Abstract
Background
Moderate alcohol consumption is cardioprotective but the mechanism of action remains unclear. Nuclear Factor kappa-B (NFκB) regulates the expression of genes involved in inflammation, stress and apoptosis. We used a swine model of diet-induced metabolic syndrome to investigate the effects of red wine and vodka on NFκB signaling and cytokine activity in chronically ischemic myocardium.
Methods
Yorkshire swine were given a high-fat diet for four weeks; an ameroid constrictor was then placed on the left circumflex artery. The high-fat diet was continued and the swine were divided into three groups for 7 weeks: hypercholesterolemic diet alone (Control, n=8), hypercholesterolemic diet with vodka (Vodka, n=8), and hypercholesterolemic diet with wine (Wine, n=8). Ischemic myocardium was analyzed by Western blot and cytokine array.
Results
Administration of alcohol was associated with decreased expression of IKKα, IKKβ and p-IκBα in the ischemic myocardium compared to the control group. Alcohol administration demonstrated an increase in NFκB in the ischemic myocardium. Both wine and vodka demonstrated a significant decrease in leptin, IL-1α, IL-13, IL-15 and IFN-γ. Vodka demonstrated a significant decrease in phosphorylated BCL-2 and caspase-9.
Conclusions
In ischemic myocardium, alcohol modulates the NFκB pathway, which may contribute to the adaptive response of tissues to the stress of ischemia. Furthermore, both wine and vodka decreased multiple pro-inflammatory cytokines. This study provides a mechanism by which alcohol may be cardioprotective in ischemic myocardium.
Introduction
Diabetes and the associated metabolic syndrome are highly prevalent and growing problem in the world, affecting 422 million people with a global prevalence of 8.5% in 2014, already surpassing past predictions of reaching 7.7% by 2030.1 Diabetes alone was responsible for 1.5 million deaths in 2012,2 eighty-percent of which were due to conditions related to atherosclerosis including stroke, peripheral arterial disease, and most commonly, coronary artery disease.3 Diabetes is known to increase the risk of acute coronary events by three to five-fold over patients without elevated glucose levels.3 The metabolic syndrome associated with type 2 diabetes and insulin resistance further increases the cardiac risks of an already-high risk population. Furthermore, patients with diabetes often have other comorbidities even beyond those of the metabolic syndrome, rendering them even higher risk for coronary revascularization and poorer candidates for percutaneous coronary intervention.4
Besides tight glucose control, there are few therapeutic preventative options for decreasing the risks of coronary artery disease in patients with diabetes. Our lab has previously demonstrated the beneficial effects of wine and vodka on insulin signaling in a swine model of metabolic syndrome. 5 The health benefits of daily alcohol consumption follows a J-shaped curve in which low to moderate doses (1–2 drinks or 15–30g ethanol/day) have the highest benefit, specifically with a reduction of risk of diabetes (30–40%) and cardiovascular disease when compared to those who abstain.6
The up-regulation of the inflammatory response is evolutionarily intended to help heal damaged tissue; however this process is often over-active and as a result, causes irreversible damage to cells and can result in apoptosis. Inflammatory pathways are aberrantly expressed in ischemic myocardial tissue. Nuclear factor kappa B (NFκB) is a transcription factor which regulates numerous genes involved in inflammation. Many of these are cytokines such as tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin-1α (IL-1α), transforming growth factor-beta (TGF-β), IL-13, and IL-15). Cardiomyocytes which have undergone necrosis from ischemic insult have been shown to release IL-1α as a damage signal to activate fibroblasts and leukocytes. IL-1α furthermore leads to induction of cytokines and molecules involved in cellular matrix degradation.7 This signaling appears to be a primary innate immune response which signals via the MyD88-dependent pathway.8 Interferon gamma (IFN-γ) is another well-characterized cytokine involved in inflammation and has been shown to be associated with poor outcomes and increased mortality in patients following myocardial infarctions.9
Beyond the innate inflammatory response, signaling of the adaptive immune response involving monocytes and B-cells is activated by myocardial ischemia. Ischemia triggers an immediate increase in levels of IL-13 and causes up-regulation of transforming growth factor-beta (TGF-β). IL-13 is known to activate the alternative macrophage pathway and activate fibroblasts via TGF-β. In IL-13 knockout mice, recovery from an ischemic event is delayed or does not occur, leading to early demise.10 However, it is not clear what level of IL-13 and TGF-β is optimal for enhanced fibrosis and healing after an event of myocardial ischemia.
Leptin has been identified as an important component of cardiovascular health as it has been shown to be involved in angiogenesis, vascular and endothelial function, and insulin sensitivity.11 Not surprisingly, previous studies have shown that leptin is increased in ischemic heart disease, especially in patients with metabolic syndrome/type 2 diabetes.12 In fact, leptin levels have been shown to be 1.5–2-fold higher in patients with insulin resistance than those without.13
When initial steps of inflammation continue to progress, cells begin to undergo movement towards apoptosis. SDF-1 has been shown to be a regulator of stem cell migration to sites of injury, and is a chemoattractant for endogenous cardiac stem cells.14 Akt (protein kinase B) is a unique signaling kinase active in numerous pathways, and when activated, promotes cell survival.15 It can be phosphorylated at serine 473 by PI3K, which can be activated by various molecules including SDF-1. Another molecule in the Akt pathway is p-BCL-2, which is a proto-oncogene which, when phosphorylated, seems to be correlated with anti-apoptotic activity.16 Therefore, mechanisms to decrease inflammation and minimizing apoptosis in the setting of ischemia are an attractive therapeutic option to improve cardiac healing.
Interestingly, alcohol has been found to attenuate inflammatory signaling. It remains unclear why red wine is often associated with greater cardiovascular benefits than other types of alcohol. Some data tout the antioxidant properties of resveratrol; however others suggest the absorption of resveratrol is often too low to make a significant impact.6 Here, we use a swine model of diet-induced metabolic syndrome and myocardial ischemia to investigate the effects of alcohol with resveratrol in the form of red wine and alcohol without resveratrol in the form of vodka. Given the known cardioprotective effects of alcohol, we hypothesized that alcohol modulates the NFκB pathway and down regulates inflammation and apoptosis signaling in the setting of chronic myocardial ischemia.
Methods
Twenty-four intact male Yorkshire swine were fed a 500g/day high-cholesterol diet consisting of 4% cholesterol, 17.2% coconut oil, 2.3% corn oil, 1.5% sodium cholate, and 75% regular chow (Sinclair Research, Columbia, MO) for four weeks to induce metabolic syndrome.5 Previous data from our group has demonstrated this diet induces obesity, hyperlipidemia, hypertension, and insulin resistance which models metabolic syndrome.17 An ameroid constrictor was then placed on the left circumflex artery to induce chronic myocardial ischemia. The high-fat diet was continued and swine were subdivided into three groups with supplementation of wine or vodka for 7 weeks: hypercholesterolemic diet alone (hypercholesterolemic control [Control], n=8), hypercholesterolemic diet with vodka (hypercholesterolemic vodka [Vodka], n=8), and hypercholesterolemic diet with wine (hypercholesterolemic wine [Wine], n=8). The wine supplementation consisted of 375mL of red wine daily (Black Mountain pinot noir, 12.5% alcohol v/v, 0.3–0.5μg/mL resveratrol, Haro Hills, CA). The vodka supplementation consisted of 112mL of vodka daily (Rubinoff vodka, 40% alcohol v/v, Somerville, MA). The alcohol in each group was designed to be nearly equivalent in daily content; i.e., daily wine intake is 375mL x 12.5% = 46.8, and for daily vodka intake: 112mL x 40% = 44.8. Animals underwent euthanasia and ischemic myocardium was harvested for analysis. Sections from the ischemic area of myocardium were used for all analysis except where expression of NFκB was compared between the ischemic and non-ischemic sections of myocardium.
SURGICAL INTERVENTIONS
Anesthesia was induced with an intramuscular injection of telazol (4.4 mg/kg). Animals were endotracheally intubated, mechanically ventilated at 12 – 20 breaths per minute, and general anesthesia was maintained with a gas mixture of oxygen at 1.5 – 2 liters/min and isoflurane at 0.75 – 3.0% concentration.
Ameroid Constrictor Placement
Animals were given a single dose of antibiotic prophylaxis, intravenous enrofloxacin 5 mg/kg, and general anesthesia was induced and maintained. Animals were prepped and draped in the usual sterile fashion. The heart was exposed through a left mini-thoracotomy. The left atrial appendage was retracted and the proximal left circumflex artery was dissected at the takeoff of the left main coronary artery. The ameroid constrictor was placed around the left circumflex artery (Research Instruments SW, Escondito, CA) approximately 1cm distal to the takeoff from the left main coronary artery. The pericardium was loosely re-approximated followed by a layered closure of the surgical incision. Postoperative pain was controlled with a single dose of intramuscular Buprenorphine (0.03 mg/kg) and 72-hour Fentanyl patch (4 μg/kg). All animals received 325 mg of aspirin daily for thromboembolic prophylaxis starting 1 day pre-operatively and continuing for a total of 5 days. All animals continued perioperative antibiotics: enrofloxacin 68 mg orally daily for 5 days. Experiments using these animals were done to evaluate myocardial perfusion in the chronically ischemic and normal myocardium, insulin signaling, proteomics, endothelial dysfunction, and collateral vessel formation were previously reported.5,18–21
Cardiac Harvest
Under general anesthesia, the heart was exposed through a median sternotomy and animals were euthanized by exsanguination. Cardiac tissue from the ischemic territory in the left circumflex artery distribution as well as from non-ischemic territory was collected for further analysis.
The Institutional Animal Care and Use Committee of the Rhode Island Hospital approved all experiments. Animals were cared for in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” (NIH publication no. 5377-3 1996).
PROTEIN EXPRESSION
Whole-tissue lysates were prepared from homogenized samples using bead homogenization. Protein concentration was determined using a radio-immunoprecipitation assay; Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA).
Western blot analysis
Whole-tissue lysates were fractionated on 4–20% Tris-Glycine gels (Novex™ Midi Protein Gels, Invitrogen, Carlsbad, CA) and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA). Primary antibodies [nuclear factor kappa B (NFκB), phosphorylated nuclear factor kappa B (p-NFκB), inhibitor of kappa-B kinase complex alpha (IKKα), inhibitor of kappa-B kinase complex beta (IKKβ), phosphorylated IKKα/β, inhibitor of kappa-B alpha (IκBα), phosphorylated inhibitor of kappa-B beta (p-IκBα), phosphorylated Akt, heat shock protein of 90kDa (HSP-90), phosphorylated BCL-2, stromal cell-derived factor 1 (SDF-1), caspase-9 and cleaved caspase-9, all from Cell Signaling, Danvers, MA] were diluted 1:1000 and incubated on membranes overnight at 4°C. GAPDH was added to all membranes to correct for loading error. After washing, a secondary anti-rabbit or anti-mouse antibody (Cell Signaling, Danvers, MA) was added and incubated for one hour at room temperature. Visualization was performed using enhanced chemiluminescence and images were captured with a digital camera system (GBox, Syngene, Cambridge, England). Image-J software (National Institutes of Health, Bethesda, MD) was used to quantify band densitometry as arbitrary light units. Protein density data were normalized to GAPDH and reported as fold-change values +/− standard error of the mean compared to control samples.
Cytokine array
Samples from each group (i.e., Control, Wine, Vodka; n=8/group) were pooled together with 25ug of protein in each sample for a total protein amount of 200ug. A protease and phosphatase inhibitor (Thermo Scientific, Rockford, IL) was then added. These pooled samples were then applied to an NFκB Antibody Array membrane (Abcam, Cambridge, MA) and incubated overnight at 4°C. Biotin-conjugated anti-cytokines were applied to the membranes and incubated for 2 hours at room temperature. Horseradish peroxidase-conjugated streptavidin was then added to the membranes and incubated overnight at 4°C. The arrays were visualized using enhanced chemiluminescence and images were captured with a digital camera system (GBox, Syngene, Cambridge, England). Image-J software (National Institutes of Health, Bethesda, MD) was used to quantify band densitometry as arbitrary light units.
STATISTICAL ANALYSES
Data was analyzed using GraphPad Prism 5.0 Software (GraphPad Software Inc., San Diego, CA) with a Kruskal Wallis ANOVA and a Dunn’s post-hoc test for between group comparisons. Results are listed as mean ± SEM with a p value of <0.05 for statistical significance.
Results
Protein Expression
Western Blot Analysis
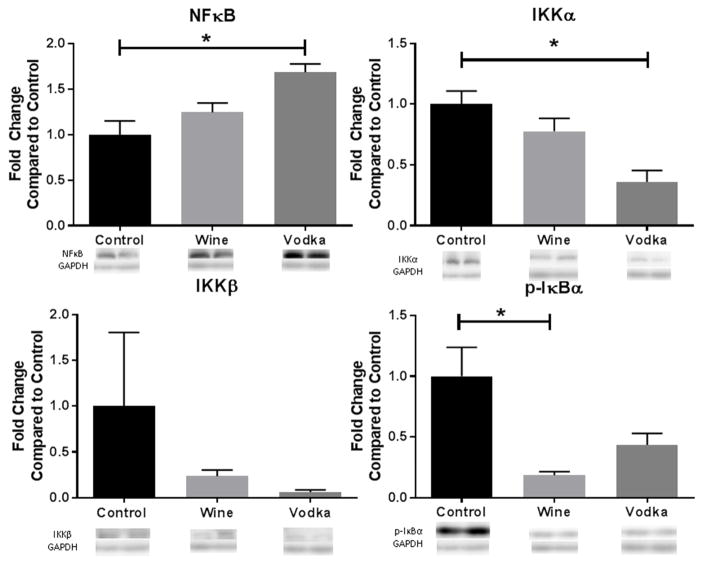
Alcohol supplementation down regulated expression of IKKα, IKKβ and phosphorylated IκBα in the ischemic myocardium in the wine and vodka animals compared to the controls. Alcohol administration also upregulated NFκB (p=0.003) expression in the ischemic myocardium, which was higher in the hypercholesterolemic vodka group than the hypercholesterolemic wine group [Figure 1]. Expression of both IKKα and NFκB was significantly different compared to the controls in the vodka group but not the wine group (IKKα: p=0.004; NFκB: p=0.003). In contrast, phosphorylation of IκBα was significantly decreased in the hypercholesterolemic wine group compared to the control (p=0.001), while p-IκBα in the vodka group was not significantly different from the controls.
Figure 1. Modulation of NFκB Pathway.
Bar diagrams show protein expression levels as fold-change values +/− standard error of the mean compared to control samples. All samples were normalized to GAPDH prior to analysis. Control = hypercholesterolemic diet alone (n=8), Wine = hypercholesterolemic diet with wine (n=8), Vodka = hypercholesterolemic diet with vodka (n=8); * = p<0.05 by Kruskal Wallis ANOVA and a Dunn’s post-hoc test.
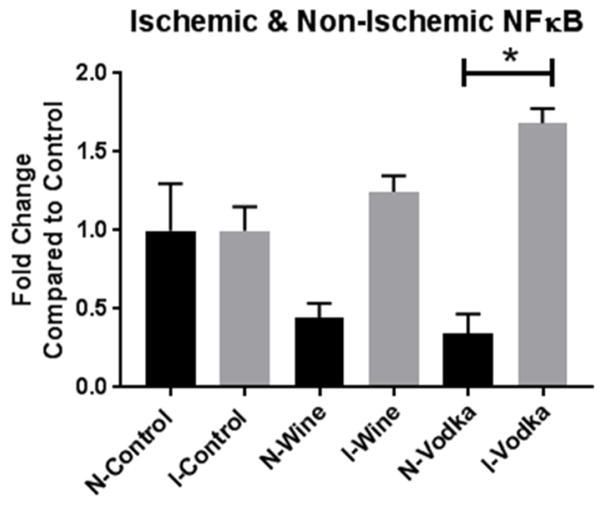
The expression of NFκB was compared between the ischemic and the non-ischemic areas of myocardium in each of the three groups. The levels of NFκB decreased with the addition of wine and vodka in the non-ischemic tissue and increased in the ischemic tissue with wine and vodka compared to the respective controls. There was a statistically significant difference between the ischemic and non-ischemic groups given vodka supplementation (p<0.0001, Figure 2). There was no significant difference between the active, phosphorylated form of NFkB expression (data not shown).
Figure 2. Ischemic versus Non-Ischemic NFκB.
Bar diagrams show NFκB expression levels as fold-change values +/− standard error of the mean compared to control samples. All samples were normalized to GAPDH prior to analysis. Control – hypercholesterolemic diet alone (n=8), Wine = hypercholesterolemic diet with wine (n=8), Vodka = hypercholesterolemic diet with vodka (n=8); * = p<0.05 by Kruskal Wallis ANOVA and a Dunn’s post-hoc test.
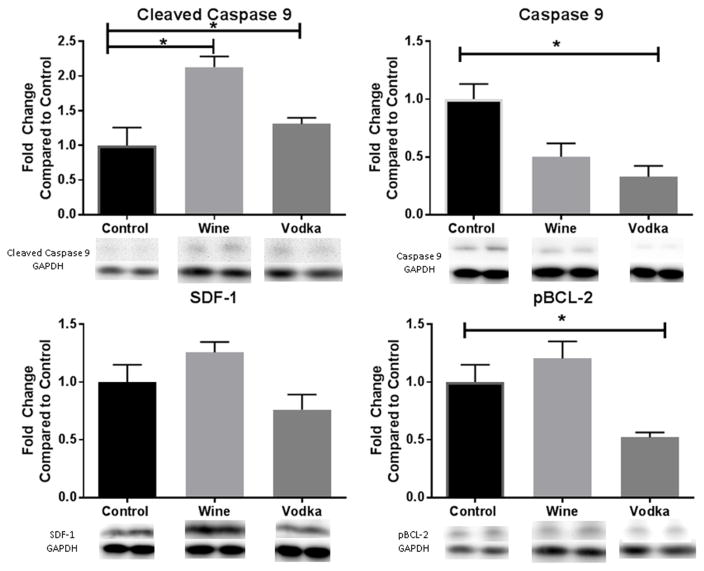
There was a non-significant decrease in both chemokine SDF-1 as well as pro-survival protein phosphorylated-Akt in the ischemic myocardium in the vodka group; however both SDF-1 and p-Akt were non-significantly increased in the wine group.
Chaperone protein heat shock protein of 90kD (HSP-90) was similarly mildly increased in the wine group but not significantly when compared to the control group. Phosphorylated BCL-2 and caspase-9 were both significantly decreased in the vodka group (p=0.0008, p=0.0052, respectively). Conversely, in the wine group, p-BCL-2 was upregulated; caspase 9 was down regulated although neither reached significance. Cleaved caspase-9 was significantly upregulated in the wine group compared to the control (p<0.05), [Figure 3].
Figure 3. Modulators of Apoptosis.
Bar diagrams show protein expression levels as fold-change values +/− standard error of the mean compared to control samples. All samples were normalized to GAPDH prior to analysis. Control – hypercholesterolemic diet alone (n=8), Wine = hypercholesterolemic diet with wine (n=8), Vodka = hypercholesterolemic diet with vodka (n=8); * = p<0.05 by Kruskal Wallis ANOVA and a Dunn’s post-hoc test.
Cytokine Array
There was significantly lower expression of several cytokines in the animals supplemented with alcohol, including pro-inflammatory cytokines leptin (Wine: p=0.02, Vodka: p=0.02), IL-1α (Wine: p=0.01, Vodka: p=0.008), IL-13 (Wine: p=0.04, Vodka: p=0.01), IL-15 (Wine: p<0.001, Vodka: p=0.001), as shown in Table 1. Interferon gamma (IFN-γ), another pro-inflammatory cytokine was also decreased in both the hypercholesterolemic wine and vodka groups when compared to the control (Wine: p=0.01, Vodka: p=0.02). Expression of IL-1β was significantly decreased in the Vodka group (p=0.02) but did not reach significance in the Wine group (p=0.06). Similarly, TGF-β was significantly decreased in the hypercholesterolemic vodka group (p=0.02) and the wine group (p=0.05). TNF-α was not significantly different with alcohol administration in either group when compared to the control group (Wine: p=0.11, Vodka: p=0.30), [Table 1].
Table 1.
Cytokine Array Table: Fold-change from comparison to control
| Cytokine | Control | Wine | Vodka |
|---|---|---|---|
| Leptin | 9807.7 | 4403.2* | 4997.7* |
| IL-1α | 7632.1 | 2298.4* | 521.7** |
| IL-1β | 9141.0 | 1317.6 | 65.0* |
| IL-13 | 4622.2 | 145.7* | 412.4* |
| IL-15 | 10697.1 | 2998.6** | 427.5** |
| IFN-γ | 7360.4 | 1063.2* | 5.0* |
| TNF-α | 1083.7 | 1927.6 | 918.8 |
| TNF-β | 3357.3 | 4482.6 | 1089.8 |
| TGF-β1 | 10083.2 | 1186.7 | 2248.6* |
| SDF-1 | 5842.8 | 98.4** | 1966.2* |
Control = hypercholesterolemic diet alone (n=8), Wine = hypercholesterolemic diet with wine (n=8), Vodka = hypercholesterolemic diet with vodka (n=8);
p<0.05,
p<0.01 when compared to control by Kruskal Wallis ANOVA and a Dunn’s post-hoc test.
Discussion
Our unique swine model of alcohol supplementation in the setting of metabolic syndrome simulates the effects of wine and vodka in a population that is particularly vulnerable to coronary artery disease. Furthermore, this patient population is often unable to undergo coronary bypass surgery as a result of multiple comorbidities which render surgical intervention high risk. Therefore, interventions which mitigate myocardial ischemia in this high-risk group may be particularly beneficial.
Alcohol Modulates the NFκB Pathway and Inflammatory Signaling
Alcohol appears to modulate the NFκB pathway in the setting of myocardial ischemia as demonstrated by the down regulation of many pro-apoptotic and pro-inflammatory cytokines, including leptin, IL-1, IFN-γ, TGF-β, SDF-1, and caspase-9. Modulation of the NFκB pathway is demonstrated by the decreased expression of IKKα after alcohol administration, which decreased phosphorylation of IκBα. When fewer molecules of IκBα are phosphorylated, it remains bound to and inhibits the passage of NFκB into the nucleus, where NFκB up regulates a variety of genes. In this study, we used NFκB p65 (RelA), which is not the active form. Therefore, the increased levels of NFκB seen under the influence of alcohol are presumed to be in the cytoplasm, bound to its inhibitor (IκBα), which is consistent with decrease of IKKα and phosphorylated IκBα we found. Furthermore, we assessed the active form of NFκB, as well as inflammatory cytokines known to be downstream of NFκB including TNF-α and found no significant change in their activity with the additional of alcohol, further suggesting the increased levels of NFκB we found are in their inactive form (i.e., bound to IkBa in the cytoplasm). The increase in NFκB seen in both the wine and vodka group compared to the control echoes previous data in a similar hamster model, in which NFκB was upregulated in animals treated with wine versus a high-fat control.22 That study also demonstrated a decrease in inflammatory signaling. The increase in levels of NFκB with the addition of wine and vodka in the ischemic tissue when compared to controls which was not seen in the non-ischemic myocardium further supports the importance of this inflammatory pathway in areas of ischemia. Many of these gene products further promote inflammation and contribute to the adaptive response of tissues such as the myocardium in response to the stress of ischemia.
Alcohol Modulates Leptin Signaling
Our findings that leptin levels were decreased in animals treated with wine and vodka suggests one mechanism for alcohol-induced cardioprotection. There is one prior study demonstrating an increase in leptin levels with red wine intake in humans, although the difference was only significant in the female population and the dose of alcohol was relatively low.23 A comparison to our data is challenging given all of our animals were male and were fed with a high-fat diet to simulate metabolic syndrome which was not examined in the study by Djurovic et al. Similar to leptin, IL-15 is associated with coronary artery disease and markers such as abdominal obesity that are positively correlated with myocardial ischemia.24 Studies have shown that after an ischemic myocardial event, IL-15 is immediately released.25 Our data demonstrates a significant decrease in IL-15 in the animals that were supplemented with wine and vodka when compared to the controls, again suggestive of a beneficial effect of alcohol on reducing inflammation after myocardial ischemia.
Stromal cell-derived factor-1 (SDF-1, aka CXCL12) is a chemokine involved in response to acute myocardial infarction, heart failure, and peripheral vascular disease. It is known to bind receptors CXCR7 and CXCR4, the latter of which is involved in a signaling cascade via PI3K which modulates reactive oxygen species in mitochondria.14 Our data suggest a decrease in SDF-1 in both the wine and vodka groups, which is unexpected, although, may suggest increased signaling of stem cells to sites in need of repair following ischemia, although further analysis of this pathway to determine significance and other factors are needed.
Our data demonstrates a decrease in IL-1α levels in both the wine and vodka groups which was statistically significant, suggesting alcohol decreases inflammation seen following myocardial ischemia seen with increased levels of IL-1α in that setting. Furthermore, it was statistically significantly lower in the vodka group compared separately to the wine group, suggesting it is not due to resveratrol. This is consistent with previous data from our group, which found administration of alcohol decreases the presence of myocardial adhesions in our swine model.26 Similarly, a statistically significant decrease was seen in IL-1β in the vodka group, but not in the wine group, which further suggests this pathway is dampened by the effects of vodka but not wine We demonstrated a significant decrease in IFN-γ levels in both the wine and vodka groups when compared to the control group, which suggests a protective effect again of the alcohol on post-myocardial ischemia myocyte recovery.
Our data demonstrates a statistically significant reduction in IL-13 from baseline in both wine and vodka groups, and a decline in TGF-β levels in the vodka group when compared to the control, which may be a decline from an over-active inflammatory state to a more normal level of inflammation seen in those without metabolic syndrome.
Alcohol Decreases Signaling in the Apoptosis Pathway
Our data demonstrated non-significantly decreased phosphorylation of Akt and SDF-1 in the group treated with vodka compared to the control. Another molecule in the Akt pathway is p-BCL-2, whose phosphorylation was significantly decreased in the vodka group when compared to the control. The decrease of these anti-apoptotic markers suggests a possible role in decreased inflammation and increased cell survival with vodka but not with wine.
Caspase-9, a pro-apoptotic marker, can be phosphorylated and inactivated by Akt, leading to a decrease in cell death.27 Our data demonstrated a decrease in caspase-9 in both the wine and vodka groups (although only significant in the vodka group) and an increase of cleaved caspase-9 (whose function is less clear) in the wine group, suggesting decreased apoptosis with the addition of wine but not with vodka.
We have previously shown that wine and vodka increase perfusion to ischemic myocardium in our animal model.18 This study provides a mechanism by which alcohol may have a protective effect on the heart via modification of post-myocardial ischemic inflammation and apoptosis. The decrease in inflammatory signaling with alcohol may be protective against scar formation and decreased future myocyte function. Furthermore, decreased apoptosis in ischemic myocardium may preserve cell mass which may preserve myocardial wall thickness and ventricular contractility.
Limitations
There are several limitations to this study. First, the number of animals in each group was kept to a minimum to achieve statistical significance. Also, this study does not address dose-dependent responses or treatment-length responses to exposure to wine or vodka as we used one dose and one fixed course of treatment. Furthermore, although porcine cardiac physiology is closely related to human cardiac physiology, there may be differences that limit extrapolation of this data to humans, including the finding that swine metabolize alcohol faster than humans. Finally, there may be a gender bias given that all of our animals used in this study were male and the effects of alcohol in the female population and effects on myocardial ischemia may vary.
Acknowledgments
We would like to thank the veterinary and animal care staff at Rhode Island Hospital for their excellent care of the animals used in this study.
FUNDING SOURCES
This work was supported in part by the National Heart, Lung, and Blood Institute (R01HL46716, R01HL69024) to Dr. Sellke; NIH T32 GM065085-12 training grant to Dr. Scrimgeour; NIH/NIGMS training grant 2T32 GM065085 to Dr. Potz, and T32 funding grant 5T32-HL094300-03 to Dr. Elmadhun.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Diabetes. WHO; [Accessed September 27, 2016]. http://www.who.int/mediacentre/factsheets/fs312/en/. Published 2016. [Google Scholar]
- 3.Lilly Leonard S. Pathophysiology of Heart Disease: A Collaborative Project of Medical Students and Faculty. 5. Baltimore, MD: Wolters Kluwer/Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 4.Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for Multivessel Revascularization in Patients with Diabetes. N Engl J Med. 2012;367(25):2375–2384. doi: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 5.Elmadhun NY, Lassaletta AD, Chu LM, Bianchi C, Sellke FW. Vodka and wine consumption in a swine model of metabolic syndrome alters insulin signaling pathways in the liver and skeletal muscle. Surgery. 2012;152(3):414–422. doi: 10.1016/j.surg.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klatsky AL. Alcohol and cardiovascular diseases: where do we stand today? J Intern Med. 2015;278(3):238–250. doi: 10.1111/joim.12390. [DOI] [PubMed] [Google Scholar]
- 7.Frangogiannis NG. Inflammation in cardiac injury, repair and regeneration. Curr Opin Cardiol. 2015;30(3):240–245. doi: 10.1097/HCO.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lugrin J, Parapanov R, Rosenblatt-Velin N, et al. Cutting edge: IL-1α is a crucial danger signal triggering acute myocardial inflammation during myocardial infarction. J Immunol. 2015;194(2):499–503. doi: 10.4049/jimmunol.1401948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prondzinsky R, Unverzagt S, Lemm H, et al. Acute myocardial infarction and cardiogenic shock: prognostic impact of cytokines: INF-γ, TNF-α, MIP-1β, G-CSF, and MCP-1β. Med Klin Intensivmed Notfmed. 2012;107(6):476–484. doi: 10.1007/s00063-012-0117-y. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann U, Knorr S, Vogel B, et al. Interleukin-13 deficiency aggravates healing and remodeling in male mice after experimental myocardial infarction. Circ Heart Fail. 2014;7(5):822–830. doi: 10.1161/CIRCHEARTFAILURE.113.001020. [DOI] [PubMed] [Google Scholar]
- 11.Ku I, Farzaneh-Far R, Vittinghoff E, Zhang M, Na B, Whooley MA. Association of low leptin with cardiovascular events and mortality in patients with stable coronary artery disease: The Heart and Soul Study. Atherosclerosis. 2011;217(2):503. doi: 10.1016/j.atherosclerosis.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vavruch C, Länne T, Fredrikson M, Lindström T, Östgren CJ, Nystrom FH. Serum leptin levels are independently related to the incidence of ischemic heart disease in a prospective study of patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:62. doi: 10.1186/s12933-015-0208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gruzdeva O, Uchasova E, Belik E, Dyleva Y, Shurygina E, Barbarash O. Lipid, adipokine and ghrelin levels in myocardial infarction patients with insulin resistance. BMC Cardiovasc Disord. 2014;14:7. doi: 10.1186/1471-2261-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penn M, Pastore J, Miller T, Aras R. SDF-1 in myocardial repair. Gene Ther. 2012;19:583–587. doi: 10.1038/gt.2012.32. [DOI] [PubMed] [Google Scholar]
- 15.Oudit GY, Penninger JM. Cardiac regulation by phosphoinositide 3-kinases and PTEN. Cardiovasc Res. 2009;82(2) doi: 10.1093/cvr/cvp014. [DOI] [PubMed] [Google Scholar]
- 16.Haldar S, Jena N, Croce CM. Inactivation of Bcl-2 by phosphorylation. Proc Natl Acad Sci. 1995;92(10):4507–4511. doi: 10.1073/pnas.92.10.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lassaletta AD, Chu LM, Robich MP, et al. Overfed Ossabaw swine with early stage metabolic syndrome have normal coronary collateral development in response to chronic ischemia. Basic Res Cardiol. 2012;107(2):243. doi: 10.1007/s00395-012-0243-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu LM, Lassaletta AD, Robich MP, et al. Effects of red wine and vodka on collateral-dependent perfusion and cardiovascular function in hypercholesterolemic swine. Circulation. 2012;126(11 Suppl 1):S65–72. doi: 10.1161/CIRCULATIONAHA.111.082172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elmadhun NY, Sadek AA, Sabe AA, Lassaletta AD, Sellke FW. Alcohol and the heart: A proteomics analysis of pericardium and myocardium in a swine model of myocardial ischemia. Ann Thorac Surg. 2015;100(5):1627–1635. doi: 10.1016/j.athoracsur.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 20.Elmadhun NY, Lassaletta AD, Burgess T, Sabe AA, Sellke FW. Alcohol consumption improves insulin signaling in the myocardium. Surgery. 2013;154(2):320–327. doi: 10.1016/j.surg.2013.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lassaletta AD, Chu LM, Elmadhun NY, et al. Cardioprotective effects of red wine and vodka in a model of endothelial dysfunction. J Surg Res. 2012;178(2):586–592. doi: 10.1016/j.jss.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romain C, Bresciani L, Gaillet S, et al. Moderate chronic administration of Vineatrol-enriched red wines improves metabolic, oxidative, and inflammatory markers in hamsters fed a high-fat diet. Mol Nutr Food Res. 2014;58(6):1212–1225. doi: 10.1002/mnfr.201300853. [DOI] [PubMed] [Google Scholar]
- 23.Djurovic S, Berge KE, Birkenes B, Braaten Ø, Retterstøl L. The effect of red wine on plasma leptin levels and vasoactive factors from adipose tissue: A randomized crossover trial. Alcohol Alcohol. 2007;42(6) doi: 10.1093/alcalc/agm056. [DOI] [PubMed] [Google Scholar]
- 24.Dozio E, Malavazos AE, Vianello E, et al. Interleukin-15 and soluble interleukin-15 receptor α in coronary artery disease patients: association with epicardial fat and indices of adipose tissue distribution. PLoS One. 2014;9(3):e90960. doi: 10.1371/journal.pone.0090960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turillazzi E, Di Paolo M, Neri M, Riezzo I, Fineschi V. A theoretical timeline for myocardial infarction: immunohistochemical evaluation and western blot quantification for Interleukin-15 and Monocyte chemotactic protein-1 as very early markers. J Transl Med. 2014;12:188. doi: 10.1186/1479-5876-12-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lassaletta AD, Chu LM, Elmadhun NY, et al. Mechanism for reduced pericardial adhesion formation in hypercholesterolemic swine supplemented with alcohol. Eur J Cardiothorac Surg. 2013;43(5):1058–1064. doi: 10.1093/ejcts/ezs488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cardone MH. Regulation of Cell Death Protease Caspase-9 by Phosphorylation. Science (80-) 1998;282(5392):1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]