Abstract
Objectives:
To assess the usefulness of contrast-enhanced ultrasound (CEUS) with peritumoral injection of microbubble contrast agent for detecting the sentinel lymph nodes for oral tongue carcinoma.
Methods:
The study was carried out on 12 patients with T1–2cN0 oral tongue cancer. A radical resection of the primary disease was planned; a modified radical supraomohyoid neck dissection was reserved for patients with larger lesions (T2, n = 8). The treatment plan and execution were not influenced by sentinel node mapping outcome. The Sonovue™ contrast agent (Bracco Imaging, Milan, Italy) was utilized. After detection, the position and radiologic features of the sentinel nodes were recorded.
Results:
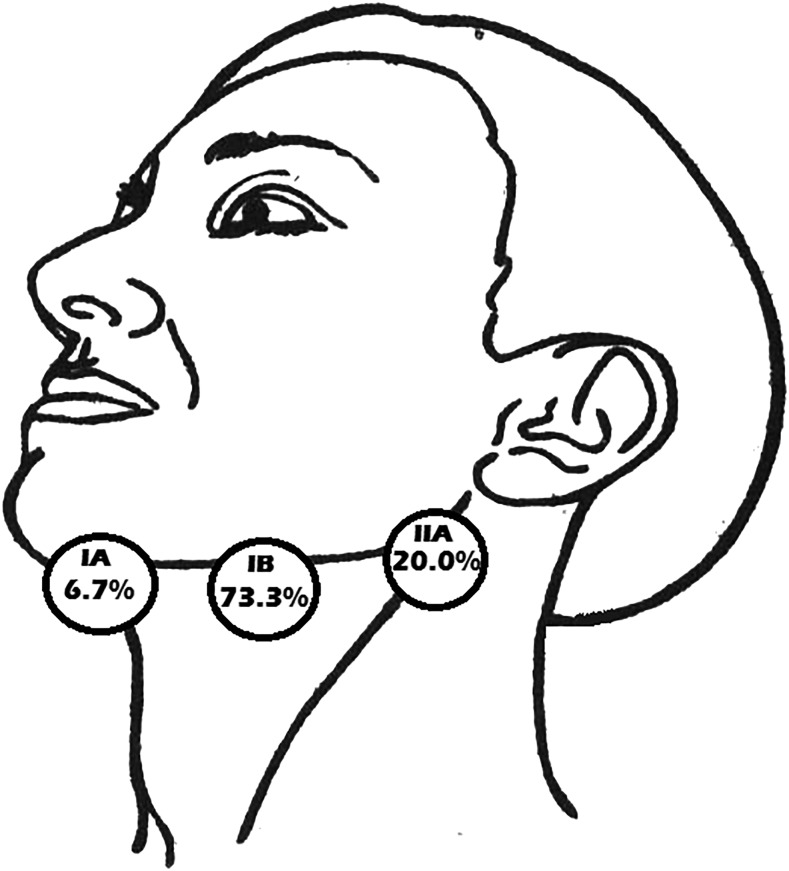
The identification rate of the sentinel nodes was 91.7%; one patient failed to demonstrate any enhanced areas. A total of 15 sentinel nodes were found in the rest of the 11 cases, with a mean of 1.4 nodes for each patient. The sentinel nodes were localized in: Level IA—1 (6.7%) node; Level IB—11 (73.3%) nodes; Level IIA—3 (20.0%) nodes. No contrast-related adverse effects were observed.
Conclusions:
For oral tongue tumours, CEUS is a feasible and potentially widely available approach of sentinel node mapping. Further clinical research is required to establish the position of CEUS detection of the sentinel nodes in oral cavity cancers.
Keywords: contrast-enhanced ultrasound, tongue squamous cell carcinoma, sentinel lymph node
Introduction
Squamous cell carcinoma (SCC) is the most common malignant tumour which arises in the oral cavity and oropharynx and accounts for >90% of the primary lesions. SCC usually has a high potency for secondary spread to the regional lymph nodes. At presentation, the existence of lymph node metastasis on the neck is the most valuable predictive factor that influences both survival prognosis and treatment plan. To date, the standard management of the majority of oral SCC is surgery alone or in combination with radiation and/or chemotherapy. The basis of surgery consists of two usually highly invasive procedures, the ablation of primary tumour and regional lymphoadenectomy or neck dissection. Depending on the stage of the disease, the extent and potential morbidity of the neck dissection varies.1 Currently, the staging of the neck is heavily relying on clinical assessment (i.e. neck palpation) and conventional imaging modalities such as CT, MRI and ultrasound. All these options exhibit low sensitivity for neck metastasis detection especially when the lesion size is <3 mm; hence, up to 40% of cN0 necks are left with unidentified occult disease. Therefore, the care of regional lymphatic basin in this group depends on a choice between possible undertreatment of 30–40% of patients with occult metastases and overtreatment of the 60–70% of those who are free of metastatic disease.2
The sentinel lymph node (SLN) was first addressed by Gould's report at the James Ewing Society in 1960 and later elaborated by Cabanas3 in 1977 in his pioneer study of penile carcinoma. Deeply investigated in melanoma and breast cancer, subsequently SLN detection gained interest in head and neck cancer research. The SLN is the first lymph node or nodes in a lymphatic basin which receives lymph from a given anatomical site and therefore is the first one to capture the metastatic cells from the primary tumour. Patients with previous surgery in the neck region, local radiotherapy or chemotherapy are unsuitable for SLN mapping. These interventions are known to distort and change normal lymph drainage patterns and hence inhibit proper SLN localization. After detection, the SLN must be biopsied for microscopic apprehension, which is the mainstay of this diagnostic measure. Analysis of the SLN for the presence of tumour cells proved itself beneficial for clinically occult regional disease exposure and more precise staging.4 It is generally agreed that if on pathology and/or immunohistochemistry the SLN turns out to be negative, the patient may be spared from the potential morbidity of the regional lymphoadenectomy and the status of SLN presents an accurate indication of the condition of the rest of the regional nodes.5 Disease-positive SLN are correlated with higher locoregional recurrence rates and a poor survival prognosis even in the presence of a therapeutic neck dissection and adjuvant therapy.6
Several techniques for SLN mapping are currently in clinical use. Their features have been evolving to achieve higher quality of anatomical visualization, less invasiveness and easy reproducibility.7 In the present article, we would like to share our preliminary experience in oral tongue SCC SLN exposure with the means of contrast-enhanced ultrasound (CEUS), a method which lately was introduced in breast cancer staging.8
Methods and materials
Institutional ethical committee approval and informed consent were obtained for every patient. A total of 12 patients, stage T1–2cN0, with morphologically proven SCC of the oral tongue were enlisted in the study. The primary tumour stage was T1 in four patients and T2 in the rest of the eight patients. Clinical staging was based on physical check-up and imaging studies such as CT and/or MRI. All patients had no history of previous treatment for the diagnosed tumour. A radical resection of the primary disease via per-oral access with or without reconstruction was planned for all of the patients; a modified radical supraomohyoid neck dissection was reserved for those who exhibited larger lesions (T2, n = 8). The treatment plan and execution were not influenced by the performance of the SLN mapping with CEUS.
Before CEUS a greyscale ultrasound examination was carried out so that the radiologist had a general idea of lymph node positions. The location, size and form of the imaged lymph nodes were documented. A single ultrasound device (MyLab™ 50, Esaote SpA, Italy) which possesses a specific contrast pulse sequence sonographic imaging regimen was used for all CEUS procedures. All patients were examined by the same experienced radiologist. The Sonovue™ contrast agent (Bracco Imaging, Milan, Italy) was used in our study. This agent is comprised of hexafluoride gas encased by phospholipid-stabilized microbubbles with a mean diameter of 2.5 μm. The dry contrast medium powder was mixed with 5 ml of normal sterile saline. The ampoule was then shaken firmly every time before injection to establish a homogeneous suspension of microbubbles. Surface anaesthesia was applied on the oral mucosa with 10% solution of lidocaine on patient demand. A 1-ml insulin syringe with a 26-gauge needle was applied for an intramucosal peritumoral injection of 0.3 ml of the contrast. The prepared contrast medium was injected three times or more in two cases when the contrast or the SLN was not visualized. The peritumoral injection was carried out chiefly under the tumour according to anatomical descriptions of the lymph flow directions (Figure 1).9 As Sonovue is presently licensed for i.v. use, possible contrast-related effects were monitored.
Figure 1.
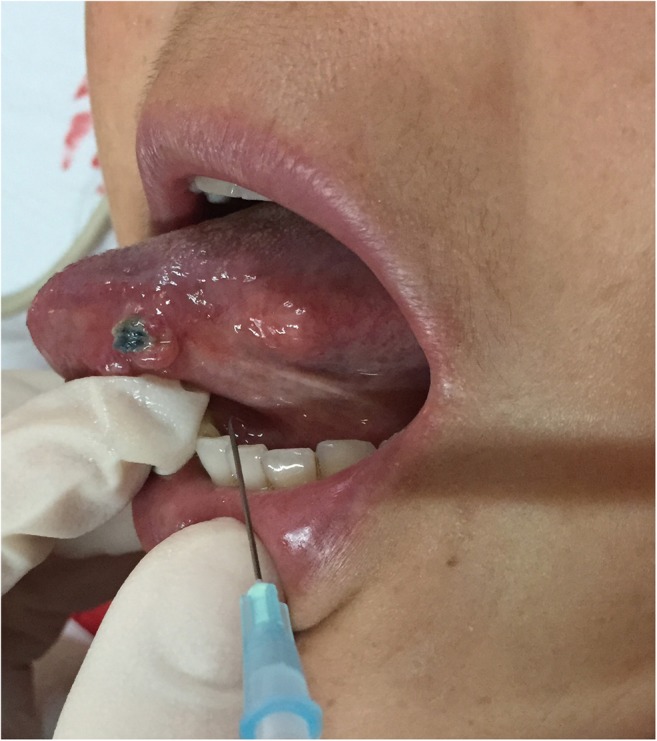
The injection of the contrast agent was performed peritumorally, mostly under the tumour and slightly posterior.
Further investigation was performed with an ultrasound device in the regimen for contrast pulse sequence imaging (B-mode). After the first injection, its site was highlighted, and the contrast identification and total enhancement times (ET) were recorded. Injection site massaging usually helps and improves enhancement. Contrast injections preceded SLN localization on the neck. The probe was moved through the neck region with special attention to Levels I, II and III looking for areas of contrast-enhancing zones and SLN. After SLN enhancement, the following parameters were recorded: the transit time from the injection site to the node; the number of visualized enhanced nodes; location; their size; and the lymph node-filling pattern, uniform or non-uniform. If after three injections no enhancement was observed (n = 1, 8.3%), the procedure was abandoned. Images and video recording were gained during the examination. The patients were all monitored for at least 1 h after the end of the CEUS procedure by the department staff. The CEUS SLN detection procedure was estimated as successful in cases which exhibited both: an identified SLN and no contrast-related adverse events.
Results
All primary tumours were located on the lateral margin of the tongue (4 tumours on the left side, 8 tumours on the right side); sagittal position: 2 (16.7%) cases closer to the tip and 5 (41.7%) cases in both the middle third and the posterior third of the oral tongue anterior to the papillae vallatae. Main observations in all patients are presented in Tables 1 and 2. In three patients, no detectable nodes were seen during pre-CEUS greyscale ultrasound in Levels I, II or III. Totally 20 nodes were pictured in the rest of the 9 patients. A mean number of 2.1 nodes in these levels were visualized for each subject. In all cases, the contrast injection was well tolerated by the patients with only two of them asking for anaesthesia. No adverse effects were observed during or after the injections. At the primary site, the contrast agent was spotted in 11 patients immediately after the injection (Figure 2). SLNs were identified in 11 of the 12 patients, which creates an identification rate (IR) of 91.7%. Overall, 15 SLNs were found in the rest of the 11 cases; the average number of SLN was 1.4 for each patient. Four patients exhibited two contrast-enhanced lymph nodes. The transit time from the injection to the SLN enhancement was 10–50 s. ET of the contrast after injection varied from 2 min 15 s to 4 min 10 s. The duration of the whole procedure, the conventional ultrasound and CEUS mapping of the SLN, did not exceed 80 min in all cases. The location of the SLN was follows: Level IA—1 (6.7%) node; Level IB—11 (73.3%) nodes; Level IIA—3 (20.0%) nodes (Figure 3). The success rate of the CEUS procedure was 91.7% (11/12 cases).
Table 1.
Disease stage and primary tumour sizes
| Case number |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tumour stage | T2cN0 | T2cN0 | T2cN0 | T2cN0 | T1cN0 | T1cN0 | T2cN0 | T2cN0 | T1cN0 | T2cN0 | T2cN0 | T1cN0 |
| Size (cm) | 2.6 × 1.7 | 2.0 × 1.0 | 2.1 × 1.2 | 2.0 × 0.6 | 1.5 × 0.5 | 1.7 × 0.7 | 2.4 × 1.3 | 1.6 × 1.0 | 1.7 × 0.7 | 2.0 × 0.7 | 2.3 × 2.2 | 1.5 × 0.5 |
Table 2.
Baseline greyscale ultrasound and contrast-enhanced ultrasound (CEUS) characteristics of the patients examined
| Case number | Greyscale ultrasound observations |
Contrast medium injection |
CEUS details |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Imaged nodes | Node location (neck level) | Node size (mm) | Node shape | Injection number | Adverse effects | Contrast spotted | SLN found | Number of SLN | Transit time (sec) | SLN location | SLN size (mm) | SLN shape | Lymph node filling pattern | ET (min) | |
| 1 | 3 | IB, IIA | 9 × 11 × 9, 8 × 5 × 7, 10 × 6 × 12 | Round, oval, oval | 4 | None | Yes | Yes | 1 | 20 | IIA | 10 × 6 × 12 | Oval | Uniform | 2 : 20 |
| 2 | 0 | – | – | – | 3 | None | No | No | 0 | – | – | – | – | – | – |
| 3 | 2 | IB | 16 × 12 × 19, 11 × 7 × 12 | Oval, round | 3 | None | Yes | Yes | 1 | 15 | IA | 6 × 7 × 8 | Round | Uniform | 2 : 50 |
| 4 | 0 | – | – | – | 3 | None | Yes | Yes | 1 | 30 | IB | 5 × 8 × 3 | Oval | Non-uniform | 2 : 40 |
| 5 | 3 | IB, IIA | 8 × 7 × 7, 6 × 9 × 5, 4 × 7 × 8 | Round, oval, oval | 3 | None | Yes | Yes | 1 | 50 | IIA | 6 × 9 × 5 | Oval | Non-uniform | 3 : 30 |
| 6 | 2 | IB | 18 × 9 × 17, 6 × 4 × 5 | Oval, round | 5 | None | Yes | Yes | 2 | 25 | IB | 18 × 9 × 17 6 × 4 × 5 | Oval, round | Both non-uniform | 4 : 10 |
| 7 | 3 | IB, IIA | 11 × 7 × 5, 17 × 13 × 11, 9 × 4 × 5 | Oval, oval, oval | 3 | None | Yes | Yes | 1 | 10 | IB | 11 × 7 × 5 | Oval | Non-uniform | 3 : 20 |
| 8 | 0 | – | – | – | 3 | None | Yes | Yes | 2 | 15 | IB, IIA | 10 × 4 × 5 9 × 8 × 7 | Oval, round | Both non-uniform | 3 : 10 |
| 9 | 2 | IB | 4 × 3 × 4, 13 × 5 × 7 | Round, oval | 3 | None | Yes | Yes | 2 | 30 | IB | 4 × 3 × 4 13 × 5 × 7 | Round, oval | Non-uniform | 2 : 50 |
| 10 | 2 | IB | 11 × 5 × 4, 1 × 1 × 0.8 | Oval, round | 3 | None | Yes | Yes | 1 | 20 | IB | 11 × 5 × 4 | Oval | Non-uniform | 2 : 30 |
| 11 | 2 | IB, IIA | 13 × 7 × 12, 19 × 12 × 10 | Oval, oval | 3 | None | Yes | Yes | 1 | 15 | IB | 13 × 7 × 12 | Oval | Non-uniform | 3 : 30 |
| 12 | 1 | IIA | 2 × 4 × 2 | Oval | 3 | None | Yes | Yes | 2 | 10 | IB | 5 × 6 × 5 4 × 3 × 5 | Round, round | Both uniform | 2 : 15 |
ET, enhancement times; SLN, sentinel lymph node.
Figure 2.

The contrast was immediately seen at the site of injection with B-mode.
Figure 3.
The location of the sentinel lymph node detected with contrast-enhanced ultrasound: Level IA—1 (6.7%) node; Level IB—11 (73.3%) nodes; Level IIA—3 (20.0%) nodes.
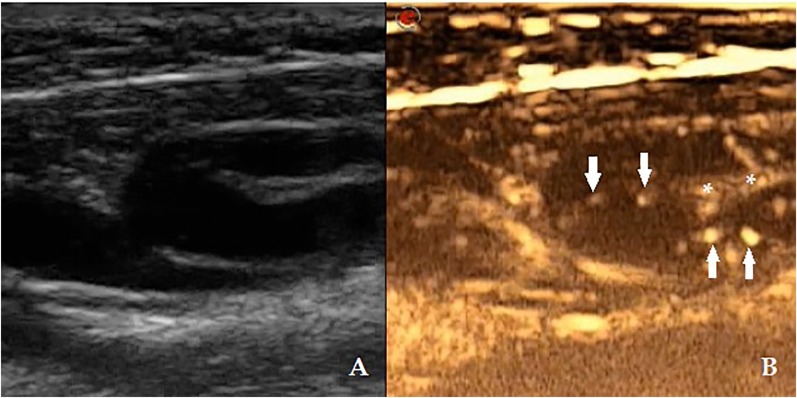
The first examined case revealed one SLN in Level IIA (Figure 4). Its detection required four injections. No lymphatic vessels were observed on CEUS, which was characteristic for all the other patients. In three cases (Cases 2, 4 and 8), no enlarged or suspicious lymph nodes were seen on the pre-CEUS greyscale assessment; CEUS visualized lymph nodes in two of these, Patient 4 had one 5 × 8 × 3-mm-sized, oval-shaped, non-uniformly filled SLN in Level IB and Patient 8 showed two enhanced SLNs: one SLN in Level IB (10 × 4 × 5 mm, oval shaped) and another in Level IIA (9 × 8 × 7 mm, round shaped), both non-uniformly filled with the contrast agent. In another pair of patients, the enhanced lymph nodes were different from those suspected during the pre-CEUS greyscale examination. In Patient 3, two nodes were seen on greyscale, one node in Level IB, 16 × 12 × 19 mm in size, oval shaped, and another node in Level IIA, 11 × 7 × 12 mm sized, round shaped. After three repetitive injections of the contrast medium, a small 6 × 7 × 8-mm-sized, round-shaped, uniformly enhanced node was imaged in Level IA. In Case 12, CEUS helped uncover two small, round-shaped, nodes both uniformly filled SLNs in Level IB while in the greyscale ultrasound, a small oval-shaped lymph node in Level IIA was suspected.
Figure 4.
The lymph node was noted during pre-contrast-enhanced ultrasound greyscale examination (a). The lymph node got enhanced after third injection: 10 × 6 × 12 mm sized, oval shaped with a clearly visualized hilum (asterisks) and uniformly filled by the contrast (arrows).
Discussion
SLN biopsy is a useful tool in regional lymph node assessment. Different implications may be used for technological support for this procedure. Lymphoscintigraphy or radioisotope technique is the most commonly used mode for SLN identification in solid malignancies of the head and neck. It involves pre-operative radioactive tracer injection with intraoperative localization of the SLN with an external radioactive detector (gamma probe). The tracer distribution is imaged in two-dimensional definition, which precludes accurate localization. A meta-analysis of SLN biopsy with lymphoscintigraphy for oral and oropharyngeal cancers proved a pooled sensitivity of 93% and negative-predictive values from 88% to 100%.10 A recent multicentre trial revealed a sensitivity of 80% and negative-predictive value rate of 88%.11 Combining radioisotope technique with single-photon emission CT allowed for three-dimensional image reconstruction and improvement of overall accuracy up to 95%. However, spatial resolution remained non-satisfactory (1–2 cm).12 Another limitation of lymphoscintigraphy is the so-called “shine-through effect”, which is encountered during SLN biopsy in Level I in cases with primary lesion situated on the floor of the mouth. The radiation counts of the primary site overshadow the SLN site background counts, which brings difficulties for its detection by the gamma probe and accordingly its proper intraoperative localization. To overcome it, several propositions were mentioned: lead shielding; removing the primary tumour prior to SLN biopsy; and mandatory Level I dissection.13 A large trial reported that the ability to identify the SLN was lower in patients with mouth floor disease compared with other oral cavity subsites (88% vs 96%; p = 0.138). The use of SLN biopsy as a single staging procedure in all oral cavity subsites except the floor of the mouth was recommended.14 Apart from this, lymphoscintigraphy yields radiation exposure to both patients and the medical staff and requires special protection and waste disposal policy.15
Conventional CT and MRI have limited ability for neck metastasis detection; they are used widely for primary tumour extension assessment and staging. CT and MRI lymphography recently showed advantages in SLN mapping.16 CT lymphography with iodine-based contrasts has the advantage of clear detection of SLN lying in the proximity of the primary tumour site with continuous anatomic visualization and was utilized for oral cancer SLN explorations.17,18 Honda et al19 conducted a study on 31 patients with cN0 with oral tongue SCC; the authors reported a high negative-predictive value of 95.8% and sensitivity value of 90.3%. MRI lymphography utilizing ultrasmall paramagnetic iron oxide or gadolinium chelates is capable of providing anatomically distinct and functional information. A meta-analysis of MRI lymphography in SLN mapping employed in different body regions showed sensitivity and specificity of 90% and 96%, respectively.20 Mizokami et al21 described an experience of using this technique in combination with lymphoscintigraphy on three patients with oral tongue SCC. The SLNs were visualized and were completely concordant with those identified by lymphoscintigraphy. Near-infrared fluorescence (NIF) with indocyanine green is another novel approach that allows optical imaging of SLN during surgery. Safety, reliability and high IR were observed in preliminary studies of NIF used for SLN identification in head and neck cancers.22 Christensen et al23 reported pre-operative NIF detection of the SLNs in all of their 30 patients with oral cancer and intraoperative IR of 97% (66/68 nodes). In total, these newly introduced means of lymphatic imaging possess prominent advantages over the traditional radiocolloid technique; still, these advanced technologies unfortunately are not too widely accessible.
Positron emission tomography (PET) complimented with CT clinically applies fluorine-18 fludeoxyglucose (18F-FDG) as a tracer. This technique makes possible the visualization of glucose metabolic activity by scrutinizing the intensity of positron emission. The injected tracer gets transferred into cells where it is not further metabolized after it gets phosphorylated to 18F-FDG-6-phosphate. This leads to cellular accumulation of fluorine-18.7 In head and neck cancers, 18FDG-PET/CT can be used in different settings with unequal efficiency. While it is highly beneficial for distant metastasis and recurrent disease detection, the role in early stage nodal assessment is doubtful. It has been postulated that great care should be taken with regard to the limited spatial resolution of 18FDG-PET/CT in identifying micrometastases of the cervical lymph nodes. A limited sensitivity of the technique with high number of false-negative results was demonstrated in patients with cN0 with metastatic nodal deposits ≤3 mm.24,25 Hence, 18FDG-PET/CT is currently not recommended for cN0 neck evaluation owing to its low negative-predictive value (around 80%).26
Greyscale ultrasound is a recognized method in the clinical work-up of patients with oral cancer. It is non-invasive, inexpensive, easy reproducible and offers multilayer and multiplanar imaging. Early clinical use of predecessors of modern ultrasound contrasts was reported by Gramiak and Shah27 in 1968. Since then, CEUS has evolved into a dynamically expanding diagnostic tool in various pathologic conditions and body regions. Experimental studies demonstrated the prospects of microbubble contrast ultrasound in SLN detection.28–30 Sulfur hexafluoride microbubble is the most widely used ultrasound contrast agent, as it acts as an inert gas in vivo with no evidence of its metabolism. The diameters of the microbubbles range from 2 µm to 10 µm (mean 2.5 µm), which is smaller than that of an erythrocyte (mean 7.2 µm). This allows the bubbles to pass through the capillaries and lymphatic microvessels, which assures rapid real-time identification and clearance. In its shell, Sonovue contains polyethylene glycol (macrogol 4000), which is known to be associated with the occurrence of allergic reactions.31 The data concerning safety of different types of contrasts used in echocardiography and abdominal imaging show that the safety profile of Sonovue is on the same level with the other agents.32,33 Still, up to now, we are not aware of any published cases describing allergic reactions during SLN mapping with microbubble contrasts.
Ultrasound lymphography with microbubbles injected intradermally for SLN identification was introduced in breast cancer staging by Sever at al in 2009.34 In a study of 80 patients, the authors employed a combo method of pre-operative CEUS and intraoperative lymphoscintigraphy and the overall sensitivity of microbubble detection of SLN was reported to be 89%. A patient satisfaction survey showed a high level of satisfaction, with 81% patients claiming no or only slight discomfort associated with the procedure of contrast injection, CEUS imaging and SLN localization.35 Recently, an overall accuracy of CEUS-guided axillary node biopsy in 54 patients was shown to be 94.4%.36 A large study on 540 patients with melanoma implementing CEUS for superficial lymph node metastases was conducted by Rubaltelli et al in 2014.37 The authors concluded that after i.v. delivery of microbubbles, the node enhancement pattern interpretation increases the diagnostic accuracy of ultrasound in the differential diagnosis of benign and malignant lymph nodes by up to 0.99 and helps avoid unnecessary fine needle biopsy. In early-stage breast cancer, Xie et al38 summarized the enhancement patterns of SLNs into three types and obtained an accuracy of 85.2%, compared with pathology.
One searching point in SLN biopsy with CEUS as well as other pre-operative SLN imaging methods is the mean of localizing the detected node(s) so that it can be quickly and properly dissected. Honda et al19 imposed on a lattice marker which was attached to the neck skin during the CT scan. The SLN location was indicated by the crossing points of the lattice marker and the CT plane light. During CEUS SLN biopsy of axillary nodes, two ways are currently utilized. A hooked 19-gauge guidewire is inserted into the tissues towards the enhanced node and then cut over the skin and kept under a dressing until the nodes are biopsied, which is usually performed the next day.34,35 Rautiainen et al36 advocate core biopsies using a special automated core needle biopsy gun; when >1 biopsy is required to gain an adequate specimen amount, tissues adjacent to the biopsy spots are marked with a breast coil for further recognition. Installed in the axilla, the wire may cause discomfort and in some cases owing to the loose axillary fat, it can get dislocated.38 Location means suitable for neck nodes are yet to be proposed.
CEUS for SLN mapping is a technique that can pre-operatively provide important diagnostic information. The size or the hydrodynamic diameter of the microbubbles allows a short imaging window, as described before and confirmed by ET and transit time parameters, which were observed in our study. This property leads to the necessity of performing >1 injection of the contrast agent, although a high patient tolerance with the procedure was observed. Also, easy reproducibility of CEUS is a sequence of this property of microbubbles. In one of our patients (Case 2), no enhancement was seen at the injection site, or on the neck. Microbubbles do not influence blood or lymph flow and behave as a red blood cell except in rare cases when they are attacked by phagocytes derived from cells of the reticuloendothelial system, which perceive microbubbles as antigents.39 On the other hand, this may be explained by possible inaccurate handling of the pharmaceutical. The main limitations of our study are a small number of patients enrolled and the absence of correlation with the post-operative morphologic picture. The latter is explained by the fact that no kind of marking of the enhanced SLN was performed during preoperative CEUS so they could be found during surgery.
Conclusions
Summarizing the experience that we gained with CEUS mapping of oral tongue SLN, it may be deemed a feasible approach. A learning curve for this technique is indisputably necessary; likewise, the establishment of close co-operation between the radiologist and the surgeon. Further clinical research must be assigned to extend the clinical use of CEUS for SLN identification in patients with oral cancer.
References
- 1.Patel KN, Shah JP. Neck dissection: past, present, future. Surg Oncol Clin N Am 2005; 14: 461–77. doi: https://doi.org/10.1016/j.soc.2005.04.003 [DOI] [PubMed] [Google Scholar]
- 2.Leusink FK, van Es RJ, de Bree R, Baatenburg de Jong RJ, van Hooff SR, Holstege FC, et al. Novel diagnostic modalities for assessment of the clinically node-negative neck in oral squamous-cell carcinoma. Lancet Oncol 2012; 13: e554–61. doi: https://doi.org/10.1016/S1470-2045(12)70395-9 [DOI] [PubMed] [Google Scholar]
- 3.Cabanas RM. An approach for the treatment of penile carcinoma. Cancer 1977; 39: 456–66. doi: https://doi.org/10.1002/1097-0142(197702)39:2<456::AID-CNCR2820390214>3.0.CO;2-I [DOI] [PubMed] [Google Scholar]
- 4.Coughlin A, Resto VA. Oral cavity squamous cell carcinoma and the clinically n0 neck: the past, present, and future of sentinel lymph node biopsy. Curr Oncol Rep 2010; 12: 129–35. doi: https://doi.org/10.1007/s11912-010-0090-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sebbesen L, Bilde A, Therkildsen M, Mortensen J, Specht L, von Buchwald C. Three-year follow-up of sentinel node-negative patients with early oral cavity squamous cell carcinoma. Head Neck 2014; 36: 1109–12. doi: https://doi.org/10.1002/hed.23414 [DOI] [PubMed] [Google Scholar]
- 6.Monroe MM, Pattisapu P, Myers JN, Kupferman ME. Sentinel lymph node biopsy provides prognostic value in thick head and neck melanoma. Otolaryngol Head Neck Surg 2015; 153: 372–8. doi: https://doi.org/10.1177/0194599815589948 [DOI] [PubMed] [Google Scholar]
- 7.Xiong L, Engel H, Gazyakan E, Rahimi M, Hunerbein M, Sun J, et al. Current techniques for lymphatic imaging: state of the art and future perspectives. Eur J Surg Oncol 2014; 40: 270–6. doi: https://doi.org/10.1016/j.ejso.2013.11.027 [DOI] [PubMed] [Google Scholar]
- 8.Sever AR, Mills P, Weeks J, Jones SE, Fish D, Jones PA, et al. Preoperative needle biopsy of sentinel lymph nodes using intradermal microbubbles and contrast-enhanced ultrasound in patients with breast cancer. AJR Am J Roentgenol 2012; 199: 465–70. doi: https://doi.org/10.2214/ajr.11.7702 [DOI] [PubMed] [Google Scholar]
- 9.Werner JA, Dunne AA, Myers JN. Functional anatomy of the lymphatic drainage system of the upper aerodigestive tract and its role in metastasis of squamous cell carcinoma. Head Neck 2003; 25: 322–32. doi: https://doi.org/10.1002/hed.10257 [DOI] [PubMed] [Google Scholar]
- 10.Govers TM, Hannink G, Merkx MA, Takes RP, Rovers MM. Sentinel node biopsy for squamous cell carcinoma of the oral cavity and oropharynx: a diagnostic meta-analysis. Oral Oncol 2013; 49: 726–32. doi: https://doi.org/10.1016/j.oraloncology.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 11.Flach GB, Bloemena E, Klop WM, van Es RJ, Schepman KP, Hoekstra OS, et al. Sentinel lymph node biopsy in clinically N0 T1–T2 staged oral cancer: the Dutch multicenter trial. Oral Oncol 2014; 50: 1020–4. doi: https://doi.org/10.1016/j.oraloncology.2014.07.020 [DOI] [PubMed] [Google Scholar]
- 12.Heuveling DA, van Weert S, Karagozoglu KH, de Bree R. Evaluation of the use of freehand SPECT for sentinel node biopsy in early stage oral carcinoma. Oral Oncol 2015; 51: 287–90. doi: https://doi.org/10.1016/j.oraloncology.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 13.Pitman KT, Ferlito A, Devaney KO, Shaha AR, Rinaldo A. Sentinel lymph-node biopsy in head and neck cancer. Oral Oncol 2003; 39: 343–9. doi: https://doi.org/10.1016/S1368-8375(02)00086-6 [DOI] [PubMed] [Google Scholar]
- 14.Alkureishi LW, Ross GL, Shoaib T, Soutar DS, Robertson AG, Thompson R, et al. Sentinel node biopsy in head and neck squamous cell cancer: 5-year follow-up of a European multicenter trial. Ann Surg Oncol 2010; 17: 2459–64. doi: https://doi.org/10.1245/s10434-010-1111-3 [DOI] [PubMed] [Google Scholar]
- 15.Miner TJ, Shriver CD, Flicek PR, Miner FC, Jaques DP, Maniscalco-Theberge ME, et al. Guidelines for the safe use of radioactive materials during localization and resection of the sentinel lymph node. Ann Surg Oncol 1999; 6: 75–82. [DOI] [PubMed] [Google Scholar]
- 16.Cousins A, Thompson SK, Wedding AB, Thierry B. Clinical relevance of novel imaging technologies for sentinel lymph node identification and staging. Biotechnol Adv 2014; 32: 269–79. doi: https://doi.org/10.1016/j.biotechadv.2013.10.011 [DOI] [PubMed] [Google Scholar]
- 17.Saito M, Nishiyama H, Oda Y, Shingaki S, Hayashi T. The lingual lymph node identified as a sentinel node on CT lymphography in a patient with cN0 squamous cell carcinoma of the tongue. Dentomaxillofac Radiol 2012; 41: 254–8. doi: https://doi.org/10.1259/dmfr/61883763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato M, Fukuda F, Okafuji M, Mano T, Ueyama Y. Computed tomographic (CT) lymphography for sentinel lymph node localization in oral cancer. Jpn J Head Neck Cancer 2006; 32: 24–8. [Google Scholar]
- 19.Honda K, Ishiyama K, Suzuki S, Oumi E, Sato T, Kawasaki Y, et al. Sentinel lymph node biopsy using computed tomographic lymphography in patients with early tongue cancer. Acta Otolaryngol 2015; 135: 507–12. doi: https://doi.org/10.3109/00016489.2015.1010126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu L, Cao Y, Liao C, Huang J, Gao F. Diagnostic performance of USPIO-enhanced MRI for lymph-node metastases in different body regions: a meta-analysis. Eur J Radiol 2011; 80: 582–9. doi: https://doi.org/10.1016/j.ejrad.2009.11.027 [DOI] [PubMed] [Google Scholar]
- 21.Mizokami D, Kosuda S, Tomifuji M, Araki K, Yamashita T, Shinmoto H, et al. Superparamagnetic iron oxide-enhanced interstitial magnetic resonance lymphography to detect a sentinel lymph node in tongue cancer patients. Acta Otolaryngol 2013; 133: 418–23. doi: https://doi.org/10.3109/00016489.2012.744143 [DOI] [PubMed] [Google Scholar]
- 22.Peng H, Wang SJ, Niu X, Yang X, Chi C, Zhang G. Sentinel node biopsy using indocyanine green in oral/oropharyngeal cancer. World J Surg Oncol 2015; 13: 278. doi: https://doi.org/10.1186/s12957-015-0691-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christensen A, Juhl K, Charabi B, Mortensen J, Kiss K, Kjær A, et al. Feasibility of real-time near-infrared fluorescence tracer imaging in sentinel node biopsy for oral cavity cancer patients. Ann Surg Oncol 2016; 23: 565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nahmias C, Carlson ER, Duncan LD, Blodgett TM, Kennedy J, Long MJ, et al. Positron emission tomography/computerized tomography (PET/CT) scanning for preoperative staging of patients with oral/head and neck cancer. J Oral Maxillofac Surg 2007; 65: 2524–35. doi: https://doi.org/10.1016/j.joms.2007.03.010 [DOI] [PubMed] [Google Scholar]
- 25.Schöder H, Carlson DL, Kraus DH, Stambuk HE, Gonen M, Erdi YE, et al. 18F-FDG PET/CT for detecting nodal metastases in patients with oral cancer staged N0 by clinical examination and CT/MRI. J Nucl Med 2006; 47: 755–62. [PubMed] [Google Scholar]
- 26.Manca G, Vanzi E, Rubello D, Giammarile F,Grassetto G, Wong KK, et al. (18)F-FDG PET/CT quantification in head and neck squamous cell cancer: principles, technical issues and clinical applications. Eur J Nucl Med Mol Imaging 2016; 43: 1360–75. doi: https://doi.org/10.1007/s00259-015-3294-0 [DOI] [PubMed] [Google Scholar]
- 27.Gramiak R, Shah PM. Echocardiography of the aortic root. Invest Radiol 1968; 3: 356–66. doi: https://doi.org/10.1097/00004424-196809000-00011 [DOI] [PubMed] [Google Scholar]
- 28.Goldberg BB, Merton DA, Liu JB, Murphy G, Forsberg F. Contrast-enhanced sonographic imaging of lymphatic channels and sentinel lymph nodes. J Ultrasound Med 2005; 24: 953–65. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg BB, Merton DA, Liu JB, Thakur M, Murphy GF, Needleman L, et al. Sentinel lymph nodes in a swine model with melanoma: contrast-enhanced lymphatic US. Radiology 2004; 230: 727–34. doi: https://doi.org/10.1148/radiol.2303021440 [DOI] [PubMed] [Google Scholar]
- 30.Nielsen KR, Grossjohann HS, Hansen CP, Nielsen MB. Use of contrast-enhanced ultrasound imaging to detect the first draining lymph node (FDLN) in a swine model: correlation of imaging findings with the distance from the injection site to the FDLN. J Ultrasound Med 2008; 27: 1203–9. [DOI] [PubMed] [Google Scholar]
- 31.Fisher AA. Immediate and delayed allergic contact reactions to polyethylene glycol. Contact Dermatitis 1978; 4: 135–8. doi: https://doi.org/10.1111/j.1600-0536.1978.tb03759.x [DOI] [PubMed] [Google Scholar]
- 32.Piscaglia F, Bolondi L; Italian Society for Ultrasound in Medicine and Biology (SIUMB) Study Group on Ultrasound Contrast Agents. The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol 2006; 32: 1369–75. doi: https://doi.org/10.1016/j.ultrasmedbio.2006.05.031 [DOI] [PubMed] [Google Scholar]
- 33.Dijkmans PA, Visser CA, Kamp O. Adverse reactions to ultrasound contrast agents: is the risk worth the benefit? Eur J Echocardiogr 2005; 6: 363–6. doi: https://doi.org/10.1016/j.euje.2005.02.003 [DOI] [PubMed] [Google Scholar]
- 34.Sever A, Jones S, Cox K, Weeks J, Mills P, Jones P. Preoperative localization of sentinel lymph nodes using intradermal microbubbles and contrast-enhanced ultrasonography in patients with breast cancer. Br J Surg 2009; 96: 1295–9. doi: https://doi.org/10.1002/bjs.6725 [DOI] [PubMed] [Google Scholar]
- 35.Sever AR, Mills P, Jones SE, Cox K, Weeks J, Fish D, et al. Preoperative sentinel node identification with ultrasound using microbubbles in patients with breast cancer. AJR Am J Roentgenol 2011; 196: 251–6. doi: https://doi.org/10.2214/AJR.10.4865 [DOI] [PubMed] [Google Scholar]
- 36.Rautiainen S, Sudah M, Joukainen S, Sironen R, Vanninen R, Sutela A. Contrast-enhanced ultrasound -guided axillary lymph node core biopsy: diagnostic accuracy in preoperative staging of invasive breast cancer. Eur J Radiol 2015; 84: 2130–6. doi: https://doi.org/10.1016/j.ejrad.2015.08.006 [DOI] [PubMed] [Google Scholar]
- 37.Rubaltelli L, Beltrame V, Scagliori E, Bezzon E, Frigo AC, Rastrelli M, et al. Potential use of contrast-enhanced ultrasound (CEUS) in the detection of metastatic superficial lymph nodes in melanoma patients. Ultraschall Med 2014; 35: 67–71. doi: https://doi.org/10.1055/s-0033-1335857 [DOI] [PubMed] [Google Scholar]
- 38.Xie F, Zhang D, Cheng L, Yu L, Yang L, Tong F, et al. Intradermal microbubbles and contrast-enhanced ultrasound (CEUS) is a feasible approach for sentinel lymph node identification in early-stage breast cancer. World J Surg Oncol 2015; 13: 319. doi: https://doi.org/10.1186/s12957-015-0736-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Novikov NE. Contrast-enhanced ultrasound. History of development and modern capabilities. REJR 2012; 2: 20–8. [Google Scholar]