Abstract
Background
Sustained genital tract inflammation caused by sexually transmitted infections (STIs) is known to increase risk of vaginal human immunodeficiency virus (HIV) infections but, to our knowledge, there are no non-human primate studies that have evaluated its link to rectal HIV acquisition.
Methods
Rhesus macaques inoculated with Chlamydia trachomatis [CT; serovars LGV-L2 and CT-E; n=7] or saline (n=7) received up to 20 rectal challenges twice a week of simian-human immunodeficiency virus (SHIVSF162p3). SHIV viremia was determined by real-time PCR and Chlamydia infection by APTIMA Combo 2 testing. The rectal cytokine-chemokine levels were evaluated by multiplex bead assays.
Results
Rectal Chlamydia infection was maintained throughout the study. We did not observe significant differences (p=1.0) in frequency of SHIV acquisition between the STI and control arms. It took fewer SHIV challenges to infect the STI animals although the difference was not significant (p=0.59). There were no significant differences in peak plasma viremia between STI and control arms (p=0.63). The association of plasma viremia with rectal shedding was significantly different by arm (p=0.038).
Conclusions
In the first such study in a macaque model, we did not observe an increased risk of SHIV acquisition due to rectal Chlamydia co-infection. This macaque model can be further developed and expanded to better investigate the impact of different rectal STIs on HIV acquisition.
Keywords: SHIV, rectal Chlamydia, HIV, co-infection
INTRODUCTION
There were more than 1.5 million cases of Chlamydia infections reported in the United States in 2015, up 5.9% from 2014 (1). The number of detected cases of rectal Chlamydia trachomatis (CT) in men who have sex with men (MSM) increased 4-fold, attributed to adoption of rectal screening and frequent use of nucleic acid amplification tests (NAATs) instead of symptom-based screening methods (2). Similarly, in Canada, the rate of detection of rectal chlamydial infection among women was 44.3% higher when specific rectal screening in STI clinics was conducted, as opposed to testing only genitourinary samples (3). In addition to better screening and testing methods, there has also been an increase in reported anal sex in heterosexual couples (4). In response to a CT infection, the mucosal epithelium recruits immune cells to the site of invasion (5), thus resulting in tissue damage that can in turn facilitate HIV acquisition by providing ports of entry and target cells. In humans, CT infections have been reported to impact human immunodeficiency virus (HIV) susceptibility and risk of transmission (6–9). Considering the increasing rates of rectal CT infections and their possible contribution to the spread of HIV, we evaluated the effect of rectal CT infections on SHIV acquisition in a macaque model.
MATERIALS AND METHODS
Macaques, study design, and sample collection/processing
Fourteen adult Indian rhesus macaques, ages 5 to 13 years old, free of SHIV or CT infection, were single-housed and studied at the Centers for Disease Control and Prevention (CDC). All procedures (see design; Figure 1) were approved by CDC’s Institutional Animal Care and Use Committee in accordance with the Guide for the Care and Use of Laboratory Animals (published by the National Academy of Science, National Academy Press, Washington, DC, USA).
Figure 1.
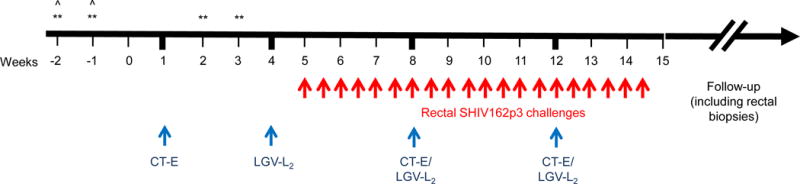
Study design. Evaluation of risk of HIV acquisition in CT co-infected animals. The design (in weeks) shows baseline collections followed by inoculation (blue arrows) with CT serovar E (CT-E) at week 1, and serovar LGV-L2 at week 4. The animals were boosted with CT (serovar E and LGV-L2) every 4 weeks to maintain infection. Starting week 5, we rectally challenged (red arrows) the animals with SHIV162p3 (10TCID50) twice a week up to 20 challenges; **=cytokines measured at these points to compare local inflammation before and after STI inoculations; ˆ=baseline rectal biopsy collections.
Chlamydia inoculations and detection
Rhesus macaques were anesthetized and inoculated rectally as previously described (10) with phosphate buffered saline (PBS; n=7) or two serovars of CT (n=7; table 1), serovar E strain E-UW/5 (1×106 IFU; 500μL volume) and LGV genotype L2 strain 434 (5×106 IFU; 500μL), obtained from University of Washington Chlamydia Laboratory. Serovar selection was based on domestic prevalence and feasibility for laboratory use (TR Henning, unpublished). The animals were first inoculated with CT-E, and three weeks later, inoculated with the second serovar LGV- L2. Infections were confirmed weekly throughout the study from rectal secretions using the GenProbe APTIMA AC2 NAAT system. Samples that were STI-negative were re-tested by Cepheid GeneXpert CT/NG (Cepheid Inc; Sunnyvale, CA) and an in-house multiplex RT-PCR (11). To maintain infection, all animals were boosted with an STI inoculation every 4 weeks. In addition, animals testing STI-negative were STI-boosted immediately. Real-time PCR was also used to differentiate the two serovars, CT-E and LGV-L2, in STI-positive samples. DNA was extracted from APTIMA swabs using the Qiagen QIAamp DNA Mini Kit (Qiagen, Valencia, CA, USA). At study completion, chlamydial infections were cleared as previously described (10).
Table 1.
Differentiating the two serovars of Chlamydia using an in-house multiplex real-time PCR (first nine weeks of the study); serovar E was inoculated in 7 animals (STI arm) at week 1 followed by inoculation of serovar LGV-L2 at week 4. The first SHIV163p3 challenge (red arrow) was in week 5; at week 8, both serovars were inoculated in the animals. Throughout the study, the animals received a boost of Chlamydia (both serovars) every 4 weeks, and up to 20 SHIV challenges two times a week (red arrows).
| E | LGV | SHIV E+LGV | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
|
| ||||||||
| Animal # | Week 1 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | Week 9 |
| 1 | ND | E | E | E | E* | M | M | E |
| 2 | ND | E | E | M | M | M* | M | M |
| 3 | ND | E | N | N | N* | E | N | E |
| 4 | ND | E | E | M | E | N | E | E |
| 5 | ND | E | E | E | E | E | E | E |
| 6 | ND | E | E | M | LGV | E | E | E |
| 7 | ND | E | E | E | E | N | E | E |
=animal SHIV-infected;
ND=not determined; E=serovar E; LGV=serovar LGV-L2; M=mixed population; N=negative result (no Chlamydia detected). Animal #4 was infected after 14 SHIV challenges (this table shows data only up to 9 viral challenges).
SHIV challenges and analyses of SHIV infections
The risk of SHIV acquisition was evaluated using a previously described repeat-exposure SHIV transmission model (12) where the animals were rectally challenged with SHIV twice a week up to 20 challenges (10TCID50; see study design Figure 1). Levels of plasma and rectal viral RNA (eluted from Merocel sponges; Medtronic, Jacksonville, FL, USA) were measured by reverse-transcriptase PCR (limit of detection: 50 copies/ml) (13). Infections were confirmed by additional in-house SHIV proviral real-time PCR assays and EIA detection of HIV envelope-specific antibodies (Bio-Rad HIV-1/HIV-2 Plus O EIA, Hercules, CA). Animals were deemed uninfected if they remained seronegative for SHIV antibodies and had undetectable SHIV RNA and DNA after 20 SHIV challenges.
Chemokine/cytokine measurements
Rectal secretions eluted from Merocel sponges were assessed for cytokines, chemokines, and other inflammatory markers (IL-1β, IL-1Ra, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-13, IL-15, IL-17, IL-18, G-CSF, GM-CSF, IFN-γ, TNF-a, MIP1α, MIP1β, MCP1, TGFA, VEGF, sCD40) using Luminex bead technology (Bioplex, Biorad, Hercules, CA) and a non-human primate cytokine bead panel (Millipore, Billerica, MA). Using Quick Start Bradford protein assay (Biorad, Hercules, CA), protein measurements were normalized against total soluble protein levels in the eluted secretions.
Histopathology and Immunohistochemical evaluation
At baseline (2–3 weeks before STI or control inoculation), rectal pinch biopsies (1–2mm; three from each animal) were collected ~5cm cranial to the anal sphincter. Biopsies were also collected one to two weeks after the last STI boost from all SHIV-uninfected animals, including the controls. Biopsies were fixed in 10% neutral buffered formalin for 72 hours and then stored in 70% ethanol at room temperature. Three to four weeks after the last STI boost, rectal tissues were collected from all SHIV-positive monkeys at necropsy. Tissue specimens were processed by routine paraffin histology, sectioned at 4 microns, and stained by hematoxylin-eosin and immunohistochemistry using an immunoalkaline phosphatase reaction with naphthol fast red substrate and the following primary antibodies: mouse monoclonal antibodies raised against CT (Washington Research Foundation, Seattle, WA), CD68 (Thermo Fisher Scientific; Fremont, CA), CD79a (Santa Cruz Biotechnology, Santa Cruz, CA), and CD20 (Dako, Carpinteria, CA), and a rabbit polyclonal antibody raised against CD3 (Dako). Positive control for the CT IHC comprised the same CT L2 or CT-E strain cultured in McCoy cells, mixed with tissue fragments and embedded in paraffin using the same protocol as for tissue specimens. Uninfected rhesus macaque lymph node and spleen tissues served as positive controls for cell marker IHC assays. Negative controls were run in parallel, and consisted of tissue sections incubated with normal rabbit or mouse serum in place of primary antibody.
Statistical analysis
Risk for SHIV acquisition in animals with and without STI was compared using Fisher’s exact test. All viral loads were log transformed before analysis. The Wilcoxon rank-sum test was used to test if peak viremia in the two arms was statistically different. We compared the magnitude of the effect of plasma viral load on rectal virus shedding in animals with and without STI. To do this, an interaction was introduced to a mixed model for repeated measures. The mixed model was used to account for dependence in the data due to multiple observations on the same monkey. The Wilcoxon rank-sum test was also used to determine whether changes in cytokine measurements were statistically different relative to baseline. SAS version 9.3 (SAS Institute, Inc., Cary, NC) or GraphPad Prism 5 for Windows (GraphPad Software, San Diego, CA) was used for all statistical analyses.
RESULTS
STI infection and viremia
Productive CT infection was confirmed by GenProbe APTIMA AC2 NAAT system before the animals were SHIV-challenged. Swabs from the first 9 weeks were further tested to determine if one Chlamydia serovar dominated. Most STI-positive rectal samples showed serovar CT-E dominating over LGV-L2 (Table 1). After the inoculation of serovar CT-E in the seven STI animals at the beginning of the study (week 1), and then serovar LGV-L2 in week 4, it was observed in the subsequent weeks that CT-E was dominant in 6/7 macaques. Also, CT-E infection was sustained in 6/7 animals for up to 3 weeks (Table 1), before LGV-L2 was introduced in week 4. One animal (#2) showed both CT serovars. It was unclear whether serovar domination affected the timing of SHIV infection because of the small sample size of infected animals; although animal #2 was infected after 4 challenges in presence of a mixed population (Table 1; CT-E and LGV-L2), animal #3 was infected after 2 challenges with serovar E dominating LGV-L2, animal #1 was infected after 2 challenges despite temporary resolution of serovar E infection. Animal #4 became infected late, after 14 challenges (not shown in table).
There was no significant difference in risk of SHIV acquisition between the two arms (Fisher’s Exact p=1.0). Four of seven animals in the STI arm (57%) became SHIV-infected, while 3/7 animals in the control arm (43%; figure 2) became infected. It took fewer viral challenges (median of 3) to infect animals in the STI arm compared to those in the control arm (median of 5), but the difference was not statistically significant (Table 2; Wilcoxon Exact p=0.59). We compared viremia in the two arms to study the effect of STI co-infection, and found that the difference in peak plasma viremias in the two arms was not statistically significant (Wilcoxon Exact p=0.63; table 2; figure 2). The levels of viremia (Table 2) in the STI arm reached 7.15 log10 RNA copies/mL (median: 6.5 log10 RNA copies/mL), compared to 7.51 log10 RNA copies/mL in the control arm (median: 6.76 log10 RNA copies/mL). To determine if the presence of STIs was associated with increased rectal shedding, we compared the levels of rectal viremia in the two arms and observed no differences (data not shown). We also analyzed rectal SHIV shedding in the context of plasma viremia (Figure 3A, B), and found that the magnitude of the effect of plasma viremia on rectal shedding was different by arm (p=0.038). It was observed in the scatterplot that the STI animals shed more virus at lower viremias (between 3.0 and 5.0 log10 RNA copies/mL; figure 3B). When we compared the viremias in this range, the STI animals had significantly higher rectal shedding (Figure 3C; p=0.049, one-sided t-test with Welch’s correction). However, this comparison lost statistical significance after applying a correction for repeated measures (p=0.089).
Figure 2.
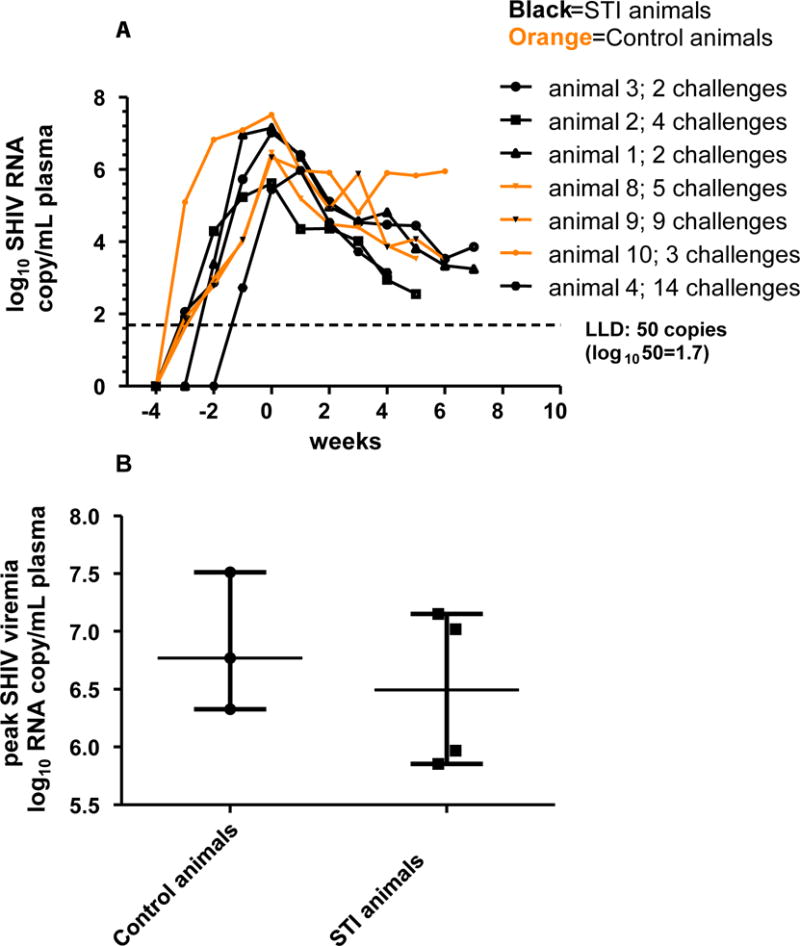
Viremia in the SHIV-infected infected STI (black) and control (orange) animals; 0=time of peak plasma viremia; plasma viral RNA was measured by RT PCR (limit of detection: 50 copies/ml)
Table 2.
SHIV infections and viremia in the two study arms. There were n=7 animals in each arm.
| Control arm | STI arm | |
|---|---|---|
| Number of SHIV infections | 3/7 | 4/7 |
| Median number of SHIV challenges | 5.0 | 3.0 |
| Median plasma viremia (log10 RNA copies/mL) | 6.75 | 6.49 |
| Peak plasma viremia (log10 RNA copies/mL) | 7.51 | 7.15 |
Figure 3.
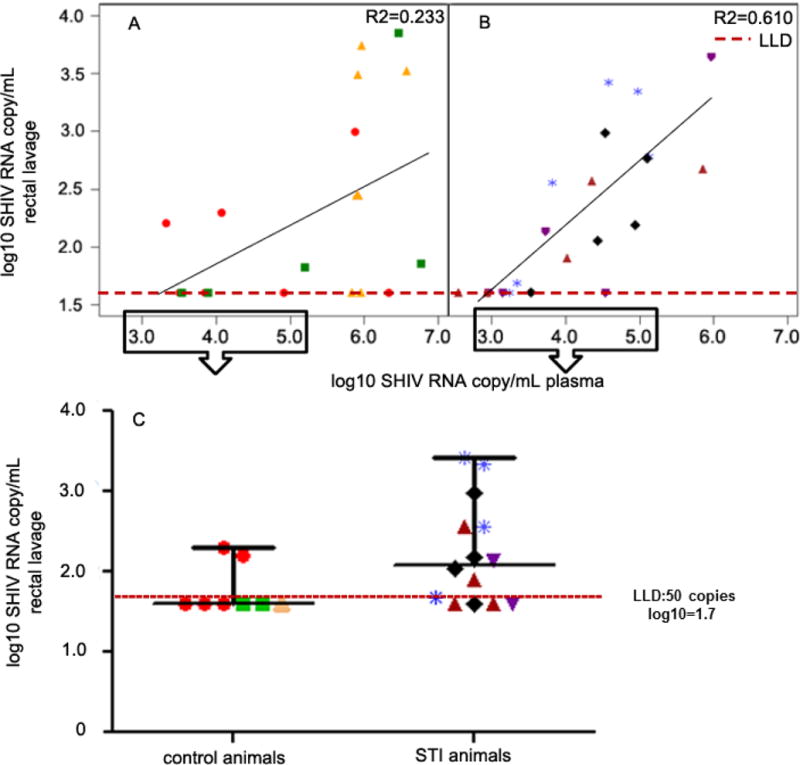
Scatterplot showing plasma and rectal viremia (paired readings) in the two arms (A, control; B, STI). The data points represent readings from infected animals where both plasma and rectal viremia data are available; there were time points where we had insufficient quantities of eluted rectal secretions; C: comparison of rectal viremia in the two arms at plasma viral loads <5.0 and >3.0 log10 RNA copies/mL; we selected data points in this range to emphasize the higher levels of rectal shedding observed in the STI animals at lower viremia; lower limit of detection (LLD) was 50 copies/mL (log10 =1.7) so for purposes of statistical analyses and graphical representation, we changed all undetected viral loads to 40, represented as data points below the LLD; 6/8 data points (75%) were below LLD in the control arm and 4/14 (29%) were below LLD in the STI arm; p=0.049, unpaired two-tailed t-test with Welch’s correction; p=0.089 after applying repeated measures.
Cytokine analyses
To test our hypothesis that the levels of local inflammation would be higher in presence of STI infection, we compared rectal cytokine levels, including pro-inflammatory proteins, in both arms just before SHIV challenges. Our results showed no differences in pro-inflammatory rectal cytokine levels (supplemental digital content 1). To ascertain the effects of SHIV infection on rectal inflammation, we then compared baseline (STI- and SHIV-naïve) and post-SHIV infection cytokine levels in each arm. We found that, post-SHIV infection, the levels of pro-inflammatory cytokines like IL-8 (one-sided Fisher’s Exact Test; p=0.046) and G-CSF (p=0.025) were significantly higher in the control arm (data not shown); this was not observed in the STI arm. Levels of other cytokines including IL-6 (p=0.090), TNF-α (p=0.090), and IFN-γ (p=0.070) were also higher post-SHIV infection in the control animals but without statistical significance.
Histopathology and immunohistochemistry
To test our hypothesis that there would be significant structural and/or inflammatory changes in the rectal mucosa post-CT infection, we compared tissue samples for all STI animals pre- and post-CT infections. We found neither significant histopathologic changes, nor CT antigen detected in any animal by IHC (data not shown), despite demonstration of cross-reactivity of the antibody with the CT-E and CT-LGV-L2 strains in formalin-fixed paraffin-embedded cell cultures, and known broad reactivity with CT in general. One STI animal had acute inflammation in baseline (pre-inoculation) rectal biopsies. Others showed intact surface mucosa and mononuclear cell populations in the lamina propria within the normal range for this species at all tissue collection time points (data not shown). Our findings are similar to those previously observed in CT-infected rhesus macaques (TR Henning, unpublished).
DISCUSSION
We used a previously developed co-infection macaque model (TR Henning, unpublished) (10) to investigate whether there would be an increased risk of rectal SHIV acquisition in the presence of Chlamydia co-infection. To our knowledge, this is the first such rectal SHIV challenge study in CT/SHIV co-infected rhesus macaques. Since superinfection with multiple CT serovars is quite common (14), especially among those with multiple sexual partners, we used two CT serovars, CT-E and LGV- L2, hypothesizing that this would render the local milieu more susceptible to SHIV acquisition. We employed a low virus dose conjecturing that the presence of CT would diminish the animals’ innate resistance to SHIV and accelerate the acquisition of infections in the STI arm compared to the control arm. Our results showed that the risk of SHIV acquisition is not significantly enhanced by rectal CT, although there were 4 animals in the STI arm that were infected compared to 3 in the control arm. Additionally, the average number of SHIV challenges required for infection was lower (but not statistically significant) in the STI arm compared to the control arm. Although we boosted the animals regularly with CT to maintain rectal STI infection and confirmed the infections by multiple molecular assays, variations in Chlamydia detection are possible due to inadequate swabbing of the rectum leading to sub-optimal sample collection, which could be responsible for some negative results.
Our findings are in contrast to vaginal studies in pig-tailed macaques (15), which showed increased risk of intra-vaginal SHIV acquisition in presence of CT and Trichomonas vaginalis infection. The difference between the two studies could be the result of quicker resolution of local tissue inflammation in the rectum likely contributed by the absence of Trichomonas infection. It could also be linked to serovar CT-E dominating in the rectum over the more pathogenic LGV- L2 serovar. This was reflected in the rectal samples in the first nine weeks of our study but more data are needed to confirm these observations throughout the length of study. Also, we did not collect lymph node biopsies or visually monitor lymph node enlargement in the Chlamydia-infected animals, which might have indicated the spread of LGV- L2 infection. It is unlikely the number of viable LGV- L2 inoculated in the macaque rectum was lower than that of CT-E, given that the original high-inoculum stocks were not subjected to thawing and freezing cycles, which could impact cell viability. We first inoculated animals with CT-E in the rectum, and three weeks later, introduced the second serovar LGV- L2 so it is possible that this allowed serovar CT-E to establish dominance in the rectum by the time LGV- L2 was introduced. Quinn et al. had shown in cynomolgus macaques that LGV-L2 had induced stronger inflammatory responses and histopathology compared to CT-E (16). In a previous study (10) (Henning et al; unpublished), the authors looked at rectal cytotoxicity markers in animals infected with LGV- L2 only, CT-E followed by LGV- L2, or CT-E followed by treatment then LGV- L2. They found higher levels of IL-1β, a vital cytokine responsible for CT-related inflammatory mediation and pathology (17, 18) in the LGV-L2 only animals. We did not observe such inflammatory responses in our STI-infected animals but it is of note that we looked at local inflammation after CT-E inoculation and before LGV-L2, and our findings could be the result of CT-E causing less inflammation (16). It is also possible that the inflammatory responses were not higher because the CT load was insufficient despite positive NAAT results. Additionally, it is possible that the levels of pro-inflammatory cytokines would have increased with time in STI-infected animals, but this could not be verified because rectal samples were not collected during SHIV challenges. Compared to Quinn et al. (16) our IHC staining didn’t show inflammatory responses or antibodies against CT but this could be attributed to the difference in macaque species used. Additionally, it’s conceivable that CT colonized a different anatomical region like the small intestine as seen in mouse models (19) but this aspect is unclear in macaques. Such collection of biopsy samples was beyond the scope of our current study.
We were not able to conclusively demonstrate higher SHIV shedding due to rectal Chlamydia infections. These observations are consistent with clinical findings by Kelley et al. who reported no significant enhancement of HIV shedding due to rectal STI co-infection (20). It is possible that rectal HIV shedding may be different from vaginal shedding since Lewis et al., after analyzing data from 39 different studies, concluded that the odds of detecting HIV-1 in the genital tract were significantly increased in the presence of CT and other infections (21). Our data also showed that post-SHIV infection, certain pro-inflammatory cytokines like IL-8 and G-CSF were higher in the control arm compared to the baseline levels, a finding not observed in the STI arm. Although we expected high levels of rectal cytokines in the control animals post-SHIV infection, it was intriguing that the STI animals did not exhibit such a response; it might reflect elevated levels of inflammation that the immune system would have already mounted in chlamydial presence. Long-term effects of Chlamydia on cytokine levels could not be established because no rectal samples were collected during SHIV challenges. Although our results did not show an acute increase in levels of cytokines immediately post-STI infection, past studies (TR Henning, unpublished) have shown elevated levels several days after initial STI infection.
Overall, we found no effect of rectal Chlamydia co-infection on SHIV acquisition. Our results cannot be extrapolated to other rectal STIs particularly ulcerative infections, including syphilis or herpes, which might have an impact on risk of HIV acquisition. In light of increasing numbers of detected cases of rectal CT in the United States, Canada, and the United Kingdom, the use of an animal co-infection model for investigation of susceptibility and potential HIV transmission risk is relevant for both MSM populations and women who engage in unprotected anal intercourse. This macaque model can be further expanded to better investigate the impact of different rectal STIs on HIV acquisition under well-controlled conditions that are difficult to achieve in human clinical studies.
Supplementary Material
Supplemental digital content 1
Comparison of levels of pro-inflammatory cytokines after STI (green) or buffer (black) inoculation (before SHIV challenges). We found no significant differences in the two arms.
SHORT SUMMARY.
Our study in rhesus macaques finds no effect of rectal Chlamydia co-infection on SHIV acquisition.
Acknowledgments
The authors thank Dr. David Garber for programmatic support, James Mitchell and Shanon Ellis for animal procedure assistance; our veterinarians and colleagues in the Animal Resources Branch (ARB) for help with macaque-related work; we appreciate Dominique Rollin for providing cell culture controls for IHC; Heather Hayes for histology and immunohistochemistry support; Chi Kai-Hua for PCR support; Drs. Tara Henning and John Papp for helpful input, and sharing the data from her unpublished paper, which informed the design of this study. SHIV SF162P3 was obtained through the NIH AIDS Reagent Program, NIAID, NIH from Drs. Janet Harouse, Cecilia Cheng-Mayer, Ranajit Pal, and the DAIDS/NIAID. A portion of the animal studies was funded by an interagency agreement (Y1-A1-0681-02) between CDC and NIH.
Source of support: This work was supported by the Centers for Disease Control and Prevention (CDC) and by the National Institutes of Health and CDC (interagency agreement AAI 12041-001-03000).
Footnotes
Conflict of interest: The authors report no conflict of interest.
Disclaimer: The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the Centers for Disease Control and Prevention or the authors’ affiliated institutions.
Author contributions
Conceived and designed the experiments: RDA ENK SAV JMM. Performed the experiments: MRM RDA SAV CZ CP JMR JRP KHC CYC. Analyzed the data: SAV CZ MRM. Wrote the paper: SAV. Statistical analyses: GMK.
References
- 1.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2015. Atlanta: Department of Health and Human Services; 2016. [Google Scholar]
- 2.Gratrix J, Singh AE, Bergman J, et al. Prevalence and characteristics of rectal chlamydia and gonorrhea cases among men who have sex with men after the introduction of nucleic acid amplification test screening at 2 Canadian sexually transmitted infection clinics. Sex Transm Dis. 2014;41(10):589–91. doi: 10.1097/OLQ.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 3.Gratrix J, Singh AE, Bergman J, et al. Evidence for increased Chlamydia case finding after the introduction of rectal screening among women attending 2 Canadian sexually transmitted infection clinics. Clin Infect Dis. 2015;60(3):398–404. doi: 10.1093/cid/ciu831. [DOI] [PubMed] [Google Scholar]
- 4.Mercer CH, Tanton C, Prah P, et al. Changes in sexual attitudes and lifestyles in Britain through the life course and over time: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal) Lancet. 2013;382(9907):1781–94. doi: 10.1016/S0140-6736(13)62035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darville T, Hiltke TJ. Pathogenesis of genital tract disease due to Chlamydia trachomatis. J Infect Dis. 2010;201(Suppl 2):S114–25. doi: 10.1086/652397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner AN, Reese PC, Ervin M, Davis JA, Fields KS, Bazan JA. HIV, rectal chlamydia, and rectal gonorrhea in men who have sex with men attending a sexually transmitted disease clinic in a midwestern US city. Sex Transm Dis. 2013;40(6):433–8. doi: 10.1097/OLQ.0b013e31828fd163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelley CF, Vaughan AS, Luisi N, et al. The Effect of High Rates of Bacterial Sexually Transmitted Infections on HIV Incidence in a Cohort of Black and White Men Who Have Sex with Men in Atlanta, Georgia. AIDS Res Hum Retroviruses. 2015;31(6):587–92. doi: 10.1089/aid.2015.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward H, Ronn M. Contribution of sexually transmitted infections to the sexual transmission of HIV. Curr Opin HIV AIDS. 2010;5(4):305–10. doi: 10.1097/COH.0b013e32833a8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75(1):3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henning T, Butler K, Mitchell J, et al. Development of a rectal sexually transmitted infection–HIV coinfection model utilizing Chlamydia trachomatis and SHIVSF162p3. J Med Primatol. 2014;43(3):135–43. doi: 10.1111/jmp.12103. [DOI] [PubMed] [Google Scholar]
- 11.Chen CY, Chi KH, Alexander S, Ison CA, Ballard RC. A real-time quadriplex PCR assay for the diagnosis of rectal lymphogranuloma venereum and non-lymphogranuloma venereum Chlamydia trachomatis infections. Sex Transm Infect. 2008;84(4):273–6. doi: 10.1136/sti.2007.029058. [DOI] [PubMed] [Google Scholar]
- 12.Otten RA, Adams DR, Kim CN, et al. Multiple vaginal exposures to low doses of R5 simian-human immunodeficiency virus: strategy to study HIV preclinical interventions in nonhuman primates. J Infect Dis. 2005;191(2):164–73. doi: 10.1086/426452. [DOI] [PubMed] [Google Scholar]
- 13.Parikh UM, Dobard C, Sharma S, et al. Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. J Virol. 2009;83(20):10358–65. doi: 10.1128/JVI.01073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnes RC, Suchland RJ, Wang SP, Kuo CC, Stamm WE. Detection of multiple serovars of Chlamydia trachomatis in genital infections. J Infect Dis. 1985;152(5):985–9. doi: 10.1093/infdis/152.5.985. [DOI] [PubMed] [Google Scholar]
- 15.Henning TR, Butler K, Hanson D, et al. Increased susceptibility to vaginal simian/human immunodeficiency virus transmission in pig-tailed macaques coinfected with Chlamydia trachomatis and Trichomonas vaginalis. J Infect Dis. 2014;210(8):1239–47. doi: 10.1093/infdis/jiu240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quinn TC, Taylor HR, Schachter J. Experimental proctitis due to rectal infection with Chlamydia trachomatis in nonhuman primates. J Infect Dis. 1986;154(5):833–41. doi: 10.1093/infdis/154.5.833. [DOI] [PubMed] [Google Scholar]
- 17.Prantner D, Darville T, Sikes JD, et al. Critical role for interleukin-1beta (IL-1beta) during Chlamydia muridarum genital infection and bacterial replication-independent secretion of IL-1beta in mouse macrophages. Infect Immun. 2009;77(12):5334–46. doi: 10.1128/IAI.00883-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdul-Sater AA, Said-Sadier N, Padilla EV, Ojcius DM. Chlamydial infection of monocytes stimulates IL-1beta secretion through activation of the NLRP3 inflammasome. Microbes Infect. 2010;12(8–9):652–61. doi: 10.1016/j.micinf.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perry LL, Hughes S. Chlamydial colonization of multiple mucosae following infection by any mucosal route. Infect Immun. 1999;67(7):3686–9. doi: 10.1128/iai.67.7.3686-3689.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelley CF, Haaland RE, Patel P, et al. HIV-1 RNA rectal shedding is reduced in men with low plasma HIV-1 RNA viral loads and is not enhanced by sexually transmitted bacterial infections of the rectum. J Infect Dis. 2011;204(5):761–7. doi: 10.1093/infdis/jir400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson LF, Lewis DA. The effect of genital tract infections on HIV-1 shedding in the genital tract: a systematic review and meta-analysis. Sex Transm Dis. 2008;35(11):946–59. doi: 10.1097/OLQ.0b013e3181812d15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content 1
Comparison of levels of pro-inflammatory cytokines after STI (green) or buffer (black) inoculation (before SHIV challenges). We found no significant differences in the two arms.


