Abstract
Objectives:
To analyze the influence of Type 2 diabetes on bone mineral density (BMD) and panoramic radiomorphometry in postmenopausal females, comparing with results from non-diabetic postmenopausal females.
Methods:
A total of 228 postmenopausal females (mean age: 59.51 ± 11.08 years) were included in this study. Demographics, T scores and Z scores from peripheral dual X-ray absorptiometry (DXA) and mandibular cortical index (MCI) from panoramic radiographs were assessed. Mean comparison between results for diabetics and non-diabetics was carried out with the Student's t-test. In addition, non-parametric correlations between MCI and DXA results were carried out with Spearman's test, at a level of significance of 5%.
Results:
Mean Z score values were significantly higher in diabetics than in non-diabetics (p = 0.001). T and Z score values were also significantly correlated with MCI (r = 0.428, p = 0.001, and r = 0.356, p = 0.022, respectively).
Conclusions:
Within the limitations of this study, the present results suggest that Type 2 diabetes might increase BMD in postmenopausal females.
Keywords: osteoporosis, diabetes, panoramic radiography
Introduction
Osteoporosis is described as a systemic disease characterized by reduction of bone mass and its microarchitectural deterioration, leading to bone fragility and increased fracture risk.1,2 Prevalence of osteoporosis is predicted to rise exponentially with the increase of elderly population.3 Osteoporosis-related fractures have a damaging impact on patient life quality and have high healthcare costs. Considered a silent illness, osteoporosis is usually detected after the occurrence of a bone fracture,4 and it affects mostly postmenopausal females, owing to the significant decrease of oestrogen, a sex hormone which plays a significant role in bone remodelling physiology.5 The foremost method for diagnosing osteoporosis is dual X-ray absorptiometry (DXA), which measures bone mineral density (BMD) with high precision and minimal radiation.6 Furthermore, DXA is useful to predict fracture risk and follow-up treatment effects. Metabolic diseases such as diabetes, hypercortisolemia, hyperthyroidism and primary hyperparathyroidism are also related to osteoporosis, as they indirectly affect bone turnover.7
Diabetes is a chronic metabolic disorder characterized by hyperglycaemia.8 It has been described that diabetics are 12 times more likely to experience osteoporotic fractures than non-diabetics.9,10 This occurs in Type 1 diabetes owing to full insulin deficiency resulting in reduced BMD. Furthermore, the osteoblast deficit is strongly related to diabetic osteopenia.11
Despite the fact that the relationship between Type 2 diabetes and osteoporosis has been broadly studied, some details remain controversial.12 Type 2 diabetes could influence bone metabolism through manifold mechanisms.13 Studies regarding the association between Type 2 diabetes and bone quality have presented different results: some studies reported that diabetes increased BMD,14–16 while others reported that it either decreased17 or did not affect the BMD18 in patients with osteoporosis.
Assessment of BMD with DXA is appropriate for patients at risk of osteoporosis. However, access to DXA is significantly limited in a number of countries.19 On the other hand, it is possible to obtain evidences of osteoporotic alterations by using panoramic radiographs, widely used as an initial dental examination at first patient attendance, with low radiation doses. The morphological changes in mandibular cortical bone layer occurring during development of osteoporosis may be identified by assessing the mandibular cortical index (MCI).20
According to previous reports, MCI is a useful index for screening osteoporotic postmenopausal females and it is significantly inversely correlated with BMD.21,22 In addition, satisfactory diagnostic performances have been previously reported for this index.23,24 In contrast, other studies suggested that MCI does not provide sufficient accuracy for osteoporosis screening25 owing to its poor to moderate ability to differentiate between patients who are normal and patients with osteopenia/osteoporosis.
Thus, the aim of this study was to analyze the influence of Type 2 diabetes on BMD and MCI in postmenopausal females, comparing with results from non-diabetic postmenopausal females.
Methods and materials
Study participants
This study was conducted with patients referred for dental treatment at the School of Dentistry of the university of this study. Approval was obtained from this university ethics committee. All patients willing to participate in this study signed an informed consent form. The Declaration of Helsinque were followed in this investigation.
Inclusion and exclusion criteria
Consecutive postmenopausal females (minimum age of 45 years) who had undergone panoramic radiographic examination (at the beginning of dental treatment) and forearm DXA (for screening osteoporosis) between 2010 and 2014 were included in this study. Patients were classified as either diabetics or non-diabetics, according to clinical history confirmation (which included glycated haemoglobin measurements).
The presence of other metabolic bone diseases such as hyperthyroidism or history of medication intake affecting bone metabolism (e.g. bisphosphonate or glucocorticoids) were considered as exclusion criteria.
Dual X-ray absorptiometry
Bone densitometry measurements were carried out with peripheral dual-energy X-ray absorptiometry (Norland; Norland Medical Systems, Inc., White Plains, NY). The scanning resolution was 1.00 × 1.00 mm, prior to scanning. The radiation dose was <0.03 mSv for each examination. The region of choice for scanning was the median forearm. The region of interest considered was defined as a rectangle with a fixed longitudinal size of 20 mm and a lateral extension large enough to cover both the radius and ulna. Its distal margin was defined to coincide with the location where the ulna and the radius start to superimpose. Patients were diagnosed based on BMD values of the forearm, measured according to World Health Organization criteria, as normal (T score > −1.0), osteopenic (T score, −1.0 to −2.5) and osteoporotic (T score ≤ −2.5 standard deviation).26,27 Furthermore, Z score values were also recorded and used to classify the patients as either normal (Z score > 2) or with low bone density (Z score < 2).28
Panoramic radiographs
All digital panoramic radiographic images were taken using the same device (Veraviewepocs® 2D; Morita, Tokyo, Japan) and with the same exposure conditions (60 kV, 4 mA, 0.5-mm copper filter). All images were processed on the same software (ImageJ; National Institute of Health, Bethesda, MD).
Mandibular cortical index
The MCI was assessed by evaluating the appearance of the cortical bone below the mandibular foramen, using a previously described classification.21 Briefly, the inferior mandibular cortex was classified as follows: C1 = normal, when presenting an even and distinct endosteal margin; C2 = moderately eroded, when presenting evidence of lacunar resorption or endosteal cortical residues; and C3 = severely eroded, when unequivocal porosity was observed.
Statistical analysis
Sample size was determined to give the study a power of 80%, at a level of significance of 5%. All panoramic radiomorphometric measurements were performed in random order by two trained observers (i.e. dentists with expertise in oral radiology). Intraobserver reliability was assessed between measurements performed 2 weeks apart to eliminate memory bias. Intraobserver and interobserver agreement were assessed using the kappa test for MCI.
Normality was assessed for continuous variables using the Shapiro–Wilk test. Differences in age, T and Z scores between diabetics and non-diabetics were assessed with the Student's t-test. In addition, non-parametric correlations between MCI and T scores and Z scores were carried out with Spearman's test.
All statistical analyses were performed at a level of significance of 5%, using IBM SPSS® v. 17 (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL).
Results
A total of 228 patients were analyzed, 190 non-diabetics (mean age 58.61 ± 11 years) and 38 diabetics (mean age 64.03 ± 10.49 years). Mean body mass index for participants was 25.04 ± 4.61 kg m−2. Normality was confirmed for T scores and Z scores, according to the Shapiro–Wilk test (p > 0.05). Intraobserver reproducibility (kappa = 0.85, 95% confidence interval = 0.73–0.90; p = 0.01) and interobserver reliability were confirmed for MCI categorical measurements (kappa = 0.83, 95% confidence interval = 0.75–0.88; p = 0.01).
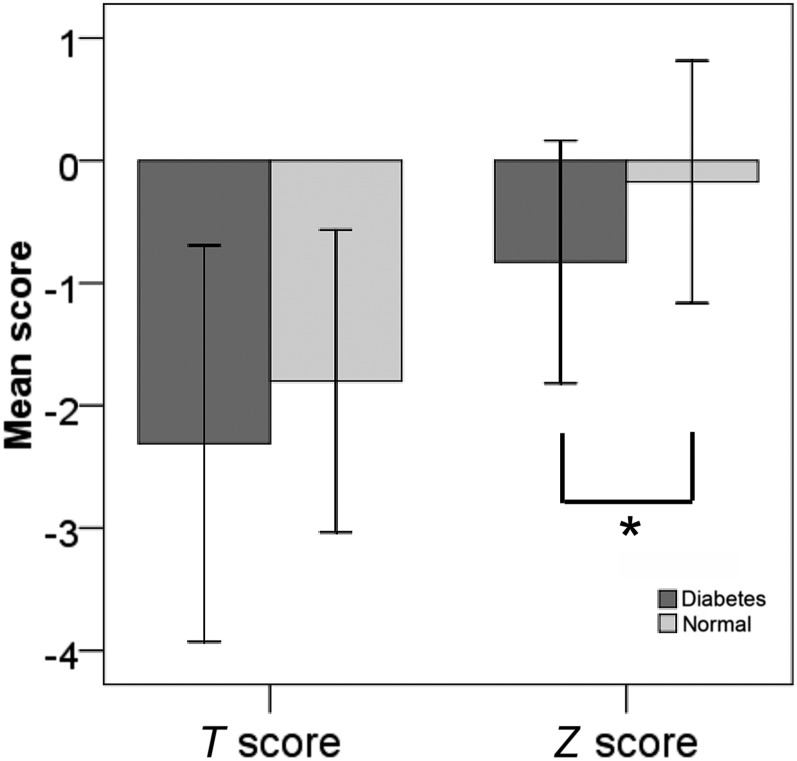
Mean Z score values were significantly higher in diabetics than in non-diabetics (p = 0.001) (Figure 1). The same difference, however, was not detected for T scores. In addition, T and Z score values were also significantly correlated with MCI (r = 0.428, p = 0.001, and r = 0.356, p = 0.022, respectively) (Table 1).
Figure 1.
Mean differences in T and Z scores between diabetics and non-diabetics. The asterisk indicates significant difference (p < 0.05), according to the Student's t-test.
Table 1.
Demographic and radiographic data of the study
| Group | n | Mean age (SD) (years) | Mean (95% CI) T score | p-valuea | T score × MCI | Mean (95% CI) Z score | p-valuea | Z score × MCI |
|---|---|---|---|---|---|---|---|---|
| Non-diabetics | 190 | 58.61 ± 11 | 1.77 (0.64–3.05) | 0.074 | r = 0.428, p = 0.001 | 0.72 (0.18–1.19) | 0.001 | r = 0.356, p = 0.022 |
| Diabetics | 38 | 64.03 ± 10.49 | 2.34 (0.67–3.96) | 0.84 (0.16–1.91) |
CI, confidence interval; MCI, mandibular cortical index; SD, standard deviation.
Significant if p < 0.05.
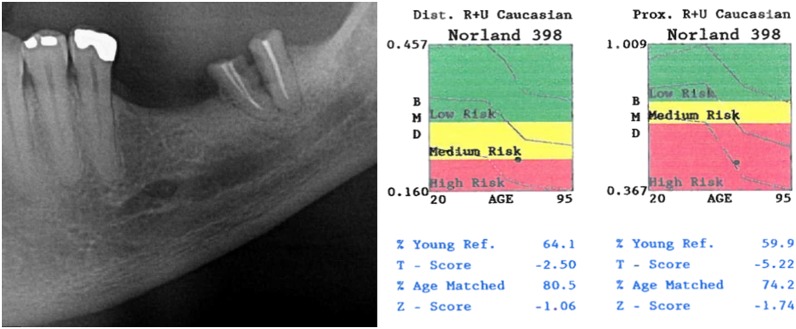
To demonstrate this correlation, we selected a panoramic radiographic of a case with inferior mandibular cortex of MCI classified as C3 and its respective DXA result (Figure 2).
Figure 2.
A panoramic radiographic image of a mandibular cortical index C3 (the severely eroded appearance of the inferior mandibular cortex can be noted) and dual X-ray absorptiometry from a patient with osteoporosis from this study.
Discussion
It is known that metabolic diseases such as diabetes affect bone turnover. However, the relationship between Type 2 diabetes and osteoporosis has been described inconsistently across studies. Osteoporosis causes generalized reduction in the amount of bone tissue, being considered a major health problem for the elderly people, especially postmenopausal females.29 Although DXA has been regarded as the gold standard analysis to diagnose osteoporosis, other imaging examinations, such as panoramic radiographs, have also been extensively described in literature as screening tools that can contribute to the early diagnosis of osteoporosis.20,30 This finding is supported by the present results, since a significant correlation was found between MCI and DXA results (p < 0.05).
In our study, mean Z scores values were significantly higher in postmenopausal females with Type 2 diabetes when compared with non-diabetics, confirming previous similar evidences.31 This finding is in agreement with previous studies using both axial32 and peripheral DXA,33 including two meta-analyses in elderly patients of both genders.12,34 However, the above-mentioned findings contrast with those from a study that found lower Z scores for patients with diabetes using peripheral34 and lumbar DXA in both genders.35
Nevertheless, in the present study, differences in mean T score values between diabetics and non-diabetics were not statistically significant (Figure 1). This other finding is also in agreement with a prior report assessing the distal radius, femoral neck and lumbar spine of only postmenopausal females36 and with a study assessing the femoral neck and spine in both genders.37 On the other hand, our results contrast with other studies reporting higher T scores in elderly diabetic males and females at the femoral neck,38 spine and forearm39 as compared with non-diabetic controls,38–40 and lower femoral neck and lumbar spine T scores.40
The above-mentioned controversy also occurs among studies focusing on actual BMD values (measured in g cm−2). Higher BMD was found among patients with diabetes in studies including both males and females using peripheral and axial DXA simultaneously,14,41 and hip and lumbar BMD.42 In contrast, lower BMD values were also found by another study.35
In the present study, the rationale for assessing both T and Z scores of postmenopausal females was to emphasize the significance of results for patients with diabetes—assessed with Z score, which is appropriate to assess the influence of other metabolic diseases on osteoporosis,28,43,44 as compared with results from the method generally used for postmenopausal females (T score).45
The present study also strove to correlate T and Z score values with MCI. The significant results observed herein demonstrate the usefulness of panoramic radiography—a commonly used dental examination—as an auxiliary tool for assessing risk of osteoporosis. Similar correlations have been previously described by studies on postmenopausal females,2,22,46,47 including those using other radiomorphometric indices.20,45,48
To our knowledge, this is one of a few studies assessing MCI in patients with diabetes. In addition, the present study demonstrates for the first time a moderate significant correlation between peripheral DXA and MCI in patients with Type 2 diabetes. On the other hand, the moderate correlation found herein contrasts with results in non-diabetic postmenopausal females, where a high accuracy of MCI was observed for detecting osteoporosis.29 One of the possible reasons is the fact that Z scores for patients with diabetes with osteoporosis were higher than those for patients who were non-diabetic.
Among the limitations of the present study are the retrospective design and the relatively small sample size. In addition, the influence of diabetes on BMD of younger patients could not be addressed. Further larger population-based prospective studies would be recommended to validate and clarify the influence of diabetes on BMD of patients of different ages.
Conclusions
In conclusion, within the limitations of this study, the present results suggest that Type 2 diabetes is associated with increased BMD in postmenopausal females. Furthermore, MCI from panoramic radiographs are only moderately correlated with DXA in patients with diabetes with osteoporosis.
Contributor Information
Luciana Munhoz, Email: dra.lucimunhoz@usp.br.
Arthur R G Cortes, Email: arthuro@usp.br.
Emiko S Arita, Email: esarita@usp.br.
References
- 1.Osteoporosis prevention, diagnosis, and therapy. NIH Consens Statement 2000; 17: 1–45. [PubMed] [Google Scholar]
- 2.Kim OS, Shin MH, Song IH, Lim IG, Yoon SJ, Kim OJ, et al. Digital panoramic radiographs are useful for diagnosis of osteoporosis in Korean postmenopausal women. Gerodontology 2016; 33: 185–92. doi: https://doi.org/10.1111/ger.12134 [DOI] [PubMed] [Google Scholar]
- 3.Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int 1997; 7: 407–13. doi: https://doi.org/10.1007/pl00004148 [DOI] [PubMed] [Google Scholar]
- 4.Lewiecki EM, Borges JL. Bone density testing in clinical practice. Arq Bras Endocrinol Metabol 2006; 50: 586–95. doi: https://doi.org/10.1590/s0004-27302006000400004 [DOI] [PubMed] [Google Scholar]
- 5.Zhao R. Immune regulation of bone loss by Th17 cells in oestrogen-deficient osteoporosis. Eur J Clin Invest 2013; 43: 1195–202. doi: https://doi.org/10.1111/eci.12158 [DOI] [PubMed] [Google Scholar]
- 6.Njeh CF, Fuerst T, Hans D, Blake GM, Genant HK. Radiation exposure in bone mineral density assessment. Appl Radiat Isot 1999; 50: 215–36. [DOI] [PubMed] [Google Scholar]
- 7.Sheu A, Diamond T. Secondary osteoporosis. Aust Prescr 2016; 39: 85–7. doi: https://doi.org/10.18773/austprescr.2016.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care 2016; 39(Suppl. 1): S13–22. doi: https://doi.org/10.2337/dc16-S005 [DOI] [PubMed] [Google Scholar]
- 9.Nicodemus KK, Folsom AR; Iowa Women's Health Study. Type 1 and type 2 diabetes and incident hip fractures in postmenopausal women. Diabetes Care 2001; 24: 1192–7. doi: https://doi.org/10.2337/diacare.24.7.1192 [DOI] [PubMed] [Google Scholar]
- 10.Leidig-Bruckner G, Ziegler R. Diabetes mellitus a risk for osteoporosis? Exp Clin Endocrinol Diabetes 2001; 109(Suppl. 2): S493–514. doi: https://doi.org/10.1055/s-2001-18605 [DOI] [PubMed] [Google Scholar]
- 11.Inaba M, Terada M, Koyama H, Yoshida O, Ishimura E, Kawagishi T, et al. Influence of high glucose on 1,25-dihydroxyvitamin D3-induced effect on human osteoblast-like MG-63 cells. J Bone Miner Res 1995; 10: 1050–6. doi: https://doi.org/10.1002/jbmr.5650100709 [DOI] [PubMed] [Google Scholar]
- 12.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporos Int 2007; 18: 427–44. [DOI] [PubMed] [Google Scholar]
- 13.Abdulameer SA, Sulaiman SA, Hassali MA, Subramaniam K, Sahib MN. Osteoporosis and type 2 diabetes mellitus: what do we know, and what we can do? Patient Prefer Adherence 2012; 6: 435–48. doi: https://doi.org/10.2147/PPA.S32745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melton LJ, Riggs BL, Leibson CL, Achenbach SJ, Camp JJ, Bouxsein ML, et al. A bone structural basis for fracture risk in diabetes. J Clin Endocrinol Metab 2008; 93: 4804–9. doi: https://doi.org/10.1210/jc.2008-0639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonds DE, Larson JC, Schwartz AV, Strotmeyer ES, Robbins J, Rodriguez BL, et al. Risk of fracture in women with type 2 diabetes: the Women's Health Initiative Observational Study. J Clin Endocrinol Metab 2006; 91: 3404–10. doi: https://doi.org/10.1210/jc.2006-0614 [DOI] [PubMed] [Google Scholar]
- 16.Kao WH, Kammerer CM, Schneider JL, Bauer RL, Mitchell BD. Type 2 diabetes is associated with increased bone mineral density in Mexican-American women. Arch Med Res 2003; 34: 399–406. [DOI] [PubMed] [Google Scholar]
- 17.Bauer DC, Browner WS, Cauley JA, Orwoll ES, Scott JC, Black DM, et al. Factors associated with appendicular bone mass in older women. The Study of Osteoporotic Fractures Research Group. Ann Intern Med 1993; 118: 657–65. doi: https://doi.org/10.7326/0003-4819-118-9-199305010-00001 [DOI] [PubMed] [Google Scholar]
- 18.Tuominen JT, Impivaara O, Puukka P, Rönnemaa T. Bone mineral density in patients with type 1 and type 2 diabetes. Diabetes Care 1999; 22: 1196–200. doi: https://doi.org/10.2337/diacare.22.7.1196 [DOI] [PubMed] [Google Scholar]
- 19.Kanis JA, Johnell O. Requirements for DXA for the management of osteoporosis in Europe. Osteoporos Int 2005; 16: 229–38. doi: https://doi.org/10.1007/s00198-004-1811-2 [DOI] [PubMed] [Google Scholar]
- 20.Klemetti E, Kolmakov S, Kröger H. Pantomography in assessment of the osteoporosis risk group. Scand J Dent Res 1994; 102: 68–72. [DOI] [PubMed] [Google Scholar]
- 21.Taguchi A, Sanada M, Krall E, Nakamoto T, Ohtsuka M, Suei Y, et al. Relationship between dental panoramic radiographic findings and biochemical markers of bone turnover. J Bone Miner Res 2003; 18: 1689–94. doi: https://doi.org/10.1359/jbmr.2003.18.9.1689 [DOI] [PubMed] [Google Scholar]
- 22.Bhatnagar S, Krishnamurthy V, Pagare SS. Diagnostic efficacy of panoramic radiography in detection of osteoporosis in post-menopausal women with low bone mineral density. J Clin Imaging Sci 2013; 3: 23. doi: https://doi.org/10.4103/2156-7514.113140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marandi S, Bagherpour A, Imanimoghaddam M, Hatef M, Haghighi A. Panoramic-based mandibular indices and bone mineral density of femoral neck and lumbar vertebrae in women. J Dent (Tehran) 2010; 7: 98–106. [PMC free article] [PubMed] [Google Scholar]
- 24.Taguchi A, Suei Y, Sanada M, Ohtsuka M, Nakamoto T, Sumida H, et al. Validation of dental panoramic radiography measures for identifying postmenopausal women with spinal osteoporosis. AJR Am J Roentgenol 2004; 183: 1755–60. [DOI] [PubMed] [Google Scholar]
- 25.Devlin H, Karayianni K, Mitsea A, Jacobs R, Lindh C, van der Stelt P, et al. Diagnosing osteoporosis by using dental panoramic radiographs: the OSTEODENT project. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007; 104: 821–8. [DOI] [PubMed] [Google Scholar]
- 26.Kanis JA, Melton LJ, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res 1994; 9: 1137–41. doi: https://doi.org/10.1002/jbmr.5650090802 [DOI] [PubMed] [Google Scholar]
- 27.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser 1994; 843: 1–129. [PubMed] [Google Scholar]
- 28.Monegal A, Navasa M, Guañabens N, Peris P, Pons F, Martinez de Osaba MJ, et al. Osteoporosis and bone mineral metabolism disorders in cirrhotic patients referred for orthotopic liver transplantation. Calcif Tissue Int 1997; 60: 148–54. doi: https://doi.org/10.1007/s002239900205 [DOI] [PubMed] [Google Scholar]
- 29.Taguchi A, Tanimoto K, Suei Y, Ohama K, Wada T. Relationship between the mandibular and lumbar vertebral bone mineral density at different postmenopausal stages. Dentomaxillofac Radiol 1996; 25: 130–5. [DOI] [PubMed] [Google Scholar]
- 30.Erdogan O, Incki KK, Benlidayi ME, Seydaoglu G, Kelekci S. Dental and radiographic findings as predictors of osteoporosis in postmenopausal women. Geriatr Gerontol Int 2009; 9: 155–64. doi: https://doi.org/10.1111/j.1447-0594.2009.00518.x [DOI] [PubMed] [Google Scholar]
- 31.Ay S, Gursoy UK, Erselcan T, Marakoglu I. Assessment of mandibular bone mineral density in patients with type 2 diabetes mellitus. Dentomaxillofac Radiol 2005; 34: 327–31. doi: https://doi.org/10.1259/dmfr/52540810 [DOI] [PubMed] [Google Scholar]
- 32.Zhou Y, Li Y, Zhang D, Wang J, Yang H. Prevalence and predictors of osteopenia and osteoporosis in postmenopausal Chinese women with type 2 diabetes. Diabetes Res Clin Pract 2010; 90: 261–9. doi: https://doi.org/10.1016/j.diabres.2010.09.013 [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto M, Yamaguchi T, Yamauchi M, Kaji H, Sugimoto T. Diabetic patients have an increased risk of vertebral fractures independent of BMD or diabetic complications. J Bone Miner Res 2009; 24: 702–9. [DOI] [PubMed] [Google Scholar]
- 34.Majima T, Komatsu Y, Yamada T, Koike Y, Shigemoto M, Takagi C, et al. Decreased bone mineral density at the distal radius, but not at the lumbar spine or the femoral neck, in Japanese type 2 diabetic patients. Osteoporos Int 2005; 16: 907–13. [DOI] [PubMed] [Google Scholar]
- 35.Dutta M, Pakhetra R, Garg M. Evaluation of bone mineral density in type 2 diabetes mellitus patients before and after treatment. Med J Armed Forces India 2012; 68: 48–52. doi: https://doi.org/10.1016/S0377-1237(11)60120-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anaforoglu I, Nar-Demirer A, Bascil-Tutuncu N, Ertorer ME. Prevalence of osteoporosis and factors affecting bone mineral density among postmenopausal Turkish women with type 2 diabetes. J Diabetes Complications 2009; 23: 12–17. doi: https://doi.org/10.1016/j.jdiacomp.2007.06.004 [DOI] [PubMed] [Google Scholar]
- 37.Agius R, Galea R, Fava S. Bone mineral density and intervertebral disc height in type 2 diabetes. J Diabetes Complications 2016; 30: 644–50. doi: https://doi.org/10.1016/j.jdiacomp.2016.01.021 [DOI] [PubMed] [Google Scholar]
- 38.Schwartz AV, Vittinghoff E, Bauer DC, Hillier TA, Strotmeyer ES, Ensrud KE, et al. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA 2011; 305: 2184–92. doi: https://doi.org/10.1001/jama.2011.715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rakic V, Davis WA, Chubb SA, Islam FM, Prince RL, Davis TM. Bone mineral density and its determinants in diabetes: the Fremantle Diabetes Study. Diabetologia 2006; 49: 863–71. doi: https://doi.org/10.1007/s00125-006-0154-2 [DOI] [PubMed] [Google Scholar]
- 40.Mathen PG, Thabah MM, Zachariah B, Das AK. Decreased bone mineral density at the femoral neck and lumbar spine in South Indian patients with type 2 diabetes. J Clin Diagn Res 2015; 9: 8–12. doi: https://doi.org/10.7860/JCDR/2015/14390.6450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shu A, Yin MT, Stein E, Cremers S, Dworakowski E, Ives R, et al. Bone structure and turnover in type 2 diabetes mellitus. Osteoporos Int 2012; 23: 635–41. doi: https://doi.org/10.1007/s00198-011-1595-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamalanathan S, Nambiar V, Shivane V, Bandgar T, Menon P, Shah N. Bone mineral density and factors influencing it in Asian Indian population with type 2 diabetes mellitus. Indian J Endocrinol Metab 2014; 18: 831–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewiecki EM, Watts NB, McClung MR, Petak SM, Bachrach LK, Shepherd JA, et al. Official positions of the international society for clinical densitometry. J Clin Endocrinol Metab 2004; 89: 3651–5. [DOI] [PubMed] [Google Scholar]
- 44.Arita ES, Pippa MG, Marcucci M, Cardoso R, Cortes AR, Watanabe PC, et al. Assessment of osteoporotic alterations in achondroplastic patients: a case series. Clin Rheumatol 2013; 32: 399–402. doi: https://doi.org/10.1007/s10067-012-2126-x [DOI] [PubMed] [Google Scholar]
- 45.Nagi R, Devi BK, Rakesh N, Reddy SS, Santana N, Shetty N. Relationship between femur bone mineral density, body mass index and dental panoramic mandibular cortical width in diagnosis of elderly postmenopausal women with osteoporosis. J Clin Diagn Res 2014; 8: 36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moeintaghavi A, Pourjavad M, Dadgar S, Tabbakh NS. Evaluation of the association between periodontal parameters, osteoporosis and osteopenia in post menopausal women. J Dent (Tehran) 2013; 10: 443–8. [PMC free article] [PubMed] [Google Scholar]
- 47.Valerio CS, Trindade AM, Mazzieiro ET, Amaral TP, Manzi FR. Use of digital panoramic radiography as an auxiliary means of low bone mineral density detection in post-menopausal women. Dentomaxillofac Radiol 2013; 42: 20120059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horner K, Devlin H, Harvey L. Detecting patients with low skeletal bone mass. J Dent 2002; 30: 171–5. [DOI] [PubMed] [Google Scholar]