Abstract
Teleosts are the largest and most diverse group of vertebrates, and many species undergo morphological, physiological, and behavioral transitions, “metamorphoses,” as they progress between morphologically divergent life stages. The larval metamorphosis that generally occurs as teleosts mature from larva to juvenile involves the loss of embryo-specific features, the development of new adult features, major remodeling of different organ systems, and changes in physical proportions and overall phenotype. Yet, in contrast to anuran amphibians, for example, teleost metamorphosis can entail morphological change that is either sudden and profound, or relatively gradual and subtle. Here, we review the definition of metamorphosis in teleosts, the diversity of teleost metamorphic strategies and the transitions they involve, and what is known of their underlying endocrine and genetic bases. We suggest that teleost metamorphosis offers an outstanding opportunity for integrating our understanding of endocrine mechanisms, cellular processes of morphogenesis and differentiation, and the evolution of diverse morphologies and life histories.
1. INTRODUCTION AND DEFINITIONS
Nearly half of all described vertebrates are teleosts (see Volff, 2005), and the more than 23,500 known species of teleosts exhibit a vast diversity of phenotypes, ecologies, and developmental life histories. Moreover, teleosts exhibit a tremendous range of metamorphic processes, sometimes undergoing major phenotypic and physiological transitions that allow a single species to exploit different habitats and niches during different life stages. This diversity in teleost life history has long fascinated comparative embryologists and marine ecologists, but the molecular mechanisms of these transitions remain almost entirely unexplored. With the methods of modern genetics and developmental biology, we can begin to elucidate the molecular, endocrinological, and morphogenetic processes underlying this incredibly diverse array of postembryonic transitions.
Several families of teleosts undergo spectacular morphological metamorphoses, comparable to the metamorphosis of anuran amphibians. Other teleosts undergo comparatively subtle phenotypic transformations, more analogous to the fetal-to-adult transition of mammals. Accordingly, workers in this area have adopted sometimes conflicting definitions of what should rightly constitute a “metamorphosis.” For example, some count only the most dramatic of transitions to be metamorphoses, requiring profound morphological and ecological differences between life stages, and rapid transitions between these stages (Balon, 1990, 1999; Just, Kraus-Just, & Check, 1981; Manzon, 2011; Youson, 1988, 2004). Such restrictive definitions dismiss more subtle morphological, physiological, or ecological changes. Alternatively, others have suggested that metamorphosis of teleosts and other chordates can be defined as a conserved period of postembryonic remodeling, how extensive it might be, that is regulated specifically by thyroid hormone (TH; Laudet, 2011; Paris et al., 2008; Paris & Laudet, 2008; Power, Silva, & Campinho, 2008). Yet, this definition precludes transformations that may rely on other hormonal axes.
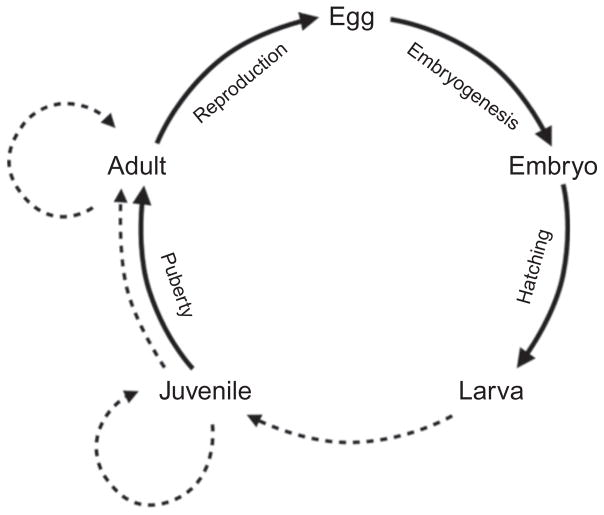
For the purposes of this chapter, we define metamorphosis to be an irreversible developmental and physiological change that affects multiple traits during post-embryonic development and is brought about by one or more systematically acting endocrine mediators, but is independent of sexual maturation, sex-specific modifications, or senescence. This definition focuses on intrinsic features of an organism, or how the environment impacts organismal functioning, and accommodates what is clearly a phylogenetic continuum in the magnitude of postembryonic remodeling. By excluding habitat changes per se, this definition allows for studies of how metamorphic processes have evolved in association with particular ecological conditions or life history modes. By our definition, metamorphosis is not necessarily a specific event, but, rather, any nonsexual, nonsenescent transition entailing developmental or physiological remodeling that is systemically controlled. Thus, our criteria allow one to pose testable hypotheses for whether any particular morphological or physiological transformation constitutes a metamorphosis; that is, whether or not it is controlled by one or more endocrine mediators. The definition deliberately excludes morphological changes (e.g., acquisition of nuptial coloration) that may be temporary, sex-specific, or both, and also does not specify any requirement for overall somatic growth. Moreover, as we have defined it, metamorphoses could occur over a range of time spans, from less than a day to periods of several months, and could, in principle, occur between a variety of life history phases, though metamorphosis is generally most prevalent during the larva-to-juvenile transition (Fig. 5.1). Finally, in being agnostic to mechanism, our definition acknowledges that additional factors mediating such transformations still may await discovery or characterization. In proposing this definition, and in the review that follows, our goal is to facilitate the study of postembryonic cellular, developmental, and physiological processes, how they intersect with endocrine mechanisms, and how they have evolved.
Figure 5.1.
Generalized life cycle of a teleost. Dashed lines indicate potential periods of metamorphic transformations within the life cycle; the transformation from larva to juvenile represents larval metamorphosis.
2. METAMORPHIC DIVERSITY IN TELEOSTS
In this section, we briefly survey the astonishing diversity of metamorphic remodeling among teleosts, set in the context of the ecological and life history transitions that they accompany (Table 5.1). The majority of described metamorphic transitions, sensu our definition, are metamorphoses from larva to juvenile. These larval metamorphoses commonly include the formation of adult fins (pectoral fins generally develop during embryogenesis) and ossification of fin rays, maturation of internal organs and sensory systems, formation of scales, modifications to pigment pattern, and allometric changes in body proportions. Accompanying these alterations are a multitude of group- and species-specific morphological and physiological changes, some of which we outline below.
Table 5.1.
Characteristics of larval metamorphosis in different teleost groups
| Group | Habitat | Niche shift | Duration | Additional morphological changes* | Species and references |
|---|---|---|---|---|---|
| Carp | Freshwater | None | Days–weeks | None | Zebrafish Danio rerio: gross morphology (Parichy, Elizondo, Mills, Gordon, & Engeszer, 2009); fin development (Cubbage & Mabee, 1996; Goldsmith, Iovine, O’Reilly-Pol, & Johnson, 2006); pigmentation (Budi, Patterson, & Parichy, 2011; Parichy & Turner, 2003a); and lateral line (Nuñez et al., 2009; Webb & Shirey, 2003) |
| Catfish | Freshwater | Pelagic to benthic | Days–weeks | Growth of barbels | Indian catfish Mystus punctatus (Ramanathan, Natarajan, & Sukumran, 1985) |
| Coral reef fish | Marine | Pelagic to demersal | Hours–weeks | Dramatic adult pigmentation changes in many species | Multiple species (McCormick & Makey, 1997; McCormick, Makey, et al., 2002) |
| Eels | Catadromous | Marine to freshwater | Months–years | Leptocephalus/glass eel to elver/silver eel transition: Salinity tolerance | American eel Anguilla rostrata otolith morphology (Cieri & McCleave, 2000) Japanese eel Anguilla japonica otolith morphology (Arai, Otake, & Tsukamoto, 1997) Australian shortfin eels Anguilla australis (De Silva, Gunasekera, Gooley, & Ingram, 2001) Multiple species (Raju, 1974) |
| Flatfish | Marine | Pelagic to benthic | Weeks | Cranial development and eye migration, asymmetrical pigmentation | Atlantic halibut Hippoglossus hippoglossus (Sæle et al., 2004) Japanese flounder Paralichthys olivaceus (Tanaka, Kawai, Seikai, & Burke, 1996) Senegalese sole Solea senegalensis (Fernández-Díaz et al., 2001) Starry flounder Platichthys stellatus (Policansky & Sieswerda, 1979) Summer flounder Paralichthys dentatus (Keefe & Able, 1993; Martinez & Bolker, 2003) Multiple species (Schreiber, 2001) |
| Gobies | Amphidromous | Marine to freshwater | Days–weeks | Change in mouth position and fin shape | Freshwater goby Sicyopterus lagocephalus (Keith et al., 2008; Taillebois et al., 2011) |
| Groupers | Marine | Pelagic to benthic | Months | Loss of larval spines, dorsoventral flattening | Spotted grouper Epinephelus tauvina (Hussain & Higuchi, 1980) Leopard grouper Mycteroperca rosacea (Martínez-Lagos & Gracia-López, 2009) Hong Kong grouper Epinephelus akaara (Fukuhara & Fushimi, 1988) |
| Medaka | Amphidromous | None | Days–weeks | None | Medaka Oryzias latipes (Iwamatsu, 1994) |
| Sea Breams | Marine | Pelagic to benthic | Months | None | Gilt-head sea bream Sparus saurata (Fukuhara, 1991) |
| Salmonids | Anadromous | Freshwater to marine | Days–weeks | Parr to smolt transition: Changes in lipid content and salinity tolerance; erythrocyte modifications | Atlantic salmon Salmo salar: Morphology (Dêbowski et al., 1999); physiology (McCormick & Saunders, 1987; Virtanen, 1987) Coho salmon Oncorhynchus kisutch physiology (Sullivan, Dickhoff, Mahnken, & Hersbberger, 1985) Multiple species (McCormick & Saunders, 1987; Woo, Bern, & Nishioka, 1978) |
| Tilapia | Freshwater | None | Days–weeks | None | Tilapia Oreochromis mossambicus (Ramanathan et al., 1985) |
| Tuna | Marine | Planktonic to pelagic | Days–weeks | None | Pacific bluefin tuna Thunnus thynnus (Kaji, Tanaka, Takahashi, Oka, & Ishibashi, 1996; Kawakami, Nozaki, Seoka, Kumai, & Ohta, 2008; Miyashita, Sawada, Okada, Murata, & Kumai, 2001; Tanaka, Satoh, Iwahashi, & Yamada, 2006) Yellowfin tuna Thunnus albacares (Kaji et al., 1999) |
At metamorphosis, all groups acquire adult fins and fin rays, internal organs and sensory systems mature, scales and adult pigment pattern form and body proportions change; additional morphological changes lists specific modifications in addition to these common changes
2.1. Marine teleosts
Marine teleosts exhibit some of the most dramatic morphological transitions seen in vertebrates. These changes in phenotype facilitate ecological transitions: most marine fish—and indeed the majority of marine organisms—exhibit a dispersive planktonic or pelagic (open water) larval stage, then undergo metamorphosis and are recruited to an adult habitat (Thorson, 1950). Pelagic larvae often posses morphological specializations that maximize survival and dispersal potential: large larval fin folds, bony plates, and long spines frequently characterize dispersive larvae. Such larval-specific features are resorbed at metamorphosis (as in the grouper, Fig. 5.2; Moser, 1981; Webb, 1999). The larval stage can last several years in some fish species, allowing larvae to disperse over long distances (Webb, 1999).
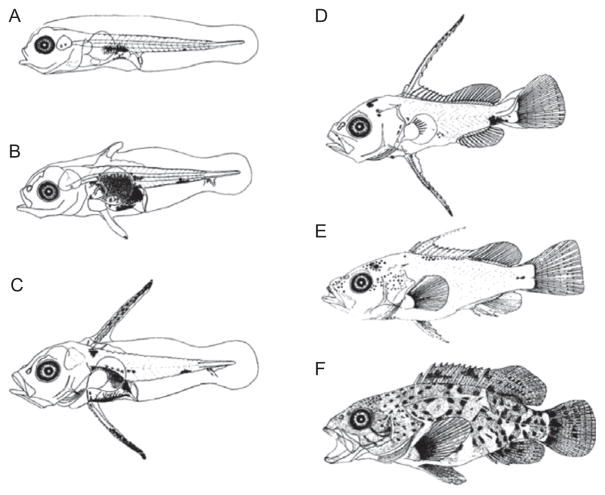
Figure 5.2.
Metamorphosis of the brown-spotted grouper (Epinephelus tauvina). (A) Newly hatched larva, 2 mm; (B) 10-day-old larva; (C) 13-day-old larva; (D) 31-day-old metamorphosing individual; (E) 31 day old, 18 mm; and (F) 50 dpf juvenile, 31 mm. Images reprinted with permission from (Hussain & Higuchi, 1980)
In addition to the loss of larval features, larval metamorphosis of marine fishes often involves dramatic changes in pigmentation and overall morphology, with specific alterations to head shape and body depth (de Jesus, Toledo, & Simpas, 1998; Fukuhara & Fushimi, 1988; Wittenrich et al., 2010). Flattening in the dorsoventral plane prepares many species to become “demersal” adults, which inhabit the bottom of the water column, or “benthic” adults, which live close to or in contact with the substrate or the sea floor (Webb, 1999). One remarkable group of benthic teleosts flattens laterally rather than dorsoventrally during metamorphosis (flatfish morphology citations, Table 5.1). As Pleuronectiform flatfish mature, bilaterally symmetric larvae become markedly asymmetrical as one eye translocates across the dorsal midline to the opposite side of the head, resulting in an ocular side that will face the water column, and a blind side that will face the substrate (Fig. 5.3). During the transitional metamorphic period, fish swim at an increasing angle to ultimately settle onto the substrate. As in other teleosts, a new pattern of pigmentation develops during metamorphosis, but only on the ocular surface of the body (Table 5.1).
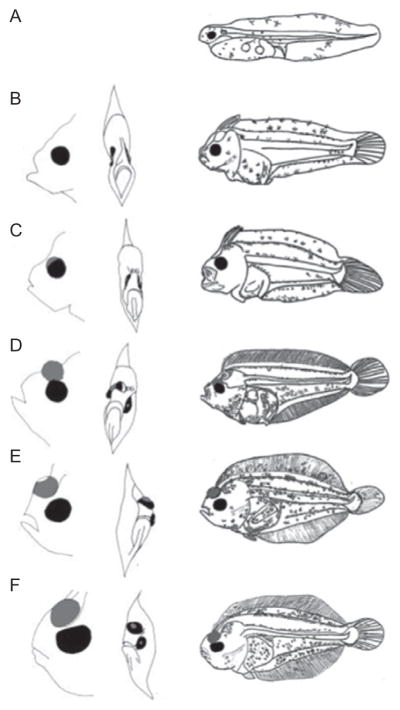
Figure 5.3.
Metamorphosis of the summer flounder Paralichthys dentatus. (A) Hatched yolk-sac larva. (B) Pretransformation larva before eye migration commences. (C) Early metamorphosis and the beginning of eye migration. (D) Mid-metamorphosis. (E) Metamorphic climax, right eye has migrated over the dorsal midline. (F) Young juvenile. Left column in B–D shows the migration of the eye across the skull; migrating right eye is shaded in gray. Rightmost column shows whole-body morphological changes at each stage. Images reprinted with permission from (Martinez & Bolker, 2003)
Particularly rapid and dramatic remodeling also occurs among coral reef fishes, which undergo changes in body shape and acquire conspicuous adult pigmentation (Leis & McCormick, 2006). Metamorphosis in reef fish enables the transition from a pelagic dispersal stage to a demersal, reef-associated adult form. This metamorphosis can be extremely rapid, with some species undergoing their metamorphic changes in pigmentation in as little as 6 h, or can last several weeks and include several intermediate stages; for example, goatfish and damselfish undergo 2–3 week metamorphic processes that include several intermediate shifts in habitat and morphology (McCormick & Makey, 1997; McCormick, Makey, & Dufour, 2002). Thus, marine teleosts exhibit an astonishing range of metamorphic transformations that often include substantial morphological changes allowing the exploitation of different spatial niches within an oceanic environment.
2.2. Diadromous teleosts
Diadromous species migrate between salt water and fresh water environments; metamorphosis can prepare these fish to survive in their new habitat. The physical demands of a marine environment differ significantly from those of fresh water, and diadromous teleosts undergo substantial changes in morphology. These include changes in body shape, muscle, skin, and pigmentation; changes in the structure and function of numerous organs, including the kidneys, gut, eyes, and lateral line; and physiological changes in osmoregulation and metabolism.
The best-studied example of a fresh-to-salt water (anadromous) migration is that of salmon. Salmon spawn in freshwater breeding grounds, where embryonic “alevin” hatch into larval “fry.” After months to several years of development, larval fry develop bars of pigmentation and are called “parr” (Fig. 5.4). Swimming downstream, parr undergo smoltification, which morphologically and physiologically prepares young “smolt” juveniles for the marine habitat. By our definition, the entire parr-to-smolt period constitutes a protracted larval metamorphosis, with different morphogenetic and physiological processes stimulated by different hormones (insulin, prolactin, TH, growth hormone (GH), and cortisol) that spike at different points during the months of parr development (see Björnsson, Einarsdottir, & Power, 2012; Dickhoff, Brown, Sullivan, & Bern, 1990). As fish metamorphose into smolts, purines are deposited in the skin, producing a silvery appearance, body fat decreases, gas bladder size increases, and body shape and condition change to become leaner and sleeker (Björnsson, Einarsdottir, et al., 2012; Johnston & Saunders, 1981; McCormick & Saunders, 1987; Winans & Nishioka, 1987). In preparation for the hyperosmotic environment, smolts become able to absorb increased amounts of water through the intestine, and expression of ion channels (aquaporins) and ion pumps (Na+/K+-ATPase) increases, allowing active excretion of salt (D’Cotta, Valotaire, le Gac, & Prunet, 2000; Tipsmark, Sørensen, & Madsen, 2010). During smoltification, salmon also begin synthesizing additional hemoglobin isoforms with enhanced oxygen affinity (Seear et al., 2010; Sullivan et al., 1985). If smolts do not reach salt water during a critical “smolt window,” they undergo desmoltification, which includes a loss of hypo-osmoregulatory abilities and metabolic adaptations, and a darkening of skin color (Stefansson, Björnsson, Ebbesson, & McCormick, 2008). Although desmoltification includes the regression of certain physiological functions, fish do not revert to an overall smolt morphology; thus, desmoltification is not a reversal of metamorphosis. Returning to freshwater environments to spawn, marine salmonids undergo yet another transformation into the life stage known as “grilse,” preparing them for migration in fresh water and breeding. This transformation is controlled predominantly by the gonadotropic axes (Youngson, McLay, Wright, & Johnstone, 1988), and the majority of the morphological changes are sex-specific, so by our definition, this transformation primarily constitutes a puberty rather than a metamorphosis.
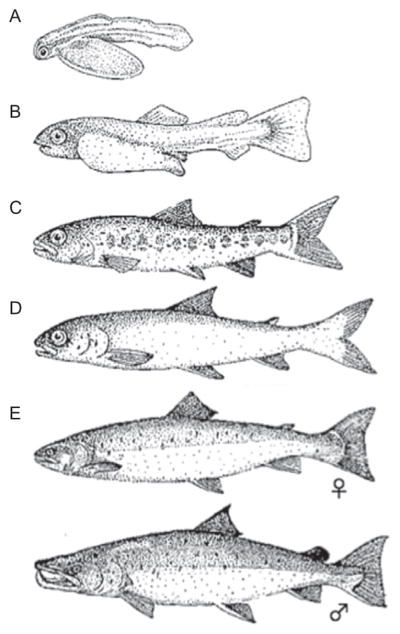
Figure 5.4.
Life stages of Atlantic Salmon, Salmosalmar.(A)Alevin;(B) fry, 3–6 weeks old;(C) parr; (D) smolt, 1–3 years old; and (E) Breeding female and male. Images from public domain.
Eels represent a second well-studied instance of a diadromous life history. In contrast to salmonids, eels develop and disperse as marine larvae, then begin to metamorphose in preparation for a salt-to-fresh water (catadromous) migration. The leaf-shaped marine larvae (leptocephali) are dispersed by ocean currents and after up to 18 months of larval development, transform into transparent “glass eels” (Fig. 5.5; Wang & Tzeng, 2000). Glass eels undergo numerous morphological and physiological changes to preadapt them to freshwater environments, suggesting that the entire glass eel stage represents the metamorphic period. As glass eels are recruited to a freshwater habitat, they complete metamorphosis to become juvenile “elvers.” To return to oceanic environments for spawning, eels undergo the process of silvering and regain saltwater tolerance. Silvering has been described as a secondary metamorphic event, although it is stimulated by sex hormones and involves maturation of the gonads (Aroua et al., 2005; Rousseau, Aroua, Schmitz, Elie, & Dufour, 2009). This event may represent a second metamorphosis simultaneous with puberty.
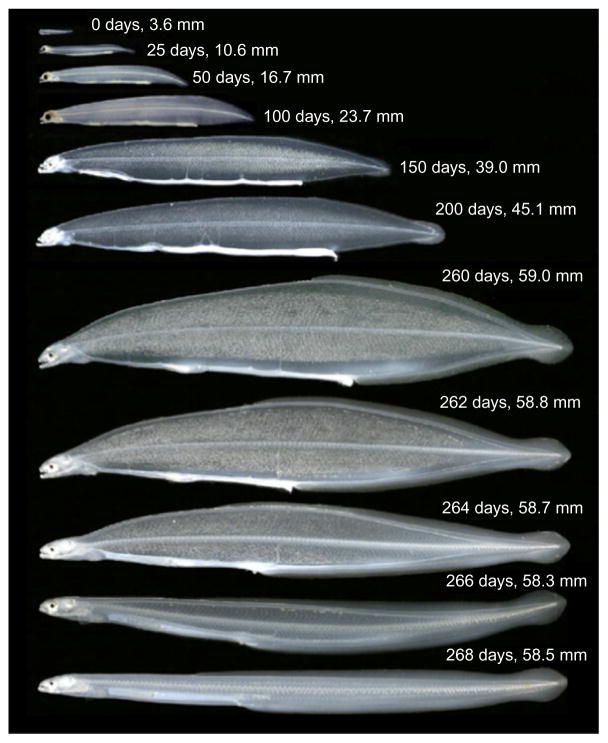
Figure 5.5.
Developmental growth series of the Japanese eel Anguilla japonica. Larval leptocephali transform into the glass eel stage (bottom image shows a young glass eel). Reprinted with permission from (Tsukamoto et al., 2009).
2.3. Freshwater teleosts
In contrast with their marine and diadromous counterparts, freshwater teleosts generally undergo more phenotypically subtle metamorphoses. Few larva-specific adaptations are found in freshwater teleosts, reflecting the fact that freshwater fish do not generally have an explicitly dispersive larval stage. Nonetheless, freshwater teleosts undergo numerous morphological and physiological changes during larval metamorphosis, modifications that have been described most extensively in the developmental genetic model species zebrafish Danio rerio (Fig. 5.6; Parichy et al., 2009). Like other teleosts, zebrafish undergo significant changes in body shape, losing larval fin folds, developing or completing development of adult fins and fin rays (Cubbage & Mabee, 1996; Goldsmith et al., 2006; Patterson, Mook, & Devoto, 2008), ossifying the axial skeleton (Bird & Mabee, 2003; Elizondo et al., 2005), forming an adult pigment pattern (Budi, Patterson, & Parichy, 2008; Budi et al., 2011; Johnson, Africa, Walker, & Weston, 1995; Parichy & Turner, 2003b), acquiring scales (Sire & Akimenko, 2004), and exhibiting maturation and remodeling of many organs, including the lateral line (Ledent, 2002; Nuñez et al., 2009; Webb & Shirey, 2003), central and peripheral nervous systems (Larson, Gordon, Lau, & Parichy, 2010), gut, kidneys, and swimbladder (Parichy et al., 2009; Robertson, McGee, Dumbarton, Croll, & Smith, 2007). Freshwater teleosts tend to have less distinct periods of larval metamorphosis than marine or diadromous species, but this postembryonic process still unequivocally occurs, transforming the larval organism into a morphologically distinct juvenile form. There are at least a few identified cases of paedomorphism in freshwater species, with miniature Paedocypris and Danionella fish failing to undergo normal metamorphosis and becoming reproductively mature while maintaining a larva-like overall morphology (Britz, Conway, & Rüber, 2009; Mayden & Chen, 2010; Rüber et al., 2007). These paedomorphic genera illustrate ways in which even relatively subtle metamorphic processes may be modified to effect major morphological change.
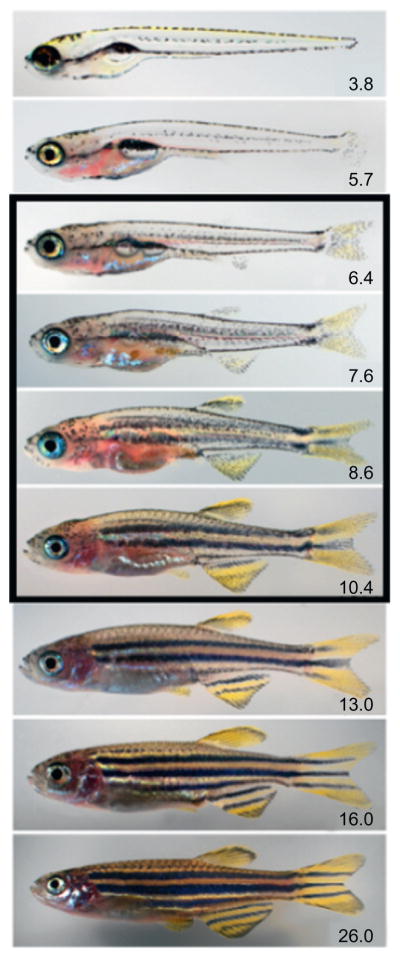
Figure 5.6.
Postembryonic development in the zebrafish (Danio rerio). Numbers indicate standard length in mm, with the period of peak metamorphic remodeling during the larva-to-juvenile transition indicated by the black box. At standard temperature and typical rearing densities, this transition begins at about 10–14 days and takes about 2 weeks. As postembryonic fish can develop and grow at markedly different rates, days of development is an inadequate staging criterion; stages based on morphological criteria, and their size equivalents have been defined for this species and are preferable for developmental studies (Parichy et al., 2009).
3. ENDOCRINE CONTROLS OF TELEOST METAMORPHOSIS
The coordinated occurrence of multiple disparate organ- and tissue-specific events during teleost life-stage transitions suggests that “local” processes are likely to be coordinated by “global” signals (and so are likely to constitute bona fide metamorphoses, as defined above). Research into the endocrine controls of postembryonic transitions in teleosts has focused almost exclusively on spectacular examples of such metamorphoses; for example, salmonid smoltification and flatfish metamorphosis. Yet, there is evidence that more subtle transitional processes are controlled by conserved endocrine mechanisms as well. Here, we outline what is known about the hormonal contributions to teleost metamorphosis. TH appears to be a key regulator of teleost metamorphosis, and most endocrine studies of metamorphosis have focused on this factor. Thus, we briefly review the metabolism of TH in teleosts, and the expression of TH-associated factors before outlining what is known about other endocrine contributions to metamorphosis.
3.1. Thyroid hormone
It became clear in the late 1980s that TH, which stimulates the metamorphosis of amphibians, also promotes metamorphosis of flatfish. Application of exogenous TH accelerates metamorphic events, including fin resorption, eye migration, and fin ray shortening (Inui & Miwa, 1985; Inui, Tagawa, Miwa, & Hirano, 1989; Klaren, Wunderink, Yüfera, Mancera, & Flik, 2008; Miwa, Yamano, & Inui, 1992). TH is also necessary for flatfish metamorphosis: treatment with TH-inhibiting goitrogens arrests metamorphosis, resulting in oversized pelagic larvae (Inui & Miwa, 1985).
Further studies showed that spikes in thyroid activity or circulating TH levels are associated with the larval metamorphosis of many teleost species, including those with spectacular transformations (salmonids, eels, flatfish) and those with more subtle metamorphoses (fatheads, greenlings, groupers, minnow, sea breams, tilapia, tuna, zebrafish; see Fig. 5.7 and hormone titer citations in Table 5.2). Treatment with exogenous TH stimulates metamorphic processes, while inhibition of TH synthesis with goitrogens inhibits metamorphosis in numerous groups: eels, gobies, greenlings, groupers, and salmon (morphological responses to TH manipulation citations, Table 5.2). Metamorphic changes in fins, pigmentation, and body size in zebrafish are inhibited by goitrogen treatment as well (Brown, 1997; D.M. Parichy, unpublished data). Evolutionary modifications of TH signaling effected by cis-regulatory changes at the thyroid-stimulating hormone β2 locus have also been implicated in the adaptive divergence of marine and freshwater stickleback populations (Kitano et al., 2010). Together, these studies suggest that TH involvement in metamorphosis is widespread among teleosts, and that changes in this pathway can contribute to evolutionary diversification. Interestingly, lampreys, relatives of early teleosts, undergo a metamorphosis in which TH plays an inhibitory rather than a stimulatory role (see Youson, 1997); the mechanistic bases for this divergent TH effect, and whether other factors substitute for TH in promoting metamorphosis, remain unknown.
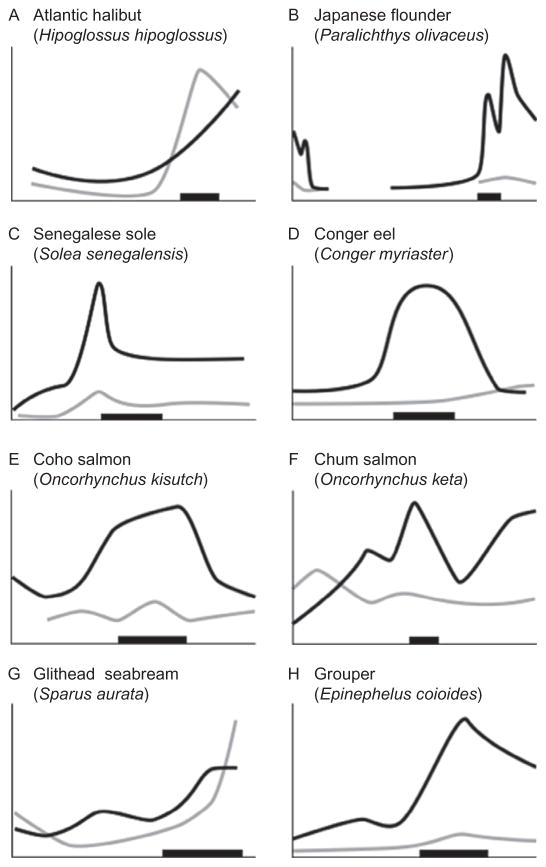
Figure 5.7.
Relative whole-body concentrations of thyroid hormone in different teleosts during larval and juvenile development. Developmental stage is indicated by the x-axis and the horizontal black bar shows the approximate climax of the metamorphic period. Black line indicates relative level of thyroxine (T4); gray line indicates relative level of tri-iodothyronine (T3); lines redrawn from original figures. Relative concentrations shown for three flatfish: (A) Halibut (Galay-Burgos, Power, Llewellyn, & Sweeney, 2008); (B) Flounder (Yamano & Miwa, 1998); and (C) Sole (Klaren et al., 2008). Also shown are (D) Eel (Kawakami, Tanda, Adachi, & Yamauchi, 2003), two species of salmon: (E) Coho salmon (Harada, Yoshinaga, Ojima, & Iwata, 2008) and (F) Chum salmon (Parhar & Iwata, 1996); (G) Seabream (Szisch, Papandroulakis, Fanouraki, & Pavlidis, 2005); and (H) Grouper (de Jesus et al., 1998).
Table 5.2.
Selected studies that have examined hormone involvement or gene expression during teleost larval metamorphosis
TH synthesis and metabolism in teleosts are similar to that of tetrapods (Yen, 2001). The genes that contribute to thyroid follicle patterning and development are highly conserved between teleosts and mammals (Alt et al., 2006; Porazzi, Calebiro, Benato, Tiso, & Persani, 2009), as are the deiodinases that regulate TH activity (Itoh et al., 2010; Orozco & Valverde-R, 2005; Power et al., 2008). In response to pituitary thyrotropin (TSH), thyroid follicles produce TH in the form of thyroxine (T4). Circulating plasma levels of T4 increase markedly at the onset of metamorphosis in several teleost species (Fig. 5.7), suggesting that the thyroid is highly active during this period. This is further consistent with the results of radioiodide uptake assays (Brown, 1997; D.M. Parichy, unpublished) and enzyme-linked immunoassays performed in zebrafish (Chang et al., 2012). T4 is converted in peripheral tissues by deiodinase enzymes (DIO1 and DIO2) into the genomically active form, tri-iodothyronine (T3). Both T3 and T4 are inactivated by a third deiodinase, DIO3. Thus, the expression and activity of deiodinases regulate TH bioactivity and availability. In teleosts, these genes are expressed in spatially and temporally specific manners during postembryonic development, with dio1 and dio2 increasing in expression immediately before and during metamorphic climax of several species (Campinho et al., 2010; Itoh et al., 2010).
T3 serves as the ligand for nuclear TH receptors (TRs), which typically activate expression of target genes in the presence of the hormone, and repress expression in its absence. All teleosts examined possess at least two isoforms of THRs: TRα and TRβ, and many posses additional copies of these loci, possibly owing to an ancient, teleost-specific genome duplication (Hoegg, Brinkmann, Taylor, & Meyer, 2004; Ravi & Venkatesh, 2008); for example, zebrafish exhibit two TRα loci, thraa and thrab, though only a single TRβ locus, thrb. In species that have been examined so far, TRs increase in expression prior to and during metamorphic climax (Fig. 5.8). Thus, TR-mediated TH signaling appears to play a critical role in the metamorphosis of teleosts examined. Nevertheless, although TH is by far the best-studied hormonal factor in teleosts, TSH and TH titers and expression of DIOs and TRs remain undocumented for the vast majority of teleost families. Thus, the requirement or sufficiency for TH in metamorphosis remains unknown in most fish; moreover, the genetic targets and molecular mechanisms of hormonal action remain largely unclear.
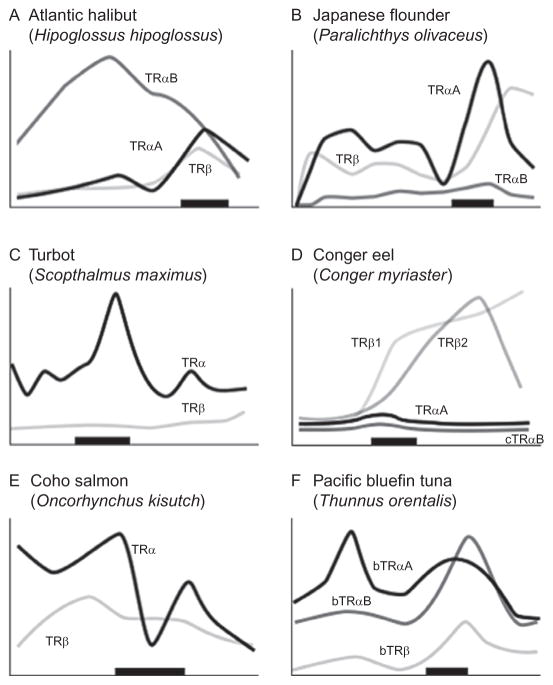
Figure 5.8.
Relative different thyroid hormone receptor genes during larval and juvenile development. Expression is from whole-body assays unless otherwise noted; lines redrawn from original figures. The x-axis shows developmental progression and the thick black bar shows the approximate climax of the metamorphic period. Relative gene expression in three flatfish: (A) Halibut (Galay-Burgos et al., 2008), (B) Flounder (gene expression in head; Yamano & Miwa, 1998), and (C) Turbot (Marchand et al., 2004), and three other teleosts: (D) Eel (Kawakami et al., 2003), (E) Salmon (gene expression in brain; Harada et al., 2008), and (F) Tuna (Kawakami, Nozaki, et al., 2008). The absence of a particular receptor subtype on a graph does not necessarily mean that the species does not possess that isoform.
3.2. Non-TH mediators of metamorphosis
In addition to TH, other endocrine factors contribute to metamorphosis as well. GH activates insulin-like growth factor (IGF) pathways, stimulating cellular proliferation and increasing basal metabolic rate. Spikes in plasma GH and IGF-I are observed during salmonid parr-to-smolt metamorphosis (Table 5.2). Further, treating parr with exogenous GH induces metamorphic changes, including changes in body shape, pigmentation, and seawater tolerance (Boeuf, 1993; Donaldson, Fagerlund, Higgs, & McBride, 1979; Dufour & Rousseau, 2007). Genetic loss of GH likewise delays metamorphic stage transitions in zebrafish; though this may be a secondary effect of delayed growth (McMenamin and Parichy, unpublished). In flatfish, GH receptors peak in expression immediately prior to the onset of metamorphosis, as do concentrations of IGF (Hildahl et al., 2007). The hormone prolactin antagonizes GH, and appears to have a negative influence on salinity tolerance and overall smoltification in salmonids (Björnsson, Stefansson, & McCormick, 2011; Madsen & Bern, 1992). The GH/IGF axis thus plays important roles in promoting growth and regulating metabolism during postembryonic development in teleosts (Yousefian & Shirzad, 2011), and its potential roles in directly stimulating morphogenetic events merit further investigation.
Another factor that may contribute to metamorphic progression is cortisol, known primarily for its roles in stress response. Cortisol titer increases during salmon smoltification, and may directly contribute to physiological metamorphosis (Richman, de Diaz, Nishioka, & Bern, 1985; Specker, 1982). Flatfish also exhibit peak cortisol levels at metamorphic climax, potentially indicating a stimulatory role in this process (de Jesus et al., 1991). Indeed, stress hormones are known to contribute to metamorphosis of some anuran amphibians (Denver, 1993, 1997). In eel leptocephali, however, cortisol levels decrease prior to metamorphosis and remain low throughout metamorphosis (Yamano, Tagawa, et al., 1991). Thus, stress hormones may play synergistic roles with TH during the metamorphosis of some teleosts (Dufour & Rousseau, 2007), but these roles require further investigation and particularly experimental manipulation.
In summary, it is clear that several hormonal axes are activated immediately before, during, and following metamorphic climax in the teleosts that have been studied to date. These hormonal axes, and specifically TH pathways, likely play roles in orchestrating metamorphosis, and may integrate external environmental cues into coordinated sets of disparate morphogenetic events. Nevertheless, the evolutionary conservation of these roles, the interactions between the hormonal axes, and the proximate mechanisms of endocrine response remain unclear.
4. LOCAL MECHANISMS OF MORPHOGENETIC CHANGES
The morphogenetic changes that occur at metamorphosis include major remodeling of existing features as well as the formation of entirely new tissues and organs; thus, metamorphosis requires extensive differentiation as well as the morphogenetic processes of cellular migration, proliferation, growth, and death. For the overwhelming majority of metamorphic events, however, the underlying cellular mechanics and genetic mechanisms remain unexplored by modern methods. Here, we discuss the relatively few areas in which the cellular or genetic bases underlying metamorphic changes are being thoroughly investigated, and we highlight some of the many outstanding questions that remain to be answered.
4.1. Flatfish cranial asymmetry
The most thoroughly studied teleost metamorphic process is undoubtedly the unique cranial transformation of the flatfish. Although flatfishes are exceptional in their external morphological asymmetry, the internal organs of all vertebrates are asymmetric to different degrees. This internal asymmetry originates during early embryogenesis with the asymmetric expression of genetic cascades that initiate asymmetric expression of the homeobox-containing transcription factor Pitx2 (Ryan et al., 1998). Flatfishes exploit this internal asymmetry to ultimately achieve external asymmetry at metamorphosis (Hashimoto et al., 2002; Suzuki et al., 2009). Pitx2 is reexpressed at metamorphosis in a fundamentally asymmetric portion of the brain, and this reexpression appears to initiate eye migration (Suzuki et al., 2009). Cellular proliferation in the suborbital tissue of one side of the cranium may “push” one eye across the dorsal midline (Bao et al., 2011). Whether this proliferation is stimulated by TH directly or whether intermediary signals are involved remains unknown, but GH and IGF pathways likely serve as more proximal factors (Hildahl et al., 2008). In several flatfish mutants, cranial asymmetry is decoupled from internal organ asymmetry, suggesting that the two are regulated by independent mechanisms (Hashimoto et al., 2002). Elucidating the local pattern-forming and morphogenetic cues that determine the definitive form of the adult craniofacial skeleton, and how these factors depend on global hormonal effectors, clearly represents an exciting area for future research.
4.2. Skin
Larval teleosts have a simple integumentary structure, composed primarily of epidermis; at metamorphosis, the skin becomes increasingly stratified and complex (Chang & Hwang, 2011; Hawkes, 1974; Rakers et al., 2010). Collagen fibrils are deposited in orthogonal arrays under the epidermis (Le Guellec, Morvan-Dubois, & Sire, 2004) and the acellular stroma is then invaded by fibroblasts of unknown origin (but potentially originating from the neural crest; Matsumoto et al., 1983). Later, in metamorphosis, these fibroblasts initiate scale formation, potentially regulated by expression of sonic hedgehog among other factors (Sire & Akimenko, 2004). The processes of skin stratification have been studied in greatest detail in zebrafish, but appear at least structurally similar in cichlids (Sire & Géraudie, 1983). Keratins mediate some of the skin restructuring events, and in flatfish, keratin expression is regulated directly by TH (Infante et al., 2007), decreasing after metamorphic climax (Campinho, Silva, Sweeney, & Power, 2007). The metamorphic transformation of flatfish skin resembles the metamorphic restructuring seen in amphibians (Power et al., 2008), in which keratin loci are also under TH control (Page et al., 2007). Nevertheless, further research is needed to determine the precise roles of TH in promoting integumentary metamorphosis of teleosts. In this regard, genetically tractable species such as zebrafish offer outstanding potential for studying skin metamorphosis; indeed, a variety of mutants with defects in the formation of scales and other postembryonic integumentary cell lineages have been identified (Harris et al., 2008; Lang, Patterson, Gordon, Johnson, & Parichy, 2009). As many features of skin development are conserved even with mammals, and mammalian skin undergoes a similar period of increased stratification and barrier function acquisition during fetal stages, studies of teleost skin metamorphosis may have translational relevance as well (Hoath & Maibach, 2003; Rakers et al., 2010).
4.3. Pigmentation
The formation of adult pigmentation is another common feature of teleost metamorphosis. Some of the genetic mechanisms underlying metamorphic pigmentation have been dissected in zebrafish, in which adult pigment patterns result from the spatial arrangements of neural crest-derived black melanophores, yellow xanthophores, and iridescent iridophores. During embryonic stages of neural crest migration, precursors to adult pigment cells are established in part owing to ErbB signaling (Budi et al., 2008; Hultman et al., 2009). Subsequently, these precursors are associated with peripheral nerves and, at metamorphosis, migrate to the skin along stereotypical pathways to form the adult pigment pattern, a process that can be directly visualized owing to the existence of fluorescent lineage reporters and the relative transparency of even metamorphic stage zebrafish (Budi et al., 2011). Embryonic and adult pigment cells have partially nonoverlapping genetic requirements, demonstrated by the phenotypes of several zebrafish mutants in which normal embryonic pigment cells develop yet adult precursors either fail to be established or fail to be recruited. In these mutants, pigment pattern metamorphosis is effectively decoupled from overall somatic metamorphosis (e.g., Budi et al., 2008; Larson et al., 2010; Parichy & Turner, 2003b). Once latent precursors have been recruited, a variety of genes acting both within the pigment cells and in the tissue environments through which these cells migrate are required to organize the different pigment cell classes into distinct juvenile and adult stripes (Eom et al., 2012; Iwashita et al., 2006; Lang et al., 2009; Parichy, Rawls, Pratt, Whitfield, & Johnson, 1999; Parichy & Turner, 2003a; Watanabe et al., 2006).
Phylogenetic comparisons reveal that adult pigment patterns of zebrafish relatives similarly depend on the recruitment of latent pigment cell precursors at metamorphosis. Interestingly, however, these cells have been mostly lost in the closely related Danio nigrofasciatus, in which the adult pigment pattern arises at metamorphosis largely through the rearrangement of embryonic/early larval melanophores that persist into the adult. Interspecific cell transplantation shows that the difference in adult melanophore development between zebrafish and D. nigrofasciatus lies extrinsic to the pigment cells, implicating a change in a still-unidentified tissue or cell type that influences metamorphic processes within the pigment cell lineage (Quigley et al., 2004). This example highlights the potential for comparative studies to reveal the cellular, and ultimately genetic, bases underlying evolutionary changes in metamorphic transformations.
In contrast to other teleosts, flatfish develop pigmentation in an asymmetric manner at metamorphosis, with only the ocular (upper) side normally developing adult melanophores. In flounder, pigment cell precursors migrate symmetrically to both lateral sides from the bases of fins at the dorsal and ventral margins of the flank, yet these cells differentiate as melanophores only on the ocular side (Watanabe et al., 2008; Yamada, Okauchi, & Araki, 2010). The genetic bases for this differential response of pigment cells remain unknown.
Development of metamorphic pigmentation in zebrafish is retarded by goitrogens that prevent TH synthesis (Brown, 1997). Whether TH is directly required by pigment cells or their precursors, or whether the hormone exerts an influence indirectly through other cellular intermediaries, is an active area of investigation. Treatment with high (hyper-physiological) levels of T4 actually inhibits adult melanophore development in flatfish (Yoo et al., 2000), eels (Jegstrup & Rosenkilde, 2003) and zebrafish (D.M. Parichy, unpublished data), but the biological significance of these observations remains obscure. Likewise, the genetic and cellular mechanisms of metamorphosis giving rise to the many and varied adult pigment patterns of other teleosts are largely unknown, though mutational and transgenic resources for some (Kelsh, 2004; Kelsh et al., 2004; Odenthal et al., 1996; Parichy, 2006), and evolutionary genetic strategies for others (Miller et al., 2007; Roberts, Ser, & Kocher, 2009), offer significant potential for future advances in this area.
4.4. Fin ray formation
Before and during metamorphosis, mesenchymal cells give rise to the fin endoskeleton, with rays growing from proximal to distal. Numerous pathways regulate positioning and outgrowth of these rays and the joints within the rays (Marí-Beffa & Murciano, 2010; Sims, Eble, & Iovine, 2009). Early proximo-distal patterning in the fin bud, largely controlled by Fgf signaling, is thought to establish a “prepattern” for ray positioning. Sonic hedgehog receptors are expressed in the proximal blastema, potentially inducing the differentiation of bone-forming cells (Laforest et al., 1998). Many of these morphogenetic processes are reactivated during fin regeneration (Iovine, 2007), and studying the normal metamorphic processes can lend insight into regenerative processes. Interestingly, fin regeneration in zebrafish is accompanied by enhanced expression of DIO3 and regenerative progress is retarded when DIO3 activity is blocked pharmacologically (Bouzaffour, Rampon, Ramaugé, Courtin, & Vriz, 2010), suggesting that local T3 degradation promotes regeneration. By contrast, growth cone formation of the regenerating lateral line is stimulated by TH (Bouzaffour et al., 2010). Remaining unknown is the extent to which hormonal mediators associated with normal metamorphosis are required during regeneration of other tissues and how precisely these contribute to particular cellular behaviors. The roles of these factors during normal fin development remain largely unknown, but will be interesting to uncover, particularly in light of the diversity of fin morphologies among teleosts more generally.
In summary, larval metamorphosis in teleosts involves coordinated morphological changes to many different organs; we now understand only a few of the genetic requirements for these transitions. The mechanisms by which these cascades are hormonally activated and coordinated remain largely unknown. Particularly, considering the growing number of molecular resources for nonmodel organisms (Sarropoulou, Nousdili, Magoulas, & Kotoulas, 2008; Volff, 2005), the genetics of metamorphic events and transitions in teleosts represents a promising field for future study.
5. CONCLUSIONS
Teleosts undergo a spectacular diversity of postembryonic metamorphic transitions, but most of these transitions have been described only superficially. Even in the most familiar teleosts, the proximate endocrine and genetic mechanisms for different elements of metamorphosis have yet to be described. Nonetheless, this period of profound remodeling encompasses many inherently interesting developmental phenomena involving fundamental processes of morphogenesis and differentiation. Moreover, this period of coordinated tissue modification offers the opportunity to examine differential tissue responses to global endocrine signals. Genetic modifications to these postembryonic processes have contributed to the spectacular diversity in adult morphology seen within teleost lineages, and elucidating their attendant molecular mechanisms will lend considerable additional insight into this diversification (Harris, 2012). Although the weight of evidence supports roles for TH signaling in promoting metamorphosis in teleosts, roles for other factors remain likely but uncertain, and the precise mechanisms by which TH effects particular morphogenetic or physiological outcomes remain largely unknown. Given the diversity of teleost metamorphoses and the particular tractability of some species (such as zebrafish) for analyses of developmental, genetic, and endocrine mechanisms, it seems certain that further studies of teleosts will provide important insights into the evolution of metamorphosis and, more generally, the diversification of physiology, behavior, and form.
GLOSSARY
- Adult
Reproductively mature form of an organism, possessing all adult organs and mature gonads. Adults are not always in breeding condition as many species are seasonal spawners.
- Amphidromous
Fish that migrate back and forth between fresh and salt water habitats, not necessarily for the purposes of breeding.
- Anadromous
Fish that spawn in fresh water, then migrate to marine habitats.
- Benthic
Associated with the lower layers of the water column, just above the substrate.
- Catadromous
Fish that spawn in marine environments, then migrate to fresh water habitats.
- Demersal
Associated with the very bottom of the water column, close to or in physical contact with the substrate.
- Diadromous
Fish that migrate between salt water and fresh water environments (catadromous and anadromous).
- Embryo
Developmental stage characterized by yolk sac and chorion. Non-feeding; acquires nutrients exclusively from yolk. Sometimes called alevin, yolk sac fry, or sac fry.
- Juvenile
Developmental stage at which the majority of adult organs have developed and organism is proportionally similar to an adult, but is not yet sexually mature. Salmonid juveniles are called smolts; eel juveniles are called elvers and yellow eels.
- Larva
Developmental stage characterized by free swimming and active feeding. Larvae are able to obtain and digest exogenous food and are often transparent or lightly pigmented. Generally show substantial morphological differences from adults. Larvae may also be called fry, salmonid larvae may be called fingerlings or parr, eel larvae are called leptocephali.
- Metamorphosis
An irreversible developmental and physiological change that affects multiple traits during postembryonic development and is brought about by one or more systematically acting endocrine mediators, but is independent of sexual maturation, sex-specific modifications, or senescence. May occur multiple times throughout during a life cycle, and may be simultaneous with puberty. The most common type of metamorphosis is larval metamorphosis, during which time a teleost transforms from larva to juvenile.
- Pelagic
Associated with the upper layers of the water column and open water. Many marine organisms have a pelagic open ocean larval stage of life.
- Puberty
The process of transforming from juvenile to adult, involving the maturation of the gonads and reproductive organs. Controlled by sex hormones in the gonadotropic axis.
References
- Ágústsson T, Sundell K, Sakamoto T, Johansson V, Ando M, Bjornsson BT. Growth hormone endocrinology of Atlantic salmon (Salmo salar): Pituitary gene expression, hormone storage, secretion and plasma levels during parr–smolt transformation. The Journal of Endocrinology. 2001;170:227–234. doi: 10.1677/joe.0.1700227. [DOI] [PubMed] [Google Scholar]
- Alt B, Reibe S, Feitosa NM, Elsalini OA, Wendl T, Rohr KB. Analysis of origin and growth of the thyroid gland in zebrafish. Developmental Dynamics. 2006;235:1872–1883. doi: 10.1002/dvdy.20831. [DOI] [PubMed] [Google Scholar]
- Andersen Ø, Dahle SW, van Nes S, Bardal T, Tooming-Klunderud A, Kjørsvik E, et al. Differential spatio-temporal expression and functional diversification of the myogenic regulatory factors MyoD1 and MyoD2 in Atlantic halibut (Hippoglossus hippoglossus) Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology. 2009;154:93–101. doi: 10.1016/j.cbpb.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Arai T, Otake T, Tsukamoto K. Drastic changes in otolith microstructure and microchemistry accompanying the onset of metamorphosis in the Japanese eel Anguilla japonica. Marine Ecology Progress Series. 1997;161:17–22. [Google Scholar]
- Aroua S, Schmitz M, Baloche S, Vidal B, Rousseau K, Dufour S. Endocrine evidence that silvering, a secondary metamorphosis in the eel, is a pubertal rather than a metamorphic event. Neuroendocrinology. 2005;82:221–232. doi: 10.1159/000092642. [DOI] [PubMed] [Google Scholar]
- Baggerman B. Salinity preference, thyroid activity and the seaward migration of four species of Pacific salmon (Oncorhynchus) Journal of the Fisheries Research Board of Canada. 1960;17:295–322. [Google Scholar]
- Balon EK. Epigenesis of an epigeneticist: The development of some alternative concepts on the early ontogeny and evolution of fishes. Guelph Ichthyology Reviews. 1990;1:1–48. [Google Scholar]
- Balon EK. Alternative ways to become a juvenile or a definitive phenotype (and on some persisting linguistic offenses) Environmental Biology of Fishes. 1999;56:17–38. [Google Scholar]
- Bao B, Ke Z, Xing J, Peatman E, Liu Z, Xie C, et al. Proliferating cells in suborbital tissue drive eye migration in flatfish. Developmental Biology. 2011;351:200–207. doi: 10.1016/j.ydbio.2010.12.032. [DOI] [PubMed] [Google Scholar]
- Bird NC, Mabee PM. Developmental morphology of the axial skeleton of the zebrafish, Danio rerio (Ostariophysi: Cyprinidae) Developmental Dynamics. 2003;228:337–357. doi: 10.1002/dvdy.10387. [DOI] [PubMed] [Google Scholar]
- Björnsson BT, Einarsdottir IE, Power D. Is salmon smoltification an example of vertebrate metamorphosis? Lessons learnt from work on flatfish larval development. Aquaculture. 2012;28:264–272. [Google Scholar]
- Björnsson BT, Stefansson SO, McCormick SD. Environmental endocrinology of salmon smoltification. General and Comparative Endocrinology. 2011;170:290–298. doi: 10.1016/j.ygcen.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Boeuf G. Salmonid smolting: A pre-adaptation to the oceanic environment. In: Rankin JC, Jensen FB, editors. Fish ecophysiology. Vol. 9. London: Chapman & Hall; 1993. pp. 105–125. [Google Scholar]
- Boeuf G, Le Bail P, Prunet P. Growth hormone and thyroid hormones during Atlantic salmon, Salmo salar L., smolting, and after transfer to seawater. Aquaculture. 1989;82:257–268. [Google Scholar]
- Bouzaffour M, Rampon C, Ramaugé M, Courtin F, Vriz S. Implication of type 3 deiodinase induction in zebrafish fin regeneration. General and Comparative Endocrinology. 2010;168:88–94. doi: 10.1016/j.ygcen.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Britz R, Conway KW, Rüber L. Spectacular morphological novelty in a miniature cyprinid fish, Danionella dracula n. sp. Proceedings of the Royal Society B: Biological Sciences. 2009;276:2179. doi: 10.1098/rspb.2009.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CL. Thyroid hormones in early development, with special reference to teleost fishes. In: Schreibman MP, Scanes CG, editors. Hormones in development, maturation and senescence of neuroendocrine systems. San Diego: Academic Press; 1989. pp. 289–306. [Google Scholar]
- Brown DD. The role of thyroid hormone in zebrafish and axolotl development. Proceedings of the National Academy of Sciences of United States of America. 1997;94:13011–13016. doi: 10.1073/pnas.94.24.13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budi EH, Patterson LB, Parichy DM. Embryonic requirements for ErbB signaling in neural crest development and adult pigment pattern formation. Development (Cambridge, England) 2008;135:2603–2614. doi: 10.1242/dev.019299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budi EH, Patterson LB, Parichy DM. Post-embryonic nerve-associated precursors to adult pigment cells: Genetic requirements and dynamics of morphogenesis and differentiation. PLoS Genetics. 2011;7:e1002044. doi: 10.1371/journal.pgen.1002044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campinho MA, Galay-Burgos M, Sweeney GE, Power DM. Coordination of deiodinase and thyroid hormone receptor expression during the larval to juvenile transition in sea bream (Sparus aurata, Linnaeus) General and Comparative Endocrinology. 2010;165:181–194. doi: 10.1016/j.ygcen.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Campinho MA, Silva N, Sweeney GE, Power DM. Molecular, cellular and histological changes in skin from a larval to an adult phenotype during bony fish metamorphosis. Cell and Tissue Research. 2007;327:267–284. doi: 10.1007/s00441-006-0262-9. [DOI] [PubMed] [Google Scholar]
- Chang WJ, Hwang PP. Development of zebrafish epidermis. Birth Defects Research. Part C, Embryo Today: Reviews. 2011;93:205–214. doi: 10.1002/bdrc.20215. [DOI] [PubMed] [Google Scholar]
- Chang J, Wang M, Gui W, Zhao Y, Yu L, Zhu G. Changes in thyroid hormone levels during zebrafish development. Zoological Science. 2012;29:181–184. doi: 10.2108/zsj.29.181. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhang Q, Qi J, Wang Z, Wang X, Sun Y, et al. Cloning and stage-specific expression of CK-M1 gene during metamorphosis of Japanese flounder, Para-lichthys olivaceus. Chinese Journal of Oceanology and Limnology. 2010;28:558–564. [Google Scholar]
- Cieri M, McCleave J. Discrepancies between otoliths of larvae and juveniles of the American eel: Is something fishy happening at metamorphosis? Journal of Fish Biology. 2000;57:1189–1198. [Google Scholar]
- Crane HM, Pickford DB, Hutchinson TH, Brown JA. Developmental changes of thyroid hormones in the fathead minnow, Pimephales promelas. General and Comparative Endocrinology. 2004;139:55–60. doi: 10.1016/j.ygcen.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Cubbage CC, Mabee PM. Development of the cranium and paired fins in the zebrafish Danio rerio (Ostariophysi, Cyprinidae) Journal of Morphology. 1996;229:121–160. doi: 10.1002/(SICI)1097-4687(199608)229:2<121::AID-JMOR1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- D’Cotta H, Valotaire C, le Gac F, Prunet P. Synthesis of gill Na+-K+-ATPase in Atlantic salmon smolts: Differences in α-mRNA and α-protein levels. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2000;278:R101–R110. doi: 10.1152/ajpregu.2000.278.1.R101. [DOI] [PubMed] [Google Scholar]
- Deane EE, Woo N. Ontogeny of thyroid hormones, cortisol, hsp70 and hsp90 during silver sea bream larval development. Life Sciences. 2003;72:805–818. doi: 10.1016/s0024-3205(02)02334-2. [DOI] [PubMed] [Google Scholar]
- Dêbowski P, Glogowski J, Robak S, Dobosz S. Smoltification of hatchery-reared Atlantic salmon (Salmo salar L.)—Indices and methods of estimation. Archives of Polish Fisheries/Archiwum Rybactwa Polskiego. 1999;7:267–279. [Google Scholar]
- de Jesus EG, Hirano T, Inui Y. Changes in cortisol and thyroid hormone concentrations during early development and metamorphosis in the Japanese flounder, Paralichthys olivaceus. General and Comparative Endocrinology. 1991;82:369–376. doi: 10.1016/0016-6480(91)90312-t. [DOI] [PubMed] [Google Scholar]
- de Jesus EG, Hirano T, Inui Y. Flounder metamorphosis: Its regulation by various hormones. Fish Physiology and Biochemistry. 1993;11:323–328. doi: 10.1007/BF00004581. [DOI] [PubMed] [Google Scholar]
- de Jesus EG, Inui Y, Hirano T. Cortisol enhances the stimulating action of thyroid hormones on dorsal fin-ray resorption of flounder larvae in vitro. General and Comparative Endocrinology. 1990;79:167–173. doi: 10.1016/0016-6480(90)90101-q. [DOI] [PubMed] [Google Scholar]
- de Jesus EGT, Toledo JD, Simpas MS. Thyroid hormones promote early metamorphosis in grouper (Epinephelus coioides) larvae. General and Comparative Endocrinology. 1998;112:10–16. doi: 10.1006/gcen.1998.7103. [DOI] [PubMed] [Google Scholar]
- De Silva S, Gunasekera R, Gooley G, Ingram B. Growth of Australian shortfin eel (Anguilla australis) elvers given different dietary protein and lipid levels. Aquaculture Nutrition. 2001;7:53–57. [Google Scholar]
- Denver RJ. Acceleration of anuran amphibian metamorphosis by corticotropin-releasing hormone-like peptides. General and Comparative Endocrinology. 1993;91:38–51. doi: 10.1006/gcen.1993.1102. [DOI] [PubMed] [Google Scholar]
- Denver RJ. Environmental stress as a developmental cue: Corticotropin-releasing hormone is a proximate mediator of adaptive phenotypic plasticity in amphibian metamorphosis. Hormones and Behavior. 1997;31:169–179. doi: 10.1006/hbeh.1997.1383. [DOI] [PubMed] [Google Scholar]
- Dickhoff WW, Brown CL, Sullivan CV, Bern HA. Fish and amphibian models for developmental endocrinology. The Journal of Experimental Zoology. 1990;256:90–97. [Google Scholar]
- Dickhoff WW, Folmar LC, Gorbman A. Changes in plasma thyroxine during smoltification of coho salmon, Oncorhynchus kisutch. General and Comparative Endocrinology. 1978;36:229–232. doi: 10.1016/0016-6480(78)90027-8. [DOI] [PubMed] [Google Scholar]
- Dickhoff WW, Folmar LC, Mighell JL, Mahnken CVW. Plasma thyroid hormones during smoltification of yearling and underyearling coho salmon and yearling chinook salmon and steelhead trout. Aquaculture. 1982;28:39–48. [Google Scholar]
- Donaldson E, Fagerlund U, Higgs D, McBride J. Hormonal enhancement of growth. In: Hoar WS, Randall DJ, Brett RJ, editors. Fish physiology. Vol. 8. New York: Academic Press; 1979. [Google Scholar]
- Dufour S, Rousseau K. Neuroendocrinology of fish metamorphosis and puberty: Evolutionary and ecophysiological perspectives. Journal of Marine Science and Technology. 2007;68:55–68. [Google Scholar]
- Elizondo MR, Arduini BL, Paulsen J, MacDonald EL, Sabel JL, Henion PD, et al. Defective skeletogenesis with kidney stone formation in dwarf zebrafish mutant for trpm7. Current Biology. 2005;15:667–671. doi: 10.1016/j.cub.2005.02.050. [DOI] [PubMed] [Google Scholar]
- Eom DS, Inoue S, Patterson LB, Gordon TN, Slingwine R, Kondo S, et al. Melanophore migration and survival during zebrafish adult pigment stripe development require the immunoglobulin superfamily adhesion molecule Igsf11. PLoS Genetics. 2012;8:e1002899. doi: 10.1371/journal.pgen.1002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Díaz C, Y’yfera M, Cañavate J, Moyano F, Alarcón F, Díaz M. Growth and physiological changes during metamorphosis of Senegal sole reared in the laboratory. Journal of Fish Biology. 2001;58:1086–1097. [Google Scholar]
- Fukuhara O. Size and age at transformation in red sea bream, Pagrus major, reared in the laboratory. Aquaculture. 1991;95:117–124. [Google Scholar]
- Fukuhara O, Fushimi T. Fin differentiation and squamation of artificially reared grouper, Epinephelus akaara. Aquaculture. 1988;69:379–386. [Google Scholar]
- Galay-Burgos M, Power DM, Llewellyn L, Sweeney GE. Thyroid hormone receptor expression during metamorphosis of Atlantic halibut (Hippoglossus hippoglossus) Molecular and Cellular Endocrinology. 2008;281:56–63. doi: 10.1016/j.mce.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Goldsmith M, Iovine M, O’Reilly-Pol T, Johnson S. A developmental transition in growth control during zebrafish caudal fin development. Developmental Biology. 2006;296:450–457. doi: 10.1016/j.ydbio.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Grau EG, Specker JL, Nishioka RS, Bern HA. Factors determining the occurrence of the surge in thyroid activity in salmon during smoltification. Aquaculture. 1982;28:49–57. [Google Scholar]
- Harada M, Yoshinaga T, Ojima D, Iwata M. cDNA cloning and expression analysis of thyroid hormone receptor in the coho salmon Oncorhynchus kisutch during smoltification. General and Comparative Endocrinology. 2008;155:658–667. doi: 10.1016/j.ygcen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Harris M. Comparative genetics of postembryonic development as a means to understand evolutionary change. Journal of Applied Ichthyology. 2012;28:306–315. [Google Scholar]
- Harris MP, Rohner N, Schwarz H, Perathoner S, Konstantinidis P, Nüsslein-Volhard C. Zebrafish eda and edar mutants reveal conserved and ancestral roles of ectodysplasin signaling in vertebrates. PLoS Genetics. 2008;4:e1000206. doi: 10.1371/journal.pgen.1000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H, Mizuta A, Okada N, Suzuki T, Tagawa M, Tabata K, et al. Isolation and characterization of a Japanese flounder clonal line, reversed, which exhibits reversal of metamorphic left-right asymmetry. Mechanisms of Development. 2002;111:17–24. doi: 10.1016/s0925-4773(01)00596-2. [DOI] [PubMed] [Google Scholar]
- Hawkes JW. The structure of fish skin. Cell and Tissue Research. 1974;149:159–172. doi: 10.1007/BF00222271. [DOI] [PubMed] [Google Scholar]
- Hildahl J, Power DM, Björnsson BT, Einarsdóttir IE. Involvement of growth hormone-insulin-like growth factor I system in cranial remodeling during halibut metamorphosis as indicated by tissue-and stage-specific receptor gene expression and the presence of growth hormone receptor protein. Cell and Tissue Research. 2008;332:211–225. doi: 10.1007/s00441-007-0568-2. [DOI] [PubMed] [Google Scholar]
- Hildahl J, Sweeney G, Galay-Burgos M, Einarsdóttir IE, Björnsson BT. Cloning of Atlantic halibut growth hormone receptor genes and quantitative gene expression during metamorphosis. General and Comparative Endocrinology. 2007;151:143–152. doi: 10.1016/j.ygcen.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Hoath SB, Maibach HI. Neonatal skin: Structure and function. New York, NY: CRC Press; 2003. [Google Scholar]
- Hoegg S, Brinkmann H, Taylor JS, Meyer A. Phylogenetic timing of the fish-specific genome duplication correlates with the diversification of teleost fish. Journal of Molecular Evolution. 2004;59:190–203. doi: 10.1007/s00239-004-2613-z. [DOI] [PubMed] [Google Scholar]
- Hultman KA, Budi EH, Teasley DC, Gottlieb AY, Parichy DM, Johnson SL. Defects in ErbB-dependent establishment of adult melanocyte stem cells reveal independent origins for embryonic and regeneration melanocytes. PLoS Genetics. 2009;5:e1000544. doi: 10.1371/journal.pgen.1000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain NA, Higuchi M. Larval rearing and development of the brown spotted grouper, Epinephelus tauvina (Forska °l) Aquaculture. 1980;19:339–350. [Google Scholar]
- Infante C, Asensio E, Cañavate JP, Manchado M. Molecular characterization and expression analysis of five different elongation factor 1 alpha genes in the flatfish Senegalese sole (Solea senegalensis Kaup): Differential gene expression and thyroid hormones dependence during metamorphosis. BMC Molecular Biology. 2008;9:19. doi: 10.1186/1471-2199-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante C, Manchado M, Asensio E, Cañavate JP. Molecular characterization, gene expression and dependence on thyroid hormones of two type I keratin genes (sseKer1 and sseKer2) in the flatfish Senegalese sole (Solea senegalensis Kaup) BMC Developmental Biology. 2007;7:118. doi: 10.1186/1471-213X-7-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui Y, Miwa S. Thyroid hormone induces metamorphosis of flounder larvae. General and Comparative Endocrinology. 1985;60:450–454. doi: 10.1016/0016-6480(85)90080-2. [DOI] [PubMed] [Google Scholar]
- Inui Y, Tagawa M, Miwa S, Hirano T. Effects of bovine TSH on the tissue thyroxine level and metamorphosis in prometamorphic flounder larvae. General and Comparative Endocrinology. 1989;74:406–410. doi: 10.1016/s0016-6480(89)80038-3. [DOI] [PubMed] [Google Scholar]
- Iovine MK. Conserved mechanisms regulate outgrowth in zebrafish fins. Nature Chemical Biology. 2007;3:613–618. doi: 10.1038/nchembio.2007.36. [DOI] [PubMed] [Google Scholar]
- Itoh K, Watanabe K, Wu X, Suzuki T. Three members of the iodothyronine deiodinase family, dio1, dio2 and dio3, are expressed in spatially and temporally specific patterns during metamorphosis of the flounder, Paralichthys olivaceus. Zoological Science. 2010;27:574–580. doi: 10.2108/zsj.27.574. [DOI] [PubMed] [Google Scholar]
- Iwamatsu T. Stages of normal development in the medaka Oryzias latipes. Zoological Science. 1994;11:825–839. doi: 10.1016/j.mod.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Iwashita M, Watanabe M, Ishii M, Chen T, Johnson SL, Kurachi Y, et al. Pigment pattern in jaguar/obelix zebrafish is caused by a Kir7.1 mutation: Implications for the regulation of melanosome movement. PLoS Genetics. 2006;2:e197. doi: 10.1371/journal.pgen.0020197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegstrup I, Rosenkilde P. Regulation of post-larval development in the European eel: Thyroid hormone level, progress of pigmentation and changes in behaviour. Journal of Fish Biology. 2003;63:168–175. [Google Scholar]
- Johnson SL, Africa D, Walker C, Weston JA. Genetic control of adult pigment stripe development in zebrafish. Developmental Biology. 1995;167:27–33. doi: 10.1006/dbio.1995.1004. [DOI] [PubMed] [Google Scholar]
- Johnston C, Saunders R. Parr-smolt transformation of yearling Atlantic salmon (Salmo salar) at several rearing temperatures. Canadian Journal of Fisheries and Aquatic Sciences. 1981;38:1189–1198. [Google Scholar]
- Just JJ, Kraus-Just J, Check DA. Survey of chordate metamorphosis. In: Gilbert LI, Frieden E, editors. Metamorphosis: A problem in developmental biology. Vol. 2. New York: Plenum; 1981. pp. 265–326. [Google Scholar]
- Kaji T, Tanaka M, Oka M, Takeuchi H, Ohsumi S, Teruya K, et al. Growth and morphological development of laboratory-reared yellowfin tuna Thunnus albacares larvae and early juveniles, with special emphasis on the digestive system. Fisheries Science. 1999;65:700–707. [Google Scholar]
- Kaji T, Tanaka M, Takahashi Y, Oka M, Ishibashi N. Preliminary observations on development of Pacific bluefin tuna Thunnus thynnus (Scombridae) larvae reared in the laboratory, with special reference to the digestive system. Marine and Freshwater Research. 1996;47:261–269. [Google Scholar]
- Kawakami Y, Nozaki J, Seoka M, Kumai H, Ohta H. Characterization of thyroid hormones and thyroid hormone receptors during the early development of Pacific bluefin tuna (Thunnus orientalis) General and Comparative Endocrinology. 2008;155:597–606. doi: 10.1016/j.ygcen.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Tanda M, Adachi S, Yamauchi K. Characterization of thyroid hormone receptor α and β in the metamorphosing Japanese conger eel, Conger myriaster. General and Comparative Endocrinology. 2003;132:321–332. doi: 10.1016/s0016-6480(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Yokoi K, Kumai H, Ohta H. The role of thyroid hormones during the development of eye pigmentation in the Pacific bluefin tuna (Thunnus orientalis) Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology. 2008;150:112–116. doi: 10.1016/j.cbpb.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Keefe M, Able K. Patterns of metamorphosis in summer flounder, Paralichthys dentatus. Journal of Fish Biology. 1993;42:713–728. [Google Scholar]
- Keith P, Hoareau T, Lord C, Ah-Yane O, Gimonneau G, Robinet T, et al. Characterisation of post-larval to juvenile stages, metamorphosis and recruitment of an amphidromous goby, Sicyopterus lagocephalus (Pallas) (Teleostei: Gobiidae: Sicydiinae) Marine and Freshwater Research. 2008;59:876–889. [Google Scholar]
- Kelsh RN. Genetics and evolution of pigment patterns in fish. Pigment Cell Research. 2004;17:326–336. doi: 10.1111/j.1600-0749.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- Kelsh RN, Inoue C, Momoi A, Kondoh H, Furutani-Seiki M, Ozato K, et al. The Tomita collection of medaka pigmentation mutants as a resource for understanding neural crest cell development. Mechanisms of Development. 2004;121:841–859. doi: 10.1016/j.mod.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Kitajima C, Sato T, Kawanishi M. On the effect of thyroxine to promote the metamorphosis of a conger eel-preliminary report. Bulletin of the Japanese Society of Scientific Fisheries. 1967;33:919–922. [Google Scholar]
- Kitano J, Lema SC, Luckenbach JA, Mori S, Kawagishi Y, Kusakabe M, et al. Adaptive divergence in the thyroid hormone signaling pathway in the stickleback radiation. Current Biology. 2010;20:2124–2130. doi: 10.1016/j.cub.2010.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaren PHM, Wunderink YS, Yúfera M, Mancera JM, Flik G. The thyroid gland and thyroid hormones in Senegalese sole (Solea senegalensis) during early development and metamorphosis. General and Comparative Endocrinology. 2008;155:686–694. doi: 10.1016/j.ygcen.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Laforest L, Brown CW, Poleo G, Geraudie J, Tada M, Ekker M, et al. Involvement of the sonic hedgehog, patched 1 and bmp2 genes in patterning of the zebrafish dermal fin rays. Development (Cambridge, England) 1998;125:4175–4184. doi: 10.1242/dev.125.21.4175. [DOI] [PubMed] [Google Scholar]
- Lam T, Sharma R. Effects of salinity and thyroxine on larval survival, growth and development in the carp, Cyprinus carpio. Aquaculture. 1985;44:201–212. [Google Scholar]
- Lang MR, Patterson LB, Gordon TN, Johnson SL, Parichy DM. Basonuclin-2 requirements for zebrafish adult pigment pattern development and female fertility. PLoS Genetics. 2009;5:e1000744. doi: 10.1371/journal.pgen.1000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson TA, Gordon TN, Lau HE, Parichy DM. Defective adult oligodendrocyte and Schwann cell development, pigment pattern, and craniofacial morphology in puma mutant zebrafish having an alpha tubulin mutation. Developmental Biology. 2010;346:296–309. doi: 10.1016/j.ydbio.2010.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudet V. The origins and evolution of vertebrate metamorphosis. Current Biology. 2011;21:R726–R737. doi: 10.1016/j.cub.2011.07.030. [DOI] [PubMed] [Google Scholar]
- Le Guellec D, Morvan-Dubois G, Sire JY. Skin development in bony fish with particular emphasis on collagen deposition in the dermis of the zebrafish (Danio rerio) International Journal of Developmental Biology. 2004;48:217–232. doi: 10.1387/ijdb.15272388. [DOI] [PubMed] [Google Scholar]
- Ledent V. Postembryonic development of the posterior lateral line in zebrafish. Development (Cambridge, England) 2002;129:597–604. doi: 10.1242/dev.129.3.597. [DOI] [PubMed] [Google Scholar]
- Leis JM, McCormick MI. The biology, behavior and ecology of the pelagic, larval stage of coral reef fishes. In: Sale PF, editor. Coral reef fishes: Dynamics and diversity in a complex ecosystem. Burlington, MA: Elsevier, Inc; 2006. pp. 171–200. [Google Scholar]
- Lin RJ, Cross TF, Mills CPR, Nishioka RS, Grau EG, Bern HA. Changes in plasma thyroxine levels during smoltification in hatchery-reared one-year and two-year Atlantic salmon (Salmo salar) Aquaculture. 1988;74:369–378. [Google Scholar]
- Madsen SS, Bern HA. Antagonism of prolactin and growth hormone—impact on seawater adaptation in two salmonids, Salmotrutta and Oncorhynchus mykiss. Zoological Science. 1992;9:775–784. [Google Scholar]
- Manchado M, Infante C, Asensio E, Crespo A, Zuasti E, Cañavate JP. Molecular characterization and gene expression of six trypsinogens in the flatfish Senegalese sole (Solea senegalensis Kaup) during larval development and in tissues. Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology. 2008;149:334–344. doi: 10.1016/j.cbpb.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Manchado M, Infante C, Asensio E, Planas JV, Cañavate JP. Thyroid hormones down-regulate thyrotropin β subunit and thyroglobulin during metamorphosis in the flatfish Senegalese sole (Solea senegalensis Kaup) General and Comparative Endocrinology. 2008;155:447–455. doi: 10.1016/j.ygcen.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Manchado M, Infante C, Rebordinos L, Cañavate JP. Molecular characterization, gene expression and transcriptional regulation of thyroid hormone receptors in Senegalese sole. General and Comparative Endocrinology. 2009;160:139–147. doi: 10.1016/j.ygcen.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Manchado M, Salas-Leiton E, Infante C, Ponce M, Asensio E, Crespo A, et al. Molecular characterization, gene expression and transcriptional regulation of cytosolic HSP90 genes in the flatfish Senegalese sole (Solea senegalensis Kaup) Gene. 2008;416:77–84. doi: 10.1016/j.gene.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Manzon RG. Thyroidal regulation of life history transitions in fish. In: Flatt T, Heyland A, editors. Mechanisms of life history evolution: The genetics and physiology of life history traits and trade-offs. Oxford: OUP; 2011. pp. 72–86. [Google Scholar]
- Marchand O, Duffraisse M, Triqueneaux G, Safi R, Laudet V. Molecular cloning and developmental expression patterns of thyroid hormone receptors and T3 target genes in the turbot (Scophtalmus maximus) during post-embryonic development. General and Comparative Endocrinology. 2004;135:345–357. doi: 10.1016/j.ygcen.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Marí-Beffa M, Murciano C. Dermoskeleton morphogenesis in zebrafish fins. Developmental Dynamics. 2010;239:2779–2794. doi: 10.1002/dvdy.22444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez GM, Bolker JA. Embryonic and larval staging of summer flounder (Paralichthys dentatus) Journal of Morphology. 2003;255:162–176. doi: 10.1002/jmor.10053. [DOI] [PubMed] [Google Scholar]
- Martínez-Lagos R, Gracia-López V. Morphological development and growth patterns of the leopard grouper Mycteroperca rosacea during larval development. Aquaculture Research. 2009;41:120–128. [Google Scholar]
- Matsumoto J, Lynch TJ, Grabowski S, Richards CM, Lo SL, Clark C, et al. Fish tumor pigment cells: Differentiation and comparison to their normal counterparts. American Zoologist. 1983;23:569–580. [Google Scholar]
- Matsumoto S, Tanaka M. The influence of thyroid hormone on development and settlement of spottybelly greenling (Hexagrammos agrammus; Pisces) Marine and Freshwater Behaviour and Physiology. 1996;28:33–44. [Google Scholar]
- Mayden RL, Chen WJ. The world’s smallest vertebrate species of the genus Paedocypris: A new family of freshwater fishes and the sister group to the world’s most diverse clade of freshwater fishes (Teleostei: Cypriniformes) Molecular Phylogenetics and Evolution. 2010;57:152–175. doi: 10.1016/j.ympev.2010.04.008. [DOI] [PubMed] [Google Scholar]
- McCormick M, Makey L. Post-settlement transition in coral reef fishes: Overlooked complexity in niche shifts. Marine Ecology Progress Series. 1997;153:247–257. [Google Scholar]
- McCormick M, Makey L, Dufour V. Comparative study of metamorphosis in tropical reef fishes. Marine Biology. 2002;141:841–853. [Google Scholar]
- McCormick SD, Moriyama S, Björnsson BT. Low temperature limits photoperiod control of smolting in Atlantic salmon through endocrine mechanisms. The American Journal of Physiology. 2000;278:R1352–R1361. doi: 10.1152/ajpregu.2000.278.5.R1352. [DOI] [PubMed] [Google Scholar]
- McCormick SD, Saunders RL. Preparatory physiological adaptations for marine life of salmonids: Osmoregulation, growth, and metabolism. American Fisheries Society Symposium. 1987;1:211–229. [Google Scholar]
- McCormick SD, Shrimpton JM, Moriyama S, Björnsson BT. Effects of an advanced temperature cycle on smolt development and endocrinology indicate that temperature is not a zeitgeber for smolting in Atlantic salmon. The Journal of Experimental Biology. 2002;205:3553–3560. doi: 10.1242/jeb.205.22.3553. [DOI] [PubMed] [Google Scholar]
- Miller CT, Beleza S, Pollen AA, Schluter D, Kittles RA, Shriver MD, et al. cis-Regulatory changes in Kit ligand expression and parallel evolution of pigmentation in sticklebacks and humans. Cell. 2007;131:1179–1189. doi: 10.1016/j.cell.2007.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa S, Inui Y. Thyroid hormone stimulates the shift of erythrocyte populations during metamorphosis of the flounder. The Journal of Experimental Zoology. 1991;259:222–228. [Google Scholar]
- Miwa S, Tagawa M, Inui Y, Hirano T. Thyroxine surge in metamorphosing flounder larvae. General and Comparative Endocrinology. 1988;70:158–163. doi: 10.1016/0016-6480(88)90105-0. [DOI] [PubMed] [Google Scholar]
- Miwa S, Yamano K, Inui Y. Thyroid hormone stimulates gastric development in flounder larvae during metamorphosis. The Journal of Experimental Zoology. 1992;261:424–430. [Google Scholar]
- Miyashita S, Sawada Y, Okada T, Murata O, Kumai H. Morphological development and growth of laboratory-reared larval and juvenile Thunnus thynnus (Pisces: Scombridae) Fishery Bulletin. 2001;99:601–616. [Google Scholar]
- Moser HG. Morphological and functional aspects of marine fish larvae. In: Lasker R, editor. Marine fish larvae, Washington Sea Grant Program. Seattle: University Washington Press; 1981. pp. 89–131. [Google Scholar]
- Nuñez VA, Sarrazin AF, Cubedo N, Allende ML, Dambly-Chaudiére C, Ghysen A. Postembryonic development of the posterior lateral line in the zebrafish. Evolution & Development. 2009;11:391–404. doi: 10.1111/j.1525-142X.2009.00346.x. [DOI] [PubMed] [Google Scholar]
- Odenthal J, Rossnagel K, Haffter P, Kelsh RN, Vogelsang E, Brand M, et al. Mutations affecting xanthophore pigmentation in the zebrafish, Danio rerio. Development (Cambridge, England) 1996;123:391–398. doi: 10.1242/dev.123.1.391. [DOI] [PubMed] [Google Scholar]
- Okimoto K, Weber M, Grau G. The effects of thyroxine and propylthiouracil treatment on changes in body form associated with a possible developmental thyroxine surge during post-hatching development of the tilapia, Oreochromis mossambicus. Zoological Science. 1993;10:803–811. [Google Scholar]
- Orozco A, Valverde-R C. Thyroid hormone deiodination in fish. Thyroid. 2005;15:799–813. doi: 10.1089/thy.2005.15.799. [DOI] [PubMed] [Google Scholar]
- Page RB, Monaghan JR, Samuels AK, Smith JJ, Beachy CK, Voss SR. Microarray analysis identifies keratin loci as sensitive biomarkers for thyroid hormone disruption in the salamander Ambystoma mexicanum. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 2007;145:15–27. doi: 10.1016/j.cbpc.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Parhar IS, Iwata M. Intracerebral expression of gonadotropin-releasing hormone and growth hormone-releasing hormone is delayed until smoltification in the salmon. Neuroscience Research. 1996;26:299–308. doi: 10.1016/s0168-0102(96)01108-x. [DOI] [PubMed] [Google Scholar]
- Parichy DM. Evolution of danio pigment pattern development. Heredity. 2006;97:200–210. doi: 10.1038/sj.hdy.6800867. [DOI] [PubMed] [Google Scholar]
- Parichy DM, Elizondo MR, Mills MG, Gordon TN, Engeszer RE. Normal table of postembryonic zebrafish development: Staging by externally visible anatomy of the living fish. Developmental Dynamics. 2009;238:2975–3015. doi: 10.1002/dvdy.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parichy DM, Rawls JF, Pratt SJ, Whitfield TT, Johnson SL. Zebrafish sparse corresponds to an orthologue of c-kit and is required for the morphogenesis of a subpopulation of melanocytes, but is not essential for hematopoiesis or primordial germ cell development. Development (Cambridge, England) 1999;126:3425–3436. doi: 10.1242/dev.126.15.3425. [DOI] [PubMed] [Google Scholar]
- Parichy DM, Turner JM. Temporal and cellular requirements for Fms signaling during zebrafish adult pigment pattern development. Development (Cambridge, England) 2003a;130:817–833. doi: 10.1242/dev.00307. [DOI] [PubMed] [Google Scholar]
- Parichy DM, Turner JM. Zebrafish puma mutant decouples pigment pattern and somatic metamorphosis. Developmental Biology. 2003b;256:242–257. doi: 10.1016/s0012-1606(03)00015-0. [DOI] [PubMed] [Google Scholar]
- Paris M, Escriva H, Schubert M, Brunet F, Brtko J, Ciesielski F, et al. Amphioxus postembryonic development reveals the homology of chordate metamorphosis. Current Biology. 2008;18:825–830. doi: 10.1016/j.cub.2008.04.078. [DOI] [PubMed] [Google Scholar]
- Paris M, Laudet V. The history of a developmental stage: Metamorphosis in chordates. Genesis (New York, NY : 2000) 2008;46:657–672. doi: 10.1002/dvg.20443. [DOI] [PubMed] [Google Scholar]
- Patterson SE, Mook LB, Devoto SH. Growth in the larval zebrafish pectoral fin and trunk musculature. Developmental Dynamics. 2008;237:307–315. doi: 10.1002/dvdy.21400. [DOI] [PubMed] [Google Scholar]
- Policansky D, Sieswerda P. Early life history of the starry flounder, Platichthys stellatus, reared through metamorphosis in the laboratory. Transactions of the American Fisheries Society. 1979;108:326–327. [Google Scholar]
- Porazzi P, Calebiro D, Benato F, Tiso N, Persani L. Thyroid gland development and function in the zebrafish model. Molecular and Cellular Endocrinology. 2009;312:14–23. doi: 10.1016/j.mce.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Power D, Silva N, Campinho MA. Metamorphosis. In: Finn RN, Kapoor BG, editors. Fish larval physiology. Enfield, New Hampshire: Science Publishers; 2008. pp. 607–638. [Google Scholar]
- Prunet P, Boeuf G, Bolton JP, Young G. Smoltification and seawater adaptation in Atlantic salmon (Salmo salar): Plasma prolactin, growth hormone, and thyroid hormones. General and Comparative Endocrinology. 1989;74:355–364. doi: 10.1016/s0016-6480(89)80031-0. [DOI] [PubMed] [Google Scholar]
- Quigley DTG, Harvey M, Hayden T, Dowling C, O’Keane M. A comparative study of smoltification in sea trout (Salmo trutta L.) and Atlantic salmon (Salmo salar L.): Seawater tolerance and thyroid hormone titres. Biology & Environment: Proceedings of the Royal Irish Academy. 2006;106:35–47. [Google Scholar]
- Quigley IK, Turner JM, Nuckels RJ, Manuel JL, Budi EH, MacDonald EL, et al. Pigment pattern evolution by differential deployment of neural crest and post-embryonic melanophore lineages in Danio fishes. Development (Cambridge, England) 2004;131:6053–6069. doi: 10.1242/dev.01526. [DOI] [PubMed] [Google Scholar]
- Raju SN. Distribution, growth and metamorphosis of leptocephali of the garden eels, Taenioconger sp. and Gorgasia sp. Copeia. 1974;2:494–500. [Google Scholar]
- Rakers S, Gebert M, Uppalapati S, Meyer W, Maderson P, Sell AF, et al. ‘Fish matters’: The relevance of fish skin biology to investigative dermatology. Experimental Dermatology. 2010;19:313–324. doi: 10.1111/j.1600-0625.2009.01059.x. [DOI] [PubMed] [Google Scholar]
- Ramanathan N, Natarajan P, Sukumran N. Studies on the induced spawning and larval rearing of a freshwater catfish, Mystus punctatus (Jerdon) Proceedings of the Indian Academy of Sciences. 1985;94:389–398. [Google Scholar]
- Ravi V, Venkatesh B. Rapidly evolving fish genomes and teleost diversity. Current Opinion in Genetics & Development. 2008;18:544–550. doi: 10.1016/j.gde.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Richman NH, de Diaz ST, Nishioka RS, Bern HA. Developmental study of coho gill functional morphology and the effects of cortisol. Aquaculture. 1985;45:386–387. [Google Scholar]
- Roberts RB, Ser JR, Kocher TD. Sexual conflict resolved by invasion of a novel sex determiner in Lake Malawi cichlid fishes. Science. 2009;326:998–1001. doi: 10.1126/science.1174705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson G, McGee C, Dumbarton T, Croll R, Smith F. Development of the swimbladder and its innervation in the zebrafish, Danio rerio. Journal of Morphology. 2007;268:967–985. doi: 10.1002/jmor.10558. [DOI] [PubMed] [Google Scholar]
- Rousseau K, Aroua S, Schmitz M, Elie P, Dufour S. Silvering: Metamorphosis or puberty. In: van den Thillart G, Dufour S, Rankin JC, editors. Spawning migration of the European Eel. Amsterdam: Springer; 2009. pp. 39–63. [Google Scholar]
- Rüber L, Kottelat M, Tan H, Ng P, Britz R. Evolution of miniaturization and the phylogenetic position of Paedocypris, comprising the world’s smallest vertebrate. BMC Evolutionary Biology. 2007;7:38. doi: 10.1186/1471-2148-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan AK, Blumberg B, Rodriguez-Esteban C, Yonei-Tamura S, Tamura K, Tsukui T, et al. Pitx2 determines left-right asymmetry of internal organs in vertebrates. Nature. 1998;394:545–551. doi: 10.1038/29004. [DOI] [PubMed] [Google Scholar]
- Sæle Ø, Solbakken J, Watanabe K, Hamre K, Power D, Pittman K. Staging of Atlantic halibut (Hippoglossus hippoglossus L.) from first feeding through metamorphosis, including cranial ossification independent of eye migration. Aquaculture. 2004;239:445–465. [Google Scholar]
- Sarropoulou E, Nousdili D, Magoulas A, Kotoulas G. Linking the genomes of nonmodel teleosts through comparative genomics. Marine Biotechnology. 2008;10:227–233. doi: 10.1007/s10126-007-9066-5. [DOI] [PubMed] [Google Scholar]
- Saunders R, McCormick S, Henderson E, Eales J, Johnston C. The effect of orally administered 3, 5, 3′-triiodo-L-thyronine on growth and salinity tolerance of atlantic salmon (Salmo salar L.) Aquaculture. 1985;45:143–156. [Google Scholar]
- Schreiber AM. Metamorphosis and early larval development of the flatfishes (Pleuronectiformes): An osmoregulatory perspective. Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology. 2001;129:587–595. doi: 10.1016/s1096-4959(01)00346-3. [DOI] [PubMed] [Google Scholar]
- Schreiber AM, Specker JL. Metamorphosis in the summer flounder (Paralichthys dentatus): Stage-specific developmental response to altered thyroid status. General and Comparative Endocrinology. 1998;111:156–166. doi: 10.1006/gcen.1998.7095. [DOI] [PubMed] [Google Scholar]
- Seear PJ, Carmichael SN, Talbot R, Taggart JB, Bron JE, Sweeney GE. Differential gene expression during smoltification of Atlantic salmon (Salmo salar L.): A first large-scale microarray study. Marine Biotechnology. 2010;12:126–140. doi: 10.1007/s10126-009-9218-x. [DOI] [PubMed] [Google Scholar]
- Shiao JC, Wu SM, Hwang YP, Wu DP, Hwang PP. Evaluation of thyroid-mediated otolith growth of larval and juvenile tilapia. The Journal of Experimental Biology. 2008;211:1919–1926. doi: 10.1242/jeb.013748. [DOI] [PubMed] [Google Scholar]
- Sims K, Eble DM, Iovine MK. Connexin43 regulates joint location in zebrafish fins. Developmental Biology. 2009;327:410–418. doi: 10.1016/j.ydbio.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sire JY, Akimenko MA. Scale development in fish: A review, with description of sonic hedgehog (shh) expression in the zebrafish (Danio rerio) The International Journal of Developmental Biology. 2004;48:233–248. doi: 10.1387/ijdb.15272389. [DOI] [PubMed] [Google Scholar]
- Sire JY, Géraudie J. Fine structure of the developing scale in the cichlid Hemichromis bimaculatus (Pisces, Teleostei, Perciformes) Acta Zoologica. 1983;64:1–8. [Google Scholar]
- Solbakken JS, Norberg B, Watanabe K, Pittman K. Thyroxine as a mediator of metamorphosis of Atlantic halibut, Hippoglossus hippoglossus. Environmental Biology of Fishes. 1999;56:53–65. [Google Scholar]
- Specker JL. Interrenal function and smoltification. Aquaculture. 1982;28:59–66. [Google Scholar]
- Specker JL, Schreck CB. Changes in plasma corticosteroids during smoltification of coho salmon, Oncorhynchus kisutch. General and Comparative Endocrinology. 1982;46:53–58. doi: 10.1016/0016-6480(82)90162-9. [DOI] [PubMed] [Google Scholar]
- Stefansson SO, Björnsson BT, Ebbesson LO, McCormick SD. Smoltification. In: Finn RN, Kapoor BG, editors. Fish larval physiology. Enfield, New Hampshire: Science Publishers; 2008. [Google Scholar]
- Sullivan CV, Dickhoff WW, Mahnken CVW, Hersbberger WK. Changes in the hemoglobin system of the coho salmon Oncorhynchus kisutch during smoltification and triiodothyronine and propylthiouracil treatment. Comparative Biochemistry and Physiology. Part A, Physiology. 1985;81:807–813. doi: 10.1016/0300-9629(85)90911-9. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Washio Y, Aritaki M, Fujinami Y, Shimizu D, Uji S, et al. Metamorphic pitx2 expression in the left habenula correlated with lateralization of eye-sidedness in flounder. Development, Growth & Differentiation. 2009;51:797–808. doi: 10.1111/j.1440-169X.2009.01139.x. [DOI] [PubMed] [Google Scholar]
- Sweeting R, McKeown B. Changes in plasma growth hormone and various metabolic factors during smoltification of coho salmon, Oncorhynchus kisutch. Aquaculture. 1989;82:279–295. [Google Scholar]
- Szisch V, Papandroulakis N, Fanouraki E, Pavlidis M. Ontogeny of the thyroid hormones and cortisol in the gilthead sea bream, Sparus aurata. General and Comparative Endocrinology. 2005;142:186–192. doi: 10.1016/j.ygcen.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Tagawa M, Aritaki M. Production of symmetrical flatfish by controlling the timing of thyroid hormone treatment in spotted halibut Verasper variegatus. General and Comparative Endocrinology. 2005;141:184–189. doi: 10.1016/j.ygcen.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Taillebois L, Keith P, Valade P, Torres P, Baloche S, Dufour S, et al. Involvement of thyroid hormones in the control of larval metamorphosis in Sicyopterus lagocephalus (Teleostei: Gobioidei) at the time of river recruitment. General and Comparative Endocrinology. 2011;173:281–288. doi: 10.1016/j.ygcen.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Kawai S, Seikai T, Burke J. Development of the digestive organ system in Japanese flounder in relation to metamorphosis and settlement. Marine & Freshwater Behaviour & Physiology. 1996;28:19–31. [Google Scholar]
- Tanaka Y, Satoh K, Iwahashi M, Yamada H. Growth-dependent recruitment of Pacific bluefin tuna Thunnus orientalis in the northwestern Pacific Ocean. Marine Ecology Progress Series. 2006;319:225–235. [Google Scholar]
- Thorson G. Reproductive and larval ecology of marine bottom invertebrates. Biological Reviews. 1950;25:1–45. doi: 10.1111/j.1469-185x.1950.tb00585.x. [DOI] [PubMed] [Google Scholar]
- Tipsmark CK, Sørensen KJ, Madsen S. Aquaporin expression dynamics in osmoregulatory tissues of Atlantic salmon during smoltification and seawater acclimation. The Journal of Experimental Biology. 2010;213:368–379. doi: 10.1242/jeb.034785. [DOI] [PubMed] [Google Scholar]
- Trijundo DD, Yoseda K, Hirokawa J, Tagawa M, Tanaka M. Effects of thyroxine and thiourea on the metamorphosis of coral trout grouper Plectropomus leopardus. Fisheries Science. 2002;68:282–289. [Google Scholar]
- Tsukamoto K, Yamada Y, Okamura A, Kaneko T, Tanaka H, Miller MJ, et al. Positive buoyancy in eel leptocephali: An adaptation for life in the ocean surface layer. Marine Biology. 2009;156:835–846. [Google Scholar]
- Virtanen E. Correlations between energy metabolism, osmotic balance and external smolt indices in smolting young salmon, Salmo salar L. Annales Zoologici Fennici. 1987;24:71–78. [Google Scholar]
- Virtanen E, Soivio A. The patterns of T3, T4, cortisol and Na +/K+-ATPase during smoltification of hatchery-reared Salmo salar and comparison with wild smolts. Aquaculture. 1985;45:97–109. [Google Scholar]
- Volff JN. Genome evolution and biodiversity in teleost fish. Heredity. 2005;94:280–294. doi: 10.1038/sj.hdy.6800635. [DOI] [PubMed] [Google Scholar]
- Wang C, Tzeng W. The timing of metamorphosis and growth rates of American and European eel leptocephali: A mechanism of larval segregative migration. Fisheries Research. 2000;46:191–205. [Google Scholar]
- Watanabe M, Iwashita M, Ishii M, Kurachi Y, Kawakami A, Kondo S, et al. Spot pattern of leopard Danio is caused by mutation in the zebrafish connexin41. 8 gene. EMBO Reports. 2006;7:893–897. doi: 10.1038/sj.embor.7400757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Washio Y, Fujinami Y, Aritaki M, Uji S, Suzuki T. Adult-type pigment cells, which color the ocular sides of flounders at metamorphosis, localize as precursor cells at the proximal parts of the dorsal and anal fins in early larvae. Development, Growth & Differentiation. 2008;50:731–741. doi: 10.1111/j.1440-169X.2008.01071.x. [DOI] [PubMed] [Google Scholar]
- Webb J. Larvae in fish development and evolution. In: Hall BK, Wake MH, editors. The origin and evolution of larval forms. San Diego: Academic Press; 1999. [Google Scholar]
- Webb J, Shirey J. Postembryonic development of the cranial lateral line canals and neuromasts in zebrafish. Developmental Dynamics. 2003;228:370–385. doi: 10.1002/dvdy.10385. [DOI] [PubMed] [Google Scholar]
- Winans GA, Nishioka RS. A multivariate description of change in body shape of coho salmon (Oncorhynchus kisutch) during smoltification. Aquaculture. 1987;66:235–245. [Google Scholar]
- Wittenrich ML, Baldwin CC, Turingen RG. Larval development of laboratory-reared Green Mandarinfish, Synchiropus splendidus (Teleostei: Callionymidae) aqua, International Journal of Ichthyology. 2010;16:7–18. [Google Scholar]
- Woo N, Bern H, Nishioka R. Changes in body composition associated with smoltification and premature transfer to seawater in coho salmon (Oncorhynchus kisutch) and king salmon (O. tschawytscha) Journal of Fish Biology. 1978;13:421–428. [Google Scholar]
- Yamada T, Okauchi M, Araki K. Origin of adult-type pigment cells forming the asymmetric pigment pattern, in Japanese flounder (Paralichthys olivaceus) Developmental Dynamics. 2010;239:3147–3162. doi: 10.1002/dvdy.22440. [DOI] [PubMed] [Google Scholar]
- Yamano K, Miwa S. Differential gene expression of thyroid hormone receptor α and β in fish development. General and Comparative Endocrinology. 1998;109:75–85. doi: 10.1006/gcen.1997.7011. [DOI] [PubMed] [Google Scholar]
- Yamano K, Miwa S, Obinata T, Inui Y. Thyroid hormone regulates developmental changes in muscle during flounder metamorphosis. General and Comparative Endocrinology. 1991;81:464–472. doi: 10.1016/0016-6480(91)90174-5. [DOI] [PubMed] [Google Scholar]
- Yamano K, Nomura K, Tanaka H. Development of thyroid gland and changes in thyroid hormone levels in Leptocephali of Japanese Eel (Anguilla japonica) Aquaculture. 2007;270:499–504. [Google Scholar]
- Yamano K, Tagawa M, Jesus EG, Hirano T, Miwa S, Inui Y. Changes in whole body concentrations of thyroid hormones and cortisol in metamorphosing conger eel. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 1991;161:371–375. [Google Scholar]
- Yen PM. Physiological and molecular basis of thyroid hormone action. Physiological Reviews. 2001;81:1097–1142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- Yoo JH, Takeuchi T, Tagawa M, Seikai T. Effect of thyroid hormones on the stage-specific pigmentation of the Japanese flounder Paralichthys olivaceus. Zoological Science. 2000;17:1101–1106. doi: 10.2108/zsj.17.1101. [DOI] [PubMed] [Google Scholar]
- Young G, Bjornsson BT, Prunet P, Lin RJ, Bern HA. Smoltification and seawater adaptation in coho salmon (Oncorhynchus kisutch): Plasma prolactin, growth hormone, thyroid hormones, and cortisol. General and Comparative Endocrinology. 1989;74:335–345. doi: 10.1016/s0016-6480(89)80029-2. [DOI] [PubMed] [Google Scholar]
- Youngson A, McLay H, Wright R, Johnstone R. Steroid hormone levels and patterns of growth in the early part of the reproductive cycle of adult Atlantic salmon (Salmo salar L.) Aquaculture. 1988;69:145–157. [Google Scholar]
- Yousefian M, Shirzad E. The review of the effect of growth hormone on immune system, metabolism and osmoregulation of fish. Australian Journal of Basic and Applied Sciences. 2011;5:467–475. [Google Scholar]
- Youson J. First metamorphosis. In: Hoar W, Randall D, editors. Fish physiology. 1lB. New York: Academic Press; 1988. pp. 125–196. [Google Scholar]
- Youson JH. Is lamprey metamorphosis regulated by thyroid hormones? American Zoologist. 1997;37:441–460. [Google Scholar]
- Youson JH. The impact of environmental and hormonal cues on the evolution of fish metamorphosis. In: Hall BK, Pearson RD, Müller GB, editors. Environment, development, and evolution: Toward a synthesis. Vol. 1. Cambridge: The MIT Press; 2004. pp. 239–278. [Google Scholar]