Abstract
Sensation of mechanical forces is critical for normal function of the gastrointestinal (GI) tract and abnormalities in mechanosensation are linked to GI pathologies. In the GI tract there are several mechanosensitive cell types—epithelial enterochromaffin cells, intrinsic and extrinsic enteric neurons, smooth muscle cells and interstitial cells of Cajal. These cells use mechanosensitive ion channels that respond to mechanical forces by altering transmembrane ionic currents in a process called mechanoelectrical coupling. Several mechanosensitive ionic conductances have been identified in the mechano-sensory GI cells, ranging from mechanosensitive voltage-gated sodium and calcium channels to the mechanogated ion channels, such as the two-pore domain potassium channels K2P (TREK-1) and nonselective cation channels from the transient receptor potential family. The recently discovered Piezo channels are increasingly recognized as significant contributors to cellular mechanosensitivity. Piezo1 and Piezo2 are nonselective cationic ion channels that are directly activated by mechanical forces and have well-defined biophysical and pharmacologic properties. The role of Piezo channels in the GI epithelium is currently under investigation and their role in the smooth muscle syncytium and enteric neurons is still not known. In this review, we outline the current state of knowledge on mechanosensitive ion channels in the GI tract, with a focus on the known and potential functions of the Piezo channels.
1. THE GASTROINTESTINAL TRACT MECHANOSENSITIVITY
Electromechanical organs, such as the heart and GI tract, are electrically excitable tissues with a primary mechanical function. These organs generate, and are subject to, mechanical forces that need to be detected as physiologic stimuli and as feedback signals to maintain normal function. Mechanosensitivity is critical for normal GI function and abnormalities in mechanosensitivity lead to disease. For example, in the stomach, distention is a critical determinant not only of gastric motility but also of satiety. Consequently, abnormalities in mechanosensation are associated with diseases such as obesity (Acosta et al., 2015). In the colon, alterations in mechanosensitivity lead to disorders of defecation, such as constipation (Neshatian et al., 2015), and may also be involved in the pathogenesis of colon cancer (Eisenhoffer et al., 2012; Fernandez-Sanchez et al., 2015).
2. MECHANOSENSITIVE CELLS IN THE GASTROINTESTINAL TRACT
At a fundamental level, all cells are mechanosensitive because of the need to sense normal physiologic forces (Sachs & Morris, 1998), such as cell crowding in the epithelium (Eisenhoffer et al., 2012). Given the importance of mechanosensation and ubiquity of mechanical forces, cells have developed several mechanisms of mechanosensation, ranging from integrins for mechanical interaction with substrates to mechanosensitivity of the nuclear envelope to modulate gene expression (reviewed in Eyckmans, Boudou, Yu, & Chen, 2011).
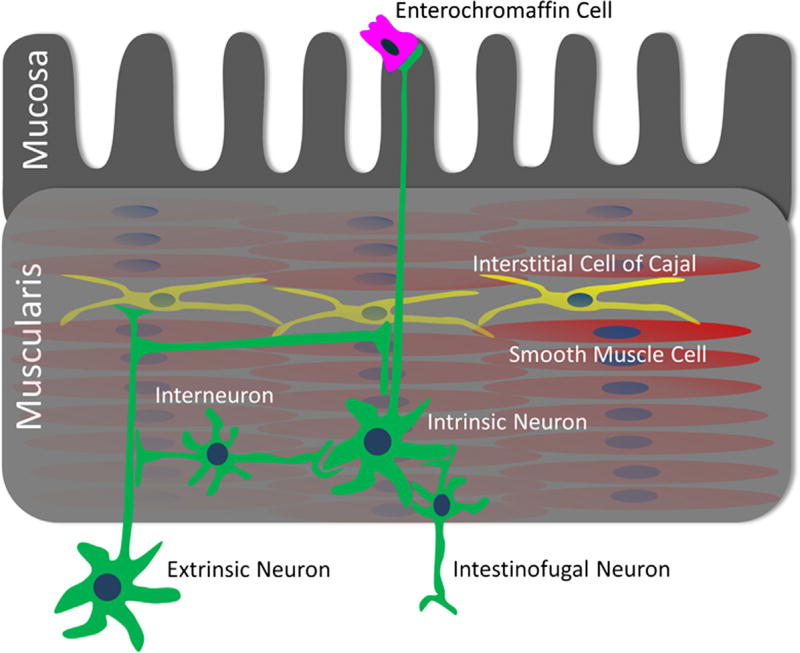
In the mechanically active tissues such as the gut, bladder, and heart, there are tissue-specific forces that are important for normal function. In these tissues, specific cells sense acute mechanical deformation. In the gastrointestinal (GI) tract several cell types are mechanosensitive (Fig. 1). The mechanosensitive cells in the GI tract are present within all layers of the wall: mucosa, submucosa, smooth muscle, and the submucosal and myenteric (between circular and longitudinal muscle) plexuses. In the epithelium, in response to mechanical forces, enterochromaffin (EC) cells release serotonin, which has well-documented effects on motility and secretion (reviewed by Mawe & Hoffman, 2013). In the mucosa, submucosa, and muscle layers, both intrinsic enteric and extrinsic nerves respond to mechanical stimuli. In the smooth muscle syncytium, both the smooth muscle pacemakers—the interstitial cells of Cajal (ICC) and smooth muscle cells (SMC) are mechanosensitive.
Figure 1.
Mechanosensitive cells in the gastrointestinal (GI) tract. Mechanosensitive cells in the GI tract include epithelial enterochromaffin cells, smooth muscle cells (SMCs), interstitial cells of Cajal (ICCs), intrinsic neurons, including interneurons, intesti-nofugal neurons, and extrinsic neurons.
3. MECHANOSENSITIVE ION CHANNELS
Mechanosensory cells detect mechanical forces and transduce them into electrical signals using mechanosensitive ion channels (reviewed in Hamill & Martinac, 2001). Mechanosensitive ion channels are transmem-brane proteins that form ion conduction pores with gates that are strongly altered by mechanical forces (reviewed in Arnadottir & Chalfie, 2010; Hamill & Martinac, 2001; Ranade, Syeda, & Patapoutian, 2015). Previous work has identified mechanosensitive ionic currents in GI mechanosensory cells, and in some cases the molecular identities of the mechanosensitive ion channels are known. However, in majority of cases, the identities of the GI tract mechanosensitive ion channels are not known. The Piezo family of ion channels are an example of mechanosensitive ion channels whose molecular identity was only recently determined. Piezo1 and Piezo2 mechanosensitive ion channels are expressed throughout the length of the GI tract (Coste et al., 2010) and have distinct biophysical properties, such as nonselective cationic permeability, kinetics described by fast activation, and inactivation that is slow-to-medium (Piezo1) (Coste et al., 2010, 2012; Gottlieb & Sachs, 2012) or fast (Piezo2) (Coste et al., 2010, 2012). Pharmacologically, the Piezo channels are inhibited by the mechanosensitive channel blockers gadolinium (Gd3+) and ruthenium red (RR), and specifically by the peptide blocker GsMTx-4 (Bae, Sachs, & Gottlieb, 2011; Coste et al., 2010, 2012; Gottlieb & Sachs, 2012). The Piezo channels have quickly found significance in several mechanosensitive systems, ranging from the sensation of light touch to preventing red blood cells from deformation-induced rupture.
In the following sections, we summarize the state of knowledge on mechanosensitive ion channels in the specific GI mechanosensitive cells, starting with the GI epithelium and working toward the extrinsic nerves that have their cell bodies outside the GI tract. Where possible, we will focus on the potential roles of the Piezo channels in GI mechanotransduction.
3.1 Gastrointestinal epithelium mechanosensitivity
GI epithelium is a sensor of both static and acute forces. Sensation of static forces is critical for cell density homeostasis, while sensation of acute forces is important for the processes of digestion and motility.
3.1.1 Static force detection by the gastrointestinal epithelium
Similar to other epithelia, such as skin and bladder, the GI epithelium is mechanosensitive. The GI epithelium serves as an important first point of interaction with the external environment (Fig. 1). In the intestinal epithelium, static forces are important for normal epithelial cell development and turnover. Studies show that mechanical forces are important for epithelial health, since abnormalities lead to diseases, including cancer (Fernandez-Sanchez et al., 2015; Yang et al., 2014). For example, mechanical pressure caused by hyperproliferative adjacent crypts overexpressing active Notch or by exogenous force application was associated with increased Ret and β-catenin signaling, leading to aberrant crypt foci (Fernandez-Sanchez et al., 2015). The link between mechanical force and downstream biochemical pathway activation is not known. However, recent work shows that Piezo1 channels in the GI epithelium are important sensors of cell crowding (Eisenhoffer et al., 2012) and migration (Yang et al., 2014). During epithelial apoptosis, extrusion occurs when dying cells send signals to surrounding epithelial cells to contract and remove the dying cell (Slattum & Rosenblatt, 2014). In normal homeostasis, overcrowding due to proliferation and migration induces extrusion of live cells that helps control the number of cells in the epithelia, which occurs at sites where there is highest crowding. In vivo studies showed that disruption of Piezo1 channels by pharmacological agents (Gd3+) or genetic knock down prevents extrusion and induces epithelial cell mass formation (Eisenhoffer et al., 2012). Formation of cell masses can promote tumorigenesis. Therefore, Piezo1 may provide the link between mechanical force and intracellular biochemical pathway activation, suggesting an important role of Piezo1 channels in the epithelium tumor-suppressive mechanisms (Slattum & Rosenblatt, 2014). These data are exciting, but further work is required to determine the molecular mechanism linking Piezo channels cellular phenotype and cancer formation.
3.1.2 Acute force detection by the gastrointestinal epithelium
The sensation of acute mechanical forces―such as intestinal contents deforming the epithelium is critical to normal day-to-day GI function. Mechanosensitive ion channels are important for acute force sensation in epithelia. In renal tubular epithelial cells, Piezo1 is responsible for a mechanosensitive ionic current (Peyronnet et al., 2013) and in urothelium, Piezo1 mediates mechanosensitive Ca2+ influx and ATP release (Miyamoto et al., 2014). In the gut epithelium, acute force sensation is performed by the specialized mechanosensory epithelial cells called EC cells (reviewed by Mawe & Hoffman, 2013) (Fig. 1). The EC cells have significant developmental and functional similarities to the Merkel cells in the skin that contribute to light touch sensation via Piezo2 channels (Ikeda & Gu, 2014; Ranade, Woo, et al., 2014; Woo et al., 2014). EC cells synthesize, store, and release a large amount of serotonin (5-HT) (Cote et al., 2003) in response to mechanical (Bulbring & Crema, 1959) and chemical (Racke & Schworer, 1991; Racke, Reimann, Schworer, & Kilbinger, 1996; Schworer, Katsoulis, & Racke, 1992) stimuli. In turn, EC cell 5-HT is critical for normal GI secretion (Brown, 1996), motility (Bulbring & Lin, 1958), and sensation and is also an important hormone involved in cardiac function (Cote et al., 2003), metabolic health (Crane et al., 2015), and bone health (Yadav et al., 2010).
Mucosa forces, such as intraluminal pressure, produce stimulus-dependent 5-HT release from EC cells (Bertrand, 2006; Bertrand, Hu, Mach, & Bertrand, 2008; Bulbring & Crema, 1959; Patel, Bian, Quaiserova-Mocko, Galligan, & Swain, 2007), within milliseconds of mechanical stimulation (Bertrand, 2004). This mucosal 5-HT stimulates peristalsis (Bulbring & Crema, 1958) and secretion (Frieling, Wood, & Cooke, 1992) via several 5-HT receptors on mucosa-projecting neurons (Bertrand, Kunze, Furness, & Bornstein, 2000; Foxx-Orenstein, Kuemmerle, & Grider, 1996; Kadowaki, Wade, & Gershon, 1996; Neya, Mizutani, & Yamasato, 1993) and epithelium (Hoffman et al., 2012). Intriguingly similar to the Merkel cell, which forms Merkel cell―neurite complex (Haeberle et al., 2004), the enteroendocrine cells akin to EC cells may make direct contacts with submucosal nerves (Bohorquez et al., 2015).
While the concept of EC cell mechanosensitivity is well known, the molecular mechanisms of EC cell mechanosensitivity remain elusive mainly due to difficulties with identification, isolation, and culture of primary epithelial cells. Therefore, most of the current knowledge is derived from pharmacologic manipulation of GI tissues and immortalized cell lines. The pancreatic carcinoid tumor BON cell line produces 5-HT and releases it in response to rotational shaking. Serotonin release in response to this mechanical stimulus was associated with an intracellular Ca2+ increase (Christofi et al., 2004; Kim, Javed, Yu, Christofi, & Cooke, 2001) and depended on Gαq (Kim et al., 2001), adenosine (Chin et al., 2012; Christofi et al., 2004), and purine P2X and P2Y (Christofi et al., 2004; Linan-Rico et al., 2013) receptors. These studies suggest a central role for purines, and specifically ATP, in mechanically stimulated 5-HT release from the EC cells (reviewed in Cooke, Wunderlich, & Christofi, 2003). However, it is unknown whether these receptors serve as primary mechanosensors.
Several ion channels have been reported in EC cells, but none in relation to EC mechanosensitivity. Specifically, studies have described TRPA1 (Cho, Callaghan, Bron, Bravo, & Furness, 2014; Nozawa et al., 2009) and L-type calcium channels (Lomax, Gallego, Novalbos, Garcia, & Warhurst, 1999; Raghupathi et al., 2013). It is accepted that cytoplasmic Ca2+ is important for 5-HT exocytosis in EC cells. However, whether mechanical activation of these ion channels is important for modulating 5-HT release is unknown.
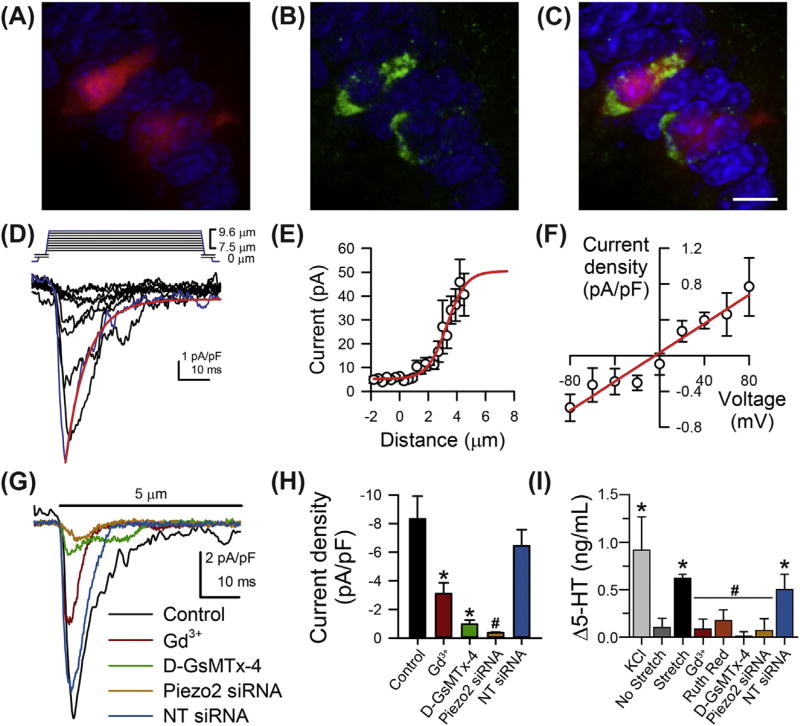
In our recent work, we examined EC cell mechanosensitivity by leveraging the similarities between EC cells and Merkel cells in both function (Nakatani, Maksimovic, Baba, & Lumpkin, 2014; Raybould, Cooke, & Christofi, 2004) and development (Li, Ray, Singh, Johnston, & Leiter, 2011; Roach et al., 2013; Wright et al., 2015; Yang, Bermingham, Finegold, & Zoghbi, 2001). Merkel cells are the specialized skin epithelium light touch sensors, and recent studies have shown that Piezo2 is important for Merkel cell mechanotransduction (Ikeda et al., 2014; Maksimovic et al., 2014; Woo et al., 2014). We discovered that Piezo2 channels are important for EC cell mechanosensitivity (Wang et al., 2016) (Fig. 2). We found Piezo2 mRNA in both human and murine small bowel epithelia. Piezo2 immunolabeling showed that these channels were specifically distributed in 5-HT positive EC cells in human jejunum. We also showed that in a transgenic mouse model TPH1-CFP, where CFP expression is encoded in EC cells, Piezo2 label localizes specifically within the CFP positive cells in the epithelium (Fig. 2A―C). We then used an EC cell model and showed that single cell mechanical stimulation elicited nonselective rapidly inactivating currents consistent with Piezo2 (Fig. 2D–F). These currents were blocked by Gd3+, GsMTx-4, and Piezo2 siRNA but not nontargeted siRNA (Fig. 2G and H). Pressure application resulted in 5-HT release, which was also prevented by treatment with pharmacological blockers (Gd3+ and D-GsMT-4) and Piezo2 siRNA. Importantly, mucosal pressure increased 5-HT release and mucosal secretion in mouse small bowel and pressure-induced increase in secretion was significantly reduced by the Piezo2 blockers Gd3+ and RR and the specific Piezo blocker D-GsMtx-4 (Wang et al., 2016) (Fig. 2I).
Figure 2.
Piezo2 is important for enterochromaffin (EC) cell mechanotransduction. Immunohistochemistry showing that mouse (A) TPH1-CFP positive EC cells [red] label with (B) Piezo2 [green] (C). Nuclei labeled with DAPI, scale bar 10 µm. (D) Inward currents evoked by mechanical stimulation of EC cell model (QGP-1) are rapidly activating and inactivating [red line]. Blue trace represents peak current. (E) Current-deformation data are fit with two-state Boltzmann [red line] and (F) current―voltage relationship is linear [red line]. (G) The mechanically induced inward currents are blocked by Gd3+, D-GsMTx-4, and Piezo2 siRNA. (H) Averaged peak current in control QGP-1 cells is inhibited by Gd3+, D-GsMTx-4, and Piezo2 siRNA but not nontargeting (NT) siRNA. *p < .05 compared to no stretch. #p < .05 compared to NT siRNA. (I) Blockade of stretch-dependent 5-HT release from EC cell model QGP-1 by Piezo channel blockers Gd3+, D-GsMTx-4, RR, and Piezo2 siRNA but not NT siRNA. *p < .05. #p > .05 compared to stretch. Modified with permission from Wang, F., Knutson, K., Alcaino, C., Linden, D. R., Gibbons, S. J., Kashyap, P. K., . Beyder, A. (2016). Mechano-sensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. The Journal of Physiology. http://dx.doi.org/10.1113/JP272718.
Further work is required to determine the EC cell mechanotransduction mechanism linking Piezo2 activation by force to 5-HT release and downstream effects on tissue physiology and in vivo effects. Functional studies on primary EC cells will be critical to confirm and extend the findings that implicate Piezo2 in EC mechanosensation. Further, given the lethality of constitutive Piezo2 mouse knockouts, cell-specific Piezo2 knockout models will be required to unequivocally determine the Piezo2-dependent mechanism of mechanotransduction in EC cells.
3.2 Gastrointestinal smooth muscle mechanosensitivity
Bayliss (1902) first described the active contractile responses to counteract increased transmural pressure in arterial wall smooth muscle more than a century ago. GI smooth muscle mechanosensitivity was suggested by Bulbring (1955), when she measured muscle responses to stretch in the absence of neuronal input, the so-called myogenic contractions. The smooth muscle syncytium contains SMCs and their pacemakers—the ICC. Both cell types are mechanosensitive and little is currently known about how much each of these cell types contributes toward myogenic responses.
3.2.1 Gastrointestinal smooth muscle cell mechanosensitivity
The SMC is the workhorse of the smooth muscle organs like the gut (Fig. 1). SMC uses voltage-gated ion channels to convert electrical energy into contractions in a process called excitation—contraction coupling (Bolton, Prestwich, Zholos, & Gordienko, 1999). Therefore, voltage-gated ion channels are critical for normal SMC function. Studies show that both the mechanosensitive voltage-gated ion channels and mechanogated ion channels are expressed in SMCs.
3.2.2 Voltage-gated ion channel mechanosensitivity
Voltage-gated channels are important determinants of smooth muscle membrane potential and excitability. Both CaV and NaV channels are expressed in SMC and have been found to respond to mechanical stimulation. The L-type voltage-gated calcium channels (CaV1.2) are sine qua non requirement for the contraction of SMC (Sanders, Koh, & Ward, 2006). A robust set of evidence indicates that CaV1.2 channels mediate SMC mechanosensitivity (Farrugia et al., 1999). GI L-type channel was sensitive to mechanical shear stress (Farrugia et al., 1999; Strege, Holm, et al., 2003) and osmotic stress (Kim, Rhee, & Kang, 2007). Mechanical stimulation increase peak current and speed up activation and inactivation, with no effect on voltage dependency (Farrugia et al., 1999; Lyford et al., 2002). CaV1.2 mechanosensitivity depended on the lipid bilayer (Kraichely, Strege, Sarr, Kendrick, & Farrugia, 2009) and not cytoskeleton (Strege, Holm, et al., 2003). In the gastric smooth muscle, while cytoskeletal disruption by cytochalasin significantly diminished L-type currents in isotonic solutions, cytoskeletal disruption did not alter L-type currents when cells were stimulated by hypoosmotic solutions (Kim et al., 2007). Similarly, a dihydropyridine-sensitive inward current increase has been found in rat myocytes in response to application of negative and positive pressure and after cell swelling. These currents were thought to be voltage-dependent Ca2+ currents (Langton, 1993). In the rat, uterine SMC stretch induced contractions due to Ca2+ influx, which modulates oxytocin-induced rhythmic contractions (Kasai, Tsutsumi, Taketani, Endo, & Iino, 1995).
A mechanosensitive voltage-gated sodium channel NaV1.5 (encoded by SCN5A), as in ICC, is also found in SMC (Holm et al., 2002). Although multiple NaV isoforms have been reported in GI SMC and ICC, the TTX-insensitive NaV1.5 has been identified in human jejunum and colon and sodium currents have been shown to be activated by shear stress in SMC in a cytoskeleton-dependent manner, with membrane lipids also playing a role (Ou et al., 2002; Strege, Holm, et al., 2003). Pharmacological block of NaV1.5 by the local anesthetic lidocaine reduced the rate of slow wave rise and increased slow wave duration, resulting in an overall decrease of the slow wave frequency (Strege, Ou, et al., 2003). There is also an important link to disease. SCN5A mutations from patients with irritable bowel syndrome (IBS) have been shown to generate NaV1.5 currents of smaller density and reduced mechanosensitivity (Saito et al., 2009).
3.2.3 Potassium channel mechanosensitivity
Mechanogated K+ channels TREK-1 were found in stomach (Ordway, Petrou, Kirber, Walsh, & Singer, 1995) and colonic SMC (Koh & Sanders, 2001). These channels are thought to contribute to membrane hyperpolarization and relaxation in certain regions of the GI tract, where relaxation is required upon mechanical stimulation, such as in gastric fundus and ascending colon that serve an important storage function (Sanders & Koh, 2006). The two-pore domain mechanosensitive K+ channel TREK-1, known to be mechanosensitive (Berrier et al., 2013), is present and functionally relevant for mechanosensation in GI smooth muscle (Hwang et al., 2008; Sanders & Koh, 2006). Patch-clamp experiments in freshly dissociated SMC from toad stomach have shown an increase in channel activation after pressure, which was increased with the application of fatty acids (Ordway et al., 1995). Other potassium channels such as BKCa are also activated by mechanical stretch (Wang, Huang, et al., 2010), generating a large outward current that hyperpolarizes the cell membrane and reduces SMC excitability in the colon.
3.2.4 Nonselective cation channel mechanosensitivity
Piezo channels are known to be nonselective cationic channels (Coste et al., 2010), with transient receptor potential (TRP) channels like TRPA1 and TRPC1 also fitting into this category (Maroto et al., 2005; Paulsen, Armache, Gao, Cheng, & Julius, 2015). Mechanosensitive nonselective cation channels are present in intestinal smooth muscle (Davis, Donovitz, & Hood, 1992). Pressurized patches from single SMCs from toad stomach showed nonselective cation current (Kirber, Walsh, & Singer, 1988). These hyperpolarization-activated currents were found to be mostly K+ and Na+ permeable and are thought to stabilize the membrane resting potential or control spontaneous electrical activity (Hisada, Ordway, Kirber, Singer, & Walsh, 1991; Hisada, Singer, & Walsh, 1993). Calcium entry has been visualized through single stretch-activated cationic channels in SMCs while applying negative pressure in the patch pipette (Zou, Lifshitz, Tuft, Fogarty, & Singer, 2002).
Gd3+, which blocks many stretch-activated ion channels (Yang & Sachs, 1989), stopped muscle contraction and prevented action potential firing in stretched tissue (Kunze, Clerc, Bertrand, & Furness, 1999). Guinea pig gastric SMCs also express mechanosensitive nonselective cation ion channels inhibited by Gd3+ and with kinetic and conductance properties similar to Piezo1 channels (Yamamoto & Suzuki, 1996). In vascular smooth muscle, stretch-activated single channels resembling Piezo1 can be blocked by Gd3+, GsMTx-4, and streptomycin (Ducret et al., 2010). GsMTx-4-inhibited mechanosensitive cation current was modulated by the balance of polycystins TRPP1 and TRPP2, but this mechanism has not been explored in intestinal smooth muscle (Sharif-Naeini et al., 2009).
Activation of nonselective cation channels in smooth muscle regulates electrical excitability, but recent evidence from vascular smooth muscle suggests that in addition to this role, Piezo1 plays a role in smooth muscle development via regulation of vascular endothelium shear stress sensitivity (Ranade, Qiu, et al., 2014) and facilitating secretion of the cross-linking enzymes required for smooth muscle remodeling (Retailleau et al., 2015). The noncanonical roles of the Piezo proteins in GI smooth muscle are currently unexplored.
In summary, mechanosensitive ion channels play an important role in GI SMC mechanotransduction. Voltage-gated Ca2+ and Na+ and non-voltage-gated K2P and nonselective cationic channels have all been described as mechanosensors. Piezo channels have been found in vascular smooth muscle and similar currents that are inhibited by the mechanosensitive channel blockers such as Gd3+ have been shown in GI SMCs. Based on this evidence, it is likely that Piezo channels are involved in GI SMC mechanosensitivity. However, additional studies are needed to determine both canonical and noncanonical roles of Piezo channels in GI smooth muscle.
3.2.5 Interstitial cell of Cajal mechanosensitivity
The ICC are found throughout the length of the gut―from esophagus to anus and are classified according to their location in the GI wall (Komuro, 2006) (Fig. 1). The ICC located in the myenteric plexus (ICC-MY) in the small bowel and submucosal border (ICC-SM) in the colon are known as pacemakers because they generate and spread an electrical rhythm, termed the “slow wave” (Huizinga et al., 1995). This ICC-generated electrical slow wave leads to the SMC depolarization that culminates in the excitation― contraction coupling by the SMC. ICC are implicated in the myogenic stretch responses, since, for example, stretching of mouse gastric muscle (Won, Sanders, & Ward, 2005) and human jejunum (Strege, Ou, et al., 2003) increased slow wave frequency independent of neuronal mechanisms. Importantly, responses to stretch were absent in transgenic mice that lack intramuscular ICC, suggesting a role of ICC in stretch-dependent responses in the GI tract, but the mechanisms of mechanosensation are not fully established (Won et al., 2005).
ICC have several ion channel types, some of which are known in other systems to be mechanosensitive (reviewed in Kraichely & Farrugia, 2007). The SCN5A encoded mechanosensitive voltage-gated sodium channel Nav1.5 (Gellens et al., 1992) has been identified in intestinal ICC and experiments using shear stress in isolated ICC increased and accelerated NaV currents (Strege, Ou, et al., 2003). A volume-activated chloride current has been found in murine-cultured jejunum ICC after cell swelling (Park, McKay, Zhu, & Huizinga, 2005). In cultured bladder ICC from guinea pig, chronic stretch leads to an increase in stretch-induced calcium transients (Wang, Fang, et al., 2010). Here, a stretch-dependent increase in intracellular calcium has been involved in bladder excitatory regulation (Wang, Fang, et al., 2010). Expression of the TRP channel subtypes TRPC4 and TRPM7 has been found in murine ICC (Kim et al., 2005; Walker, Koh, Sergeant, Sanders, & Horowitz, 2002). However, while some TRP channels are known to be mechanosensitive, TRPC4 and TRPM7 are not. Overall, our current knowledge on mechanosensitive channels in GI ICC is still very limited.
3.3 Mechanosensitivity of the enteric neurons
The enteric nervous system includes both intrinsic and extrinsic neurons and it is critical for autonomous GI function (Fig. 1). The intrinsic neurons are contained completely within the GI tract and in general they regulate regional GI motor and secretory/absorptive functions, while the extrinsic neurons have their soma outside the GI tract (Fig. 1). Extrinsic nerves also regulate GI motility, but in addition supply the GI tract with sensory capabilities. Both intrinsic and extrinsic neurons are mechanosensitive.
3.3.1 Intrinsic enteric neuron mechanosensitivity
Mechanosensitive enteric neurons control reflex activity by responding to mechanical stress in the gut wall. A particular type of enteric neurons, termed intrinsic primary afferent neurons (IPANs), is mechanosensitive (Furness, Kunze, Bertrand, Clerc, & Bornstein, 1998) (Fig. 1). The IPANs project to the mucosa and actively communicate with each other and other neuronal types (Kunze, Furness, Bertrand, & Bornstein, 1998). IPANs fire action potentials after mechanical stimulation of their soma or processes, and their responses can be abolished pharmacologically preventing muscle contraction (Kunze et al., 1999, 1998).
Recent studies have challenged the concept that IPANs alone are mechanosensitive, instead work has suggested that enteric neurons are more broadly mechanosensitive (Schemann & Mazzuoli, 2010; Smith, Spencer, Hennig, & Dickson, 2007). Several studies have reported distinct mechanosensitive responses in enteric neurons to tensile stress (stretch), compressive stress (volume injection, glass probe, or von Frey hair), shear stress, and cell swelling (hypoosmotic solutions) (Dong, Jiang, Dong, & Mittal, 2014; Hibberd, Zagorodnyuk, Spencer, & Brookes, 2012; Kugler et al., 2015; Kunze et al., 1999; Kunze, Clerc, Furness, & Gola, 2000; Kunze et al., 1998; Mayer & Wood, 1975; Mazzuoli & Schemann, 2009, 2012; Spencer & Smith, 2004). While these studies suggest roles in mechanosen-sitivity sensing and control of muscle activity, as well as a servo-feedback loop (Mazzuoli-Weber & Schemann, 2015), the identities of the mechano-sensitive ion channels responsible for mechanotransduction are largely unknown. Only one study identified a molecular transduction mechanism, which involved the activation of a large conductance BK-like potassium channel (Kunze et al., 2000). In this study, enteric neurons were subjected to patch clamp in cell-attached and whole cell configurations while pressing the cell processes with a fine probe. Pressure on the cell surface initiated generator potentials and depolarization. These potentials persisted when synaptic transmission was blocked by solutions containing low Ca2+ (0.2 mM) and high Mg2+ (10 mM). In patches, application of intraelectrode pressure increased the open probability of BK channels.
3.3.2 Extrinsic enteric neuron mechanosensitivity
Piezo channels are important mechanotransducers in the somatosensory system. Piezo2 ion channels are critical for mechanotransduction of light touch sensation by the Merkel cell―neurite complex formed between the epithelial Merkel cells and the sensory neurons that innervate the skin (Dubin et al., 2012; Ranade, Woo, et al., 2014), (reviewed in Woo, Lumpkin, & Patapoutian, 2015). Mechanical forces are sensed directly by Piezo2 channels in both Merkel cells and somatosensory neurons, which allow for a complex encoding of mechanical forces (Dubin et al., 2012; Ranade, Woo, et al., 2014).
While the role of Piezo channels in somatosensation is being established, their full role in the autonomic nervous system is still unclear. Sensory information from the GI tract is acquired by sensory afferent nerves that have their cell bodies outside the GI tract, although a unique type of afferents called intestinofugal afferent neurons (IFANS) have their bodies and processes within the gut but their axons project away from the bowel (Fig. 1). IFANS communicate with prevertebral ganglion neurons known as PVG (reviewed in Szurszewski, Ermilov, & Miller, 2002). These neurons respond to stretch of circular muscle but not tension and colonic distension activates their slow adapting mechanoreceptors, generating acetylcholine release in the PVG and evoking action potentials (Parkman, Ma, Stapelfeldt, & Szurszewski, 1993). Functionally, their role relies on relaxation of the colonic wall during filling, opposing the depolarization and contraction of colonic SMC, since the intraluminal content generates distention. This mechanism regulates the motor activity of the intestine (reviewed in Szurszewski et al., 2002).
Vagal and spinal sensory neurons have been shown to be mechanosensitive, where neuron deformation modulates reflex activity (Berthoud, Blackshaw, Brookes, & Grundy, 2004). Vagal afferent nerves detect tension at physiological levels (Andrews, 1986), where they are important in the control of normal GI function. Mechanoreceptors in the vagal afferents can be divided in two groups: those responding to distension or contraction of the gut wall and those that can be activated by chemical or mechanical stimulation of the mucosa (Page, Martin, & Blackshaw, 2002).
Spinal afferents have high threshold of distension detection and can respond to a wide range of stimuli extending to the noxious range (Blackshaw, Brookes, Grundy, & Schemann, 2007; Rong et al., 2004). Their cell bodies lie on the dorsal root ganglia (DRG) and their peripheral projections extend to the muscle layers, mucosal epithelia and enteric ganglia (Blackshaw et al., 2007).
3.3.3 Role of mechanosensitive ion channels in visceral mechanosensation
Direct activation of ion channels on the afferent endings could mediate visceral mechanosensation (Fig. 1). In guinea pig, mechanosensitive ion channels are likely involved (Zagorodnyuk, Chen, Costa, & Brookes, 2003; Zagorodnyuk, Lynn, Costa, & Brookes, 2005). The vagal mechanoreceptors responses were not affected by Ca2+ depletion and mechanotransduction was thought to be mediated via benzamil-sensitive, stretch-activated ion channels, which were not affected by high concentrations of Gd3+ (300 µM), with no involvement of chemical transmission (Zagorodnyuk et al., 2003, 2005).
The acid-sensing ion channel (ASIC) family has been proposed to play a role as a mechanosensory molecule because of its homology to the invertebrate cationic channel DEG/ENaC, known to be involved in touch sensation (Price et al., 2001). Expression of ASIC channels has been found in splanchnic DRG and vagal gastroesophageal neurons (Hughes, Brierley, Young, & Blackshaw, 2007; Page et al., 2005). All three types of ASIC channels expressed in visceral neurons are known to be required for normal mechanosensation, but their specific role in modulation of visceral mechanosensitivity is masked by the variability of positive and negative effects elicited by knocking down and mutating these channels (reviewed in Brierley, 2010). TRP channels have been involved in gut hypersensitivity (Brierley et al., 2009). TRPA1 expresses in enteric neurons (Cattaruzza et al., 2010; Poole et al., 2011). In TRPA1 KO mice behavioral responses to noxious colonic distension were reduced, with TRPA1 agonists causing mechanical hypersensitivity (Brierley et al., 2009). TRPV1 and TRPV4 also contributed to gut mechanosensitivity. The capsaicin-activated TRPV1 is expressed in colonic neurons (Brierley, Jones, Xu, Gebhart, & Blackshaw, 2005; Christianson, McIlwrath, Koerber, & Davis, 2006; Robinson, McNaughton, Evans, & Hicks, 2004). TRPV1 deletion has been shown to reduce the mechanosensitivity of distension-sensitive afferent neurons from the gastroesophageal region, jejunum, and pelvic colon (Bielefeldt & Davis, 2008; Jones, Xu, & Gebhart, 2005; Rong et al., 2004). Interestingly, TRPV1 is not a mechanogated channel and its effects on mechanosensation are thought to be due to indirect interactions with other TRP channels, such as TRPA1 or effects on neuron excitability. In colonic serosal afferents, capsaicin-dependent desensitization of TRPV1 was prevented in TRPA1 KO mice (Brierley et al., 2009; Levine & Alessandri-Haber, 2007). TRPV4 was also found along DRG neurons in the gut (Brierley et al., 2008). TRPV4 agonists potentiated mechanosensory responses in wild-type mice, whereas the nonselective blocker RR reduces afferents mechanosensitivity, with no effect on TRPV4 KO mice (Brierley et al., 2008; Sipe et al., 2008). Additionally, TRPV4 was also thought to play a role in gut hypersensitivity (Cenac et al., 2010).
Piezo channels represent a good candidate for mechanotransduction of extrinsic signals in the gut. Recent work suggests that in rat visceral afferent (DRG) neurons rely on Piezo2 channels for visceral sensation (Yang et al., 2016). In control rats, Piezo2 knockdown by shRNA by intrathecal injection resulted in a decreased visceromotor response to innocuous stimuli but not noxious stimuli. In a model of IBS the increase in visceromotor responses to both innocuous and noxious stimuli was inhibited by Piezo2 knockdown in DRGs. Since the alterations of the visceromotor responses in response to irritation occurred without an increase in Piezo2 mRNA or protein, the authors suggested that other channels or chemical mediators are involved in noxious responses. Further work is required to confirm these findings and to extend these discoveries toward mechanistic understanding.
In summary, several TRP and ASIC channels are known to be expressed in extrinsic nerves and may be required for normal mechanosensation, but knocking down of these channels does not knock down mechanosensitivity in extrinsic afferents. Recent work suggests that Piezo2 is involved in visceral sensitivity but the extent of involvement in response to innocuous versus noxious stimuli is unclear. Given the apparently shared role of ASIC and TRP channel subtypes in regulation of mechano responses, one could speculate these channels form complexes and Piezo channels could also be interacting.
4. SUMMARY AND CONCLUSIONS
Mechanosensation is critical for normal GI tract function. Multiple cell types throughout the wall of the GI tract are mechanosensitive, from the epithelial EC cells to SMCs and their pacemakers and to neurons, both extrinsic and intrinsic. These cells use mechanosensitive ion channels to convert mechanical forces into chemical signals. In the GI tract both the mechanosensitive voltage-gated ion channels and mechanogated ion channels are important for normal physiology.
The mammalian Piezo1 and Piezo2 proteins are recently discovered eukaryotic mechanosensitive cationic channels. Piezo channels are known to be critical for a wide set of mechanotransduction processes, including nociception, light touch, proprioception, volume regulation of erythrocytes, and vascular function. Piezo expression has been found along the GI tract in stomach, small bowel, and colon but currently little is known about their specific localization within the GI mechanosensory cells. Mechanosensitive cells in GI have been shown to express a wide range of mechanosensitive channels and nonselective cationic currents with properties that could match those of Piezo channels have been described. Moreover, mechanosensitive channel blockers such as Gd3+ and RR have been shown to block mechanical responses in SMC and ICC. In the GI epithelium, EC cells express Piezo2 and the recent findings suggest the role of Piezo2 as the principal EC cell mechanotransducer, where mechanically stimulated 5-HT release is Piezo2 dependent. However, further work is needed to understand the downstream effects of mechanical activation of Piezo2 in EC cells. The role of Piezo2 in mechanotransduction of mechanical forces in GI epithelium represents only one of the potential roles of Piezo channels in GI mechanosensitivity.
Acknowledgments
The authors thank Mrs Jennifer Rud for administrative assistance and Mr. Peter Strege who contributed to figure preparation. Financial support to AB from NIH K08 (DK106456) and 2015 American Gastroenterological Association Research Scholar Award (AGA RSA), Pilot and Feasibility Grant from Mayo Clinic Center for Cell Signaling in Gastroenterology (NIH P30DK084567), and to GF from NIH R01 (DK52766).
References
- Acosta A, Camilleri M, Shin A, Vazquez-Roque MI, Iturrino J, Burton D, Zinsmeister AR. Quantitative gastrointestinal and psychological traits associated with obesity and response to weight-loss therapy. Gastroenterology. 2015;148(3):537–546. doi: 10.1053/j.gastro.2014.11.020. http://dx.doi.org/10.1053/j.gastro.2014.11.020. e534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PL. Vagal afferent innervation of the gastrointestinal tract. Progress in Brain Research. 1986;67:65–86. doi: 10.1016/s0079-6123(08)62757-0. [DOI] [PubMed] [Google Scholar]
- Arnadottir J, Chalfie M. Eukaryotic mechanosensitive channels. Annual Review of Biophysics. 2010;39:111–137. doi: 10.1146/annurev.biophys.37.032807.125836. http://dx.doi.org/10.1146/annurev.biophys.37.032807.125836. [DOI] [PubMed] [Google Scholar]
- Bae C, Sachs F, Gottlieb PA. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry. 2011;50(29):6295–6300. doi: 10.1021/bi200770q. http://dx.doi.org/10.1021/bi200770q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss WM. On the local reactions of the arterial wall to changes of internal pressure. The Journal of Physiology. 1902;28(3):220–231. doi: 10.1113/jphysiol.1902.sp000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrier C, Pozza A, de Lacroix de Lavalette A, Chardonnet S, Mesneau A, Jaxel C, Ghazi A. The purified mechanosensitive channel TREK-1 is directly sensitive to membrane tension. The Journal of Biological Chemistry. 2013;288(38):27307–27314. doi: 10.1074/jbc.M113.478321. http://dx.doi.org/10.1074/jbc.M113.478321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, Blackshaw LA, Brookes SJ, Grundy D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterology and Motility. 2004;16(Suppl. 1):28–33. doi: 10.1111/j.1743-3150.2004.00471.x. http://dx.doi.org/10.1111/j.1743-3150.2004.00471.x. [DOI] [PubMed] [Google Scholar]
- Bertrand PP. Real-time detection of serotonin release from enterochromaffin cells of the Guinea-pig ileum. Neurogastroenterology and Motility. 2004;16(5):511–514. doi: 10.1111/j.1365-2982.2004.00572.x. http://dx.doi.org/10.1111/j.1365-2982.2004.00572.x. [DOI] [PubMed] [Google Scholar]
- Bertrand PP. Real-time measurement of serotonin release and motility in Guinea pig ileum. The Journal of Physiology. 2006;577(Pt 2):689–704. doi: 10.1113/jphysiol.2006.117804. http://dx.doi.org/10.1113/jphysiol.2006.117804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand PP, Hu X, Mach J, Bertrand RL. Serotonin (5-HT) release and uptake measured by real-time electrochemical techniques in the rat ileum. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2008;295(6):G1228–G1236. doi: 10.1152/ajpgi.90375.2008. http://dx.doi.org/10.1152/ajpgi.90375.2008. [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Kunze WA, Furness JB, Bornstein JC. The terminals of myenteric intrinsic primary afferent neurons of the Guinea-pig ileum are excited by 5-hydroxytryptamine acting at 5-hydroxytryptamine-3 receptors. Neuroscience. 2000;101(2):459–469. doi: 10.1016/s0306-4522(00)00363-8. [DOI] [PubMed] [Google Scholar]
- Bielefeldt K, Davis BM. Differential effects of ASIC3 and TRPV1 deletion on gastroesophageal sensation in mice. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2008;294(1):G130–G138. doi: 10.1152/ajpgi.00388.2007. http://dx.doi.org/10.1152/ajpgi.00388.2007. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterology and Motility. 2007;19(Suppl. 1):1–19. doi: 10.1111/j.1365-2982.2006.00871.x. http://dx.doi.org/10.1111/j.1365-2982.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- Bohorquez DV, Shahid RA, Erdmann A, Kreger AM, Wang Y, Calakos N, Liddle RA. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. Journal of Clinical Investigation. 2015 doi: 10.1172/JCI78361. http://dx.doi.org/10.1172/JCI78361. [DOI] [PMC free article] [PubMed]
- Bolton TB, Prestwich SA, Zholos AV, Gordienko DV. Excitation-contraction coupling in gastrointestinal and other smooth muscles. Annual Review of Physiology. 1999;61:85–115. doi: 10.1146/annurev.physiol.61.1.85. http://dx.doi.org/10.1146/annurev.physiol.61.1.85. [DOI] [PubMed] [Google Scholar]
- Brierley SM. Molecular basis of mechanosensitivity. Autonomic Neuroscience. 2010;153(1–2):58–68. doi: 10.1016/j.autneu.2009.07.017. http://dx.doi.org/10.1016/j.autneu.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Hughes PA, Page AJ, Kwan KY, Martin CM, O’Donnell TA, Blackshaw LA. The ion channel TRPA1 is required for normal mechanosensation and is modulated by algesic stimuli. Gastroenterology. 2009;137(6):2084–2095. doi: 10.1053/j.gastro.2009.07.048. http://dx.doi.org/10.1053/j.gastro.2009.07.048. e2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley SM, Jones RC, 3rd, Xu L, Gebhart GF, Blackshaw LA. Activation of splanchnic and pelvic colonic afferents by bradykinin in mice. Neurogastroenterology and Motility. 2005;17(6):854–862. doi: 10.1111/j.1365-2982.2005.00710.x. http://dx.doi.org/10.1111/j.1365-2982.2005.00710.x. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Page AJ, Hughes PA, Adam B, Liebregts T, Cooper NJ, Blackshaw LA. Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology. 2008;134(7):2059–2069. doi: 10.1053/j.gastro.2008.01.074. http://dx.doi.org/10.1053/j.gastro.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DR. Mucosal protection through active intestinal secretion: Neural and paracrine modulation by 5-hydroxytryptamine. Behavioural Brain Research. 1996;73(1–2):193–197. doi: 10.1016/0166-4328(96)00095-2. [DOI] [PubMed] [Google Scholar]
- Bulbring E. Correlation between membrane potential, spike discharge and tension in smooth muscle. The Journal of Physiology. 1955;128(1):200–221. doi: 10.1113/jphysiol.1955.sp005299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulbring E, Crema A. Observations concerning the action of 5-hydroxytryptamine on the peristaltic reflex. British Journal of Pharmacology and Chemotherapy. 1958;13(4):444–457. doi: 10.1111/j.1476-5381.1958.tb00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulbring E, Crema A. The release of 5-hydroxytryptamine in relation to pressure exerted on the intestinal mucosa. The Journal of Physiology. 1959;146(1):18–28. doi: 10.1113/jphysiol.1959.sp006175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulbring E, Lin RC. The effect of intraluminal application of 5-hydroxytryptamine and 5-hydroxytryptophan on peristalsis; the local production of 5-HT and its release in relation to intraluminal pressure and propulsive activity. The Journal of Physiology. 1958;140(3):381–407. [PMC free article] [PubMed] [Google Scholar]
- Cattaruzza F, Spreadbury I, Miranda-Morales M, Grady EF, Vanner S, Bunnett NW. Transient receptor potential ankyrin-1 has a major role in mediating visceral pain in mice. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2010;298(1):G81–G91. doi: 10.1152/ajpgi.00221.2009. http://dx.doi.org/10.1152/ajpgi.00221.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenac N, Altier C, Motta JP, d’Aldebert E, Galeano S, Zamponi GW, Vergnolle N. Potentiation of TRPV4 signalling by histamine and serotonin: An important mechanism for visceral hypersensitivity. Gut. 2010;59(4):481–488. doi: 10.1136/gut.2009.192567. http://dx.doi.org/10.1136/gut.2009.192567. [DOI] [PubMed] [Google Scholar]
- Chin A, Svejda B, Gustafsson BI, Granlund AB, Sandvik AK, Timberlake A, Kidd M. The role of mechanical forces and adenosine in the regulation of intestinal enterochromaffin cell serotonin secretion. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2012;302(3):G397–G405. doi: 10.1152/ajpgi.00087.2011. http://dx.doi.org/10.1152/ajpgi.00087.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, Callaghan B, Bron R, Bravo DM, Furness JB. Identification of enteroendocrine cells that express TRPA1 channels in the mouse intestine. Cell and Tissue Research. 2014 doi: 10.1007/s00441-013-1780-x. http://dx.doi.org/10.1007/s00441-013-1780-x. [DOI] [PubMed]
- Christianson JA, McIlwrath SL, Koerber HR, Davis BM. Transient receptor potential vanilloid 1-immunopositive neurons in the mouse are more prevalent within colon afferents compared to skin and muscle afferents. Neuroscience. 2006;140(1):247–257. doi: 10.1016/j.neuroscience.2006.02.015. http://dx.doi.org/10.1016/j.neuroscience.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Christofi FL, Kim M, Wunderlich JE, Xue J, Suntres Z, Cardounel A, Cooke HJ. Endogenous adenosine differentially modulates 5-hydroxytryptamine release from a human enterochromaffin cell model. Gastroenterology. 2004;127(1):188–202. doi: 10.1053/j.gastro.2004.04.070. [DOI] [PubMed] [Google Scholar]
- Cooke HJ, Wunderlich J, Christofi FL. “The force be with you”: ATP in gut mechanosensory transduction. News in Physiological. 2003;18:43–49. doi: 10.1152/nips.01411.2002. [DOI] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330(6000):55–60. doi: 10.1126/science.1193270. http://dx.doi.org/10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, Patapoutian A. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483(7388):176–181. doi: 10.1038/nature10812. http://dx.doi.org/10.1038/nature10812nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote F, Thevenot E, Fligny C, Fromes Y, Darmon M, Rpoche MA, Vodjdani G. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(23):13525–13530. doi: 10.1073/pnas.2233056100. http://dx.doi.org/10.1073/pnas.2233056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JD, Palanivel R, Mottillo EP, Bujak AL, Wang H, Ford RJ, Steinberg GR. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nature Medicine. 2015;21(2):166–172. doi: 10.1038/nm.3766. http://dx.doi.org/10.1038/nm.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, Donovitz JA, Hood JD. Stretch-activated single-channel and whole cell currents in vascular smooth muscle cells. The American Journal of Physiology. 1992;262(4 Pt 1):C1083–C1088. doi: 10.1152/ajpcell.1992.262.4.C1083. [DOI] [PubMed] [Google Scholar]
- Dong H, Jiang Y, Dong J, Mittal R. Inhibitory motor neurons of the esophageal myenteric plexus are mechanosensitive. American Journal of Physiology. Cell Physiology. 2014 doi: 10.1152/ajpcell.00159.2014. http://dx.doi.org/10.1152/ajpcell.00159.2014. [DOI] [PubMed]
- Dubin AE, Schmidt M, Mathur J, Petrus MJ, Xiao B, Coste B, Patapoutian A. Inflammatory signals enhance piezo2-mediated mechanosensitive currents. Cell Reports. 2012;2(3):511–517. doi: 10.1016/j.celrep.2012.07.014. http://dx.doi.org/10.1016/j.celrep.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducret T, El Arrouchi J, Courtois A, Quignard JF, Marthan R, Savineau JP. Stretch-activated channels in pulmonary arterial smooth muscle cells from normoxic and chronically hypoxic rats. Cell Calcium. 2010;48(5):251–259. doi: 10.1016/j.ceca.2010.09.011. http://dx.doi.org/10.1016/j.ceca.2010.09.011. pii:S0143-4160(10)00147-8. [DOI] [PubMed] [Google Scholar]
- Eisenhoffer GT, Loftus PD, Yoshigi M, Otsuna H, Chien CB, Morcos PA, Rosenblatt J. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature. 2012;484(7395):546–549. doi: 10.1038/nature10999. http://dx.doi.org/10.1038/nature10999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyckmans J, Boudou T, Yu X, Chen CS. A hitchhiker’s guide to mechanobiology. Developmental Cell. 2011;21(1):35–47. doi: 10.1016/j.devcel.2011.06.015. http://dx.doi.org/10.1016/j.devcel.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrugia G, Holm AN, Rich A, Sarr MG, Szurszewski JH, Rae JL. A mechanosensitive calcium channel in human intestinal smooth muscle cells. Gastroenter-ology. 1999;117(4):900–905. doi: 10.1016/s0016-5085(99)70349-5. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sanchez ME, Barbier S, Whitehead J, Bealle G, Michel A, Latorre-Ossa H, Farge E. Mechanical induction of the tumorigenic beta-catenin pathway by tumour growth pressure. Nature. 2015 doi: 10.1038/nature14329. http://dx.doi.org/10.1038/nature14329. [DOI] [PubMed]
- Foxx-Orenstein AE, Kuemmerle JF, Grider JR. Distinct 5-HT receptors mediate the peristaltic reflex induced by mucosal stimuli in human and Guinea pig intestine. Gastroenterology. 1996;111(5):1281–1290. doi: 10.1053/gast.1996.v111.pm8898642. [DOI] [PubMed] [Google Scholar]
- Frieling T, Wood JD, Cooke HJ. Submucosal reflexes: Distension-evoked ion transport in the Guinea pig distal colon. The American Journal of Physiology. 1992;263(1 Pt 1):G91–G96. doi: 10.1152/ajpgi.1992.263.1.G91. [DOI] [PubMed] [Google Scholar]
- Furness JB, Kunze WA, Bertrand PP, Clerc N, Bornstein JC. Intrinsic primary afferent neurons of the intestine. Progress in Neurobiology. 1998;54(1):1–18. doi: 10.1016/s0301-0082(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Gellens ME, George AL, Jr, Chen LQ, Chahine M, Horn R, Barchi RL, Kallen RG. Primary structure and functional expression of the human cardiac tetrodotoxin-insensitive voltage-dependent sodium channel. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(2):554–558. doi: 10.1073/pnas.89.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb PA, Sachs F. Piezo1: Properties of a cation selective mechanical channel. Channels. 2012;6(4):214–219. doi: 10.4161/chan.21050. http://dx.doi.org/10.4161/chan.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeberle H, Fujiwara M, Chuang J, Medina MM, Panditrao MV, Bechstedt S, Lumpkin EA. Molecular profiling reveals synaptic release machinery in Merkel cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(40):14503–14508. doi: 10.1073/pnas.0406308101. http://dx.doi.org/10.1073/pnas.0406308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiological Reviews. 2001;81:685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- Hibberd TJ, Zagorodnyuk VP, Spencer NJ, Brookes SJ. Identification and mechanosensitivity of viscerofugal neurons. Neuroscience. 2012;225:118–129. doi: 10.1016/j.neuroscience.2012.08.040. http://dx.doi.org/10.1016/j.neuroscience.2012.08.040. [DOI] [PubMed] [Google Scholar]
- Hisada T, Ordway RW, Kirber MT, Singer JJ, Walsh JV., Jr Hyperpolarization-activated cationic channels in smooth muscle cells are stretch sensitive. Pflugers Archiv. 417(5):493–499. doi: 10.1007/BF00370945. [DOI] [PubMed] [Google Scholar]
- Hisada T, Singer JJ, Walsh JV., Jr Aluminofluoride activates hyperpolarization- and stretch-activated cationic channels in single smooth muscle cells. Pflugers Archiv. 1993;422(4):397–400. doi: 10.1007/BF00374297. [DOI] [PubMed] [Google Scholar]
- Hoffman JM, Tyler K, MacEachern SJ, Balemba OB, Johnson AC, Brooks EM, Mawe GM. Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenter-ology. 2012;142(4):844–854. e844. doi: 10.1053/j.gastro.2011.12.041. http://dx.doi.org/10.1053/j.gastro.2011.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm AN, Rich A, Miller SM, Strege P, Ou Y, Gibbons S, Farrugia G. Sodium current in human jejunal circular smooth muscle cells. Gastroenterology. 2002;122(1):178–187. doi: 10.1053/gast.2002.30346. [DOI] [PubMed] [Google Scholar]
- Hughes PA, Brierley SM, Young RL, Blackshaw LA. Localization and comparative analysis of acid-sensing ion channel (ASIC1, 2, and 3) mRNA expression in mouse colonic sensory neurons within thoracolumbar dorsal root ganglia. The Journal of Comparative Neurology. 2007;500(5):863–875. doi: 10.1002/cne.21204. http://dx.doi.org/10.1002/cne.21204. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373(6512):347–349. doi: 10.1038/373347a0. http://dx.doi.org/10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, O’Kane N, Singer C, Ward SM, Sanders KM, Koh SD. Block of inhibitory junction potentials and TREK-1 channels in murine colon by Ca2+ store-active drugs. The Journal of Physiology. 2008;586(4):1169–1184. doi: 10.1113/jphysiol.2007.148718. http://dx.doi.org/10.1113/jphysiol.2007.148718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda R, Cha M, Ling J, Jia Z, Coyle D, Gu JG. Merkel cells transduce and encode tactile stimuli to drive abeta-afferent impulses. Cell. 2014;157(3):664–675. doi: 10.1016/j.cell.2014.02.026. http://dx.doi.org/10.1016/j.cell.2014.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda R, Gu JG. Piezo2 channel conductance and localization domains in Merkel cells of rat whisker hair follicles. Neuroscience Letters. 2014;583:210–215. doi: 10.1016/j.neulet.2014.05.055. [DOI] [PubMed] [Google Scholar]
- Jones RC, Xu GF., 3rd The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. The Journal of Neuroscience. 2005;25(47):10981–10989. doi: 10.1523/JNEUROSCI.0703-05.2005. http://dx.doi.org/10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki M, Wade PR, Gershon MD. Participation of 5-HT3, 5-HT4, and nicotinic receptors in the peristaltic reflex of Guinea pig distal colon. The American Journal of Physiology. 1996;271(5 Pt 1):G849–G857. doi: 10.1152/ajpgi.1996.271.5.G849. [DOI] [PubMed] [Google Scholar]
- Kasai Y, Tsutsumi O, Taketani Y, Endo M, Iino M. Stretch-induced enhancement of contractions in uterine smooth muscle of rats. The Journal of Physiology. 1995;486(Pt 2):373–384. doi: 10.1113/jphysiol.1995.sp020819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Javed NH, Yu JG, Christofi F, Cooke HJ. Mechanical stimulation activates Galphaq signaling pathways and 5-hydroxytryptamine release from human carcinoid BON cells. Journal of Clinical Investigation. 2001;108(7):1051–1059. doi: 10.1172/JCI12467. http://dx.doi.org/10.1172/JCI12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BJ, Lim HH, Yang DK, Jun JY, Chang IY, Park CS, Kim KW. Melastatin-type transient receptor potential channel 7 is required for intestinal pacemaking activity. Gastroenterology. 2005;129(5):1504–1517. doi: 10.1053/j.gastro.2005.08.016. http://dx.doi.org/10.1053/j.gastro.2005.08.016. pii:S0016-5085(05)01731-2. [DOI] [PubMed] [Google Scholar]
- Kim JH, Rhee PL, Kang TM. Actin cytoskeletons regulate the stretch-induced increase of Ca2+ current in human gastric myocytes. Biochemical and Biophysical Research Communications. 2007;352(2):503–508. doi: 10.1016/j.bbrc.2006.11.051. [DOI] [PubMed] [Google Scholar]
- Kirber MT, Walsh JV, Jr, Singer JJ. Stretch-activated ion channels in smooth muscle: A mechanism for the initiation of stretch-induced contraction. Pflugers Archiv. 1988;412(4):339–345. doi: 10.1007/BF01907549. [DOI] [PubMed] [Google Scholar]
- Koh SD, Sanders KM. Stretch-dependent potassium channels in murine colonic smooth muscle cells. The Journal of Physiology. 2001;533(Pt 1):155–163. doi: 10.1111/j.1469-7793.2001.0155b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro T. Structure and organization of interstitial cells of Cajal in the gastrointestinal tract. The Journal of Physiology. 2006;576(Pt 3):653–658. doi: 10.1113/jphysiol.2006.116624. http://dx.doi.org/10.1113/jphysiol.2006.116624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraichely RE, Farrugia G. Mechanosensitive ion channels in interstitial cells of Cajal and smooth muscle of the gastrointestinal tract. Neurogastroenterology and Motility. 2007;19(4):245–252. doi: 10.1111/j.1365-2982.2006.00880.x. [DOI] [PubMed] [Google Scholar]
- Kraichely RE, Strege PR, Sarr MG, Kendrick ML, Farrugia G. Lysophosphatidyl choline modulates mechanosensitive L-type Ca2+ current in circular smooth muscle cells from human jejunum. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2009;296(4):G833–G839. doi: 10.1152/ajpgi.90610.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler EM, Michel K, Zeller F, Demir IE, Ceyhan GO, Schemann M, Mazzuoli-Weber G. Mechanical stress activates neurites and somata of myenteric neurons. Frontiers in Cellular Neuroscience. 2015;9:342. doi: 10.3389/fncel.2015.00342. http://dx.doi.org/10.3389/fncel.2015.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze WA, Clerc N, Bertrand PP, Furness JB. Contractile activity in intestinal muscle evokes action potential discharge in Guinea-pig myenteric neurons. The Journal of Physiology. 1999;517(Pt 2):547–561. doi: 10.1111/j.1469-7793.1999.0547t.x. pii:PHY_8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze WA, Clerc N, Furness JB, Gola M. The soma and neurites of primary afferent neurons in the Guinea-pig intestine respond differentially to deformation. The Journal of Physiology. 2000;526(Pt 2):375–385. doi: 10.1111/j.1469-7793.2000.00375.x. pii:PHY_0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze WA, Furness JB, Bertrand PP, Bornstein JC. Intracellular recording from myenteric neurons of the Guinea-pig ileum that respond to stretch. The Journal of Physiology. 1998;506(Pt 3):827–842. doi: 10.1111/j.1469-7793.1998.827bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton PD. Calcium channel currents recorded from isolated myocytes of rat basilar artery are stretch sensitive. The Journal of Physiology. 1993;471:1–11. doi: 10.1113/jphysiol.1993.sp019887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JD, Alessandri-Haber N. TRP channels: Targets for the relief of pain. Biochimica et Biophysica Acta. 2007;1772(8):989–1003. doi: 10.1016/j.bbadis.2007.01.008. http://dx.doi.org/10.1016/j.bbadis.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Li HJ, Ray SK, Singh NK, Johnston B, Leiter AB. Basic helix-loop-helix transcription factors and enteroendocrine cell differentiation. Diabetes, Obesity & Metabolism. 2011;13(Suppl. 1):5–12. doi: 10.1111/j.1463-1326.2011.01438.x. http://dx.doi.org/10.1111/j.1463-1326.2011.01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linan-Rico A, Wunderlich JE, Grants IS, Frankel WL, Xue J, Williams KC, Christofi FL. Purinergic autocrine regulation of mechano-sensitivity and serotonin release in a human EC model: ATP-gated P2X3 channels in EC are downregulated in ulcerative colitis. Inflammatory Bowel Diseases. 2013;19(11):2366–2379. doi: 10.1097/MIB.0b013e31829ecf4d. http://dx.doi.org/10.1097/MIB.0b013e31829ecf4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax RB, Gallego S, Novalbos J, Garcia AG, Warhurst G. L-Type calcium channels in enterochromaffin cells from Guinea pig and human duodenal crypts: An in situ study. Gastroenterology. 1999;117(6):1363–1369. doi: 10.1016/s0016-5085(99)70286-6. [DOI] [PubMed] [Google Scholar]
- Lyford GL, Strege PR, Shepard A, Ou Y, Ermilov L, Miller SM, Farrugia G. alpha(1C) (Ca(V)1.2) L-type calcium channel mediates mechanosensitive calcium regulation. American Journal of Phyisology. Cell Physiology. 2002;283(3):C1001–C1008. doi: 10.1152/ajpcell.00140.2002. [DOI] [PubMed] [Google Scholar]
- Maksimovic S, Nakatani M, Baba Y, Nelson AM, Marshall KL, Wellnitz SA, Lumpkin EA. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature. 2014;509(7502):617–621. doi: 10.1038/nature13250. http://dx.doi.org/10.1038/nature13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nature Cell Biology. 2005;7(2):179–185. doi: 10.1038/ncb1218. [DOI] [PubMed] [Google Scholar]
- Mawe GM, Hoffman JM. Serotonin signalling in the gut-functions, dysfunctions and therapeutic targets. Nature Reviews. Gastroenterology and Hepatology. 2013;10(8):473–486. doi: 10.1038/nrgastro.2013.105. http://dx.doi.org/10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer CJ, Wood JD. Properties of mechanosensitive neurons within Auerbach’s plexus of the small intestine of the cat. Pflugers Archiv. 1975;357(1–2):35–49. doi: 10.1007/BF00584543. [DOI] [PubMed] [Google Scholar]
- Mazzuoli G, Schemann M. Multifunctional rapidly adapting mechanosensitive enteric neurons (RAMEN) in the myenteric plexus of the Guinea pig ileum. The Journal of Physiology. 2009;587(Pt 19):4681–4694. doi: 10.1113/jphysiol.2009.177105. http://dx.doi.org/10.1113/jphysiol.2009.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzuoli G, Schemann M. Mechanosensitive enteric neurons in the myenteric plexus of the mouse intestine. PLoS One. 2012;7(7):e39887. doi: 10.1371/journal.pone.0039887. http://dx.doi.org/10.1371/journal.pone.0039887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzuoli-Weber G, Schemann M. Mechanosensitivity in the enteric nervous system. Frontiers in Cellular Neuroscience. 2015;9:408. doi: 10.3389/fncel.2015.00408. http://dx.doi.org/10.3389/fncel.2015.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Mochizuki T, Nakagomi H, Kira S, Watanabe M, Takayama Y, Tominaga M. Functional role for Piezo1 in stretch-evoked Ca2+ influx and ATP release in urothelial cell cultures. Journal of Biological Chemistry. 2014;289(23):16565–16575. doi: 10.1074/jbc.M113.528638. http://dx.doi.org/10.1074/jbc.M113.528638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani M, Maksimovic S, Baba Y, Lumpkin EA. Mechanotransduction in epidermal Merkel cells. Pflugers Archiv. 2014;467(1):101–108. doi: 10.1007/s00424-014-1569-0. http://dx.doi.org/10.1007/s00424-014-1569-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neshatian L, Strege PR, Rhee P-L, Kraichely RE, Mazzone AM, Bernard CE, Farrugia G. Ranolazine inhibits voltage-gated mechanosensitive sodium channels in human colon circular smooth muscle cells. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2015;309:G506–G512. doi: 10.1152/ajpgi.00051.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neya T, Mizutani M, Yamasato T. Role of 5-HT3 receptors in peristaltic reflex elicited by stroking the mucosa in the canine jejunum. The Journal of Physiology. 1993;471:159–173. doi: 10.1113/jphysiol.1993.sp019895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa K, Kawabata-Shoda E, Doihara H, Kojima R, Okada H, Mochizuki S, Ito H. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(9):3408–3413. doi: 10.1073/pnas.0805323106. http://dx.doi.org/10.1073/pnas.0805323106. pii:0805323106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordway RW, Petrou S, Kirber MT, Walsh JV, Jr, Singer JJ. Stretch activation of a toad smooth muscle K+ channel may be mediated by fatty acids. The Journal of Physiology. 1995;484(Pt 2):331–337. doi: 10.1113/jphysiol.1995.sp020668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Y, Gibbons SJ, Miller SM, Strege PR, Rich A, Distad MA, Farrugia G. SCN5A is expressed in human jejunal circular smooth muscle cells. Neurogastroen-terology and Motility. 2002;14(5):477–486. doi: 10.1046/j.1365-2982.2002.00348.x. [DOI] [PubMed] [Google Scholar]
- Page AJ, Brierley SM, Martin CM, Price MP, Symonds E, Butler R, Blackshaw LA. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut. 2005;54(10):1408–1415. doi: 10.1136/gut.2005.071084. http://dx.doi.org/10.1136/gut.2005.071084. pii:gut.2005.071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AJ, Martin CM, Blackshaw LA. Vagal mechanoreceptors and chemo-receptors in mouse stomach and esophagus. Journal of NeuroPhysiology. 2002;87(4):2095–2103. doi: 10.1152/jn.00785.2001. http://dx.doi.org/10.1152/jn.00785.2001. [DOI] [PubMed] [Google Scholar]
- Park SJ, McKay CM, Zhu Y, Huizinga JD. Volume-activated chloride currents in interstitial cells of Cajal. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2005;289(5):G791–G797. doi: 10.1152/ajpgi.00050.2005. [DOI] [PubMed] [Google Scholar]
- Parkman HP, Ma RC, Stapelfeldt WH, Szurszewski JH. Direct and indirect mechanosensory pathways from the colon to the inferior mesenteric ganglion. The American Journal of Physiology. 1993;265(3 Pt 1):G499–G505. doi: 10.1152/ajpgi.1993.265.3.G499. [DOI] [PubMed] [Google Scholar]
- Patel BA, Bian X, Quaiserova-Mocko V, Galligan JJ, Swain GM. In vitro continuous amperometric monitoring of 5-hydroxytryptamine release from enterochro-maffin cells of the Guinea pig ileum. The Analyst. 2007;132(1):41–47. doi: 10.1039/b611920d. http://dx.doi.org/10.1039/b611920d. [DOI] [PubMed] [Google Scholar]
- Paulsen CE, Armache JP, Gao Y, Cheng Y, Julius D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature. 2015 doi: 10.1038/nature14871. http://dx.doi.org/10.1038/nature14367. [DOI] [PubMed]
- Peyronnet R, Martins JR, Duprat F, Demolombe S, Arhatte M, Jodar M, Patel A. Piezo1-dependent stretch-activated channels are inhibited by polycystin-2 in renal tubular epithelial cells. EMBO Reports. 2013;14(12):1143–1148. doi: 10.1038/embor.2013.170. http://dx.doi.org/10.1038/embor.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole DP, Pelayo JC, Cattaruzza F, Kuo YM, Gai G, Chiu JV, Bunnett NW. Transient receptor potential ankyrin 1 is expressed by inhibitory motoneurons of the mouse intestine. Gastroenterology. 2011;141(2):565–575. doi: 10.1053/j.gastro.2011.04.049. http://dx.doi.org/10.1053/j.gastro.2011.04.049, 575 e561-564. [DOI] [PubMed] [Google Scholar]
- Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Welsh MJ. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron. 2001;32(6):1071–1083. doi: 10.1016/s0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- Racke K, Schworer H. Regulation of serotonin release from the intestinal mucosa. Pharmacological Research. 1991;23(1):13–25. doi: 10.1016/s1043-6618(05)80101-x. [DOI] [PubMed] [Google Scholar]
- Racke K, Reimann A, Schworer H, Kilbinger H. Regulation of 5-HT release from enterochromaffin cells. Behavioural Brain Research. 1996;73(1–2):83–87. doi: 10.1016/0166-4328(96)00075-7. [DOI] [PubMed] [Google Scholar]
- Raghupathi R, Duffield MD, Zelkas L, Meedeniya A, Brookes SJ, Sia TC, Keating DJ. Identification of unique release kinetics of serotonin from Guinea-pig and human enterochromaffin cells. The Journal of Physiology. 2013;591(Pt 23):5959–5975. doi: 10.1113/jphysiol.2013.259796. http://dx.doi.org/10.1113/jphysiol.2013.259796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranade SS, Qiu Z, Woo SH, Hur SS, Murthy SE, Cahalan SM, Patapoutian A. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(28):10347–10352. doi: 10.1073/pnas.1409233111. http://dx.doi.org/10.1073/pnas.1409233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranade SS, Syeda R, Patapoutian A. Mechanically activated ion channels. Neuron. 2015;87(6):1162–1179. doi: 10.1016/j.neuron.2015.08.032. http://dx.doi.org/10.1016/j.neuron.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Patapoutian A. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature. 2014;516(7529):121–125. doi: 10.1038/nature13980. http://dx.doi.org/10.1038/nature13980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybould HE, Cooke HJ, Christofi FL. Sensory mechanisms: Transmitters, modulators and reflexes. Neurogastroenterology and Motility. 2004;16(Suppl. 1):60–63. doi: 10.1111/j.1743-3150.2004.00477.x. http://dx.doi.org/10.1111/j.1743-3150.2004.00477.x. [DOI] [PubMed] [Google Scholar]
- Retailleau K, Duprat F, Arhatte M, Ranade SS, Peyronnet R, Martins JR, Honore E. Piezo1 in smooth muscle cells is involved in hypertension-dependent arterial remodeling. Cell Reports. 2015;13(6):1161–1171. doi: 10.1016/j.celrep.2015.09.072. http://dx.doi.org/10.1016/j.celrep.2015.09.072. [DOI] [PubMed] [Google Scholar]
- Roach G, Heath Wallace R, Cameron A, Emrah Ozel R, Hongay CF, Baral R, Wallace KN. Loss of ascl1a prevents secretory cell differentiation within the zebrafish intestinal epithelium resulting in a loss of distal intestinal motility. Developmental Biology. 2013;376(2):171–186. doi: 10.1016/j.ydbio.2013.01.013. http://dx.doi.org/10.1016/j.ydbio.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DR, McNaughton PA, Evans ML, Hicks GA. Characterization of the primary spinal afferent innervation of the mouse colon using retrograde labelling. Neurogastroenterology and Motility. 2004;16(1):113–124. doi: 10.1046/j.1365-2982.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- Rong W, Hillsley K, Davis JB, Hicks G, Winchester WJ, Grundy D. Jejunal afferent nerve sensitivity in wild-type and TRPV1 knockout mice. The Journal of Physiology. 2004;560(Pt 3):867–881. doi: 10.1113/jphysiol.2004.071746. http://dx.doi.org/10.1113/jphysiol.2004.071746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs F, Morris CE. Mechanosensitive ion channels in nonspecialized cells. Reviews of Physiology. Biochemistry and Pharmacology. 1998;132:1–77. doi: 10.1007/BFb0004985. [DOI] [PubMed] [Google Scholar]
- Saito YA, Strege PR, Tester DJ, Locke GR, Talley NJ, 3rd, Bernard CE, Farrugia G. Sodium channel mutation in irritable bowel syndrome: Evidence for an ion channelopathy. American Journal of Physiology Gastrointestinal and Liver Physiology. 2009;296(2):G211–G218. doi: 10.1152/ajpgi.90571.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Koh SD. Two-pore-domain potassium channels in smooth muscles: New components of myogenic regulation. The Journal of Physiology. 2009;570(Pt 1):37–43. doi: 10.1113/jphysiol.2005.098897. http://dx.doi.org/10.1113/jphysiol.2005.098897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annual Review of Physiology. 2006;68:307–343. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- Schemann M, Mazzuoli G. Multifunctional mechanosensitive neurons in the enteric nervous system. Autonomic Neuroscience. 2010;153(1–2):21–25. doi: 10.1016/j.autneu.2009.08.003. http://dx.doi.org/10.1016/j.autneu.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Schworer H, Katsoulis S, Racke K. Histamine inhibits 5-hydroxytryptamine release from the porcine small intestine: Involvement of H3 receptors. Gastroenterology. 1992;102(6):1906–1912. doi: 10.1016/0016-5085(92)90312-m. [DOI] [PubMed] [Google Scholar]
- Sharif-Naeini R, Folgering JH, Bichet D, Duprat F, Lauritzen I, Arhatte M, Honore E. Polycystin-1 and -2 dosage regulates pressure sensing. Cell. 2009;139(3):587–596. doi: 10.1016/j.cell.2009.08.045. http://dx.doi.org/10.1016/j.cell.2009.08.045. pii: S0092-8674(09)01125-8. [DOI] [PubMed] [Google Scholar]
- Sipe WE, Brierley SM, Martin CM, Phillis BD, Cruz FB, Grady EF, Bunnett NW. Transient receptor potential vanilloid 4 mediates protease activated receptor 2-induced sensitization of colonic afferent nerves and visceral hyperalgesia. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2008;294(5):G1288–G1298. doi: 10.1152/ajpgi.00002.2008. http://dx.doi.org/10.1152/ajpgi.00002.2008. [DOI] [PubMed] [Google Scholar]
- Slattum GM, Rosenblatt J. Tumour cell invasion: An emerging role for basal epithelial cell extrusion. Nature Reviews. Cancer. 2014;14(7):495–501. doi: 10.1038/nrc3767. http://dx.doi.org/10.1038/nrc3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Spencer NJ, Hennig GW, Dickson EJ. Recent advances in enteric neurobiology: Mechanosensitive interneurons. Neurogastroenterology and Motility. 2007;19(11):869–878. doi: 10.1111/j.1365-2982.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Smith TK. Mechanosensory S-neurons rather than AH-neurons appear to generate a rhythmic motor pattern in Guinea-pig distal colon. The Journal of Physiology. 2004;558(Pt 2):577–596. doi: 10.1113/jphysiol.2004.063586. http://dx.doi.org/10.1113/jphysiol.2004.063586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strege PR, Holm AN, Rich A, Miller SM, Ou Y, Sarr MG, Farrugia G. Cytoskeletal modulation of sodium current in human jejunal circular smooth muscle cells. American Journal of Physiology: Cell Physiology. 2003;284(1):C60–C66. doi: 10.1152/ajpcell.00532.2001. [DOI] [PubMed] [Google Scholar]
- Strege PR, Ou Y, Sha L, Rich A, Gibbons SJ, Szurszewski JH, Farrugia G. Sodium current in human intestinal interstitial cells of Cajal. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2003;285(6):G1111–G1121. doi: 10.1152/ajpgi.00152.2003. [DOI] [PubMed] [Google Scholar]
- Szurszewski JH, Ermilov LG, Miller SM. Prevertebral ganglia and intesti-nofugal afferent neurons. Gut. 2002;51(Suppl. 1):i6–10. doi: 10.1136/gut.51.suppl_1.i6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RL, Koh SD, Sergeant GP, Sanders KM, Horowitz B. TRPC4 currents have properties similar to the pacemaker current in interstitial cells of Cajal. American Journal of Physiology. Cell Physiology. 2002;283(6):C1637–C1645. doi: 10.1152/ajpcell.00266.2002. http://dx.doi.org/10.1152/ajpcell.00266.2002. [DOI] [PubMed] [Google Scholar]
- Wang Y, Fang Q, Lu Y, Song B, Li W, Li L. Effects of mechanical stretch on interstitial cells of cajal in Guinea pig bladder. The Journal of Surgical Research. 2010 doi: 10.1016/j.jss.2010.04.040. http://dx.doi.org/10.1016/j.jss.2010.04.040. pii:S0022-4804(10)00427-0. [DOI] [PubMed]
- Wang W, Huang H, Hou D, Liu P, Wei H, Fu X, Niu W. Mechanosen-sitivity of STREX-lacking BKCa channels in the colonic smooth muscle of the mouse. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2010 doi: 10.1152/ajpgi.00268.2010. http://dx.doi.org/10.1152/ajpgi.00268.2010. pii:ajpgi.00268.2010. [DOI] [PubMed]
- Wang F, Knutson K, Alcaino C, Linden DR, Gibbons SJ, Kashyap PK, Beyder A. Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. The Journal of Physiology. 2016 doi: 10.1113/JP272718. http://dx.doi.org/10.1113/JP272718. [DOI] [PMC free article] [PubMed]
- Won KJ, Sanders KM, Ward SM. Interstitial cells of Cajal mediate mecha-nosensitive responses in the stomach. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(41):14913–14918. doi: 10.1073/pnas.0503628102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SH, Lumpkin EA, Patapoutian A. Merkel cells and neurons keep in touch. Trends in Cell Biology. 2015;25(2):74–81. doi: 10.1016/j.tcb.2014.10.003. http://dx.doi.org/10.1016/j.tcb.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Patapoutian A. Piezo2 is required for Merkel-cell mechanotransduction. Nature. 2014;509(7502):622–626. doi: 10.1038/nature13251. http://dx.doi.org/10.1038/nature13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MC, Reed-Geaghan EG, Bolock AM, Fujiyama T, Hoshino M, Maricich SM. Unipotent, Atoh1+ progenitors maintain the Merkel cell population in embryonic and adult mice. The Journal of Cell Biology. 2015;208(3):367–379. doi: 10.1083/jcb.201407101. http://dx.doi.org/10.1083/jcb.201407101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VK, Balaji S, Suresh PS, Liu XS, Lu X, Li Z, Ducy P. Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nature Medicine. 2010;16(3):308–312. doi: 10.1038/nm.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Suzuki H. Two types of stretch-activated channel activities in Guinea-pig gastric smooth muscle cells. The Japanese Journal of Physiology. 1996;46(4):337–345. doi: 10.2170/jjphysiol.46.337. [DOI] [PubMed] [Google Scholar]
- Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294(5549):2155–2158. doi: 10.1126/science.1065718. http://dx.doi.org/10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- Yang XN, Lu YP, Liu JJ, Huang JK, Liu YP, Xiao CX, Guleng B. Piezo1 is as a novel trefoil factor family 1 binding protein that promotes gastric cancer cell mobility in vitro. Digestive Diseases and Sciences. 2014;59(7):1428–1435. doi: 10.1007/s10620-014-3044-3. http://dx.doi.org/10.1007/s10620-014-3044-3. [DOI] [PubMed] [Google Scholar]
- Yang XC, Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science. 1989;243(4894 Pt 1):1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]
- Yang J, Zhang J, Yang H, Li K, Lei X, Xu C. The potential role of Piezo2 in the mediation of visceral sensation. Neuroscience Letters. 2016;630:158–163. doi: 10.1016/j.neulet.2016.07.058. http://dx.doi.org/10.1016/j.neulet.2016.07.058. [DOI] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Chen BN, Costa M, Brookes SJ. Mechanotransduction by intraganglionic laminar endings of vagal tension receptors in the Guinea-pig oesophagus. The Journal of Physiology. 2003;553(Pt 2):575–587. doi: 10.1113/jphysiol.2003.051862. http://dx.doi.org/10.1113/jphysiol.2003.051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Lynn P, Costa M, Brookes SJ. Mechanisms of mecha-notransduction by specialized low-threshold mechanoreceptors in the Guinea pig rectum. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2005;289(3):G397–G406. doi: 10.1152/ajpgi.00557.2004. http://dx.doi.org/10.1152/ajpgi.00557.2004. [DOI] [PubMed] [Google Scholar]
- Zou H, Lifshitz LM, Tuft RA, Fogarty KE, Singer JJ. Visualization of Ca2+ entry through single stretch-activated cation channels. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(9):6404–6409. doi: 10.1073/pnas.092654999. [DOI] [PMC free article] [PubMed] [Google Scholar]