Abstract
Objectives:
To undertake a systematic review on quality assurance (QA) phantoms for CBCT imaging, including studies on the development and application of phantoms.
Methods:
The MEDLINE (PubMed) bibliographic database was searched until May 2016 for studies evaluating the development and use of phantoms in CBCT image QA. The search strategy was restricted to English language publications using the following combined terms: (Cone Beam CT) OR (Cone Beam Computed Tomography) OR (Cone-Beam Computed Tomography) OR (CBCT) AND (quality OR phantom). It was assessed which of the six image quality parameters stated by the European Commission could be evaluated with each phantom and which of them actually were.
Results:
The search strategy yielded 37 studies, which had developed and used (25 studies) or only used (12 studies) a phantom in CBCT image QA. According to the literature, in 7 phantoms, it is possible to evaluate 4 or more image quality parameters while in 11 phantoms, merely 1 parameter can be evaluated. Only two phantoms permit the evaluation of the six image quality parameters stated by the European Commission. The parameters, which can most often be evaluated using a phantom, are image density values, spatial resolution and geometric accuracy. The SEDENTEXCT phantom was used most frequently. In two studies, all quality parameters suggested by the European Commission were evaluated.
Conclusions:
QA phantoms rarely allow all image quality parameters stated by the European Commission to be evaluated. Furthermore, alternative phantoms, which allow all image quality parameters to be evaluated in a single exposure, even for a small field of view, should be developed.
Keywords: quality assurance, quality control, image quality, CBCT
Introduction
CBCT is a potentially low-dose CT technique for the visualization of mineralized tissues in the head and neck region.1–4 Owing to the relative novelty of this diagnostic method, there is an ongoing effort to define quality assurance (QA) processes for CBCT.5,6 Although the European guidelines on radiation protection in dental radiology7 include strategies for image QA, they do not cover CBCT imaging. To overcome this deficiency, the Safety and Efficacy of a New Emerging Dental X-Ray (SEDENTEXCT) project in 2008 pursued to define a QA process for CBCT imaging. The preliminary results were presented in 2009, and the final outcome was adopted and described by the European Commission in 2012.5 The American Academy of Oral and Maxillofacial Radiology6 also made recommendations on the topic, stating that a QA programme should include, among other things, a documentation of the evaluated image quality parameters.
An adequate phantom is an essential part of an image QA programme. A phantom is a device containing materials that are able to mimic the density of the tissues in the human body.8 In 2012, the European Commission suggested that the Catphan phantoms, which are commonly used for QA in multidetector tomography (fan beam CT), could be used for CBCT as well.9 But, owing to the rather large size of the Catphan phantom and to the limited field of view (FOV) available in the CBCT units, this solution is not ideal. The SEDENTEXCT group was the first to suggest guidelines for the use of a CBCT-specific phantom, describing its development and application.5
QA should not be based solely on subjective image evaluation.10 The European Commission has stated six parameters to be included in the QA programme (in the order given in the guidelines): image density values, image uniformity and the presence of artefacts, noise, spatial resolution, contrast detail and geometric accuracy.5 Action should be taken when there is a variation, when comparing a test and a reference image, >10%.5 If the variation exceeds 25%, the British Health Protection Agency11 advocates that the use of the unit should be suspended. As a matter of fact, no studies have reported on the clinical relevance of such pre-established values.12
No systematic information regarding the QA standards specifically developed for CBCT exists in the literature. The objective of the present study was to undertake a systematic review on QA phantoms for CBCT imaging, including studies on the development and application of phantoms.
Methods and materials
Literature search and systematic review
Electronic literature search included the MEDLINE (Medical Literature Analysis and Retrieval System Online via PubMed) bibliographic database (searched from 2000 to July 2016) for studies evaluating the development and use of phantoms in CBCT image QA. The search strategy was restricted to English language publications using the following combined terms: (Cone Beam CT OR Cone Beam Computed Tomography OR Cone-Beam Computed Tomography OR CBCT) AND (quality OR phantom). Systematic reviews, reviews, conference abstracts, case reports and articles merely on dosimetry were excluded. Studies which assessed the development and use or only the use of phantoms in CBCT QA programmes qualified for inclusion.
Unpublished data were sought by searching a database listing unpublished studies (OpenGray—www.opengrey.eu). Electronic databases of the following journals were also searched: Dentomaxillofacial Radiology, Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology and Medical Physics. A manual search was additionally conducted based on the reference lists of the selected articles and other previous reviews.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was followed during data assessment and extraction.13 Data extraction included information regarding the (1) physical characteristics of the phantom(s); (2) image quality parameters, which could potentially be evaluated by each phantom; (3) parameters for image acquisition (FOV and voxel size); and (4) image quality parameters, which were actually evaluated using each phantom. All studies were screened by two authors (MVLO and RSN), and data extraction was performed individually. In cases of disagreement regarding study inclusion or data extraction, a consensus between the authors was reached during discussion.
Results
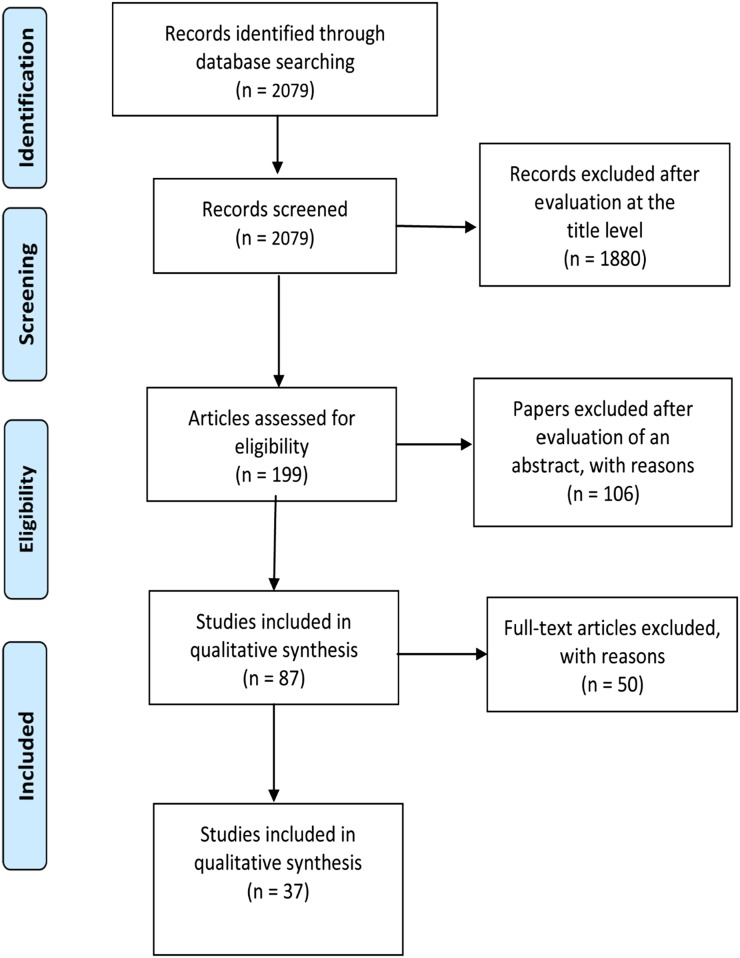
A total of 2079 titles, which fit the designated search strategy, were found in MEDLINE (PubMed). The initial screening, based solely on the publication titles, yielded 199 studies, which potentially met the inclusion criteria. Studies using CBCT outside the dentomaxillofacial diagnostic context (e.g. guidance for radiotherapy) and/or the imaging of anatomical structures other than those of the dentomaxillofacial region were excluded. The second screening (based on abstract reading) revealed 87 studies, which were potentially eligible and therefore selected for full-text reading. After full-text reading, 50 studies were excluded. The main reasons for exclusion were the fact that they did not use a standardized phantom, and/or image quality was based solely on subjective assessment (i.e. did not quantify any of the image quality parameters). Further, there were 10 studies evaluating only the overall influence of artefacts on image quality, and they were also excluded. Eventually, 37 studies were identified as eligible to be included in this systematic review. The PRISMA 200913 flow diagram shows the sequence for achieving these studies in Figure 1.
Figure 1.
Workflow for achieving of studies.
Initially, only the studies presenting the development of new phantoms to be used in CBCT image QA programmes, as well as the phantom characteristics (i.e. design, material and content), were considered. There were 25 studies, which fit these criteria, describing the development of 25 different phantoms. According to the guidelines,5 an ideal phantom should allow the evaluation of six image quality parameters: (1) image density values, (2) image uniformity and the presence of artefacts, (3) noise, (4) spatial resolution, (5) contrast detail and (6) geometric accuracy.5 However, some of the phantoms have separate features for image uniformity and the presence of artefacts. Therefore, seven instead of six image quality parameters were quoted in Tables 1 and 2, to fit the characteristics of the included phantoms in a didactic manner. In Table 1, the characteristics and the image quality parameters, which can be evaluated, are described for the 25 phantoms.10,14–37
Table 1.
Phantoms available for CBCT image quality assurance, their characteristics and the image quality parameters which can be evaluated
| Study (chronological order) | Phantom name | Phantom ID | Phantom size (diameter/side, height) (mm) | Phantom characteristics and materials | Image quality
parameters |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Density values | Image uniformity | Presence of artefacts | Noise | Spatial resolution | Contrast | Geometric accuracy | Others | |||||
| Araki et al14 (2004) | nd | I | 165, nd | One acrylic cylinder with a hollow body filled with water containing 61 acrylic “pipes” (8 mm in diameter), and one consisting of a 50-µm Cu foil, placed between 20-mm thick acrylic plates | x | x | x | |||||
| Marmulla et al15 (2005) | nd | II | 120, 120 | PMMA cubic-shaped body with 36 openings (originating air-filled cylinder bores, 5 mm in diameter and 5 mm in height) on each side. A total of 108 cylinder bores (distance of 20 mm among them) forming an orthogonal three-dimensional grid | x | |||||||
| Lagravere et al16 (2006) | nd | III | 260/246, 220 | Plexiglass cubic-shaped body with six cylindrical inserts (20 mm in diameter and 20 mm in height) made of acetal, acrylic, nylon, cork, celfortic pink foam and spruce) | x | |||||||
| Ballrick et al17 (2008) | CIC, C | IV | nd | One acrylic body phantom (nd shape) with embedded chromium spheres (0.3 mm in diameter) placed every 5 mm in a row. There are 3 layers containing spheres organized as such, in which the spheres of one layer have a common central point of intersection with the spheres of the others. One phantom consisting of acrylic and metal plates in a cylinder container filled with distilled water. 9 series of 4 plates placed in parallel at decreasing, but not specified, distances | x | x | FOV size accuracy | |||||
| Loubele et al18 (2008) | nd | V | nd | One PMMA body phantom (nd shape) containing 4 cylindrical inserts of aluminium, PMMA, bone-equivalent plastic and air. One PMMA cylinder body containing an insert of folded aluminium plates, shaped as a “mushroom” | x | x | x | |||||
| Bryant et al19 (2008) | nd | VI | nd | One polygonal phantom alternating continuous layers of acrylic and precision plastic grills, one polygonal phantom of continuous layers of acrylic mounted in a “terraced” shape and one polygonal phantom of continuous layers of acrylic mounted in a “pyramidal” shape | x | x | x | x | x | |||
| Suomalainen et al20 (2009) | nd | VII | 40, 30 | Perspex cylinder body (40 mm in diameter and 30 mm in height) covered with 5-mm-thick top and bottom acrylic plates. Three cylindrical recesses (10 mm in diameter and 30 mm in height) were drilled at a distance of 8 mm from the outer phantom surface. One of the recesses filled with PTFE, one with silicon gel in which a human premolar was embedded for possible future use and one air-filled. One additional cylinder, smaller in diameter, was drilled in the very centre of the phantom, providing space dose measurement devices | x | x | x | |||||
| Watanabe et al21 (2010) | X001-99520-400 | VIII | nd | PMMA cylinder body containing a 100-mm tungsten wire in the centre | x | |||||||
| Vassileva and Stoyanov22 (2010) | nd | VIX | 160, nd | Cylinder body containing water | x | x | x | |||||
| Pauwels et al23 (2011) | SEDENTEXCT | X | 160, 162 | PMMA cylinder body containing seven defined sections, the top six containing seven cylindrical recesses (35 × 20 mm) each, in which diverse inserts containing diverse materials (metal—aluminium and titanium; polymers—PTFE, LDPE, acetal; air) can be placed. The lower section of the phantom is uniform PMMA | x | x | x | x | x | x | x | |
| Nackaerts et al24 (2011) | QCT-Bone Mineral | XI | 76/38, 12 | Solid cubic body housing homogeneous bars of water-equivalent material, hydroxyapatite-equivalent material at 75 mg cm−3 and hydroxyapatite-equivalent material at 150 mg cm−3 | x | |||||||
| Tsutsumi et al25 (2011) | nd | XII | 100, 75 | Acrylic cylinder hollow body filled with water and containing an aluminium body with 12 “steps” with a height ranging from 1 to 12 mm, in 1-mm increments. Each step has seven recesses (1 mm in diameter), with a depth ranging from 0.1 to 0.7 mm | x | |||||||
| Panzarella et al26 (2011) | nd | XIII | 50, 100 | Nylon cylinder body, with 4 “channels” (depth of 1 mm), organized in a cross-like design in the longitudinal axis of the cylinder, and 10 “channels” (depth of 1 mm), displayed transversally, 10 mm away from each other. The intersections among the channels are filled with zinc oxide and eugenol paste | x | |||||||
| Reeves et al27 (2012) | nd | XIV | nd | Acrylic body, shaped as an intraoral biting plate, containing cubes (5 mm side) of aluminium, cortical bone-equivalent material, trabecular bone-equivalent material, PMMA and water-equivalent material | x | |||||||
| Thongvigitmanee et al28 (2013) | nd | XV | nd | Plastic object containing 9 “positions” with known distances among them | x | |||||||
| Ozaki et al29 (2013) | nd | XVI | 100, 95 | Acrylic cylinder with a hollow body with a tungsten wire (0.1 mm in diameter) stretched by 2 coil springs from the top to the bottom of the phantom | x | |||||||
| Batista et al30 (2013) | nd | XVII | 150, 100 | PMMA cylinder body filled with water and with recesses for the inclusion of diverse material inserts (PVC, PTFE, acetal, PP, PMMA) | x | x | x | x | x | |||
| Ludlow and Walker31 (2013) | QUART DVT_AP | XVIII | 160, 150 | PMMA cylinder body with PVC and air-filled elements | x | x | x | x | Nyquist frequency | |||
| Torgersen et al10 (2014) | nd | XIX | 160, 70 | PMMA cylinder body with PE, nylon, acetal and PTFE inserts. Tantalum beads are placed into holes drilled in the bottom-centre of each rod. The bottom part has a hollow base containing a stainless steel wire | x | x | x | x | x | x | Nyquist frequency | |
| Steiding et al32 (2014) | QRM-dental CBCT-QA | XX | 100, 100 or 160, 100 | PMMA cylinder body with five defined sections: (I) four inserts in water (air, −3% contrast, +3% contrast, and bone), (II) centrally located aluminium sphere (12 mm in diameter), (III) water-equivalent plastic, (IV) PMMA structures of 0.3–1.0 mm in diameter positioned in water-equivalent plastic and (V) titanium and tissue-equivalent resin inserts (9 or 13 × 17.5 mm) | x | x | x | x | x | x | ||
| Oliveira et al33 (2014) | nd | XXI | 90 , 60 | 28 PP tubes filled with aqueous solution of dipotassium hydrogen phosphate placed within a cylindrical PP container filled with water. Tubes were positioned vertically and symmetrically arranged in three circles from the phantom centre. Diverse concentrations of dipotassium hydrogen phosphate were tested (1200, 1000, 800, 600, 400 and 200 mg ml−1) | x | |||||||
| Dillenseger et al34 (2015) | vmCT | XXII | 63, 70 | Acrylic cylinder with a hollow body containing: one section with 4 (alternated) aluminium and Mylar coils with thicknesses of 150, 200, 300 and 500 μm, embedded in a polycarbonate plastic plate (4.8-mm thick); one section with 4 metallic beads placed 35-mm apart, forming the corners of an ideally centred square, while 1 bead is placed at the centre of the square; and one section containing 7 vials filled with air, increasing concentrations of iodine (0.94, 1.87, 3.75, 7.5, 15 and 30 mg ml−1) or water | x | x | x | x | x | |||
| Plachtovics et al35 (2015) | ICAT | XXIII | 150, 120 | Plexiglass cylinder body containing 4 other smaller cylinders filled with air, LDPE, acrylic or PTFE (12 mm in diameter) and a “bar pattern” | x | x | ||||||
| Pauwels et al36 (2015) | nd | XXIV | 160, nd | Homogeneous PMMA cylinder body with a central air hole (10 mm in diameter) at the bottom | x | |||||||
| Elkhateeb et al37 (2016) | QAT | XXV | 160, 40.7 | Plastic cylinder, in which the bottom 20 mm is composed of PMMA (tissue equivalent), and the upper 20 mm is composed of three elements: PMMA, PVC and air | x | x | x | x | ||||
Acetal, polyoxymethylene; FOV, field of view; LDPE, low-density polyethylene; nd, not declared; PE, polyethylene; plexiglass, polymethyl 2-methylpropenoate; PMMA, polymethyl methacrylate; PP, polypropylene; PTFE, polytetrafluoroethylene; PVC, polyvinylchloride.
Table 2.
Studies using the phantoms available for CBCT image quality assurance, their characteristics, and the image quality parameters which were evaluated
| Study (chronological order) | Phantom ID | Test units | Voxel sizes (range) (mm) | FOVa (small, medium and large) | Evaluated image quality
parameters |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Density values | Image uniformity | Presence of artefacts | Noise | Spatial resolution | Contrast | Geometric accuracy | Others | |||||
| Araki et al14 (2004) | I | MercuRay (Hitachi Medical Systems America, Twinsburg, OH) | 0.1–0.376 | Small, medium and large | x | x | x | |||||
| Marmulla et al15 (2005) | II | NewTom 9000 (QR srl, Verona, Italy) | 0.29 | nd | x | |||||||
| Lagravere et al16 (2006) | III | NewTom QR-DVT 9000 (QR srl, Verona, Italy) | nd | nd | x | |||||||
| Ballrick et al17 (2008) | IV | i-CAT (Imaging Sciences International, Hatfield, PA) | 0.2–0.4 | Small and medium | x | x | FOV size accuracy | |||||
| Loubele et al18 (2008) | V | NewTom 3G (QR srl, Verona, Italy), Accuitomo 3D (J. Morita Mfg Corp., Kyoto, Japan), MercuRay (Hitachi Medical Systems America, Twinsburg, OH) | 0.13–0.38 | nd | x | x | x | |||||
| Loubele et al38 (2008) | V | NewTom 3G (QR srl), i-CAT (Imaging Sciences International), MercuRay (Hitachi Medical Systems America), Accuitomo 3D (J. Morita Mfg Corp.) | 0.18–0.38 | Small, medium and large | x | |||||||
| Bryant et al19 (2008) | VI | i-CAT (Imaging Sciences International) | 0.4 | Large | x | x | x | x | x | |||
| Suomalainen et al20 (2009) | VII | 3D Accuitomo (J. Morita Mfg Corp.), Promax 3D (Planmeca, Helsinki, Finland), Scanora 3D (Soredex, Tuusula, Finland) | 0.12–0.36 | Small | x | x | x | |||||
| Watanabe et al21 (2010) | VIII | 3D Accuitomo (J. Morita Mfg Corp.) | 0.125 | Small | x | |||||||
| Vassileva and Stoyanov22 (2010) | IX | Iluma (IMTEC, Ardmore, OK) | 0.1–0.4 | Large | x | x | x | |||||
| Pauwels et al23 (2011) | X | Scanora 3D (Soredex), Galileos (Sirona, Bensheim, Germany), i-CAT Classic (Imaging Sciences International), Iluma Elite (IMTEC), ProMax 3D (Planmeca), SkyView (MyRay, Imola, Italy), Veraviewepocs 3D (J. Morita Mfg Corp, Kyoto, Japan) | 0.13–0.35 | Small, medium and large | x | x | x | x | x | x | ||
| Nackaerts et al24 (2011) | XI | Accuitomo 3D XYZ (J. Morita Mfg Corp.), Galileos Comfort (Sirona), Kodak 9000 3D (Kodak Dental Systems, Carestream Health, Rochester, NY), Picasso Duo (Vatech/E-WOO Technology, Seoul, Republic of Korea), Scanora 3D (Soredex) | 0.0765–0.289 | Small and medium | x | |||||||
| Tsutsumi et al25 (2011) | XII | MercuRay 12 (Hitachi Medical Systems America) | 0.2–0.377 | Medium and large | x | |||||||
| Panzarella et al26 (2011) | XIII | NewTom 3G (QR srl) and i-CAT (Imaging Sciences International) | 0.16–0.4 | Medium and large | x | |||||||
| Watanabe et al9 (2011) | VIII | 3D Accuitomo (J. Morita Mfg Corp.) | 0.125 | Medium and large | x | |||||||
| Reeves et al27 (2012) | XIV | Asahi Alphard 3030 (Belmont Takara, Kyoto, Japan) and Planmeca ProMax 3D (Planmeca, Helsinki, Finland) | 0.1–0.39 | Small, medium and large | x | |||||||
| Pauwels et al39 (2012) | X | 3D Accuitomo 170 (J. Morita Mfg Corp.), 3D Accuitomo XYZ (J. Morita Mfg Corp.), Veraviewepocs 3D (J. Morita Mfg Corp.), Galileos Comfor (Sirona), i-CAT Next Generation (Imaging Sciences International), Kodak 9000 3D (Kodak Dental Systems, Carestream Health), Kodak 9500 (Kodak Dental Systems, Carestream Health), NewTom VGi (QR srl), Pax-Uni3D (Value Added Technologies, Yongin, Republic of Korea), Picasso Trio (Value Added Technologies, Yongin, Republic of Korea), ProMax 3D (Planmeca), Scanora 3D (Soredex) and SkyView (Cefla Dental Group, Imola, Italy) | 0.076–0.4 | Small, medium and large | x | x | ||||||
| Thongvigitmanee et al28 (2013) | XV | DentiiScan (NECTEC and MTEC, Pathum Thani, Thailand) | 0.4 | Medium | x | |||||||
| Ozaki et al29 (2013) | XVI | 3DXFPD8 (Hitachi Medical Systems America, Twinsburg, OH) and FineCube v. 12 (Yoshida Dental Mfg Co. Ltd, Tokyo, Japan) | 0.08–0.157 | Small | x | |||||||
| Batista et al30 (2013) | XVII | Kodak 9000 (Kodak Dental Systems, Carestream Health), Kodak 9500 (Kodak Dental Systems, Carestream Health), and i-CAT Classic (Imaging Sciences International) | 0.076–1.0 | Small, medium and large | x | x | x | x | x | |||
| Ludlow and Walker31 (2013) | XVIII | i-CAT Next Generation (Imaging Sciences International) | 0.125–0.4 | Small | x | x | x | x | Nyquist frequency | |||
| Bamba et al40 (2013) | X | CS9300 3D (Kodak Dental Systems, Carestream Health, Rochester, NY), Accuitomo 80 (J. Morita Mfg Corp.) and Veraviewepocs 3Df (J. Morita Mfg Corp.) | 0.08–0,125 | Small | x | x | x | x | x | x | x | |
| Torgersen et al10 (2014) | XIX | 3D Accuitomo 170 (J. Morita Mfg Corp.) | nd | Small | x | x | x | x | x | x | Nyquist frequency | |
| Steiding et al32 (2014) | XX | Kavo 3D eXam (KaVo Dental GmbH, Biberach, Germany) | 0.3 | Medium | x | x | x | x | x | x | ||
| Oliveira et al33 (2014) | XXI | Picasso-Trio (Vatech/E-WOO Technology, Seoul, Republic of Korea) | nd | Small | x | |||||||
| Pauwels et al41 (2014) | X | 3D Accuitomo 170 (J. Morita Mfg Corp.) | 0.16 | Small and medium | x | |||||||
| Dillenseger et al34 (2015) | XXII | Newtom 5G (QR srl, Verona, Italy) | 0.075–0.25 | Small and medium | x | x | x | x | x | |||
| Plachtovics et al35 (2015) | XXIII | iCAT Classic (Imaging Sciences International) | 0.2 | Small | x | x | ||||||
| Pauwels et al36 (2015) | XXIV | 3D Accuitomo 170 (J. Morita Mfg Corp.), Cranex 3D (Soredex, Tuusula, Finland), Scanora 3D (Soredex), and Galileos Comfort (Sirona) | 0.125–0.35 | Small, medium and large | x | |||||||
| Kosalagood et al42 (2015) | X | 3D Accuitomo 170 (J. Morita Mfg Corp.), Galileos Comfort (Sirona), Scanora 3D (Soredex), CB MercuRay (Hitachi Medical Systems America), i-CAT Next Gen (Imaging Sciences International), Kodak CS 9300 (Kodak Dental Systems, Carestream Health), WhiteFox (Acteon Group, Mérignac, France), Dentiiscan (NECTEC and MTEC, Pathum Thani, Thailand) | 0.25–0.4 | Small, medium and large | x | |||||||
| Steiding et al12 (2015) | XX | KaVo 3D eXam (KaVo Dental GmbH) | 0.3 | Medium | x | x | x | x | x | |||
| Abouei et al43 (2015) | X | Carestream 9300 (Kodak Dental Systems, Carestream Health, Rochester, NY) | 0.18–0.25 | Small and medium | x | x | x | x | x | x | ||
| Ali et al44 (2015) | VIII | Scanora 3D (Soredex) and 3D Accuitomo 80 (J. Morita Mfg Corp.) | 0.08 | Small | x | x | ||||||
| Choi et al45 (2015) | X | Dinnova3 (HDXwill Inc., Seoul, Republic of Korea) | 0.3 | Large | x | x | x | |||||
| Pauwels et al46 (2016) | X | 3D Accuitomo 170 (J. Morita Mfg Corp.) | nd | Small and medium | x | |||||||
| Taylor et al47 (2016) | X | 3D Accuitomo 170 (J. Morita Mfg Corp.) | nd | Medium | x | x | x | |||||
| Elkhateeb et al37 (2016) | XXV | CS 9300 PREMIUM (Kodak Dental Systems, Carestream Health, Rochester, NY) | 0.18–0.5 | Small and medium | x | x | x | x | ||||
FOV, field of view; nd, not declared.
Small FOV = Height ≤ 80 mm, Medium FOV = Height > 80 ≤ 130 mm, Large FOV = Height > 130 mm.
The phantoms were mainly built of polymethyl methacrylate, solid or shallow and filled with water. The only exception was one phantom containing an object for evaluation of the accuracy for linear measurements, in which the used materials were not stated.28 In phantoms dedicated to image density measurements, various polymers, such as polyvinylchloride, polytetrafluoroethylene, polypropylene, polyoxymethylene (acetal), polyethylene, low-density polyethylene and nylon, were used. Some phantoms also presented metal parts, mainly used for artefact or geometric accuracy evaluation.
According to the studies of the 25 phantoms, 7 phantoms possess features for evaluating 4 or more image quality parameters,10,23,30–32,34,37 while 11 phantoms can be used to test merely 1 parameter. Only two phantoms permit the evaluation of the six image quality parameters as stated by the European Commission.10,23 Nevertheless, one of the described phantoms does not allow the evaluation of the parameter image uniformity and the presence of artefacts in a complete manner, since it focuses only on image uniformity.10 The parameters which can most often be evaluated using a phantom are image density values (15 phantoms), spatial resolution (12 phantoms) and geometric accuracy (11 phantoms). The presence of artefacts can be evaluated by three phantoms.18,23,32 Three phantoms included additional parameters, such as Nyquist frequency assessment10,31 and accuracy of FOV size.17 The evaluation of spatial resolution was solved in diverse manners. In two of the phantoms,17,35 this parameter is evaluated from a pattern bar while in nine phantoms, it is evaluated using the modulation transfer function from wires or spheres with a high atomic number.10,14,20,21,23,31,32,34,37 In one of the phantoms,29 both methods can be used.
In a second step, the actual use of the phantoms in a QA programme was assessed. There were 37 studies, in which a phantom was used for CBCT image QA: the 25 studies, which also presented the development of the phantoms, and 12 studies, which used phantoms previously described in the literature. These studies are presented in Table 2. Image quality was evaluated in 45 CBCT units in total (range in 1 study: 1–13 units). The SEDENTEXCT phantom was the most frequently used one (nine studies). In only two of the included studies, all parameters suggested by the European Commission were evaluated.10,40 However, in one of these studies, focus was only on image uniformity and the presence of artefacts was not mentioned.10 The parameters most frequently evaluated were contrast detail (in 19 studies), spatial resolution (in 18 studies) and image density (in 17 studies).
Discussion
QA programmes promote the effective use of radiation for diagnostic purposes through achieving and maintaining appropriate image quality.5 Within QA, an evaluation of image quality (also classified as “constancy” test by the International Electrotechnical Commission) is intended to test the components of a given radiological system and to verify that the equipment is operating satisfactorily.48 CBCT image quality evaluation must be carried out regularly. Guidelines suggest that the evaluation must be performed at a yearly and/or monthly interval.5,11 The evaluation is frequently performed by the manufacturer or regulatory authorities and allows for detection of deterioration of accuracy and differences in contrast between tissue densities over time.48
A QA programme should cover all aspects of the imaging process, including objective measures of image quality. Consequently, six specific parameters suggested by the European Commission must be objectively evaluated, ensuring that the equipment is in accord with prevailing standards.5 Through image density evaluation, it is possible to evaluate the system's ability to distinguish between different tissues and materials in an image. The image uniformity assessment ensures that there are no significant areas of damage in the images (e.g. artefacts), nor problems with detector calibration.5,48 Possible image artefacts, which are not uniquely related to changes in image uniformity, must also be evaluated.5 Noise has significant relevance in image quality and at high levels will compromise the display of low-contrast objects. Therefore, when noise level is low, viewing of low-contrast lesions is improved.49 Spatial resolution is checked since it affects the system's ability to discriminate two adjacent high-contrast objects.29 This is an important parameter, since the spatial resolution may determine the accuracy to which anatomic details can be measured. Inadequate spatial resolution may affect procedures such as planning of dental implants or measuring endodontic file length.50 Moreover, contrast detail allows the evaluation of a system to display details of contrast in various objects. It will provide important information about possible deterioration of image quality over time.5 In the present review, some of the included studies considered one of the six quality parameters, “image uniformity and the presence of artefacts”, as two separate entities. This division was also used in our tables based on the fact that there are some artefacts (e.g. motion and aliasing artefacts) which are not necessarily or solely related to image uniformity. We therefore advocate that future image quality tests should account for seven separate parameters.
In 18 of the 25 phantoms included in the present review, it is possible to evaluate <4 of the 6 image quality parameters stated by the European Commission. The phantoms most often allowed the evaluation of image density values, spatial resolution and geometric accuracy. Pauwels et al23 were the first to suggest a phantom which allows the evaluation of all suggested image quality parameters, as part of the SENDENTEXCT project. However, the first study describing this phantom did not include all parameters, which could have been evaluated.23 This was first accomplished in a study of Bamba et al,40 which presented the final results and complete application of the SEDENTEXCT phantom. Considering the studies using phantoms as part of a CBCT QA programme, the parameters most often evaluated were spatial resolution, contrast detail and image density. This is directly linked to basic image quality characteristics described by four fundamental parameters (spatial resolution, contrast, noise and artefacts) in accordance with Pauwels et al.51 In addition, the geometric accuracy defines how accurately the CBCT apparatus displays a distance between two objects,17 reflecting true measures.5
An important parameter is the presence of artefacts, such as metal-induced artefacts. This has shown to be one of the main causes of interferences with diagnostic quality in CBCT, since artefacts will also affect other image quality parameters, such as spatial resolution.50,52–54 Through regular image quality assessment using phantoms, it is possible to monitor the presence of metal artefacts over time. However, in only three of the described phantoms18,23,32 it is possible to perform this evaluation. In our opinion, future guidelines should be clear on which type of image artefacts must be evaluated (e.g. beam hardening, cupping, movement).
CBCT guidelines regarding QA programmes are yet sparse. In a review by Horner et al55 on guidelines for clinical use of CBCT, the authors identified 11 guidelines supporting the proper use of CBCT, including image quality evaluation. Most of them were, however, supported only by expert opinions. Although two guidelines5,11 suggest methodologies and criteria for the evaluation of CBCT image quality, a detailed step-by-step guide, including characteristics of the CBCT units (e.g. FOV size, resolution etc.), would be a major improvement. In the present review, only 10 studies had presented results of QA performed in 3 CBCT units or more. This is a relevant issue, since it is evident that findings from one unit cannot be extrapolated to all units in the market.
The Journal of the American Dental Association6 declares that the staff working with the CBCT unit (i.e. medical physicists, radiology technicians, dentists and medical doctors) should establish a QA programme based on the manufacturer recommendations. However, in many cases, few image quality parameters would be evaluated following only the recommendations of the manufacturer. The manufacturer typically controls image quality during the initial installation of the unit, but the final user must also pay attention to performing regular QA evaluations.56
It would be ideal to adapt QA programmes to fit the diverse FOV sizes and resolutions of the CBCT units available in the market. However, these issues were not properly considered, as it can be seen from this review. Most studies included in the present review conducted tests in CBCT units with a small FOV (24 studies), while only 13 studies were performed using a large FOV also.57 But, when a small FOV was used, several exposures were performed to evaluate all image quality parameters. In fact, the two phantoms, which permit the evaluation of all six quality parameters as stated by the European Commission,10,23 are quite large; this means that, to proceed with the evaluation of all parameters, multiple exposures are needed.
From the present review, it is clear that more effort should be put in developing QA phantoms. Ideally, these phantoms should also be easily accessible and user-friendly, allowing all image quality parameters to be evaluated in a single exposure, even for small FOVs. The use of low-cost plastic materials to simulate a wide contrast range should be encouraged. The use of a phantom with such characteristics would, in our opinion, help make QA protocols more standardized and facilitate QA monitoring by clinicians and radiation protection authorities.
Conclusions
QA phantoms for CBCT imaging rarely allow all image quality parameters stated by the European Commission to be evaluated. Of the 25 described phantoms, only 2 phantoms provide features for the evaluation of all 6 image quality parameters stated by the European Commission. Furthermore, alternative phantoms, which allow all image quality parameters to be evaluated in a single exposure, even for a small FOV, should be developed.
Contributor Information
Marcus V L de Oliveira, Email: marcusradiology@gmail.com.
Ann Wenzel, Email: awenzel@odont.au.dk.
Paulo S F Campos, Email: paulo@radiologia.odo.br.
Rubens Spin-Neto, Email: rsn@odont.au.dk.
References
- 1.Miracle AC, Mukherji SK. Conebeam CT of the head and neck, part 1: physical principles. Am J Neuroradiol 2009; 30: 1088–95. doi: https://doi.org/10.3174/ajnr.A1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miracle AC, Mukherji SK. Conebeam CT of the head and neck, part 2: clinical applications. Am J Neuroradiol 2009; 30: 1285–92. doi: https://doi.org/10.3174/ajnr.A1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scarfe WC, Li Z, Aboelmaaty W, Scott SA, Farman AG. Maxillofacial cone beam computed tomography: essence, elements and steps to interpretation. Aust Dent J 2012; 57: 46–60. doi: https://doi.org/10.1111/j.1834-7819.2011.01657.x [DOI] [PubMed] [Google Scholar]
- 4.White SC. Cone-beam imaging in dentistry. Health Phys 2008; 95: 628–37. doi: https://doi.org/10.1097/01.HP.0000326340.81581.1a [DOI] [PubMed] [Google Scholar]
- 5.SEDENTEXCT Project. Radiation protection n° 172: cone beam CT for dental and maxillofacial radiology. Luxembourg: European Commission; 2012. [Google Scholar]
- 6.Carter L, Farman AG, Geist J. American academy of oral and maxillofacial radiology executive opinion statement on performing and interpreting diagnostic cone beam computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008; 106: 561–2. doi: https://doi.org/10.1016/j.tripleo.2008.07.007 [DOI] [PubMed] [Google Scholar]
- 7.European Commission for Radiation Protection. Radiation protection 136: European guidelines on radiation protection in dental radiology. The safe use of radiographs in dental practice, Belgium; 2004.
- 8.Larry A, de Werd MK. The phantoms of medical and health physics. New York, NY: Springer; 2014. [Google Scholar]
- 9.Watanabe H, Honda E, Tetsumura A, Kurabayashi T. A comparative study for spatial resolution and subjective image characteristics of a multi-slice CT and a cone-beam CT for dental use. Eur J Radiol 2011; 77: 397–402. doi: https://doi.org/10.1016/j.ejrad.2009.09.023 [DOI] [PubMed] [Google Scholar]
- 10.Torgersen GR, Hol C, Moystad A, Hellen-Halme K, Nilsson M. A phantom for simplified image quality control of dental cone beam computed tomography units. Oral Surg Oral Med Oral Pathol Oral Radiol 2014; 118: 603–11. doi: https://doi.org/10.1016/j.oooo.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 11.Health Protection Agency. Guidance on the safe use of dental cone beam CT (computed tomography) equipment—HPA-CRCE-010. Chilton, WI: Health Protection Agency; 2010.
- 12.Steiding C, Kolditz D, Kalender W. Comparison of methods for acceptance and constancy testing in dental cone-beam computed tomography. Rofo 2015; 187: 283–90. doi: https://doi.org/10.1055/s-0034-1385333 [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009; 62: 1006–12. doi: https://doi.org/10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 14.Araki K, Maki K, Seki K. Characteristics of a newly developed dentomaxillofacial X-ray cone beam CT scanner (CB MercuRay): system configuration and physical properties. Dentomaxillofac Radiol 2004; 33: 51–9. doi: https://doi.org/10.1259/dmfr/54013049 [DOI] [PubMed] [Google Scholar]
- 15.Marmulla R, Wortche R, Muhling J, Hassfeld S. Geometric accuracy of the NewTom 9000 cone beam CT. Dentomaxillofac Radiol 2005; 34: 28–31. doi: https://doi.org/10.1259/dmfr/31342245 [DOI] [PubMed] [Google Scholar]
- 16.Lagravere MO, Fang Y, Carey J, Toogood RW, Packota GV, Major PW. Density conversion factor determined using a cone-beam computed tomography unit NewTom QR-DVT 9000. Dentomaxillofac Radiol 2006; 35: 407–9. [DOI] [PubMed] [Google Scholar]
- 17.Ballrick JW, Palomo JM, Ruch E, Amberman BD, Hans MG. Image distortion and spatial resolution of a commercially available cone-beam computed tomography machine. Am J Orthod Dentofacial Orthop 2008; 134: 573–82. doi: https://doi.org/10.1016/j.ajodo.2007.11.025 [DOI] [PubMed] [Google Scholar]
- 18.Loubele M, Maes F, Jacobs R, van Steenberghe D, White SC, Suetens P. Comparative study of image quality for MSCT and CBCT scanners for dentomaxillofacial radiology applications. Radiat Prot Dosimetry 2008; 129: 222–6. doi: https://doi.org/10.1093/rpd/ncn154 [DOI] [PubMed] [Google Scholar]
- 19.Bryant JA, Drage NA, Richmond S. Study of the scan uniformity from an i-CAT cone beam computed tomography dental imaging system. Dentomaxillofac Radiol 2008; 37: 365–74. doi: https://doi.org/10.1259/dmfr/13227258 [DOI] [PubMed] [Google Scholar]
- 20.Suomalainen A, Kiljunen T, Kaser Y, Peltola J, Kortesniemi M. Dosimetry and image quality of four dental cone beam computed tomography scanners compared with multislice computed tomography scanners. Dentomaxillofac Radiol 2009; 38: 367–78. doi: https://doi.org/10.1259/dmfr/15779208 [DOI] [PubMed] [Google Scholar]
- 21.Watanabe H, Honda E, Kurabayashi T. Modulation transfer function evaluation of cone beam computed tomography for dental use with the oversampling method. Dentomaxillofac Radiol 2010; 39: 28–32. doi: https://doi.org/10.1259/dmfr/27069629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vassileva J, Stoyanov D. Quality control and patient dosimetry in dental cone beam CT. Radiat Prot Dosimetry 2010; 139: 310–12. doi: https://doi.org/10.1093/rpd/ncq011 [DOI] [PubMed] [Google Scholar]
- 23.Pauwels R, Stamatakis H, Manousaridis G, Walker A, Michielsen K, Bosmans H, et al. Development and applicability of a quality control phantom for dental cone-beam CT. J Appl Clin Med Phys 2011; 12: 3478. doi: https://doi.org/10.1120/jacmp.v12i4.3478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nackaerts O, Maes F, Yan H, Couto Souza P, Pauwels R, Jacobs R. Analysis of intensity variability in multislice and cone beam computed tomography. Clin Oral Implants Res 2011; 22: 873–9. doi: https://doi.org/10.1111/j.1600-0501.2010.02076.x [DOI] [PubMed] [Google Scholar]
- 25.Tsutsumi K, Chikui T, Okamura K, Yoshiura K. Accuracy of linear measurement and the measurement limits of thin objects with cone beam computed tomography: effects of measurement directions and of phantom locations in the fields of view. Int J Oral Maxillofac Implants 2011; 26: 91–100. [PubMed] [Google Scholar]
- 26.Panzarella FK, Junqueira JL, Oliveira LB, de Araujo NS, Costa C. Accuracy assessment of the axial images obtained from cone beam computed tomography. Dentomaxillofac Radiol 2011; 40: 369–78. doi: https://doi.org/10.1259/dmfr/88722046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reeves TE, Mah P, McDavid WD. Deriving Hounsfield units using grey levels in cone beam CT: a clinical application. Dentomaxillofac Radiol 2012; 41: 500–8. doi: https://doi.org/10.1259/dmfr/31640433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thongvigitmanee SS, Pongnapang N, Aootaphao S. Radiation dose and accuracy analysis of newly developed cone-beam CT for dental and maxillofacial imaging. Conf Proc IEEE Eng Med Biol Soc 2013; 2013: 2356–9. doi: https://doi.org/10.1109/EMBC.2013.6610011 [DOI] [PubMed] [Google Scholar]
- 29.Ozaki Y, Watanabe H, Nomura Y, Honda E, Sumi Y, Kurabayashi T. Location dependency of the spatial resolution of cone beam computed tomography for dental use. Oral Surg Oral Med Oral Pathol Oral Radiol 2013; 116: 648–55. doi: https://doi.org/10.1016/j.oooo.2013.07.009 [DOI] [PubMed] [Google Scholar]
- 30.Batista WO, Navarro MV, Maia AF. Development and implementation of a low-cost phantom for quality control in cone beam computed tomography. Radiat Prot Dosimetry 2013; 157: 552–60. doi: https://doi.org/10.1093/rpd/nct177 [DOI] [PubMed] [Google Scholar]
- 31.Ludlow JB, Walker C. Assessment of phantom dosimetry and image quality of i-CAT FLX cone-beam computed tomography. Am J Orthod Dentofacial Orthop 2013; 144: 802–17. doi: https://doi.org/10.1016/j.ajodo.2013.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steiding C, Kolditz D, Kalender WA. A quality assurance framework for the fully automated and objective evaluation of image quality in cone-beam computed tomography. Med Phys 2014; 41: 031901. doi: https://doi.org/10.1118/1.4863507 [DOI] [PubMed] [Google Scholar]
- 33.Oliveira ML, Freitas DQ, Ambrosano GM, Haiter-Neto F. Influence of exposure factors on the variability of CBCT voxel values: a phantom study. Dentomaxillofac Radiol 2014; 43: 20140128. doi: https://doi.org/10.1259/dmfr.20140128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dillenseger JP, Matern JF, Gros CI. MSCT versus CBCT: evaluation of high-resolution acquisition modes for dento-maxillary and skull-base imaging. Eur Radiol 2015; 25: 505–15. doi: https://doi.org/10.1007/s00330-014-3439-8 [DOI] [PubMed] [Google Scholar]
- 35.Plachtovics M, Goczan J, Nagy K. The effect of calibration and detector temperature on the reconstructed cone beam computed tomography image quality: a study for the workflow of the iCAT classic equipment. Oral Surg Oral Med Oral Pathol Oral Radiol 2015; 119: 473–80. doi: https://doi.org/10.1016/j.oooo.2014.12.009 [DOI] [PubMed] [Google Scholar]
- 36.Pauwels R, Seynaeve L, Henriques JC. Optimization of dental CBCT exposures through mAs reduction. Dentomaxillofac Radiol 2015; 44: 20150108. doi: https://doi.org/10.1259/dmfr.20150108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elkhateeb SM, Torgersen GR, Arnout EA. Image quality assessment of clinically-applied CBCT protocols using a QAT phantom. Dentomaxillofac Radiol 2016; 45: 20160075. doi: https://doi.org/10.1259/dmfr.20160075 [DOI] [PubMed] [Google Scholar]
- 38.Loubele M, Jacobs R, Maes F. Image quality vs radiation dose of four cone beam computed tomography scanners. Dentomaxillofac Radiol 2008; 37: 309–18. doi: https://doi.org/10.1259/dmfr/16770531 [DOI] [PubMed] [Google Scholar]
- 39.Pauwels R, Beinsberger J, Stamatakis H. Comparison of spatial and contrast resolution for cone-beam computed tomography scanners. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 114: 127–35. doi: https://doi.org/10.1016/j.oooo.2012.01.020 [DOI] [PubMed] [Google Scholar]
- 40.Bamba J, Araki K, Endo A, Okano T. Image quality assessment of three cone beam CT machines using the SEDENTEXCT CT phantom. Dentomaxillofac Radiol 2013; 42: 20120445. doi: https://doi.org/10.1259/dmfr.20120445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pauwels R, Silkosessak O, Jacobs R, Bogaerts R, Bosmans H, Panmekiate S. A pragmatic approach to determine the optimal kVp in cone beam CT: balancing contrast-to-noise ratio and radiation dose. Dentomaxillofac Radiol 2014; 43: 20140059. doi: https://doi.org/10.1259/dmfr.20140059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kosalagood P, Silkosessak OC, Pittayapat P, Pisarnturakit P, Pauwels R, Jacobs R. Linear measurement accuracy of eight cone beam computed tomography scanners. Clin Implant Dent Relat Res 2015; 17: 1217–27. doi: https://doi.org/10.1111/cid.12221 [DOI] [PubMed] [Google Scholar]
- 43.Abouei E, Lee S, Ford NL. Quantitative performance characterization of image quality and radiation dose for a CS 9300 dental cone beam computed tomography machine. J Med Imaging (Bellingham) 2015; 2: 044002. doi: https://doi.org/10.1117/1.jmi.2.4.044002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ali AS, Fteita D, Kulmala J. Comparison of physical quality assurance between Scanora 3D and 3D Accuitomo 80 dental CT scanners. Libyan J Med 2015; 10: 28038. doi: https://doi.org/10.3402/ljm.v10.28038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi JW, Lee SS, Choi SC. Relationship between physical factors and subjective image quality of cone-beam computed tomography images according to diagnostic task. Oral Surg Oral Med Oral Pathol Oral Radiol 2015; 119: 357–65. doi: https://doi.org/10.1016/j.oooo.2014.11.010 [DOI] [PubMed] [Google Scholar]
- 46.Pauwels R, Jacobs R, Bogaerts R, Bosmans H, Panmekiate S. Reduction of scatter-induced image noise in cone beam computed tomography: effect of field of view size and position. Oral Surg Oral Med Oral Pathol Oral Radiol 2016; 121: 188–95. doi: https://doi.org/10.1016/j.oooo.2015.10.017 [DOI] [PubMed] [Google Scholar]
- 47.Taylor C. Evaluation of the effects of positioning and configuration on contrast to noise ratio in the quality control of a 3D Accuitomo 170 dental cone beam CT system. Dentomaxillofac Radiol 2016; 45: 20150430. doi: https://doi.org/10.1259/dmfr.20150430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.International Atomic Energy Agency. Quality assurance programme for computed tomography: diagnostic and therapy applications. Vienna, Austria: IAEA Human Health Series; 2012.
- 49.Scarfe WC, Farman AG. What is cone-beam CT and how does it work? Dent Clin North Am 2008; 52: 707–30. doi: https://doi.org/10.1016/j.cden.2008.05.005 [DOI] [PubMed] [Google Scholar]
- 50.Brullmann D, Schulze RK. Spatial resolution in CBCT machines for dental/maxillofacial applications—what do we know today? Dentomaxillofac Radiol 2015; 44: 20140204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pauwels R, Araki K, Siewerdsen JH, Thongvigitmanee SS. Technical aspects of dental CBCT: state of the art. Dentomaxillofac Radiol 2015; 44: 20140224.doi: https://doi.org/10.1259/dmfr.20140224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bechara BB, Moore WS, McMahan CA, Noujeim M. Metal artefact reduction with cone beam CT: an in vitro study. Dentomaxillofac Radiol 2012; 41: 248–53. doi: https://doi.org/10.1259/dmfr/80899839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bechara B, McMahan CA, Moore W, Noujeim M, Teixeira F, Geha H. Cone beam CT scans with and without artefact reduction in root fracture detection of endodontically treated teeth. Dentomaxillofac Radiol 2013; 42: 20120245. doi: https://doi.org/10.1259/dmfr.20120245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schulze R, Heil U, Gross D. Artefacts in CBCT: a review. Dentomaxillofac Radiol 2011; 40: 265–73. doi: https://doi.org/10.1259/dmfr/30642039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horner K, Jacobs R, Schulze R. Dental CBCT equipment and performance issues. Radiat Prot Dosimetry 2013; 153: 212–18. doi: https://doi.org/10.1093/rpd/ncs289 [DOI] [PubMed] [Google Scholar]
- 56.Kiljunen T, Kaasalainen T, Suomalainen A, Kortesniemi M. Dental cone beam CT: a review. Phys Med 2015; 31: 844–60. doi: https://doi.org/10.1016/j.ejmp.2015.09.004 [DOI] [PubMed] [Google Scholar]
- 57.American Academy of Oral and Maxillofacial Radiology. Clinical recommendations regarding use of cone beam computed tomography in orthodontics. Position statement by the American Academy of Oral and Maxillofacial Radiology. Oral Surg Oral Med Oral Pathol Oral Radiol 2013; 116: 238–57. [DOI] [PubMed] [Google Scholar]