Abstract
Background
Gastric adenocarcinoma is an aggressive disease with frequent lymph node (LN) metastases for which lymphadenectomy results in a survival benefit. In the United States, the NCCN guidelines recommend D2 lymphadenectomy or a minimum of 15 LNs retrieved. However, retrieval of only 15 LNs is considered by most international guidelines as inadequate. We seek to evaluate the survival benefits associated with a more complete lymphadenectomy.
Study Design
An international database was constructed by combining gastric cancer cases from the SEER database (n=13,932) and the Yonsei University Gastric Cancer database (n=11,358)(total n=25,289). Kaplan-Meier survival analysis was performed along with Joinpoint analysis to obtain the optimal number of LNs to retrieve based upon survival. Prognostic significance of number of nodes retrieved was then confirmed with uni- and multivariate analyses.
Results
Analysis for both mean and median survival yielded 29 LNs removed as the Joinpoint. This was further confirmed with multivariate analysis, where 15 retrieved LNs cutoff fell out of the model while 29 retrieved LNs remained intact with an hazard ratio (HR): 0.799(95%CI 0.759–0.842, p<0.001). Stage-stratified Kaplan- Meier analysis for a cutoff point of 29 also demonstrated a statistically significant improvement in survival.
Conclusion
Joinpoint analysis has allowed for the creation of a model demonstrating the point at which additional dissection would not provide further benefit. Thus, this large international dataset analysis demonstrates that the maximal survival advantage is seen by performing a lymphadenectomy with a minimum of 29 LNs retrieved.
Introduction
Surgical resection remains the primary curative therapy for gastric adenocarcinoma (GC), which is an aggressive disease requiring multimodality treatment. While survival benefit of additional chemotherapy has been established (MAGIC, McDonald, CLASS, ACT trials) (1–4), the timing and extent of surgical treatment continues to be investigated. As one of the most important determinants of recurrence and long-term survival in patients undergoing radical gastrectomy is nodal metastases, management of nodal disease is a key component to ensuring the best patient outcome (5). Specifically, the proper extent of lymph node dissection and the specific number of nodes required for adequate staging has generated decades of discourse with variable worldwide practice (6, 7).
The prognostic importance of nodal positivity is reflected in the 7th edition of the TNM-Staging System of gastric cancer (8). Treatment planning is guided by predicted nodal metastases and prognosis guided by the number of pathologically positive lymph nodes and subsequent accurate staging of the disease. Moreover, D2 lymphadenectomy, which allows for clearance of the nodal stations likely harboring metastatic disease and increased number of nodes for evaluation, is now internationally accepted as the standard procedure with demonstrated decreased regional recurrence and improved long-term survival for patients undergoing curative surgery (9–13). Thus, many guidelines (Italian Gastric Cancer Study Group, the German Gastric Carcinoma Study Group, the Brazilian Gastric Cancer Association and the Chinese Gastric Cancer Association) support D2 lymphadenectomy as the optimum extent of lymph node dissection (14–16). While D2 lymph node dissection has long been advocated as the surgical standard for radical gastrectomy in Japan and South Korea by the Japanese Research Society for Gastric Cancer and Korean Gastric Cancer Association (17, 18), D2 lymphadenectomy in Western studies has until recently been associated with forbidding morbidity and mortality.
In the United States, the National Comprehensive Cancer Network (NCCN) Guidelines have adopted D2 lymphadenectomy as the standard surgical treatment, with an additional goal of removing a minimum of 15 lymph nodes (19). This recommendation was based on a study that identified 15 as the minimal number of lymph nodes retrieved that was associated with a survival benefit. This study along with several others, have demonstrated that there is a continued incremental increase in survival per stage up to 40 lymph nodes. At present, the oncologic quality of the lymph node dissection is measured by the removal and evaluation of at least 15 lymph nodes in order to meets the NCCN guidelines for proper staging according to the TNM-staging system. Of note, the expected lymph node count obtained by a D2 lymphadenectomy is generally significantly higher than 15. A study examining mean number of lymph nodes obtained during dissection of cadavers predicted an average of 31.1 lymph nodes would be retrieved during a D2 lymph node dissection for a total gastrectomy and 29.1 for a distal gastrectomy (20). Results of many clinical trials where data for D2 lymph node numbers are available indicate that an adequate nodal dissection for D2 would yield at least 33 to 47 lymph nodes (10, 21, 22).
Our study aimed to define the optimum number of lymph nodes needed to be retrieved during radical gastrectomy for maximum survival benefit using the largest combined international dataset created to date.
Methods
To develop an international dataset with a both Western and East Asian gastric cancer patients, data was obtained from the Surveillance, Epidemiology, and End Results (SEER) database (23) and combined with data from Yonsei University, Seoul, Korea. The following factors were obtained from the data: age, sex, ethnicity, histology, surgery performed, T-classification, N-classification, M-classification, stage, total number of lymph nodes examined, and total number of positive lymph nodes, vital status, and survival. The Yonsei University gastric cancer database is prospectively maintained and all information was pulled directly from the database after meticulous verification through internal quality control measures. All staging data within the database were updated and coded to confirm to the AJCC TNM 7th edition staging system.
Due to changes in coding, specifically AJCC TNM staging, only the years 2002 – 2012 were extracted for use from the SEER database. The SEER database was searched identifying ICD-0-3 site recode for “stomach” and then further narrowed down using the behavior code “malignant”, initially obtaining 57,237 potentially patients. ICD-0-3 histology/behavior codes were then used to identify only the cases of gastric adenocarcinoma, eliminating other gastric tumors (neuroendocrine, GIST, unknown, metastatic disease). We then obtained data from the following categories: “sex”, “age recode with single ages and 85+”, “grade”, “icd-o-3 hist/behav”, “derived ajcc t, 6th ed (2004+)”, “derived ajcc t, 7th ed (2010+)”, “derived ajcc m, 6th ed (2004+)”, “derived ajcc m, 7th ed (2010+)”, “rx sum—surg prim site (1998+)”, “regional nodes examined (1988+)”, “regional nodes positive (1988+)”, “vital status recode (study cutoff used)”, “survival months”.
All patients with incomplete or unknown information contained within the data were eliminated from the dataset. All patients with stage IV disease were also removed from the dataset. Data regarding age, sex, nodes examined, nodes positive, vital status, and survival were taken directly from the original data with no need for further recoding. Histology data was obtained from “grade” and “icd-o-3 hist/behav” and recoded to be: adenocarcinoma well differentiated, adenocarcinoma moderately differentiated, adenocarcinoma poorly differentiated, mucinous adenocarcinoma, or signet ring carcinoma. Depth of invasion was determined by AJCC TNM 7th edition T-classification with “derived ajcc m, 6th ed (2004+)” being recoded accordingly to represent the appropriate depth. Presence of metastatic disease was determined from M-classification. Type of surgery performed was divided into total gastrectomy and subtotal gastrectomy, using “rx sum—surg prim site (1998+)” to determine this. If exact surgery could not be determined and fit into the above categories, the patient was removed from the dataset along with any patient who did not receive a gastrectomy.
Following collection of the data, IBM SPSS statistics version 21 was used to obtain comparative statistics, using chi square analysis and the z-test with significance considered for p-values >0.05. The datasets were then combined to perform the Joinpoint analysis. Kaplan-Meier survival analysis was then performed on the entire dataset as a whole as well as the individual datasets, with mean and median survival obtained for individual number of lymph nodes removed (node numbers 1–45). The decision to use nodal levels of 1–45 was chosen as those levels had a minimum of 1% of the overall sample (≥252 patients).
Joinpoint Trend Analysis software was downloaded from the National Cancer Institute, Division of Cancer Control and Population Sciences website (http://surveillance.cancer.gov/joinpoint) (24). The mean and median survival at each level was then entered into the Joinpoint analysis software using the number of lymph nodes removed as the independent variable and the survival (in months) as the dependent variable. Standard error obtained from the Kaplan-Meier analysis was also entered into the model. Joinpoint analysis was performed for the individual datasets as well as the dataset as a whole using both mean and median survival as the dependent variable. The optimal number of Joinpoints was calculated by the statistical software so that the addition of an additional Joinpoint does not improve the statistical significance of the model.
After obtaining the Joinpoint, multivariate Cox analysis was performed using the following factors: age, sex, ethnicity, extent of surgical resection, histology, lymph nodes removed and lymph nodes positive, AJCC T/N/M-stage, overall stage as well as cutoff of 15 (≥ 15 or < 15 lymph nodes removed), and cutoff of 29 (≥ 29 or < 29 lymph nodes removed) to obtain hazard ratios (HR). Univariate analysis with stage stratified Kaplan-Meier curves was also performed for a cutoff of 29 or more lymph nodes removed, with comparisons made within each stage by log rank test.
Results
The analysis of the SEER dataset (2014 release) identified 57,237 patients with gastric malignancies. After selection of only gastric adenocarcinomas, the following groups of patients were eliminated: patients less than 18 years of age, less than one month survival, patients with multiple primary tumors, patients with incomplete datasets, and removal of patients with stage IV disease, 13,932 patients remained. Data from the Yonsei University gastric cancer database was obtained in the same methods yielding 11,358 patients. The two databases combined gave 25,290 patients.
The patient and tumor characteristics of the two databases as well as the combined dataset are presented in Table 1. There are significant differences demonstrated in mean age, frequency of gender, ethnic breakdown, tumor histologic type, TNM and overall staging, mean number of lymph nodes retrieved, and mean number of lymph nodes harvested. There was no statistical difference in the histologic subtype mucinous adenocarcinoma, T-stage-T2, and the extent of gastrectomy performed. The patient age ranged between 20 years old to 101 years old with the average age of the American gastric cancer patients being 9.6 years older than that of the Korean group. The most common histologic type in both groups was poorly differentiated adenocarcinoma (SEER: 43.6%; Yonsei: 34.2%; p-value <0.05) and the SEER dataset had a notably smaller percentage of well-differentiated tumors. Another significant difference between the two groups is in nodal staging. Patients who did not have adequate nodal dissections by the NCCN guidelines were considered stage “Nx” and overall stage were considered “unable to be stage”. The SEER dataset had 52.1% of the patients with “Nx” due to inadequate nodal evaluation, in comparison to only 2.4% in the Yonsei dataset. When evaluating overall stage, only 9.2% of patients from the SEER dataset presented with stage I disease in comparison to 47.4% of the patients in the Yonsei dataset.
Table 1.
Demographics of Patient Population
| Category | SEER | Yonsei | p Value | Overall |
|---|---|---|---|---|
| Mean age, y | 65.9 | 55.9 | <0.001 | 61.4 |
| Sex, n (%) | ||||
| Male | 8524 (61.2) | 7527 (66.3) | <0.001 | 16051(63.5) |
| Female | 5408 (38.8) | 3831 (33.7) | 9239 (36.5) | |
| Ethnicity, n (%) | ||||
| White | 6553 (47) | 0 | <0.05 | 6553 (25.9) |
| Asian | 2904 (20.8) | 11358 (100) | <0.05 | 14262 (56.4) |
| Black | 1829 (13.1) | 0 | <0.05 | 1829 (7.2) |
| Hispanic | 2482 (17.8) | 0 | <0.05 | 2482 (9.8) |
| Native American | 123 (0.9) | 0 | <0.05 | 123 (0.5) |
| Unknown | 41 (0.3) | 0 | <0.05 | 41 (0.2) |
| Histology, n (%) | ||||
| AWD | 563 (4) | 1541 (13.6) | <0.05 | 2104 (8.3) |
| AMD | 3281 (23.6) | 3160 (27.8) | <0.05 | 6441 (25.5) |
| APD | 6075 (43.6) | 3883 (34.2) | <0.05 | 9958 (39.4) |
| MUC | 392 (2.8) | 334 (2.9) | NS | 726 (2.9) |
| SRC | 1844 (13.2) | 2198 (19.4) | <0.05 | 4042 (16) |
| Unknown | 1777 (12.8) | 242 (2.1) | <0.05 | 2019 (8) |
| T-classification, n (%) | ||||
| T1a | 548 (3.9) | 2626 (23.1) | <0.05 | 3174 (12.6) |
| T1b | 2089 (15) | 2290 (20.2) | <0.05 | 4379 (17.3) |
| T2 | 1927 (13.8) | 1509 (13.3) | NS | 3436 (13.6) |
| T3 | 3097 (22.2) | 1567(13.8) | <0.05 | 4664 (18.4) |
| T4a | 5213 (37.4) | 3063 (27) | <0.05 | 8276 (32.7) |
| T4b | 1058 (7.6) | 303 (2.7) | <0.05 | 1361 (5.4) |
| N-Classification, n (%) | ||||
| N0 | 2099 (15.1) | 6198 (54.6) | <0.05 | 8297 (32.8) |
| N1 | 997 (7.2) | 1419 (12.5) | <0.05 | 2416 (0.6) |
| N2 | 1121 (8) | 1346 (11.9) | <0.05 | 2467 (9.8) |
| N3 | 2459 (17.7) | 2128 (18.7) | <0.05 | 4587 (18.1) |
| Nx | 7259 (52.1) | 267 (2.4) | <0.05 | 7523 (29.7) |
| Stage, n (%) | ||||
| Ia | 747 (5.4) | 4235 (37.3) | <0.05 | 4982 (19.7) |
| Ib | 530 (3.8) | 1142 (10.1) | <0.05 | 1672 (6.6) |
| IIa | 712 (5.1) | 964 (8.5) | <0.05 | 1676 (6.6) |
| IIb | 891 (6.4) | 1142 (10.1) | <0.05 | 2033 (8) |
| IIIa | 830 (6) | 889 (7.8) | <0.05 | 1719 (6.8) |
| IIIb | 1177 (8.4) | 1131 (10) | <0.05 | 2308 (9.1) |
| IIIc | 1789 (12.8) | 1588 (14) | <0.05 | 3377 (13.4) |
| Unable to stage | 7256 (52.1) | 267 (2.4) | <0.05 | 7523 (29.7) |
| Extent of gastrectomy, n (%) | ||||
| Sub-total | 10410 (74.7) | 84.3 (74.2) | NS | 18843 (74.5) |
| Total | 3522 (25.3) | 2925 (25.8) | 6447 (25.5) | |
| Lymph nodes examined, mean | 16.6 | 39.7 | <0.001 | 26.9 |
| Lymph nodes positive, mean | 4.32 | 3.87 | <0.001 | 4.12 |
AWD, adenocarcinoma well differentiated; AMD, adenocarcinoma moderately differentiated; APD, adenocarcinoma poorly differentiated; MUC, mucinous adenocarcinoma; SRC, signet ring cell adenocarcinoma; NS, not statistically significant; SEER, Surveillance, Epidemiology, and End Results database
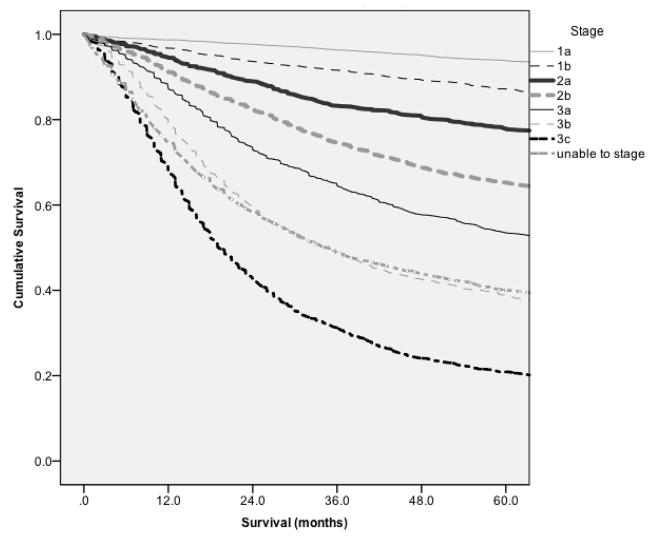
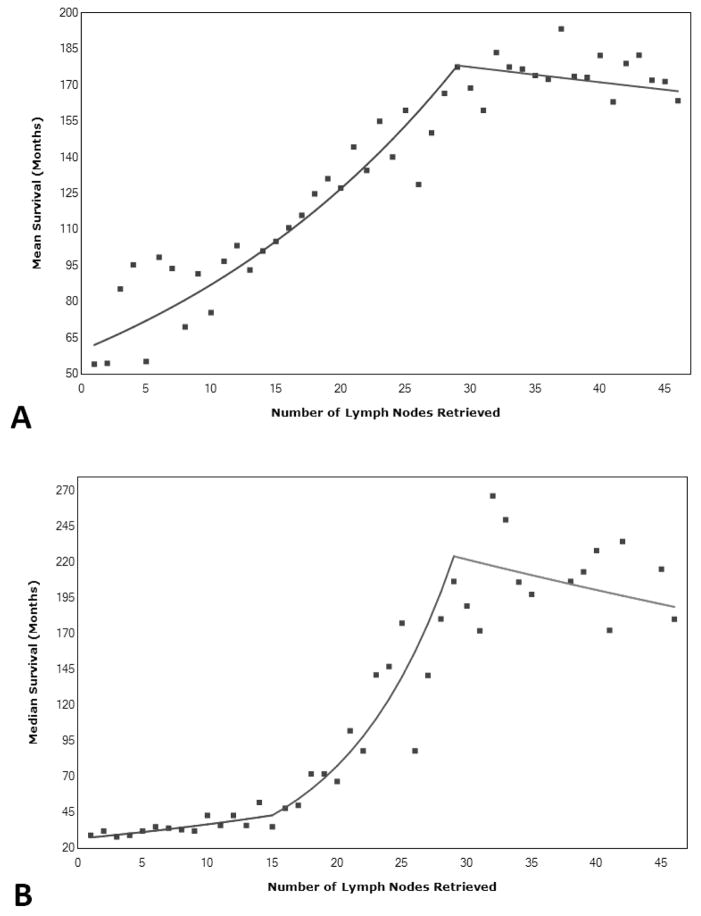
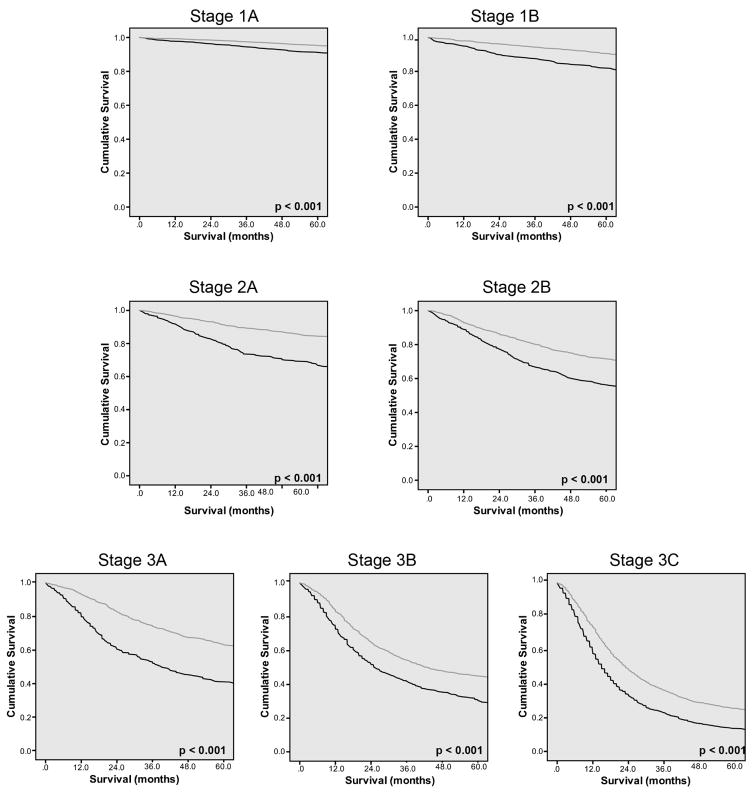
Kaplan-Meier analysis was performed comparing the number of lymph nodes removed and survival, giving us mean and median survival at each possible number of lymph nodes removed (Figure 1). When using both mean and median survival, the Joinpoint was found to be 29 lymph nodes. The Joinpoint model based upon the mean survival was found to be most significant based upon having 1 Joinpoint. The 2 slopes noted on Figure 2a are 3.7% and −0.3% with the change of slope occurring at the Joinpoint. Kaplan-Meier analysis compared patients with less than 29 lymph nodes removed versus those with greater than or equal 29 lymph nodes removed in a stage stratified fashion. Stage by state, survival was seen to be statistically improved for all stages, p<0.001 (Figure 3).
Figure 1.
Kaplan-Meier curve demonstrating stage specific survival.
Figure 2.
Joinpoint analysis demonstrating a Joinpoint at 29 lymph nodes removed for (A) mean survival, and (B) median survival.
Figure 3.
Kaplan-Meier analysis comparing survival for those with <29 lymph nodes removed and those with > 29 lymph nodes removed for Stage 1a–3c. All comparisons of <29 nodes retrieved and >29 nodes retrieved, p<001.
A multivariate Cox analysis was performed. The model started with the following variables: Age, Sex, Ethnicity, Histology, T-classification, N-classification, Extent of Surgery, Cutoff of 15 (≥ 15 or < 15 lymph nodes removed), and Cutoff of 29 (≥ 29 or < 29 lymph nodes removed) (Table 2). The model revealed a cutoff of 15 lymph nodes removed to no longer be in the multivariate model (a non- significant factor), whereas the cutoff of 29 lymph nodes removed demonstrated a hazard ratio (HR) of 0.799 (95% CI 0.759–0.842, p<0.001). Increasing T and N classification were associated with statistically significant increasing HR. Total gastrectomy was associated with worse survival, HR 1.296 (95% CI 1.244–1.351, p<0.001). Male gender was found to have a HR of 0.932 (95% CI 0.897–0.969, p<0.001). Other significant factors included ethnicity, age, and histology. The assumption of proportional hazards was tested using a graph of survival function versus survival time, demonstrating parallel curves for the following: sex, ethnicity, histology, surgery performed, cutoff of 29, T-classification, N- classification, and overall stage.
Table 2.
Cox Logistical Regression Multivariate Analysis.
| Variable (comparison variable) | p Value | HR | 95% CI for HR | |
|---|---|---|---|---|
| Lower | Upper | |||
| Ethnicity (White) | <.001 | |||
| Asian | <.001 | 0.66 | 0.62 | .69 |
| Black | 0.047 | 1.07 | 1.00 | 1.15 |
| Hispanic | <.001 | 0.87 | 0.82 | .93 |
| Native American | 0.007 | 1.34 | 1.08 | 1.67 |
| Unknown | 0.018 | 0.45 | 0.24 | .87 |
| Sex (female) | <.001 | 0.93 | 0.90 | .97 |
| Male | ||||
| Age | <.001 | 1.03 | 1.026 | 1.03 |
| Histology (AWD) | <.001 | |||
| AMD | 0.424 | 1.04 | 0.95 | 1.15 |
| APD | 0.001 | 1.17 | 1.066 | 1.29 |
| MUC | 0.670 | 1.03 | 0.90 | 1.18 |
| SRC | <.001 | 1.22 | 1.10 | 1.35 |
| Unknown | <.001 | 1.27 | 1.13 | 1.42 |
| T-classification (T1a) | <.001 | |||
| T1b | <.001 | 1.48 | 1.30 | 1.68 |
| T2 | <.001 | 1.79 | 1.55 | 2.08 |
| T3 | <.001 | 2.54 | 2.20 | 2.93 |
| T4a | <.001 | 3.01 | 2.62 | 3.46 |
| T4b | <.001 | 4.37 | 3.75 | 5.07 |
| N-classification (N0) | <.001 | |||
| N1 | <.001 | 1.48 | 1.39 | 1.58 |
| N2 | <.001 | 1.80 | 1.69 | 1.92 |
| N3 | <.001 | 3.02 | 2.81 | 3.25 |
| Surgery (distal) | ||||
| Total | <.001 | 1.30 | 1.24 | 1.35 |
| Minimal lymph nodes retrieved (<29) | ||||
| ≥29 nodes retrieved | <.001 | 0.80 | 0.76 | 0.84 |
AWD, adenocarcinoma well differentiated; AMD, adenocarcinoma moderately differentiated; APD, adenocarcinoma poorly differentiated; MUC, mucinous adenocarcinoma; SRC, signet ring cell adenocarcinoma; HR, hazard ratio
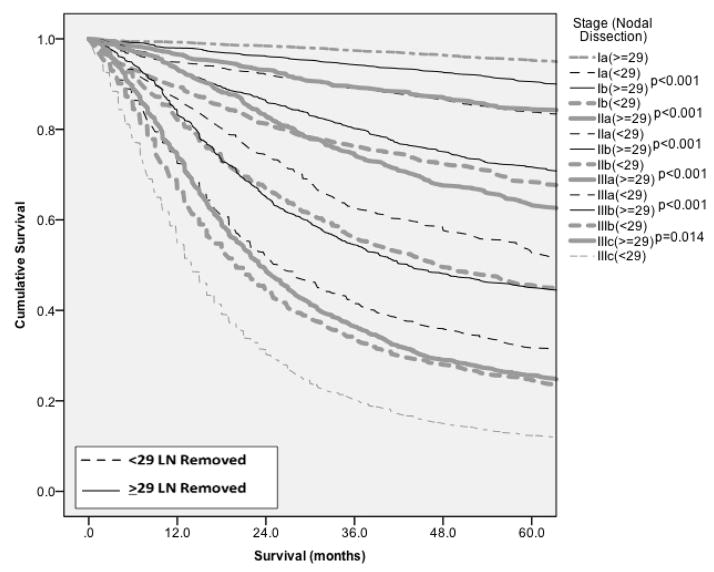
Adequate lymphadenectomy as defined by ≥ 29 lymph nodes (LN) and inadequate lymphadenectomies with < 29 LN harvested were compared stage by stage using Kaplan-Meier analysis for the entire dataset (Figure 4). The data also demonstrated that when an inadequate lymphadenectomy was performed (<29 lymph nodes removed), stage migration occurred: the patients exhibited outcome a stage at the next higher stage with an adequate lymphadenectomy. Stage migration was present in stages Ia, Ib, IIa, IIb, IIIa, and IIIb. In all of these stages, those patients with inadequate lymphadenectomy exhibited statistically inferior survival when compared to those with adequate lymphadenectomy, p < 0.05 (Figure 4), and similar survival compared to the next stage of disease for patients with adequate lymphadenectomy.
Figure 4.
Stage migration analysis. Data demonstrates stage migration for patients with inadequate lymphadenectomy (<29 nodes retrieved) to the next worse stage.
Discussion
Lymph node involvement is one of the strongest predictors of survival in gastric cancer with the anatomic and numerical extent of nodal dissection during radical surgical resection prognostic of oncologic outcome. While D2 lymphadenectomy has become part of the international gold standard in gastric cancer surgery and included in the recommendations by the NCCN guidelines, the total number of lymph nodes for proper extent of surgery and accurate staging of the disease remain unresolved. This present study utilizing an international dataset of 25,289 gastric cancer patients has identified 29 lymph nodes retrieved as the number associated with optimum survival benefit in patients undergoing surgery for gastric cancer.
The lymph node count of 29 is almost two-fold that of the 15 lymph node minimum currently recommended by the NCCN guidelines and 13 lymph nodes more than the 16 lymph nodes considered adequate for proper staging by the AJCC (6, 25, 26). While minimum number to gain survival benefit was defined by a previous study at least 15 (6), our study demonstrates the maximum number beyond which the survival benefit no longer exists to be at 29 lymph nodes highlighting the importance of our study results. Moreover, under staging is seen along with distinct patterns of stage migration with less than 29 lymph nodes evaluated indicating that 29 lymph nodes should be removed for more accurate staging and prognostication of curatively resected gastric cancer patients.
Nodal positivity of gastric cancer marks regional spread of disease and portents increased risk of recurrence and poorer survival when compared to those without nodal positive disease. Identifying the presence of nodal metastases and clearance of these nodal basins can lead to proper staging and planning appropriate treatment strategies. Studies have demonstrated the importance of both the numerical and anatomic extent of lymph node assessment in its impact on proper identification of nodal metastases as well as an important factor in achieving an R0 resection (27). A number of studies including clinical trials has clearly demonstrated that within the same TNM-Stage, the greater the number of lymph node assessed the better the prognosis (19, 28). Incremental number of lymph nodes assessed is directly related to better survival and inadequate lymph node dissection is implicated in understaging of patients.
Stage migration
Our study also clearly demonstrates that inadequate lymphadenectomies is associated with stage migration and poorer survival. In this study, all stages (Ia–IIIb) were noted to be equivalent to or worse survival for the next higher stage if inadequate lymphadenectomy measured (<29 LN) was performed. Consistent with the findings of the meta-analysis by Seevaratnam et al.(29), which reviewed twenty-five articles involving 74,228 patients and found greatest survival impact of lymph node count in Stage II and III disease, our study also revealed that there was the greatest difference in survival in the Stage II and Stage IIIa and IIIb groups. This is expected as T1 cancers are the least likely to have nodal involvement. Thus, limited nodal evaluation is more likely to result in underestimating the N-classification of a T2–T4 cancers, since these have a high likelihood of nodal metastases.
Nodal dissection
Lymphadenectomies were defined by the nomenclature D0, D1, D2 by the Japanese Research Society for the Study of Gastric Cancer, which has established the N1–4 groupings for gastric lymph nodes (30). Lymph node stations 1–6 are referred to as N1, stations 7–11 as N2, stations 12–14 as N3, and stations 15–16 as N4. Per the NCCN guidelines the Japanese classification is used and a D0 is determined as incomplete resection of N1 lymph nodes (25). D1 is considered as removal of all the N1 perigastric lymph nodes, while D2 includes all of the D1 nodes with the addition of nodes along the left gastric, common hepatic, celiac, splenic artery, and splenic hilum.
Since our study included patients irrespective of the extent of lymph node dissection, a significant number of patients undergoing all levels of lymph node dissection D0, D1, and D2 were included in the study. Most of the patients in the SEER dataset did not receive D2 lymphadenectomy, in contrast to the patients in the Yonsei dataset who did. The extent of nodal dissection and the number of lymph nodes retrieved are directly related. When D2 lymphadenectomy is properly performed, an average of 31.1 lymph nodes retrieved during a total gastrectomy and 29.1 for a distal gastrectomy are predicted (20). The results of well conducted clinical trials of D2 lymphadenectomy in Italy, Korea, China, and Japan report an average of 33–47 lymph nodes (10, 31–35). In fact, the oncologic benefit of greater number of lymph nodes continues to be achieved at various cut off numbers greater than 15(28, 29, 36). However, our study has identified the maximum number beyond which the survival advantage no longer exists as 29 lymph nodes.
Recommendations
Based on our results and supported by current literature, we recommend an extended lymphadenectomy with a new goal of 29 lymph nodes retrieved for evaluation. A properly performed D2 lymphadenectomy is expected to yield greater than 29 lymph nodes and should be recommended. While changing of guidelines to include a cutoff of 29 lymph nodes will take time to implement and become fully accepted, the data on stage migration can still be applied. Patients who received inadequate lymphadenectomy could be identified and be considered in the next stage for consideration of adjuvant therapies.
Significance of International Datasets
The success of this study lies in the statistical power afforded by combining distinctly different datasets from two different countries, which not only increases the numbers for analysis but also diversifies the range of independent variables for analysis. This combined analysis has led to the ability to determine where the maximal survival benefit is seen for nodal dissection. This newly created international database of gastric cancer patients is likely to allow evaluation of many other questions.
Limitations
Our study has two major limitations. The use of the SEER dataset does not allow for identification of the extent of lymph node dissection performed, therefore the direct impact of D2 lymphadenectomy cannot be assessed in this study. In addition, the effects of adjuvant treatment such as chemotherapy or radiation were not evaluated.
Conclusion
Lymphadenectomy with an optimum of 29 lymph nodes retrieved improves staging and overall survival in patients undergoing radical surgical resection for gastric cancer. To improve the surgical outcome of our gastric cancer patients and assist in the standardization of the extent of lymph node dissection, a D2 lymphadenectomy with the goal of assessing 29 lymph nodes is recommended.
Footnotes
Disclosure Information: Nothing to disclose
Presented at the Southern Surgical Association 128th Annual Meeting, Palm Beach, FL, December 2016.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006 Jul 6;355(1):11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001 Sep 6;345(10):725–30. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 3.Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012 Jan 28;379(9813):315–21. doi: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- 4.Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007 Nov 1;357(18):1810–20. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 5.Choi YY, An JY, Katai H, et al. A Lymph Node Staging System for Gastric Cancer: A Hybrid Type Based on Topographic and Numeric Systems. PLoS One. 2016;11(3):e0149555. doi: 10.1371/journal.pone.0149555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karpeh MS, Leon L, Klimstra D, Brennan MF. Lymph node staging in gastric cancer: is location more important than Number? An analysis of 1,038 patients. Ann Surg. 2000 Sep;232(3):362–71. doi: 10.1097/00000658-200009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mocellin S, McCulloch P, Kazi H, Gama-Rodrigues JJ, Yuan Y, Nitti D. Extent of lymph node dissection for adenocarcinoma of the stomach. Cochrane Database Syst Rev. 2015 Aug;12(8):CD001964. doi: 10.1002/14651858.CD001964.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakagawa M, Choi YY, An JY, et al. Staging for Remnant Gastric Cancer: The Metastatic Lymph Node Ratio vs. the UICC 7th Edition System. Ann Surg Oncol. 2016 Dec;23(13):4322–31. doi: 10.1245/s10434-016-5390-1. [DOI] [PubMed] [Google Scholar]
- 9.Bonenkamp JJ, Hermans J, Sasako M, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999 Mar 25;340(12):908–14. doi: 10.1056/NEJM199903253401202. [DOI] [PubMed] [Google Scholar]
- 10.Degiuli M, Sasako M, Ponti A, et al. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg. 2014 Jan;101(2):23–31. doi: 10.1002/bjs.9345. [DOI] [PubMed] [Google Scholar]
- 11.Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999 Mar;79(9–10):1522–30. doi: 10.1038/sj.bjc.6690243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010 May;11(5):439–49. doi: 10.1016/S1470-2045(10)70070-X. [DOI] [PubMed] [Google Scholar]
- 13.Wu CW, Hsiung CA, Lo SS, et al. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol. 2006 Apr;7(4):309–15. doi: 10.1016/S1470-2045(06)70623-4. [DOI] [PubMed] [Google Scholar]
- 14.Zilberstein B, Mucerino DR, Yagi OK, et al. Results of D2 gastrectomy for gastric cancer: lymph node chain dissection or multiple node resection? Arq Bras Cir Dig. 2012 Jul-Sep;25(3):161–4. doi: 10.1590/s0102-67202012000300005. [DOI] [PubMed] [Google Scholar]
- 15.Ji J. Chinese guidelines for diagnosis and treatment of gastric cancer (2011 edition) Translational Gastrointestinal Cancer. 2012;1(1):103–14. [Google Scholar]
- 16.De Manzoni G, Marrelli D, Baiocchi GL, et al. The Italian Research Group for Gastric Cancer (GIRCG) guidelines for gastric cancer staging and treatment: 2015. Gastric Cancer. 2016 Jun 2; doi: 10.1007/s10120-016-0615-3. [DOI] [PubMed] [Google Scholar]
- 17.Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer. 2016 Jun 24; doi: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hur H, Lee HY, Lee HJ, et al. Efficacy of laparoscopic subtotal gastrectomy with D2 lymphadenectomy for locally advanced gastric cancer: the protocol of the KLASS-02 multicenter randomized controlled clinical trial. BMC Cancer. 2015 May 05;15:355. doi: 10.1186/s12885-015-1365-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YI. Is retrieval of at least 15 lymph nodes sufficient recommendation in early gastric cancer? Ann Surg Treat Res. 2014 Oct;87(4):180–4. doi: 10.4174/astr.2014.87.4.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner PK, Ramaswamy A, Ruschoff J, Schmitz-Moormann P, Rothmund M. Lymph node counts in the upper abdomen: anatomical basis for lymphadenectomy in gastric cancer. Br J Surg. 1991 Jul;78(7):825–7. doi: 10.1002/bjs.1800780719. [DOI] [PubMed] [Google Scholar]
- 21.Lee J, Kim YM, Woo Y, Obama K, Noh SH, Hyung WJ. Robotic distal subtotal gastrectomy with D2 lymphadenectomy for gastric cancer patients with high body mass index: comparison with conventional laparoscopic distal subtotal gastrectomy with D2 lymphadenectomy. Surg Endosc. 2015 Nov;29(11):3251–60. doi: 10.1007/s00464-015-4069-1. [DOI] [PubMed] [Google Scholar]
- 22.Yonemura Y, Wu CC, Fukushima N, et al. Randomized clinical trial of D2 and extended paraaortic lymphadenectomy in patients with gastric cancer. Int J Clin Oncol. 2008 Apr;13(2):132–7. doi: 10.1007/s10147-007-0727-1. [DOI] [PubMed] [Google Scholar]
- 23.SEER. Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov)SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2014 Sub (1973–2012) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2013. 2014.
- 24.Joinpoint. Joinpoint Regression Program, Version 4.3.1.0 - April 2016; Statistical Methodology and Applications Branch, Surveillance Research Program. National Cancer Institute; 2016. [Google Scholar]
- 25.NCCN GC. NCCN Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network; 2016. [Google Scholar]
- 26.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010 Dec;17(12):3077–9. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 27.Biondi A, Persiani R, Cananzi F, et al. R0 resection in the treatment of gastric cancer: room for improvement. World J Gastroenterol. 2010 Jul 21;16(27):3358–70. doi: 10.3748/wjg.v16.i27.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng G, Feng F, Guo M, et al. Harvest of at Least 23 Lymph Nodes is Indispensable for Stage N3 Gastric Cancer Patients. Ann Surg Oncol. 2016 Nov 9; doi: 10.1245/s10434-016-5667-4. [DOI] [PubMed] [Google Scholar]
- 29.Seevaratnam R, Bocicariu A, Cardoso R, et al. A meta-analysis of D1 versus D2 lymph node dissection. Gastric Cancer. 2012 Sep;15( Suppl 1):S60–9. doi: 10.1007/s10120-011-0110-9. [DOI] [PubMed] [Google Scholar]
- 30.Kajitani T. The general rules for the gastric cancer study in surgery and pathology. Part I. Clinical classification. Jpn J Surg. 1981 Mar;11(2):127–39. doi: 10.1007/BF02468883. [DOI] [PubMed] [Google Scholar]
- 31.Hu Y, Huang C, Sun Y, et al. Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J Clin Oncol. 2016 Apr 20;34(12):1350–7. doi: 10.1200/JCO.2015.63.7215. [DOI] [PubMed] [Google Scholar]
- 32.Inaki N, Etoh T, Ohyama T, et al. A Multi-institutional, Prospective, Phase II Feasibility Study of Laparoscopy-Assisted Distal Gastrectomy with D2 Lymph Node Dissection for Locally Advanced Gastric Cancer (JLSSG0901) World J Surg. 2015 Nov;39(11):2734–41. doi: 10.1007/s00268-015-3160-z. [DOI] [PubMed] [Google Scholar]
- 33.Galizia G, Lieto E, De Vita F, et al. Modified versus standard D2 lymphadenectomy in total gastrectomy for nonjunctional gastric carcinoma with lymph node metastasis. Surgery. 2015 Feb;157(2):285–96. doi: 10.1016/j.surg.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Glehen O, Passot G, Villeneuve L, et al. GASTRICHIP: D2 resection and hyperthermic intraperitoneal chemotherapy in locally advanced gastric carcinoma: a randomized and multicenter phase III study. BMC Cancer. 2014 Mar 14;14:183. doi: 10.1186/1471-2407-14-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim W, Kim HH, Han SU, et al. Decreased Morbidity of Laparoscopic Distal Gastrectomy Compared With Open Distal Gastrectomy for Stage I Gastric Cancer: Short-term Outcomes From a Multicenter Randomized Controlled Trial (KLASS-01) Ann Surg. 2016 Jan;263(1):28–35. doi: 10.1097/SLA.0000000000001346. [DOI] [PubMed] [Google Scholar]
- 36.Schwarz RE, Smith DD. Clinical impact of lymphadenectomy extent in resectable esophageal cancer. J Gastrointest Surg. 2007 Nov;11(11):1384–93. doi: 10.1007/s11605-007-0264-2. discussion 93–4. [DOI] [PubMed] [Google Scholar]