Abstract
Background
There is disagreement regarding the utility of urinary albumin excretion as a marker for capillary injury in patients with severe burn injuries. We examined protein components in urine specimens from patients with burn injury.
Methods
Detailed analysis was performed for a set of 5 urine specimens selected based on a high ratio of albumin-sized molecules by size-exclusion HPLC (AccuminR) versus albumin by immunoassay methods. Specimens were analyzed for total protein, α1-microglobulin, α1-acid glycoprotein, cystatin C, and retinol-binding protein. Urine components were analyzed by chromatographic and electrophoretic methods. Major components were identified by mass spectrometry of tryptic peptides.
Results
A subset of urine specimens had increased total protein with slight increases in albumin by immunoassay or by polyacrylamide gel electrophoresis. Albumin values by size-exclusion HPLC were more than 10-fold higher. Immunoassays for α1-microglobulin and α1-acid glycoprotein yielded concentrations 5–10 fold higher than for albumin. Other major components identified included zinc-α2-glycoprotein and leucine-rich- α2-glycoprotein.
Conclusions
A subset of patients with burn injury had increased total urinary protein resulting primarily from increased excretion of proteins such as α1-microglobulin and α1-acid glycoprotein with little increase in albumin excretion. The unusual composition of urinary proteins in these patients may relate to decreased filtered load of albumin and increased filtered load of acute phase reactants or to alterations in renal tubular protein processing. Thus, measurement of urinary albumin may have decreased sensitivity for detecting kidney injury in burn patients.
Keywords: Urine albumin, burn injury, albuminuria, proteinuria, acute phase proteins
1. Introduction
Increased urinary excretion of albumin is a potential early indicator both of glomerular injury and of systemic endothelial injury in chronic disease processes such as diabetes and atherosclerosis [1,2] and in acute illness or surgery [3–6]. Consequently, there has been interest in examining whether urinary albumin excretion can serve as a marker for kidney injury or risk of capillary leak syndromes in disorders such as adult respiratory distress syndrome or severe burn injury [7–9]. Kidney injury can occur following burn injury, possibly as a consequence of hemodynamic compromise or exposure to increased products of tissue degradation in the circulation [10–12]. For patients with burn injuries, there is disagreement, however, regarding whether urine albumin excretion serves as an indicator of injury severity or resuscitation demands [7–9]. This suggested that there might be complicating factors in the application of urine albumin measurements to evaluation of burn patients.
Of potential significance for the interpretation of albuminuria in specimens from burn patients, there has been recent controversy regarding the measurement of urinary albumin. A new procedure for measurement of urinary albumin by size-exclusion high-performance liquid chromatography (HPLC) obtains substantially higher albumin values than immunoassay procedures that have been in common usage [13–18]. Two different hypotheses have been proposed to explain the difference between methods: 1) Immunoassays fail to detect some conformationally-modified forms of albumin that are detected by the size-exclusion assay [13–15]. 2) the size-exclusion chromatographic method responds to other molecules that co-elute with albumin [19,20]. We found that some specimens from patients with burn injury had a >10-fold difference in values of albumin determined by size-exclusion chromatography vs immunoassay methods. Considering that major tissue injury might be a source of degraded or conformationally-modified albumin, we investigated the nature of proteinuria and the profound difference in albumin measurement by two methods applied to specimens obtained from patients with severe burn injury.
2. Materials and Methods
Specimens from patients with burn injury were collected with a protocol approved by the local institutional review board as previously described [9]. Urine total protein (pyrogallol red-molybdate), albumin (immunoturbidimetric), and creatinine (alkaline picrate method) were determined on a Beckman-Coulter LX20 analyzer. A second immunoturbidimetric method for urine albumin used reagents from Wako Diagnostics. Analysis of albumin by size-exclusion HPLC used the AccuminR method from AusAm Biotechnologies. Quantitative analysis of cystatin C, α1-acid glycoprotein, α1-microglobulin, and retinol-binding protein were performed on a Siemens BN-II nephelometer. Polyacrylamide gels for sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS PAGE), molecular weight standards, and SimpleBlue stain were from Invitrogen. Urine specimens were concentrated using Amicon Ultra-15 concentrators with regenerated cellulose membranes of 10 kDa cutoff. Immunodepletion of albumin was performed using spun columns of microparticles bearing polyclonal chicken antiserum directed against human albumin, as previously described for immunodepletion of albumin from serum. Preparative size-exclusion HPLC was performed on a 9.4 × 250 mm Zorbax GF-250 column from Agilent eluted with buffered saline as in a previous report [19]. Proteins detected in polyacrylamide gels stained with Coomassie blue were subjected to trypsin digestion and analysis of tryptic peptides by matrix-assisted laser desorption tandem time-of-flight (MALDI TOF-TOF) mass spectrometry using a Bruker UltraFlex mass spectrometer. Fragment spectra for peptides were submitted to a Mascot search engine (Matrix Science Inc; www.matrixscience.com) for protein identifications.
3. Results
A subset of 5 urine specimens from burn patients was selected based on a low ration of albumin (by immunoassay) versus total protein and a high ratio of albumin by HPLC versus immunoassay (albumin by HPLC > 10-fold higher than mean albumin value by 2 immunoassay methods). This was considered as a potential means to select specimens where immunoassay results might be falsely low due to partially degraded or conformationally-modified albumin, and might account for problems in detecting albuminuria in burn patients. As listed in Table 1, these specimens were obtained from 3 patients at timepoints between 2 and 11 days from admission to the hospital. Although total protein was increased to a mean of >900 mg/l and 680 mg/g creatinine in this subset of specimens, albumin concentrations by immunoassay were only about 20 mg/l and represented only about 2% of the total protein. The size-exclusion HPLC method for albumin yielded a mean value about 20-fold higher, representing about 40% of the total protein. Concentrations of α1-microglobulin and α1-acid glycoprotein were about 10-fold higher than albumin measured by immunoassay. A notable characteristic of patients at the time of these urine collections was severe hypoalbuminemia, with a mean albumin concentration of 17 g/l.
Table 1.
Quantitative analysis of a subset of 5 urine specimens from 3 patients with burn injury. 1
| Patient A | Patient B | Patient C | ||||
|---|---|---|---|---|---|---|
| Component | 66h | 216 h | 264 h | 48 h | 72h | Mean |
| Urine | ||||||
| Total protein, mg/l | 410 | 1,520 | 1,030 | 710 | 1,120 | 958 |
| Albumin (LX20), mg/l | 35 | 15 | 6 | 7 | 11 | 15 |
| Albumin (Wako), mg/l | 23 | 33 | 19 | 16 | 22 | 23 |
| Albumin (HPLC), mg/l | 1 | 604 | 320 | 325 | 443 | 402 |
| α1-acid glycoprotein | ND | 220 | 120 | 210 | 250 | 160 |
| α1-microglobulin | ND | 266 | 155 | 79 | 149 | 130 |
| Retinol-binding prot., mg/l | ND | 0.4 | 0.6 | 11.9 | 7.9 | 4.2 |
| Cystatin C, mg/l | ND | 29 | 7 | 5 | 15 | 11 |
| Creatinine, g/l | 2.0 | 0.9 | 0.5 | 1.8 | 2.0 | 1.4 |
| Plasma | ||||||
| Albumin, g/l | 22 | 15 | 15 | 17 | 17 | 17 |
Specimens were selected for high ratio of albumin by HPLC versus immunoassay methods ( >10). Time of collection following hospital admission is indicated. ND indicates not determined. Plasma albumin at the time of urine collection also is indicated.
A second subset of specimens listed in Table 2 was identified with lower total protein excretion, a higher ratio of albumin (by immunoassay) vs total protein, and a lower ratio of albumin by immunoassay versus HPLC assay. These specimens were collected from patients with burn injury 0–3 days from admission to the hospital. Albumin was present in much higher concentrations than other proteins such as α1-microglobulin and α1-acid glycoprotein. Hypoalbuminemia was less severe in this group, with a mean plasma albumin concentration of 26 g/l.
Table 2.
Quantitative analysis of a subset of 5 urine specimens from 5 patients with burn injury.1
| Patient A | Patient D | Patient E | Patient F | Patient G | ||
|---|---|---|---|---|---|---|
| Component | 6 h | 0 h | 30 h | 24 h | 72h | Mean |
| Urine | ||||||
| Total protein, mg/l | 70 | 60 | 170 | 260 | 50 | 122 |
| Albumin (LX20), mg/l | <3 | 13 | 47 | 54 | 15 | 26 |
| Albumin (Wako), mg/l | 31 | 26 | 62 | 61 | 23 | 41 |
| Albumin (HPLC), mg/l | 35 | 42 | 104 | 132 | 51 | 73 |
| α1-acid glycoprotein | ND | <5 | <5 | <5 | <5 | <5 |
| α1-microglobulin | ND | 1.0 | 0.6 | 6.3 | 0.6 | 4.1 |
| Retinol-binding prot., mg/l | ND | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Cystatin C, mg/l | ND | <0.1 | <0.1 | 1.4 | <0.1 | 0.4 |
| Creatinine, g/l | 0.4 | 0.3 | 0.9 | 0.4 | 0.2 | 0.4 |
| Plasma | ||||||
| Albumin, g/l | 16 | 32 | 34 | 29 | 20 | 26 |
Specimens were selected for low ratio of albumin by HPLC versus immunoassay methods ( >3). Time of collection following hospital admission is indicated. ND indicates not determined. Plasma albumin at the time of urine collection also is indicated.
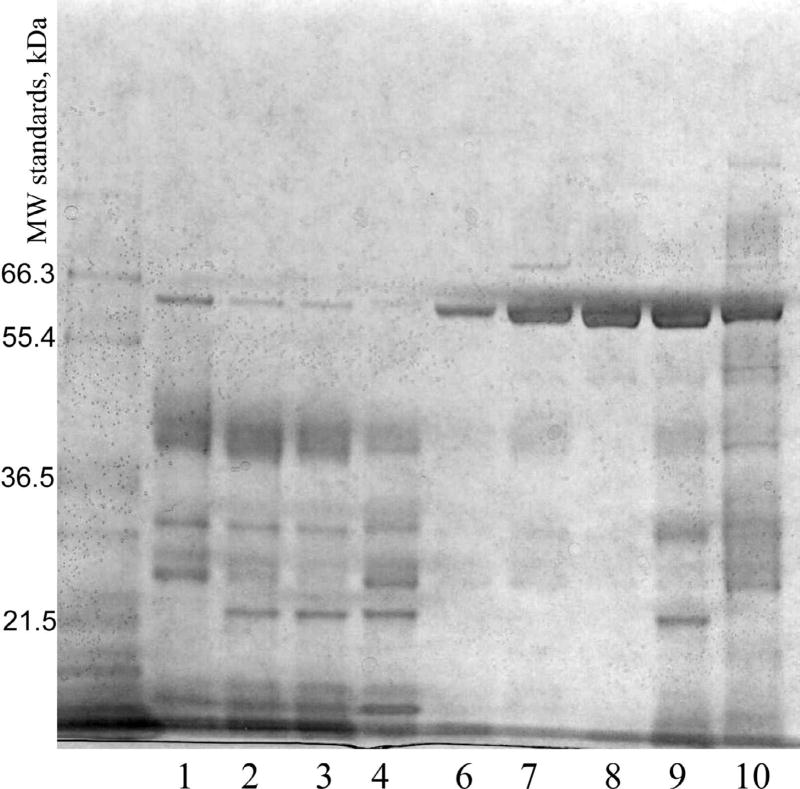
Specimens were concentrated by ultrafiltration and analyzed by SDS PAGE. Results for nine specimens run under reducing conditions are shown in Figure 1. One specimen had insufficient volume for analysis. The specimens with a low proportion of albumin by immunoassay showed very small amounts of albumin, while specimens with high proportions of albumin by immunoassay found albumin to be the major protein component. The specimens with low proportions of albumin had many components more abundant than albumin, and these had lower masses than albumin. Similar results were obtained for SDS PAGE under nonreducing conditions (data not shown).
Fig. 1.
SDS PAGE of urine specimens from patients with burn injury. Lanes 1–4 represent specimens from patients A, B, and C with low proportions of albumin. Lanes 6–10 represent specimens from patients A, D, E, F, and G with high proportions of albumin. Specimens were analyzed after reduction with dithiothreitol. Molecular weight standards are indicated.
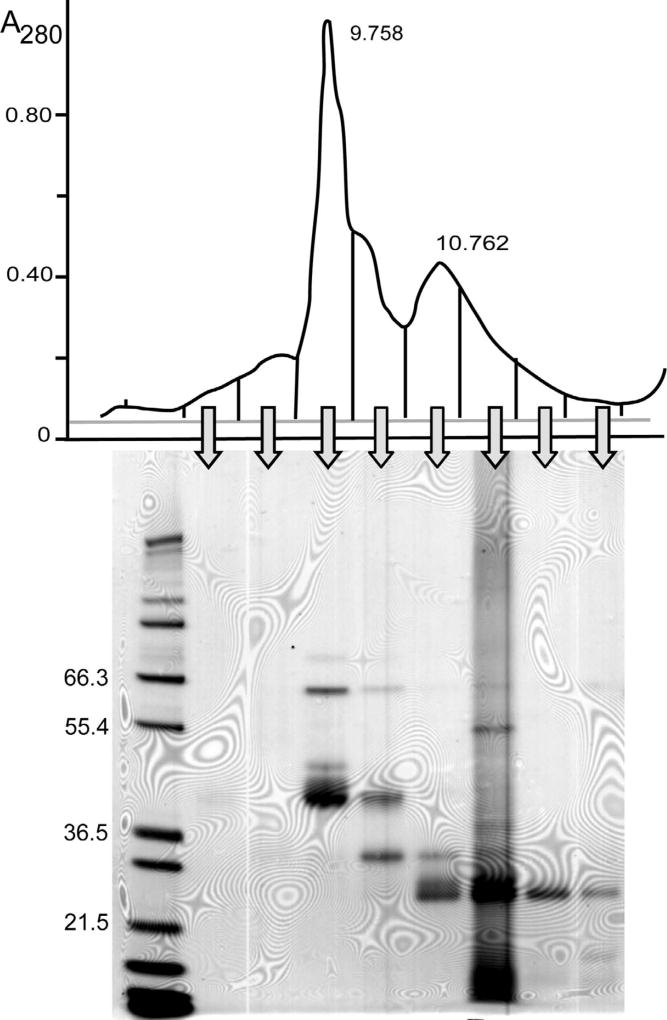
Specimens with a low proportion of albumin were pooled for further analysis. The pooled specimen was subjected to size-exclusion HPLC and fractions collected from this analysis were analyzed by SDS PAGE (Fig. 2). The major peak on size-exclusion HPLC eluted at 9.758 min in the position expected of albumin, but SDS PAGE indicated that albumin was a minor component in this fraction. Based on previous reports the other major components in this fraction might be either partially degraded forms of albumin [13] or other proteins that coelute with albumin during size-exclusion HPLC [19].
Fig. 2.
Analysis of pooled urine specimens with low albumin:total protein. The pooled specimen was first analyzed by size-exclusion HPLC with elution time on the x-axis and absorbance at 280 nm on the y-axis. Fractions collected as indicated were analyzed by SDS PAGE under reducing conditions. The peak at 9.758 min on the size-exclusion HPLC corresponds to the elution position of an albumin standard.
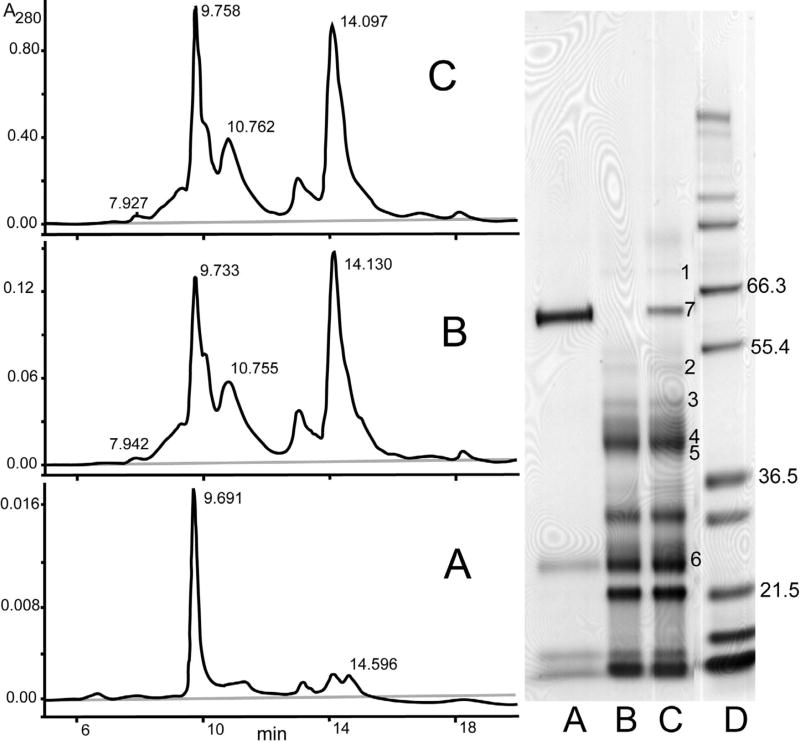
To address the identities of the major protein components in the urine pool with a low proportion of albumin, further analysis was performed. In particular, there was an interest in ascertaining whether any of the major components were degraded forms of albumin. An affinity column with a polyclonal antiserum versus human albumin was used to capture albumin in the urine pool. Analyses of the total pool, albumin-depleted pool, and eluate of proteins captured by the albumin affinity column are shown in Figure 3. SDS PAGE indicated that the albumin depletion step effectively removed albumin (lane B), but albumin depletion yielded little change versus the total pool in the pattern observed by size-exclusion HPLC; there was just a small decrease in the size of the peak in the position of albumin. The eluate of proteins bound to the affinity column consisted predominantly of intact albumin, with smaller amounts of 3 components <30 kDa that may represent proteins adsorbed on the column. Some previous reports have suggested that there may be modified forms of albumin with decreased immunoreactivity [13]. Therefore, several of the major bands on SDS PAGE analysis were identified by mass spectrometry of tryptic digests as listed in Table 3. Protein identifications were based on tandem mass spectrometry of multiple peptides, except in the case of α1-microglobulin, where identification was based on a single peptide. Electrophoretic mobilities on SDS PAGE of identified proteins were all consistent with expected values. None of the major bands were identified as fragments of albumin. One of the bands recovered partially with proteins bound to the albumin affinity column corresponded to α1-microglobulin. Recovery of this protein could represent either nonspecific binding to the column or disulfide linkage of some molecules to albumin to form a heterodimer captured by the antibody.
Fig. 3.
SDS PAGE of a pooled specimen with a low proportion of albumin following fractionation on an albumin affinity column. Shown are total pooled specimen (C), specimen after depletion of albumin by an anti-ablumin affinity column (B), and bound components eluted from the albumin affinity column (A). Protein identities of numbered bands on the gel were determined by excising the bands, digesting them with trypsin, and analyzing the digests by mass spectrometry: 1) transferrin, 2) α1-proteinase inhibitor (α1-antitrypsin), 3) leucine-rich α2-glycoprotein, 4) α1-acid glycoprotein, 5) zinc- α2-glycoprotein, 6) α1-microglobulin, 7) albumin. Peptides used for identifications are listed in Table 3.
Table 3.
Identification of major protein bands on SDS PAGE. The observed mass was estimated by SDS PAGE.
| Protein Identification/ SwissProt Accession |
Protein Mass | Tryptic Peptides Identified |
Mowse Score | |
|---|---|---|---|---|
| Observed | Reported | |||
| 1) Transferrin P02787 | 80,000 | 79,600 | DLLFK | 337 |
| DGAFDVAFVK | ||||
| SASDLTWDNIK | ||||
| MYGYEYVTAIR | ||||
| 2) α1-Proteinase Inhibitor P01009 | 50,000 | 50,300 | IVDLVK | 173 |
| SPLFMGK | ||||
| SASLHLPK | ||||
| VFSNGADLSGVTEEAPLK | ||||
| 3) Leucine-rich α2-glycoprotein P02750 | 48,000 | 50,000 | VLDLTR | 128 |
| GPLQLER | ||||
| GQTLLAVAK | ||||
| VAAGAFQGLR | ||||
| 4) α1-Acid glycoprotein P02763 | 40,000 | 40,000 | SDVVYTDWK | 108 |
| EQLGEFTEALDCLR | ||||
| YVGGQEHFAHLLILR | ||||
| 5) Zinc-α2-glycoprotein P25311 | 39,000 | 38,500 | SSGAFWK | 495 |
| IDVHWTR | ||||
| AGEVQEPELR | ||||
| AYLEEECPATLR | ||||
| 6) α1-Microglobulin P02760 | 25,000 | 27,000 | VVAQGVGIP | 33 |
Mowse scores > 46 indicate identity or extensive homology with the specified protein (p < 0.05). For α1-microglobulin, mass spectrometric data was combined with electrophoretic mobility to confirm the identification.
4. Discussion
A number of reports have identified degraded or modified forms of albumin detected in serum or urine by techniques such as 1- or 2-dimensional electrophoresis, Western blotting, or mass spectrometry [22–30]. We considered that burn injury might be a disorder with enhanced albumin degradation or modification due to enhanced inflammation and extravasation of albumin into extracellular spaces. This was considered a possible explanation for the previously-reported lack of measured increases in albumin excretion in burn injury [9], if modified forms of urinary albumin fail to be detected by immunoassays as hypothesized in some reports [13–18]. However, that hypothesis is controversial, and it is not clear that there are any modified forms of albumin that cannot be detected by immunoassays [19,20,31]. In the present report, a subset of urine specimens in which size-exclusion HPLC values for albumin were >10-fold than immunoassay values for albumin were subjected to further analysis to determine whether modified forms of albumin account for the difference. By 1-dimensional SDS PAGE, it was clear that this subset of specimens contained low amounts of intact albumin. Analysis of major bands on gels did not find any major bands resulting from proteolytic degradation of albumin. Instead, other major components were identified such as α1-acid glycoprotein, α1-microglobulin, α1-antitrypsin, leucine-rich-α2-glycoprotein, and zinc-α2-glycoprotein. Product information for the HPLC albumin assay states that it is not suitable for analysis of specimens from patients with inflammatory disorders, and results presented here clarify why this is the case, considering that acute-phase proteins such as α1-acid glycoprotein become increased substantially to concentrations above albumin. Previous work has shown that the relatively low resolution size-exclusion HPLC method has difficulty resolving proteins such as α1-acid glycoprotein and α1-antitrypsin from albumin [19]. Burn injury is recognized to have a strong inflammatory component and induction of acute phase responses [32,33], and positive acute phase reactants have been identified in increased concentrations in urine in a variety of disease states [34,35].
Although burn injury, is recognized as a potent stimulator of acute-phase responses [32,33], there may be a timelag of 1–2 days for induction of many acute-phase reactants. This is consistent with the collection time of specimens with high concentrations of α1-acid glycoprotein (2–11 days after admission) compared with specimens not showing a high concentration of α1-acid glycoprotein (0–3 days). A previous study of urinary α1-acid glycoprotein excretion in post-surgical patients observed that peak levels of α1-acid glycoprotein may not be reached for several days [34]. However, not all of the increased protein excretion is explained by an overflow proteinuria of small proteins that are positive acute-phase reactants. Retinol-binding protein is considered to be a negative acute-phase reactant and cystatin C is not recognized as an acute phase reactant. Increases in excretion of these small protein components suggests a coincident impairment of tubular uptake at the timepoints of these specimen collections and not observed at earlier timepoints.
Results presented here may help explain the variable findings and controversy regarding the value of urine albumin measurements as a marker for kidney injury in patients with burn injury [7–9]. It is striking that, in some urine specimens containing >1 g/l total protein and substantially increased protein:creatinine ratios, there was little apparent increase in albumin excretion. Perhaps, this can be explained by the diminished glomerular filtration of albumin in the setting of severe hypoalbuminemia. In severe hypoalbuminemia, urine albumin measurements may have decreased sensitivity as an indicator of kidney injury. In clinical disorders with severe hypoalbuminemia it may be necessary to rely more on measurements of total urinary protein or proteins other than albumin as markers of kidney injury.
Acknowledgments
Studies were supported by the intramural research program of the Clinical Center, National Institutes of Health, Department of Health and Human Services, and by the ARUP Institute for Clinical and Experimental Pathology.
Abbreviations
- MALDI TOF
matrix-assisted laser desorption/ionization time-of-flight
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Viberti GC, Hill RD, Jarret RJ, Argyropoulos A, Mahmud U, Keen H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet. 1982;1:1430–1432. doi: 10.1016/s0140-6736(82)92450-3. [DOI] [PubMed] [Google Scholar]
- 2.Arnlov J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112:969–975. doi: 10.1161/CIRCULATIONAHA.105.538132. [DOI] [PubMed] [Google Scholar]
- 3.Abid O, Sun Q, Sugimoto K, Mercan D, Vincent JL. Predictive value of microalbuminuria in medical ICU patients: results of a pilot study. Chest. 2001;120:1984–1988. doi: 10.1378/chest.120.6.1984. [DOI] [PubMed] [Google Scholar]
- 4.Sarti A, Raffaele-De Gaudio A, Messineo A, Cuttini M, Ventura A. Glomerular permeability after surgical trauma in children: relationship between microalbuminuria and surgical stress score. Crit Care Med. 2001;29:1626–1629. doi: 10.1097/00003246-200108000-00021. [DOI] [PubMed] [Google Scholar]
- 5.MacKinnon K, Lowe ZMD, Watson I, Shearer E. Use of microalbuminuria as a predictor of outcome in critically ill patients. Br J Anaesth. 2000;84:239–241. doi: 10.1093/oxfordjournals.bja.a013409. [DOI] [PubMed] [Google Scholar]
- 6.Gosling P, Brudney S, McGrath LS, Riseboro S, Manji M. Mortality prediction at admission to intensive care: a comparison of microalbuminuria with acute physiology scores after 24 hours. Crit Care Med. 2003;31:98–103. doi: 10.1097/00003246-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Vlachou E, Gosling P, Moiemen NS. Microalbuminuria: a marker of endothelial dysfunction in thermal injury. Burns. 2006;32:1009–1016. doi: 10.1016/j.burns.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 8.Vlachou E, Gosling P, Moiemens NS. Microalbuminuria: a marker of systemic endothelial dysfunction during burn excision. Burns. 2008;34:241–246. doi: 10.1016/j.burns.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Cochran A, Dong L, Edelman LS, et al. Microalbuminuria in acute burn injury. J Burn Care Res. 2008;29:176–179. doi: 10.1097/BCR.0b013e31815f5a28. [DOI] [PubMed] [Google Scholar]
- 10.Kim GH, Oh KH, Yoon YW, et al. Impact of burn size and initial serum albumin level on acute renal failure occurring in major burn. Am J Nephrol. 2003;23:55–60. doi: 10.1159/000066299. [DOI] [PubMed] [Google Scholar]
- 11.Coca SG, Bauling P, Schifftner T, Howard CS, Teitelbaum I, Parikh CR. Contribution of acute kidney injury toward morbidity and mortality in burns: a contemporary analysis. Am J Kidney Dis. 2007;49:517–523. doi: 10.1053/j.ajkd.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Mariano F, Cantaluppi V, Stell M, et al. Circulating plasma factors induce tubular and glomerular alterations in septic burn patients. Crit Care Med. 2008;12(2) doi: 10.1186/cc6848. epub ahead of print Mar 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osicka TM, Comper WD. Characterization of immunochemically nonreactive urinary albumin. Clin Chem. 2004;50:2286–2291. doi: 10.1373/clinchem.2004.039743. [DOI] [PubMed] [Google Scholar]
- 14.Clavant SP, Sastra SA, Osicka TM, Comper WD. The analysis and characterization of immuno-unreactive urinary albumin in healthy volunteers. Clin Biochem. 2006;39:143–151. doi: 10.1016/j.clinbiochem.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Brinkman JW, Bakker SJL, Gansevoort RT, et al. Which method for quantifying urinary albumin excretion gives what outcome? A comparison of immunonephelometry with HPLC. Kidney Int. 2004;66(Suppl 92):S69–S75. doi: 10.1111/j.1523-1755.2004.09219.x. [DOI] [PubMed] [Google Scholar]
- 16.Owen WE, Roberts WL. Performance characteristics of an HPLC assay for urinary albumin. Am J Clin Pathol. 2005;124:219–225. doi: 10.1309/F6WV-K152-5KLQ-GXR4. [DOI] [PubMed] [Google Scholar]
- 17.Contois JH, Hartigan C, Rao LV, Snyder LM, Thompson MJ. Analytical validation of an HPLC assay for urinary albumin. Clin Chim Acta. 2006;367:150–155. doi: 10.1016/j.cca.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Polkinghorne KR, Su Q, Chadban SJ, Shaw JE, Zimmet PZ, Atkins RC. Population prevalence of albuminuria in the Australian Diabetes, Obesity, and Lifestyle (AusDiab) Study: immunonephelometry compared with high-performance liquid chromatography. Am J Kidney Dis. 2006;47:604–613. doi: 10.1053/j.ajkd.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 19.Sviridov D, Meilinger B, Drake SK, Hoehn GT, Hortin GL. Co-elution of other proteins with albumin during size-exclusion HPLC: implications for urine albumin analysis. Clin Chem. 2006;52:389–397. doi: 10.1373/clinchem.2005.057323. [DOI] [PubMed] [Google Scholar]
- 20.Sviridov D, Drake SK, Hortin GL. Reactivity of urine albumin (microalbumin) assays with fragmented or modified albumin. Clin Chem. 2008;54:61–68. doi: 10.1373/clinchem.2007.092825. [DOI] [PubMed] [Google Scholar]
- 21.Seam N, Gonzales DA, Kern SJ, Hortin GL, Hoehn GT, Suffredini AF. Quality control of serum albumin depletion for proteomic analysis. Clin Chem. 2007;53:1915–1920. doi: 10.1373/clinchem.2007.091736. [DOI] [PubMed] [Google Scholar]
- 22.Wiggins RC, Kshrisagar B, Kelsch RC, Wilson BS. Fragmentation and polymeric complexes of albumin in human urine. Clin Chim Acta. 1985;149:155–163. doi: 10.1016/0009-8981(85)90329-8. [DOI] [PubMed] [Google Scholar]
- 23.Kausler E, Spiteller G. Fragments of albumin and β2-microglobulin--constituents of the middle molecule fraction in hemofiltrate. Biol Chem Hoppe-Seyler. 1991;372:849–855. [PubMed] [Google Scholar]
- 24.Heine G, Raida M, Forssmann WG. Mapping of peptides and protein fragments in human urine using liquid chromatography-mass spectrometry. J Chromatogr A. 1997;776:117–124. doi: 10.1016/s0021-9673(97)00440-8. [DOI] [PubMed] [Google Scholar]
- 25.Lafitte D, Dussol B, Andersen S, et al. Optimized preparation of urine samples for two-dimensional electrophoresis and initial application to patient samples. Clin Biochem. 2002;35:581–589. doi: 10.1016/s0009-9120(02)00362-4. [DOI] [PubMed] [Google Scholar]
- 26.Candiano G, Musante L, Bruschi M, et al. Repetitive fragmentation products of albumin and α1-antitrypsin in glomerular diseases associated with nephritic syndromes. J Am Soc Nephrol. 2006;17:3139–3148. doi: 10.1681/ASN.2006050486. [DOI] [PubMed] [Google Scholar]
- 27.Bar-Or D, Rael LT, Bar-Or R, Slone DS, Craun ML. The formation and rapid clearance of a truncated albumin species in a critically ill patient. Clin Chim Acta. 2006;365:346–349. doi: 10.1016/j.cca.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Musante L, Candiano G, Petretto A, et al. Active focal segmental glomerulosclerosis is associated with massive oxidation of plasma albumin. J Am Soc Nephrol. 2007;18:799–810. doi: 10.1681/ASN.2006090965. [DOI] [PubMed] [Google Scholar]
- 29.Hortin GL, Sviridov D. Analysis of molecular forms of albumin in urine. Proteomics Clin Appl. 2008;2:950–955. doi: 10.1002/prca.200780145. [DOI] [PubMed] [Google Scholar]
- 30.Miller WG, Narva A, Bruns DE, et al. Current issues in measurement and reporting of urinary albumin excretion. Clin Chem. 2009;55:24–38. doi: 10.1373/clinchem.2008.106567. [DOI] [PubMed] [Google Scholar]
- 31.Shaikh A, Seegmiller JC, Borland TM, et al. Comparison between immunoturbidimetry, size-exclusion chromatography, and LC-MS to quantify urinary albumin. Clin Chem. 2008;54:1504–1510. doi: 10.1373/clinchem.2008.107508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas S, Wolf SE, Chinkes DL, Herndon DN. Recovery from the hepatic acute phase response in severely burned and the effects of long-term growth hormone treatment. Burns. 2004;30:675–679. doi: 10.1016/j.burns.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Jeschke MG, Finnerty CC, Kulp GA, Przkora R, Mlcak RP, Herndon DN. Combination of recombinant human growth hormone and propanolol decreases hypermetabolism and inflammation in severely burned children. Pediatr Crit Care Med. 2008;9:209–216. doi: 10.1097/PCC.0b013e318166d414. [DOI] [PubMed] [Google Scholar]
- 34.Magid E, Guldager H, Hesse D, Christiansen MS. Monitoring urinary orosomucoid in acute inflammation: observations on urinary excretion of orosomucoid, albumin, α1-microglobulin, and IgG. Clin Chem. 2005;51:2052–2058. doi: 10.1373/clinchem.2005.055442. [DOI] [PubMed] [Google Scholar]
- 35.Hortin GL, Sviridov D. Diagnostic potential for urinary proteomics. Pharmacogenomics. 2007;8:237–255. doi: 10.2217/14622416.8.3.237. [DOI] [PubMed] [Google Scholar]