Abstract
The aim of this study was to systematically review the existing scientific literature and evidence about (a) the validation of masseter muscle ultrasonography for accurate assessment of muscle thickness and (b) the reproducibility of masseter muscle thickness measures. An electronic literature search was conducted using determined keywords on specific databases. Preliminary search revealed 298 articles listed in Medline, Scopus and Web of Science. 60 duplicates were rejected, leaving 238 articles for review. After reading titles and abstracts, 31 articles remained. 23 articles were assessed for eligibility. These articles were categorized as follows: thickness, cross-section, volume and the length of the masseter muscle measured by ultrasonography. It is possible to verify the thickness of the masseter muscle in males and females in relaxation (10–15 and 9–13 mm, respectively) and contraction (14–19 and 12–15 mm, respectively). A similar tendency can also be evidenced in other measurements. Many studies evaluate masseter muscle dimensions to relate it to cephalometric analysis as such to evaluate morphological variations. It can be concluded that ultrasound is a reliable clinical tool for masseter muscle measurements, yet there is a need for standardization of methods and parameters to be recorded.
Introduction
Masseter muscle thickness has been extensively studied as it relates to masticatory function and craniofacial functional mechanisms. Indeed, growth, facial morphology, and muscle thickness can be influenced by occlusal morphology and bite force.1–3
Several studies have reported that muscle anatomy is related to individuals' physiognomy and other anthropometric variables. Some researchers believe that masseter thickness is related with facial morphology.1,4–7 Other authors stressed that measurement of cross-sectional distances of the head and neck muscles can be correlated with muscle palpation pain, facial morphology, bite force and occlusal factors.2,8–10 Furthermore, there seems to be an association between masseter muscle thickness and several features of the dental arches, such as alveolar process thickness and maxillary dental arch width.8,11
Ultrasound imaging has been used to measure in vivo masseter muscle thickness as an indicator of muscle size.3,4 Owing to its numerous advantages comparing to CT and MRI, it is appropriate for larger scale studies.5 It allows a reproducible and economical method to measure muscle thickness,3,12 without the use of ionizing radiation.5,13 Also, the ultrasound equipment can be easily handled and transported and is accurate for soft-tissue assessment.12,14
Linear and volume cross-sectional measurements of isolated masticatory muscle have already been shown to correlate with facial variables, although the sites and dimensions at which the masseter muscle measurements have been taken varied between investigators, which makes direct comparison difficult.7,12,15 Furthermore, given this fact, it reinforces the need for further studies to provide technique-related data for intra- and interobserver reliability, to gain consistent diagnosis for evaluation of masseter muscle dimensions.
The aim of this study was to systematically review the existing scientific literature and evidence about (a) the validation of masseter muscle ultrasonography for accurate assessment of muscle thickness and (b) the reproducibility of masseter muscle thickness measures performed by ultrasonography.
Methods and materials
Information sources
A wide electronic database search was conducted to identify relevant publications. Additionally, reference lists of pertinent articles were searched manually. We did not apply language limitations, and we also explored informally published literature: conference proceedings and research abstracts presented at conferences and dissertations. The following databases were searched: Ovid Medline (1946–November 2014), Scopus (1960–November 2014) and Web of Science (1945–November 2014).
Search strategy
The search strategy was designed having an information specialist as an advisor. The searches did not have a date limit nor were restricted to certain types of studies. The keywords used were: Masseter muscle AND (ultrasonography OR ultrasound OR ultra-sound OR ultrasonographic OR ecography OR ecographic) AND (thickness OR volume OR size OR measure).
Study selection
At the first stage, two reviewers (experienced dentomaxillofacial radiologists) individually selected the retrieved records titles. Only the titles related to masseter muscle thickness measured in ultrasound were included. In a second phase, the retrieved articles' abstracts were read by both observers and categorized according to the subject of study. In order to be included for the second selection phase, a publication had merely to be accepted by one observer. All relevant articles were included, regardless of language.
The analysis had to be based on primary materials or include an effectiveness evaluation. If the abstract was relevant for at least one reviewer, it was read in full text. At the second step, the full texts were retrieved and critically examined. The relevant reference lists of publications which were found relevant in the first phase were carried out manually. Articles which were selected mentioned the words “masseter muscle ultrasound”, “masseter muscle ultrasonography”, together with “size”, “thickness” and “volume”. Book chapters and reviews were excluded, since the objective of this systematic review was to evaluate primary studies focused in ultrasonography.
Data extraction
Data were obtained with the aid of Protocol 1 (Supplementary Table S1). It was prepared by reading the pertinent literature on how to critically assess studies about diagnostic methods. To diminish bias, two trained observers independently assessed the validity of the original studies according to the quality of diagnostic accuracy study tool using Protocol 2 (quality evaluation of studies of diagnostic accuracy included in systematic reviews—QUADAS) (Supplementary Table S2).16 To resolve any divergence between observers regarding an article, a conversation was held, where each observer presented their ideas, until an agreement was achieved. Previously, the protocols were tested in 10 publications. Moreover, five publications were read to standardize the principles of the two reviewers regarding the criteria in Protocol 2. Only publications that were believed to be pertinent in both Protocols 1 (diagnostic efficacy) and 2 (level of evidence) were finally included. The quality and internal validity (level of evidence) of each publication was rated as high, moderate or low according to the criteria in the following subsection.17 It is important to note that a number of publications in this systematic review can be included in several categories.
Levels of evidence and criteria for evidence synthesis
High level of evidence
A study was classified with high level of evidence if it fulfilled all the following:
– There was an independent blind comparison between the methods of test and reference.
– The study population was described from the status, prevalence and severity of the condition, also the criteria were clearly described. The spectrum of patients was analogous to the spectrum of patients on whom the test method would be applied in clinical practice.
– The results of the evaluation method being tested did not influence the choice to perform the reference method(s).
– The test procedures and measurements and reference methods were well described.
– The observations and measurements were well described regarding diagnostic criteria applied and information and instructions for evaluators.
– The reproducibility of the assay technique was described for one evaluator (intraobserver performance) and for several (minimum three) evaluators (interobserver performance).
– The results were shown as of relevant data required for accurate calculations.
Moderate level of evidence
A study was judged to have moderate level if any of the above criteria were not found. Furthermore, the study fitted this criterion if it had none of the deficiencies that are described below for studies with a low level of evidence.
Low level of evidence
An article was considered to have a low level of evidence if:
– the evaluation of the test and reference technique was not independent
– the population has not been clearly described, and the spectrum of patients was modified
– the results of the test method influenced the decision to make the reference method
– the test or reference method or both were not exactly described
– the relevant observations were not well described
– the reproducibility of the assay technique was not described or was described by only one evaluator
– the results could provide a systematic bias
– the results were not shown in a manner that allowed efficient calculations.
Rating conclusions according to evidence grade
Evaluation of the scientific evidence on diagnostic efficacy was considered to be strong, moderately strong, limited or insufficient dependent on the quality and internal validity (level of evidence) of the publications assessed:17,18
– Strong research-based evidence: at least two of the publications or a systematic review must have a high-level of evidence;
– Moderately strong research-based evidence: one of the publications should show a high level of evidence and two more of the articles must have a moderate level of evidence;
– Limited research-based evidence: at least two of the publications must have a moderate level of evidence;
– Insufficient research-based evidence: scientific evidence is insufficient or missing according to the criteria defined in this study.
Synthesis of evidence
The results of this review were presented descriptively. No meta-analyses were attempted due to the deficiency on original studies.
Results
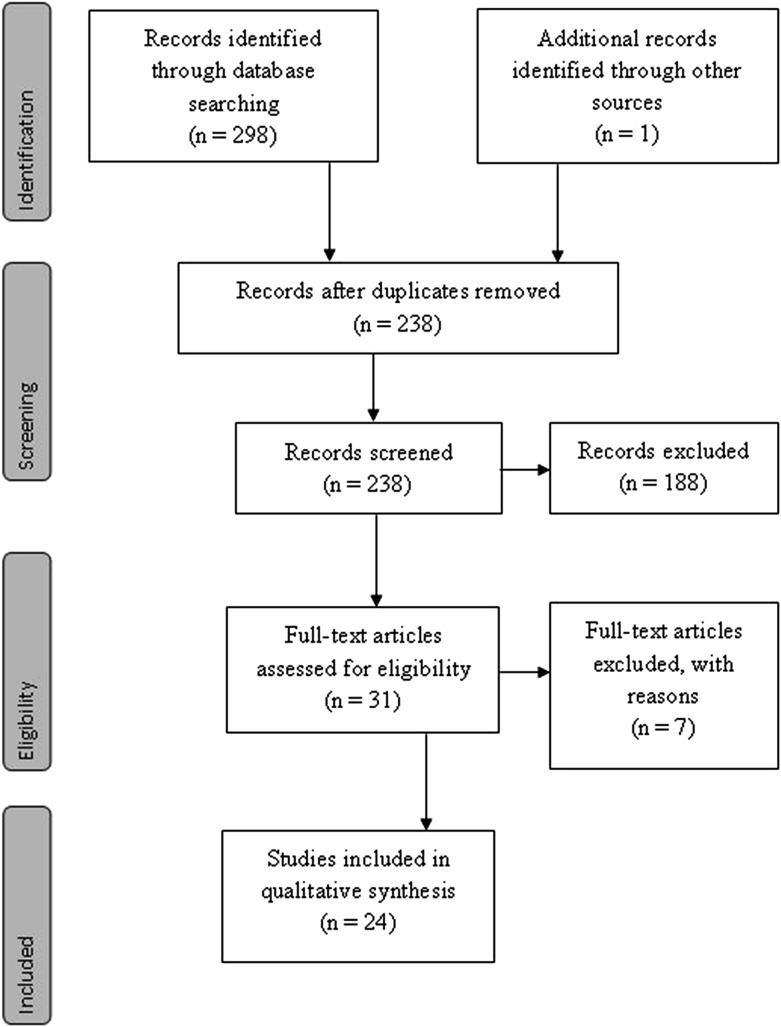
The number of articles reviewed in each phase of this systematic review is presented in the PRISMA flow diagram (Figure 1).19 Preliminary search revealed 84 articles listed in Medline (Ovid), 103 articles in Scopus, 110 articles in the Web of Science and 1 additional record identified by other sources, totalling 298 articles. The second stage was to evaluate the reference lists of selected articles, which added one article of interest. 60 duplicates were rejected, leaving 238 articles for review. In the first phase of selection, the observers screened the articles by reading titles and abstracts. Articles that were not eligible because of irrelevant aims and not directly related to this systematic review were excluded, thus 31 articles remained for further reading. 23 articles were assessed for eligibility. These were obtained after the two observers' agreement.
Figure 1.
Flow diagram of information followed in the article selection process. Adapted from Moher et al.19
After article selection using Protocols 1 and 2, a final 23 articles met the inclusion criteria and were screened for qualitative synthesis and appraised for their level of evidence. All articles that remained after screening passed the qualitative synthesis. These articles were categorized as follows: 3 articles related to the length of the masseter muscle, 18 articles referred to the muscle thickness, 5 articles mentioned cross-sectional measurements and 3 articles discussed the volume of the masseter muscle. Among the publications selected in this systematic review, several were included in various categories such as Benington et al7 (Tables 1 and 4), Naser-ud-Din et al20 (Tables 1–4) and Naser-ud-Din et al21 (Tables 1, 2 and 4).
Table 1.
Publications on the length of the masseter muscle measured by ultrasonography.
| Authors | Subjects (M/F) | Age (years) | Methods | Length of the masseters |
Level of evidence | |
|---|---|---|---|---|---|---|
| Relaxation (mm) | Contraction (mm) | |||||
| Benington et al7 | 4/6 | 15–31/20–26 | Linear probe:
7 MHz Three alternate slices from the muscle belly middle (length and volume) |
Not performed | M: 53.8
(±5.8) F: 46.5 (±8.2) |
Moderate |
| Naser-ud-Din et al20 | 8 | 22–30 | Linear probe:
5–13 MHz Unilateral Length: between zygomatic tubercule and gonial angle |
Not performed | Length: 64.7 (±SD 6.8) |
Low |
| Naser-ud-Din et al21 | 3/8 | 22–30 | Linear probe:
5–13 MHz Length: distance between zygomatic tubercule and gonial angle |
Not performed | Pearson's correlation
coefficients Length: 0.86 Thickness: 0.95 Volume: 0.14 |
Low |
F, female; M, male; SD, standard deviation.
Table 4.
Publications on volume of masseter muscle measured by ultrasonography
| Authors | Subjects (M/F) | Age (years) | Methods | Volume of the masseters |
Level of evidence | |
|---|---|---|---|---|---|---|
| Relaxation (mm3) | Contraction (mm3) | |||||
| Benington et al7 | 4/6 | 15–31/20–26 | Linear probe:
7 MHz Three alternate slices from the muscle belly middle (volume) |
F:
9.5 ± 1.2 M: 11.1 ± 1.3 Width F: 34.2 ± 4.1 M: 40.8 ± 4.3 |
Moderate | |
| Naser-ud-Din et al20 | 8 F | 22–30 | Linear probe:
5–13 MHz Unilateral Volume:MM thickness and length |
Not performed | Volume: 3189.0 (±973.4) (cm3) | Low |
| Naser-ud-Din et al21 | 3/8 | 22–30 | Linear probe: 5–13 MHz | Not performed | Pearson's correlation
coefficients Volume: 0.14 |
Low |
F, female; M, male; MM, Material and Methods.
Table 2.
Publications related to the thickness of masseter muscle seen in ultrasonography
| Authors | Subjects (M/F) | Age (years) | Methods | Thickness of the
masseters |
Level of evidence | |
|---|---|---|---|---|---|---|
| Relaxation (mm) | Contraction (mm) | |||||
| Kiliaridis and Kälebo3 | 20/20 | 20–35 | Linear probe:
7 MHz Occlusal plane, middle of ramus mediolateral distance |
M: 9.7
(±1.5) F: 8.7 (±1.6) |
M: 15.1
(±1.9) F: 13.0 (±1.8) |
Moderate |
| Bakke et al4 | 13 F | 21–28 | Curvilinear probe: 7.5 MHz | 11.08 | 12.97 | Moderate |
| Raadsheer et al5 | 15 M | 25–51 | Linear probe:
7.5-MHz Thickness Large distance between ramus and superficial muscle surface, ┴ to underlying ramus |
Measurement 1 | Measurement 1 | Low |
| Right side: | Right side: | |||||
| Upper: 13.7 ± 2.5 | Upper: 16.2 ± 2.7 | |||||
| Middle: 13.3 ± 2.7 | Middle: 15.4 ± 2.6 | |||||
| Lower: 11.3 ± 3.3 | Lower: 13.3 ± 3.2 | |||||
| Measurement 1 | Measurement 1 | |||||
| Left side: | Left side: | |||||
| Upper: 13.5 ± 2.1 | Upper: 15.8 ± 1.8 | |||||
| Middle 13.8 ± 2.6 | Middle: 16.0 ± 2.0 | |||||
| Lower: 11.1 ± 2.8 | Lower: 14.1 ± 2.9 | |||||
| Measurement 2 | Measurement 2 | |||||
| Right side: | Right side: | |||||
| Upper: 14.1 ± 2.5 | Upper: 15.8 ± 3.0 | |||||
| Middle: 13.7 ± 2.8 | Middle: 15.7 ± 2.6 | |||||
| Lower: 11.4 ± 2.5 | Lower: 13.4 ± 3.2 | |||||
| Measurement 2 | Measurement 2 | |||||
| Left side: | Left side: | |||||
| Upper: 14.3 ± 2.0 | Upper: 16.1 ± 1.4 | |||||
| Middle: 13.8 ± 2.4 | Middle: 16.3 ± 2.3 | |||||
| Lower: 12.1 ± 2.3 | Lower: 13.2 ± 2.8 | |||||
| Kubota et al6 | 80 M | Mean age 23.8 (±1.9) | Linear probe:
7.5 MHz Crosses a line connecting the labial corner to the intertragic notch of the ear |
15.8 (±3.0) | 16.7 (±2.7) | Low |
| Emshoff et al12 | 30 subjects | 19–56 | Linear probe: 7.5 MHz | 13.5 | Not performed | Low |
| Jonasson and Kiliaridis11 | 62 F | 40–75 | Linear probe: 7 MHz | 13.1 ± 1.9 (left side) | Low | |
| Raadsheer et al10 | 57/64 | 18–36 | Linear probe:
7.5 MHz Halfway between the zygomatic arch and gonion |
Right side:
12.0/−1.9 Left side: 12.2/−1.9 |
Not performed | Low |
| Satiroğlu et al13 | 24/23 | Mean age of 24.96 (±3.57) | Linear probe:
7.5–9.0 MHz Part of the MM, close to occlusal plane level, in the mediolateral middle ramus distance |
High angle M: 15.50 ± 1.99 F: 12.08 ± 1.89 Total: 13.29 ± 2.52 Low angle M: 15.87 ± 1.83 F: 13.55 ± 0.74 Total: 15.20 ± 1.90 Normal M: 14.92 ± 1.59 F: 12.74 ± 1.69 Total: 13.56 ± 1.95 |
High angle M: 17.19 ± 2.05 F: 13.37 ± 1.82 Total: 14.72 ± 2.63 Low angle M: 17.01 ± 2.15 F: 14.57 ± 0.98 Total: 16.31 ± 2.18 Normal M: 15.92 ± 1.89 F: 13.76 ± 1.24 Total: 14.57 ± 1.83 |
Low |
| Georgiakaki et al22 | 52 | Mean age of 23.7 (±2.5) | Linear probe: 7.5 MHz occlusal plane level, between zygomatic arch, gonial angle, centre of the mediolateral ramus | 13.9 ± 1.55 (right
side) 13.3 ± 1.4 (left side) |
Low | |
| Ngom et al23 | 55/46 | 17–43 | Linear probe: 8 MHz | M: 14.40 F: 12.79 |
M: 16.14 F: 14.45 |
Moderate |
| Mangilli et al24 | 5 subjects | 20–30 | Linear probe: No
referred Occlusal plane to ramus mediolateral distance |
Pearson's correlation
coefficient: 0.948742179 |
Pearson's correlation
coefficient 0.85684586 |
Low |
| Naser-ud-Din et al20 | 8 F | 22–30 | Linear probe:
5–13 MHz Unilateral thickness: between superficial and deep aspects of the MM |
Not performed | Thickness: 13.7 (±SD 2.2) | Low |
| Rani and Ravi25 | 72 subjects | 18–25 | Linear probe: 7.5–11.0 MHz | Class I:
10.4 ± 1.1 M: 11.2 ± 0.9 F: 9.6 ± 0.5 |
Class I:
12.8 ± 1.3 M: 13.7 ± 1.1 F: 11.9 ± 0.8 |
Moderate |
| Naser-ud-Din et al21 | 3/8 | 22–30 | Linear
probe: 5–13 MHz |
Not performed | Pearson's correlation
coefficients Thickness: 0.95 |
Low |
| Egwu et al14 | 30/30 | 19–30 | Linear probe: 7.5 MHz—line from mouth lateral commissure to ear intertragic notch | M:
14.86 ± 3.42 F: 11.94 ± 1.81 |
M:
19.07 ± 3.69 F: 15.00 ± 1.66 |
Low |
| Liao et al27 | 42 subjects | No referred | Linear probe:
5–12 MHz MM anterior border to mandibular ramus. 2.5 cm above mandibular inferior border |
9.0 ± 1.9 | 11.8 ± 2.8 | Low |
| Rohila et al26 | 30/30 | 18–24 | Linear probe:
7.5–9.0 MHz Mouth commissure to ear intertragic notch, crossing the MM |
Hypodivergent group:
13.94 ± 1.51 Normodivergent group: 12.53 ± 1.21 Hyperdivergent group: 11.13 ± 1.18 |
Hypodivergent group:
15.46 ± 1.33 Normodivergent group: 13.81 ± 1.38 Hyperdivergent group: 2.27 ± 1.26 |
Low |
| Strini et al28 | 12/26 | 18–32 | Linear probe:
10 MHz Between zygomatic arch and gonial angle |
M:
13.3 ± 1.4 F: 10.9 ± 1.3 |
M:
15.5 ± 1.8 F: 13.0 ± 1.2 |
Moderate |
F, female; M, male; MM, Material and Methods; SD, standard deviation.
Length of the masseter muscle measurements by ultrasonography
Three articles examined the length of the masseter muscle measured in ultrasound were found (Table 1). One was rated as a moderate level of evidence,7 whereas the other two were found to have a low level of evidence.20,21 The masseter muscle length was on average 54 mm for males and 46.5 mm for females.7
Thickness of masseter muscle measured by ultrasonography
18 articles were selected as eligible in this category (Table 2). Five publications presented a moderate level of evidence,3,4,23,25,28 whereas the others were identified as having a low level of evidence.5,8,10–14,20–22,24,26,27 In these articles, the mean values for masseter muscle thickness ranged from 12.0 to 17.2 mm.
Cross-sectional measurement of masseter muscle measured by ultrasonography
Five publications with low-level evidence were found2,9,15,20,29 (Table 3). The mean values found in the literature for the cross-sectional measurement of the masseter muscle, considering males and females, ranged from 3.0 to 16.1 mm2.
Table 3.
Publications on cross-sectional measurements of the masseter muscle performed by ultrasonography
| Authors | Subjects (M/F) | Age (years) | Methods | Cross-section of the
masseters |
Level of evidence | |
|---|---|---|---|---|---|---|
| Relaxation (mm2) | Contraction (mm2) | |||||
| Close et al9 | 19/20 | 21–47 | Linear probe:
5 MHz Cross-sectional |
M:
4.3 ± l.5 F: 3.0 ± 1.2 |
Not performed | Low |
| Bertram et al15 | 10/32 | 18–59 | Linear probe:
7.5 MHz Middle section of MM |
Cross-section 6.8–12.9 | 9.0–16.1 | Low |
| Naser-ud-Din et al20 | 8 F | 22–30 | Linear probe:
5–13 MHz Unilateral Cross-sectional: manually trace around muscle boundaries with electronic tools |
Not performed | 6.2 (±1.7) | Low |
| Uchida et al2 | 11/24 | Mean age of 27.6 ± 5.6 | Linear probe:
7.5 MHz Cross-sectional |
414.7 ± 74.3 | 492.1 ± 7.8 | Low |
| Hernandez et al29 | 45/45 | 18–60 | Linear probe:
12 MHz Cross-sectional |
Brachyfacial: M: 11.5 ± 2.08 F: 8.8 ± 1.4 Mesiofacial: M : 11.4 ± 1.6 F: 7.8 ± 1.6 Dolichofacial: M: 10.08 ± 1.2 F : 7.7 ± 1.4 |
Not performed | Low |
F, female; M, male; MM, Material and Methods.
Volume of masseter muscle measured by ultrasonography
Three articles met the inclusion criteria (Table 4). One was rated as a moderate level of evidence, whereas the other two were classified as having a low evidence level.7,20,21 No articles could be identified in the category of high levels of evidence. To measure the masseter muscle volume, the mean values observed between the publications ranged from 9.5 to 11.1 mm.3,7
In the present review, we found a range of measures of normality of masseter muscle thickness according to gender with values for males between 10 and 15 mm in relaxation and 14–19 mm in contraction. For females, ranges were between 9 and 13 mm in relaxation and 12–15 mm in contraction (Table 2).3,13,14,23,25,28
Discussion
Ultrasonography has been described as a medical imaging technique which provides accurate measurement of masticatory muscles dimensions. Several authors suggest that ultrasound is a feasible method of diagnosis, measuring and detecting changes in the face and neck muscle dimensions. The following are among the possible measurements: length,7,20,21 thickness,3–5,8,11–14,20–28 cross-sectional area2,9,15,20,29 and volume.7,20,21 In this systematic review, ultrasonography was evaluated as a tool used to measure masseter muscle dimensions in healthy patients. It is a simple technique that is inexpensive, with rapid diagnosis; is non-invasive and atraumatic; and does not use ionizing radiation.
With ultrasound, it is possible to obtain well-defined masticatory muscle images, especially for the masseter and anterior temporal muscles, in establishing thickness with high reproducibility and speed and without exposure to ionizing radiation. Thus, it makes it a suitable technique for evaluation of perioral muscles. The discrepancies observed in muscle measurements obtained in different studies are thought to be due to differences in sampling, diverse transducer location to capture images, ultrasound systems, varied transducers design and frequencies and the various techniques used by different workers.7 However, it is possible to measure accurately the thickness of the masseter muscle by ultrasound, following a specific protocol and avoiding excessive pressure to the transducer.3,12 In some studies, the transducer position is determined by palpation of muscles in relaxation and contraction.15 The correct position can be confirmed by observing the muscle image on the ultrasound device screen because images of the surface of the mandibular ramus are very clear. If the transducer is positioned obliquely, the image could artificially show an increase in muscle size.3
Studies consider measurements performed by ultrasound as a potential source of error, in comparison with measurements performed by MRI or CT. This fact may be explained, considering the difficulty to identify muscle edge and pressure applied on the transducer.5,30 This error caused by the pressure on the skin would presumably be higher for thickness measurements than for cross-sectional area estimation because the reduction in a dimension can be compensated by bulging in another dimension, with the muscle volume remaining constant. These authors suggest that the choice of ultrasound in muscle thickness evaluations is associated with lower costs, greater availability of equipment and fewer contraindications than for MRI or CT examinations.30
Methodologies for masseter muscle ultrasound thickness measurements in young adults have been described.3,4,6,13,20–22,24–26,28 The heterogeneity of the measures observed in the literature could be minimized with the subdivision of the masseter muscle into segments and the standardization of the number of measurements for a morphometric analysis. The use of multiple measurements at the same point3,4,12,15 or measures of more than one location3,4,15 represents a limitation in this study.
Unfortunately, in this systematic review no articles met all criteria for classification of high levels of evidence and criteria for evidence synthesis. This could justify the need for more research with larger samples and primarily determine a means for standardization to measure thickness of the masseter muscle using ultrasound or even CT and MRI. For more precise measures, there is a need for a standardization of measurement sites. It is also suggested that in future studies, not only thickness but also works that are concomitantly related to the dimensions and area of the masseter muscle, gender and age group should be considered. In addition, another aspect that should also be considered is that in most studies, the measurements of the masseter were evaluated in millimetres. It is believed that this is a unit of measurement that offers more precision.
Accordingly, the reduced muscle thickness measurements that are obtained can be due to the movement of the transducer, placed horizontally, from the middle level towards the most upper or lower levels which may reflect a gradual reduction in cross-sectional area as the muscle tapers towards its origin and insertion.12 Additionally, ultrasound evaluation of the masseter muscle performed by Close et al9 demonstrated that there is an important gender variation in muscle thickness and volume. Benington et al7 found the mean masseter muscle volume for males was 23.0 ± 7.1 cm3 and the mean volume for females was 11.3 ± 0.8 cm3.
Close et al9 reported that cross-sectional area values for males of 4.6 ± 1.0 cm2 is broadly consistent. Muscle thickness tends to be larger in males than in females. This observed difference may be partly the result of natural sexual dimorphism but probably has been heightened by differences in facial morphology between genders. Males tend to have short facial features, whereas females have long facial characteristics.3,7
A significant correlation has been shown to exist between gender, age and thickness of the masseter muscle. It has been reported to be greater in males and elderly individuals.12,14,26 According to Raadsheer et al,5 the thickness of the masseter muscle decreases significantly with age in both sexes. In this review, it was observed that similar results were described by Kiliaridis and Kälebo,3 Bakke et al,4 Close et al9 and Hernandez et al29 in relaxed and/or clenched positions.
Ultrasound examinations have been used for several decades. Several authors provide measurement of subcutaneous muscles and other anatomical structures in adult patients, using frequency of transducers from 5 to 13 MHz.3–5,8,15 Satiroğlu et al13 used a transducer of 7.5 MHz in their researches and Strini et al28 used that of 10 MHz. High-frequency probes produce a sharper image in the superficial tissues.8
Ultrasound measurement of the masseter muscle has been applied to diverse observations, for example, to correlate muscle thickness with the facial morphology and width of the dental arch.2–4,7,8,13,20,21,23,25,26 This technique has also been used for thickness and/or cross-sections to evaluate the relationship with temporomandibular joint dysfunction, muscle palpation pain, facial morphology, bite force and occlusal factors, specifically of the incisors and molars.4 However, owing to phenotypic variability of masseter muscle thickness between genders, ultrasonographic images might not detect these changes caused by temporomandibular dysfunction.3,9
Non-standardized methods and measurement error variation observed may possibly have had a confounding effect on masseter length reported in literature.3,5,12,15
In the published literature, reports about muscle actions in craniofacial growth are controversial. For Kubota et al,8 craniofacial morphology may be the result, not the cause, of variations in masticatory function, including muscle size and bite force, proposing that bone growth is influenced by muscle growth. Several studies have found this relationship by cephalometry and ultrasound in adults, determining linear and angular craniofacial variables on radiographs. It was observed that the greatest thickness of the masseter muscle is related to longer mandibular ramus,7,8 lower mandibular inclination and gonial angle less obtuse.4,7 Kubota et al8 found no statistically significant correlation between muscle thickness and anterior facial height.
Measurements performed with the contracted masseter muscle showed greater thickness than the relaxed muscle.3–5,8,15 Bakke et al4 observed in healthy adults that thickness of the masseter muscle in contraction was strongly correlated with the number of teeth in contact, i.e. the occlusal contacts were associated with the parameters related to the maximum muscular action.
Low reproducibility of measurements in the relaxed position when compared with contraction is because the masseter during relaxation is more susceptible to pressure caused by the transducer. Other factors can also interfere with the technique, such as variation in transducer orientation, anatomical marks used for guidance, patient position relative to the ultrasonographer and patient occlusal forces.3,5,12
Masseter muscle cross-sectional dimensions during relaxation ranged from 6.8 to 12.9 mm in the study by Bertram et al;15 these figures corroborate with the previous study by Emshoff et al.12 More accurate and reliable values were ascertained by other authors, showing thickness values results ranging from 10 to 13 mm.4,5
Several anatomical reference sites to obtain measurements of thickness of the masseter muscle are described in literature,3,13,22,24,28 such as the part of the masseter close to the level of the occlusal plane, a point halfway between the zygomatic arch and gonial angle, at the centre of the mediolateral distance of the ramus. Raadsheer et al5 measured between the ramus and superficial muscle surface, perpendicular to the underlying ramus. The reference point used by Kubota et al,8 Egwu et al14 and Rohila et al26 crossed the muscle in a line joining the lateral commissure of the mouth to the intertragic notch of the ear. Another site was reported from the anterior border of the muscle and the surface of the mandibular ramus at 2.5 cm above the inferior border of the mandible.27
Some authors20,21 conducted measurements of the thickness, length and volume of the masseter muscle. To measure the length of the masseter muscle, the authors used the distance between the zygomatic tubercule and the gonial angle.
To obtain the cross-sectional area, Bertram et al15 measured the middle section of the masseter muscle while Naser-ud-Din et al20 made a manual trace around the boundaries of the muscle with electronic tools.
Bertram et al15 evaluated the anterior portion of the masseter while Bakke et al4 examined the most prominent portion located above the base of the jaw, and Raadsheer et al5 performed measurements on the middle portion between the zygomatic arch and the lower border of the mandible.
According to Emshoff et al,12 the cross-sectional dimensions are not similar through the masseter muscle. Consequently, the anatomical and functional heterogeneities of the masseter muscle may also influence the spatial differences in muscle thickness observed. The same authors reported that the cross-sectional dimension assessment by ultrasound can be highly susceptible to factors related to the technique performed. Variables such as the pressure applied by the transducer on the muscle, transducer orientation and lack of standardization of points for muscle measurement may significantly vary measurement values achieved. These factors indicate the need for further studies to determine intra- and interobserver reliability, for consistency in cross-sectional dimension assessment for the masticatory muscles. Variations in thickness of the masseter muscle across different populations may also be associated with racial factors and the relative indulgence in masticatory activities that may have led to adaptive increase in size. It may also be associated with the orientation and size of the muscle fibres that may be influenced by genetic and environmental factors.14
Conclusions
Based on this systematic review, conclusions are that (a) the methods of measurement involved vary among authors, and there is no standardization of specific site for each measurement parameter (thickness, cross-sectional area, volume and length); there is unanimity among authors on the fact that ultrasound is a reliable tool; (b) as for reproducibility, authors considered the importance of reducing the errors when performing ultrasound; several studies evaluated masseter muscle dimensions and related it to cephalometric analysis to evaluate morphological variations.
Contributor Information
Ana Paula Reis Durão, Email: paula.o.reis@gmail.com.
Aline Morosolli, Email: aline.morosolli@pucrs.br.
Jackie Brown, Email: jackie.brown@kcl.ac.uk.
Reinhilde Jacobs, Email: reinhilde.jacobs@uzleuven.be.
References
- 1.Weijs WA, Hillen B. Correlations between the cross-sectional area of the jaw muscles and craniofacial size and shape. Am J Phys Anthropol 1986; 70: 423–31. doi: https://.doi.org/10.1002/ajpa.1330700403 [DOI] [PubMed] [Google Scholar]
- 2.Uchida Y, Motoyoshi M, Shigeeda T, Shinohara A, Igarashi Y, Sakaguchi M, et al. Relationship between masseter muscle size and maxillary morphology. Eur J Orthod 2011; 33: 654–9. doi: https://.doi.org/10.1093/ejo/cjq152 [DOI] [PubMed] [Google Scholar]
- 3.Kiliaridis S, Kälebo P. Masseter muscle thickness measured by ultrasonography and its relation to facial morphology. J Dent Res 1991; 70: 1262–5. doi: https://.doi.org/10.1177/00220345910700090601 [DOI] [PubMed] [Google Scholar]
- 4.Bakke M, Tuxen A, Vilmann P, Jensen BR, Vilmann A, Toft M. Ultrasound image of human masseter muscle related to bite force, electromyography, facial morphology, and occlusal factors. Scand J Dent Res 1992; 100: 164–71. doi: https://.doi.org/10.1111/j.1600-0722.1992.tb01734.x [DOI] [PubMed] [Google Scholar]
- 5.Raadsheer MC, van Eijden TM, van Spronsen PH, van Ginkel FC, Kiliaridis S, Prahl-Andersen B. A comparison of human masseter muscle thickness measured by ultrasonography and magnetic resonance imaging. Arch Oral Biol 1994; 39: 1079–84. doi: https://.doi.org/10.1016/0003-9969(94)90061-2 [DOI] [PubMed] [Google Scholar]
- 6.Weijs WA, Hillen B. Relationships between masticatory muscle cross-section and skull shape. J Dent Res 1984; 63: 1154–7. doi: https://.doi.org/10.1177/00220345840630091201 [DOI] [PubMed] [Google Scholar]
- 7.Benington PC, Gardener JE, Hunt NP. Masseter muscle volume measured using ultrasonography and its relationship with facial morphology. Eur J Orthod 1999; 21: 659–70. doi: https://.doi.org/10.1093/ejo/21.6.659 [DOI] [PubMed] [Google Scholar]
- 8.Kubota M, Nakano H, Sanjo I, Satoh K, Sanjo T, Kamegai T, et al. Maxillofacial morphology and masseter muscle thickness in adults. Eur J Orthod 1998; 20: 535–42. doi: https://.doi.org/10.1093/ejo/20.5.535 [DOI] [PubMed] [Google Scholar]
- 9.Close PJ, Stokes MJ, L'Estrange PR, Rowell J. Ultrasonography of masseter muscle size in normal young adults. J Oral Rehabil 1995; 22: 129–34. doi: https://.doi.org/10.1111/j.1365-2842.1995.tb00246.x [DOI] [PubMed] [Google Scholar]
- 10.Raadsheer MC, Van Eijden TM, Van Ginkel FC, Prahl-Andersen B. Human jaw muscle strength and size in relation to limb muscle strength and size. Eur J Oral Sci 2004; 112: 398–405. doi: https://.doi.org/10.1111/j.1600-0722.2004.00154.x [DOI] [PubMed] [Google Scholar]
- 11.Jonasson G, Kiliaridis S. The association between the masseter muscle, the mandibular alveolar bone mass and thickness in dentate women. Arch Oral Biol 2004; 49: 1001–6. doi: https://.doi.org/10.1016/j.archoralbio.2004.07.005 [DOI] [PubMed] [Google Scholar]
- 12.Emshoff R, Emshoff I, Rudisch A, Bertram S. Reliability and temporal variation of masseter muscle thickness measurements utilizing ultrasonography. J Oral Rehabil 2003; 30: 1168–72. [DOI] [PubMed] [Google Scholar]
- 13.Satiroğlu F, Arun T, Işik F. Comparative data on facial morphology and muscle thickness using ultrasonography. Eur J Orthod 2005; 27: 562–7. [DOI] [PubMed] [Google Scholar]
- 14.Egwu OA, Njoku CO, Ewunonu EO, Ukoha U, Eteudo AN, Mgbachi CE. Assessment of masseter muscle thickness in an adult Nigerian population: an ultrasound based study. Int J Biomed Res 2012; 3: 143–6. [Google Scholar]
- 15.Bertram S, Brandlmaier I, Rudisch A, Bodner G, Emshoff R. Cross-sectional characteristics of the masseter muscle: an ultrasonographic study. Int J Oral Maxillofac Surg 2003; 32: 64–8. doi: https://.doi.org/10.1054/ijom.2002.0259 [DOI] [PubMed] [Google Scholar]
- 16.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003; 3: 25–37. doi: https://.doi.org/10.1186/1471-2288-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. CBEM-Centre for evidence-based medicine. Critical Appraisal Worksheet Diagnosis. Available from: http://www.cebm.net/critical-appraisal/
- 18.Jaeschke R, Guyatt GH, Sackett DL. Users' guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. JAMA 1994; 271: 703–7. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009; 62: 1006–12. doi: https://.doi.org/10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 20.Naser-Ud-Din S, Sampson WJ, Dreyer CW, Thoirs K. Ultrasound measurements of the masseter muscle as predictors of cephalometric indices in orthodontics: a pilot study. Ultrasound Med Biol 2010; 36: 1412–21. doi: https://.doi.org/10.1016/j.ultrasmedbio.2010.05.019 [DOI] [PubMed] [Google Scholar]
- 21.Naser-Ud-Din S, Thoirs K, Sampson WJ. Ultrasonography, lateral cephalometry and 3D imaging of the human masseter muscle. Orthod Craniofac Res 2011; 14: 33–43. doi: https://.doi.org/10.1111/j.1601-6343.2010.01505.x [DOI] [PubMed] [Google Scholar]
- 22.Georgiakaki I, Tortopidis D, Garefis P, Kiliaridis S. Ultrasonographic thickness and electromyographic activity of masseter muscle of human females. J Oral Rehabil 2007; 34: 121–8. doi: https://.doi.org/10.1111/j.1365-2842.2006.01677.x [DOI] [PubMed] [Google Scholar]
- 23.Ngom PI, Ly BA, Diagne F, Diouf JS, Chakib O, Hennequin M. Masseter muscle thickness in relation to craniofacial morphology. Int Orthod 2008; 6: 251–67. doi: https://.doi.org/10.1016/s1761-7227(08)75162-3 [Google Scholar]
- 24.Mangilli LD, Sassi FC, Sernik RA, Tanaka C, de Andrade CR. Electromyographic and ultrasonographic assessment of the masseter muscle in normal individuals: a pilot study. Pro Fono 2009; 21: 261–4. doi: https://.doi.org/10.1590/s0104-56872009000300014 [DOI] [PubMed] [Google Scholar]
- 25.Rani S, Ravi MS. Masseter muscle thickness in different skeletal morphology: an ultrasonographic study. Indian J Dent Res 2010; 21: 402–7. [DOI] [PubMed] [Google Scholar]
- 26.Rohila AK, Sharma VP, Shrivastav PK, Nagar A, Singh GP. An ultrasonographic evaluation of masseter muscle thickness in different dentofacial patterns. Indian J Dent Res 2012; 23: 726–31. doi: https://.doi.org/10.4103/0970-9290.111247 [DOI] [PubMed] [Google Scholar]
- 27.Liao LJ, Lo WC. High-resolution sonographic measurement of normal temporomandibular joint and masseter muscle. J Med Ultrasound 2012; 20: 96–100. doi: https://.doi.org/10.1016/j.jmu.2012.04.003 [Google Scholar]
- 28.Strini PJ, Strini PJ, Barbosa Tde S, Gavião MB. Assessment of thickness and function of masticatory and cervical muscles in adults with and without temporomandibular disorders. Arch Oral Biol 2013; 58: 1100–8. doi: https://.doi.org/10.1016/j.archoralbio.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 29.Hernandez CA, Frugone ZR, Valenzuela PH, Retamal VV. Masseter muscle deep measured by ultrasound per facial index related to sex. Int J Morphol 2012; 30: 964–9. [Google Scholar]
- 30.Dupont AC, Sauerbrei EE, Fenton PV, Shragge PC, Loeb GE, Richmond FJ. Real-time sonography to estimate muscle thickness: comparison with MRI and CT. J Clin Ultrasound 2001; 29: 230–6. doi: https://.doi.org/10.1002/jcu.1025 [DOI] [PubMed] [Google Scholar]