi: 10.1002/gcc.20548. [DOI] [PubMed] [Google Scholar]
- 21.Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Urashima M, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bignell GR, Greenman CD, Davies H, Butler AP, Edkins S, Andrews JM, Buck G, Chen L, Beare D, Latimer C, et al. Signatures of mutation and selection in the cancer genome. Nature. 2010;463:893–898. doi: 10.1038/nature08768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krummel KA, Roberts LR, Kawakami M, Glover TW, Smith DI. The characterization of the common fragile site FRA16D and its involvement in multiple myeloma translocations. Genomics. 2000;69:37–46. doi: 10.1006/geno.2000.6321. [DOI] [PubMed] [Google Scholar]
- 24.Jenner MW, Leone PE, Walker BA, Ross FM, Johnson DC, Gonzalez D, Chiecchio L, Dachs Cabanas E, Dagrada GP, Nightingale M, et al. Gene mapping and expression analysis of 16q loss of heterozygosity identifies WWOX and CYLD as being important in determining clinical outcome in multiple myeloma. Blood. 2007;110:3291–3300. doi: 10.1182/blood-2007-02-075069. [DOI] [PubMed] [Google Scholar]
- 25.Agnelli L, Mosca L, Fabris S, Lionetti M, Andronache A, Kwee I, Todoerti K, Verdelli D, Battaglia C, Bertoni F, et al. A SNP microarray and FISH-based procedure to detect allelic imbalances in multiple myeloma: An integrated genomics approach reveals a wide gene dosage effect. Genes Chromosomes Cancer. 2009;48:603–614. doi: 10.1002/gcc.20668. [DOI] [PubMed] [Google Scholar]
- 26.Bednarek AK, Keck-Waggoner CL, Daniel RL, Laflin KJ, Bergsagel PL, Kiguchi K, Brenner AJ, Aldaz CM. WWOX, the FRA16D gene, behaves as a suppressor of tumor growth. Cancer Res. 2001;61:8068–8073. [PubMed] [Google Scholar]
- 27.Driouch K, Prydz H, Monese R, Johansen H, Lidereau R, Frengen E. Alternative transcripts of the candidate tumor suppressor gene, WWOX, are expressed at high levels in human breast tumors. Oncogene. 2002;21:1832–1840. doi: 10.1038/sj.onc.1205273. [DOI] [PubMed] [Google Scholar]
- 28.Gourley C, Paige AJ, Taylor KJ, Scott D, Francis NJ, Rush R, Aldaz CM, Smyth JF, Gabra H. WWOX mRNA expression profile in epithelial ovarian cancer supports the role of WWOX variant 1 as a tumour suppressor, although the role of variant 4 remains unclear. Int J Oncol. 2005;26:1681–1689. doi: 10.3892/ijo.26.6.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yendamuri S, Kuroki T, Trapasso F, Henry AC, Dumon KR, Huebner K, Williams NN, Kaiser LR, Croce CM. WW domain containing oxidoreductase gene expression is altered in non-small cell lung cancer. Cancer Res. 2003;63:878–881. [PubMed] [Google Scholar]
- 30.Kuroki T, Trapasso F, Shiraishi T, Alder H, Mimori K, Mori M, Croce CM. Genetic alterations of the tumor suppressor gene WWOX in esophageal squamous cell carcinoma. Cancer Res. 2002;62:2258–2260. [PubMed] [Google Scholar]
- 31.Iliopoulos D, Guler G, Han SY, Johnston D, Druck T, McCorkell KA, Palazzo J, McCue PA, Baffa R, Huebner K. Fragile genes as biomarkers: Epigenetic control of WWOX and FHIT in lung, breast and bladder cancer. Oncogene. 2005;24:1625–1633. doi: 10.1038/sj.onc.1208398. [DOI] [PubMed] [Google Scholar]
- 32.Iliopoulos D, Fabbri M, Druck T, Qin HR, Han SY, Huebner K. Inhibition of breast cancer cell growth in vitro and in vivo: Effect of restoration of Wwox expression. Clin Cancer Res. 2007;13:268–274. doi: 10.1158/1078-0432.CCR-06-2038. [DOI] [PubMed] [Google Scholar]
- 33.Cantor JP, Iliopoulos D, Rao AS, Druck T, Semba S, Han SY, McCorkell KA, Lakshman TV, Collins JE, Wachsberger P, et al. Epigenetic modulation of endogenous tumor suppressor expression in lung cancer xenografts suppresses tumorigenicity. Int J Cancer. 2007;120:24–31. doi: 10.1002/ijc.22073. [DOI] [PubMed] [Google Scholar]
- 34.Ng MH, Chung YF, Lo KW, Wickham NW, Lee JC, Huang DP. Frequent hypermethylation of p16 and p15 genes in multiple myeloma. Blood. 1997;89:2500–2506. [PubMed] [Google Scholar]
- 35.Guillerm G, Gyan E, Wolowiec D, Facon T, Avet-Loiseau H, Kuliczkowski K, Bauters F, Fenaux P, Quesnel B. p16 (INK4a) and p15 (INK4b) gene methylations in plasma cells from monoclonal gammopathy of undetermined significance. Blood. 2001;98:244–246. doi: 10.1182/blood.V98.1.244. [DOI] [PubMed] [Google Scholar]
- 36.Stanganelli C, Arbelbide J, Fantl DB, Corrado C, Slavutsky I. DNA methylation analysis of tumor suppressor genes in monoclonal gammopathy of undetermined significance. Ann Hematol. 2010;89:191–199. doi: 10.1007/s00277-009-0818-3. [DOI] [PubMed] [Google Scholar]
- 37.Braggio E, Maiolino A, Gouveia ME, Magalhães R, Souto Filho JT, Garnica M, Nucci M, Renault IZ. Methylation status of nine tumor suppressor genes in multiple myeloma. Int J Hematol. 2010;91:87–96. doi: 10.1007/s12185-009-0459-2. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez M, Mateos MV, García-Sanz R, Balanzategui A, López-Pérez R, Chillón MC, González D, Alaejos I, San Miguel JF. De novo methylation of tumor suppressor gene p16/INK4a is a frequent finding in multiple myeloma patients at diagnosis. Leukemia. 2000;14:183–187. doi: 10.1038/sj.leu.2401617. [DOI] [PubMed] [Google Scholar]
- 39.Heuck CJ, Mehta J, Bhagat T, Gundabolu K, Yu Y, Khan S, Chrysofakis G, Schinke C, Tariman J, Vickrey E, et al. Myeloma is characterized by stage-specific alterations in DNA methylation that occur early during myelomagenesis. J Immunol. 2013;190:2966–2975. doi: 10.4049/jimmunol.1202493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaiser MF, Johnson DC, Wu P, Walker BA, Brioli A, Mirabella F, Wardell CP, Melchor L, Davies FE, Morgan GJ. Global methylation analysis identifies prognostically important epigenetically inactivated tumor suppressor genes in multiple myeloma. Blood. 2013;122:219–226. doi: 10.1182/blood-2013-03-487884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka H, Shimada Y, Harada H, Shinoda M, Hatooka S, Imamura M, Ishizaki K. Methylation of the 5′ CpG island of the FHIT gene is closely associated with transcriptional inactivation in esophageal squamous cell carcinomas. Cancer Res. 1998;58:3429–3434. [PubMed] [Google Scholar]
- 42.Zöchbauer-Müller S, Fong KM, Maitra A, Lam S, Geradts J, Ashfaq R, Virmani AK, Milchgrub S, Gazdar AF, Minna JD. 5′ CpG island methylation of the FHIT gene is correlated with loss of gene expression in lung and breast cancer. Cancer Res. 2001;61:3581–3585. [PubMed] [Google Scholar]
- 43.Ishii H, Vecchione A, Furukawa Y, Sutheesophon K, Han SY, Druck T, Kuroki T, Trapasso F, Nishimura M, Saito Y, et al. Expression of FRA16D/WWOX and FRA3B/FHIT genes in hematopoietic malignancies. Mol Cancer Res. 2003;1:940–947. [PubMed] [Google Scholar]
- 44.Uehara E, Takeuchi S, Tasaka T, Matsuhashi Y, Yang Y, Fujita M, Tamura T, Nagai M, Koeffler HP. Aberrant methylation in promoter-associated CpG islands of multiple genes in therapy-related leukemia. Int J Oncol. 2003;23:693–696. [PubMed] [Google Scholar]
- 45.Nakayama S, Semba S, Maeda N, Matsushita M, Kuroda Y, Yokozaki H. Hypermethylation-mediated reduction of WWOX expression in intraductal papillary mucinous neoplasms of the pancreas. Br J Cancer. 2009;100:1438–1443. doi: 10.1038/sj.bjc.6604986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, Chao L, Jin G, Ma G, Zang Y, Sun J. Association between CpG island methylation of the WWOX gene and its expression in breast cancers. Tumour Biol. 2009;30:8–142. doi: 10.1159/000197911. [DOI] [PubMed] [Google Scholar]
- 47.Yan H, Sun J. Methylation status of WWOX gene promoter CpG islands in epithelial ovarian cancer and its clinical significance. Biomed Rep. 2013;1:375–378. doi: 10.3892/br.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.International Myeloma Working Group: Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: A report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–757. doi: 10.1046/j.1365-2141.2003.04355.x. [DOI] [PubMed] [Google Scholar]
- 49.Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Bladé J, Boccadoro M, Child JA, Avet-Loiseau H, Kyle RA, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 50.Le Tallec B, Koundrioukoff S, Wilhelm T, Letessier A, Brison O, Debatisse M. Updating the mechanisms of common fragile site instability: How to reconcile the different views? Cell Mol Life Sci. 2014;71:4489–4494. doi: 10.1007/s00018-014-1720-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abu-Odeh M, Salah Z, Herbel C, Hofmann TG, Aqeilan RI. WWOX, the common fragile site FRA16D gene product, regulates ATM activation and the DNA damage response. Proc Natl Acad Sci USA. 2014;111:E4716–E4725. doi: 10.1073/pnas.1409252111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gourzones-Dmitriev C, Kassambara A, Sahota S, Rème T, Moreaux J, Bourquard P, Hose D, Pasero P, Constantinou A, Klein B. DNA repair pathways in human multiple myeloma: Role in oncogenesis and potential targets for treatment. Cell Cycle. 2013;12:2760–2773. doi: 10.4161/cc.25951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aqeilan RI, Trapasso F, Hussain S, Costinean S, Marshall D, Pekarsky Y, Hagan JP, Zanesi N, Kaou M, Stein GS, et al. Targeted deletion of Wwox reveals a tumor suppressor function. Proc Natl Acad Sci USA. 2007;104:3949–3954. doi: 10.1073/pnas.0609783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ludes-Meyers JH, Kil H, Nuñez MI, Conti CJ, Parker-Thornburg J, Bedford MT, Aldaz CM. WWOX hypomorphic mice display a higher incidence of B-cell lymphomas and develop testicular atrophy. Genes Chromosomes Cancer. 2007;46:1129–1136. doi: 10.1002/gcc.20497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ludes-Meyers JH, Kil H, Parker-Thornburg J, Kusewitt DF, Bedford MT, Aldaz CM. Generation and characterization of mice carrying a conditional allele of the Wwox tumor suppressor gene. PloS One. 2009;4:e7775. doi: 10.1371/journal.pone.0007775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bouteille N, Driouch K, Hage PE, Sin S, Formstecher E, Camonis J, Lidereau R, Lallemand F. Inhibition of the Wnt/beta-catenin pathway by the WWOX tumor suppressor protein. Oncogene. 2009;28:2569–2580. doi: 10.1038/onc.2009.120. [DOI] [PubMed] [Google Scholar]
- 57.Fu J, Qu Z, Yan P, Ishikawa C, Aqeilan RI, Rabson AB, Xiao G. The tumor suppressor gene WWOX links the canonical and noncanonical NF-κB pathways in HTLV-I Tax-mediated tumorigenesis. Blood. 2011;117:1652–1661. doi: 10.1182/blood-2010-08-303073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ekizoglu S, Bulut P, Karaman E, Kilic E, Buyru N. Epigenetic and genetic alterations affect the WWOX gene in head and neck squamous cell carcinoma. PloS One. 2015;10:e0115353. doi: 10.1371/journal.pone.0115353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang C, Tian Y, Peng R, Zhang C, Wang D, Han S, Jiao C, Wang X, Zhang H, Wang Y, Li X. Association of downregulation of WWOX with poor prognosis in patients with intrahepatic cholangiocarcinoma after curative resection. J Gastroenterol Hepatol. 2015;30:421–433. doi: 10.1111/jgh.12722. [DOI] [PubMed] [Google Scholar]