Abstract
We estimated the prevalence of recent HIV testing (i.e., having an HIV test during the last 12 months and knew the results) among 1,295 HIV-negative Iranian female sex workers (FSW) in 2015. Overall, 70.4% (95% confidence intervals: 59.6, 79.3) of the participants reported a recent HIV testing. Concerns about their HIV status (83.2%) was reported as the most common reason for HIV testing. Incarceration history, having >5 paying partners, having >1 non-paying partner, receiving harm reduction services, utilizing healthcare services, and knowing an HIV testing site were significantly associated with recent HIV testing. In contrast, outreach participants, having one non-paying sexual partner, and self-reported inconsistent condom use reduced the likelihood of recent HIV testing. HIV testing uptake showed a ~2.5 times increase among FSW since 2010. While these findings are promising and show improvement over a short period, HIV testing programs should be expanded particularly through mobile and outreach efforts.
Keywords: HIV testing, Female sex workers, Surveillance, Harm reduction, Iran
Introduction
Although the risk of HIV infection and AIDS-related deaths are decreasing in all regions [1], the Middle East and North Africa (MENA) is one of the two regions with a growing HIV epidemic [2, 3]. One of the main challenges faced by HIV prevention and treatment programs in the MENA is the low HIV diagnosis rate; only 37% of people living with HIV were diagnosed in 2016 [4]. Female sex workers (FSW) are highly vulnerable to HIV due to their high-risk sexual behaviors, limited access to HIV and other sexually transmitted infections (STIs) prevention, diagnosis, and treatment services, high level of stigma associated with sex work [5–7], as well as social and economic marginalization. In comparison with other women in low- and middle-income countries (LMIC), FSW were found to be 13.5 times more likely to be living with HIV infection [5]. The overall HIV prevalence among FSW was found to be 11.8% in LMIC and 17.3% in the US as a high-income country [8].
Iran has the largest number of people living with HIV (~ 75,700) in MENA [2], only one-third of whom have been diagnosed [9]. While the HIV epidemic in Iran is still driven by injection drug use, HIV transmission via unsafe heterosexual sex is on the rise, claiming for 36.8% of all HIV-diagnosed cases [9]. HIV testing uptake is low among key populations at risk of HIV in Iran; for example, in 2010, about half (49.8%) of the people who inject drugs (PWID) reported having ever tested for HIV. Only 24.9% of PWID [10], and 27.5% of FSW [11] reported having a recent HIV test result.
HIV diagnosis is the entry point of HIV care continuum [12] and one of the UNAIDS 90-90-90 targets to be met by 2020 [13]. Iran has recently implemented several interventions to improve the HIV testing uptake among key populations. For example, HIV rapid tests have been introduced as a routine part of counseling services in numerous health services and harm reduction settings (e.g., voluntary counselling and testing (VCTs) centers, antenatal public and private clinics, drop-in centers, methadone clinics, TB centers [9], and vulnerable women facilities [14]). The HIV testing protocol was also revised by adapting the WHO guideline for provider-initiated HIV testing and counseling (PITC), in which health care providers recommend HIV testing uptake as a part of routine medical care to all people attending such facilities [15]. Moreover, several mobile HIV testing units have become available to increase HIV testing availability for vulnerable populations and communities with limited access to healthcare facilities [9].
In light of the above-mentioned interventions, the current study aims to see whether HIV testing uptake among FSW has been improved over the past five years. We compare the results from the two recent FSW national surveys (round 2010 vs. 2015) and investigate the individual-level factors associated with having a recent HIV test result among this vulnerable population.
Methods
Study Design and Setting
We recruited 1,337 FSW (1,185 from facilities catered towards FSW and 152 through outreach efforts) from 13 cities (20 sites) between January and August 2015. These facilities provide harm reduction services to the vulnerable women (i.e., FSW, women who use or inject drugs, and women with a history of incarceration) [14]. These sites are mostly run by non-governmental organizations (NGOs) under the supervision of the Ministry of Health through provincial and regional medical universities. Some centers for vulnerable women are also operated by the Social Welfare Organization (SWO). The study sites represented different geographical locations of the country, most of which were included in the previous round of the study conducted in 2010 (Figure 1) [16].
Fig. 1.
Cities included in the bio-behavioral surveillance survey of female sex workers in Iran, 2015
Eligibility Criteria
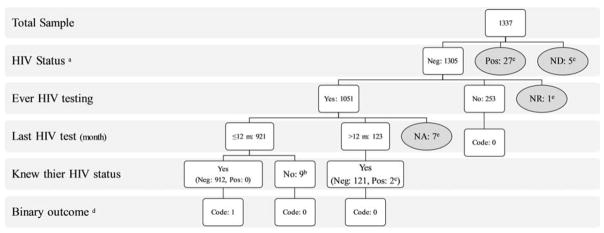
The eligibility criteria included: Female sex, self-reported sexual intercourse (vaginal, anal or oral) in exchange for money or goods with more than one male client in the last 12 months, ≥18 years of age, holding Iranian citizenship, and residing or working in the city of the study. For the purpose of the current paper, participants were excluded if they were HIV-positive, had unknown HIV status, or did not report their HIV testing history or the time of HIV testing. The flowchart on how the final analytic sample was reached is outlined in Figure 2. Overall, 42 cases out of 1,337 FSW were excluded.
Fig. 2. Final analytic sample flowchart.
Neg: HIV Negative; Pos: HIV Positive; ND: No Data; NR: Not Remember; NA: No Answer; a) Checked via rapid test; b) Did not receive, did not know, no answer; c) These two people reported they were positive while their rapid test was negative, then excluded; Grey color circles were excluded from the analysis; d) This is the outcome variable of the current study under consideration: Code 1: Those who self-reported having a recent HIV test and knew the result of the test (i.e., had a recent HIV test result); Code 0: Those who either had never tested for HIV or had tested for HIV but not in the past year, or had tested for HIV in the past year but did not know their test results (i.e., did not have a recent HIV test result); e) Removed
Data Collection
The study was led by one principal investigator and one project manager who were responsible for maintaining the scientific rigor of the project. In each city, a university-affiliated supervisor oversaw the sampling, data collection, as well as data quality and assurance based on the study protocol. FSW who visited the study site or those who were approached by our peer-led outreach team were invited to participate in the study after receiving a brief introduction on the study aims, and the potential risks and benefits of participating in the study. FSW who provided verbal informed consent were interviewed in a private room by a trained female interviewer using a standardized risk-assessment questionnaire. During the implementation phase, each study site was visited at least once by the project manager or a member of the study supervisory team for quality assessment purposes.
The working language of the study documents was Farsi. The study questionnaire consisted of sections on demographic, sexual and drug use practices, and HIV testing. The interviews took less than an hour. HIV status was determined by HIV rapid test (HIV/syphilis Dual) and confirmed by another rapid test (Unigold). Participants received separate monetary incentives for interview (70,000 Rials equivalent to ~2 USD) and for HIV test (30,000 Rials equivalent to ~1 USD). The study protocol and procedures were reviewed and approved by the Research Review Board at Kerman University of Medical Sciences (Ethics Code: K/93/209).
Measures
Dependent Variable – Having A Recent HIV Test Result
FSW with a history of HIV testing were asked about the date of their last HIV test followed by a question on whether they knew their test results. The primary outcome, having a recent (i.e., past year) HIV test result, was treated as a binary variable: Those who reported having a recent HIV test and knew their test result (i.e., had a recent HIV test result) versus those who had never tested for HIV or had tested for HIV but not in the past year, or had tested for HIV in the past year but did not know their test results (i.e., did not have a recent HIV test result).
Independent Variables
Data was collected on demographic and baseline characteristics including age, type of recruitment (outreach or facilities), duration of sex work, marital status, highest level of education, having other source of income than sex work, housing status, primary solicitation venue, lifetime history of incarceration, lifetime history of sexual violence, and HIV comprehensive knowledge measured by asking five questions on HIV transmission and misconceptions [17]. We measured their HIV risk perception by asking the question “Are you considering yourself to be at risk for HIV?”, with four response categories: No, low risk, moderate risk and high risk. For the analysis, we grouped low, moderate and high risks as having the minimum risk perception versus others who reported no self-perceived risk for HIV. Data were also collected on number of paying and non-paying partners (last month), history of group sex, frequency of male condom use with all sex partners (last year), and history of injection drug use. Furthermore, we obtained information on participants’ use of healthcare services including access to harm reduction services (last year), health service utilization (last year) defined as referring to health and treatment centers or clinics in last year, knowing an HIV testing site, and perceived healthcare stigma (last year).
Statistical Analysis
Descriptive statistics including absolute and relative frequencies (n [%]) of the main outcome of the study, reasons for having a recent HIV testing, and the correlates of HIV testing were reported. Pearson Chi-square test was used to compare the prevalence of recent HIV testing result for the current round of surveillance study, 2015, and the previous round, 2010. Rao-Scott modified Chi-Square test (RS χ2) – in order to adjust for the clustering effect of the survey design [18] – was performed to examine the relative differences in the proportion of HIV testing across subgroups of independent variables. RS χ2 is a design-adjusted Pearson Chi-Square in which the ordinary test statistic is converted to the F distribution with two degrees of freedom (df): Fadj ~ F (numerator df, denominator df) [18]. The cities were considered as clusters in the analysis. Next, we applied univariate and multivariable regression analyses to determine the correlates of having a recent HIV test result. We used the univariate and multivariable modified Poisson regression approaches using a generalized linear model (GLM) with Poisson as family, and log link function. This regression method allowed us to report crude and adjusted prevalence ratios (PRs) along with 95% confidence intervals (CIs) for the association between study main outcome and covariates. [19]. Using the survey data analysis package in Stata, the Tylor linearization method was used to approximate the standard errors and calculate the 95%Cis [20]. Correlates with a moderate association with recent HIV testing (a P-value of 0.20 or less derived from Table 1) were entered into the multivariable regression model [21].
Table 1.
Prevalence of HIV testing, reasons for being and not being tested for HIV and self-perceived risk of for HIV among female sex workers in Iran, 2015
| N | % (95% CI) | |
|---|---|---|
| Lifetime HIV testing | ||
| Yes | 1,051 | 80.6 (71.8, 87.1) |
| No | 253 | 19.4 (12.9, 28.2) |
|
| ||
| Past-year HIV testing | ||
| Tested and knew or receive the results | 912 | 70.4 (59.6, 79.3) |
| Not tested or tested but did not know or receive the result | 383 | 29.6 (20.7, 40.4) |
|
| ||
| Reasons for testing for HIV among testersa | ||
| Wanted to know my HIV status | 860 | 83.2 (74.5, 89.1) |
| Partner requested | 32 | 3.1 (1.2, 7.5) |
| Wanted to start sexual relations with a new partner | 11 | 1.1 (0.4, 3.0) |
| Wanted to get married | 10 | 1.0 (0.3, 3.1) |
| Pregnancy | 11 | 1.1 (0.6, 2.0) |
| Advised by friends | 27 | 2.6 (1.2, 5.7) |
| Advised by a Health Worker | 382 | 36.9 (21.5, 55.6) |
| Advised by a peer educator | 31 | 3.0 (1.4, 6.5) |
| Thought I am infected | 69 | 6.7 (3.3, 12.9) |
| Others | 34 | 3.3 (2.0, 5.5) |
|
| ||
| Reasons for not testing for HIV among non-testersb | ||
| Not knowing an HIV testing sitec | 69 | 28.3 (20.4, 37.7) |
| Consistent condom use | 14 | 5.7 (2.9, 11.0) |
| No self-perceived risk for HIV | 80 | 32.8 (21.6, 46.3) |
| No time | 51 | 20.9 (12.8, 32.3) |
| Trust in partner | 15 | 6.1 (2.8, 13.1) |
| Afraid of testing positive | 22 | 9.0 (4.1, 18.6) |
| Confidentiality concerns | 13 | 5.3 (2.7, 10.2) |
| Inconvenient testing location or hours | 1 | 0.4 (0.1, 3.0) |
| Expensive HIV tests | 34 | 13.9 (8.7, 21.6) |
| Others | 27 | 11.1 (5.4, 21.5) |
These frequencies are among those who had past-year HIV testing and received the results back;
These frequencies are among those who did not have past-year HIV testing or did not received the result of the test;
participants could choose more than one choice
Handling Missing Data
In the multivariable regression analysis, our dataset had 936 records (72.28% of all recruited samples) with complete responses to all variables we included in the analysis. The percentage of missing ranged from less than 2% for housing status, incarceration, sexual violence, number of non-paying partners, health service utilization, and knowing an HIV testing site, to 11.1% for receiving free condom and 14.9% for HIV risk perception. To minimize the effect of the missing values, we generated ten imputed datasets by multiple imputations using chained equation algorithm under the assumption of missing at random (MAR) mechanism [22, 23]. This algorithm replaced the missing values by repeatedly drawing values from conditional probability distributions of the non-missing values of the observed data. All statistical analyses were performed in Stata 13 using survey estimation command (StataCorp, College Station, Texas, USA). P-values less than 0.05 were considered statistically significant.
Results
Most participants were recruited from facilities (88.6%), were young (50.1% <30 years old), had less than diploma education (93.5%), were not married (66.6%), had no income other than sex work (58.8%), and were involved in sex work for less than 10 years (68.3%).
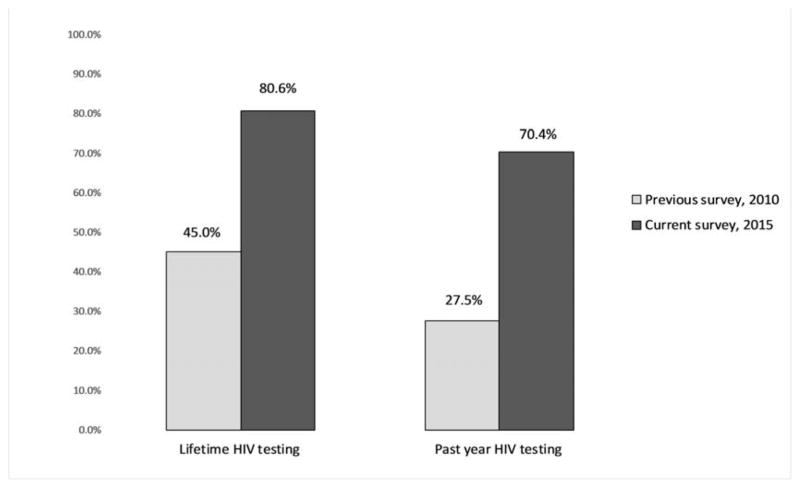
Overall, 80.6% (95% CI: 71.8, 87.1) reported having ever tested HIV, and 70.4% (95% CI: 59.6, 79.3) reported having a recent HIV test result. Common reasons for HIV testing were an intention to know their HIV status (83.2%) and being offered an HIV test by a health care provider (36.9%). Among non-testers, the most common reasons for not being tested for HIV were a lack of self-perceived risk for HIV (32.8%), not knowing an HIV testing site (28.3%), and not having enough time (20.9%) (Table 1). Having ever tested for HIV as well as a recent HIV test result for the current research were significantly higher than that observed in the previous bio-behavioral surveillance survey (BBSS) study in 2010 (Figure 3). Given the similar study designs in both surveys, having a recent HIV test result in the 2015 BBSS was approximately 2.5 times that of the 2010 BBSS.
Fig. 3.
The prevalence of “lifetime HIV testing” and “past year HIV testing” among female sex workers in Iran in the two bio-behavioral surveillance surveys in 2010 (n=715; Ref:[23]) and 2015 (n=1295).
Table 2 shows the frequency of recent HIV testing by different subgroups of demographics and risk behaviors. Having a recent HIV test result was significantly higher among FSW who were recruited from facilities (75.1% vs. 34.0%, RS χ2 = 24.9, P< 0.001), had higher educational levels (74.8% vs. 58.1% for illiterate FSW, RS χ2 = 3.2, P = 0.038), had been ever incarcerated (78.0% vs. 67.8%, RS χ2 = 4.8, P = 0.041), had sufficient knowledge of HIV (77.9% vs. 68.5%, RS χ2 = 7.2, P = 0.014), perceived themselves at risk of HIV (72.5% vs. 65.5%, RS χ2 = 5.7, P = 0.027), had more than five paying partner (80.2% vs. 64.6%, RS χ2 = 3.2, P = 0.046), had more than one non-paying sexual partners (78.5% vs. 66.7% for only one partner vs. 72.1% for no partners, RS χ2 = 3.9, P = 0.036), were consistent condom users (79.2% vs. 69.2%, RS χ2 = 10.8, P = 0.004), received harm reduction services (80.3% vs. 43.1%, RS χ2 = 52.2, P <0.001), reported health service utilization (76.9% vs. 46.2%, RS χ2 = 23.1, P < 0.001), and knew an HIV testing site (77.7% vs. 10.2%, RS χ2 = 235.0, P < 0.001).
Table 2.
Recent HIV test result by demographic and risk characteristics of female sex workers in Iran, 2015
| Variables | N | HIV testing‡ | x2 (df)† | x2 (df1,df2) †† | P-value |
|---|---|---|---|---|---|
| Type of study sample | 105.5(1) | 24.9 (1, 19) | <0.001 | ||
| Facility-based | 1148 | 75.1 (62.7,84.4) a | |||
| Outreach | 147 | 34.0 (22.8,47.4) | |||
|
| |||||
| Age categories | 1.4 (2) | 0.4 (1.5, 28.9) | 0.620 | ||
| <25 years old | 135 | 74.8 (57.4,86.7) | |||
| 25–34 years old | 513 | 70.4 (60.1,78.9) | |||
| 35+ | 645 | 69.6 (57.4,79.5) | |||
|
| |||||
| Duration of sex work involvement | 2.8 (3) | 0.5 (2.3, 44.2) | 0.660 | ||
| ≤2 yrs | 231 | 69.7 (61.4,76.9) | |||
| 3 to 5 yrs | 300 | 74.3 (61.4,84.1) | |||
| 6 to 10 yrs | 325 | 68.6 (53.6,80.5) | |||
| >10 yrs | 397 | 69.8 (56.5,80.4) | |||
|
| |||||
| Current Marital status | 12.0 (3) | 2.3 (2.7, 50.8) | 0.095 | ||
| Single | 83 | 74.7 (57.6,86.5) | |||
| Married | 431 | 65.0 (49.9,77.5) | |||
| Others (widow, divorced) | 580 | 71.7 (61.3,80.2) | |||
| Temporary marriage (Sigheh) | 198 | 77.3 (67.8,84.6) | |||
|
| |||||
| Education | 15.0 (3) | 3.2 (2.5, 46.9) | 0.038 | ||
| Illiterate | 124 | 58.1 (39.4,74.7) | |||
| Primary school or less | 363 | 67.5 (52.8,79.4) | |||
| Middle and high school | 455 | 72.7 (62.8, 80.9) | |||
| Diploma and above | 353 | 74.8 (66.0, 81.9) | |||
|
| |||||
| Having income other than sex work | 0.4 (1) | 0.1 (1, 19) | 0.735 | ||
| Yes | 531 | 69.7 (55.3,81.0) | |||
| No | 758 | 71.2 (61.5,79.4) | |||
|
| |||||
| Housing status | 4.5 (1) | 2.3 (1, 19) | 0.145 | ||
| Stable | 1168 | 69.5 (57.9,79.1) | |||
| Unstable | 126 | 78.6 (68.0,86.3) | |||
|
| |||||
| Primary Solicitation venue | 0.4 (1) | 0.2 (1, 19) | 0.665 | ||
| Street-based | 637 | 71.4 (58.9,81.3) | |||
| Independent and home -based/indoor | 644 | 69.9 (59.7,78.4) | |||
|
| |||||
| Incarceration history * | 12.3 (1) | 4.8 (1, 19) | 0.041 | ||
| No | 954 | 67.8 (56.2,77.6) | |||
| Yes | 336 | 78.0 (65.4,86.9) | |||
|
| |||||
| Experienced sexual violence * | 5.4 (1) | 2.5 (1, 19) | 0.128 | ||
| No | 781 | 68.0 (55.9,78.0) | |||
| Yes | 512 | 74.0 (63.3,82.5) | |||
|
| |||||
| HIV Comprehensive knowledge b | 12.3 (1) | 7.2 (1, 19) | 0.014 | ||
| Insufficient | 819 | 68.5 (57.7,77.6) | |||
| Sufficient | 434 | 77.9 (67.4,85.7) | |||
|
| |||||
| Self-perceived risk for HIV | 4.3 (1) | 5.7 (1, 19) | 0.027 | ||
| No | 207 | 65.2 (52.6,76.0) | |||
| Yes (low, moderate, high) | 895 | 72.5 (61.6,81.3) | |||
|
| |||||
| Number of paying partners *** | 25.5 (3) | 3.2 (2.3, 42.9) | 0.046 | ||
| 0 | 285 | 64.6 (53.2,74.5) | |||
| 1 | 182 | 70.3 (56.0,81.5) | |||
| 2–5 | 415 | 66.5 (52.1,78.4) | |||
| >5 | 384 | 80.2 (68.7,88.2) | |||
|
| |||||
| Number of non-paying partners *** | 9.3 (2) | 3.9 (1.7, 33.0) | 0.036 | ||
| 0 | 606 | 72.1 (62.2,80.3) | |||
| 1 | 519 | 66.7 (52.7,78.2) | |||
| >1 | 158 | 78.5 (70.3,84.9) | |||
|
| |||||
| Lifetime group sex ** | 0.5 (1) | 0.4 (1, 19) | 0.544 | ||
| No | 1130 | 70.0 (59.0,79.1) | |||
| Yes | 161 | 72.7 (59.3,82.9) | |||
|
| |||||
| Male condom use with all partners *** | 11.8 (1) | 10.8 (1, 19) | 0.004 | ||
| Consistent | 331 | 79.2 (69.1,86.5) | |||
| Inconsistent | 930 | 69.2 (59.1,77.8) | |||
|
| |||||
| Drug injection * | 0.7 (2) | 0.2 (1.6, 30.7) | 0.752 | ||
| No drug injection history | 1219 | 70.2 (59.3,79.2) | |||
| Yes – only injection | 45 | 75.6 (57.3,87.7) | |||
| Yes – shared injection | 26 | 73.1 (39.6,91.8) | |||
|
| |||||
| Harm reduction service use ** | 117.0 (1) | 52.2 (1, 19) | <0.001 | ||
| No | 195 | 43.1 (31.8,55.2) | |||
| Yes | 956 | 80.3 (72.5,86.4) | |||
|
| |||||
| Health service utilization ** | 94.6 (1) | 23.1 (1, 19) | <0.001 | ||
| No | 260 | 46.2 (29.6,63.6) | |||
| Yes | 1022 | 76.9 (68.6,83.5) | |||
|
| |||||
| Knowing a site for HIV testing | 255.0 (1) | 235.0 (1, 19) | <0.001 | ||
| No | 128 | 10.2 (4.7,20.4) | |||
| Yes | 1156 | 77.7 (69.4,84.2) | |||
|
| |||||
| Perceived health-care stigma ** | 0.3 (1) | 0.5 (1, 19) | 0.470 | ||
| No | 1253 | 70.6 (59.8,79.4) | |||
| Yes | 42 | 66.7 (50.2,79.9) | |||
Defined as last year HIV test and knew the results;
% (95% CI);
Measured by 1) condoms can prevent HIV transmission, 2) restricting sexual activities to only one faithful, but uninfected partner can prevent HIV transmission, 3) mosquito bites can transmit HIV, 4) sharing meal with a person living with HIV can transmit HIV, 5) a healthy looking individual can have HIV;
Uncorrected Chi-Square statistic, df: degree of freedom;
Design-based (Rao-Scott correction) Chi-Square statistic with F distribution, df1: numerator degree of freedom and df2: denominator degree of freedom;
Duration: lifetime;
Duration: past year;
Duration: past month
In the multivariable model, we found that having a recent HIV test result was less likely among FSW who were recruited from outreach (PR = 0.64; 95% CI: 0.47, 0.87, P = 0.007), had only one non-paying sexual partner (PR = 0.89; 95% CI: 0.84, 0.95, P = 0.001), and were inconsistent condom users (PR = 0.93; 95% CI: 0.87, 0.99, P = 0.028). In contrast, having a recent HIV test result was more likely among FSW with an incarceration history (PR = 1.1; 95% CI: 1.0, 1.20, P = 0.048), >5 paying partners (PR = 1.20; 95% CI: 1.05, 1.35, P = 0.007), and >1 non-paying partner (PR = 1.07; 95% CI: 1.0, 1.16, P = 0.048). Having a recent HIV test result was also more likely among FSW who had received harm reduction services (PR = 1.34; 95% CI: 1.10, 1.61, P = 0.005), utilized health services (PR = 1.39; 95% CI: 1.14, 1.70, P = 0.002), and knew an HIV testing site (PR=4.80; 95% CI: 2.6, 8.6, P < 0.001). Our findings remained unchanged after imputing the missing data (Table 3).
Table 3.
Factors associated with prevalence of recent HIV testing using three regression models on original (n=936) and imputed (1295) data of female sex workers in Iran, 2015
| Variables | Crude Analysis | Adjusted Modela | Adjusted Imputed Modelb |
|---|---|---|---|
|
| |||
| PR* (95% CI) | PR (95% CI) | PR (95% CI) | |
| Type of sample | |||
| Facility-based | 1 | 1 | 1 |
| Outreach | 0.45 (0.30, 0.67)e | 0.64 (0.47, 0.87)e | 0.55 (0.38,0.80)e |
|
| |||
| Education | |||
| Illiterate | 1 | 1 | 1 |
| Primary school or less | 1.16 (0.95, 1.42) | 0.91 (0.80, 1.04) | 0.99 (0.85,1.16) |
| Middle and high school | 1.25 (0.98, 1.62)c | 0.94 (0.85, 1.06) | 1.05 (0.87,1.26) |
| Diploma and above | 1.35 (0.98, 1.86)c | 0.96 (0.89, 1.04) | 1.08 (0.93,1.25) |
|
| |||
| Unstable housing | |||
| Stable | 1 | 1 | 1 |
| Unstable | 1.13 (0.95, 1.34) | 1.02 (0.92, 1.14) | 1.08 (0.97,1.20) |
|
| |||
| Incarceration history * | |||
| No | 1 | 1 | 1 |
| Yes | 1.15 (1.01, 1.31)d | 1.1 (1.00, 1.20)d | 1.08 (0.98,1.20)c |
|
| |||
| Experienced sexual violence * | |||
| No | 1 | 1 | 1 |
| Yes | 1.09 (0.97, 1.22) | 1.01 (0.89, 1.12) | 1.02 (0.91,1.14) |
|
| |||
| HIV Comprehensive knowledge | |||
| Insufficient | 1 | 1 | 1 |
| Sufficient | 1.13 (1.03, 1.26)d | 1.01 (0.95, 1.08) | 1.02 (0.96,1.08) |
|
| |||
| Self-perceived risk for HIV | |||
| No risk | 1 | 1 | 1 |
| Yes (low, moderate, high) | 1.11 (1.00, 1.23)d | 0.99 (0.92, 1.06) | 1.05 (0.96,1.16) |
|
| |||
| Number of paying partners *** | |||
| 0 | 1 | 1 | 1 |
| 1 | 1.08 (0.97, 1.22) | 1.01 (0.92, 1.10) | 1.01 (0.92,1.12) |
| 2–5 | 1.03 (0.85, 1.25) | 0.99 (0.86, 1.50) | 0.96 (0.84,1.10) |
| >5 | 1.24 (1.03, 1.50)d | 1.20 (1.05, 1.35)e | 1.16 (1.02,1.33)e |
|
| |||
| Number of non-paying partners *** | |||
| 0 | 1 | 1 | 1 |
| 1 | 0.92 (0.83, 1.02) | 0.89 (0.84, 0.95)e | 0.94 (0.87,1.00)d |
| >1 | 1.08 (0.96, 1.23) | 1.07 (1.00, 1.16)d | 1.05 (0.96,1.14) |
|
| |||
| Male condom use with all partners *** | |||
| Consistent | 1 | 1 | 1 |
| Inconsistent | 0.87 (0.80, 0.95)e | 0.93 (0.87, 0.99)d | 0.92 (0.85,0.99)d |
|
| |||
| Harm reduction service use ** | |||
| No | 1 | 1 | 1 |
| Yes | 1.86 (1.44, 2.41)f | 1.34 (1.10, 1.61)e | 1.35 (1.15,1.58)e |
|
| |||
| Health service utilization ** | |||
| No | 1 | 1 | 1 |
| Yes | 1.66 (1.18, 2.34)e | 1.39 (1.14, 1.70)e | 1.38 (1.11,1.72)e |
|
| |||
| Knowing a site for HIV testing | |||
| No | 1 | 1 | 1 |
| Yes | 7.64 (3.86, 15.10)f | 4.80 (2.60, 8.60)f | 5.77 (3.04,10.94)f |
Prevalence ratio;
Those variables with P-value less than 0.02 in univariate analysis were entered to this adjusted model (the univariate results of other variables are not shown here);
Regression model using the imputed variables;
P-value < 0.01;
P-value < 0.05;
P-value < 0.01;
P-value < 0.001;
Duration: lifetime;
Duration: past year;
Duration: past month
Discussion
In this cross-sectional study FSW, we observed that more than two-third of study participants had a recent HIV test result in 2015, indicating a significant increase in HIV testing uptake since 2010 [11]. However, there is still further room for improvement to meet the UNAIDS 90-90-90 targets [13] for HIV diagnosis and treatment in Iran. The current Iranian national HIV testing guidelines require individuals to visit HIV counseling and testing clinics [9] which could create significant structural barriers for sub-populations involved with stigmatizing and illegal behaviors. Such a barrier to HIV testing could be greatest for FSW due to the elevated stigma associated with sex work compared to other high-risk behaviors (e.g., injection drug use) in Iran. Moreover, the rising contribution of unprotected heterosexual in spreading HIV across the country [9], calls for specific programs for HIV testing and preventions targeting high-risk hetrosexual populations including FSW.
About one-third of outreach FSW had a recent HIV test result, which reflects a better estimation of HIV testing uptake among FSW in the community (i.e. those who do not visit the health facilities). So far, about 9,000 FSW have been provided prevention services and HIV testing in 39 active centers for vulnerable women in Iran (Unpublished Data, Iran’s Centre for Disease Control). Considering the estimated number of FSW to be more than 200,000 in Iran [24], a substantial proportion of FSW seem to have been missed by the existing HIV prevention programs. More innovative HIV prevention approaches are needed to reach this population.
In addition to PITC strategy for HIV testing [25], other types of HIV testing such as community-based outreach HIV testing as well as supervised (i.e., test is provided by a health care provider) and unsupervised (i.e., test is done by the patients while having access to a counselor) HIV self-testing have been shown to be highly acceptable and feasible approaches in increasing HIV testing uptake [26]. Despite WHO recommendation and studies that have shown the potentials for HIV self-testing [27], the oral fluid- or blood-based HIV self-testing is not yet available in Iran and studies to assess its feasibility and acceptability among FSW, particularly those not linked to existing services would be beneficial.
The overall HIV prevalence among FSW remains as low as 2.1% [28]. Efficient HIV monitoring and evaluation programs would benefit from scaling up HIV testing programs in regions with a higher prevalence of HIV, larger FSW populations, sub-populations with dual risks for HIV infection (e.g., FSW who inject drugs, sexual partners of people who inject drugs). An accurate programmatic mapping of FSW and services catered towards them could help provide such strategic information [29].
Another opportunity to increase HIV testing uptake among FSW could be through referrals made to STIs clinics for STIs other than HIV. In comparison with HIV, testing for other STIs are perceived to be less stigmatized [30]; therefore, individuals are more likely to accept the offer to test for HIV when the test is offered alongside other STIs. Moreover, lessons could be learned from the successful integration of HIV testing programs and routine harm reduction and screening programs for incarcerated populations in Iran [27], given that FSW with a history of incarceration had a higher chance of having tested for HIV.
We found that knowing a center for HIV testing increased the chance of having a recent HIV test result up to six times; a significant correlate of HIV testing across national [23] and international settings [31]. The effect of knowing an HIV testing site was prominent in both facility-based and outreach FSW. Since many FSW did not know where to get tested for HIV, it is critical to find appropriate and culturally-sensitive ways to promote HIV testing and make the HIV testing sites more visible to them. For many FSW, the Internet has become as an important tool for communicating with friends and sexual partners. Given the considerable number of mobile- and Internet-based sex workers, developing and piloting educational programs to promote HIV testing in social media, smart phones, and cyberspace could be a viable option for future interventions [32, 33]. Moreover, strategies to simplify and improve access to health centers where HIV testing is provided could increase HIV testing among FSW [34].
We acknowledge the limitations of our study. Since most participants were recruited from facilities serving vulnerable women, we may have overestimated the true rate of HIV testing. Our findings may not be generalizable to all FSW in Iran as participants were recruited from the main cities of the most populated provinces where access to healthcare services would be better than smaller cities or rural settings. HIV testing status was assessed by self-report which may lead to an overestimate of HIV testing rate. Also, we did not collect data on the frequency of HIV testing.
Conclusion
Since 2010, having a recent HIV test result among Iranian FSW has considerably increased. Making the HIV testing sites more visible to FSW, expanding the peer-led HIV testing and mobile clinics that offer HIV testing, integrating HIV testing services in all health and social support programs targeting FSW and feasibility studies of adding HIV self-testing to Iran’s HIV testing program could further improve HIV diagnosis and access to HIV treatment in this marginalized and under-severed population in Iran.
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS) [Accessed 16 Oct 2016];Global AIDS Update. 2016 http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf.
- 2.Gokengin D, Doroudi F, Tohme J, Collins B, Madani N. HIV/AIDS: trends in the Middle East and North Africa region. Int J Infect Dis. 2016;44:66–73. doi: 10.1016/j.ijid.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Joint United Nations Programme on HIV/AIDS (UNAIDS) [Accessed 8 Aug 2015];The gap report. 2014 http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_en.pdf.
- 4.UNAIDS. [Accessed 15 Feb 2017];Prevention Gap Report. 2016 http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf.
- 5.Baral S, Beyrer C, Muessig K, Poteat T, Wirtz AL, Decker MR, et al. Burden of HIV among female sex workers in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:538–549. doi: 10.1016/S1473-3099(12)70066-X. [DOI] [PubMed] [Google Scholar]
- 6.Platt L, Jolley E, Rhodes T, Hope V, Latypov A, Reynolds L, et al. Factors mediating HIV risk among female sex workers in Europe: a systematic review and ecological analysis. BMJ Open. 2013:3. doi: 10.1136/bmjopen-2013-002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shannon K, Strathdee SA, Goldenberg SM, Duff P, Mwangi P, Rusakova M, et al. Global epidemiology of HIV among female sex workers: influence of structural determinants. Lancet. 2015;385:55–71. doi: 10.1016/S0140-6736(14)60931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paz-Bailey G, Noble M, Salo K, Tregear SJ. Prevalence of HIV Among U.S. Female Sex Workers: Systematic Review and Meta-analysis. AIDS Behav. 2016;20:2318–31. doi: 10.1007/s10461-016-1332-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iran AIDS Progress Report. Islamic Republic of Iran AIDS Progress Report: On Monitoring of the United Nations General Assembly Special Session on HIV and AIDS. National AIDS Committee Secretariat, Ministry of Health and Medical Education; Mar, 2015. [Accessed 15 Oct 2016]. http://www.unaids.org/sites/default/files/country/documents/IRN_narrative_report_2015.pdf. [Google Scholar]
- 10.Shokoohi M, Karamouzian M, Osooli M, Sharifi H, Fahimfar N, Haghdoost A, et al. Low HIV testing rate and its correlates among men who inject drugs in Iran. Int J Drug Policy. 2016;32:64–69. doi: 10.1016/j.drugpo.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Shokoohi M, Karamouzian M, Khajekazemi R, Osooli M, Sharifi H, Haghdoost AA, et al. Correlates of HIV Testing among Female Sex Workers in Iran: Findings of a National Bio-Behavioural Surveillance Survey. PLoS One. 2016;11:e0147587. doi: 10.1371/journal.pone.0147587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization (WHO) Prevention and treatment of HIV and other sexually transmitted infections for sex workers in low- and middle-income countries – Recommendations for a public health approach. World Health Organization; Geneva, Switzerland: 2012. [Accessed 15 Oct 2016]. http://apps.who.int/iris/bitstream/10665/77745/1/9789241504744_eng.pdf?ua=1. [PubMed] [Google Scholar]
- 13.Baggaley R, Dalal S, Johnson C, Macdonald V, Mameletzis I, Rodolph M, et al. Beyond the 90-90-90: refocusing HIV prevention as part of the global HIV response. J Int AIDS Soc. 2016;19(1):21348. doi: 10.7448/IAS.19.1.21348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fahimfar N, Sedaghat A, Hatami H, Kamali K, Gooya M. Counseling and Harm Reduction Centers for Vulnerable Women to HIV/AIDS in Iran. Iran J Public Health. 2013;42:98–104. [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization (WHO) Guidance on provider-initiated HIV testing and counselling in health facilities. World Health Organization; Geneva, Switzerland: 2007. [Accessed 15 Oct 2016]. http://www.who.int/hiv/topics/vct/PITCguidelines.pdf. [Google Scholar]
- 16.Sajadi L, Mirzazadeh A, Navadeh S, Osooli M, Khajehkazemi R, Gouya MM, et al. HIV prevalence and related risk behaviours among female sex workers in Iran: results of the national biobehavioural survey, 2010. Sex Transm Infect. 2013;89(Suppl 3):iii37–40. doi: 10.1136/sextrans-2013-051028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.UNGASS. [Accessed 16 Oct 2016];United Nations General Assembly Special Session on HIV/AIDS: Monitoring the Declaration of Commitment on HIV/AIDS Guidelines on Construction of Core Indicators. 2010 http://www.unaids.org/sites/default/files/sub_landing/files/jc1676_core_indicators_2009_en.pdf.
- 18.Rao JNK, Scott AJ. The analysis of categorical data from complex sample surveys: Chi-squared tests for goodness of fit and independence in two-way tables. Journal of the American Statistical Association. 1981;76:221–230. [Google Scholar]
- 19.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 20.Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models:in chapter 12: complex survey. 2. New York: Springer; 2012. p. 474. [Google Scholar]
- 21.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 22.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 23.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: Wiley; 1987. [Google Scholar]
- 24.Sharifi H, Karamouzian M, Baneshi MR, Shokoohi M, Haghdoost AA, McFarland W, et al. Population Size Estimation of Female Sex Workers in Iranian Cities: Synthesis of Methods and Results. 2017 doi: 10.1371/journal.pone.0182755. Submitted to PLoS One. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iran AIDS Progress Report. Islamic Republic of Iran AIDS Progress Report: On Monitoring of the United Nations General Assembly Special Session on HIV and AIDS; March 2014. National AIDS Committee Secretariat, Ministry of Health and Medical Education; [Accessed 15 Oct 2016]. http://files.unaids.org/en/dataanalysis/knowyourresponse/countryprogressreports/2014countries/IRN_narrative_report_2014_en.pdf. [Google Scholar]
- 26.Pant Pai N, Sharma J, Shivkumar S, Pillay S, Vadnais C, Joseph L, et al. Supervised and unsupervised self-testing for HIV in high- and low-risk populations: a systematic review. PLoS Med. 2013;10:e1001414. doi: 10.1371/journal.pmed.1001414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. WHO issues new guidance on HIV self-testing ahead of World AIDS Day 29 Nov 2016. Geneva: 2017. [Google Scholar]
- 28.Mirzazadeh A, Shokoohi M, Haghdoost AA, et al. The Iran Vulnerable Women’s Study 2015, Regional Knowledge Hub, and WHO Collaborating Centre for HIV Surveillance: Tehran [unpublished report] 2015 [Google Scholar]
- 29.PEPFAR. Using Programmatic Mapping to Improve Program Access and Coverage for Key Populations - Guidelines for Countries. 2015 Mar; [Google Scholar]
- 30.Gwadz M, Cleland CM, Jenness SM, Silverman E, Hagan H, Ritchie AS, et al. Exploring Factors Associated with Recent HIV Testing among Heterosexuals at High Risk for HIV Infection Recruited with Venue-based Sampling. J AIDS Clin Res. 2016:7. doi: 10.4172/2155-6113.1000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsereteli N, Chikovani I, Chkhaidze N, Goguadze K, Shengelia N, Rukhadze N. HIV testing uptake among female sex workers and men who have sex with men in Tbilisi, Georgia. HIV Med. 2013;14(Suppl 3):29–32. doi: 10.1111/hiv.12065. [DOI] [PubMed] [Google Scholar]
- 32.Rhodes SD, Vissman AT, Stowers J, Miller C, McCoy TP, Hergenrather KC, et al. A CBPR partnership increases HIV testing among men who have sex with men (MSM): outcome findings from a pilot test of the CyBER/testing internet intervention. Health Educ Behav. 2011;38:311–320. doi: 10.1177/1090198110379572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson D. HIV Programs for Sex Workers: Lessons and Challenges for Developing and Delivering Programs. PLoS Med. 2015;12:e1001808. doi: 10.1371/journal.pmed.1001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenness SM, Murrill CS, Liu KL, Wendel T, Begier E, Hagan H. Missed opportunities for HIV testing among high-risk heterosexuals. Sex Transm Dis. 2009;36:704–710. doi: 10.1097/OLQ.0b013e3181ab375d. [DOI] [PubMed] [Google Scholar]