Abstract
Setting
IMPAACT P1041 was a TB prevention trial in children enrolled from 2004–2008, during the combination antiretroviral therapy (ART) rollout in South Africa.
Objective
To estimate TB incidence and mortality and the effect of ART.
Design
Children were pre-screened to exclude TB disease and exposure, screened 3-monthly, and provided post-exposure isoniazid prophylaxis therapy (IPT). TB diagnoses were definite, probable, or possible and mortality all-cause. Testing was at the 5% significance level.
Results
In 539 children age 3–4 months at entry followed a median (interquartile range) of 74 (48, 116) weeks, incidence/100 person-years was 10.67 (95% confidence interval (CI): 8.47, 13.26) for any TB and 2.89 (95% CI: 1.85, 4.31) for definite/probable TB. Any TB incidence was 9.39, 13.59, and 9.83/100 person-years before, < 6 months after, and ≥6 months after ART initiation, respectively. Adjusted analysis showed a non-significant increase in any TB (hazard ratio=1.32 (95% CI: 0.71, 2.52)), p=0.38) and significant mortality decrease after ART initiation (hazard ratio=0.39 (95% CI: 0.17, 0.82), p=0.017).
Conclusions
ART reduced mortality but not TB incidence in P1041, possibly attributable to regular active monitoring for TB exposure with post-exposure IPT; studying this as a strategy for TB prevention in HIV-infected children may be warranted.
Keywords: TB, perinatal HIV infection, antiretroviral therapy, mortality
INTRODUCTION
World Health Organization (WHO) estimated 450,000 incident tuberculosis (TB) cases in South Africa in 2014, 60% occurring in HIV co-infected individuals.1 Diagnosing TB in young children is challenging, particularly in areas with high burden.2 Less than 15% of childhood TB cases are sputum acid-fast bacilli (AFB) smear positive, as sampling is difficult and samples often paucibacillary.3 Nevertheless, several reports have quantified the burden of TB in South African children. Hesseling et al. showed that the risk of culture-confirmed TB in HIV-infected infants (≤12 months) was 24 times that in HIV-uninfected infants in the Western Cape prior to widespread use of ART.4 Dangor et al. noted a temporal association during expanding antiretroviral therapy (ART) coverage between 2004 and 2009 in South Africa and reduction in hospitalizations for culture-confirmed TB in HIV-infected (70.6%) and HIV-uninfected (41.3%) children 3 month to <15 years.5 The CHER trial comparing immediate vs deferred ART showed a 59% lower TB incidence with immediate ART in children a median of 7.4 weeks of age at enrollment.6
Among HIV-infected adults, the decrease in TB incidence with ART is well documented. A 2012 meta-analysis showed that ART was associated with a 65% reduction in TB incidence with the greatest benefits observed in those with the lowest CD4 counts.7 In adults, the benefits of ART on TB incidence have been observed in both high and low TB burden settings.8,9 Studies in South African children with median age of 23.5 months (Walters et al5,10,11) and 6.3 year (Martinson et al.5,10,11) have shown the benefits of ART on TB5,10,11, but data in young children are sparse.
Contact investigation, with IPT for TB-exposed, asymptomatic children who are HIV-infected or age<5 years, is often inadequately implemented in low-income countries.12 In a 1997 South African study of household contacts of TB cases <5 years of age, while only 4% were taken to hospital because of symptoms, 34% upon examination were symptomatic and had TB disease and 14% had latent infection.13 While contact tracing, where index cases with TB identify their contacts for investigation, differs from screening of presumably unexposed individuals for symptoms and contact to an active case, these results suggest a potential differing role of ART for TB prevention in the setting of active screening.14
IMPAACT P1041 was a trial that enrolled during the public sector rollout of ART in South Africa. Using P1041 data, we describe the incidence of TB and estimate the effect of ART on TB incidence and all-cause mortality in a setting of active screening.
STUDY POPULATION AND METHODS
Population
This study is a secondary analysis of IMPAACT P1041, a phase II/III, trial to evaluate the efficacy of primary isoniazid (INH) prophylaxis in HIV-infected and HIV-exposed, uninfected (HEU), BCG-vaccinated infants. Infants ages 91–120 days received either daily INH or placebo for 96 weeks and were followed for an additional 96 weeks. No differences were found between INH and placebo groups.15 We included children enrolled at South African sites and studied participants according to their confirmed HIV status at baseline.
Screening for TB disease, infection, and exposure prior to study entry
Infants with a history of TB infection/disease or contact with a smear- or culture-positive TB case, including the mother if treatment had not been completed by delivery, were excluded from participation in P1041. Further details of the enrollment screening process were previously reported.16
Screening for TB disease and exposure during the study
Children were monitored for symptoms of TB disease and contact with a smear- or culture-positive TB case at 3-month intervals. For those exposed to TB, study drug was discontinued and open-label INH given following South African guidelines.17
TB disease definition
Evaluations for children suspected of having TB are detailed elsewhere.15 Briefly, participants were evaluated according to an algorithm to screen for and diagnose TB, and diagnoses classified as either definite, probable, or possible (see Supplemental Materials) and verified by an endpoint review group. We studied: (1) “any TB” defined as definite, probable, or possible TB; (2) definite/probable TB; and (3) death (all-cause).
Use of antiretroviral therapy
ART was provided according to South African HIV treatment guidelines. History of ART usage and initiation or changes during follow-up were collected. ART must have been composed of at least three antiretroviral drugs from at least two classes.
Statistical methods
Overall incidence rates for TB were calculated for both HIV-infected and HEU children, the latter to serve as a reference point rather than for statistical comparison. Among HIV-infected children, TB incidence was estimated by baseline WHO HIV immunological classification18 and Centers for Disease Control and Prevention (CDC) HIV disease classification, by timing from ART initiation (<180 days and ≥180 days; the latter should exclude most HIV-associated immune reconstitution inflammatory syndrome (IRIS)19).
Cox proportional hazards models were used to assess TB incidence and mortality by ART-use status, HIV disease severity, sex, birth weight, weight-for-age z-score, WHO immunological classification, breastfed status, maternal history of TB, P1041 study arm, and household characteristics: ≥3people sleeping/room, persons aged ≥55 years, and smoking status. ART-use status was time-updated; once ART was initiated (including before study entry), the participant was assumed treated for the duration of follow-up. Multivariable Cox models for TB outcomes adjusted for fixed or time-updated covariates selected based on univariate baseline covariate analysis with p≤0.2 supplemented with CDC HIV disease and WHO immunological classifications. Multivariable Cox models for the death outcome were similarly developed.
Marginal structural models were used to model the association of ART-use status and TB disease in the presence of time-varying confounding.20,21 This required exclusion of participants initiating ART at or before study entry due to inadequate covariate histories. Details of the marginal structural model analysis are provided in the Supplementary Materials.
Among those receiving post-exposure IPT, we calculated TB incidence with and without ART using 6-month follow-up post-IPT initiation (WHO recommended prophylaxis period) and assessed time-updated ART-use status using Cox models.
Analyses were carried out in SAS 9.2. Testing was two-sided at the 5% significance level. Reported p-values are nominal.
Ethical Considerations
IMPAACT P1041 was approved by the institutional review boards of participating sites, the Medicines Control Council of South Africa, and the Division of AIDS at NIAID. Written informed consent for participation was obtain from the legal guardians before randomization.
RESULTS
Figure 1 shows the participant disposition by HIV status and enumerates study outcomes. Table 1 shows demographic characteristics of participants. Enrollment was from December 2004 to June 2008. The 539 confirmed HIV-infected children were enrolled at a median age of 14 weeks and followed for a median of 74 weeks, i.e., until they were a median of 89 weeks of age (IQR from 61 to 201 weeks). Fifty-six percent were female, 63% lived in formal houses, and 21% accessed water from communal taps. Seven percent of children had mothers previously treated for TB. Thirteen percent of children had been breastfed.
Figure 1.
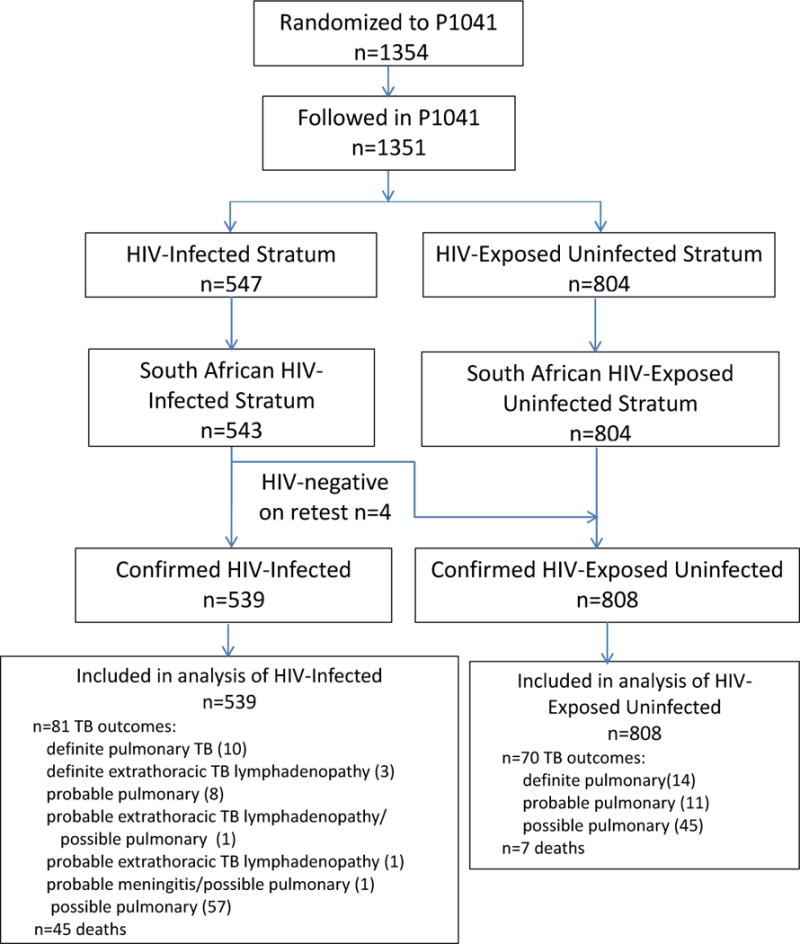
Disposition of Study Participants
Table 1.
Characteristics of HIV-infected and HIV-exposed uninfected infants from South African sites in IMPAACT P1041
| Characteristic | HIV-infected (N=539)* n (%) |
HIV-uninfected (N=808)* | |
|---|---|---|---|
| Female | 304 (56%) | 396 (49%) | |
|
| |||
| INH randomized group | 271 (50%) | 404 (50%) | |
|
| |||
| Site | Johannesburg | 358 (66%) | 520 (64%) |
| Cape Town | 129 (24%) | 280 (35%) | |
| Durban | 52 (10%) | 8 (1%) | |
|
| |||
| Housing Type | Formal (brick house) | 340 (63%) | 470 (58%) |
| Informal (shack/wooden) | 196 (36%) | 338 (42%) | |
| Dorm/hostel | 2 (<1%) | 0 (0%) | |
|
| |||
| Water access in household | Tap inside house | 187 (35%) | 259 (32%) |
| Tap on plot, single use | 238 (44%) | 315 (39%) | |
| Communal tap | 114 (21%) | 234 (29%) | |
|
| |||
| Ever breastfed | 72 (13%) | 48 (6%) | |
|
| |||
| Mother ever had TB diagnosis | 39 (7%) | 58 (7%) | |
|
| |||
| Study participant birth weight (grams): mean (SD) | 2,819 (565) | 3,026 (471) | |
|
| |||
| WHO weight-for-age z-score: mean (SD) | −1.4 (1.5) | −0.2 (1.1) | |
|
| |||
| CDC clinical HIV category | N | 351 (66%) | |
| A | 141 (26%) | ||
| B | 37 (7%) | ||
| C | 5 (1%) | ||
|
| |||
| CD4%: median (IQR) | 28 (21, 35) | ||
|
| |||
| WHO immunological classification | None | 121 (24%) | |
| Mild | 111 (22%) | ||
| Advanced | 90 (18%) | ||
| Severe | 190 (37%) | ||
|
| |||
| Plasma HIV-1 RNA (copies/ml): median (IQR) | 612,000 (46,000,750,000) | ||
|
| |||
| On ART at or before baseline | 168 (31%) | ||
|
| |||
| Ever initiated ART during P1041 | 450 (83%) | ||
|
| |||
| Age at ART initiation (months): median (IQR) | 4.4 (2.7, 7.2) | ||
|
| |||
| Duration pre-ART during follow-up (weeks): median (IQR) | 8 (0, 23) | ||
|
| |||
| Duration on ART during follow-up (weeks): median (IQR) | 71 (46, 108) | ||
|
| |||
| ART Regimen | 3TC, LPV-r, RTV | 2 (0%) | |
| 3TC, ZDV, LPV-r | 99 (22%) | ||
| 3TC, ZDV, LPV-r, RTV | 5 (1%) | ||
| 3TC, ZDV, NVP | 25 (6%) | ||
| 3TC, ZDV, RTV | 2 (0%) | ||
| d4T, 3TC, EFV | 1 (0%) | ||
| d4T, 3TC, LPV-r | 302 (67%) | ||
| d4T, 3TC, LPV-r, RTV | 7 (2%) | ||
| d4T, 3TC, LPV-r | 1 (0%) | ||
| d4T, 3TC, NVP | 1 (0%) | ||
| d4T, 3TC, RTV | 3 (1%) | ||
| ZDV, 3TC, NVP | 1 (0%) | ||
| ZDV, NVP, LPV-r | 1 (0%) | ||
n (%) for categorical variables, mean (SD) or median (IQR) as indicated for continuous variables.
n (%): frequency (percentage of non-missing); SD: standard deviation; IQR: interquartile range, 3TC: lamivudine, LPV-r: lopinavir-ritonavir, RTV: full-dose ritonavir, ZDV: zidovudine, NVP: nevirapine; d4T: stavudine, ART: combination antiretroviral therapy composed of at least 3 drugs from a minimum of 2 classes.
Absolute CD4 counts and CD4% were not measured in HIV-uninfected participants.
CDC HIV clinical classification and ART-use status are not applicable to HIV-uninfected participants.
At entry, HIV-infected participants had WHO weight-for-age z-score mean (range) was 1.4 (−6.1 to 2.6), 65% of were CDC class N and 26% class A, 37% were classified as having severe and 18% advanced WHO-defined HIV-immunodeficiency, and median plasma HIV-1 viral load was >500,000 copies/ml.
ART regimens taken were predominantly stavudine, lamivudine, and Kaletra and lamivudine, zidovudine, and Kaletra. ART was initiated at or before entry in 31%; the median age at initiation was 4.4 months. By end of study, 83% had initiated ART. The median (IQR) weeks of follow-up pre-ART was 8 (0, 23) and on ART was 71 (46, 108). Total person-years of follow-up pre-ART was 199 and on ART was 674. The median age at the end of follow-up for children receiving ART was 22 months.
Of the 539 HIV-infected children, 81 (15%) were diagnosed with TB (76 pulmonary, 5 extrathoracic lymphadenopathy, 1 meningitis) (Table 2). Incidence of “any TB” was 10.67 cases/100 person-years (p-y) (95% confidence interval [CI]: 8.47, 13.26). Twenty-four (4%) children had definite/probable TB, incidence of 2.89 cases/100 p-y (95% CI: 1.85, 4.31). Forty-five deaths occurred, incidence 5.22/100 p-y (95% CI: 3.18, 7.00).
Table 2.
Incidence rates of TB for HIV-infected South African infants enrolled in IMPAACT P1041 cohort overall and stratified by current age*
| Outcome/category | Number with outcome | Total person-years of follow-up | Incidence rate (per 100 person-years) (95% CI)* |
|---|---|---|---|
| Any TB/age category: | 81 | 759 | 10.67 (8.47, 13.26) |
| <1 year old | 45 | 347 | 12.96 (9.45, 17.36) |
| 1–2 years old | 25 | 283 | 8.83 (5.72, 13.05) |
| >2 years old | 11 | 129 | 8.50 (4.24, 15.22) |
| Probable or definite | |||
| TB/age category: | 24 | 830 | 2.89 (1.85, 4.31) |
| <1 year old | 14 | 358 | 3.91 (2.14, 6.57) |
| 1–2 years old | 7 | 312 | 2.25 (0.90, 4.63) |
| >2 years old | 3 | 160 | 1.88 (0.38, 5.49) |
| Death/age category: | 45 | 860 | 5.22 (3.18, 7.00) |
| <1 year old | 39 | 364 | 10.72 (7.63, 14.66) |
| 1–2 years old | 6 | 324 | 1.85 (0.68, 4,02) |
| >2 years old | 0 | 172 | 0.0 (0.0, 2.14) |
assumes a Poisson distribution for the number of events.
CI: exact confidence interval
“Any TB” incidence was 12.96 for those <1 year, 8.83 for those 1–2 years, and 8.50 cases/100 p-y for those >2 years of age (Table 2). For definite/probable TB, the rates were 3.91, 2.25, and 1.88 cases/100 p-y for ages <1, 1–2, and >2 years of age, respectively. Death rates also declined with age, with rates 10.72, 1.85, and 0.0 cases/100 p-y for ages <1, 1–2, and >2 years of age, respectively.
We also assessed TB incidence rates by baseline WHO immunological and CDC HIV disease classifications (Table 3). By WHO immunological classification, the lowest “all TB” incidence rate was among those with no significant/no HIV-associated immunodeficiency, and highest among those with mild HIV-associated immunodeficiency; a trend test was not significant (p=0.17 any TB, p=0.61 definite/probable TB). Children with severe HIV CDC classification had a higher TB incidence rate, significant for definite/probable TB (p=0.033) but not “any TB” (p=0.57) (trend test with linear score for 4 categories).
Table 3.
Incidence rates of TB and death for HIV-infected South African infants enrolled in IMPAACT P1041 stratified by baseline WHO immune classification, CDC classification, and timing from initiation of combination of antiretroviral therapy (ART)
| Outcome/category | Number with outcome | Total number of person-years | Incidence rates (per 100 person-years) (95% CI) |
|---|---|---|---|
| Any TB/WHO immunological classification: | |||
| None | 10 | 178 | 5.62 (2.69, 10.33) |
| Mild | 21 | 140 | 14.99 (9.28, 22.91) |
| Advanced | 13 | 129 | 10.07 (5.36, 17.21) |
| Severe | 31 | 264 | 11.80 (8.02, 16.75) |
|
Any TB/CDC HIV disease classification: | |||
| CDC class N | 55 | 511 | 10.76 (8.10, 14.01) |
| CDC class A | 17 | 188 | 9.06 (5.27, 14.51) |
| CDC class B or C | 9 | 57 | 15.71 (7.18, 29.84) |
|
Any TB/Timing of ART: | |||
| Not on ART | 18 | 192 | 9.39 (5.56, 14.84) |
| <180 days after ART initiation | 26 | 191 | 13.59 (8.87, 19.92) |
| ≥180 days after ART initiation | 37 | 376 | 9.83 (6.92, 13.56) |
|
Probable or definite TB/WHO immunological classification: | |||
| None | 3 | 183 | 1.64 (0.34, 4.79) |
| Mild | 6 | 157 | 3.81 (1.40, 8.29) |
| Advanced | 6 | 140 | 4.28 (1.57, 5.84) |
| Severe | 9 | 293 | 3.08 (1.41, 5.84) |
|
Probable or definite TB/CDC HIV disease classification: | |||
| CDC class N | 15 | 561 | 2.67 (1.49, 4.41) |
| CDC class A | 3 | 204 | 1.47 (0.30, 4.29) |
| CDC class B or C | 6 | 60 | 9.97 (3.65, 21.70) |
|
Probable or definite TB/Timing of ART: | |||
| Not on ART | 6 | 196 | 3.06 (1.12, 6.66) |
| <180 days after ART initiation | 8 | 200 | 4.01 (1.73, 7.90) |
| ≥180 days after ART initiation | 10 | 434 | 2.31 (1.10, 4.24) |
|
Death/WHO immunological classification: | |||
| None | 9 | 183.52 | 4.90 (2.24, 9.31) |
| Mild | 7 | 166.85 | 4.20 (1.69, 8.64) |
| Advanced | 6 | 147.89 | 4.06 (1.49, 8.83) |
| Severe | 20 | 306.35 | 6.53 (3.99, 10.08) |
|
Death/CDC HIV disease classification: | |||
| CDC class N | 25 | 578 | 4.33 (2.80, 6.39) |
| CDC class A | 14 | 210 | 6.66 (3.64, 11.17) |
| CDC class B or C | 5 | 69 | 7.25 (2.35, 16.91) |
|
Death/Timing of ART: | |||
| Not on ART | 33 | 195 | 16.94 (11.66, 23.80) |
| <180 days after ART initiation | 8 | 202 | 3.96 (1.71, 7.80) |
| ≥180 days after ART initiation | 4 | 452 | 0.89 (0.24, 2.27) |
assumes a Poisson distribution for the number of events.
CI: exact confidence interval
Before ART initiation, incidence of “any TB” was estimated at 9.39 cases/100 p-y (95% CI: 5.56, 14.84). Within 180 days after initiating ART, there was a trend to increased incidence of “any TB” to 13.59 cases/100 p-y (95% CI: 8.87, 19.92), decreased to 9.83 cases/100 p-y (95% CI: 6.92, 13.56) thereafter. This pattern of incidence with respect to timing of ART was mirrored for definite/probable TB; 3.06 cases/100 p-y pre-ART, 4.01 within 180 days, and decreased to 2.31 ≥180 days after initiation, although differences were not statistically significant.
In unadjusted Cox regression models, ≥3 people sleeping in a room, lower weight-for-age z-score, never been breastfed, and ART-use at/before entry were associated with increased risk of “any TB” but not definite/probable TB, the latter possibly underpowered. Of time-updated covariates, lower weight-for-age z-score was significantly associated with increased “any TB” incidence (hazard ratio [HR] = 0.70, p<0.001). Time-updated ART-use status was not a statistically significant predictor of TB incidence (HR=1.51, p=0.15). Time-updated covariates, including ART-use status (HR=1.50, p=0.42), were not significantly associated with definite/probable TB (Table 4), possibly due to low power. In baseline adjusted models, the estimated HR for ART-use status attenuated, remaining statistically non-significant for “any TB” (1.33; p=0.36) and definite/probable TB, (HR =1.198, p=0.74). The marginal structural model used to assess TB risk before and after initiation of ART included data from the 371 children who initiated ART on study. The smaller sample size precluded use of marginal structural models with the definite/probable TB and death outcomes but we evaluated the association of ART with “any TB”. Again, we found no significant association between ART-use status and “any TB” (odds ratio=1.63, p=0.25) (Table 4).
Table 4.
Results from Cox proportional hazards and marginal structural models for estimating and testing the association of combination antiretroviral therapy (ART) use on tuberculosis incidence in HIV-infected South African infants enrolled in IMPAACT P1041
| Model (outcome)/Characteristic | Hazard Ratio (95% CI*) | P-value |
|---|---|---|
| Cox model (any TB)/ | ||
| ART use status (time-updated unadjusted) | 1.51 (0.88, 2.70) | 0.15 |
| ART use status (baseline adjusted) | 1.33 (0.74, 2.51) | 0.36 |
| ART use status (time-updated adjusted) | 1.32 (0.71, 2.52) | 0.38 |
|
Cox model (probable or definite TB)/ | ||
| ART use status (time-updated unadjusted) | 1.50 (0.59, 4.34) | 0.42 |
| ART use status (baseline adjusted) | 1.19 (0.44, 3.55) | 0.74 |
| ART use status (time-updated adjusted) | 1.08 (0.34, 3.57) | 0.90 |
|
Cox model (all-cause mortality)/ | ||
| ART use status (time-updated unadjusted) | 0.22 (0.11, 0.44) | <0.001 |
| ART use status (baseline adjusted) | 0.20 (0.08, 0.45) | <0.001 |
| ART use status (time-updated adjusted) | 0.39 (0.17, 0.82) | 0.017 |
|
Marginal structural model (any TB) | ||
| ART use status | 1.63 (0.71, 3.75) | 0.25 |
CI: confidence interval
Cox models are adjusted for ever breastfed status, WHO weight-for-age z-score, and WHO immunological classification, and ≥3 persons sleeping per room.
Cox model with all-cause mortality outcomes are adjusted for CDC HIV disease classification, WHO immunological classification, log10 viral load, and WHO weight-for-age z-score.
Forty-five HIV-infected children died during follow-up. The mortality rate declined with ART from pre-ART of 16.94/100 p-y (95% CI: 11.66, 23.80) to on ART of 1.81 (95% CI: 0.94, 3.17) (<180 days incidence of 9.96 and ≥180 days incidence of 0.89/100 p-y). When the ≥180 day post-ART initiation period was restricted to after viral load dropped to <400 copies/ml, incidence decreased to 0.0 (95% CI: 0.0, 1.11)/100 p-y. Time-updated ART-use status was associated with reduced mortality, unadjusted (HR=0.22, p<0.001) and adjusted for baseline covariates (HR=0.20 (95% CI: 0.08, 0.45), p<0.001), and adjusted for time-updated covariates (HR=0.39 (95% CI: 0.17, 0.82), p=0.017). In the time-updated adjusted model, weight-for-age z-score (HR=0.58 per unit increase (95% CI: 0.47, 0.71), p<0.001) and viral load (HR=1.79 per log10 increase (95% CI: 1.19, 2.92), p=0.009) were also associated with death.
Seventy-three HIV-infected children received post-exposure IPT. For the 6-month period beginning IPT initiation, 10 children had “any TB”; children had incidence/100 p-y of 109 (95% CI: 30, 279) if not on ART and 25 (95% CI: 9, 55) on ART; HR=0.25 (95% CI: 0.52, 1.09) (p=0.057). Six children had definite/probable TB during the 6 month period, with incidence per 100 p-y of 54 (95% CI: 6, 197) if not on ART and 17 (95% CI: 5, 43) on ART; HR=0.16 (95% CI: 0.02, 1.52) (p=0.088). No children died within 6 months of IPT initiation.
The incidence rates among HEU children were 3.22 cases/100 p-y (95% CI: 2.51, 4.06) and 1.11 cases/100 p-y (95% CI: 0.72, 1.64), for “any TB” and definite/probable TB, respectively, and for death was 0.30 (95% CI: 0.12, 0.64)/100 p-y.
DISCUSSION
We explored the relationship between ART and TB incidence among South African HIV-infected children in whom pre-study exposure to TB had been excluded. The CHER trial, conducted in HIV-infected children similar in age (median 7 weeks) and demographic characteristics but with higher CD4%, demonstrated that children receiving immediate ART had lower TB incidence than those with ART deferred until clinical or CD4 criteria were met.6 The CHER study reported 8.3 TB cases/100 p-y for immediate and 20.2 cases/100 p-y for deferred ART. We did not show a significant difference in TB incidence pre- (9.39 cases/100 p-y) versus180 days post- ART initiation (9.83 cases/100 p-y); incidence rates similar to those in the CHER immediate ART arm.
Prior studies have shown marked reductions in TB incidence following ART initiation. One study involving 298 HIV-infected children whose median age was two years and >80% WHO clinical stage 3 or 4, ART conferred a 78% decrease in TB incidence, but ART in combination with IPT had a larger decrease (89%).22 We found trends toward efficacy of ART for “any TB”, and definite/probable TB during post-exposure IPT but ART was not effective in reducing TB incidence more generally. Exclusion of TB-exposed children or careful monitoring for TB exposure and provision of post-exposure prophylaxis during study follow-up may have been responsible for lack of efficacy of primary INH prophylaxis for TB incidence in P10412,23,24 and may similarly be responsible our inability to demonstrate an effect of ART on TB incidence.
In contrast, ART significantly reduced all-cause mortality, consistent with a wide body of literature. Brady et al in study PACTG 219/219c reported a halving in mortality after initiating a 3-drug regimen from at least two drug classes25. In baseline adjusted models we observed an 80% reduction in mortality hazard associated with current ART-use status.
Our study had several limitations. Few definite/probable TB cases limited our power to study this outcome. Seventy percent of TB cases were “possible” TB perhaps suggesting overdiagnosis, but the proportion was similar among the HEU (64%). Our primary comparisons used models adjusted for potential confounders but residual bias may exist. Our data was limited for applying marginal structural models. We did not collect data to sensitively identify IRIS cases and post-ART initiation follow-up ≥180 days post-ART initiation may inadequately for eliminate IRIS.
CONCLUSIONS
ART use among HIV-infected children in the first two years of life reduced mortality but not TB in the P1041 TB prevention trial. Our results may be explained by excluding TB exposed infants at baseline and active monitoring for TB exposure and symptoms with post-exposure IPT. A study to assess the benefits of systematic periodic screening for TB for children living in high HIV burden settings receiving ART may be warranted. Screenings could coincide with scheduled HIV care visits.
Supplementary Material
Acknowledgments
Sources of funding: Overall support for the International Maternal–Pediatric–Adolescent AIDS Clinical Trials (IMPAACT) Group was provided by grants from the NIAID (U01 AI068632), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (AI068632). This work was supported by the Statistical and Data Analysis Center at the Harvard School of Public Health, under NIAID cooperative agreements with the Pediatric AIDS Clinical Trials Group (5 U01 AI41110) and the IMPAACT Group (1 U01 AI068616). Support for the sites was provided by NIAID and the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network (NICHD contract number N01-DK-9-001/HHSN267200800001C). Support for Dr. Jean-Philippe comes from NIAID Contract No. HHSN272200800014C. The study was also funded by a grant from the Secure the Future Fund, a philanthropy program sponsored by Bristol-Myers Squibb.
Footnotes
Roles: Conception and design: BZ, SK, GM, SN, SAM, CM; drafting article or revision for intellectual content: BZ, SK, GM, MFC, PJ-P, AV, RB, SN, LM, SAM, CM; and all authors approved the final version
Conflicts of interest: Drs Violari and Cotton report receiving lecture fees from Abbott Laboratories, Dr Madhi reports receiving fees from Pfizer, GSK, Sanofi Pasteur and Novartis for participation in expert-boards and/or for supporting PhD students, and Dr Mitchell reports receiving lecture fees from MedImmune, GSK, and Novartis Vaccines. No other potential conflict of interest relevant to this article was reported.
References
- 1.WHO. Global Tuberculosis Report 2015. 2015 http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf?ua=1. Accessed December 22, 2015.
- 2.Perez-Velez CM, Marais BJ. Tuberculosis in children. N Engl J Med. 2012;367(4):348–361. doi: 10.1056/NEJMra1008049. [DOI] [PubMed] [Google Scholar]
- 3.Swaminathan S, Rekha B. Pediatric tuberculosis: global overview and challenges. Clin Infect Dis. 2010;50(Suppl 3):S184–194. doi: 10.1086/651490. [DOI] [PubMed] [Google Scholar]
- 4.Hesseling AC, Cotton MF, Jennings T, et al. High incidence of tuberculosis among HIV-infected infants: evidence from a South African population-based study highlights the need for improved tuberculosis control strategies. Clin Infect Dis. 2009;48(1):108–114. doi: 10.1086/595012. [DOI] [PubMed] [Google Scholar]
- 5.Dangor Z, Izu A, Hillier K, et al. Impact of the Antiretroviral Treatment Program on the Burden of Hospitalization for Culture-confirmed Tuberculosis in South African Children: A Time-series Analysis. Pediatr Infect Dis J. 2013;32(9):972–977. doi: 10.1097/INF.0b013e31828d9aa4. [DOI] [PubMed] [Google Scholar]
- 6.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359(21):2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suthar AB, Lawn SD, del Amo J, et al. Antiretroviral therapy for prevention of tuberculosis in adults with HIV: a systematic review and meta-analysis. PLoS Med. 2012;9(7):e1001270. doi: 10.1371/journal.pmed.1001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pettit AC, Jenkins CA, Stinnette SE, et al. Tuberculosis risk before and after highly active antiretroviral therapy initiation: does HAART increase the short-term TB risk in a low incidence TB setting? J Acquir Immune Defic Syndr. 2011;57(4):305–310. doi: 10.1097/QAI.0b013e3182182e2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Impact of antiretroviral therapy on tuberculosis incidence among HIV-positive patients in high-income countries. Clin Infect Dis. 2012;54(9):1364–1372. doi: 10.1093/cid/cis203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walters E, Cotton MF, Rabie H, Schaaf HS, Walters LO, Marais BJ. Clinical presentation and outcome of tuberculosis in human immunodeficiency virus infected children on anti-retroviral therapy. BMC Pediatr. 2008;8:1. doi: 10.1186/1471-2431-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinson NA, Moultrie H, van Niekerk R, et al. HAART and risk of tuberculosis in HIV-infected South African children: a multi-site retrospective cohort. Int J Tuberc Lung Dis. 2009;13(7):862–867. [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson LJ, Wells CD. Global epidemiology of childhood tuberculosis. Int J Tuberc Lung Dis. 2004;8(5):636–647. [PubMed] [Google Scholar]
- 13.Beyers N, Gie RP, Schaaf HS, et al. A prospective evaluation of children under the age of 5 years living in the same household as adults with recently diagnosed pulmonary tuberculosis. Int J Tuberc Lung Dis. 1997;1(1):38–43. [PubMed] [Google Scholar]
- 14.Ayles H, Muyoyeta M, Du Toit E, et al. Effect of household and community interventions on the burden of tuberculosis in southern Africa: the ZAMSTAR community-randomised trial. Lancet. 2013 doi: 10.1016/S0140-6736(13)61131-9. [DOI] [PubMed] [Google Scholar]
- 15.Madhi SA, Nachman S, Violari A, et al. Primary isoniazid prophylaxis against tuberculosis in HIV-exposed children. N Engl J Med. 2011;365(1):21–31. doi: 10.1056/NEJMoa1011214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cotton MF, Schaaf HS, Lottering G, Weber HL, Coetzee J, Nachman S. Tuberculosis exposure in HIV-exposed infants in a high-prevalence setting. Int J Tuberc Lung Dis. 2008;12(2):225–227. [PubMed] [Google Scholar]
- 17.South African Department of Health. The South African National Tuberculosis Control Programme Practical Guidelines 2004. 2004 [Google Scholar]
- 18.World Health Organization. WHO Case Definitinos of HIV for Surveillance and Revised Clinical Staging and Immunological Classification of HIV-Related Disease in Adults and Children. 2007 [Google Scholar]
- 19.Haddow LJ, Moosa MY, Mosam A, Moodley P, Parboosing R, Easterbrook PJ. Incidence, clinical spectrum, risk factors and impact of HIV-associated immune reconstitution inflammatory syndrome in South Africa. PLoS One. 2012;7(11):e40623. doi: 10.1371/journal.pone.0040623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11(5):561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Frigati LJ, Kranzer K, Cotton MF, Schaaf HS, Lombard CJ, Zar HJ. The impact of isoniazid preventive therapy and antiretroviral therapy on tuberculosis in children infected with HIV in a high tuberculosis incidence setting. Thorax. 2011;66(6):496–501. doi: 10.1136/thx.2010.156752. [DOI] [PubMed] [Google Scholar]
- 23.Zar HJ, Eley B, Nicol MP, Figaji A, Hawkridge A. Advances in childhood tuberculosis - contributions from the University of Cape Town. S Afr Med J. 2012;102(6):518–521. doi: 10.7196/samj.5552. [DOI] [PubMed] [Google Scholar]
- 24.Zar HJ, Lombard C. Isoniazid prophylaxis against tuberculosis in children. N Engl J Med. 2011;365(16):1543. doi: 10.1056/NEJMc1109603. author reply 1543–1544. [DOI] [PubMed] [Google Scholar]
- 25.Brady MT, Oleske JM, Williams PL, et al. Declines in mortality rates and changes in causes of death in HIV-1-infected children during the HAART era. J Acquir Immune Defic Syndr. 2010;53(1):86–94. doi: 10.1097/QAI.0b013e3181b9869f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


