Abstract
Objectives
We tested whether a sleep and circadian-based treatment shown to improve circadian adaptation to night shifts and attenuate negative effects on alertness, performance, and sleep in young adults would also be effective in older adults.
Methods
We assessed subjective alertness, sustained attention (psychomotor vigilance task, PVT), sleep duration (actigraphy), and circadian timing (salivary dim-light melatonin onset, DLMO) in eighteen older adults (57.2±3.8 y; mean±SD) in a simulated shift work protocol. Four day shifts were followed by three night shifts in the laboratory. Participants slept at home and were randomized to either the Treatment Group (scheduled evening sleep and enhanced lighting during the latter half of night shifts), or Control Group (ad lib sleep and typical lighting during night shifts).
Results
Compared to day shifts, alertness and sustained attention declined on the first night shift in both groups, and was worse in the latter half of the night shifts. Alertness and attention improved on nights 2 and 3 for the Treatment Group but remained lower for the Control Group. Sleep duration in the Treatment Group remained similar to baseline (6–7 h) following night shifts, but was shorter (3–5 h) following night shifts in the Control Group. Treatment Group circadian timing advanced by 169.3±16.1 min (mean±SEM) but did not shift (−9.7±9.9 min) in the Control Group.
Conclusions
The combined treatment of scheduled evening sleep and enhanced lighting increased sleep duration and partially aligned circadian phase with sleep and work timing, resulting in improved night shift alertness and performance.
Keywords: aging, shiftwork, sleep, circadian rhythms, neurobehavioral effects
INTRODUCTION
The prevalence of shift work has increased in recent decades and more than 21 million U.S. workers perform night, rotating, or alternative shifts outside of typical daytime hours.[1] However, there are significant consequences of shift work on health, safety, productivity, and quality-of-life, including insufficient or disturbed sleep,[2–6] alertness and performance decrements,[2, 7–9] occupational injuries and accidents,[7, 8, 10] drowsy driving accidents on home commutes,[11] cardiovascular disease,[12] cancer,[13] and early mortality.[13, 14] Greater exposure to night or rotating shifts, diagnosis of shift work disorder (SWD, affecting >10% of shift workers), and older age are associated with even greater risks to performance, alertness, quality-of-life, morbidity, and mortality compared to day workers.[3–5, 13–16]
Strategies to improve adaptation to night shift schedules based on sleep and circadian principles commonly manipulate sleep timing and light exposure patterns. In previous studies conducted by our colleagues, scheduled evening sleep and enhanced lighting during the latter half of night shifts improved night shift alertness and performance in young adults.[17] The light-dark exposure pattern was designed to produce circadian phase advance (earlier) shifts and thus partial circadian alignment to the night work and evening sleep schedule.[17, 18] An evening sleep schedule also maintains the typical temporal relationship between sleep and work seen in day workers, i.e. work begins within just a few hours after awakening, minimizing the buildup of homeostatic sleep pressure prior to beginning night shifts. In contrast, typical night shift schedules in which workers sleep following work and then awaken in the early afternoon result in night shifts beginning after 8 or more hours awake. Furthermore, shift workers have high rates of impaired and insufficient sleep that is largely related to sleeping at adverse circadian times.[2–5] Sleep is longest and least disturbed when it occurs at the biological times when the circadian system is promoting sleep,[19, 20] therefore treatments that result in shifting the timing of circadian sleep promotion closer to the sleep schedule of shift workers should produce the longest and least disrupted sleep.
The U.S. population is rapidly aging and an estimated 2 million+ adults age 55 and older work night, rotating, or alternative shift schedules.[1] Although older adults represent a sizeable and at-risk sector of the shift worker population, previous shift work treatment strategies were predominantly developed in young adults. In general, older adults show lower sleep duration and continuity during both nighttime and daytime sleep episodes compared to young adults.[20–22] Circadian rhythms of melatonin and core body temperature are less robust, and the timing of circadian rhythms and sleep are earlier in older versus young adults.[20, 23, 24] These age-related physiological changes in sleep and circadian rhythms impair the ability of older adults to cope with shift work schedules, and may underlie the shorter sleep duration, greater sleep difficulties, worse on-shift alertness, more serious occupational accidents and injuries, and increased use of hypnotic medications reported in older shift workers.[3, 5, 10, 25–28]
Despite age-related changes to sleep and circadian rhythms that may impact the efficacy of treatment strategies, to our knowledge no studies have tested sleep and circadian-based shift work adaptation treatments in older adults. Therefore, in the current study we used a simulated shift work protocol and tested the hypotheses that in older adults the combined treatment of scheduled evening sleep and enhanced lighting in the latter half of night shifts would improve night shift alertness and performance, increase sleep duration following night shifts, and result in significant circadian phase advance shifts compared to ad lib sleep timing and typical room lighting during night shifts.
METHODS
Participants
Eighteen adults (12 men, 6 women) aged 57.2±3.8 y (mean±SD) participated in the study. They were recruited from the Boston area through internet and print advertisements. All were in good health and free from medical and psychiatric disorders as determined by medical history, psychological and sleep disorders questionnaires, clinical blood and urine tests, and physical, psychological, and ophthalmological examinations. Female participants were post-menopausal by ≥1 y. Exclusion criteria included age <50 or >65 y, body mass index >32 kg/m2 (25.7±3.3 kg/m2; mean±SD), average self-reported sleep duration <6 or >9 h, caffeine intake >500mg/day, alcohol intake >14 drinks/week, recent or extensive history of performing overnight or rotating shift work, travel across >1 time zone <3 months before study, eye injury or disease, colorblindness, and current use of nicotine or medications that affect sleep or circadian physiology. Participants gave written informed consent prior to study and the protocol was approved by the Partners HealthCare System Institutional Review Board and was in accordance with the Declaration of Helsinki. Participants were compensated for their participation.
Pre-study conditions
Participants maintained self-selected ~8-h sleep schedules without naps for ≥10 days immediately prior to study. Adherence to the sleep schedule was verified via wrist actigraphy. Participants were instructed to abstain from caffeine, alcohol, and non-prescribed or over-the-counter medications from the start of the pre-study monitoring until the end of the 10-day protocol.
Study protocol
The 10-day protocol simulated rotating shift work, beginning with four consecutive 8-h daytime shifts from 07:00–15:00 followed by three consecutive overnight shifts from 23:00–07:00 (figure 1). Participants were studied individually in sound and light-attenuated research rooms in the Center for Clinical Investigation at Brigham and Women’s Hospital. Following all shifts, participants left the laboratory and, other than instructions about scheduled sleep times (see below), their activities were unrestricted. Participants slept at home and were not given bedroom preparation instructions.
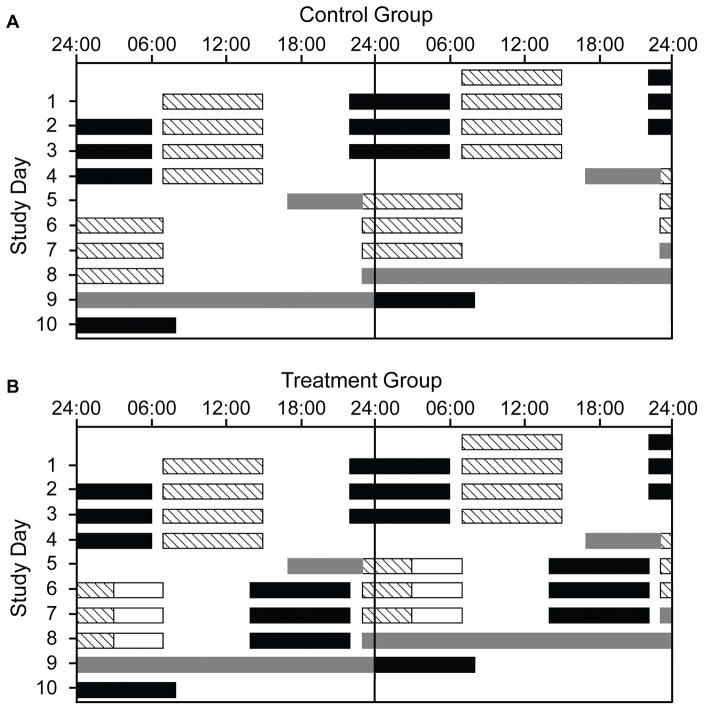
Figure 1.
Double-raster plots of the 10-day simulated shift work protocol for the Control Group (A) and Treatment Group (B). Clock hour is indicated across the x-axis and study day on the y-axis. Consecutive study days are shown both to the right of and beneath the previous study day. Hatched bars indicate work shifts under typical room lighting (mean: ~89 lux, ~0.23 W/m2; maximum: ~150 lux, ~0.48 W/m2)) in the laboratory. The four Day shifts were from 07:00–15:00 and the three Night shifts were from 23:00–07:00. White open bars in Panel B indicate times of enhanced lighting (~2,209±342 lux; mean±SD, ~4.87 W/m2; 03:00–07:00) during the Night shifts. Black bars indicate scheduled sleep episodes at home (except Day 10, when the final sleep episode occurred in the laboratory). The Control Group slept ad lib after the three night shifts, so sleep episodes are not indicated for the Control Group on Days 6–8. Grey bars indicate dim-light (mean: ~3.3 lux, ~0.0087 W/m2; maximum: ~15 lux, ~0.048 W/m2) circadian phase (CP) estimation procedures in the laboratory, when hourly saliva samples were collected. CP1 took place on Day 5 from 17:00–23:00 (immediately before the first Night shift), and CP2 took place from 23:00 on Day 8 though 24:00 on Day 9. Participants were outside the laboratory at all other times.
Following Day shifts 1–3, participants were instructed not to nap, and to maintain a self-selected ~8-h sleep time that would allow them sufficient time to return for the next day shift. Following Day 4, participants were allowed to sleep ad lib on the transition night prior to Night 1 and were then randomized into one of two study groups: Control Group (6 men, 3 women) or Treatment Group (6 men, 3 women). All participants were exposed to typical indoor room lighting (mean: ~89 lux, ~0.23 W/m2) throughout all day shifts. Control Group participants (figure 1A) were allowed to sleep ad lib following the night shifts (i.e., at any time or duration, for any number of bouts) and were exposed to typical indoor lighting throughout the night shifts. Treatment Group participants (figure 1B) were instructed to maintain ~8-h sleep schedules beginning between 13:00–14:00 following each night shift, and were exposed to typical indoor lighting from 23:00–03:00 and enhanced lighting (~2,209±342 lux; mean±SD, ~4.87 W/m2) from 03:00–07:00 during the three night shifts. All lighting was administered from ceiling-mounted fluorescent lamps (4100K; Philips Lighting, Eindhoven, The Netherlands) and measured from 137 cm above the floor in the direction the participant was facing while sitting at the desk where they spent most of their shift.
Subjective sleepiness and reaction time assessments
Hourly repeating cognitive test batteries (lasting ~45 min of every hour) began 45 min into each shift. Participants were seated at a desk in a consistent posture and location during testing, and research assistants monitored tests from inside the room. Here we report results from the Karolinska Sleepiness Scale (KSS) and Psychomotor Vigilance Task (PVT). The KSS is a 9-point Likert scale in which participants rate their sleepiness for the preceding 5-min interval. The PVT tests the ability to maintain attention, and we used a 10-min PVT in which participants were tasked with pressing a response button with the thumb of their dominant hand at the onset of a visual stimulus presented on a desktop computer screen. Inter-stimulus interval was 1–9 sec, resulting in ~100 trials per 10-min test. Two outcomes from the PVT were analyzed, reaction time (RT; which was converted to mean 1/RT, the rate of mean RT) and number of lapses of attention (RTs>500 ms). Participants completed the KSS about twice per hour and the PVT once per hour (totaling 14 KSS and 8 PVT sessions per shift). A 30-min meal break was scheduled 4 h into each shift.
Sleep duration assessment
Actigraphy data were collected from a device worn on the participant’s non-dominant wrist throughout the study. Eleven participants wore the MotionWatch 8 (CamNTech Ltd., Cambridge, UK), five wore the Actiwatch-L (MiniMitter Respironics, Bend, OR, USA), and two wore the Actiwatch-Spectrum (Philips Respironics, Eindhoven, The Netherlands). Actigraphy data were collected in 60-sec epochs and sleep-wakefulness was scored in each sleep episode per epoch by MotionWare (version 1.1.20, CamNTech Ltd., Cambridge, UK) using the high-sensitivity setting. Bed and wake times were determined from voicemail call-ins and sleep diaries and input into the software for sleep-wakefulness analysis. Total sleep time (TST) was calculated as minutes of sleep in each sleep episode. For Control Group participants who slept ad lib following night shifts, TST was summed across sleep episodes if they slept in >1 bout.
Circadian phase assessment
Circadian phase of the salivary dim-light melatonin onset (DLMO) was assessed following Day 4 and again following Night 3. During these circadian phase (CP) estimation procedures, hourly saliva samples were collected while the participant remained in dim (mean: ~3.3 lux, ~0.0087 W/m2) lighting. CP1 was on Day 5 from 17:00–23:00 (just before Night 1) and CP2 was from 23:00 on Day 8 (Night 4) through 24:00 on Day 9 (figure 1). Saliva samples were frozen and shipped to Solidphase, Inc (Portland, ME, USA) where melatonin levels were assayed via direct saliva melatonin radioimmunoassay (Bühlmann Laboratories AG, Schönenbuch, Switzerland). DLMO was calculated as the clock time at which melatonin levels reached a 3.0 pg/ml threshold, using linear interpolation between adjacent samples. Melatonin levels for two Control Group participants and one Treatment Group participant never reached 3.0 pg/ml; in those participants, a 1.0 pg/ml DLMO threshold was used. Phase shifts were calculated as the difference in clock time of DLMO from CP1 to CP2.
Data analysis
Baseline PVT and KSS measures were calculated as the average of testing sessions on Days 3 and 4, so as to minimize the impact of laboratory acclimation effects from the first two study days. Shapiro-Wilk tests were used to confirm that KSS data and mean 1/RT PVT data were normally-distributed. Linear mixed models were used to analyze main and interaction effects for factors Condition (Control vs Treatment Group), Shift (Baseline, Night 1, Night 2, Night 3), and Half-of-shift (1st vs 2nd-Half-of-shift), with Participant as a random effect. PVT lapses were analyzed using a general linear mixed model with Poisson distribution, with non-integer averaged baseline lapses rounded up to the nearest integer.
Baseline TST was calculated as the average TST during the nights preceding Days 3 and 4. TST was analyzed with a linear mixed model for main and interaction effects for factors Condition and Shift, with Participant as a random effect. Planned post-hoc comparisons for PVT, KSS, and TST measures were computed with t-tests after Bonferroni corrections for multiple testing between each night shift and baseline, and between groups within each shift. Models for KSS and PVT were fit over each shift and also separately within each half-of-shift. DLMO phase shifts were assessed with t-tests, and correlations between DLMO clock time and phase shift were calculated with Pearson correlation coefficients. Unless otherwise stated, data are presented as mean±SEM. Statistical analyses were performed with SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
PVT data from the first test session on Night 3 was excluded from analysis in one Treatment Group participant because they used the wrong response button. Melatonin data were outside the assay’s range in one Treatment Group participant and saliva samples were contaminated with blood for another Treatment Group participant, and they were therefore excluded from phase shift analyses. TST data following Nights 2 and 3 were excluded in one Control Group participant because they did not consistently wear the actigraphy device during those sleep episodes, and TST data following Night 2 was excluded in another Control Group participant because of a device malfunction.
RESULTS
Subjective sleepiness
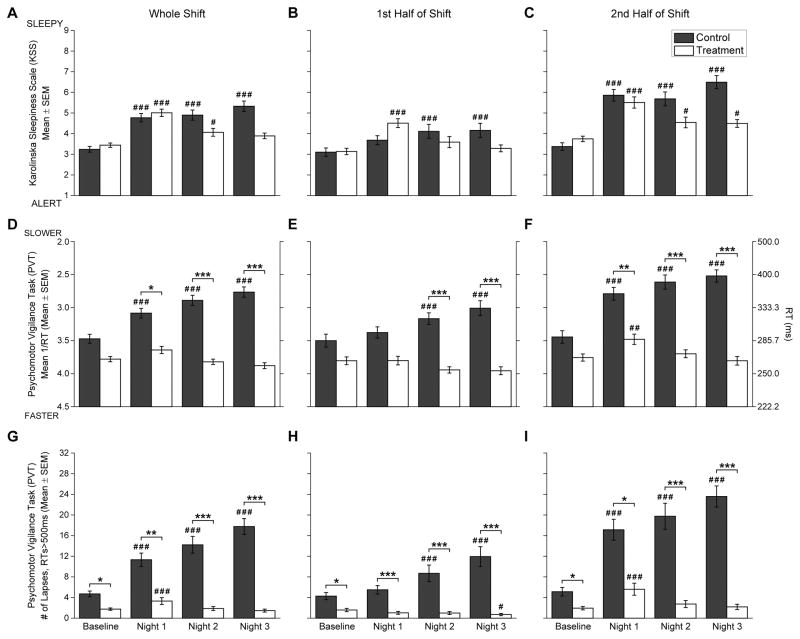
Overall, subjective sleepiness (figures 2A–C) differed significantly by Shift (F3,976=59.74; p<0.001) and Half-of-shift (F1,976=205.82; p<0.001) with greater sleepiness in the 2nd-Half-of-shifts. There was also a significant interaction between Shift and Half-of-shift (F3,976=11.13; p<0.001). Overall, subjective sleepiness did not differ by Condition (F1,976=0.41; p=0.520), but there was a significant interaction between Condition and Shift (F3,976=21.64; p<0.001). The Control Group showed greater sleepiness on all Nights compared to Baseline (all p<0.001), while the Treatment Group showed greater sleepiness than Baseline on Nights 1 (p<0.001) and 2 (p=0.017) but not on Night 3 (figure 2A). There was a significant interaction between Condition and Half-of-shift (F1,976=13.37; p<0.001), and a significant 3-way interaction (Condition and Shift and Half-of-shift: F3,976=3.98; p=0.008). Compared to Baseline, on Nights 2 and 3 the Control Group was more sleepy during the 1st-Half-of-shifts (all p<0.001; figure 2B), but not on Night 1 (p=0.062), while the Treatment Group showed the opposite pattern, with greater sleepiness compared to Baseline on Night 1 (p<0.001) but not on Nights 2 and 3. During the 2nd-Half-of-shifts (figure 2C), the Control Group was more sleepy compared to Baseline for all Nights (all p<0.001). The Treatment Group was also more sleepy on all Nights, although the level of significance was diminished on Nights 2 (p=0.026) and 3 (p=0.047) compared to Night 1 (p<0.001). There were no significant between-group differences for sleepiness within either half-of-shift.
Figure 2.
Subjective sleepiness (upper row, A–C), reaction time (RT) performance (middle row, D–F), and lapses of attention (lower row, G–I) during the work shifts. The left panels (A, D, G) show data from the whole shift, the middle panels (B, E, H) show data from the first half of the shifts, and right panels (C, F, I) show data from the second half of the shifts. Black bars indicate Control Group and white bars indicate Treatment Group. Data presented are mean±SEM. For all measures, Baseline data were averaged over testing sessions on Day shifts 3 and 4. The right y-axis for RT performance (middle row, D–F) indicates the equivalent RT (ms) as a reference for the left y-axis Mean 1/RT values. Number symbols (#) above bars indicate Night shifts that were significantly different versus the day shift Baseline, and asterisk symbols (*) indicate significantly different between Control and Treatment Groups. Level of significance is indicated by the number of symbols: one symbol = p<0.05; two symbols = p<0.01; three symbols = p<0.001.
PVT reaction time (RT) and lapses of attention
There were overall significant differences in PVT RT (calculated as mean 1/RT, figures 2D–F) by Condition (F1,543=17.03; p<0.001) with slower RTs in the Control Group; Shift (F3,543=25.04; p<0.001); and Half-of-shift (F1,543=116.60; p<0.001) with slower RTs in the 2nd-Half-of-shifts. There was also a significant interaction between Condition and Shift (F3,543=41.06; p<0.001), with RTs in the Control Group significantly slower on all Nights compared to Baseline (all p<0.001; figure 2D) and compared to the Treatment Group (Night 1: p=0.027, Nights 2 and 3: p<0.001). Treatment Group RTs did not differ from Baseline on any Night. There were also significant interactions between Condition and Half-of-shift (F1,543=16.85; p<0.001), and Shift and Half-of-shift (F3,543=9.97; p<0.001), but not for the 3-way interaction Condition, Shift, and Half-of-shift (F3,543=1.84; p=0.139). During the 1st-Half-of-shifts (figure 2E) the Control Group showed similar RTs on Night 1 compared with Baseline, but on Nights 2 and 3 showed slower RTs compared to Baseline and compared to the Treatment Group (all p<0.001). RTs during the 1st-Half-of-shifts in the Treatment Group were similar to Baseline on all Nights. During the 2nd-Half-of-shifts (figure 2F) the Control Group had slower RTs on all Nights compared to Baseline (all p<0.001) and compared to the Treatment Group (Night 1; p=0.007, Nights 2 and 3; p<0.001), whereas the Treatment Group only showed slower RTs during the 2nd-Half-of-shift on Night 1 (p=0.004) but recovered to Baseline levels by Night 2 and maintained those levels during Night 3 (both p=1.000).
The pattern of results for PVT lapses of attention (RTs>500 ms, figures 2G–I) were similar to the RT results, showing significant effects of Condition (F1,543=26.06; p<0.001) with greater lapses in the Control Group; Shift (F3,543=22.49; p<0.001) with greater lapses in Night shifts; and Half-of-shift (F1,543=259.07; p<0.001) with greater lapses in the 2nd-Half-of-shifts. There was also a significant interaction between Condition and Shift (F3,543=41.92; p<0.001), with greater lapses in the Control Group on all Nights (all p<0.001; figure 2G) compared to Baseline, but in the Treatment Group greater lapses only on Night 1 (p<0.001) versus Baseline. The Control Group showed greater lapses compared to the Treatment Group during Baseline (p=0.016) and on all Nights (Night 1: p=0.002, Nights 2 and 3: p<0.001). There was a significant interaction between Condition and Half-of-shift (F1,543=8.05; p=0.005) with a greater increase in lapses between the 1st-Half and 2nd-Half-of-shifts in the Control Group versus the Treatment Group. Similar to whole-shift averages, lapses at Baseline were higher in the Control Group versus the Treatment Group during both the 1st-Half (p=0.017; figure 2H) and 2nd-Half-of-shifts (p=0.038; figure 2I). There was also a significant interaction between Shift and Half-of-shift (F3,543=24.32; p<0.001). During the 1st-Half-of-shifts (figure 2H), lapses did not differ between Baseline and Night 1, but on Nights 2 and 3 the Control Group showed more lapses than Baseline (all p<0.001). In contrast, the Treatment Group had similar lapses between Baseline and Nights 1 and 2, and by Night 3 showed fewer lapses than Baseline (p=0.012). There was not a significant 3-way interaction between Condition, Shift, and Half-of-shift (F3,543=1.37; p=0.250). On all nights, during the 1st-Half-of-shifts the Control Group had more lapses than the Treatment Group (all p<0.001). During the 2nd-Half-of-shifts (figure 2I), the Control Group had greater lapses on all Nights compared to Baseline (all p<0.001) and the Treatment Group (Night 1; p=0.011, Nights 2 and 3; p<0.001), whereas the Treatment Group only showed more lapses during the 2nd-Half-of-shifts compared to Baseline on Night 1 (p<0.001).
Sleep duration
Actigraphy-measured TST was not significantly different between groups during the final week of the pre-study period (Control: 395.33±6.13 min; Treatment: 384.89±5.57 min, p=0.210; data not shown), nor was TST significantly different during Baseline (Control: 366.83±15.23 min; Treatment: 383.22±12.18 min, p=1.000; figure 3). TST showed significant overall effects for Condition (F1,45=27.23; p<0.001) and Shift (F3,45=8.61; p<0.001), and their interaction (F3,45=7.66; p<0.001). Control Group Night shift ad lib TSTs were between 3–5 h (Night 1: 235.00±29.23 min, Night 2: 221.43±31.90 min, Night 3: 267.75±25.32 min), significantly shorter compared to their Baseline (Nights 1 and 2: p<0.001; Night 3: p=0.001) and the Treatment Group (Nights 1 and 2: p<0.001; Night 3: p=0.004). Treatment Group TSTs following Night shifts were similar to their Baseline, and were maintained between 6–7 h (Night 1: 379.33±22.18 min, Night 2: 379.00±11.66 min, Night 3: 378.22±16.49 min).
Figure 3.
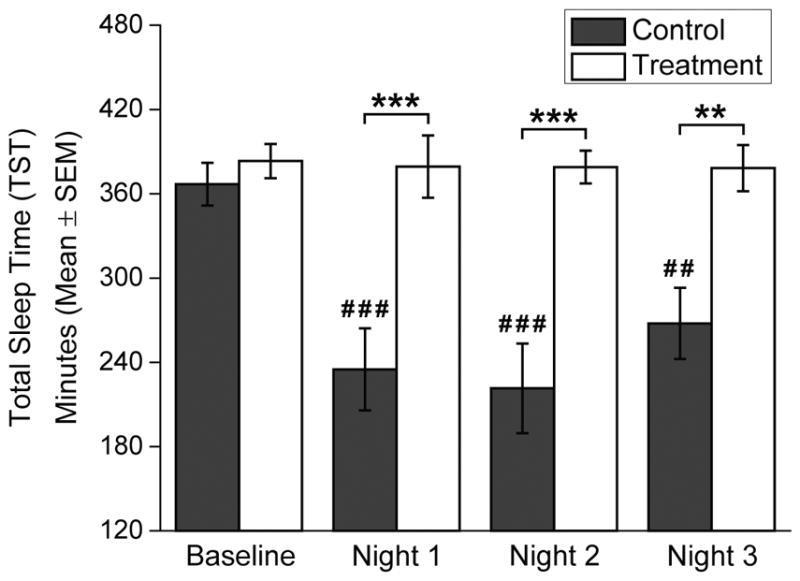
Total sleep time (TST, mean±SEM) in minutes as determined from wrist actigraphy. Black bars indicate Control Group and white bars indicate Treatment Group. Baseline TST was averaged over the final two scheduled nighttime sleep episodes (those immediately prior to Day shifts 3 and 4), and TST for Nights 1–3 represents the amount of sleep following the respective night shifts. Control Group participants slept ad lib following each night shift, so if participants slept in more than one episode prior to the subsequent night shift the amount of all sleep since the last night shift was summed. Number symbols (#) above bars indicate that TST is significantly different versus the Baseline TST, and asterisk symbols (*) indicate a significant difference in TST between the Control and Treatment Groups. Level of significance is indicated by the number of symbols shown: one symbol = p<0.05; two symbols = p<0.01; three symbols = p<0.001.
Circadian phase
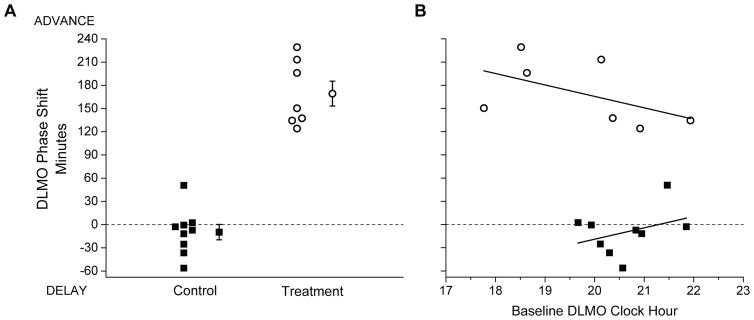
The initial DLMO (CP1) timing was not significantly different between Conditions (Control: 20:38±00:14 h; Treatment: 19:45±00:34 h, t14=1.56; p=0.141). Following the night shifts, Treatment Group participants all showed robust phase advance (earlier) shifts (mean: 169.27±16.07 min, range: 124.15 to 229.19 min; figure 4A) whereas 7 of 9 Control Group participants showed phase delay (later) shifts and overall the Control Group showed very small changes in circadian timing (mean: −9.73±9.91 min, range: −56.24 to +50.74 min; figure 4A). By the final DLMO (CP2), circadian timing of the Treatment Group had shifted significantly more than the Control Group (t14=9.93; p<0.001; figure 4A). We also tested whether the phase shift magnitude was related to the initial DLMO timing, and found it was not significantly correlated for either group (Control: r=0.35; p=0.349, Treatment: r=−0.52; p=0.233; figure 4B).
Figure 4.
Panel A: Circadian phase shifts of the salivary dim-light melatonin onset (DLMO). Panel B: Correlation of DLMO phase shift with Baseline DLMO time. Black squares indicate Control Group participants and white circles indicate Treatment Group participants. In (A), the amount (in minutes) that the DLMO timing shifted between the first assessment (before the first Night shift) and second assessment (after the third Night shift) is shown for each individual, as well as for the group (group mean±SEM symbol to the right of the individual participant data). The dotted horizontal line at zero indicates no change in timing of the DLMO between assessments. By convention, phase delay shifts (to a later hour) are presented as negative values and phase advance shifts (to an earlier hour) are presented as positive values. The Treatment Group shifted significantly more than the Control Group (+169.27±16.07 min vs. −9.73±9.91 min; t14=9.93; p<0.001). In (B), the individual DLMO phase shifts are plotted on the y-axis against each participant’s Baseline DLMO timing on the x-axis, along with the linear best-fit line fit to the data for each group. Neither group showed a significant correlation between the Baseline DLMO timing and phase shift of DLMO (Control: r=0.35, p=0.349; Treatment: r=−0.52, p=0.233).
DISCUSSION
We found that the combined treatment of scheduled evening sleep and enhanced lighting during the latter half of three night shifts was effective in improving on-shift alertness and attention, increasing sleep duration, and better aligning circadian timing to a night work and evening sleep schedule compared to an ad lib sleep schedule and typical room lighting during night shifts. Overall, these findings in older adults are similar to those in studies of young adults and indicate that modifications of sleep timing combined with enhanced workplace lighting can result in robust improvements in alertness, performance, sleep duration, and circadian timing for older adults when transitioning to a night work schedule.
Over three consecutive night shifts, subjective alertness improved in the Treatment Group but worsened in the Control Group, indicating that the combined treatment was able to attenuate the increased sleepiness that normally occurs across night shifts. In fact, by Night 3 alertness in the Treatment Group had improved to Day shift levels, in contrast with that of the Control Group whose alertness continued to decline across night shifts. The Treatment Group alertness effect may have, in part, resulted from light-induced melatonin suppression but was not solely due to an acute enhancement by the brighter light during the latter half of shifts, because alertness also improved during the first half of shifts after Night 1. Studies testing consecutive night shifts in young adults show similar alertness improvements after Night 1, both with[17] and without[29] the same combined treatment used in the current study. Thus subjective alertness improvements over consecutive night shifts do not occur in older adults without treatment, but with combined treatment alertness improves in a similar time course as seen in young adults.
Attention as assessed with the PVT was also improved by the combined treatment. While Control Group RTs and lapses increased significantly compared with Baseline starting in the latter half of Night 1, the Treatment Group’s RTs and lapses were similar to Baseline throughout the first half of all Nights, and during the latter half of Nights 2 and 3. During the latter half of Night 1, the Treatment Group had significantly faster RTs and fewer lapses compared with the Control Group. This finding indicates that prior to the imposition of scheduled evening sleep, the acute effects of enhanced lighting alone can effectively attenuate the decline of vigilance performance in the latter half of the night when alertness levels are lowest. There were a greater number of lapses (~2–3) in the Control Group at Baseline. However, given the large change in RTs and lapses between Baseline and night shifts, and the large difference between Control and Treatment Groups on the night shifts, we do not believe that the small difference in Baseline lapses can explain the larger difference in PVT performance across nights. In young adults, long RT lapses (>90th percentile RT) were shown to improve after Night 1 with[17] and without[29] the same combined treatment used in the current study. Overall, these results indicate that with the combined treatment vigilance performance is maintained at high levels after Night 1, but without treatment will progressively worsen across three night shifts in both young and older adults.
The Treatment Group participants were compliant with the imposed ~8-h evening time-in-bed following night shifts, and were thus able to maintain sleep durations between 6–7 h, similar to their Baseline sleep durations. In contrast, the Control Group ad lib sleep durations (~3–5 h) were significantly shorter, similar to those reported in shift worker populations[3–6, 26] who attempt sleep at adverse biological times.[19, 20] Prior studies also found that sleep of older shift workers is shorter and of poorer quality than that of younger workers.[3, 5, 25–28] Therefore, our Treatment Group evening sleep duration findings are notable because they demonstrate that older night workers can maintain typical nighttime sleep durations when their sleep opportunity occurs in the evening as long as time-in-bed is of sufficient duration.
After working three successive nights, the Control Group circadian timing moved later on average by only 9.7 minutes, and individual changes were <1 hour, indicating they did not show circadian adaptation to the night shift schedule. In contrast, Treatment Group participants showed circadian timing shifts of 2–4 hours, averaging 2.8 hours earlier. Therefore, the combined treatment resulted in partial circadian adjustment after only three nights. This circadian adjustment is similar to that shown in young adults given the same combined treatment.[17] In that same study, young adults randomized to scheduled evening sleep alone (without enhanced lighting during night shifts) showed a ~1 hour earlier average shift in circadian timing.[18] Thus, our current findings together with those prior findings suggest that: 1) the circadian phase shifting effects of each treatment component are likely additive; 2) the enhanced lighting component may be more efficacious for producing circadian phase shifts compared to the scheduled evening sleep component; and 3) the enhanced lighting used in our treatment appears to be of sufficient intensity to overcome any age-related physiological changes or reductions in circadian sensitivity to light[24, 30–34] and therefore is able to shift circadian rhythm timing in both young and older adults.
Shift work simulation studies that shift circadian timing to later hours have also reported improved on-shift alertness and performance that correlated with partial circadian alignment.[19, 35–37] In those studies, participants slept in the morning following night shifts, a sleep schedule commonly used by shift workers. However, when sleep occurs in the morning directly following night shifts, the individual is awake for longer and has greater homeostatic sleep pressure before the next night shift. This may limit alertness and performance improvements, especially towards the end of shifts. In young adults, Santhi et al.[17] conducted a direct comparison of phase delay (scheduled morning sleep and enhanced lighting in first half of night shifts) versus phase advance (scheduled evening sleep and enhanced lighting in latter half of night shifts) combined treatments. They found equivalent sleep durations between the morning and evening sleep schedules, but greater alertness and performance impairments on Nights 2 and 3 in the phase delay versus the phase advance treatment. Therefore, the treatment strategy tested in our study (a phase advance model) is preferred from the standpoint of interaction effects between sleep homeostasis and circadian rhythmicity. This is especially the case for older adults, who in general show greater tendency toward early sleep and circadian timing than young adults,[20, 23, 24, 32] and therefore may more easily shift their sleep and circadian timing to earlier hours. The chronotype of night workers has been reported to modulate disease risk,[38] and matching shift schedule to chronotype improved sleep and well-being in a real-world shift work intervention.[6] Thus, older workers, who are more likely to be “morning types,” would be expected to benefit the most from a phase advance shift work treatment. An estimated <10% of night workers report regularly initiating sleep after 13:00.[39] However, we found that four of the nine Control Group participants chose to begin their ad lib sleep after 13:00 following at least one night shift, and two of those participants did so after all three night shifts. Older workers may also have fewer family demands that limit their ability to sleep in the evening than do young workers, making the treatment model used in our study a more practical option for them.
In this initial study we elected to test the combined treatment of scheduled evening sleep and enhanced lighting, but future studies should test each treatment alone to determine their relative contributions to the overall effects we observed. In addition, future studies should test how the timing, duration, and spectral composition of enhanced lighting impacts alertness, performance, sleep, and circadian timing in older workers, because not all occupations or work settings can accommodate the light treatment used in our study. We required Treatment Group participants to remain in bed attempting to sleep for 8 hours each evening; however, this duration and timing may present a barrier to compliance. Therefore, variations on the scheduled evening sleep treatment should also be tested, as it is arguably the most modifiable treatment for individual workers. Other countermeasures should also be tested in conjunction with sleep schedule and enhanced lighting treatments, including napping and widely-used stimulants such as caffeine.
In summary, we found that a combined treatment of scheduled evening sleep and enhanced lighting in the latter half of three night shifts produced improvements in alertness, attention, sleep duration, and circadian timing in older adults compared to an ad lib sleep schedule and typical room lighting. The combined treatment we tested shows great promise for improving adaptation to shift work and for treatment of SWD in older workers, who may be at increased health, safety, and performance risk compared to young workers. Therefore these and similar sleep and circadian rhythm-based shift work treatments should be further evaluated and implemented in operational settings.
WHAT THIS PAPER ADDS.
Shift work reduces sleep duration and quality, impairs on-shift alertness and performance, and increases occupational health and safety risks.
Interventions based on sleep and circadian rhythm treatments in young adults have been shown to improve adaptation to night shift work, however age-related changes in sleep and circadian physiology may alter the efficacy of shift work treatments in older adults, although this has not yet been tested.
We found that a combined treatment of scheduled evening sleep and enhanced lighting in the latter half of night shifts was able to significantly lengthen sleep duration and shift circadian rhythm timing, thereby improving alertness and reaction time, in older adults after only three nights.
This is the first study to demonstrate that sleep and circadian-based shift work treatments are effective and improve adaptation to shift work in older adults.
Sleep and circadian rhythm-based shift work treatments should be implemented and further evaluated in operational settings.
Acknowledgments
The authors wish to thank the study participants; Joyce Hong and Diane Ihebom for assistance with participant recruitment and data processing; the staff of the Brigham and Women’s Hospital Center for Clinical Investigation and the Partners Chronobiology Core for assistance with data collection; Joseph M. Ronda, M.S. for technical assistance with data collection; and Dr. Charles A. Czeisler for helpful advice in designing the study.
FUNDING
The studies were supported by NIH grant R01 AG044416 and were carried out in the Brigham and Women’s Hospital Center for Clinical Investigation, part of Harvard Catalyst (Harvard Clinical and Translational Science Center) and supported by NIH Award UL1 TR001102 and financial contributions from Brigham and Women’s Hospital and from Harvard University and its affiliated academic health care centers. EDC was supported by NIH fellowship T32 HL007901. MJK was supported by Howard OsanDongtan Hospital, Osan, Korea.
Footnotes
COMPETING INTERESTS
None
CONTRIBUTORS
JFD designed the research. EDC, MPH, and MJK collected the data. EDC, MPH, WW, and JFD analyzed the data. EDC, MPH, WW, and JFD wrote the paper. All authors reviewed and approved the paper.
References
- 1.McMenamin TM. A time to work: recent trends in shift work and flexible schedules. Mon Labor Rev. 2007;130:3–15. [Google Scholar]
- 2.Åkerstedt T, Wright KP., Jr Sleep loss and fatigue in shift work and shift work disorder. Sleep Med Clin. 2009;4:257–71. doi: 10.1016/j.jsmc.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonnefond A, Härmä M, Hakola T, et al. Interaction of age with shift-related sleep-wakefulness, sleepiness, performance, and social life. Exp Aging Res. 2006;32:185–208. doi: 10.1080/03610730600553968. [DOI] [PubMed] [Google Scholar]
- 4.Drake CL, Roehrs T, Richardson G, et al. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27:1453–62. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 5.Marquié JC, Foret J. Sleep, age, and shiftwork experience. J Sleep Res. 1999;8:297–304. doi: 10.1046/j.1365-2869.1999.00170.x. [DOI] [PubMed] [Google Scholar]
- 6.Vetter C, Fischer D, Matera JL, et al. Aligning work and circadian time in shift workers improves sleep and reduces circadian disruption. Curr Biol. 2015;25:907–11. doi: 10.1016/j.cub.2015.01.064. [DOI] [PubMed] [Google Scholar]
- 7.Barger LK, Lockley SW, Rajaratnam SM, et al. Neurobehavioral, health, and safety consequences associated with shift work in safety-sensitive professions. Curr Neurol Neurosci Rep. 2009;9:155–64. doi: 10.1007/s11910-009-0024-7. [DOI] [PubMed] [Google Scholar]
- 8.Folkard S, Tucker P. Shift work, safety and productivity. Occup Med (Lond) 2003;53:95–101. doi: 10.1093/occmed/kqg047. [DOI] [PubMed] [Google Scholar]
- 9.Vetter C, Juda M, Roenneberg T. The influence of internal time, time awake, and sleep duration on cognitive performance in shiftworkers. Chronobiol Int. 2012;29:1127–38. doi: 10.3109/07420528.2012.707999. [DOI] [PubMed] [Google Scholar]
- 10.Folkard S. Shift work, safety, and aging. Chronobiol Int. 2008;25:183–98. doi: 10.1080/07420520802106694. [DOI] [PubMed] [Google Scholar]
- 11.Lee ML, Howard ME, Horrey WJ, et al. High risk of near-crash driving events following night-shift work. Proc Natl Acad Sci U S A. 2016;113:176–81. doi: 10.1073/pnas.1510383112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knutsson A. Health disorders of shift workers. Occup Med (Lond) 2003;53:103–8. doi: 10.1093/occmed/kqg048. [DOI] [PubMed] [Google Scholar]
- 13.Lin X, Chen W, Wei F, et al. Night-shift work increases morbidity of breast cancer and all-cause mortality: a meta-analysis of 16 prospective cohort studies. Sleep Med. 2015;16:1381–7. doi: 10.1016/j.sleep.2015.02.543. [DOI] [PubMed] [Google Scholar]
- 14.Gu F, Han J, Laden F, et al. Total and cause-specific mortality of U.S. nurses working rotating night shifts. Am J Prev Med. 2015;48:241–52. doi: 10.1016/j.amepre.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramin C, Devore EE, Wang W, et al. Night shift work at specific age ranges and chronic disease risk factors. Occup Environ Med. 2015;72:100–7. doi: 10.1136/oemed-2014-102292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalmbach DA, Pillai V, Cheng P, et al. Shift work disorder, depression, and anxiety in the transition to rotating shifts: the role of sleep reactivity. Sleep Med. 2015;16:1532–8. doi: 10.1016/j.sleep.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santhi N, Aeschbach D, Horowitz TS, et al. The impact of sleep timing and bright light exposure on attentional impairment during night work. J Biol Rhythms. 2008;23:341–52. doi: 10.1177/0748730408319863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santhi N, Duffy JF, Horowitz TS, et al. Scheduling of sleep/darkness affects the circadian phase of night shift workers. Neurosci Lett. 2005;384:316–20. doi: 10.1016/j.neulet.2005.04.094. [DOI] [PubMed] [Google Scholar]
- 19.Crowley SJ, Lee C, Tseng CY, et al. Complete or partial circadian re-entrainment improves performance, alertness, and mood during night-shift work. Sleep. 2004;27:1077–87. doi: 10.1093/sleep/27.6.1077. [DOI] [PubMed] [Google Scholar]
- 20.Dijk DJ, Duffy JF, Riel E, et al. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999;516:611–27. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klerman EB, Davis JB, Duffy JF, et al. Older people awaken more frequently but fall back asleep at the same rate as younger people. Sleep. 2004;27:793–8. doi: 10.1093/sleep/27.4.793. [DOI] [PubMed] [Google Scholar]
- 22.Ohayon MM, Carskadon MA, Guilleminault C, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 23.Duffy JF, Zeitzer JM, Rimmer DW, et al. Peak of circadian melatonin rhythm occurs later within the sleep of older subjects. Am J Physiol Endocrinol Metab. 2002;282:E297–303. doi: 10.1152/ajpendo.00268.2001. [DOI] [PubMed] [Google Scholar]
- 24.Duffy JF, Zitting KM, Chinoy ED. Aging and circadian rhythms. Sleep Med Clin. 2015;10:423–34. doi: 10.1016/j.jsmc.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torsvall L, Åkerstedt T, Gillberg M. Age, sleep and irregular workhours: A field study with electroencephalographic recordings, catecholamine excretion and self-ratings. Scand J Work Environ Health. 1981;7:196–203. doi: 10.5271/sjweh.3112. [DOI] [PubMed] [Google Scholar]
- 26.Åkerstedt T, Torsvall L. Age, sleep and adjustment to shiftwork. In: Koella WP, editor. Sleep 1980. Basel: S. Karger; 1981. pp. 190–5. [Google Scholar]
- 27.Härmä M, Tenkanen L, Sjöblom T, et al. Combined effects of shift work and life-style on the prevalence of insomnia, sleep deprivation and daytime sleepiness. Scand J Work Environ Health. 1998;24:300–7. doi: 10.5271/sjweh.324. [DOI] [PubMed] [Google Scholar]
- 28.Härmä MI, Hakola T, Åkerstedt T, et al. Age and adjustment to night work. Occup Environ Med. 1994;51:568–73. doi: 10.1136/oem.51.8.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santhi N, Horowitz TS, Duffy JF, et al. Acute sleep deprivation and circadian misalignment associated with transition onto the first night of work impairs visual selective attention. PLoS One. 2007;2:e1233. doi: 10.1371/journal.pone.0001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kessel L, Siganos G, Jorgensen T, et al. Sleep disturbances are related to decreased transmission of blue light to the retina caused by lens yellowing. Sleep. 2011;34:1215–9. doi: 10.5665/SLEEP.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duffy JF, Zeitzer JM, Czeisler CA. Decreased sensitivity to phase-delaying effects of moderate intensity light in older subjects. Neurobiol Aging. 2007;28:799–807. doi: 10.1016/j.neurobiolaging.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klerman EB, Duffy JF, Dijk DJ, et al. Circadian phase resetting in older people by ocular bright light exposure. J Investig Med. 2001;49:30–40. doi: 10.2310/6650.2001.34088. [DOI] [PubMed] [Google Scholar]
- 33.Kim SJ, Benloucif S, Reid KJ, et al. Phase-shifting response to light in older adults. J Physiol. 2014;592:189–202. doi: 10.1113/jphysiol.2013.262899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kripke DF, Elliott JA, Youngstedt SD, et al. Circadian phase response curves to light in older and young women and men. J Circadian Rhythms. 2007;5:4. doi: 10.1186/1740-3391-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith MR, Fogg LF, Eastman CI. Practical interventions to promote circadian adaptation to permanent night shift work: study 4. J Biol Rhythms. 2009;24:161–72. doi: 10.1177/0748730409332068. [DOI] [PubMed] [Google Scholar]
- 36.Boudreau P, Dumont GA, Boivin DB. Circadian adaptation to night shift work influences sleep, performance, mood and the autonomic modulation of the heart. PLoS One. 2013;8:e70813. doi: 10.1371/journal.pone.0070813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lamond N, Dorrian J, Roach GD, et al. The impact of a week of simulated night work on sleep, circadian phase, and performance. Occup Environ Med. 2003;60:e13. doi: 10.1136/oem.60.11.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vetter C, Devore EE, Ramin CA, et al. Mismatch of sleep and work timing and risk of type 2 diabetes. Diabetes Care. 2015;38:1707–13. doi: 10.2337/dc15-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tepas DI. Shiftworker sleep strategies. J Hum Ergol (Tokyo) 1982;11(Suppl):325–36. [PubMed] [Google Scholar]