Abstract
Drosha and Dicer are important regulators of microRNA (miRNA) biogenesis, and it has been suggested that their aberrant regulation may cause colorectal cancer (CRC). The aim of the present study was to evaluate the mRNA expression levels of these two important RNase III nucleases and their association with clinical features in CRC specimens from South Korean patients. The expression levels of Drosha and Dicer mRNA were investigated in 77 CRC tissues and adjacent histologically non-neoplastic tissues using the quantitative polymerase chain reaction. The expression levels of Drosha and Dicer mRNA were identified to be upregulated in CRC. Neither the Drosha nor the Dicer mRNA expression level was associated with any clinical parameter, including sex, age, TNM stage, body mass index and carcinoembryonic antigen titer in patients with CRC. Furthermore, the expression levels of Drosha and Dicer mRNA were not associated with each other. The miRNA biogenesis-associated RNase III nucleases Drosha and Dicer are significantly upregulated in CRC, suggesting their importance in the pathobiology of colorectal carcinogenesis.
Keywords: microRNA biogenesis, colorectal adenocarcinoma, Drosha, Dicer
Introduction
Colorectal carcinoma (CRC) is the third most common cancer in Western countries and South Korea (1,2). CRC is also one of the primary causes of cancer mortality worldwide (1,3). The pathogenesis of CRC has been reported to be complicated and tightly controlled by various mechanisms, including genome structural rearrangements, chromatin remodeling, genetic mutations and epigenetic alterations (4,5).
MicroRNAs (miRNAs), which are small RNA molecules that serve an essential role in fine-tuning gene expression, regulate a range of biological processes, including cellular development, differentiation, proliferation, K+ channel modulation, stress responses, DNA repair, cell adhesion, cell death, inflammation, metabolism and tumor development (6–9). It has been identified that appropriate and physiological miRNA biogenesis is controlled by an elaborate and well-regulated process, referred to as the ‘miRNA machinery’ pathway (10). This signaling pathway is regulated by various components, including Drosha, DiGeorge syndrome critical region gene 8 (DGCR8), exportin-5 (Xpo5), Dicer, transactivation-responsive RNA-binding protein (TRBP) and Argonaute (AGO). Among these, Drosha and Dicer are important regulators of miRNA biogenesis. In the nucleus, primary miRNAs (RNA extensions several hundred nucleotides long) are processed into precursor miRNAs (pre-miRNAs; stem-loop structures of between 70 and 100 nucleotides) by RNase III Drosha (11). These pre-miRNAs are then processed by another RNase III, Dicer, into mature miRNAs within the cytoplasm (12).
As disruption of the miRNA machinery pathway has been suggested to be involved in cancer (13), a number of studies have demonstrated the expression and the clinical implications of the components of the miRNA machinery pathway in CRC. For instance, Faber et al (14) identified that overexpressed Dicer is significantly associated with poor survival and decreased progression-free survival rates. Furthermore, Papachristou et al (10) demonstrated an association between upregulated Dicer and stage III, but not stage II, tumors. However, downregulated Dicer reveals statistically significant association with disease (World Health Organization) stage, tumor stage, tumor grade and nodal metastasis (15). By contrast, it has been identified that dysregulated DGCR8 and AGO2 are not associated with tumor-node-metastasis (TNM) stage or carcinoembryonic antigen (CEA) titer in CRC (16). In spite of these results, investigation of the mRNA expression levels of Drosha and Dicer, and their clinicopathological associations in Korean patients with CRC has been limited.
In the present study, the expression levels of Drosha and Dicer mRNA in CRC tissues and their corresponding adjacent non-neoplastic tissues from the same South Korean patients were investigated, and the association between the expression levels of Drosha and Dicer mRNA with various clinicopathological characteristics, including sex, age, TNM stage, body mass index (BMI) and CEA titer, were evaluated.
Materials and methods
Patients and tissues
In total, 77 patients (52 male and 25 female) diagnosed with colorectal adenocarcinoma were included in the present study. The mean age was 64.2±9.8 years. Colorectal adenocarcinomas and adjacent non-neoplastic tissues were obtained from the patients undergoing surgery in Dongsan Medical Center (Daegu, Korea) between June 2008 and November 2010. Tissue specimens were immediately frozen in liquid nitrogen and stored at −80°C until RNA isolation. Tissue specimens were provided from the Keimyung Human Bio-Resource Bank (Keimyung, Korea). The purpose of the present study was explained to each patient who each provided written informed consent. The present study was approved by the Institutional Review Board of Keimyung University Dongsan Medical Center (approval #2015-10-031-002).
RNA and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from tissues using TRIzol reagent (Molecular Research Center, Inc., Cincinnati, OH, USA). RNAs were quantified using a NanoDrop 1000 instrument (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Each cDNA was synthesized from 2 µg total RNA using Moloney murine leukemia virus reverse transcriptase (Promega Corporation, Madison, WI, USA), according to the manufacturer's protocol. qPCR was performed on a LightCycler® 480 Real-Time PCR system (Roche Diagnostics GmbH, Mannheim, Germany) using SYBR Green Premix (Roche Diagnostics GmbH) and specific primer pairs (listed in Table I). The following thermocycling conditions were maintained: Pre-incubation, 5 min at 95°C; amplification, 45 cycles with 10 sec at 95°C, 10 sec at 55°C and 15 sec at 72°C; melting curve, gradually from 65 to 95°C; cooling, 10 min at 37°C. β-actin was used as a loading control, and a no-template sample was used as a negative control. qPCR data were analyzed with the ΔCq values using Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) (17). A total of three replicates per experiment were performed.
Table I.
Primer sequences of microRNA machinery components used in quantitative polymerase chain reaction.
| Gene | Direction | Sequence |
|---|---|---|
| Drosha | Forward | 5′-CTGTCGATGCACCAGATT-3′ |
| Reverse | 5′-TGCATAACTCAACTGTGCAGG-3′ | |
| Dicer | Forward | 5′-TTAACCTTTTGGTGTTTGATGAGTGT-3′ |
| Reverse | 5′-AGGACATGATGGACAATT-3′ | |
| β-actin | Forward | 5′-CAGCCATGTACGTTGCTATCCAGG-3′ |
| Reverse | 5′-AGGTCCAGACGCAGGATGGCATG-3′ |
Statistical analysis
Statistical analysis was performed using SPSS software (version 22.0; IBM SPSS, Armonk, NY, USA). Statistical comparisons for significance were performed using Wilcoxon's signed-rank test for paired samples. Differences between the groups were analyzed statistically using the paired Students t-test. The association between Drosha and Dicer expression was assessed using Pearson's correlation coefficient analysis for continuous variables and Fisher's exact test for categorical variables. Clinicopathological associations with the mRNA expression levels of Drosha and Dicer were analyzed using a linear-by-linear association, Pearson's χ2 test and Fisher's exact test for categorical variables. The mean value was used as threshold value (low and high) for categorical variables. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression levels of Drosha and Dicer mRNA in CRC tissues and adjacent non-neoplastic colorectal tissues of patients with CRC
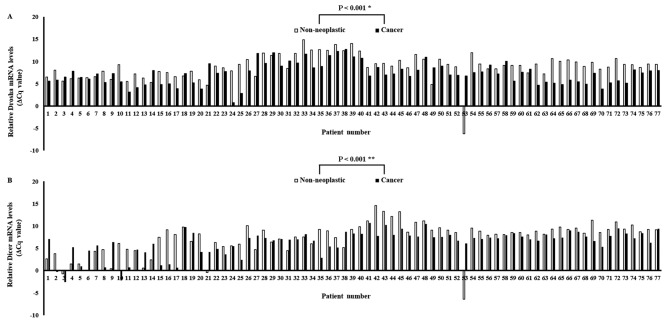
The expression levels of Drosha and Dicer mRNA were quantified using qPCR in paired specimens of human cancerous colorectal tissues and their corresponding non-neoplastic colorectal tissues from 77 patients with CRC. The Drosha and Dicer mRNA levels were normalized to the level of β-actin mRNA. The results identified that Drosha mRNA expression was significantly upregulated in carcinomatous tissues compared with in the corresponding non-neoplastic tissues in 59/77 patients with CRC (P<0.001; Fig. 1A). Furthermore, Dicer mRNA expression was also significantly upregulated in carcinomatous tissues compared with in the corresponding non-neoplastic tissues in 59/77 patients with CRC (P<0.001; Fig. 1B). The mean levels of Drosha and Dicer mRNA expression in cancerous tissues were significantly upregulated compared with in non-neoplastic colorectal tissues (P<0.001 and P=0.006, respectively; Fig. 2).
Figure 1.
Relative Drosha and Dicer mRNA expression levels in tumor specimens. (A) Relative Drosha mRNA level (normalized to the corresponding β-actin mRNA) in tumor tissues compared with adjacent non-cancerous colorectal tissues. (B) Relative Dicer mRNA level (normalized to the corresponding β-actin mRNA) in tumor tissues compared with adjacent non-cancerous colorectal tissues. */**P-values were calculated using the Wilcoxon's signed rank test.
Figure 2.
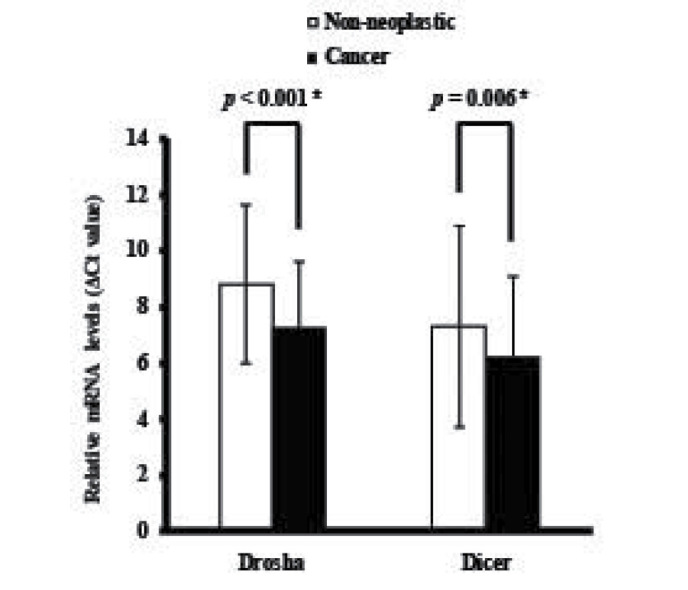
Relative Drosha and Dicer mRNA expression in the colorectal cancer and control groups. P-values were calculated using the paired Student's t-test.
Association between Drosha and Dicer mRNA expression levels and their clinicopathological features in patients with CRC
To investigate the association between mRNA levels of Drosha and Dicer in CRC specimens, Pearson's correlation coefficient analysis was performed. No significant association was identified between Drosha and Dicer, with a Pearson's correlation coefficient value of −0.007 (P=0.953; Fig. 3). Furthermore, the expression levels of Drosha and Dicer mRNA were not associated with each other, as determined by Fisher's exact test (Table II). To investigate the biological roles of Drosha and Dicer in CRC, the association between their mRNA expression levels and the clinicopathological parameters that are used to describe tumor progression and aggressiveness in CRC were evaluated. Prior to the statistical analysis, the patients were classified according to each clinical characteristic. The clinicopathological parameters in the 77 patients with CRC according to Drosha and Dicer mRNA expression levels are presented in Table II. Drosha and Dicer mRNA expression levels were not significantly associated with clinical parameters, including sex, age, TNM stage, BMI and CEA titer in the CRC specimens.
Figure 3.
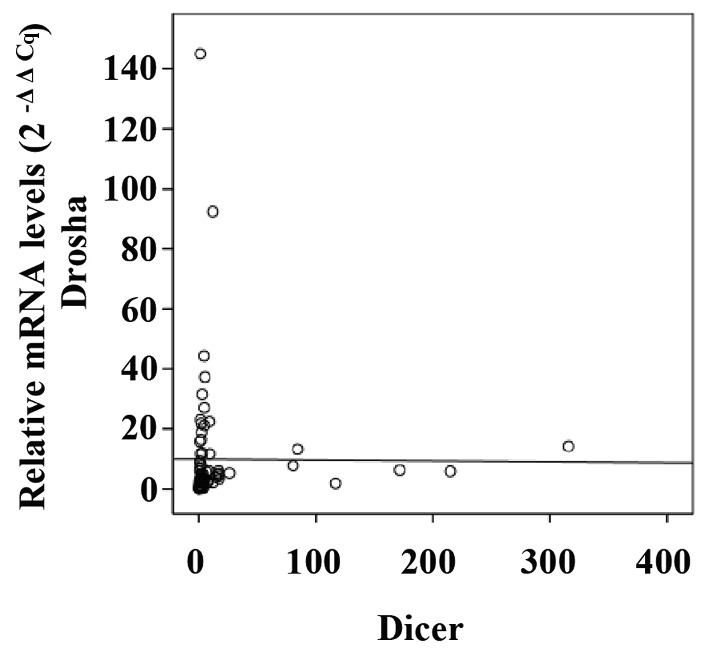
Association between mRNA expression levels of interindividual components in CRC. No significant association between Drosha and Dicer mRNA expression was identified (P=0.953, calculated using Pearson's correlation coefficient analysis).
Table II.
Association between Drosha and Dicer mRNA expression levels and clinicopathological parameters.
| Drosha | Dicer | |||||
|---|---|---|---|---|---|---|
| Characteristic | Low | High | P-value | Low | High | P-value |
| Sex | 0.070c | 0.092b | ||||
| Male | 43 | 9 | 42 | 10 | ||
| Female | 16 | 9 | 24 | 1 | ||
| Age, years | 0.190b | 0.056b | ||||
| ≤50 | 7 | 0 | 4 | 3 | ||
| >50 | 52 | 18 | 62 | 8 | ||
| T stage | 0.308a | 0.366a | ||||
| T1 | 3 | 2 | 4 | 1 | ||
| T2 | 11 | 2 | 10 | 3 | ||
| T3 | 36 | 14 | 44 | 6 | ||
| T4 | 9 | 0 | 8 | 1 | ||
| N stage | 0.542a | 0.957a | ||||
| N0 | 35 | 11 | 39 | 7 | ||
| N1 | 13 | 4 | 15 | 1 | ||
| N2 | 9 | 6 | 10 | 3 | ||
| N3 | 2 | 0 | 2 | 0 | ||
| M stage | 0.585b | 0.146b | ||||
| Negative | 54 | 18 | 63 | 9 | ||
| Positive | 5 | 0 | 3 | 2 | ||
| BMI | 0.614a | 0.872a | ||||
| ≤18.5 | 1 | 1 | 1 | 1 | ||
| 18.5–24.9 | 37 | 12 | 44 | 5 | ||
| 25-29.9 | 20 | 4 | 19 | 5 | ||
| >30 | 1 | 1 | 2 | 0 | ||
| CEA (ng/ml) | 0.268c | 1.000b | ||||
| >5 | 15 | 7 | 19 | 3 | ||
| ≤5 | 44 | 11 | 47 | 8 | ||
| Dicer | 1.000b | |||||
| Low | 50 | 16 | ||||
| High | 9 | 2 | ||||
| Drosha | 1.000b | |||||
| Low | 50 | 9 | ||||
| High | 16 | 2 | ||||
Linear-by-linear association;
Fisher's exact test;
Pearson's χ2 test. T, tumor; N, node; M, metastasis; CEA, carcinoembryonic antigen.
Discussion
Gene expression is affected by various genetic and epigenetic factors, including transcriptional or post-transcriptional regulation, miRNA, long non-coding RNA, DNA methylation, DNA-binding proteins and histone acetylation. Among the factors involved in gene expression-regulating processes, miRNAs mediate post-transcriptional gene silencing by the degradation and/or translational suppression of target mRNA as a result of binding to their complementary sequence in the 3′ untranslated region of target mRNA (18,19). The biogenesis of miRNA is a well-organized, finely tuned and complicated process, which is regulated by a number of miRNA machinery components, including Drosha, DGCR8, Xpo5, Dicer, TRBP and AGO (20). Although miRNAs and their biogenesis serve important and diverse roles in development and pathogenesis, the underlying molecular mechanisms by which miRNA machinery components regulate miRNA production remain unclear. Kim et al (21), using a knockout procedure, demonstrated that Drosha, Xpo5 and Dicer are required for the miRNA biogenesis pathway. In particular, this study demonstrated an essential role for Drosha and a contributory role for Dicer in the canonical miRNA signaling pathway.
Dysregulation of the miRNA machinery components is hypothesized to be associated with tumorigenic processes, including tumor initiation, development and metastasis (13,22). According to this aspect, the majority of the research in the miRNA biogenesis field has focused on the regulation and clinical association of miRNA machinery components in various human cancer specimens (13,22). For example, the upregulated expression of Drosha in gastric cancer is associated with patient survival rates (23) and its downregulated expression in endometrial cancer is associated with histological grade (24). Furthermore, the upregulated expression of Dicer in serous ovarian carcinoma is associated with advanced tumor stages (25) and its downregulated expression in breast cancer is associated with cancer progression and recurrence (26). Particularly in CRC, previous studies have identified that dysregulation of the miRNA machinery may be involved in carcinogenesis (10,14,16,27,28). Drosha mRNA levels were not identified to be significantly associated with tumor stages (10). However, contradictory results regarding the association between the expression levels of Dicer and clinicopathological parameters were identified (14,27,28).
These results were the motivation for the present study exploring the expression levels of Drosha and Dicer mRNA in CRC tissues compared with those in adjacent non-neoplastic colorectal tissues and to evaluate their associations with specific clinicopathological parameters. The expression levels of Drosha and Dicer mRNA were identified to be significantly upregulated in 59/77 CRC specimens. However, no significant association was identified between altered mRNA expression levels of Drosha and Dicer and any clinicopathological characteristic in CRC, including sex, age, TNM stage, BMI and CEA titer.
Accumulating evidence has identified that interindividual miRNA biogenesis-associated components are significantly associated with each other in paired samples of cancer and adjacent non-neoplastic tissues: AGO2 and DGCR8 in CRC specimens (16); Drosha and Dicer in triple negative breast cancer specimens (29); and AGO2 and DGCR8 in invasive ductal breast carcinoma specimens (30). Therefore, the association between the expression levels of Drosha and Dicer was investigated using Pearson's correlation coefficient analysis and Fisher's exact test. In contrast with previous studies, the results of the present study indicated that the expression levels of Drosha and Dicer mRNA were not associated with each other in CRC.
The results of the present study indicate that the expression levels of Drosha and Dicer mRNA are significantly upregulated in cases of CRC in the Korean population, suggesting that increased expression of Drosha and Dicer may serve an important role in the pathogenesis of CRC.
Acknowledgements
The present study was supported by Bumsuk Academic Scholarship Foundation, 2013. The biospecimens for the present study were provided by the Keimyung Human Bio-Resource Bank, a member of the National Biobank of Korea, which is supported by the Ministry of Health and Welfare.
Glossary
Abbreviations
- miRNA
microRNA
- CRC
colorectal cancer
- DGCR8
DiGeorge syndrome critical region gene 8
- Xpo5
exportin-5
- TRBP
transactivation-responsive RNA-binding protein
- AGO
Argonaute
- pre-miRNA
precursor miRNAs
References
- 1.Ministry of Health & Welfare KCCR, National Cancer Center: Annual report of cancer statistics in Korea in 2012. Journal. 2014 [Google Scholar]
- 2.Janakiram NB, Rao CV. Molecular markers and targets for colorectal cancer prevention. Acta Pharmacol Sin. 2008;29:1–20. doi: 10.1111/j.1745-7254.2008.00742.x. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-I. [DOI] [PubMed] [Google Scholar]
- 5.Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479–507. doi: 10.1146/annurev-pathol-011110-130235. [DOI] [PubMed] [Google Scholar]
- 6.Pilmore E, Hamilton KL. The role of microRNAs in the regulation of K(+) channels in epithelial tissue. Front Physiol. 2015;6:352. doi: 10.3389/fphys.2015.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tuna M, Machado AS, Calin GA. Genetic and epigenetic alterations of microRNAs and implications for human cancers and other diseases. Genes Chromosomes Cancer. 2016;55:193–214. doi: 10.1002/gcc.22332. [DOI] [PubMed] [Google Scholar]
- 8.Horsham JL, Ganda C, Kalinowski FC, Brown RA, Epis MR, Leedman PJ. MicroRNA-7: A miRNA with expanding roles in development and disease. Int J Biochem Cell Biol. 2015;69:215–224. doi: 10.1016/j.biocel.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Xie Y, Zhang L, Gao Y, Ge W, Tang P. The multiple roles of microrna-223 in regulating bone metabolism. Molecules. 2015;20:19433–19448. doi: 10.3390/molecules201019433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papachristou DJ, Korpetinou A, Giannopoulou E, Antonacopoulou AG, Papadaki H, Grivas P, Scopa CD, Kalofonos HP. Expression of the ribonucleases Drosha, Dicer, and Ago2 in colorectal carcinomas. Virchows Arch. 2011;459:431–440. doi: 10.1007/s00428-011-1119-5. [DOI] [PubMed] [Google Scholar]
- 11.Lee Y, Han J, Yeom KH, Jin H, Kim VN. Drosha in primary microRNA processing. Cold Spring Harb Symp Quant Biol. 2006;71:51–57. doi: 10.1101/sqb.2006.71.041. [DOI] [PubMed] [Google Scholar]
- 12.Tijsterman M, Plasterk RH. Dicers at RISC; the mechanism of RNAi. Cell. 2004;117:1–3. doi: 10.1016/S0092-8674(04)00293-4. [DOI] [PubMed] [Google Scholar]
- 13.Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer. 2015;15:321–333. doi: 10.1038/nrc3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faber C, Horst D, Hlubek F, Kirchner T. Overexpression of Dicer predicts poor survival in colorectal cancer. Eur J Cancer. 2011;47:1414–1419. doi: 10.1016/j.ejca.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Faggad A, Kasajima A, Weichert W, Stenzinger A, Elwali NE, Dietel M, Denkert C. Down-regulation of the microRNA processing enzyme Dicer is a prognostic factor in human colorectal cancer. Histopathology. 2012;61:552–561. doi: 10.1111/j.1365-2559.2011.04110.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim B, Lee JH, Park JW, Kwon TK, Baek SK, Hwang I, Kim S. An essential microRNA maturing microprocessor complex component DGCR8 is up-regulated in colorectal carcinomas. Clin Exp Med. 2014;14:331–336. doi: 10.1007/s10238-013-0243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 18.Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 19.Siomi H, Siomi MC. Posttranscriptional regulation of microRNA biogenesis in animals. Mol Cell. 2010;38:323–332. doi: 10.1016/j.molcel.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Mockenhaupt S, Schürmann N, Grimm D. When cellular networks run out of control: Global dysregulation of the RNAi machinery in human pathology and therapy. Prog Mol Biol Transl Sci. 2011;102:165–242. doi: 10.1016/B978-0-12-415795-8.00006-4. [DOI] [PubMed] [Google Scholar]
- 21.Kim YK, Kim B, Kim VN. Re-evaluation of the roles of DROSHA, Export in 5 and DICER in microRNA biogenesis. Proc Natl Acad Sci USA. 2016;113:E1881–E1889. doi: 10.1073/pnas.1602532113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hata A, Kashima R. Dysregulation of microRNA biogenesis machinery in cancer. Crit Rev Biochem Mol Biol. 2016;51:121–134. doi: 10.3109/10409238.2015.1117054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tchernitsa O, Kasajima A, Schäfer R, Kuban RJ, Ungethüm U, Györffy B, Neumann U, Simon E, Weichert W, Ebert MP, Röcken C. Systematic evaluation of the miRNA-ome and its downstream effects on mRNA expression identifies gastric cancer progression. J Pathol. 2010;222:310–319. doi: 10.1002/path.2759. [DOI] [PubMed] [Google Scholar]
- 24.Torres A, Torres K, Paszkowski T, Jodłowska-Jędrych B, Radomański T, Książek A, Maciejewski R. Major regulators of microRNAs biogenesis Dicer and Drosha are down-regulated in endometrial cancer. Tumour Biol. 2011;32:769–776. doi: 10.1007/s13277-011-0179-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaksman O, Hetland TE, Trope CG, Reich R, Davidson B. Argonaute, Dicer, and Drosha are up-regulated along tumor progression in serous ovarian carcinoma. Hum Pathol. 2012;43:2062–2069. doi: 10.1016/j.humpath.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 26.Yan M, Huang HY, Wang T, Wan Y, Cui SD, Liu ZZ, Fan QX. Dysregulated expression of dicer and drosha in breast cancer. Pathol Oncol Res. 2012;18:343–348. doi: 10.1007/s12253-011-9450-3. [DOI] [PubMed] [Google Scholar]
- 27.Faggad A, Kasajima A, Weichert W, Stenzinger A, Elwali NE, Dietel M, Denkert C. Down-regulation of the microRNA processing enzyme Dicer is a prognostic factor in human colorectal cancer. Histopathology. 2012;61:552–561. doi: 10.1111/j.1365-2559.2011.04110.x. [DOI] [PubMed] [Google Scholar]
- 28.Stratmann J, Wang CJ, Gnosa S, Wallin A, Hinselwood D, Sun XF, Zhang H. Dicer and miRNA in relation to clinicopathological variables in colorectal cancer patients. BMC Cancer. 2011;11:345. doi: 10.1186/1471-2407-11-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Passon N, Gerometta A, Puppin C, Lavarone E, Puglisi F, Tell G, Di Loreto C, Damante G. Expression of Dicer and Drosha in triple-negative breast cancer. J Clin Pathol. 2012;65:320–326. doi: 10.1136/jclinpath-2011-200496. [DOI] [PubMed] [Google Scholar]
- 30.Kwon SY, Lee JH, Kim B, Park JW, Kwon TK, Kang SH, Kim S. Complexity in regulation of microRNA machinery components in invasive breast carcinoma. Pathol Oncol Res. 2014;20:697–705. doi: 10.1007/s12253-014-9750-5. [DOI] [PubMed] [Google Scholar]