Abstract
Background
Lactobacillus species produce biosurfactants that can contribute to the bacteria’s ability to prevent microbial infections associated with urogenital and gastrointestinal tracts and the skin. Here, we described the biological and physicochemical properties of biosurfactants produced by Lactobacillus jensenii P6A and Lactobacillus gasseri P65.
Results
The biosurfactants produced by L. jensenii P6A and L. gasseri P65 reduced the water surface tension from 72 to 43.2 mN m−1 and 42.5 mN m−1 as their concentration increased up to the critical micelle concentration (CMC) values of 7.1 and 8.58 mg mL−1, respectively. Maximum emulsifying activity was obtained at concentrations of 1 and 5 mg mL−1 for the P6A and P65 strains, respectively. The Fourier transform infrared spectroscopy data revealed that the biomolecules consist of a mixture of carbohydrates, lipids and proteins. The gas chromatography-mass spectrum analysis of L. jensenii P6A biosurfactant showed a major peak for 14-methypentadecanoic acid, which was the main fatty acid present in the biomolecule; conversely, eicosanoic acid dominated the biosurfactant produced by L. gasseri P65. Although both biosurfactants contain different percentages of the sugars galactose, glucose and ribose; rhamnose was only detected in the biomolecule produced by L. jensenii P6A. Emulsifying activities were stable after a 60-min incubation at 100 °C, at pH 2–10, and after the addition of potassium chloride and sodium bicarbonate, but not in the presence of sodium chloride. The biomolecules showed antimicrobial activity against clinical isolates of Escherichia coli and Candida albicans, with MIC values of 16 µg mL−1, and against Staphylococcus saprophyticus, Enterobacter aerogenes and Klebsiella pneumoniae at 128 µg mL−1. The biosurfactants also disrupted preformed biofilms of microorganisms at varying concentrations, being more efficient against E. aerogenes (64%) (P6A biosurfactant), and E. coli (46.4%) and S. saprophyticus (39%) (P65 biosurfactant). Both strains of lactobacilli could also co-aggregate pathogens.
Conclusions
This report presents the first characterization of biosurfactants produced by L. jensenii P6A and L. gasseri P65. The antimicrobial properties and stability of these biomolecules indicate their potential use as alternative antimicrobial agents in the medical field for applications against pathogens that are responsible for infections in the gastrointestinal and urogenital tracts and the skin.
Keywords: Biosurfactants, Antibiofilm, Antimicrobial, Lactobacillus jensenii, Lactobacillus gasseri
Background
Microorganisms are able to produce diverse surface-active compounds (SACs) containing both hydrophilic and hydrophobic moieties that can interact with surfaces, lower surface and interfacial tensions, form micelles, and emulsify immiscible substances [1]. Microbial SACs can be distinguished by their size, such as low-molecular-weight biosurfactants and high-molecular-weight surface-active polymers. High-molecular-weight surface-active polymers can be amphiphilic or polyphilic [2]. The former possesses one hydrophobic region at one end of the molecule; examples include lipopolysaccharides, lipoteichoic acids and lipoglycans of bacterial cell walls. In contrast, the latter have hydrophobic groups distributed across the entire molecule that are identical to the hydrophobically modified, comb-type polymers; examples include emulsan and hydrophobic polysaccharides [2]. An additional criterion for categorizing microbial SACs is the chemical nature of the molecules. The major classes of molecules consist of various structures, such as glycolipids, lipopeptides, polysaccharides or protein complexes, phospholipids, fatty acids and neutral lipids [3]. These biomolecules can be transported to the extracellular medium or remain attached to the cell surface as particulate biosurfactants [4].
In recent years, interest in SACs have increased due to their possible applications in environmental protection, crude oil drilling and the food processing and pharmaceutical industries [5, 6]. Unlike chemical surfactants, which are primarily derived from petroleum, these molecules can be produced by a wide variety of microorganisms, including bacteria, yeasts and filamentous fungi [7–11]. Furthermore, biosurfactants have several advantages over chemical surfactants, including the following: low toxicity, a lower critical micelle concentration (CMC), higher intrinsic biodegradability, greater stability at temperature, pH, and salinity extremes, the possibility of being produced from renewable substrates, and greater ecological acceptability [12].
SAC-producing Lactobacillus species has been described and are predominately found among the urogenital and gastrointestinal tract microbiota of humans. SACs derived from lactic acid bacteria (LAB) contribute to the bacteria’s ability to prevent microbial infections associated with its ecosystems [13, 14]. Lactobacilli can prevent colonization of the urogenital tract by several pathogens, including yeasts of the genera Candida albicans, C. tropicalis and C. krusei, responsible for vulvovaginal candidiasis, anaerobic bacteria responsible for bacterial vaginosis (BV), such as Gardnerella vaginalis, Mycoplasma hominis, Atopobium vaginae, Prevotella spp., Veillonella spp. and Mobiluncus spp., the uropathogens Escherichia coli, Proteus spp., Klebsiella spp., Serratia spp. and sexually transmitted viruses [13–18].
Lactobacilli modulate the microbiota at these sites via different mechanisms, such as auto-aggregation, i.e., the ability to form multi-cellular aggregates that incorporate bacteria from the same species; lactic acid, hydrogen peroxide, bacteriocin, and SAC production; co-aggregation with pathogenic microorganisms (in which different bacterial species are incorporated); and adhesion to epithelial cells excluding pathogens [13, 17, 19]. This hypothesis of microbiota modulation has stimulated research on the isolation and characterization of novel SAC-producing lactobacilli, followed by investigations of the potential of these microorganisms to control pathogens.
Many studies have previously reported the antibacterial, antifungal and antiviral activities of SACs produced by lactobacilli [11, 20–22]. However, another valuable application of SACs is their use as anti-adhesive agents to prevent pathogen adhesion to the host epithelium and solid surfaces as biomedical instruments [21–26]. Thus, these biomolecules might constitute a new and effective method to prevent host colonization by pathogenic microorganisms and the consequent development of clinical disturbs.
The production yields of bacterial SACs are relatively high (2–10 g L−1). Additionally, SACs reduce the surface tension of water to values lower than 30 mN m−1. In contrast, biosurfactants produced by lactobacilli are less effective, only reducing the surface tension of water to values of approximately 36–40 mN m−1, and are produced at lower levels (20–100 mg L−1) [7, 20–22, 27, 28]. Furthermore, the chemical compositions of these biomolecules have not been well studied, with only a few biomolecules being partially characterized [10, 20–22, 29], and these characteristics can influence the biological activities of the SACs.
In general, human vaginal communities are dominated by one of the four more common Lactobacillus species, L. gasseri, L. jensenii, L. crispatus, and L. iners [30]. In this study, we characterized the antimicrobial activity of purified SACs from two Lactobacillus species (L. jensenii P6A and L. gasseri P65) isolated from vaginal samples obtained from healthy women against clinical isolates of urogenital bacterial pathogens and the reference samples of the yeasts, C. albicans, C. krusei and C. tropicalis. These strains were previously characterized as able of antagonizing sixteen reference bacterial strains as demonstrated by in vitro assays [31]. However, the authors did not characterize the chemical nature of the active biomolecules produced by strains of Lactobacillus nor its effectiveness in controlling biofilms. Then, the physicochemical characterization of SACs was also performed, including the determination of the minimum surface tension, critical micelle concentration, stability at various pH values, temperatures and salt concentrations, as well as the evaluation of their chemical composition. It has been suggested that biofilm formation is an important virulence determinant in BV and other disorders of the genitourinary tract [32, 33]. Thus, the evaluation of the antibiofilm activities of biomolecules of these strains is important to validate their use as probiotic products to prevent urogenital infections. Besides this feature, the auto-aggregation activity and co-aggregation of these strains with pathogens were also studied.
Methods
Strains and culture conditions
In this study two strains of Lactobacillus (L. jensenii P6A and L. gasseri P65) isolated from the vaginal fluids of healthy women were employed [31]. The Lactobacillus strains are non-H2O2-producing, that show antagonistic activity against strains of Gardnerella vaginalis isolated from healthy women and from women with BV, as demonstrated by in vitro assays. In this study, the strains were evaluated in terms of their production of biosurfactants with antimicrobial and anti-adhesive activities against uropathogens. The strains were stored at −80 °C in conventional synthetic de Man, Rogosa and Sharpe (MRS) broth (Difco, Detroit, MI, USA) with 15% (v/v) glycerol until further use [34]. Bacteria from a frozen stock were streaked on MRS agar plates and incubated overnight at the optimum growing temperature (37 °C) for further culturing. The agar plates were stored at 4 °C for no longer than 2 weeks. The following strains were used for the antimicrobial and anti-adhesive assays: urogenital tract clinical isolates of E. coli, Klebsiella pneumoniae, Enterobacter aerogenes and Staphylococcus saprophyticus, and reference strains of C. albicans ATCC 18804, C. krusei ATCC 20298, and C. tropicalis ATCC 750. All the bacterial strains were cultured in brain heart infusion (BHI) broth (Difco, Detroit, MI, USA) at 37 °C for 24 h, and the yeast were cultured on Sabouraud Dextrose agar (SD) (Oxoid, Basingstoke, UK) at 30 °C for 48 h.
Biosurfactant production and isolation
For biosurfactant production, L. jensenii P6A and L. gasseri P65 were cultured at 37 °C in 1 L Erlenmeyer flasks containing 600 mL of MRS broth (Difco, Detroit, MI, USA) on a rotatory shaker at 120 rpm. Six milliliters of an overnight culture were used for inoculations. After 72 h, the cells were harvested by centrifugation (10,000×g for 5 min at 10 °C), washed twice in demineralized water, and suspended in 100 mL of phosphate-buffered saline solution (PBS; 10 mM KH2PO4/K2HPO4 and 150 mM NaCl, pH adjusted to 7.0). Cell suspensions were incubated at room temperature for 2 h with gentle stirring to release the biosurfactant, as previously described [27, 28]. The cells were then removed by centrifugation, and the supernatant was dried in an oven at 70 °C. To confirm biosurfactant production, the emulsifying activity (E24), using toluene as the hydrophobic substrate, and surfactant activity were routinely measured. The biomolecules were extracted by acid precipitation according to the protocol described by Van Hoogmoed et al. [35]. Briefly, the extracts were suspended in PBS (pH 7.0) at a concentration of 10 mg mL−1, and the pH was adjusted to 2.0 with 1 M HCl. The acidified samples were incubated at 4 °C for 2 h, and the precipitates were collected by centrifugation (10,000×g for 15 min at 4 °C) and washed twice with acidic water (pH 2.0). The precipitates were dissolved in distilled water and adjusted to pH 7.0 using 1 M NaOH.
Physicochemical properties
Surface–activity determination and critical micelle concentration (CMC)
The surface tension of the PBS extracts was measured using a KRUSS tensiometer (K10T model, Hamburg, Germany) with the plate method, and the relationship between the biosurfactant concentration and the surface tension was determined. To increase the accuracy of the surface tension measurements, the average value of triplicate determinations was calculated. All measurements were performed at room temperature (25 °C). The CMC was determined by plotting the surface tension as a function of the biosurfactant concentration and by locating the point of the intersection between the two lines that best fit through the pre- and post-CMC data. Concentrations ranging from 0.1 to 50 mg mL−1 were used in the assays.
Effects of the biosurfactant concentrations and the organic phase on the emulsifying activity
The extract was diluted in deionized water to concentrations ranging from 0.1 to 20 mg mL−1 to determine the effect of the biosurfactant concentration on emulsifying activity. In these emulsification assays, 1 mL of the solution at each concentration was added to screwcap tubes containing 1.5 mL of toluene, followed by homogenization using a vortex mixer at maximum speed for 2 min. After allowing the sample to stand for 24 h, emulsifying activity (E24) was determined using the method described by Cameron et al. [36]. The assays were performed in triplicate. The means were compared by Tukey’s test at 5% probability.
To evaluate the spectra of the emulsifying activity, E24 assays were performed using 5 mg mL−1 biosurfactant extracts and the following hydrophobic substrates: hexadecane (Sigma, St. Louis, MO), hexane (Sigma, St. Louis, MO), diesel oil (Petrobras, Brazil), gasoline (Petrobras, Brazil), kerosene (Petrobras, Brazil), olive oil (Food-Bunge), cottonseed oil (Food-Bunge), sunflower oil (Food-Bunge) and toluene (Sigma, St. Louis, MO).
Characterization of biosurfactants
The protein concentrations of the biosurfactants were determined using Lowry’s method [37]. The total carbohydrate concentrations were quantitatively determined using a colorimetric method with glucose as the standard [38]. The method developed by Piretti et al. [39] was used to quantify lipids.
Fatty acid analysis
Fatty acids were analyzed by gas chromatography–mass spectrometry after conversion to their methyl esters derivatives. To determine the fatty acids composition, crude biosurfactants (2–5 mg) were hydrolyzed with aqueous 2 mol L−1 HCl at 100 °C for 2 h in a sealed tube. The free lipids were extracted using n-hexane dried and then methylated with a 14% boron fluoride-methanol reagent (Sigma-Aldrich, St. Louis, MO, USA) at a ratio of 1 mL of the reagent per 10 mg of lipids. The resulting sample was stored in 2 mL microtubes and incubated in a 95 °C water bath for 15 min [40]. Fatty acid methyl esters (FAMEs) were extracted three times with n-hexane and analyzed by gas chromatography and mass spectrometry (GC–MS) using a Shimadzu GC–MS model QP 5050 A (Shimadzu, Kyoto, Japan) equipped with a PTE-5-Supelco column (30 m × 0.25 mm ID, 0.25 µm film) and employing He as the carrier gas at 0.8 mL min−1, split of 20 and 50 kPa pressure. The column temperature was programmed to increase from 80 °C (1 min) to 180 °C at 20 °C min−1, increases of 3 °C min−1 until 240 °C, and a following 20 °C min−1 increase until 300 °C and then to be maintained at this temperature for 2 min. Electron impact spectra in positive ionization mode were acquired between m/z 50 and 500. The identification of the compounds was performed by means comparison of the retention time, mass fragmentation profiles and molecular ion between sample and standard of FAME (Supelco 37 component FAME MIX, Bellefonte, PA, USA). The results were recorded and processed using Class 3.02 software (Shimadzu) and expressed as the relative percentages of each FAME. The mass spectrum of each fatty acid methyl ester was matched with the National Institute of Standards and Technology (NIST) database.
Monosaccharide analysis
The carbohydrate composition of the biosurfactants was determined by analyses of their polyacetal derivatives using GC–MS. A lyophilized sample of biosurfactant (1 mg) was hydrolyzed with 150 µL 2 M trifluoroacetic acid (CF3COOH) in a sealed tube at 120 °C for 4 h. After evaporation, the residue was washed twice using methanol. The sample was then reduced with 1 M aqueous sodium borohydride (NaBH4, 100 µL) and acetylated with a mixture of potassium acetate (100 µg) and acetic anhydride (100 µL) at 100 °C for 2 h. Excess reagent was removed by evaporation and the sample was washed several times with ethanol. Alditol acetates were extracted with ethyl acetate and water (1:1, v:v) and analyzed using a Shimadzu GC–MS model QP 5050 A (Shimadzu, Kyoto, Japan) equipped with a PTE-5-Supelco column (30 m × 0.25 mm ID, 0.25 µm film) employing He as the carrier gas at 0.7 mL min−1, split of 10 and 40 kPa pressure. The column temperature was programmed to increase from 100 °C (1 min) to 200 °C at 4 °C min−1, followed by a 20 °C min−1 increase until 300 °C and then to be maintained at this temperature for 5 min. Electron impact spectra in positive ionization mode were acquired between m/z 40 and 400. The identity of the sugars was first confirmed by comparing with the retention time obtained from the individual monosaccharide standard by means of addition of sample in the mixture of standard (SUPELCO, Bellefonte, PA, USA) and were further identified through GC/MS coupled to the NIST database.
Fourier transmission infrared spectroscopy (FTIR)
The biosurfactants functional groups were further analyzed using FTIR [41]. Pellets for the infrared analysis were obtained by grinding a mixture of 1 mg EPS with 100 mg of potassium bromide. FTIR spectra were recorded in the region of 4000–650 cm−1 at a resolution of 4 cm−1 on a Spectrum-One FTIR spectrometer (Perkin Elmer, Shelton, CT, USA) using an attenuated total reflectance (ATR) system.
Stability assays
The stability of the biosurfactants under different physicochemical conditions was evaluated using a solution of 5 mg mL−1 crude biosurfactant. To examine the influence of pH on the emulsification index, the biomolecule was eluted in different buffer solutions with different pH values. The sample was then subjected to the emulsification assay (E24) using toluene as the organic layer. A 200 mM potassium chloride/hydrochloric acid buffer was used for measurements at pH 1 and 2; a 200 mM sodium acetate/acetic acid buffer for measurements at pH 3, 4 and 5; a 100 mM sodium phosphate buffer for measurements at pH 6, 7 and 8; and a 100 mM glycine/sodium hydroxide buffer for measurements at pH 9 and 10.
Furthermore, the heat stability of the crude biosurfactants was determined by incubating the biosurfactant solution (50 mg mL−1) in a 100 °C water bath for 60 min, and then cooling at room temperature. The emulsifying activity (E24) of each sample was determined as described above.
The effects of different sodium chloride (NaCl), potassium chloride (KCl) and sodium bicarbonate (NaHCO3) concentrations on the biosurfactant activity were evaluated by adding different concentrations of the salts (800, 1200 and 2000 µg mL−1) as the emulsion formed. The solutions were allowed to stand for 20 min, and then the emulsification indexes (E24) of the biosurfactants were measured. All assays were performed in triplicates.
Antimicrobial activity assays
Culture media and inocula
Mueller–Hinton broth (Himedia, Maharashtra, India) was prepared in accordance with the CLSI document M7-A10 for minimal inhibitory concentration (MIC) bacterial assays [42]. The inocula of all bacteria at final concentration of 10 × 105 CFU mL−1 were prepared using the spectrophotometric method. Candida cultures were freshly grown at 35 °C. For the susceptibility tests, inoculum suspensions were prepared at final concentrations of 1–5 × 103 cells mL−1 using the spectrophotometric method, in accordance with CLSI document M27-A3 [43].
Susceptibility tests
The broth microdilution method was performed in accordance with the guidelines of the CLSI document M7-A6 for bacteria and M27-A3 for yeast using flat-bottom 96-well microplates (Corning, NY, USA). Stock solutions of biosurfactants were prepared in water at a concentration of 1024 µg mL−1. The compounds were diluted 1:2 in Mueller–Hinton broth to obtain a concentration two-fold greater than the maximum concentration in the analysis. Serial dilutions were prepared from this solution using the medium as a diluent. The compounds were tested at concentrations ranging from 256 to 4 µg mL−1. For tests using yeast, the stock solutions were prepared in RPMI 1640 medium (Sigma-Aldrich, St. Louis, MO, USA). Media without the extract and the solvent were used as growth and sterility controls. Chloramphenicol (Sigma-Aldrich; 0.78–100 µg mL−1) was used as a positive antibacterial control, and amphotericin B (Sigma-Aldrich; 0.03–15 µg mL−1) was used as a positive antifungal control. After plate assembly, 100 µL of each bacterial and yeast strain was inoculated per well in order to obtain 5 × 105 CFU mL−1 (or 5 × 104 CFU per well) and 0.5–2.5 × 103 CFU mL−1 (or 0.5–2.5 × 102 CFU per well), respectively. Then, the plates were incubated at 37 °C for 24 h for bacteria and 48 h for Candida species. All tests were performed in triplicate in at least two independent experiments. The MIC was defined as the lowest concentration of biosurfactants that completely inhibited the visible growth of test microorganisms.
Biosurfactant-mediated disruption of pre-formed biofilms
The antibiofilm activity of the biosurfactants against several microbial strains was determined using the procedure described by Heinemann et al. [44]. Bacterial isolates were grown in BHI for 24 h at 37 °C and yeast were grown in RPMI for 48 h at 30 °C. After incubation, the cells were centrifuged at 7200×g for 15 min, washed twice with PBS and used to prepare an inoculum at a density equivalent to 0.5 on the McFarland scale.
180 µL of BHI broth containing 1% glucose (bacteria) or RPMI (yeast) and 20 µL of the standardized inocula were added to each well of untreated 96-well polystyrene plates (Corning, NY, USA). The plates were then incubated for 24 h at 37 °C for bacteria and at 30 °C for yeasts. After incubation, unattached cells were removed by washing the wells, and biosurfactant at concentrations ranging from 180 to 22.5 mL L−1 were then added. The plates were further incubated under the same conditions.
The assay was performed with four replicates of the control (medium without extract/biosurfactant) and four replicates of each concentration of the extract/biosurfactant studied. Non-adherent cells were removed using a multichannel pipette and the wells were washed three times with PBS. Cells that adhered to the bottoms of the wells (biofilm) were fixed with 300 µL of 99% methanol and stained for 5 min with a solution of 1% crystal violet. The excess stain was removed by placing the plate under running tap water. The plates were then air-dried and the dye that bound to the adherent cells was solubilized with 200 µL 95% ethanol. The solutions were transferred to another polystyrene plate and the absorbance was measured at 450 nm using a Multiskan MMC/340 microplate reader (Thermo Scientific GO, Waltham, MA, USA). The percentage of biofilm disruption was assessed by comparing the absorbance readings of the wells treated with the extract/biosurfactant and the control wells (not treated with the extract/biosurfactant).
Auto-aggregation and co-aggregation assays and cell surface hydrophobicity
Auto-aggregation assays were performed using the method reported by Vandevoorde et al. [45]. L. jensenii P6A and L. gasseri P65 were grown in flasks containing 100 mL MRS broth for 48 h at 37 °C and 120 rpm. The cells were harvested by centrifugation (10,000×g for 10 min at 10 °C), washed twice in demineralized water, and re-suspended in PBS (pH 7) at an OD600 of 0.6 ± 0.5 (approximately 108 CFU mL−1). The OD was measured using a spectrophotometer (Shimadzu CPS 240A) at regular intervals over a 4 h period, without disturbing the microbial suspension, and the sedimentation kinetics were obtained. The auto-aggregation coefficient (AC) was calculated at different times using the method reported by Kos et al. [46] as follows:
where ODt is the optical density at 600 nm of the microbial suspension at t time (0.5, 1, 2, 3 or 4 h), and ODi is the initial optical density.
The co-aggregation assay was performed using the same method as the auto-aggregation assay, the same pathogen isolates and two isolates of the LAB L. jensenii P6A and L. gasseri P65. Equal volumes (2 mL) of each Lactobacillus suspension were added to the following pathogens: E. coli, S. saprophyticus, E. aerogenes, K. pneumoniae, C. albicans, C. krusei, and C. tropicalis. Then, the samples were vortexed for 15 s. Control tubes containing 4 mL of each bacterial suspension were prepared simultaneously. The OD of the suspensions was measured after the initial preparation and 4 h after incubation at 25 °C. The co-aggregation percentage was calculated using the equation reported by Handley et al. [47]:
where x and y represent OD measurements of tubes containing either the lactobacilli or the pathogen suspensions, respectively, and (x + y) represents OD measurements of tubes containing a mixture of the pathogen and Lactobacillus suspensions.
The two strains were treated with LiCl (5 M) and incubated for 30 min at room temperature to remove the S layer (cell surface proteins) and to evaluate the influence of the S layer in auto-aggregation and co-aggregation capacities. Auto- and co-aggregation assays were performed and the results were compared to the assays conducted with cells containing the S layer.
Results and discussion
Production and tensoactive properties of the SACs
The production of biosurfactants by L. jensenii P6A and L. gasseri P65 during growth in MRS broth was monitored by verifying the emulsifying activity using toluene as organic phase and by measuring the surfactant activity of supernatant. The emulsifying and surfactant activities at 72 h of incubation corresponded to 63.75% and 56 mN m−1 for L. jensenii P6A and 70% and 46 mN m−1 for L. gasseri P65. The production corresponded to 0.27 g L−1 for L. jensenii P6A and 0.42 g L−1 for L. gasseri P65. This low production pattern has already been described for other LAB, with values ranging from 0.02 to 0.1 g L−1, whereas for genera as Pseudomonas and Bacillus, the yield varies from 2 to 15 g L−1 [20, 22, 27, 28, 48].
In the assays performed to establish the CMC of the crude biosurfactants isolated from L. jensenii P6A and L. gasseri P65, a progressive decrease in surface tension was observed as the biosurfactant concentration increased. The CMC values of the biosurfactants from P6A and P65 were calculated as 7.1 and 8.58 mg mL−1, respectively, as shown in Fig. 1. There are different mathematical methods based on parametric and nonparametric estimation of the regression function to CMC definition and of other features in different application fields with good results [49–51]. In this study, we used the method of simple linear regression. It was assumed that the regression function corresponds to a straight line, hence called regression line, and the estimation of this straight line is reduced to the estimation of two of its parameters, slope and intercept. The advantage of these parametric methods is that when the functional model assumed for X (CMC concentration in this case) is adequate, the estimation is reduced to a few parameters, and therefore, it is extremely efficient. For CMC determination, two lines were estimated and assumed that the intersection point of these lines indicates the precise CMC concentration and thus an indication for the biosurfactant concentration with the highest capacity for surface tension reduction. At the points corresponding to CMC, the biomolecules from L. jensenii P6A and L. gasseri P65 reduced the water surface tension from 72 mN m−1 to approximately 43.2 and 42.5 mN m−1, respectively. The ability of a biosurfactant to reduce surface and interfacial tensions determines its functionality and effectiveness. For example, a good surfactant reduces the surface tension of water from 73.20 to 35.0 mN m−1 [52]. The values observed for biosurfactants from P6A and P65 are in the range of those observed for sodium dodecylsulfate (SDS) and biosurfactants isolated from different lactobacilli strains and other lactic acid bacteria (LAB) [20, 22, 27, 28, 52].
Fig. 1.

Effects of different concentrations of biosurfactants on the surface tension of water at room temperature (25 °C). a Surface tension (mN m−1) of the biosurfactants produced by L. jensenii P6A and b L. gasseri P65. The CMC was determined from the intersection between the regression lines that better described the two parts of the curve, below and above the CMC (arrow). The results represent the average of two independent measurements
Relationships between the concentrations of the biosurfactants produced by L. gasseri P65 and L. jensenii P6A and the emulsifying activity, expressed as the emulsification index (E24), were evaluated using toluene as organic phase (Fig. 2). In general, E24 values increased as the concentration of the biosurfactant increased to 20 mg mL−1. For the biosurfactant produced by P6A, E24 values ranged from 21% in tests containing 0.5 mg mL−1 to 88.7% in tests with 20 mg mL−1 biosurfactant. No emulsifying activity was observed in tests using concentrations lower than 0.5 mg mL−1, and the differences in values between 1 and 17.5 mg mL−1 were not significantly different (p > 0.05). For the biosurfactant produced by L. gasseri P65, the emulsification index ranged from 10% in tests with 0.75 mg mL−1 biosurfactant to 77% in tests with 12.5 mg mL−1 biosurfactant. At concentrations higher than 5 mg mL−1, there was no significant difference in emulsifying activity (p > 0.05) according to Tukey’s test.
Fig. 2.

Effects of the concentration of biosurfactants produced by L. jensenii P6A and L. gasseri P65 on emulsifying activity, expressed as the emulsification index (E24), using toluene as the organic phase
The biosurfactants produced by L. jensenii P6A and L. gasseri P65 presented different emulsification activities according to the different evaluated hydrophobic substrates. The biomolecule produced by L. jensenii P6A emulsified kerosene, toluene, hexane and xylene organic solvents at values greater than 62% and diesel oil at 28.3%, but showed low levels of emulsification of gasoline and hexadecane (Fig. 3a). E24 values ranged from 61 to 70% for vegetable oils (cotton, olive, and sunflower oils). For the biosurfactant produced by L. gasseri P65 (Fig. 3b), high E24 values were observed in assays with vegetable oils (cotton, olive and sunflower oils), whereas low values were observed for gasoline, diesel oil, hexane, xylene and hexadecane. The values for kerosene and toluene were 28.0 and 64.6%, respectively.
Fig. 3.

Emulsifying activities of 5 mg mL−1 biosurfactants produced by L. jensenii P6A (a) and L. gasseri P65 (b) on the aqueous phase using different hydrophobic substrates, expressed as the emulsification index (E24)
The chemical structure of both the biosurfactants and the emulsions organic phases may explain the variations observed in the emulsifying activity saturation point and in the profile of the emulsified compounds. The different results observed for L. jensenii P6A biosurfactant with diesel oil and gasoline can be explained by the fact that these substrates consist of a complex mixture of hydrocarbons, with the predominance of hydrocarbons of shorter carbon chains in gasoline and longer in diesel. Vegetable oils have a different hydrocarbon composition, mainly consisting of triglycerides. In general, the indexes found in our study were greater than the values reported for biosurfactants produced by other LAB. Emulsifying indexes between 40 and 49% with gasoline, kerosene and octane were observed for the biosurfactant produced by L. pentosus CECT4023 [53, 54]. In addition, the biosurfactant produced by L. plantarum CFR2194 exhibited emulsifying indexes between 13.6 and 38.2% for a variety of water-immiscible substrates [55]. The biomolecules from LAB strains (L26, L35 and L61) emulsified kerosene, sunflower oil, and olive oil with indexes varying from 8.22 to 26.5%, and the emulsions formed with the edible oils were more stable than the emulsions formed with kerosene [56]. The lipopeptide from Bacillus subtilis K1 isolated from aerial roots of banyan also showed good emulsification rates for olive oil [57].
Characterization of the biosurfactant
The carbohydrate, protein and lipid concentrations of the L. jensenii P6A and L. gasseri P65 biosurfactant extracts were determined. The carbohydrate concentrations ranged from 51.49 to 38.61% and the protein and lipid concentrations ranged from 15.17 to 9.81% and 29.45 to 49.53%, respectively. These results show diverse biosurfactant structures, which are confirmed by FTIR analysis (Fig. 4). FTIR is widely used to characterize the functional groups of organic compounds based on the characteristic infrared absorption bands of specific chemical groups [58]. The presence of a broad band at 3500–3200 cm−1 in the spectra of the biosurfactants produced by L. gasseri P65 and L. jensenii P6A indicates the presence of OH groups (and, possibly, NH groups) of glycoproteins (Fig. 4; Table 1). Another band observed at approximately 1650 cm−1 corresponds to C=O stretching of peptide bonds. The absorption band observed in the region near 1720 cm−1, which was partly superimposed on the band at 1650 cm −1, may be attributed to the C=O stretching of lipid esters. The absorption bands around 1230 and ~1100 cm−1 can be attributed to ester asymmetric and symmetric C–O–C stretching, respectively. Intense bands were also observed at 1100–1000 cm−1, indicating the presence of C–O sugar linkages.
Fig. 4.
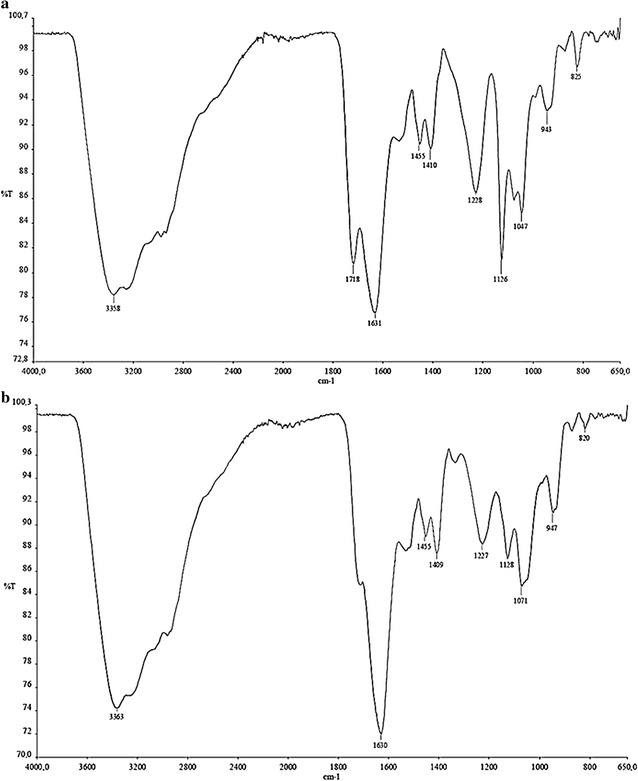
FTIR spectra of the biosurfactants produced by L. jensenii P6A (a) and L. gasseri P65 (b)
Table 1.
Correlation between FTIR spectra and functional groups detected in biosurfactants produced by L. jensenii P6A and L. gasseri P65
| Absorbance range (cm−1) | Functional groups detected |
|---|---|
| Below 1000 | OH deformation vibrations/CN |
| 1000–1300 | C–O sugar stretching |
| 1400–1460 | C–H vibrations of groups CH2 e CH3 |
| 1520 | Groups N–H in proteins |
| 1725–1675 | C=O stretching of carbonyl group |
| 3200–3600 | OH and NH stretching |
Although little is known about the chemical structures of the biosurfactants produced by lactobacilli, some researchers have reported an initial characterization [53]. Indeed, it was found that L. pentosus biosurfactants are composed of 44.7 ± 1.5% soluble protein and 13.4 ± 2.9% total sugars; those obtained from L. fermentum B54 are rich in proteins, with few polysaccharides and phosphate groups [21]. These results are similar to those described for high molecular weight biosurfactants produced by bacteria and yeast, which are characterized by the presence of primarily 16- and 18-carbon fatty acids.
The biosurfactants produced by L. gasseri P65 and L. jensenii P6A showed a different profile of fatty acids in the lipid portion, presenting only a fatty acid in common, the 14-methylpentadecanoic acid (16 carbon fatty acid) (Table 2). This fatty acid predominated on the biosurfactant produced by L. jensenii P6A, constituting 69% of the lipid fraction, while eicosanoic acid (20 carbon fatty acid) predominated in the biomolecule produced by L. gasseri P65, corresponding to 47.43%. Although the two biosurfactants contained the same sugars, the percentages varied. In addition, rhamnose was not detected in the biosurfactant produced by L. gasseri P65. These differences can explain the emulsified compounds profile, the E24 and CMC values and the stability profiles at different temperatures, pH and in the presence of different salts ions.
Table 2.
Fatty acid and monosaccharide compositions of biosurfactants produced by L. jensenii P6A and L. gasseri P65
| Chemical composition | L. jensenii P6A | L. gasseri P65 |
|---|---|---|
| Concentration (%) | ||
| Fatty acid | ||
| 9-Dodecenoic acid | 31.0 | – |
| 12-Methyltetradecanoic acid | – | 9.88 |
| 14-Methypentadecanoic acid | 69.0 | 9.70 |
| (Z)-9-Octadecenoic acid | – | 16.91 |
| Octadecanoic acid | – | 16.08 |
| Eicosanoic acid | – | 47.43 |
| Monosaccharides | ||
| Galactose | 38.12 | 25.50 |
| Glucose | 47.99 | 40.70 |
| Rhamnose | 10.44 | – |
| Ribose | 3.46 | 33.80 |
Several studies have reported similar structures for LAB. Lactobacillus helveticus produce a glycolipid-type biosurfactant closely resembling xylolipids [59]. The biosurfactants produced by Lactococcus lactis 53 are composed of glycoproteins with glucose, rhamnose, fucose and mannose [28]. The biosurfactant from L. fermentum B54 are also composed of a large amount of proteins, but fewer polysaccharides and phosphate groups [21]. The biosurfactants spectra produced by L. lactis, L. paracasei and L. pentosus showed bands at approximately 3200–3500 cm−1, characteristic of glycoprotein stretching [7, 29, 53]. Bands at approximately 1675 and 1725 cm−1, corresponding to C=O (carbonyl groups) and NH (peptides), respectively, have been observed in L. pentosus biosurfactant, whereas bands at 2900 and 1000–1200 cm−1 [53] indicate the presence of glycoproteins. The biosurfactants produced by L. rhamnosus 1825 and L. fermenti 126 CCM have also been evaluated, with bands at approximately 3285, 1635 and 1549 cm−1, typical of NH bonds and CO–N bonds in proteins found for the latter. Similar spectra were observed for L. rhamnosus. The bands at approximately 2964, 2929 and 1458 cm −1 and 2961, 2936 and 1453 cm−1 for the biosurfactants produced by L. fermenti and L. rhamnosus CCM1825 126, respectively, correspond to the CH bonds of aliphatic groups; while peaks at 1200–1000 cm−1 confirm the presence of polysaccharide fractions [60]. When comparing infrared spectroscopic data for the compounds produced by L. gasseri P65 and L. jensenii P6A with data reported for other biosurfactants produced by LAB, it can be concluded that the compounds reported in the present study are related to those produced by L. lactis and L. paracasei. These observations suggest that these substances have a complex structure composed of glycolipoproteins.
Stability of the biosurfactants
The applicability of biosurfactants in several fields depends on their stability at different temperatures, pH values and salt concentrations. We observed stability of the biosurfactants produced by L. jensenii P6A and L. gasseri P65 after 60-min incubation at 100 °C, with no loss of activity (data not shown). This profile is similar to previously reported results. Desai and Banat [4] found that heat treatment (autoclaving at 120 °C for 15 min) of Bacillus sp. biosurfactants did not cause appreciable changes in their surface and emulsifying activities, and an additional biosurfactant isolated from L. paracasei showed unaltered surfactant activity after 120 h of incubation at 60 °C [29].
The emulsions formed also remained relatively stable after standing for 24 h at pH values ranging from pH 2 to 10, maintaining values of approximately 66.34% (±1.12) (data not shown). The biomolecules are relatively more stable to pH variations than other biomolecules described for LAB. Gudiña et al. [29] observed precipitation of some of the biosurfactant components produced by L. paracasei at pH values lower than 6, which may have contributed to the alterations in the surface activities.
The addition of potassium chloride and sodium bicarbonate to the emulsions formed from toluene and the biosurfactants produced by L. jensenii P6A and L. gasseri P65 did not affect the emulsions. Indeed, the emulsifying indexes of L. jensenii P6A and L. gasseri P65 were maintained between 62 and 65.35% and between 58.43 and 65.54%, respectively, even when the salt concentration exceeded the limits of saturation (data not shown). However, when NaCl was added to the emulsions, there was an approximately 30% decrease in the height of the emulsified layer for L. jensenii P6A biosurfactant and an approximately 31% decrease for L. gasseri P65 biosurfactant. This profile is already known; the emulsifying activity of commercial surfactants tends to decrease with NaCl increasing, falling at concentration about 10–12% [61].
Antimicrobial activity
The evaluation of antimicrobial effects of biosurfactants of both L. jensenii P6A and L. gasseri P65 showed similar MIC values corresponding to 16 µg mL−1 for E. coli, and 128 µg mL−1 for K. pneumoniae, E. aerogenes and S. saprophyticus (Table 3). Furthermore, the extracts at a concentration of 16 µg mL−1 completely inhibited C. albicans growth, but were not active against the other species of Candida (C. krusei and C. tropicalis) at the highest concentration tested (256 µg mL−1). Although there are few reports regarding the antimicrobial activity of biosurfactants isolated from lactobacilli, some have been reported to exhibit activity against various microorganisms. For example, biosurfactants isolated from L. jensenii and L. rhamnosus completely inhibited the growth of Acinetobacter baumannii, E. coli and S. aureus at 25–50 mg mL−1 [62]. But the authors did not chemically characterize the biosurfactants. In another study, biosurfactants produced by Lactobacillus strains inhibited the growth of Pseudomonas spp. isolated from fresh beef, with minimal inhibitory concentrations (MIC) ranging from 25 to 50 mg mL−1 [63]. The biosurfactants studied here exhibited MIC values that support more studies objecting its use in antibacterial therapies or probiotics.
Table 3.
Antimicrobial activity of biosurfactants produced by L. jensenii P6A and L. gasseri P65 on uropathogens
| Strains | MIC of biosurfactants (µg mL−1) | |
|---|---|---|
| L. jensenii P6A | L. gasseri P65 | |
| Escherichia coli | 16 | 16 |
| Klebsiella pneumoniae | 128 | 128 |
| Enterobacter aerogenes | 128 | 128 |
| Staphylococcus saprophyticus | 128 | 128 |
| Candida albicans | 16 | 16 |
| Candida krusei | n | n |
| Candida tropicalis | n | n |
n the biosurfactants not inhibited the growth of yeasts at the highest concentration tested (256 µg mL−1)
Disruption of pre-formed biofilms
The Lactobacillus biosurfactants disrupted the biofilms of all tested microorganisms at different levels. The greatest percentages of biofilm disruption were obtained in tests with 180 µL mL−1 of L. jensenii P6A biosurfactant and E. aerogenes (64%) (Fig. 5a). For the other microorganisms, the disruption did not exceed 36% at the same concentration. The L. gasseri P65 biosurfactant decreased the formation of E. coli (46.4%) and S. saprophyticus (39%) biofilms in a high degree, but the values did not exceed 33% for the other microorganisms (Fig. 5b). Biosurfactants can adsorb to surfaces, forming a film at the interfaces by orienting polar and nonpolar groups according to the hydrophilicity/hydrophobicity of the surface. This interaction between biosurfactants and a substratum surface alters the surface hydrophobicity, thereby interfering with microbial adhesion and desorption processes [2, 28].
Fig. 5.
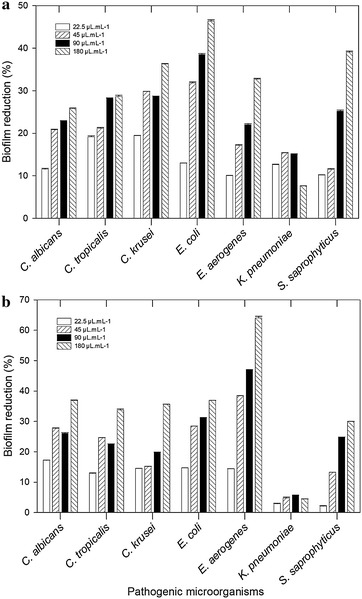
Percentage of disruption of biofilms produced by pathogenic microorganisms on the surface of polystyrene plates in the presence of different concentrations of biosurfactants produced by L. gasseri P65 (a) and L. jensenii P6A (b). The test was conducted on four replicates after 24 h of incubation
Diverse studies have highlighted the importance of biofilm formation as a virulence factor for uropathogens such as G. vaginalis and E. coli [32, 33]. The in vitro model of adherence and biofilm formation used in this study is limited by the fact that the use of polystyrene plates does not mimic the in vivo conditions in epithelial cells. However, despite their limitations, in vitro models can be very informative, and they are crucial for obtaining further understanding of activities of anti-biofilm compounds. The results showing the anti-biofilm activities of biomolecules produced by lactobacilli validate and/or explain their activities in vivo, for example, following intravaginal administration in a pharmaceutical form for the prevention and treatment of recurrent infections of the genital tract.
Auto-aggregation and co-aggregation of L. jensenii P6A and L. gasseri P65
Auto-aggregation of L. jensenii P6A and L. gasseri P65 and their co-aggregation with pathogens were evaluated. The sedimentation rate of the Lactobacillus strains was measured over a 4-h period using washed cells resuspended in PBS (pH 7.0). Low sedimentation levels after 4 h were obtained for both strains, with values corresponding to 7.38 and 10.74% for L. jensenii P6A and L. gasseri P65, respectively (data not shown). L. jensenii P6A exhibited greater aggregation with E. coli (27.4%) and C. albicans (25.9%) in co-aggregation tests with pathogenic microorganisms; for L. gasseri P65, a higher co-aggregation score was found for C. tropicalis (11.9%) (Table 4). In general, the activities were higher in the Gram-negative bacteria assays (E. coli, E. aerogenes and K. pneumoniae) when compared to the Gram-positive (S. saprophyticus) ones.
Table 4.
Co-aggregation activities of L. jensenii P6A and L. gasseri P65 after 4 h of incubation in PBS
| Pathogens | % agreggation | % agreggation after S layer removal | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Oh | 1h | 2h | 3h | 4h | Oh | 1h | 2h | 3h | 4h | |
| Lactobacillus jensenii P6A | ||||||||||
| Escherichia coli | 3.4 ± 0.7 | 26.8 ± 0.2 | 27.5 ± 0.5 | 27.8 ± 0.0 | 27.4 ± 0.1 | 1.4 ± 0.5 | 2.8 ± 0.6 | 1.5 ± 0.2 | 1.2 ± 0.0 | 1.4 ± 0.4 |
| Staphylococcus saprophytics | 5.9 ± 0.6 | 7.8 ± 0.2 | 8.6 ± 0.3 | 7.9 ± 0.0 | 7.4 ± 0.3 | 0.7 ± 0.2 | 3.5 ± 0.0 | 2.8 ± 0.0 | 8.9 ± 0.0 | 2.4 ± 0.2 |
| Enterobacter aerogenes | 6.6 ± 0.7 | 9.9 ± 4.4 | 9.7 ± 2.8 | 10.6 ± 5.2 | 10.3 ± 4.8 | 2.8 ± 0.1 | 1.5 ± 0.4 | 0.1 ± 0.8 | 2.7 ± 5.2 | 3.3 ± 0.4 |
| Klebsiella pneumoniae | 17.3 ± 0.6 | 10.2 ± 0.2 | 7.3 ± 0.4 | 10.4 ± 0.1 | 7.2 ± 0.4 | 14.8 ± 0.2 | 8.7 ± 0.2 | 6.2 ± 0.5 | 11.1 ± 0.2 | 5.2 ± 0.1 |
| Candida albicans | 10.3 ± 0.6 | 13.5 ± 0.2 | 18.5 ± 0.3 | 25.6 ± 0.1 | 25.9 ± 0.4 | 8.7 ± 0.3 | 10.5 ± 0.1 | 15.2 ± 0.2 | 17.1 ± 0.0 | 15.2 ± 0.6 |
| Candida krusei | 6.9 ± 0.7 | 16.7 ± 0.9 | 1.5 ± 0.5 | 4.5 ± 0.1 | 6.9 ± 0.5 | 6.03 ± 0.4 | 10.1 ± 0.3 | 12.2 ± 0.4 | 13.6 ± 0.6 | 9.4 ± 0.7 |
| Candida tropicalis | 7.6 ± 0.7 | 20.2 ± 0.2 | 13.7 ± 0.6 | 8.8 ± 0.2 | 3.1 ± 0.6 | 4.9 ± 0.0 | 10.3 ± 0.0 | 11.8 ± 0.0 | 6.4 ± 0.1 | 0.1 ± 0.5 |
| Lactobacillus gasseri P65 | ||||||||||
| Escherichia coli | 5.5 ± 2.8 | 3.3 ± 2.6 | 3.1 ± 3.6 | 2.6 ± 3.4 | 4.5 ± 11 | 0.5 ± 0.2 | 1.3 ± 0.2 | 2.1 ± 0.8 | 1.7 ± 0.5 | 2.5 ± 0.1 |
| Staphylococcus saprophytics | 6.5 ± 2.6 | 4.6 ± 2.4 | 6.0 ± 3.2 | 7.8 ± 3.0 | 6.6 ± 5.2 | 1.2 ± 0.6 | 13.8 ± 0.4 | 0.2 ± 0.3 | 6.4 ± 0.6 | 4.8 ± 0.5 |
| Enterobacter aerogenes | 2.6 ± 2.9 | 5.9 ± 2.7 | 5.9 ± 3.6 | 7.8 ± 3.6 | 9.7 ± 6.2 | 3.6 ± 0.2 | 2.5 ± 2.7 | 0.5 ± 0.6 | 3.5 ± 0.7 | 6.8 ± 0.6 |
| Klebsiella pneumoniae | 4.2 ± 2.9 | 3.6 ± 2.7 | 3.4 ± 3.7 | 2.6 ± 3.1 | 4.4 ± 1.5 | 5.8 ± 0.8 | 6.3 ± 0.2 | 9.9 ± 0.7 | 3.4 ± 0.3 | 2.6 ± 0.1 |
| Candida albicans | 8.9 ± 2.7 | 2.3 ± 0.9 | 8.4 ± 5.6 | 10.7 ± 5.9 | 7.5 ± 4.1 | 5.6 ± 0.6 | 1.9 ± 0.6 | 6.0 ± 0.6 | 7.2 ± 0.5 | 4.7 ± 0.6 |
| Candida krusei | 2.2 ± 1.3 | 6.2 ± 2.8 | 3.1 ± 4.2 | 6.1 ± 3.9 | 6.2 ± 0.4 | 2.5 ± 0.1 | 6.6 ± 0.7 | 1.8 ± 0.2 | 4.6 ± 0.6 | 2.1 ± 0.6 |
| Candida tropicalis | 9.0 ± 2.6 | 10.9 ± 2.4 | 25.4 ± 6.9 | 9.6 ± 5.9 | 11.9 ± 1.0 | 8.5 ± 2.6 | 9.9 ± 0.6 | 15.2 ± 0.7 | 7.8 ± 0.4 | 10.1 ± 0.5 |
The average results of two separate experiments are shown
Auto-aggregation of lactobacilli appears to be necessary for adhesion to epithelial cells and mucosal surfaces, while co-aggregation with pathogens has been considered a strategy to exclude pathogenic bacteria from their hosts [46, 64]. The very close proximity of bacteria on the aggregate permit that antimicrobial substances released by lactobacilli can directly inhibit the pathogens. The lactobacilli in our study did not show a substantial self-aggregation ability, which differs from a previous study reporting auto-aggregation indexes of 51% for Lactobacillus paracasei strains, 45% for L. acidophilus M92 and 58% for Lactobacillus kefir 2345 [29, 65]. The co-aggregation scores with pathogens also were lower as compared to other studies. As an example, a strain of L. plantarum showed a co-aggregation score of 41.5% with enterohemorrhagic E. coli (EHEC), 40.5% with Salmonella enterica serotype Typhimurium, and 37.4% with Listeria monocytogenes [66]. The co-aggregation activity of lactobacilli can be variable and appears to be dependent on the strain used in the tests, as observed for L. delbrueckii L10. This strain could co-aggregate with S. aureus 1351 and C. albicans ATCC 70014, at percentages of 48.88 and 59.37%, respectively [67]. In contrast, Pan et al. [68] reported co-aggregation capacities of L. acidophilus and L. delbrueckii with Clostridium butirycum of only 5.76% ± 6.32 and 1.2 ± 1.6, respectively.
The auto-aggregation rates of the Lactobacillus sp. strains decreased after removing the S layer, showing percentages of up to 5.0 and 6.8% for L. jensenii P6A and L. gasseri P65, respectively (data not shown). Similarly, a decrease in co-aggregation capacity with the pathogens was found. The greatest reductions after removal of the S layer were between L. jensenii P6A and E. coli, with values ranging from 27.4 to 1.4% (Table 4). Some authors have reported the importance of this protein layer for adhesion of Lactobacillus spp. For example, Kos et al. [46] showed that the removal of the protein layer decreased the auto-aggregation capacity among L. acidophilus M92 isolates. In another study, Golowczyc et al. [69] demonstrated a decrease in adhesion to Saccharomyces lipolytica cells for an L. kefir isolate after removal of the S layer. In addition, several studies have shown that pH, temperature and intergeneric interactions can influence these reactions [64].
Determination of the cell surface hydrophobicity of pathogenic microorganisms after incubation with the biosurfactants produced by L. jensenii P6A and L. gasseri P65
To examine changes in the cell surface that occurred after the pathogens were exposed to the biosurfactants, the cellular hydrophobicity of the cells was quantified. The percentages of C. krusei, S. saprophyticus and E. aerogenes cells that adhered to hexadecane were 47, 11 and 2%, respectively, after 24 h of incubation with the biosurfactant produced by L. jensenii P6A (data not shown). For the biosurfactant produced by the L. gasseri P65, the percentages for E. coli and E. aerogenes were 47 and 1.5%, respectively. These data indicate that the biosurfactants altered the cell surface of these pathogens by changing their adhesion to the hydrophobic substrate. For other microorganisms, the percentage of adherence to hexadecane was comparable to the controls. Previously, it was suggested that the hydrophobicity of the microbial cell, including viral particles can drive the adhesion of many pathogens [70].
Conclusions
In this study, biosurfactants produced by two Lactobacillus strains, L. jensenii P6A and L. gasseri P65, were characterized. The emulsifying activities of biosurfactants from these strains were stable at different pH values (2 at 10) and high temperatures (100 °C). However, emulsifying activity was destabilized in the presence of NaCl, which was not observed employing various concentrations of KCl and NaHCO3. Although the auto-aggregation and co-aggregation data obtained for these Lactobacillus strains with pathogens may not prove to be very effective in protecting the vaginal mucosa, L. jensenii P6A and L. gasseri P65 produced biosurfactants with considerable antimicrobial activities against E. coli and C. albicans and anti-adhesive activity against E. coli, S. saprophyticus and E. aerogenes. These results indicate the potential use of these biosurfactants as alternative antimicrobial agents in medicine for applications against pathogenic microorganisms that are responsible for infections and diseases in the gastrointestinal and urogenital tracts and the skin.
Authors’ contributions
IMC carried out the experimental work, analyzed and interpreted the data and wrote the manuscript. GST isolated the lactobacilli strains; ALC and VSD collaborated on biosurfactant production and antimicrobial assays; RJA and EPS performed the chemical characterization by FTIR and CG-MS and contributed to data interpretation; RMDN and ASM contributed to data interpretation and critically revised the manuscript; VLS contributed to the conception and coordination of study and writing of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The material and data supporting their findings can be found in the main paper.
Consent for publication
All the co-authors approved the publication of this work in Microbial Cell Factories.
Ethical aspects
The local Research Ethics Committee on Human Experimentation (COEP/UFMG) approved the research project that led to the initial collection of the Lactobacillus strains (Protocol ETIC 062/03).
Funding
The authors are thankful for the financial support provided by Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq), Fundação do Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and Comissão de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
I. M. C. Morais, Email: iranymcm@yahoo.com.br
A. L. Cordeiro, Email: ambiental.cordeiro@gmail.com
G. S. Teixeira, Email: andreasmont@gmail.com
V. S. Domingues, Email: domingues.vtrs@gmail.com
R. M. D. Nardi, Email: nardi@icb.ufmg.br
A. S. Monteiro, Email: andreasmont@gmail.com
R. J. Alves, Email: dylancover@gmail.com
E. P. Siqueira, Email: ezequias@cpqrr.fiocruz.br
V. L. Santos, Email: verabio@gmail.com
References
- 1.Franzetti A, Tamburini E, Banat IM. Applications of biological surface active compounds in remediation technologies. Adv Exp Med Biol. 2010;672:121–134. doi: 10.1007/978-1-4419-5979-9_9. [DOI] [PubMed] [Google Scholar]
- 2.Neu TR. Significance of bacterial surface-active compounds in interaction of bacteria with interfaces. Microbiol Rev. 1996;60:151–166. doi: 10.1128/mr.60.1.151-166.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper DG, Zajic JE. Surface active compounds from microorganisms. Adv Appl Microbiol. 1980;26:229–253. doi: 10.1016/S0065-2164(08)70335-6. [DOI] [Google Scholar]
- 4.Desai JD, Banat IM. Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev. 1997;61:47–64. doi: 10.1128/mmbr.61.1.47-64.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banat IM, Franzetti A, Gandolfi I, Bestetti G, Martinotti MG, Fracchia L, Smyth TJ, Marchant R. Microbial biosurfactants production, applications and future potential. Appl Microbiol Biotechnol. 2010;87:427–444. doi: 10.1007/s00253-010-2589-0. [DOI] [PubMed] [Google Scholar]
- 6.Liu JF, Mbadinga SM, Yang SZ, Gu JD, Mu BZ. Chemical structure, property and potential applications of biosurfactants produced by Bacillus subtilis in petroleum recovery and spill mitigation. Int J Mol Sci. 2015;16:4814–4837. doi: 10.3390/ijms16034814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodrigues L, Moldes A, Teixeira J, Oliveira R. Kinetic study of fermentative biosurfactant production by Lactobacillus strains. Biochem Eng J. 2006;28:109–116. doi: 10.1016/j.bej.2005.06.001. [DOI] [Google Scholar]
- 8.Alejandro CS, Humberto HS, María JF. Production of glycolipids with antimicrobial activity by Ustilago maydis FBD12 in submerged culture. Afr J Microbiol Res. 2011;5:2512–2523. [Google Scholar]
- 9.Monteiro AS, Miranda TT, Lula I, Denadai ÂM, Sinisterra RD, Santoro MM, Santos VL. Inhibition of Candida albicans CC biofilms formation in polystyrene plate surfaces by biosurfactant produced by Trichosporon montevideense CLOA72. Colloids Surf B Biointerfaces. 2011;84:467–476. doi: 10.1016/j.colsurfb.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Shokouhfard M, Kermanshahi RK, Shahandashti RV, Feizabadi MM, Teimourian S. The inhibitory effect of a Lactobacillus acidophilus derived biosurfactant on biofilm producer Serratia marcescens. Iran J Basic Med Sci. 2015;18:1001–1007. [PMC free article] [PubMed] [Google Scholar]
- 11.Satpute SK, Kulkarni GR, Banpurkar AG, Banat IM, Mone NS, Patil RH, Cameotra SS. Biosurfactants from Lactobacilli species: properties, challenges and potential biomedical applications. J Basic Microbiol. 2016;56:1–19. doi: 10.1002/jobm.201600143. [DOI] [PubMed] [Google Scholar]
- 12.Makkar RS, Cameotra SS. An update on the use of unconventional substrates for biosurfactant production and their new applications. Appl Microbiol Biotechnol. 2002;58:428–434. doi: 10.1007/s00253-001-0924-1. [DOI] [PubMed] [Google Scholar]
- 13.Reid G, Bruce AW, Smeianov V. The role of Lactobacilli in preventing urogenital and intestinal infections. Int Dairy J. 1998;8:555–562. doi: 10.1016/S0958-6946(98)00075-2. [DOI] [Google Scholar]
- 14.Borges S, Silva J, Teixeira P. The role of lactobacilli and probiotics in maintaining vaginal health. Arch Gynecol Obstet. 2014;289:479–489. doi: 10.1007/s00404-013-3064-9. [DOI] [PubMed] [Google Scholar]
- 15.Pendharkar S, Brandsborg E, Hammarström L, Marcotte H, Larsson PG. Vaginal colonisation by probiotic lactobacilli and clinical outcome in women conventionally treated for bacterial vaginosis and yeast infection. BMC Infect Dis. 2015;15:255. doi: 10.1186/s12879-015-0971-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoesl CE, Altwein JE. The probiotic approach: an alternative treatment option in urology. Eur Urol. 2005;47:288–296. doi: 10.1016/j.eururo.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Lepargneur JP, Rousseau V. Protective role of the Doderleïn flora. J Gynecol Obstet Biol Reprod. 2002;31:485–494. [PubMed] [Google Scholar]
- 18.Barrons R, Tassone D. Use of Lactobacillus probiotics for bacterial genitourinary infections in women: a review. Clin Ther. 2008;30:453–468. doi: 10.1016/j.clinthera.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Valore EV, Park CH, Igreti SL, Ganz T. Antimicrobial components of vaginal fluid. Am J Obstet Gynecol. 2002;187:561. doi: 10.1067/mob.2002.125280. [DOI] [PubMed] [Google Scholar]
- 20.Velraeds MMC, Van Der Mei HC, Reid G, Busscher HJ. Physicochemical and biochemical characterization of biosurfactats released by Lactobacillus strains. Colloids Surf B Biointerfaces. 1996;8:51–61. doi: 10.1016/S0927-7765(96)01297-0. [DOI] [Google Scholar]
- 21.Velraeds MMC, Van Der Mei HC, Reid G, Busscher HJ. Inhibition of initial adhesion of uropathogenic Enterococcus faecalis by biosurfactants from Lactobacillus isolates. Appl Environ Microbiol. 1996;62:1958–1963. doi: 10.1128/aem.62.6.1958-1963.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Busscher VJ, Van Hoogmoed CG, Geertsma-Doornbusch GI, Van Der Kuijl-Boolj M, Van Der Mei HC. Streptococcus thermophilus and its biosurfactants inhibit adhesion by Candida spp. on silicone rubber. Appl Environ Microbiol. 1997;63:3810–3817. doi: 10.1128/aem.63.10.3810-3817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velraeds MM, van de Belt-Gritter B, Busscher HJ, Reid G, van der Mei HC. Inhibition of uropathogenic biofilm growth on silicone rubber in human urine by lactobacilli—a teleologic approach. World J Urol. 2000;18:422–426. doi: 10.1007/PL00007084. [DOI] [PubMed] [Google Scholar]
- 24.Fracchia L, Cavallo M, Allegrone G, Martinotti MG. A Lactobacillus-derived biosurfactant inhibits biofilm formation of human pathogenic Candida albicans biofilm producers. Appl Microbiol Biotechnol. 2010;2:827–837. [Google Scholar]
- 25.Rodrigues LR, Van der Mei HC, Teixeira JA, Oliveira R. Influence of biosurfactants from probiotic bacteria on formation of biofilms on voice prosthesis. Appl Environ Microbiol. 2004;70:4408–4410. doi: 10.1128/AEM.70.7.4408-4410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van der Mei HC, Free RH, Elving GJ, van Weissenbruch R, Albers F, Busscher HJ. Effect of probiotic bacteria on probiotic organisms for infection control and prevalence of yeasts in oropharyngeal biofilms on silicone rubber voice prostheses in vitro. J Med Microbiol. 2000;49:713–718. doi: 10.1099/0022-1317-49-8-713. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigues LR, Teixeira JA, Van der Mei HC, Oliveira R. Isolation and partial characterization of a biosurfactant produced by Streptococcus thermophilus A. Colloids Surf B Biointerfaces. 2006;53:105. doi: 10.1016/j.colsurfb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Rodrigues LR, Teixeira JA, Van der Mei HC, Oliveira R. Phycochemical and functional characterization of a biosurfactant produced by Lactobacillus lactis 53. Colloids Surf B Biointerfaces. 2006;49:79–86. doi: 10.1016/j.colsurfb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Gudiña EJ, Teixeira JÁ, Rodrigues LR. Isolation and functional characterization of a biosurfactant produced by Lactobacillus paracasei. Colloids Surf B. 2010;76:298. doi: 10.1016/j.colsurfb.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Mendes-Soares H, Suzuki H, Hickey RJ, Forney LJ. Comparative functional genomics of Lactobacillus spp. reveals possible mechanisms for specialization of vaginal lactobacilli to their environment. J Bacteriol. 2014;196(7):1458–1470. doi: 10.1128/JB.01439-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teixeira GS, Carvalho FP, Arantes RM, Nunes AC, Moreira JLS, Mendonça M, Almeida RB, Farias LM, Carvalho MAR, Nicoli JR. Characteristics of Lactobacillus and Gardnerella vaginalis from women with or without bacterial vaginosis and their relationships in gnotobiotic mice. J Med Microbiol. 2012;61:1074–1081. doi: 10.1099/jmm.0.041962-0. [DOI] [PubMed] [Google Scholar]
- 32.Fattahi S, Kafil HS, Nahai MR, Asgharzadeh M, Nori R, Aghazadeh M. Relationship of biofilm formation and different virulence genes in uropathogenic Escherichia coli isolates from Northwest Iran. GMS Hyg Infect Control. 2015;10:Doc11. doi:10.3205/dgkh000254. [DOI] [PMC free article] [PubMed]
- 33.Machado A, Cerca N. Influence of biofilm formation by Gardnerella vaginalis and other anaerobes on bacterial vaginosis. J Infect Dis. 2015;212:1856–1861. doi: 10.1093/infdis/jiv338. [DOI] [PubMed] [Google Scholar]
- 34.De Man JC, Rogosa M, Sharpe ME. A medium for the cultivation of lactobacilli. J Appl Bacteriol. 1960;23:130. doi: 10.1111/j.1365-2672.1960.tb00188.x. [DOI] [Google Scholar]
- 35.Van Hoogmoed CG, Van der Kuijl-Booij M, Van der Mei HC, Busscher HJ. Inhibition of Streptococcus mutans NS adhesion to glass with and without a salivary conditioning film by biosurfactant-releasing Streptococcus mitis strain. Appl Environ Microbiol. 2000;66:659. doi: 10.1128/AEM.66.2.659-663.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cameron D, Cooper DG, Neufeld RJ. The mannoprotein of Saccharomyces cerevisiae is an effective bioemulsifier. Appl Environ Microbiol. 1988;54:1420–1425. doi: 10.1128/aem.54.6.1420-1425.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lowry OH, Rosebrouch NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 38.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- 39.Piretti MV, Zuppa F, Pagliuca G, Taioli F. Variations of fatty acid constituents in selected tissues of the bivalve mollusc Scapharia inaequivaleis. Comp Biochem Physiol. 1988;89:183–187. [Google Scholar]
- 40.Bligh EG, Dyer WJ. Um método rápido para a extração total de lipídeos e de purificação. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 41.Abu GO, Weiner RM, Rice J, Colwell RR. Properties of an extracellular adhesive polymer from the marine bacterium Shewanella colwelliana. Biofouling. 1991;3:69–84. doi: 10.1080/08927019109378163. [DOI] [Google Scholar]
- 42.CLSI—Clinical and Laboratory Standard Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 10th ed. CLSI document M07-A10. Wayne: Clinical and Laboratory Standards Institute; 2015.
- 43.CLSI—Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeast. Approved Standard M27-A3. Wayne: CLSI; 2008.
- 44.Heinemann C, Van Hylckama Vlieg JE, Janssen DB, Busscher HJ, Van der Mei HC, Reid G. Purification and characterization of a surface-binding protein from Lactobacillus fermentum RC-14 that inhibits adhesion of Enterococcus faecalis 1131. FEMS Microbiol Lett. 2000;190:177–180. doi: 10.1111/j.1574-6968.2000.tb09282.x. [DOI] [PubMed] [Google Scholar]
- 45.Vandevoorde L, Christiaens H, Verstraete W. Prevalence of coaggregation among chicken lactobacilli. J Appl Bacteriol. 1992;72:214–219. doi: 10.1111/j.1365-2672.1992.tb01826.x. [DOI] [PubMed] [Google Scholar]
- 46.Kos B, Suskovic J, Sukovic S, Simpraga M, Frece J, Matosic S. Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J Appl Microbiol. 2003;94:981–987. doi: 10.1046/j.1365-2672.2003.01915.x. [DOI] [PubMed] [Google Scholar]
- 47.Handley PS, Harty DWS, Wyatt JE, Brown CR, Doran JP, Gibbs ACC. A comparison of the adhesion, coaggregation and cell-surface hydrophobicity properties of fibrillar and fimbriate strains of Streptococcus salivarius. J Gen Microbiol. 1987;133:3207–3217. doi: 10.1099/00221287-133-11-3207. [DOI] [PubMed] [Google Scholar]
- 48.Marius H, Mareen G, Fabiola W, Rudolf H. Production of microbial biosurfactants: status quo of rhamnolipid and surfactin towards large-scale production. Biotechnol J. 2017;12:1600561. doi: 10.1002/biot.201600561. [DOI] [PubMed] [Google Scholar]
- 49.Fontán JLL, Costa J, Ruso JM, Prieto G, Sarmiento F. A nonparametric approach to calculate critical micelle concentrations: the local polynomial regression method. Eur Phys J E Soft Matter. 2004;13:133–140. doi: 10.1140/epje/e2004-00050-3. [DOI] [PubMed] [Google Scholar]
- 50.Mohammad V, Banihabib ME, Behbahani SMR. Comparison of the ARMA, ARIMA, and the autoregressive artificial neural network models in forecasting the monthly inflow of Dez dam reservoir. J Hydrol. 2013;476:433–441. doi: 10.1016/j.jhydrol.2012.11.017. [DOI] [Google Scholar]
- 51.Valipour M, Sefidkouhi MA, Raeini M. Selecting the best model to estimate potential evapotranspiration with respect to climate change and magnitudes of extreme events. Agric Water Manag. 2017;180(Part A):50–60. doi: 10.1016/j.agwat.2016.08.025. [DOI] [Google Scholar]
- 52.Mulligan CN. Environmental applications for biosurfactants. Environ Pollut. 2005;133:183–189. doi: 10.1016/j.envpol.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 53.Moldes AB, Paradelo R, Vecino X, Cruz JM, Gudina EJ, Rodrigues LR, Teixeira JA, Domínguez JM, Barral MT. Partial characterization of biosurfactant from Lactobacillus pentosus and comparison with sodium dodecyl sulphate for the bioremediation of hydrocarbon contaminated soil. Biomed Res Int. 2013;2013:961842. doi: 10.1155/2013/961842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Portilla-Rivera O, Torrado A, Dominguez JM, Moldes AB. Stability and emulsifying capacity of biosurfactants obtained from lignocellulosic sources using Lactobacillus pentosus. J Agric Food Chem. 2008;56:8074–8080. doi: 10.1021/jf801428x. [DOI] [PubMed] [Google Scholar]
- 55.Madhu N, Prapulla SG. Evaluation and functional characterization of a biosurfactant produced by Lactobacillus plantarum CFR 2194. Appl Biochem Biotechnol. 2014;172:1777–1789. doi: 10.1007/s12010-013-0649-5. [DOI] [PubMed] [Google Scholar]
- 56.Cornea CP, Roming FI, Sicuia OA, Voaideș C, Zamfir M, Grosu-Tudor SS. Biosurfactant production by Lactobacillus spp. strains isolated from Romanian traditional fermented food products. Rom Biotechnol Lett. 2016;21:2. [Google Scholar]
- 57.Pathak KV, Keharia H. Application of extracellular lipopeptide biosurfactant produced by endophytic Bacillus subtilis K1 isolated from aerial roots of banyan (Ficus benghalensis) in microbially enhanced oil recovery (MEOR) 3 Biotech. 2014;4:41–48. doi: 10.1007/s13205-013-0119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silverstein RM, Webster FX, Kiemle DJ, Bryce DL. Spectrometric identification of organic compounds. 8. New York: Wiley; 2014. [Google Scholar]
- 59.Sharma D, Saharan BS, Chauhan N, Bansal A, Procha S. Production and structural characterization of Lactobacillus helveticus derived biosurfactant. Sci World J. 2014;2014:493548. doi: 10.1155/2014/493548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brzozowski B, Bednarski W, Golek P. Physicochemical properties of Lactobacillus biosurfactants. Food Technol Biotechnol. 2011;49:177–186. [Google Scholar]
- 61.Phetrong K, Aran H, Maneerat S. Production and characterization of bioemulsifier from a marine bacterium, Acinetobacter calcoaceticus subsp. anitratus SM7. Songkl J Sci Technol. 2008;30:297–305. [Google Scholar]
- 62.Sambanthamoorthy K, Feng X, Patel R, Patel S, Paranavitana C. Antimicrobial and antibiofilm potential of biosurfactants isolated from lactobacilli against multi-drug-resistant pathogens. BMC Microbiol. 2014;14:197. doi: 10.1186/1471-2180-14-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Augustin M, Hippolyte MT, Raïssa KR. Antibacterial activity of Lactobacillus’ biosurfactants against Pseudomonas spp. isolated from fresh beef. Novus Int J Biotechnol Biosci. 2013;2:7–22. [Google Scholar]
- 64.Abdulla AA, Abed TA, Saeed AM. Adhesion, autoaggregation and hydrophobicity of six Lactobacillus strains. Br Microbiol Res J. 2014;4:381–391. doi: 10.9734/BMRJ/2014/6462. [DOI] [Google Scholar]
- 65.Golowczyc MA, Mobili P, Abraham AG, Garrote GL, De Antoni GL. Protective action of Lactobacillus kefir carrying S-layer against Salmonella enterica serovar Enteritidis. Int J Food Microbiol. 2007;118:264–273. doi: 10.1016/j.ijfoodmicro.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 66.Jankovic T, Frece J, Abram M, Gobin I. Aggregation ability of potential probiotic Lactobacillus plantarum strains. Int J Sanit Eng Res. 2012;6:19–24. [Google Scholar]
- 67.Gomaa Z. Antimicrobial and anti-adhesive properties of biosurfactant produced by lactobacilli isolates, biofilm formation and aggregation ability. J Gen Appl Microbiol. 2013;59:425–436. doi: 10.2323/jgam.59.425. [DOI] [PubMed] [Google Scholar]
- 68.Pan X, Wu T, Zhang L, Song Z, Tang H, Zhao Z. In vitro evaluation on adherence and antimicrobial properties of a candidate probiotic Clostridium butyricum CB2 for farmed fish. J Appl Microbiol. 2008;105:1623–1629. doi: 10.1111/j.1365-2672.2008.03885.x. [DOI] [PubMed] [Google Scholar]
- 69.Golowczyc MA, Mobili P, Garrote GL, Serrabell MA, Abraham AG, De Antoni GL. Interaction between Lactobacillus kefir and Saccharomyces lipolytica isolated from kefir grains. Evidence for lectin-like activity of bacterial surface proteins. J Dairy Res. 2009;76:111–116. doi: 10.1017/S0022029908003749. [DOI] [PubMed] [Google Scholar]
- 70.Duncan-Hewitt WC. Nature of the hydrophobic effect. In: Doyle RJ, Rosenberg M, editors. Microbial cell surface hydrophobicity. Washington, D.C.: ASM Publications; 1990. pp. 39–73. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The material and data supporting their findings can be found in the main paper.


