Abstract
Background
Autoinducer-2 (AI-2) is a universal signal molecule and enables an individual bacteria to communicate with each other and ultimately control behaviors of the population. Harnessing the character of AI-2, two kinds of AI-2 “controller cells” (“consumer cells” and “supplier cells”) were designed to “reprogram” the behaviors of entire population.
Results
For the consumer cells, genes associated with the uptake and processing of AI-2, which includes LsrACDB, LsrFG, LsrK, were overexpressed in varying combinations. Four consumer cell strains were constructed: Escherichia coli MG1655 pLsrACDB (NK-C1), MG1655 pLsrACDBK (NK-C2), MG1655 pLsrACDBFG (NK-C3) and MG1655 pLsrACDBFGK (NK-C4). The key enzymes responsible for production of AI-2, LuxS and Mtn, were also overexpressed, yielding strains MG1655 pLuxS (NK-SU1), and MG1655 pLuxS-Mtn (NK-SU2). All the consumer cells could decrease the environmental AI-2 concentration. NK-C2 and NK-C4 were most effective in AI-2 uptake and inhibited biofilm formation. While suppliers can increase the environmental AI-2 concentration and NK-SU2 was most effective in supplying AI-2 and facilitated biofilm formation. Further, reporter strain, MG1655 pLGFP was constructed. The expression of green fluorescent protein (GFP) in reporter cells was initiated and guided by AI-2. Mixture of consumer cells and reporter cells suggest that consumer cells can decrease the AI-2 concentration. And the supplier cells were co-cultured with reporter cells, indicating that supplier cells can provide more AI-2 compared to the control.
Conclusions
The consumer cells and supplier cells could be used to regulate environmental AI-2 concentration and the biofilm formation. They can also modulate the AI-2 concentration when they were co-cultured with reporter cells. It can be envisioned that this system will become useful tools in synthetic biology and researching new antimicrobials.
Electronic supplementary material
The online version of this article (10.1186/s12866-017-1107-2) contains supplementary material, which is available to authorized users.
Keywords: Autorinducer-2, “Consumer cells”, “Supplier cells”, Biofilm
Background
Microbial cells are widespread throughout the world. Intrinsic of bacterial plasticity to survive in diverse situations is their capacity to detect and respond to transient environments. As microorganisms usually share their environment with numerous other microorganisms, the communication amongst them plays a vital role in their survival. To view such systems as a whole “multicellular organism”, it is essential to develop effective strategies to regulate the behavior of the community [1]. Quorum sensing (QS) is a cell–cell communication that allows bacteria to sense one another and to regulate multicellular organism behaviors [2]. This system is regulated by signaling molecules known as autoinducers. Autoinducer-2 (AI-2) is a kind of biomolecule incorporating boron, which was first identified in the marine bacterium Vibrio harveyi [3, 4]. As a universal langue, AI-2 can be synthesized and recognized by both Gram-negative and Gram-positive bacteria [5, 6]. AI-2 could modulate a wide variety of cell-density dependent physiological activities including symbiosis, virulence, motility, antibiotic production and biofilm formation [7]. Based on the characteristics mentioned above, AI-2 is considered a kind of biosensor for regulating the activities dependent on quorum sensing.
QS networks dependent on AI-2 have been investigated in many bacteria such as Helicobacter pylori, Bacillus cereus and Escherichia coli K12 [8–10]. The mechanism of AI-2 uptake, processing and synthesis in E.coli has been well characterized. During active growth, LuxS produces AI-2, which is secreted into the extracellular space. AI-2 accumulates until it triggers the activation of the Lsr system in the receptor cells. LsrACDB, encoded by the genes of the lsr operon, are responsible for the internalization of AI-2 [11]. Other enzymes encoded by the lsr operon were involved in the regulation of gene expression and further intracellular metabolic degradation of AI-2 [12, 13].
For the synthesis of AI-2, previous studies suggest that under different biological contexts, the pathways for the formation of AI-2 are different [14]. One of the pathway was related to nucleic acid precursor, adenosine. The adding of glucose can modulate the metabolic flux and AI-2 synthesis [15]. However this pathway has not been illuminated. The AI-2 signal molecule is also a by-product of the activated methyl cycle (AMC). The substrate for AI-2 synthesis catalyzed by LuxS is S-ribosylhomocysteine (SRH), which derives from the toxic intermediate S-adenosylhomocysteine (SAH), a product from S-adenosylmethionine (SAM) metabolism, an important and ubiquitous central metabolite of the cell [11, 16]. During the process of AMC, SAH is hydrolyzed to SRH by 5′-methylthioadenosine/S-adenosylhomocysteine nucleosidase, which is called Pfs in E. coli W3110 and Mtn in MG1655 (Fig. 1).
Fig. 1.
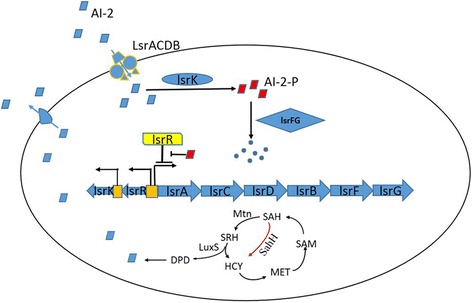
The uptake and synthesis of AI-2 in E. coli. AI-2 was transported into the cell by LsrACDB. LsrK was involved in the following phosphorylation of AI-2 signal molecule. When AI-2-P was accumulated to a specific value, the repression of lsr operon caused by LsrR was relieved. And then, the AI-2-P was degraded by LsrFG. AI-2 is the byproducts of the activated methyl cycle (AMC) and the process of AI-2 synthesis were depicted. Moreover, there is another pathway directing the conversion from SAH to HCY to complete the AMC, i.e. one-step conversion by SAH hydrolase (SahH). However, there was no AI-2 produced through the second route
Much research has focused on the QS system. Overexpression of the Pfs and LuxS could be used to AI-2 synthesis in vitro [17]. Based on the QS system in E. coli, exogenous addition of AI-2 to the QS synthase mutants was used to direct the assembly of “quantized quorums,” microbial subpopulations [18]. Furthermore, four kinds of bacterial AI-2 consumers were constructed by overexpressing components responsible for AI-2 uptake (lsrACDB), phosphorylation (lsrK), and degradation (lsrFG) [19]. These consumer cells were defined as “controller cells”, which can be deployed to control environmental AI-2 concentration. It has been previously demonstrated that consumer cells with lsrACDB overexpression are capable of silencing gene expression mediated by QS and can modulate chemotaxis and biofilm formation.
However, we thought that as the “controller cells”, they should not only include “consumer cell” capabilities, but also “supplier cell” functionality, capable of manipulating the AI-2 signaling molecule environmental concentration. In this study, the consumer cells were designed by overexpressing the components of lsr operon in varying combinations. Compared to the previous study, two new kinds of consumer strains were engineered by overexpressing lsrACDBK (NK-C2) and lsrACDBEGK (NK-C4). Both NK-C2 and NK-C4 have better AI-2 uptake capability than the consumer strains overexpressing lsrACDB and lsrACDBFG. In addition, supplier strains were constructed with luxS and/or mtn overexpression with the corresponding plasmids, pLuxS and pLuxS-Mtn. Since the controller cells were used to modulate the environmental AI-2 concentration, co-culture experiments were needed to characterize the function of controller cells. Then reporter cells were constructed. Briefly, pLGFP responsible for expression of GFP under the control of the lsr promoter (Fig. 2), was built and the expression of GFP can response to AI-2. MG1655 was transformed with pLGFP, resulting in the reporter strain (MG1655 pLGFP). The results indicated that supplier cells and consumer cells could modulate the environmental concentration of AI-2. With regards to biofilm effects, consumer cells could inhibit biofilm formation whereas supplier cells facilitate its formation. Besides that the supplier cells can activate GFP expression and the consumer cells silence GFP expression when they were co-cultured. These results suggest that supplier cells can provide more AI-2 in the environment while consumer cells can decrease the AI-2 concentration.
Fig. 2.
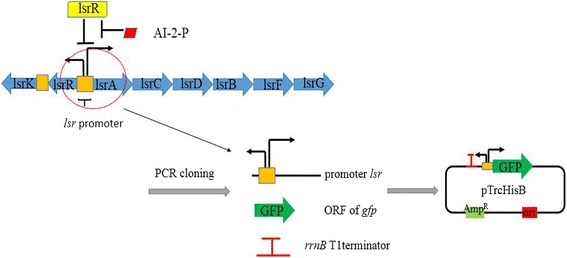
The plasmid used for the construction of reporter cells. The structure of lsr operon was depicted in the picture. The lsr is a bidirectional promoter. Three elements: lsr promoter, gfp, rrnB T1 terminator were ligated to pTrcHisB so that the plasmid pLGFP can respone to the intrecellular AI-2-P concentration
Methods
Strains and culture conditions
All strains and plasmids used in this study are listed in Table 1. E. coli MG 1655 was used as the basal strain. As needed, media were supplemented with antibiotics or IPTG with the following concentrations: 100 μg /mL ampicillin, 1 mM IPTG.
Table 1.
Strains and plasmids used in this study
| Strains and plasmids | Gene types and characteristics | Source |
|---|---|---|
| Strains | ||
| E. coli MG 1655 | F-φ80 lac ZΔM15 Δ(lacZYA-arg F) U169 endA1 recA1 hsdR17(rk − ,mk +) supE44λ- thi − 1 gyrA96 relA1 phoA | Transgene |
| E. coli NK-C1 | MG1655 contains pLsrACDB | This study |
| E. coli NK-C2 | MG1655 contains pLsrACDBK | This study |
| E. coli NK-C3 | MG1655 contains pLsrACDBFG | This study |
| E. coli NK-C4 | MG1655 contains pLsrACDBFGK | This study |
| E. coli SU1 | MG1655 contains pLuxS | This study |
| E. coli SU2 | MG1655 contains pLuxS-Mtn | This study |
| Plasmids | ||
| pTrcHisB | Cloning vector Apr | |
| pLsrACDB | pTrcHisB derivative, containing lsrACDB | This study |
| pLsrACDBFG | pTrcHisB derivative, containing lsrACDB, and lsrFG | This study |
| pLsrACDBK | pTrcHisB derivative, containing lsrACDB and lsrK | This study |
| plsrACDBFGK | pTrcHisB derivative, containing lsrACDB,lsrFG,lsrK | This study |
| pLuxS | pTrcHisB derivative, containing luxS | This study |
| pLuxS-Mtn pLGFP |
pTrcHisB derivative, containing luxS and mtn
pTrcHisB derivative, containing lsr promoter and gfp |
This study This study |
Plasmid construction
The pTrcHisB was used as the plasmid backbone. The genes, luxS, mtn, lsrACDB, were PCR-amplified from the E. coli MG 1655 genome and ligated to pTrcHisB (KpnI, EcoRI, BamHI, SacI, BglII were used in this process) using ClonExpress II (Vazyme, China), to form three plasmids: pLuxS, pLuxS-Mtn, pLsrACDB. Then the PstI and BstBI digested lsrK segment was inserted into the same restriction sites of the pLsrACDB vector and transformed into E.coli K12, resulting in pLsrACDBK. The construction of plasmids pLsrACDBFG and pLsrACDBFGK was analogous to pLsrACDBK construction. For the construction of pLGFP, and the promoter sequence of lsrR, lsr and the opening reading frame (ORF) of green fluorescent protein (GFP) were PCR-amplified. This two fragments were ligated in pTrcHisB (BamHI, SacI were used in this process) using ClonExpress MultiS (Vazyme, China). Primers used in this study are listed in Additional file 1: Table S1. Procedures of cloning, DNA purification, and transformations were performed using standard protocols. All plasmids were confirmed by restriction enzyme digestion and DNA sequencing.
Analysis of the gene expression
The levels of genes transcription and expression were analyzed using quantitative real-time PCR and Urea-PAGE. The E. coli MG 1655 pTrcHisB was used as the control. Strains harboring pLsrACDB, pLsrACDBK, pLsrACDBFG, pLsrACDBFGK, pLuxS, pLuxSMtn were cultured overnight. Strains were reinoculated and 1 mM IPTG was added into the medium when the strains reached the mid-log phase. After being induced for 4 h, the cells were collected for RNA extraction using RNApure Bacteria Kit (CWBIO, China). The target mRNAs were obtained by reverse-transcription and analyzed using the FastStart Universal SYBR Green Master (Rox). The expression of protein was evaluated by Urea-PAGE. Cell pellets were collected and resuspended with distilled water. Then cells were disrupted by a sonicator (300 W for 4 min with cycles of 5 s sonication followed by 10 s pause). Cell extracts were analyzed by Urea-PAGE following the standard protocol. Experiments were independently repeated at least three times, and the means and standard deviations were calculated.
Analysis of AI-2 extracellular concentration
To validate the function of AI-2 consumer cells, extracellular AI-2 was first prepared. Compound A (Atomax Chemiscals Co. Ltd), defined as substance 11 in the previous study, was used for the supplement of 4, 5-dihydroxy-2, 3-pentanedione (DPD). The procedure of producing synthetic DPD was performed as the manuscript description [20]. First, compound A was dissolved in double-distilled water (ddH2O) and adjusted to pH 2 by H2SO4. After the reaction proceeded for 2 h, the resultant mixture was diluted with 0.1 M potassium phosphate buffer (pH = 7) to adjust the pH to 7.3. The obtained material, synthetic DPD, could be used without further purification in the V. harveyi bioassay, and displayed an activity equal to that of enzymatically prepared DPD. The synthetic DPD undergoes spontaneous rearrangements to form AI-2.
The uptake rate of AI-2 was analyzed as previously reported [21]. AI-2 (40 μM) and IPTG (1 mM) were added into the culture during mid-logarithmic phase (when the optical density at 600 nm (OD600 nm) was about 0.4). An Alltech system controller (Alltech Associates Inc., USA) equipped with a C18 reverse-phase column and UV-visible detector was used for AI-2 analysis. Formic acid of 0.01% (V/V) and acetonitrile was used as the mobile phase at a flow rate of 1 mL/min. Absorbance at 240 nm was recorded and used to calculate AI-2 concentration. The relative concentration changes was used to assess uptake capability of consumer cells.
The resulting environmental AI-2 concentration of the supplier cells was measured using high performance liquid chromatography (HPLC). Briefly, Strains were cultured overnight and inoculated. Then IPTG was added into the cultures during mid-logarithmic phase (about 4 h after inoculated) and then the AI-2 concentration was detected. E. coli MG 1655 pTrcHisB was used as the control.
Characterization of biofilm
The MG1655 pTrcHisB base strain, four strains of AI-2 consumer cells, and two strains of supplier cells were cultured individually overnight and diluted to OD600 nm = 0.05. Each AI-2 consumer cell strain and supplier cell strain was co-cultured with MG1655 pTrcHisB at a 1:1 (v/v) ratio with a total volume of 200 μl [22]. MG1655 pTrcHisB was cultured as control. Strains were grown in polystyrene 96-well plates at 30 °C overnight without shaking. IPTG was added into the culture at an OD600 nm 0.4. To quantify the biofilm mass, the cell suspension was discarded and the plate was washed 3 times with PBS buffer (0.1 M, pH = 7). The plate was then incubated at 60 °C with the lid off for 60 min. The biofilms were stained with 0.1% crystal violet for 15 min and the excess dye was washed with water. The remaining dye (staining the biofilms) was dissolved with 95% ethanol and incubated with shaking for 30 min, and the OD at 540 nm was measured. Experiments were independently repeated at least three times, and the means and standard deviations were calculated. A two-tailed unpaired Student’s t-test was performed between the groups.
To validate the function of AI-2 “reporter cells”
The function of reporter cells (MG1655 pLGFP), were validated as following. MG1655 pLGFP were cultured overnight and inoculated into fresh medium for 2 h (OD 600 = 0.2). Exogenous AI-2 was added into the culture. The final concentration of AI-2 was 50 μM, 40 μM, 30 μM, 20 μM, 10 μM, 0 μM, respectively. The 96-well black plates which contains cell cultures were subjected to fluorescence intensity determination through EnSpire Multimode Plate Reader using the 530/60 nm filter.
Environmental concentration of AI-2 in co-cultures
Strains were cultured and inoculated at 37 °C with shaking at 180 rpm. IPTG was added into cultures at mid-log growth phase (OD600 = 0.40–0.60). Each AI-2 consumer cell strain and supplier cell strain was co-cultured with MG1655 pLGFP at a 1:1 (v/v) ratio with a total volume of 200 μl. MG1655 pLGFP co-cultured with MG1655 pTrcHisB was used as control. Fluorescence intensity and OD600 were measured every two hours. Experiments were independently repeated at least three times, and the means and standard deviations were calculated. A two-tailed unpaired Student’s t-test was performed between the groups.
Results
Construction of AI-2 “consumer cells” and “supplier cells”
Two consumer cells were constructed to overexpress either lsrACDBK, or lsrACDBFGK and were named as E. coli NK-C2 (MG1655 pLsrACDBK) and E. coli NK-C4 (MG1655 pLsrACDBFGK). Another two consumer cells were also constructed, NK-C1 (MG1655 pLsrACDB) and E. coli NK-C3 (MG1655 pLsrACDBFG). To verify whether the consumer cells played the expected role, quantitative real-time PCR and Urea-PAGE were conducted (Fig. 3, Additional file 2: Figure S1a). The results confirmed that all the target genes in consumer cells were overexpressed.
Fig. 3.
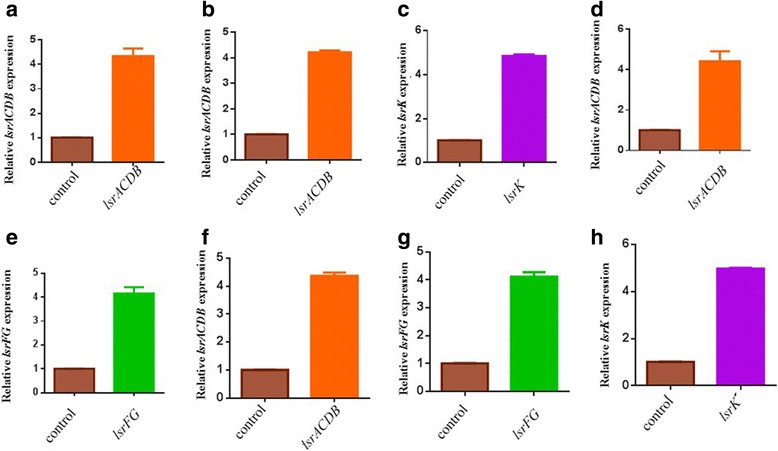
The quantitative real-time PCR of AI-2 “consumer cells”. a. The relative transcription level of lsrACDB in E. coli NK-C1. b and c. The relative transcription levels of lsrACDB and lsrK in E. coli NK-C2. d and e. The relative transcription levels of lsrACDB and lsrFG in E. coli NK-C3. f, g and h. The relative transcription levels of lsrACDB, lsrFG and lsrK in E. coli NK-C4. The relative transcription levels of lsrA and lsrF represented the transcription levels of lsrACDB and lsrFG,respectively. The strain containig pTrcHisB was defined as 1 in the expression level of corresponding genes
Along with the AI-2 consumer cells, supplier cells that could increase the environmental AI-2 concentration of were also constructed. In this study, the AI-2 synthesis genes luxS and mtn were cloned into pTrcHisB, yielding pTrcLuxS and pTrcLuxS-Mtn. The plasmids were transformed into E. coli MG1655, resulting in two supplier cell strains named E. coli NK-SU1 (MG1655 pLuxS) and NK-SU2 (MG1655 pLuxS-Mtn). The transcription and expression levels of luxS and mtn are summarized in Fig. 4 and Additional file 2: Figure S1b. In NK-SU1 and NK-SU2, luxS expression level was 3.5-fold higher than the control, whereas the mtn expression in NK-SU2 was around 4-fold higher than the control. The consumer cells and supplier cells were constructed by modulating the expression of genes associated with AI-2 synthesis and degradation. These modifications are listed in Additional file 3: Table S2.
Fig. 4.
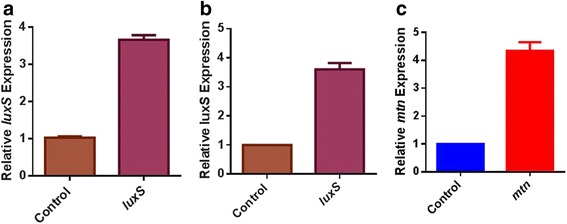
The quantitative real-time PCR of AI-2 “supplier cells”. a. The relative transcription level of luxS in E. coli NK-SU1. b and c. The relative transcription levels of luxS and mtn in E. coli NK-SU2. The strain containing pTrcHisB was defined as 1 in the expression level of corresponding genes
Environmental concentration of AI-2 controlled by “consumer cells” and “supplier cells”
It was verified that all the genes used for the construction of the six AI-2 consumer cell/supplier cell strains were expressed successfully. To further validate whether consumer cells could uptake AI-2, 40 μM exogenous AI-2 was added into the liquid culture. Then, the extracellular concentration of AI-2 was monitored by HPLC. Results showed that all consumer cells could successfully uptake the supplemented AI-2, compared to E. coli MG 1655 pTrcHisB. As shown in Fig. 5 a, environmental AI-2 concentration was significantly reduced after two hour induction with IPTG, which meant that consumer cells could “quench” the environmental AI-2 signal. Besides that the consumer cell strain NK-C2 and NK-C4, which overexpressed LsrK possessed effective AI-2 uptake capability, and the AI-2 uptake capability of NK-C4 was the greatest.
Fig. 5.
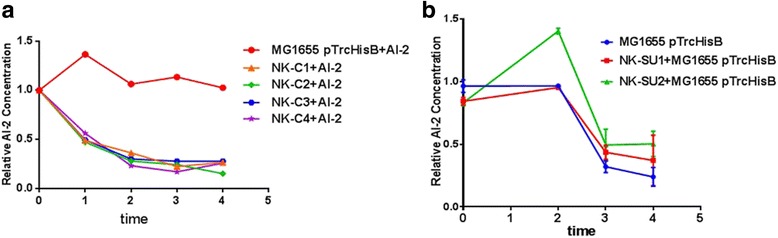
Effects of “consumer cells” and “supplier cells” on environmental AI-2 concentration. a. AI-2 uptake profiles of “consumer cells”. The relative environmental concentration of AI-2 of the control was defined as 1. b. AI-2 supply profiles of “AI-2 supplier cells”. The relative concentration of AI-2 was analyzed. The relative environmental concentration of AI-2 was defined as 1. The error bar indicated the standard deviation and each experiment was carried out by three replicates
Additionally, environmental AI-2 concentration of supplier cells were also measured (Fig. 5 b). At the first 2 h, the AI-2 concentration of all strains increased. Then AI-2 concentration decreased significantly. During the last hour the decrease rate of AI-2 concentration became slower compared to that of the third hour. During the whole process, the AI-2 concentration of NK-SU2 were higher than NK-SU1 and MG1655 pTrcHisB. After induced for 4 h, the environmental concentration of AI-2 secreted by NK-SU1 and NK-SU2 resulted in 54% and 100% increases.
Biofilm change caused by AI-2
Nearly all cells make biofilms, which are formed in aquatic environments as the result of attachment of bacteria to submerged surfaces, to the air/liquid interface, and to each other [22, 23]. AI-2, being a universal signal molecule, can regulate many bacterial behaviors, including the formation of biofilms. Since the constructed consumer cells and supplier cells could regulate the environmental concentration of AI-2, they may also play a role in the formation of bacterial biofilm. Consumer cells and supplier cells were co-inoculated in parallel with MG1655 pTrcHisB to determine their effects on biofilm formation (Fig. 6) The biofilm thickness of MG1655 pTrcHisB co-cultured with NK-C1, NK-C2, NK-C3 or NK-C4 decreased by 15%, 34%, 12% and 43%, respectively, compared to the control. In contrast, the biofilm thickness of MG1655 pTrcHisB co-cultured with NK-SU1 or NK-SU2 increased by 27% and 28%, respectively, compared to the control (Fig. 6).
Fig. 6.
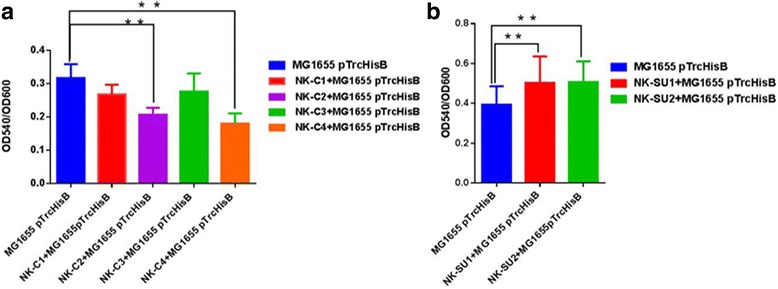
Effects of “consumer cells” and “supplier cells” on biofilm formation. a. Effects of “consumer cells” on biofilm formation. b. Effects of “supplier cells” on biofilm formation. The biofilm formation of MG1655 containing pTrcHisB was the control. The biofilm formation was characterized by OD540 nm/OD600 nm. The error bar indicated the standard deviation and each experiments was carried out by three replicates. A two-tailed unpaired Student’s t-test was performed between the groups and significant results indicated by the star. Two stars: P ≤ 0.05, three stars: P ≤ 0. 01
Manipulation of “controller cells” in co-cultures
The native QS regulon was re-engineered and AI-2 was used to guide high level expression of recombinant proteins in E. coli [15]. In this study, the native QS system was rewired to construct reporter cells that could response to AI-2. The AI-2 quorum sensing network was depicted in the Fig. 1. Promoter lsr can be repressed by LsrR, affecting the expression of lsr operon. This process can be derepressed by AI-2-P. The plasmid pLGFP involved in reporter cells construction was built (Fig. 2). The expression of GFP can be regulated by the environmental AI-2 concentration. The response ability to AI-2 of MG1655 pLGFP is shown in Fig. 7. After adding the exogenous AI-2, reporter cells can respond to variant concentration of AI-2 by emitting different fluorescence intensity. These results suggest that reporter cells can be used as a detector for the environmental AI-2 concentration.
Fig. 7.
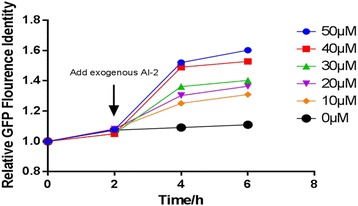
The response ability of reporter cells to exogenous AI-2. The strain containig pLGFP wihtout adding exougenous AI-2 was used as the control and its GFP flourence indensity was defined as 1. At the second hour, exogenous AI-2 were added into the culture at different concentration
AI-2 controller cells were co-cultured with reporter cells at the ratio of 1:1. Results showed that AI-2 consumer cells could significantly depress AI-2-inducible GFP fluorescence compared to the control group (Fig. 8 a), as expected. Also, at the 1:1 mixture of AI-2 reporter cells with AI-2 supplier cells, AI-2-inducible GFP fluorescence was significantly enhanced compared to the control group (Fig. 8 b). These suggest that our consumer cells do decrease the AI-2 concentration and supplier cells can increase the AI-2 concentration then regulate the GFP fluorescence. These results indicated that the controller cells could regulate the environmental AI-2 concentration.
Fig. 8.
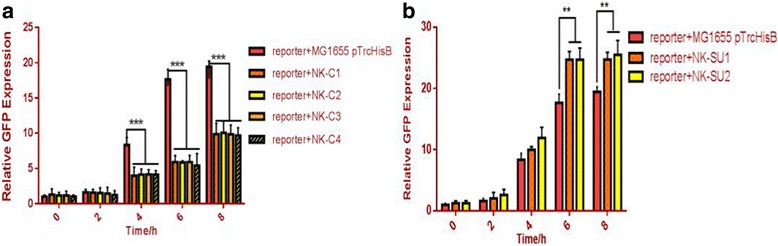
Manipulation of “controller cells” in co-cultures a. The regulation of GFP fluorescence by consumer cells. b. The regulation of GFP fluorescence by supplier cells. The strain containig pTrcHisB co-cultured with reporter cells was used as the control and its GFP flourence indensity at the beginning was defined as 1. The error bar indicated the standard deviation and each experiments was carried out by three replicates. A two-tailed unpaired Student’s t-test was performed between control and samples. And significant results indicated by the star. Two stars: P ≤ 0.05; three stars ≤0.01
Discussion
Quorum-sensing networks enable bacterial cells to sense and communicate, which was needed for orchestrating population behavior. In this work, we constructed two kinds of controllers: AI-2 consumer cells and supplier cells. Since LsrACDB is the transporter of AI-2 and constitutes the rate-limiting step of AI-2 assimilation, these genes were overexpressed to construct numerous consumer cell strains. First, two consumer cell strains, similar with previous design [19], were constructed and named NK-C1 and NK-C3. Although extracellular concentration of AI-2 decreased, the AI-2 uptake of NK-C1 was not significantly different from NK-C3. One possible explanation is that LsrK is required for the phosphorylation of AI-2, which means AI-2 could be sequestrated in the cytoplasm [19], and AI-2-P is the optimum substrate for the LsrFG. It has been proved that LsrK can phosphorylate AI-2 in vitro, and LsrK-treated AI-2 can significantly reduce the native QS response of E. coli populations [24]. Therefore, two more consumer cell strains, NK-C2 and NK-C4, were constructed. Results indicated that the AI-2 uptake capability of NK-C2 was similar with that of NK-C4, with both strains possessing higher uptakes rates than NK-C1 and NK-C3. Moreover, consumer cells with LsrACDB and LsrK co-overexpression could absorb more AI-2 and prevent AI-2 from leaking out of the cell, which resulted in reduced environmental AI-2 concentrations. Since LsrFG is responsible for cellular degradation of AI-2-P, the effects of LsrFG on the environmental AI-2 were less notable. Thus, the uptake capability of NK-C2 was similar to that of NK-C4. In addition, both NK-C2 and NK-C4 possessed a strong capability to interfere with biofilm formation, coinciding with the uptake profile of extracellular AI-2. However, luxS has not been deleted. LuxS deletion could further improve AI-2 uptake capability.
In this study, luxS and mtn (also known as mtn, pfs or yadA in E. coli MG 1655) were manipulated in supplier cell design. Results indicated that NK-SU2 was more effective in increasing environmental AI-2 than that of NK-SU1. Previous studies with regards to enhancing AI-2 concentration were mostly focused on luxS overexpression [25]. In the AMC cycle, Mtn catalyzed the conversion of toxic intermediate SAH to SRH [26]. The co-expression of mtn and luxS could provide more substrate for AI-2 synthesis, resulting in higher AI-2 concentrations. And the AI-2 concentration increased in the logarithmic phase and dropped in the state-stage.
Biofilm formation of NK-C1 and NK-C2 was enhanced compared to the control, coinciding with the supply profile with AI-2. As biofilm formation occurs in 80% of human bacterial infections [22], and pathogens within biofilms have up to 1000 times higher antibiotic resistance compared to being present in a planktonic state, consumer cells have the potential to serve as next-generation antibiotic treatments due to their biofilm formation inhibition capabilities [27, 28]. Additionally, supplier cells can increase AI-2 concentration, which could increase bacterial stress resistance, benefiting the synthesis of probiotics products [25]. Thus, AI-2 consumer cells and supplier cells could be used to regulate different bacteria population behaviors.
Conclusions
The two kinds of AI-2 controllers could be used as a tool to manipulate AI-2 concentrations which influences many cell density-dependent behaviors. Consumer cells can decrease AI-2 concentrations and repress biofilm formation. Supplier cells can increase AI-2 concentrations and facilitate biofilm formation. However, there are still some limitations in our present study. One is that consumer cells should be LuxS mutant strains to reduce the controller cells AI-2 concentration. Another is that our controller cells should be removed more convenient from the co-culture environment. Capsule or membrane can be used in the co-culture experiments.
Additional files
Primers used in this study. (DOCX 13 kb)
The expression of target genes. a. the expression of target genes in consumer cells. b. the expression of target genes in supplier cells. The masses of LsrA, LsrB, LsrC, LsrD, LsrF, LsrG, LsrK, LuxS, Mtn were 55.8kD, 36.6kD, 36.4kD, 34.4kD, 31.8kD, 57.45kD, 11.2kD, 19.4kD, 24.3kD, respectively. Compared to the control, there are three bands, 31kD, and 11.2kD in the Lane of pLsrACDBFG, which could indicate that the LsrF, LsrG were all overexpressed. The similar gel bands also indicated that LsrK, Mtn and LuxS were expressed in the target cells. Furthermore, the gel results were consistent with the qPCR results. (JPEG 294 kb)
The expression situation of corresponding genes in AI-2 “consumer cells” and “supplier cells”. (DOCX 12 kb)
Acknowledgements
We would like to thank Dr. Deon Ploessl from Department of Chemical and Biological Engineering at Iowa State University (USA) for his friendly work on the English editing and modification.
Funding
This work was supported by National High Technology Research and Development Program of China (2015BAD16B04); National Natural Science Foundation of China (31,470,213, 31,670,093 and 81,671,842); Project of Tianjin, China (15ZCZDNC00450, 16JCZDJNK-C37600, 17JCZDJC32100); the State Key Laboratory of Medicinal Chemical Biology (201603007).
Availability of data and materials
Not applicable.
Abbreviations
- AI-2
Autoinducer-2
- AMC
activated methyl cycle
- DPD
4, 5-dihydroxy-2, 3-pentanedione
- HCY
homocysteine
- QS
quorum sensing
- SAH
S-adenosyl homocysteine
- SahH
SAH hydrolase
- SAM
S-adenosylmethionine
- SRH
S-ribosyl homocysteine
Authors’ contributions
QYF, MFK, MXY and SCJ conceived and designed the study, MFK, MXY performed the study, QYF, MFK, SXH and LX analyzed and interpreted the data, QYF wrote the manuscript. GWX, DYL, MY, CMF and SCJ revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12866-017-1107-2) contains supplementary material, which is available to authorized users.
References
- 1.Purnick PE, Weiss R. The second wave of synthetic biology: from modules to systems. Nat Rev Mol Cell Biol. 2009;10(6):410–422. doi: 10.1038/nrm2698. [DOI] [PubMed] [Google Scholar]
- 2.Pereira CS, Thompson JA, Xavier KB. AI-2-mediated signalling in bacteria. FEMS Microbiol Rev. 2013;37(2):156–181. doi: 10.1111/j.1574-6976.2012.00345.x. [DOI] [PubMed] [Google Scholar]
- 3.Bassler BL, Wright M, Showalter RE, Silverman MR. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol Microbiol. 1993;9:773–786. doi: 10.1111/j.1365-2958.1993.tb01737.x. [DOI] [PubMed] [Google Scholar]
- 4.Bassler BL, Greenberg EP, Stevens AM. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J Bacteriol. 1997;179(12):4043–4045. doi: 10.1128/jb.179.12.4043-4045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller S, Xavier KB, Campagna SR, Taga ME, Semmelhack MF, Bassler BL, Hughson FM. Salmonella typhimurium recognizes a chemically distinct form of the bacterial quorum-sensing signal AI-2. Mol Cell. 2004;15(5):677–687. doi: 10.1016/j.molcel.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 6.Xavier KB, Bassler BL. Interference with AI-2-mediated bacterial cell–cell communication. Nature. 2005;437:750–753. doi: 10.1038/nature03960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller MB, Bassler BL. Quorum sensing in bacteria. Annu Rev Microbiol. 2001;55:165–199. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- 8.Armbruster CE, Pang B, Murrah K, Juneau RA, Perez AC, Weimer KE, Swords WE. RbsB (NTHI_0632) mediates quorum signal uptake in nontypeable Haemophilus influenzae strain 86-028NP. Mol Microbiol. 2011;82(4):836–850. doi: 10.1111/j.1365-2958.2011.07831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auger S, Krin E, Aymerich S, Gohar M. Autoinducer 2 affects biofilm formation by Bacillus cereus. Appl Environ Microbiol. 2006;72:937–941. doi: 10.1128/AEM.72.1.937-941.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.González Barrios AF, Zuo R, Hashimoto Y, Yang L, Bentley WE, Wood TK. Autoinducer 2 controls biofilm formation in Escherichia coli through a novel motility quorum-sensing regulator (MqsR, B3022) J Bacteriol. 2006;188(1):305–316. doi: 10.1128/JB.188.1.305-316.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heurlier K, Vendeville A, Halliday N, Green A, Winzer K, Tang CM, Hardie KR. Growth deficiencies of Neisseria meningitidis pfs and luxS mutants are not due to inactivation of quorum sensing. J Bacteriol. 2009;191(4):1293–1302. doi: 10.1128/JB.01170-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vendeville A, Winzer K, Heurlier K, Tang CM, Hardie KR. Making 'sense' of metabolism: autoinducer-2, LuxS and pathogenic bacteria. Nat Rev Microbiol. 2005;3(5):383–396. doi: 10.1038/nrmicro1146. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Attila C, Wang L, Wood TK, Valdes JJ, Bentley WE. Quorum sensing in Escherichia coli is signaled by AI-2/LsrR: effects on small RNA and biofilm architecture. J Bacteriol. 2007;189(16):6011–6020. doi: 10.1128/JB.00014-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hauck T, Hubner Y, Bruhlmann F, Schwab W. Alternative pathway for the formation of 4,5-dihydroxy-2,3-pentanedione, the proposed precursor of 4-hydroxy-5-methyl-3(2H)-furanone as well as autoinducer-2, and its detection as natural constituent of tomato fruit. Biochim Biophys Acta. 2003;1623:109–119. doi: 10.1016/j.bbagen.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Wang L, Hashimoto Y, Tsao CY, Wood TK, Valdes JJ, Zafiriou E, Bentley WE. A stochastic model of Escherichia coli AI-2 quorum signal circuit reveals alternative synthesis pathways. Mol Syst Biol. 2010;2:67. doi: 10.1038/msb4100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xavier KB, Miller ST, Lu W, Kim JH, Rabinowitz J, Pelczer I, Semmelhack MF, Bassler BL. Phosphorylation and processing of the quorum-sensing molecule autoinducer-2 in enteric bacteria. ACS Chem Biol. 2007;2(2):128–136. doi: 10.1021/cb600444h. [DOI] [PubMed] [Google Scholar]
- 17.Fernandes R, Tsao CY, Hashimoto Y, Wang L, Wood TK, Payne GF, Bentley WE. Magnetic nanofactories: localized synthesis and delivery of quorum-sensing signaling molecule autoinducer-2 to bacterial cell surfaces. Metab Eng. 2007;9(2):228–239. doi: 10.1016/j.ymben.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Servinsky MD, Terrell JL, Tsao CY, Wu HC, Quan DN, Zargar A, Allen PC, Byrd CM, Sund CJ, Bentley WE. Directed assembly of a bacterial quorum. ISME J. 10(1):158–69. [DOI] [PMC free article] [PubMed]
- 19.Zargar A, Quan DN, Emamian M, Tsao CY, Wu HC, Virgile CR, Bentley WE. Rational design of 'controller cells' to manipulate protein and phenotype expression. Metab Eng. 2015;30:61–68. doi: 10.1016/j.ymben.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Semmelhack MF, Campagna SR, Federle MJ, Bassler BL. An expeditious synthesis of DPD and boron binding studies. Org Lett. 2005;7(4):569–572. doi: 10.1021/ol047695j. [DOI] [PubMed] [Google Scholar]
- 21.Tsao CY, Wang L, Hashimoto Y, Yi H, March JC, DeLisa MP, Wood TK, Valdes JJ, Bentley WE. LuxS co-expression enhances yields of recombinant proteins in Escherichia coli in part through posttranscriptional control of GroEL. Appl Environ Microbiol. 2011;77(6):2141–2152. doi: 10.1128/AEM.02347-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood TK, Hong SH, Ma Q. Engineering biofilm formation and dispersal. Trends Biotechnol. 2011;29(2):87–94. doi: 10.1016/j.tibtech.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taga ME, Miller ST, Bassler BL. Lsr-mediated transport and processing of AI-2 in salmonella typhimurium. Mol Microbiol. 2003;50(4):1411–1427. doi: 10.1046/j.1365-2958.2003.03781.x. [DOI] [PubMed] [Google Scholar]
- 24.Roy V, Fernandes R, Tsao CY, Bentley WE Cross species quorum quenching using a native AI-2 processing enzyme. ACS Chem Biol. 2010, 19; 5(2):223-232. [DOI] [PubMed]
- 25.Gao M, Song H, Zheng H, Ren Y, Li S, Liu X, Yu W, Ma X. Culture of low density E. coli cells in alginate-chitosan microcapsules facilitates stress resistance by up-regulating luxS/AI-2 system. Carbohydr Polym. 2016;141:160–165. doi: 10.1016/j.carbpol.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 26.Brito PH, Rocha EP, Xavier KB, Gordo I. Natural genome diversity of AI-2 quorum sensing in Escherichia Coli: conserved signal production but labile signal reception. 2013; 5(1):16-30. [DOI] [PMC free article] [PubMed]
- 27.Romero R, Schaudinn C, Kusanovic JP, Gorur A, Gotsch F, Webster P, Nhan-Chang CL, Erez O, Kim CJ, Espinoza J, Gonçalves LF, Vaisbuch E, Mazaki-Tovi S, Hassan SS, Costerton JW. Detection of a microbial biofilm in intra-amniotic infection. Am J Obstet Gynecol. 2008;198(135):e1–e5. doi: 10.1016/j.ajog.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roy V, Adams BL, Bentley WE. Developing next generation antimicrobials by intercepting AI-2 mediated quorum sensing. Enzym Microb Technol. 2011;49(2):113. doi: 10.1016/j.enzmictec.2011.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers used in this study. (DOCX 13 kb)
The expression of target genes. a. the expression of target genes in consumer cells. b. the expression of target genes in supplier cells. The masses of LsrA, LsrB, LsrC, LsrD, LsrF, LsrG, LsrK, LuxS, Mtn were 55.8kD, 36.6kD, 36.4kD, 34.4kD, 31.8kD, 57.45kD, 11.2kD, 19.4kD, 24.3kD, respectively. Compared to the control, there are three bands, 31kD, and 11.2kD in the Lane of pLsrACDBFG, which could indicate that the LsrF, LsrG were all overexpressed. The similar gel bands also indicated that LsrK, Mtn and LuxS were expressed in the target cells. Furthermore, the gel results were consistent with the qPCR results. (JPEG 294 kb)
The expression situation of corresponding genes in AI-2 “consumer cells” and “supplier cells”. (DOCX 12 kb)
Data Availability Statement
Not applicable.


