Abstract
Previous attempts to identify a unified theory of brain serotonin function have largely failed to achieve consensus. In this present synthesis, we integrate previous perspectives with new and older data to create a novel bipartite model centred on the view that serotonin neurotransmission enhances two distinct adaptive responses to adversity, mediated in large part by its two most prevalent and researched brain receptors: the 5-HT1A and 5-HT2A receptors. We propose that passive coping (i.e. tolerating a source of stress) is mediated by postsynaptic 5-HT1AR signalling and characterised by stress moderation. Conversely, we argue that active coping (i.e. actively addressing a source of stress) is mediated by 5-HT2AR signalling and characterised by enhanced plasticity (defined as capacity for change). We propose that 5-HT1AR-mediated stress moderation may be the brain’s default response to adversity but that an improved ability to change one’s situation and/or relationship to it via 5-HT2AR-mediated plasticity may also be important – and increasingly so as the level of adversity reaches a critical point. We propose that the 5-HT1AR pathway is enhanced by conventional 5-HT reuptake blocking antidepressants such as the selective serotonin reuptake inhibitors (SSRIs), whereas the 5-HT2AR pathway is enhanced by 5-HT2AR-agonist psychedelics. This bipartite model purports to explain how different drugs (SSRIs and psychedelics) that modulate the serotonergic system in different ways, can achieve complementary adaptive and potentially therapeutic outcomes.
Keywords: Depression, serotonin, psychedelics
Introduction
Overview
The aim of this paper is to discuss the function of brain serotonin (5-HT) transmission by focusing on two of its major receptor subtypes, the 5-HT1AR and 5-HT2AR. Our selective focus on these receptors is justified by their dense and widespread expression in the human brain (Beliveau et al., 2016), diametrically opposite functional effects (Araneda and Andrade, 1991) and extensive evidence implicating both in psychiatric disorders and their treatment (Chattopadhyay, 2007). We believe that a fuller understanding of the function of 5-HT1A and particularly, 5-HT2A receptor signalling motivates a revision of current thinking on a well-known problem in neuropsychopharmacology, namely: what principal function is served by brain serotonin transmission? Broadly consistent with prior theories (Deakin, 2013), we maintain that a key function of brain 5-HT is to moderate anxiety and stress, and promote patience and coping (Miyazaki et al., 2012) via (postsynaptic) 5-HT1AR signalling. Crucially however, we also extend on this by proposing that a second major function of brain 5-HT is to open a window of plasticity for greater adaptation (Branchi, 2011), mediated in large part by 5-HT2AR signalling. This bipartite model is consistent with a ‘flexible coping’ model of brain serotonin function, in which postsynaptic 5-HT1ARs mediate so-called ‘passive coping’ (i.e. tolerating but not necessarily dealing with a source of psychological pain) and 5-HT2ARs mediate ‘active coping’ (actively dealing with a source of psychological pain by changing one’s relationship to it) (Puglisi-Allegra and Andolina, 2015). Note: we use the term ‘plasticity’ in a broad sense throughout this paper to refer to the capacity for change and we address our intentional neglect of the other serotonin receptors in the discussion section as well as immediately below.
The charge that our neglect of the functioning of the full range of serotonin receptors means that the present paper cannot be considered a fully comprehensive model of brain serotonin function is one we accept. However, we propose that the functioning of signalling at other serotonin receptors (than 1A and 2A) may, in several cases, be comfortably incorporated into either (or both) arms of the bipartite model we introduce below – and we encourage attempts to do this. A final introductory caveat is that signalling at serotonin receptors can have more than one function, depending on such factors as: basal serotonin efflux and related synaptic concentrations, the specific localisation of the relevant receptor subtype (e.g. whether they are pre- or postsynaptic), the temporal development or time course of a specific pharmacological manipulation, and the animal’s present behavioural state (e.g. see Mitchell, 2005 for a relevant review). As much as is possible, we have endeavoured to acknowledge such inherent complexities in the serotonin system – particularly when we feel they are critical for a proper comprehension of the relevant phenomenon – but this has had to be balanced against considerations of parsimony and focus – in any already extensive narrative review.
With these caveats entered, let us return to the main focus of this paper: brain serotonin functioning – as seen through postsynaptic 5-HT1A and 5-HT2A receptor signalling. The 5-HT1AR is highly expressed in brain regions involved in regulating stress and emotion and 5-HT has an especially high affinity for its 1A receptor (Peroutka and Snyder, 1979). We suggest that the 5-HT1AR and its associated functions dominate 5-HT transmission under normal conditions but that 5-HT2AR signalling also serves a role that becomes increasingly important during extreme states when 5-HT release is elevated. We propose that 5-HT mediates stress moderation and plasticity-mediated adaptability in response to different levels of stress and adversity, via its postsynaptic 1A and 2A receptors respectively. We acknowledge that agonism at other 5-HT receptors has also been linked with neurotrophic factors and other molecular markers of neuroplasticity (Kraus et al., 2017); however, our focus here is on the remarkable psychological and functional plasticity associated with the acute ‘psychedelic’ state – as produced by psychedelic drugs such as LSD and psilocybin (Carhart-Harris et al., 2016c) – and the enduring changes that appear to follow from exposure to these drugs’ effects (e.g. MacLean et al., 2011). We also propose that combined signalling at the 5-HT1A and 2A receptors has a generally complementary influence on mood, facilitating stress relief (5-HT1AR-mediated) but also a flexibility of mind (5-HT2AR-mediated) that under favourable conditions (Alboni et al., 2017; Branchi, 2011; Chiarotti et al., 2017; Hartogsohn, 2016), is conducive to positive mood (Hirt et al., 2008; Schmid et al., 2015). In what follows, we present evidence supporting these hypotheses and discuss their clinical significance.
The function of brain serotonin is an enigma
There have been several attempts to identify a unifying function of dopaminergic transmission in the brain (Berridge and Robinson, 1998; Schultz, 2010; Schwartenbeck et al., 2014) and similar attempts have been made for serotonin (Andrews et al., 2015; Azmitia, 2007; Branchi, 2011; Dayan and Huys, 2009; Deakin, 1998). Most researchers acknowledge that the function of the 5-HT system remains ‘elusive’ (Dayan and Huys, 2009) and ‘a puzzle’ (Cools et al., 2008; Dayan and Huys, 2015; Seymour et al., 2012) and it is argued here that this may be due to the special diversity and complexity of the serotonin system with its many receptor subtypes (Hoyer et al., 1994), extensive innervation of the brain and paracrine style of transmission (Hornung, 2003; Jennings, 2013). The notion that 5-HT is an enigma among neuromodulators (said to be ‘involved in everything but responsible for nothing’ (Muller and Homberg, 2015)) is relevant here, and it is argued that the riddle of 5-HT can only be solved by focusing on its individual receptor subtypes.
Accordingly, given the inherent complexity of the serotonin system, one strategy for understanding its functioning is to focus on a select number of receptor subtypes that have been particularly well characterised. From this foundation, one might then consider whether other serotonin receptor subtypes can be incorporated into the associated model, or whether one or more additional models are required to cover the full range of functions associated with brain serotonin transmission. Following this approach, we have chosen to concentrate on the 5-HT1A and 5-HT2A receptors. Our reasons for doing this are (at least) three-fold, and include: (1) the prevalence of their expression in the human brain and specific localisation – e.g. in stress circuitry (5-HT1AR) and high-level cortex (5-HT2AR) (e.g. Beliveau et al., 2016); (2) compelling evidence for their involvement in the pharmacology of different psychiatric disorders and medications (Celada et al., 2004); and (3) their apparent functional pre-eminence and opposition – as has been noted by others (Azmitia, 2001). Following on from this last point, the 5-HT1A and 5-HT2A receptors show diametrically opposite responses to their endogenous ligand, with 5-HT1A receptor signalling being inhibitory and 5-HT2A receptor signalling being excitatory (Araneda and Andrade, 1991; Azmitia, 2001; Charig et al., 1986; Fletcher et al., 2007). This stark functional opposition is intriguing – and motivates us to ask why this should be the case, and what purpose it serves? We suggest that inherent diversity within the serotonergic system relates to its capacity for flexibly and adaptably responding to different degrees of adversity and challenge in the organism’s environment, with distinct responses mediated by distinct serotonergic pathways.
As noted above, an obvious caveat here is that 5-HT receptors we do not specifically focus on in the present review may complement one or the other of these two pathways – and may also modulate unrelated physiological and behavioural functions. For example, signalling at 5-HT receptors other than the 2A receptor has been associated with neuroplasticity (Kraus et al., 2017) – and thus, may also feed into pathway 2 (below). Similarly blockade of certain 5-HT receptors (e.g. 5-HT2C, 5-HT7 and even 5-HT2A) may complement pathway 1 (below). However, a thorough coverage of this matter is beyond the scope of this article.
In what follows, focus is directed to 5-HT1A and 5-HT2A receptor signalling and research pertaining to their associated functions. It is argued that studying potent serotonergic compounds such as rapid-acting, highly effective 5-HT releasers (such as 3,4-methylenedioxymethamphetanine, MDMA (Baumann et al., 2008; Heifets and Malenka, 2016)) and direct 5-HT2AR agonist psychedelic drugs such as psilocybin and lysergic acid diethylamide, LSD (Glennon et al., 1984; Vollenweider et al., 1998), can be particularly informative about the function of serotonergic transmission in the brain because their acute and longer-term effects are especially marked and novel (Griffiths et al., 2008; Mithoefer et al., 2013), and there is a growing literature on human research with such drugs, including an increasing number of neuroimaging studies (Carhart-Harris et al., 2013b, 2015b; Muthukumaraswamy et al., 2013) and clinical trials (Bogenschutz et al., 2015; Carhart-Harris et al., 2016a; Gasser et al., 2014; Griffiths et al., 2016; Grob et al., 2011; Mithoefer et al., 2011; Ross et al., 2016; Sanches et al., 2016) – see Carhart-Harris and Goodwin (2017) for a review.
Note: we acknowledge that MDMA also releases dopamine (DA) and noradrenaline (NA) (Baumann et al., 2008) but its 5-HT releasing properties are many times greater than its catecholamine releasing properties, e.g. 5-HT release in the frontal cortex is approximately 5 times that of DA release (Golembiowska et al., 2016), preferential 5-HT versus DA and NA release is unusual for an amphetamine, and MDMA’s subjective effects are also distinct from those of other more conventional amphetamines (Bedi et al., 2014).
Serotonin receptor subtypes
What is the 5-HT2AR and where is it expressed?
The 5-HT2AR is one of at least 14 different 5-HT receptor subtypes expressed in the mammalian brain (Glennon, 2000), and like almost all of these, it is a G protein-coupled receptor (GPCR). In the context of neurotransmission, the principal effect of 5-HT binding to the 5-HT2AR is to increase the excitability of the host neuron, and the 5-HT2AR is the main excitatory GPCR of the serotonin receptor family (Andrade, 2011).
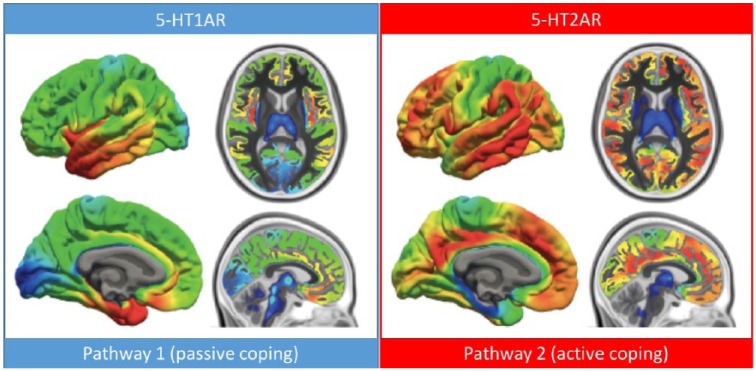
The 5-HT2AR is predominantly a cortical receptor; indeed, it is the most abundant 5-HT receptor in the cortex (Varnas et al., 2004). In humans, the density of 5-HT2AR expression is relatively high throughout the cortex and especially so in high-level associative cortex – such as regions belonging to the so-called default-mode network (see Figure 1) (Beliveau et al., 2016). 5-HT2AR expression is considerably higher in the cortex than in subcortical structures such as the thalamus, basal ganglia, and hippocampus (Gross-Isseroff et al., 1990; Hall et al., 2000) – with minimal/negligible expression in the cerebellum and brainstem (Hall et al., 2000). The predominantly cortical expression of the 5-HT2AR places it at a high evolutionary and hierarchical level and as we will discuss later (e.g. Section 4.4), this is likely to have important functional implications.
Figure 1.
Regional distribution of serotonin 1A (left) and 2A receptors (right) in healthy volunteers as measured using PET imaging and radioligands selective for the 5-HT 1A and 2A receptors. Pathway 1 refers to the ‘passive coping’ pathway hypothesised to be mediated by 5-HT1AR signalling and concerned with passive endurance, and ‘pathway 2’ refers to the ‘active coping’ pathway hypothesised to be mediated by 5-HT2AR signalling and concerned with an active change in outlook and/or behaviour. Images reproduced from (Beliveau et al., 2016) with permission. Note: The dense expression of the 5-HT1AR in medial temporal lobe regions and particularly the hippocampus is not clearly evident in the relevant maps shown here (left) but can be seen in values presented in the paper itself, as well as others (Pazos and Palacios, 1985; Pazos et al., 1987).
In terms of its cellular and laminar localisation, 5-HT2A receptors are most densely expressed on the dendrites of excitatory glutamatergic pyramidal neurons, particular in layer V of the cortex (Weber and Andrade, 2010). One study found that almost all glutamatergic neurons in layers II-V of the monkey and human prefrontal cortex (PFC) expressed 5-HT2ARs, whereas only about 30% of GABAergic interneurons within the same layers exhibited 5-HT2AR expression (de Almeida and Mengod, 2007). Thus, cortical pyramidal neurons are likely to be especially sensitive to modulation via 5-HT activating 5-HT2ARs, and furthermore, the laminar localisation of 5-HT2ARs (e.g. in layer V of the cortex) corresponds well with the localisation of axon terminals of serotonergic neurons, particularly in the cortex (Blue et al., 1988). These data imply that cortical 5-HT2ARs should be sensitive to changes in synaptic serotonin concentrations (Tyacke and Nutt, 2015). A well-demonstrated effect of (prefrontal) cortical 5-HT2AR signalling is the initiation of a negative feedback mechanism which inhibits the firing of serotonergic neurons in the dorsal raphe nucleus (Boothman et al., 2003; Quesseveur et al., 2013), suggesting that the 5-HT2AR plays a crucial role in regulating the release of serotonin in the cortex, via a top-down modulatory influence on a cortical-raphe inhibitory feedback circuit (Sharp et al., 2007; Vazquez-Borsetti et al., 2009).
What is the 5-HT1AR and where is it expressed?
Identified in the early 1980s as a distinct 5-HT receptor subtype (Pedigo et al., 1981), the 5-HT1AR is densely expressed in midbrain, limbic and cortical regions (Varnas et al., 2004). 5-HT1AR agonism causes host-cell hyperpolarisation and an inhibition of firing via G protein-mediated mechanisms (Oleskevich et al., 2005). The 5-HT1AR is highly expressed on serotonergic neurons in the dorsal and median raphe nuclei where it functions as a presynaptic autoreceptor – exerting a strong homeostatic control over 5-HT neuron firing rates and thus, 5-HT efflux in the forebrain (Lanfumey and Hamon, 2000). The majority of 5-HT1A receptors are expressed postsynaptically in many brain regions, particularly the limbic system (especially the hippocampus) and cortex (Pazos et al., 1987; Varnas et al., 2004) see Figure 1. Presynaptic 5-HT1ARs readily desensitise following exposure to increased 5-HT availability (e.g. through chronic selective serotonin reuptake inhibitors (SSRIs)) but postsynaptic 5-HT1ARs do not (Lanfumey and Hamon, 2000), although they do appear to downregulate in response to stress (Berton et al., 1998; Lopez et al., 1999) – and perhaps relatedly, to electroconvulsive shock (Burnet et al., 1955, 1999). In summary, based on its high density of expression, localisation to regions densely innervated by serotonergic projections (such as the hippocampus) and high affinity for its endogenous ligand, the postsynaptic 5-HT1AR is serotonin’s principal inhibitory receptor in the brain.
Serotonin 2A versus 1A receptor signalling
At a basic level, the principal effect of 5-HT2AR activation is to increase the excitability of the host neuron (Andrade, 2011). If the host neuron is excitatory (e.g. a pyramidal neuron), the outcome of 5-HT2AR stimulation may be to increase its firing and the firing of those cells that it projects to. If the host cell is inhibitory (e.g. a GABAergic interneuron), the net result of 5-HT2AR stimulation will be to increase its firing and so enhance its inhibitory influence onto the neurons to which it projects (Andrade, 2011). Given that 5-HT2ARs are expressed mostly on excitatory neurons (at least in the cortex – where their expression is highest) one might expect release of endogenous 5-HT in the cortex to elicit a mostly excitatory effect but this is not what is typically observed (Hajos et al., 2003; Jacobs and Azmitia, 1992; Puig et al., 2005). For example, in vivo studies investigating the effect of dorsal raphe nucleus stimulation (inducing an increase in cortical 5-HT efflux) on cellular activity in the medial PFC (mPFC) have observed a decrease in the firing rate of the majority of pyramidal cells recorded (Hajos et al., 2003; Puig et al., 2005). Importantly, this effect appears to be modulated via postsynaptic 5-HT1ARs, since it could be prevented by a selective 5-HT1AR antagonist (Hajos et al., 2003; Puig et al., 2005). Consistently, chronic dorsal raphe stimulation was found to decrease metabolism in limbic regions, alongside decreases in depressive behaviours, presumably via inhibitory postsynaptic 5-HT1ARs (Urban et al., 2016).
It is a well-replicated finding that postsynaptic 5-HT1AR and 5-HT2AR activation produces opposite effects on single cell activity, with 5-HT1AR signalling having a hyperpolarising (inhibitory) effect, and 5-HT2AR activation causing a depolarising (excitatory) effect (Andrade, 2011; Araneda and Andrade, 1991). Up to 80% of pyramidal neurons in the PFC co-express 5-HT1A and 5-HT2A receptors (Amargos-Bosch et al., 2004). Studies in the 1970s and 80s suggested that 5-HT has an appreciably higher affinity for its 1A than 2A receptor (Hoyer et al., 1985; Peroutka and Snyder, 1979) but further research with 5-HT2AR agonist ligands suggest that, like other neuromodulator receptors (Skinbjerg et al., 2012) the 5-HT2A receptor can exist in a low (G-protein uncoupled) or high affinity (G-protein coupled) state – and when in their high-affinity state, 5-HT has a higher affinity for its 5-HT2AR than previously appreciated (Sleight et al., 1996). Under normal conditions, 5-HT1AR signalling seems to dominate serotoninergic functioning in cortical as well as limbic regions (Puig et al., 2005). However, as we will discuss later (e.g. Section 4), the 5-HT2A receptor is still likely to be functionally relevant, and we predict, increasingly so during states of exceptionally high adversity (Amargos-Bosch et al., 2004; Puig et al., 2005). In this context, the possibility that high-affinity 5-HT2ARs upregulate (Benekareddy et al., 2010; Berton et al., 1998) and 5-HT1ARs downregulate during extreme adversity (Berton et al., 1998; Lopez et al., 1999) is an intriguing one, which seems deserving of further study.
The opposite effect of electroconvulsive shock on 5-HT1A and 5-HT2A receptor functioning in rats may be relevant here, with (hippocampal but not the dentate gyrus) 5-HT1AR expression appearing to decrease post ECS while 5-HT2AR functioning increases (Burnet et al., 1995, 1999). Conversely however, ECS/electroconvulsive therapy appears to downregulate 5-HT2AR binding in primates (Strome et al., 2005) and humans (Yathamet al., 2010) – an effect that is more consistent with that of conventional antidepressant medications (Yatham et al., 1999) as well as direct 5-HT2AR agonism (Buckholtz et al., 1990) – while also being the logical consequence of acutely enhanced 5-HT release with ECS/ECT (Zis et al., 1992).
Psychological functions associated with brain 5-HT
Impulsivity and aggression
One of the most reliable behavioural effects of reducing 5-HT transmission in the brain is to increase impulsive and aggressive behaviours (Audero et al., 2013; Brown et al., 1979; Duke et al., 2013; Mosienko et al., 2015; Soubrie, 1986). Indeed, some of the earliest hypotheses on the function of 5-HT in the brain proposed that it serves to suppress behavioural response to pain (Harvey et al., 1975), anxiety (Wise et al., 1970) and aversive stimuli more generally (Deakin and Graeff, 1991; Soubrie, 1986) and these ideas continue to have traction (Deakin, 2013; Yanowitch and Coccaro, 2011). The anti-aggression effects of 5-HT enhancing compounds led to them being called ‘serenics’ (Olivier and Moss, 1990), a fitting term in our view, and one that is also apt in relation to the subjective effects of MDMA, a particularly potent 5-HT releaser. Related to these hypotheses, is the notion that 5-HT transmission enables a person to better tolerate delay (Soubrie, 1986), and the patience-promoting properties of 5-HT have recently received significant experimental support (Fonseca et al., 2015; McDannald, 2015; Miyazaki et al., 2012, 2014; Ranade et al., 2014). Low concentrations of the serotonin metabolite (5-HIAA), implying low central 5-HT function, have been associated with impulsivity (Fairbanks et al., 2001), aggression (Brown and Linnoila, 1990) and suicidal behaviour (Asberg et al., 1976), and tryptophan depletion (a diet-based approach that produces a transient depletion of central 5-HT) has also been found to enhance impulsivity and aggression (Dougherty et al., 1999, 2010). In contrast, tryptophan supplementation (Duke et al., 2013), acute MDMA administration (Ramaekers and Kuypers, 2006; van Wel et al., 2012), acute fenfluramine (Cherek and Lane, 2001) and chronic 5-HT reuptake inhibitor administration (Butler et al., 2010; Wolff and Leander, 2002), all of which are known to increase central 5-HT function, have all been found to reduce impulsivity and aggression. For a more in-depth discussion of the complexities of the relationship between brain 5-HT and aggression, including some contradictory findings to the rule that low synaptic 5-HT is associated with increased aggression, see this review (Mitchell, 2005).
5-HT1AR signalling, impulsivity and aggression
There are solid grounds to believe that the anti-aggression and impulsivity effects of 5-HT are mediated by postsynaptic 5-HT1A receptor signalling (Sanchez and Hyttel, 1994; Schreiber and De Vry, 1993), with some contribution from postsynaptic 5-HT1B receptors (Ramboz et al., 1996; Sijbesma et al., 1991). Assessing the functional effects of 5-HT1A receptor manipulations is complicated, however, owing to the opposing influences of pre- and postsynaptic 1A receptor activation. Prior to a time-dependent 5-HT1A autoreceptor desensitisation by reuptake blockers (Le Poul et al., 1995), stimulation of these presynaptic 5-HT1A receptors reduces serotonin efflux, whereas postsynaptic 5-HT1A receptor activation is an important (and often clinically desirable) consequence of increased serotonin efflux (Artigas, 2013b). Moreover, selective 5-HT1AR antagonists or full 5-HT1AR agonists are not available for human use (beyond the very low doses used in PET imaging), and so cannot be used to incisively inform on this matter. With these caveats, it can be relatively safely inferred that (postsynaptic) 5-HT1AR agonism appears to reduce aggressive and impulsive behaviours (de Boer and Koolhaas, 2005; Olivier et al., 1989; Popova et al., 2007; Sanchez and Hyttel, 1994; White et al., 1991; Wolff and Leander, 2002). Note, however, that many 5-HT1A receptor agonists are in fact, only partial agonists; thus, their impact on net 5-HT1AR signalling is dependent on basal 5-HT efflux and competition with the full agonist endogenous ligand, 5-HT itself (Mitchell, 2005).
It has been claimed that the 5-HT1AR is the most prevalent and well-distributed 5-HT receptor in the brain (Paterson et al., 2013; Varnas et al., 2004). Serotonin has a high affinity for this receptor subtype (Peroutka and Snyder, 1979), serotonergic projections densely innervate 5-HT1AR-rich regions (Hornung, 2003) and 5-HT concentrations may be higher in 5-HT1AR-rich subcortical/limbic regions than in the 5-HT2AR-rich cortex during basal conditions (Bose et al., 2011; Erritzoe et al., 2010; Kirby et al., 1995; Rueter and Jacobs, 1996), although see Adell et al. (1991) and Hjorth and Sharp (1991). These factors imply that manipulation of synaptic 5-HT concentrations will significantly impact on postsynaptic 5-HT1AR signalling and limbic functioning. With this in mind, it is telling that 5-HT lesions and depletion both tend to promote impulsivity and aggression (Audero et al., 2013; Dougherty et al., 1999), whereas stimulating serotonin function tends to reduce these behaviours (Miyazaki et al., 2014). It is also relevant that the potent 5-HT releaser, MDMA, has marked pro-social, pro-empathy, anti-aggressive effects during the acute phase (Bedi et al., 2010, 2014; Frye et al., 2014; Hysek et al., 2012, 2014a; Kamboj et al., 2015; Kirilly et al., 2006; Schmid et al., 2014; Stewart et al., 2014), perhaps via an inhibitory action on activity in limbic regions (Carhart-Harris et al., 2015b), and some of these effects in rodents’ can be attenuated by pre-treatment with a 5-HT1A receptor antagonist (Hunt et al., 2011).
Table 1 summarises findings that support various associations between 5-HT, signalling at its post-synaptic 5-HT1A and 5-HT2A receptors and relevant psychological phenomena. A number of these associations require qualification, e.g. 5-HT2AR agonism can have opposite acute and longer-term effects (Carhart-Harris et al., 2016c). To account for this, we use the acronyms ‘ST’ and ‘LT’ for acute (short-term) and long-term outcomes respectively, where we feel disambiguation is required. Also, receptor signalling may increase plasticity in one region but decrease it in the other (e.g. Vaidya et al., 1997). As this matter is most relevant in relation to molecular markers of plasticity in the hippocampus and cortex, we use the acronyms ‘hip’ and ‘cx’ to provide the necessary disambiguation. Regarding plasticity, we use ‘general plasticity’ (gP) to refer simply to an increased capability for change and ‘regional plasticity’ (rP) when we are specifically referring regional changes in molecular markers of plasticity such as trophic factors. It is important to stress that the effects of 5-HT2AR agonism are highly context sensitive (see Figure 2), e.g. the effects of 5-HT2AR signalling on mood and mental health are likely highly sensitive to the quality of the environment in which a 5-HT2AR-mediated experience occurs (Johnson et al., 2008), and this rule may also apply for treatment with an SSRI (Branchi, 2011) perhaps due to increased 5-HT2A receptor signalling through increased synaptic 5-HT. For this reason, and due to the still developing evidence base for psychedelics for depression (e.g. see Carhart-Harris and Goodwin, 2017), we took the modest step of not describing the association between 5-HT2AR signalling and depression as ‘strong’ (+++). In fact, we describe all associations between 5-HT, mood and depression as resting on ‘reasonable’ (i.e. ++) evidence because we acknowledge that these associations are especially complex. Also, some aspects of cognition but not others may be enhanced by increased signalling at a specific receptor and this is not qualified in the table. The reader may therefore notice some contradictory associations, simply because the data are not straightforward in supporting one particular direction. Importantly, this table is not intended as an exhaustive nor comprehensive account of literature pertaining to brain serotonin function but rather as an overview of significant associations between 5-HT, its 1A and 2A receptors and specific psychological phenomena of interest. This table cannot be considered a substitute for a detailed reading of the surrounding text. To properly understand the relevant associations, a careful reading of the text and supporting references is encouraged. Key: to provide a qualitative index of the perceived strength of evidence for a given association, we use the symbols +, ++ and +++ to denote ‘weak’, ‘reasonable’ and ‘strong’ evidence. Moreover, strong associations are shown in bold font. The ‘↑’ symbol denotes an increase in a particular factor and ‘↓’ denotes a decrease. The ‘→’ symbol denotes that one factor causes another.
Table 1.
Functions associated with brain serotonin.
| 5-HT implicated | Post-synaptic (pst) 5-HT1AR signalling (sg) implicated | 5-HT2AR signalling (sg) implicated | |
|---|---|---|---|
| Impulsivity and aggression (I&A) | 5-HT ↓ → I&A ↑ +++ | pst5-HT1ARsg↑ → I&AG↓ +++ | 5-HT2ARsg↑ → I&A ↑(ST) ++ 5-HT2ARsg↑ → I&A ↓ (LT) ++ |
| Anxiety and stress (A&S) and punishment (Pun) |
5-HT ↓ → A&S ↑ +++
Pun ↑ → 5-HT ↑ → +++ |
pst5-HT1ARsg↑ → A&S ↓ +++ | 5-HT2ARsg↑ → A&S ↑(ST) ++ 5-HT2ARsg↑ → A&S ↓(LT) ++ |
| Learning and cognition (L&C) | 5-HT ↓ → L&C ↓ ++ | pst5-HT1ARsg↑ → L&C ↓ ++ pst5-HT1ARsg↑ → L&C ↑ + |
5-HT2ARsg↑ → L&C ↑(ST) + 5-HT2ARsg↑ → L&C ↓(ST) ++ 5-HT2ARsg↑ → L&C ↑(LT) +++ |
| Depression (D) and mood* | 5-HT ↓ → mood ↓ ++ 5-HT ↑ → mood ↑ ++ |
pst5-HT1ARsg↑ → D ↓ ++ | 5-HT2ARsg↑ → D ↓(LT) ++ |
| General plasticity (gP) and regional specific plasticity (rP) | 5-HT ↑ → gP ↑ +++ | pst5-HT1ARsg↑ → GP ↑(hip) ++ | 5-HT2ARsg↑ → rP ↑(LT, cx) ++ 5-HT2ARsg↑ → rP ↓(LT, hip) ++ 5-HT2ARsg↑ → gP ↑(ST & LT) +++ |
Figure 2.
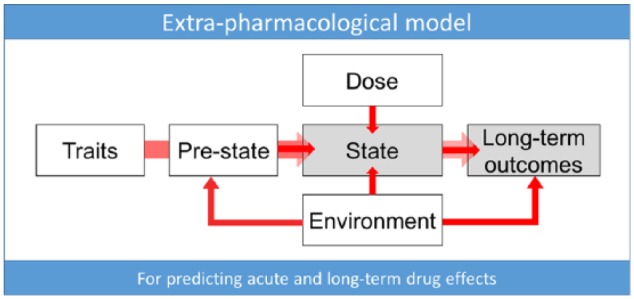
Extra-pharmacological (EP) model of drug action. This model is intended to provide a comprehensive account of the action of psychoactive drugs that takes into account important extra-pharmacological components such as trait, pre-state, dosage and environmental factors and how these interact with a given drug’s specific pharmacology to predict the quality of the acute ‘intoxicated’ or ‘medicated’ state and subsequent longer-term outcomes. The model is conceived with acute dosing in mind; however, it could also be adapted and applied to chronic dosing regimens.
5-HT2AR signalling, impulsivity and aggression
In contrast to what is typically associated with postsynaptic 5-HT1AR agonism, there is some evidence in rodents that 5-HT2AR agonism increases impulsivity (Anastasio et al., 2015; Carli et al., 2006; Winstanley et al., 2004). However, the relationship between the 5-HT2AR and impulsivity and aggression in humans is somewhat ambiguous (da Cunha-Bang et al., 2013; van Wel et al., 2012) and anti-impulsivity effects of 5-HT2AR antagonists may be an epiphenomenon of these compounds’ mild sleep-promoting/sedating properties (Ivgy-May et al., 2015; Morairty et al., 2008). Moreover, 5-HT2AR agonist psychedelics such as LSD and psilocybin are not typically associated with aggressive or impulsive behaviours in humans, and may even possess some pro-social properties in certain contexts (Dolder et al., 2016; Kraehenmann et al., 2016; Preller et al., 2016) – see also (Watts et al., 2017). Rare cases of behavioural disinhibition and even aggression have been observed with high doses of potent psychedelic 5-HT2AR agonists – but such incidences are likely to be strongly context specific (Gee et al., 2016). See Figure 2.
Anxiety and stress
5-HT1AR signalling, anxiety and stress
Related to the hypothesis that 5-HT functions to moderate aversive mental states (Deakin and Graeff, 1991) and promote patience (McDannald, 2015) is the notion that 5-HT plays an important role in negatively modulating anxiety (Piszczek et al., 2015). Selective reductions of 5-HT in the forebrain have been found to enhance anxiety-related behaviours (Pum et al., 2009; Tu et al., 2014), whereas chronically administered SSRIs have been found to reduce anxiety (Blanco et al., 2013). Like impulsivity and aggression, anxiety appears to be negatively modulated by 5-HT1AR stimulation (Heisler et al., 1998; Parks et al., 1998; Schreiber and De Vry, 1993; Toth, 2003), and although there are some contradictory findings (File et al., 1996), this effect appears to be mediated by postsynaptic 5-HT1AR signalling (Celada et al., 2013a; Gross et al., 2002; Piszczek et al., 2015; Stefanski et al., 1993; Tauscher et al., 2001; Tu et al., 2014; Zhou et al., 2008, 2014).
Postsynaptic 5-HT1A receptors are densely expressed in limbic regions and particularly the hippocampus (Pazos and Palacios, 1985; Varnas et al., 2004), which is known to be involved in anxiety (Gray, 1983; Tu et al., 2014). Serotonin 1A receptors are highly expressed on excitatory neurons in the hippocampus (Pompeiano et al., 1992) and 5-HT1AR stimulation has an inhibitory influence on pyramidal neuron activity (Andrade, 2011). Hippocampal hyperactivity is strongly associated with states of anxiety and stress (Engel et al., 2009) and 5-HT appears to quell limbic hyperactivity via the inhibitory action of postsynaptic 5-HT1ARs (Dong et al., 1998; Tada et al., 2004). This mechanism could explain the reduced metabolism and blood flow observed in limbic regions with acutely administered MDMA (Carhart-Harris et al., 2015b; Gamma et al., 2000), buspirone (Friston et al., 1991), fenfluramine (thalamus and temporal cortex (Meyer et al., 1996)) and chronically administered SSRIs (Mayberg et al., 2000) – as well as reduced cortico-limbic reactivity to negative stimuli with MDMA (Bedi et al., 2009; Carhart-Harris et al., 2014d) and SSRIs (Arnone et al., 2012; Ma, 2015). The improved ability to tolerate negative stimuli with both acute MDMA (Carhart-Harris et al., 2014d; Mithoefer et al., 2011, 2013) and chronic SSRI treatment (Corchs et al., 2009; Mineur et al., 2015) may be due to elevated levels of synaptic 5-HT activating inhibitory postsynaptic 1A receptors in stress-sensitive limbic regions. It is also likely to explain the use of SSRIs and direct 5-HT1AR agonists such as buspirone, as anxiolytic medications. There is also compelling evidence through 5-HT1AR knock out studies that this receptor is involved in the moderation of anxiety (Chattopadhyay, 2007).
Punishment, 5-HT release and 5-HT1AR signalling
Intriguingly, other than pharmacological manipulations (Bradbury et al., 2013), punishment is one of the most effective means of stimulating 5-HT release (Adell et al., 1997; Amat et al., 1998; Bland et al., 2003a, 2003b; Ferres-Coy et al., 2013; Gronli et al., 2007; Kawahara et al., 1993; Rex et al., 2005; Yoshioka et al., 1995). Several studies have demonstrated that anxiety (Rex et al., 2005) and stress (Fujino et al., 2002) can profoundly increase synaptic 5-HT. Consistent with previous theories (Deakin, 2013), it seems reasonable to suppose that brain 5-HT functions to alleviate psychological distress under adverse conditions – thereby improving coping and resilience. The moderation of aversive mental states may be evolutionarily advantageous in certain contexts, e.g. promoting a more patient, waiting and observing behavioural style, and perhaps greater sociability (or at least reduced anti-sociability). We suggest that this function is mediated by postsynaptic 5-HT1AR signalling, serving to quell hyperactivity in stress-sensitive circuits (Puig and Gulledge, 2011), particularly under conditions of mild-moderate adversity. We link this to the notion of ‘passive coping’, since the behavioural outcome is one of improved endurance of adversity via a moderation of stress and perhaps emotional responsiveness more generally (McCabe et al., 2010; Price et al., 2009).
Anxiety, stress and the 5-HT2AR
The serotonin 2A receptor has also been implicated in anxiety. Serotonin 2A receptor knock-out mice display reduced anxiety which is normalised when its functioning is recovered (Weisstaub et al., 2006). These findings suggest that 5-HT2AR signalling has an anxiogenic effect that is opposite to the anxiolytic effect of postsynaptic 5-HT1AR activation. This idea is leant support by findings of reduced anxiety with 5-HT2AR antagonism (Bressa et al., 1987). Serotonin 2A receptor agonists have complex effects on anxiety in humans (Zanoveli et al., 2005). Subjective anxiety is inconsistently and only marginally increased by the 5-HT2AR agonists psilocybin and LSD during their acute intoxication state (Carhart-Harris et al., 2012a, 2015a; Griffiths et al., 2006) (although acute panic can occur (Barrett et al., 2016; Carbonaro et al., 2016)), yet there is increasing evidence that anxiety can be significantly reduced for a prolonged period after a therapeutically mediated psychedelic drug experience (Gasser et al., 2014, 2015; Griffiths et al., 2016; Grob et al., 2011) – for a discussion of this apparent paradox see (Carhart-Harris et al., 2016c). Thus, whereas postsynaptic 5-HT1AR activation appears to moderate anxiety and stress, the effect of 5-HT2AR activation is more complex (Carhart-Harris et al., 2016c). Similarly, 5-HT2C receptor agonism has been associated with anxiety (and inversely with ‘assertiveness’ in rats) – but a more detailed discussion of 5-HT2C receptor functioning is beyond the remit of this paper (see Mitchell, 2005 for a relevant review).
The effects of 5-HT2AR signalling are highly context sensitive
In forthcoming sections, we develop the idea that 5-HT2AR signalling has a time and context sensitive effect on cognition and emotion, increasing plasticity-related processes (and often anxiety (Griffiths et al., 2006)) in the short-term while facilitating openness, learning and well-being in the longer-term (Carhart-Harris et al., 2016c; MacLean et al., 2011). If mediated properly (e.g. with appropriate psychological support and positive environmental conditions) the acute labile state can be used to facilitate emotional approach and eventual acceptance with potentially enduring beneficial effects (Roseman et al., 2017b; Watts et al., 2017); moreover, it remains possible that reduced anxiety and improved general well-being during the post-acute ‘after glow’ (Winkelman et al., 2014) of a psychedelic experience is related to agonist-induced 5-HT2AR downregulation (Buckholtz et al., 1990).
Consistent with a recent hypothesis on the function of brain 5-HT (Branchi, 2011), we predict that the plasticity-enhancing effects of 5-HT accentuate the influence of environmental factors on the individual (Branchi, 2011) but we would qualify this relationship by emphasising that it is primarily a 5-HT2AR-mediated process. Thus, we propose that 5-HT2AR signalling opens a window of plasticity during which environmental-sensitivity is enhanced and significant therapeutic work can be done. Supporting this hypothesis, central 5-HT2ARs expression is highest during key developmental periods (Sheline et al., 2002; Volgin et al., 2003) when plasticity-related learning is maximal. The quality of a 5-HT2AR dependent psychedelic experience is known to be highly sensitive to the context in which it occurs (Hartogsohn, 2016) and to be consequently predictive of long-term mental health outcomes (Carhart-Harris et al., 2017; Roseman et al., 2017a).
Extra-pharmacological model of drug effects
The extra-pharmacological or ‘EP’ model presented in Figure 2 is inspired by recent empirical and theoretical work on the psychedelic state and is conceived as a working model for testing and refining our understanding of the many determinants of the acute and longer-term effects of psychoactive drugs in general, albeit with special reference and relevance to psychedelics. Trait factors may be biological (e.g. receptor polymorphisms (Ott et al., 2006)) or psychological in nature (e.g. personality (MacLean et al., 2011) or suggestibility (Carhart-Harris et al., 2015a)). The pre-state refers to such thing as anticipatory anxiety, expectations and assumptions (which account for so-called ‘placebo’ and ‘nocebo’ effects), and readiness to surrender resistances and ‘let go’ to the drug effects (e.g. see Russ and Elliott, 2017). In the context of psychedelic research, the pre-state is traditionally referred to as the ‘set’ (Hartogsohn, 2016). State refers to the acute subjective and biological quality of the drug experience and may be measured via subjective rating scales or brain imaging (see Roseman et al., 2017). Dose relates to the drug dosage – which may be a critical determinant of state (Griffiths et al., 2011; Nour et al., 2016) – as well as long-term outcomes (Roseman et al., 2017). Environment relates to the various environmental influences. In the context of psychedelic research this is traditionally referred to as ‘setting’ (Hartogsohn, 2016). We recognise that the environment can be influential at all stages of the process of change associated with drug action. The long-term outcomes may include such things as symptoms of a specific psychiatric condition such as depression – measured using a standard rating scale (Carhart-Harris et al., 2016a) as well as relatively pathology-independent factors such as personality (MacLean et al., 2011) and outlook (Nour et al., 2017). The EP model may prove useful in future studies of psychedelics that aim to determine the weighting or relative influence of different predictor variables on the quality of the acute state and longer-term outcomes. Predictor variables such as trait, pre-state, dose and environment could be entered as independent variables in a regression model, with state as the dependent variable. Similarly, a regression model could include state as an independent ‘predictor’ variable, with a long-term outcome as the dependent variable (for example as in Roseman et al., 2017a; Russ and Elliot, 2017). This model could eventually be used to assist screening for psychedelic therapy and inform on how the therapy is to be delivered, e.g. what dose to administer and how to tune the environment to promote optimal outcomes.
Learning and cognition
5-HT1AR signalling learning and cognition
Postsynaptic 5-HT1AR stimulation is generally considered to be a desirable property of anxiolytic and antidepressant medications (Artigas, 2015), and the postsynaptic 5-HT1AR is thought to be the principal (therapeutic) site of action of SSRIs (Artigas, 2013a, 2015; Samuels et al., 2015). Chronic treatment with SSRIs has been associated with increased neurogenesis (Boldrini et al., 2009), particularly in the hippocampus (Boldrini et al., 2009, 2012) and some improvements in learning and cognition (Bui et al., 2013), albeit with some contradictory findings (Deakin et al., 2004). There is evidence to suggest that increased neurogenesis (at least in the hippocampus) is a 5-HT1AR-mediated effect (Gould, 1999; Huang and Herbert, 2005; Malberg et al., 2000; Santarelli et al., 2003); however, other 5-HT receptors (e.g. the 5-HT4 and 5-HT2A) are also thought to contribute (Azmitia, 2001; Imoto et al., 2015; Jha et al., 2008; Kraus et al., 2017).
Despite this association between 5-HT1AR signalling and neurogenesis, there is a body of evidence to suggest that postsynaptic 5-HT1AR stimulation is impairing to learning and cognition (Ogren et al., 2008), so how can we reconcile these things? One possibility is that the observed pro-cognitive effects of SSRIs are actually mediated by other (non-1A) 5-HT receptors (Boulougouris et al., 2008; Furr et al., 2012; Imoto et al., 2015), and another is that improvements in cognition in patients treated with SSRIs is an epiphenomenon of improvements in mood (Chepenik et al., 2007). It is also important to note that the evidence that SSRIs improve cognition is relatively weak (Beheydt et al., 2015; Knorr, 2012; Knorr et al., 2011; Siepmann et al., 2003) and their modest ability to address cognitive symptoms in depression is considered one of their limitations (Popovic et al., 2015).
5-HT2AR signalling, learning and cognition
The relationship between the 5-HT2AR and cognition is somewhat different to that of the 5-HT1AR. As discussed above, activation of postsynaptic 5-HT1ARs is associated with cognitive and learning impairments (Ogren et al., 2008), whereas 5-HT2AR activation is associated with improvements in certain aspects of cognition and learning (Gimpl et al., 1979; Harvey, 1996, 2003; Harvey et al., 2004, 2012; King et al., 1974; Romano et al., 2006, 2010; Welsh et al., 1998; Zhang and Stackman, 2015; Zhang et al., 2016) as well as an unlearning or ‘extinction’ learning (Zhang et al., 2013). Serotonin 2A receptor activation has also been associated with neurogenesis (Catlow et al., 2013; Cavus and Duman, 2003; Frankel and Cunningham, 2002; Gewirtz et al., 2002; Jones et al., 2009; Meller et al., 2002; Niitsu et al., 1995; Vaidya et al., 1997), particularly in the cortex (Gewirtz et al., 2002; Jones et al., 2009; Vaidya et al., 1997) (but not in the hippocampus (Vaidya et al., 1997)), which may explain the type of cognitive and learning enhancements that are associated with its functioning (e.g. associative learning). Specifically, a number of studies have shown enhancements of associative learning with 5-HT2AR agonism and impairments with its blockade (Barre et al., 2016; Harvey, 1996, 2003; Harvey et al., 2004; Romano et al., 2000, 2006; Welsh et al., 1998).
Cognitive flexibility in humans is thought to be positively modulated by 5-HT2AR functioning (Boulougouris et al., 2008) and there is evidence to suggest that 5-HT2AR agonists (such as LSD and psilocybin) enhance cognitive flexibility and creative thinking (Frecska et al., 2012; Harman et al., 1966; Janiger and Dobkin de Rios, 1989; King et al., 1974; MacLean et al., 2011; McGlothlin et al., 1967; Sessa, 2008), potentially in an enduring way (MacLean et al., 2011). Serotonin depletion and inactivation has been shown to impair cognitive flexibility (Clarke et al., 2004, 2007; Matias et al., 2017) and there is evidence that this may be due to decreased basal activation of 5-HT2ARs (Boulougouris et al., 2008; Furr et al., 2012). Serotonin neurons have been found to activate when animals experience a surprising violation of assumptions, independent of its reward-related implications (Matias et al., 2017), supporting the association between 5-HT, environmental sensitivity and adaptability (Branchi, 2011). Our argument here is that 5-HT2AR signalling is the key mediator of this effect. Promotion of plasticity via 5-HT2AR signalling is central to our thesis that, along with improving stress-tolerance, a key function of brain serotonin transmission is to engage processes necessary for change, when change is necessary. Note: although we acknowledge it would be pertinent and potentially valuable, a more in-depth discussion of the 5-HT2AR and animal and human behavioural measures of cognitive flexibility is beyond the scope of this paper.
Serotonin, depression and mood
Evidence for an association between serotonin and mood
Serotonin was first isolated and named in the late 1940s (Rapport et al., 1948) and subsequently found in the brain in the early 1950s (Gaddum, 1953; Twarog and Page, 1953). At the same time, scientists were beginning to identify interactions between serotonin and the recently discovered lysergic acid diethylamide (LSD) (Gaddum, 1953; Shaw and Woolley, 1956). Struck by LSD’s remarkable potency (psychoactive in doses as low as 20 µg) and powerful modulatory effects on mood and cognition (Busch and Johnson, 1950; Hofmann, 1980), it was speculated that abnormal serotoninergic functioning may underlie certain mental disorders (Gaddum, 1957; Woolley and Shaw, 1954). Although the ‘psychotomimetic’ (mimicking psychosis) properties of LSD and related psychedelics were recognised in the 1950s and 60s (Isbell et al., 1959), as they are today (Carhart-Harris et al., 2013a, 2016c), these compounds were also used extensively as psychotherapeutic aids for the treatment of a range of disorders, including depression and anxiety (Grinspoon and Bakalar, 1979; Sandison, 1954; Sandison and Hopkin, 1964).
The earliest and most direct evidence for the involvement of monoamines in mood regulation however, came with the observation that reserpine, which depletes 5-HT and noradrenaline in the brain (Pletscher et al., 1955), also induces depressed mood in some individuals (Achor et al., 1955) – see also (Antkiewicz-Michaluk et al., 2014). This observation was closely followed by the discovery of the antidepressant properties of the monoamine oxidase inhibitors (MAOIs) (Udenfriend et al., 1957) and subsequently the tricyclic antidepressants (TCAs) (Axelrod and Inscoe, 1963; Kuhn, 1958) – both of which increase synaptic monoamines (Gur et al., 1999; Matos et al., 1990). More specific evidence for the involvement of 5-HT in depression came from studies showing a combined antidepressant effect with an MAOI plus tryptophan, the biochemical precursor to 5-HT (Coppen et al., 1963; Hess and Doepfner, 1961; Pare, 1965).
The idea that serotonergic mechanisms are involved in the pathogenesis and treatment of depression was controversial in the 1960s (Coppen, 1969, 1967); however, it gradually gained traction in the 1980s and into the 1990s with the development and licensing of the SSRIs (Carlsson, 1981; Cowen and Browning, 2015) and particularly fluoxetine (Bremner, 1984). When chronically administered, SSRIs increase concentrations of synaptic 5-HT (Smith et al., 2000) by blocking its reuptake (Carlsson, 1981), show superior efficacy to placebo in depression (Horder et al., 2011; Hieronymus et al., 2016; Barth et al., 2016) and are safer than MAOIs and TCAs (Pletscher, 1991). Another important finding supporting the involvement of serotonin in depression was the observation that acute tryptophan depletion can induce a (transient) relapse in symptoms in formerly depressed patients (Smith et al., 1997) and plasma tryptophan levels have been found to be low in patients with severe depression (Anderson et al., 1990), potentially owing to inflammation-related mechanisms (Wichers et al., 2005).
The involvement of serotonin in mood regulation is further substantiated by the fact that the potent mood-enhancing agent, MDMA, has marked 5-HT releasing properties (Bradbury et al., 2013). In rodents, MDMA is also a noradrenaline (NA) and dopamine (DA) releaser (Kankaanpaa et al., 1998) but its 5-HT releasing properties are far more pronounced (Bradbury et al., 2013; Golembiowska et al., 2016). Blockade of the serotonin transporter by pre-treatment with the SSRI citalopram, significantly attenuated the signature positive mood effects of MDMA (Liechti and Vollenweider, 2000, 2001) – presumably via preventing MDMA from interacting with the 5-HT transporter. Pre-treatment with the D2 antagonist haloperidol also attenuated the positive mood effects of MDMA (Liechti and Vollenweider, 2001) – suggesting that combined DA and 5-HT functioning may have a synergistic influence on mood. However, in a separate study, combining the DA reuptake blocker methylphenidate with MDMA did not have a supplementary influence on positive mood (Hysek et al., 2014b) and stimulants with greater DA than 5-HT releasing properties (such as amphetamine, cocaine and methylphenidate) do not induce the same pro-empathy and pro-social sentiments as well as frank euphoria that can be attributed to MDMA (Bedi et al., 2014; Schmid et al., 2014). The sudden popularity of mephedrone as a party-drug in the early 2010s (Carhart-Harris et al., 2011), may be explained by its pronounced serotonin-releasing properties (Golembiowska et al., 2016), in conjunction with DA release (Kehr et al., 2011), with users likening its euphoric effect to that of MDMA (Carhart-Harris et al., 2011). Like MDMA, mephedrone causes massive 5-HT release that far exceeds its still considerable DA releasing properties (Golembiowska et al., 2016).
In summary, there is a wealth of evidence that 5-HT is involved in the regulation of mood but exactly how it does this is not properly understood (Dayan and Huys, 2015). A central theme of this paper is that the combination of 5-HT1A and 5-HT2A receptor signalling has a complementary effect on mood by promoting stress moderation and patience (predominantly 5-HT1AR mediated) and plasticity and open-mindedness (predominantly 5-HT2AR mediated). For the remainder of the paper, these ideas will be unpacked, first with a focus on postsynaptic 5-HT1AR signalling, before addressing the function of 5-HT2AR signalling in detail.
Postsynaptic 5-HT1AR signalling and mood
The importance of postsynaptic 5-HT1AR receptor signalling in the therapeutic action of serotonergic antidepressants has been convincingly demonstrated (Blier and Ward, 2003; Blier et al., 1997). Selective 5-HT1AR agonists appear to work in a similar way to traditional serotonergic antidepressants (Lucki, 1991), i.e. with a delayed onset of action of 7–14 days due to the gradual desensitisation of the presynaptic 5-HT1A autoreceptors (Blier and Ward, 2003). Subsequent to autoreceptor desensitisation (Le Poul et al., 1995), 5-HT1AR agonists (such as buspirone) appear to act in the same stress-reducing way as has been described for the SSRIs, and this may explain their therapeutic value as anxiolytics (Beneytez et al., 1998; Celada et al., 2013a; Chilmonczyk et al., 2015; Gordon and Hen, 2004; Jolas et al., 1995; Koek et al., 1998; Li et al., 2006; Plaznik et al., 1994; Strauss et al., 2013). Moreover, 5-HT1AR knock-out rodents exhibit greater levels of anxiety and depressive symptoms (Heisler et al., 1998; Ramboz et al., 1998), presumably due to deficient postsynaptic 5-HT1AR-signalling (e.g. in limbic regions).
Determining the importance of the 5-HT1AR to the mechanisms of action of MDMA and classic psychedelics is difficult, due to the unavailability of selective 5-HT1AR antagonists for human research which could be given as blocking agents. The non-selective weak 5-HT1AR antagonist pindolol had a negligible influence on MDMA’s positive mood effects in one study (van Wel et al., 2012) but slightly attenuated them in another (Hasler et al., 2009). Pindolol slightly augmented the psychoactive effects of the classic psychedelic and 5-HT2AR agonist dimethyltryptamine (DMT) (Strassman, 1996), and the 5-HT1AR partial agonist buspirone significantly attenuated the psychoactive effects of psilocybin (Pokorny et al., 2016). The lack of pharmacological selectivity and/or only partial agonism and weak antagonism of buspirone and pindolol (respectively) preclude us from making strong inferences about their effects in pre-treatment studies, although broadly speaking, they support a view that postsynaptic 1A receptor signalling is only mildly (Hasler et al., 2009) and unreliably (van Wel et al., 2012) involved in MDMA’s positive mood effects but may significantly attenuate some of the key psychological effects of classic psychedelics (Pokorny et al., 2016; Strassman, 1996). Supporting this latter inference, depletion of brain serotonin augments the behavioural effects of LSD in animals (Harvey et al., 1975) and humans (Resnick et al., 1965) and this effect may be explained in part by lower postsynaptic 5-HT1AR signalling enabling an exaggerated effect at the 5-HT2A receptor, although an adaptive, homeostatic upregulation of 5-HT2AR availability due to low synaptic 5-HT may be another mechanism (Jennings et al., 2008, 2016). Note also that 5-HT1AR expression is low in the visual cortex (Figure 1) which may explain why 5-HT2AR agonist psychedelics have pronounced visual perceptual effects – i.e. because the excitatory effects of 5-HT2AR agonism go unopposed (by 5-HT1AR signalling) in this region.
Further considering the contribution of 5-HT1AR signalling to MDMA’s acute effects, it is notable that marked changes in cerebral blood flow and functional connectivity in limbic structures (that exhibit the richest expression of 5-HT1A receptors in the forebrain) were observed with acute MDMA administration (Carhart-Harris et al., 2015b), and MDMA’s characteristic pro-social effects were significantly attenuated by pre-treatment with a selective 5-HT1AR antagonist in rats (Hunt et al., 2011) (although see Pitts et al., 2017). The development of new PET ligands sensitive to 5-HT release may prove useful in determining the contribution of different receptor subtypes to the psychological effects of MDMA and other potent serotonergic drugs (Jorgensen et al., 2016; Tyacke and Nutt, 2015). However, in brief, it is our assumption that the effects of MDMA reflect combined signalling at postsynaptic 5-HT1AR, 5-HT2AR and catecholamine receptors (i.e. DA and NA) to produce a state of improved stress tolerability (5-HT1AR-mediated) combined with increased cognitive flexibility and emotional lability (5-HT2AR-mediated) and enhanced focus, motivation and confidence (NA/DA receptor mediated) that in combination, is especially conducive to positive mood (Sessa, 2016).
5-HT2AR signalling, depression and mood
It has been convention in neuropsychopharmacology to view 5-HT2AR agonism as potentially harmful (or at least unconducive) to mental health. The main arguments for this are: (1) 5-HT2AR agonists, such as LSD and psilocybin, are psychotomimetics (i.e. psychosis models) (Curran et al., 2009; Gerber and Tonegawa, 2004); and (2) a number of antidepressants (Carpenter et al., 1999) as well as many antipsychotics (Meltzer, 2012) have 5-HT2AR antagonist properties. However, recent studies have begun to challenge the notion that 5-HT2AR agonism is an undesirable property for a psychotropic medication (Carhart-Harris et al., 2016c; Griffiths and Grob, 2010; Carhart-Harris et al., 2016b; Qesseveur et al., 2016; Petit et al., 2014 – see Carhart-Harris and Goodwin, 2017 for a review) – and about their harm, comparative rating scales suggest 5-HT2AR agonist psychedelics like psilocybin are among the least harmful drugs of potential misuse (Carhart-Harris and Nutt, 2013; Nutt et al., 2010; van Amsterdam et al., 2015). Moreover, an increasing number of studies are reporting enduring positive mental health outcomes (Bogenschutz et al., 2015; Bouso et al., 2012; Gasser et al., 2014; Grob et al., 2011; Hendricks et al., 2015b; Osorio Fde et al., 2015) and psychological well-being (Carhart-Harris et al., 2016c; Griffiths et al., 2008) with administration and use of 5-HT2AR agonist psychedelics. Additionally, several studies have found associations between 5-HT2AR polymorphisms and SSRI response (Kishi et al., 2010; McMahon et al., 2006; Wilkie et al., 2009), although it is unclear if alleles predicting better response are associated with more or less 5-HT2AR functioning. Potentially, resolving this, however, a recent study suggested that 5-HT2AR signalling is an important (and therefore underappreciated) component of antidepressant action of SSRIs (Qesseveur et al., 2016).
Supporting the principle that 5-HT2AR agonism is a viable antidepressant target, are the growing number of studies demonstrating the antidepressant potential of 5-HT2AR agonist psychedelics (Baumeister et al., 2014; Buchborn et al., 2014; Carhart-Harris et al., 2016b; Griffiths et al., 2016; Grob et al., 2011; Osorio Fde et al., 2015; Ross et al., 2016; Sanches et al., 2016 – see Carjart-Harris and Goodwin, 2017 for a review). For example, a recent pilot study by our team reported rapid and enduring improvements in depressive symptoms after two treatment sessions with psilocybin in patients with treatment-resistant depression (Carhart-Harris et al., 2016b). The results of this study are consistent with those of others reporting reduced depressive symptoms in depressed patients treated with ayahuasca (Osorio Fde et al., 2015; Sanches et al., 2016) and end-of-life anxiety patients treated with psilocybin (Griffiths et al., 2016; Grob et al., 2011; Ross et al., 2016), as well as a population study showing lower rates of psychological distress and suicidality in relation to psychedelic drug use (Hendricks et al., 2015b). Taken together, these findings motivate a revision of the conventional view that psychedelics are harmful to mental health (Hendricks et al., 2015b), and encourage a rethink on the role of 5-HT2AR signalling in the pharmacology of depression (see also (Petit et al., 2014; Qesseveur et al., 2016).
Further support for a positive association between 5-HT2AR signalling and (trait) psychological health comes from human PET imaging work that has shown a positive relationship between 5-HT2AR binding and trait neuroticism (Frokjaer et al., 2008), pessimism (Bhagwagar et al., 2006; Meyer et al., 2003) and personality disorder (Soloff et al., 2007; Rosell et al., 2010). Cortical 5-HT2AR expression is sensitive to basal 5-HT concentrations (Cahir et al., 2007; Jorgensen et al., 2016), with 5-HT2A receptors becoming more populous and/or available in response to reduced synaptic 5-HT (Cahir et al., 2007; Jennings et al., 2008; Jorgensen et al., 2016) and less available in response to increased synaptic 5-HT (Jorgensen et al., 2016; Meyer et al., 2001). Thus, increased 5-HT2AR binding and associated pessimistic thinking (Bhagwagar et al., 2006; Meyer et al., 2003) may be a corollary of deficient 5-HT2AR signalling – and the enduring increases in optimism that have been observed with LSD (Carhart-Harris et al., 2016c) may be viewed as evidence of extreme 5-HT2AR signalling having a lasting impact on positive thinking (Carhart-Harris et al., 2016c).
Postmortem studies showing increased 5-HT2AR availability in unmedicated depressed patients (Shelton et al., 2009) and suicide victims (Anisman et al., 2008; Pandey et al., 2002; Stanley and Mann, 1983; Turecki et al., 1999) could be viewed as consistent with the hypothesis that there is an adaptive upregulation of 5-HT2A receptors in response to deficient 5-HT2AR signalling in depression. The existent of discrepant findings (e.g. decreased 5-HT2AR availability in depression and suicide victims) that challenge this hypothesis may be explained by the confounding influence of antidepressant and other psychiatric medications – which reverse this relationship by downregulating 5-HT2AR availability (Attar-Levy et al., 1999; Dean et al., 2014; Gray and Roth, 2001; Muguruza et al., 2014; van Heeringen et al., 2003; Yatham et al., 1999).
Electroconvulsive shock and 5-HT2AR functioning
The effect of electroconvulsive shock (ECS) on 5-HT2AR densities and functioning is important to address, particularly given the notable efficacy of electroconvulsive therapy (ECT) in terms of reducing depressive symptoms for a period (UK ECT Review Group, 2003). Interestingly, we have recently found that functional brain changes one day after psilocybin for treatment-resistant depression compare best with those of ECT (Carhart-Harris et al., 2017b). For example, as with ECT (Bolwig, 2015), the post-psilocybin treatment brain changes were the inverse of what is typically seen during the acute psilocybin experience itself (Carhart-Harris et al., 2017b). More specifically, whereas resting state functional connectivity in the default-mode network is significantly decreased during the acute psychedelic experience (Carhart-Harris et al., 2016), it is increased (or ‘normalised’) one day after psilocybin for treatment-resistant depression – and this effect is greatest in treatment responders (Carhart-Harris et al., 2017b). Increased or ‘normalised’ DMN RSFC has also been seen after successful treatment with ECT (Mulders et al., 2016).
Early rat work revealed increased 5-HT2AR functioning (Moorman et al., 1996) and cortical 5-HT2AR expression after ECS (Burnet et al., 1995, 1999; Butler et al., 1993) – an effect that appeared to be relatively selective for the 5-HT2AR in relation to other serotonin receptor subtypes (Burnet et al., 1999). However, contradictory findings have since been observed in primates (Strome et al., 2005) and humans (Yatham et al., 2010) with 5-HT2AR binding showing decreased post ECS/ECT. This downregulation of 5-HT2AR densities post ECT is more consistent with the effects of conventional antidepressant medications (Yatham et al., 1999) – as well as classic psychedelics (Buckholtz et al., 1990) – and also makes more logical sense given the marked 5-HT release that is associated with ECS (Zis et al., 1992).
How do we explain the observed 5-HT2AR upregulation in rats however? Stress has been found to increase 5-HT2AR density (Katagiri et al., 2001) and affinity (Harvey et al., 2003) in rats. Extreme stress is hypothesised to engage ‘pathway 2’ in our bipartite model, which is mediated by 5-HT2AR signalling, and characterised by a rapid plasticity – serving to facilitate major change in conditions of extreme adversity. Although speculative, one interpretation of the upregulated 5-HT2AR functioning post ECS in rats, is that it is a consequence of the extreme stress (‘shock’) of the procedure in this species. It might also be worth noting that ECT has been found to promote neural plasticity (Bouckaert et al. 2014; Joshi et al. 2016), and so is consistent with pathway 2 in this regard.
5-HT2A agonists and antagonists as antidepressants: resolving a paradox
Some effective drugs for depression (such as mirtazapine) have 5-HT2AR antagonist properties (Watanabe et al., 2008) and 5-HT2AR antagonist antipsychotic drugs (such as risperidone and olanzapine) have been found to augment the antidepressant efficacy of SSRIs in treatment-resistant depression (Marangell et al., 2002; Ostroff and Nelson, 1999; Shelton and Papakostas, 2008). This has led some to consider 5-HT2AR antagonism a treatment target in depression (Pandey et al., 2010) but this matter requires some careful thought, not least because 5-HT2AR antagonism presents additional side-effects to those of first-line antidepressants such as SSRIs (Jarema, 2007; Shelton and Papakostas, 2008; Teegarden et al., 2008). To our knowledge, selective 5-HT2AR antagonists have not been trialled as stand-alone treatments for depression, and have largely failed as stand-alone treatments for schizophrenia (Ebdrup et al., 2011), so their efficacy appears to be predicated on the augmentation of other pharmacological mechanisms. For example, blocking postsynaptic 5-HT2ARs in the mPFC may lessen the ability of top-down circuits to inhibit the firing of serotonergic neurons in the midbrain (potentially leading to increased 5-HT efflux) (Artigas, 2013a), and 5-HT2AR blockade more generally, may encourage a preferential effect of 5-HT on its postsynaptic 5-HT1A receptors. Considered in this way, the effects of 5-HT2AR antagonism could be perceived as supplementing the stress moderation effects of postsynaptic 5-HT1AR agonism, and so pathway 1 in our bipartite model (Figure 3). Moreover, 5-HT2AR antagonists have mild pro-sleep/sedating properties (Idzikowski et al., 1987; Teegarden et al., 2008; Vanover and Davis, 2010) that could complement the stress moderating effects of SSRIs.
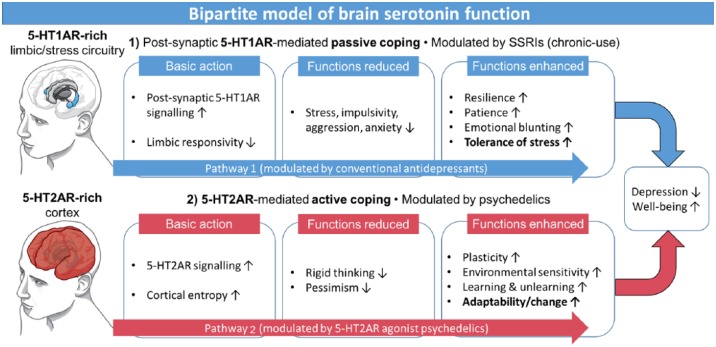
Figure 3.
A two-part or ‘bipartite’ model of brain serotonin function. Model proposes that brain serotonin mediates adaptive responses to adversity via two distinct mechanisms: one mediated by postsynaptic 5-HT1AR signalling in aid of stress moderation (pathway 1) and the other mediated by 5-HT2AR signalling is aid of more substantial adaptive changes (pathway 2). SSRIs and other conventional antidepressant medications work on and can enhance pathway 1, whereas pathway 2 can be enhanced by 5-HT2AR agonist psychedelic drugs such as psilocybin. Note: it is hypothesised that active coping can be most effectively implemented if the window of plasticity afforded by 5-HT2AR agonism is complemented by supportive psychotherapy that promotes a willingness to confront and work through sources of stress (Watts et al., 2017). Illustrations by Samantha Strong (S.L.Strong1@bradford.ac.uk).
A likely solution to the paradox that 5-HT2AR agonists and antagonists have antidepressant properties is that they achieve the same outcome but via different routes. Whereas 5-HT2AR antagonism supplements the emotionally moderating effects associated with postsynaptic 5-HT1AR signalling (pathway 1), 5-HT2AR agonism may work to enhance plasticity, adaptability and the capacity for change. Both mechanisms can be viewed as adaptive responses to adverse conditions, with potentially consistent outcomes, albeit achieved via different, perhaps even antithetical mechanisms.
Acute versus longer-term mood effects of 5-HT2AR signalling
The paradox that 5-HT2AR agonist psychedelics can be acutely psychotomimetic (Carhart-Harris et al., 2013a; Gouzoulis-Mayfrank et al., 2005) and yet have long-term beneficial effects on well-being (Griffiths et al., 2006) and mental health (Carhart-Harris et al., 2016a; Griffiths et al., 2008; Hendricks et al., 2015b) has previously been discussed (Carhart-Harris et al., 2016c). In brief, it has been proposed that the acute state produced by 5-HT2AR agonist psychedelics does not directly modulate the valence of mood, i.e. it does not directly promote either positive or negative mood (Carhart-Harris et al., 2016c). This argument could be contested on the basis that positive mood effects are often seen with acute administration of psychedelics (Schmid et al., 2015) and the positive mood effects of MDMA (van Wel et al., 2012), LSD (Preller, 2016), psilocybin (Kometer et al., 2012) and ayahuasca (Valle et al., 2016) are all attenuated by pre-treatment with a 5-HT2AR antagonist, as are the pro-social effects of MDMA (Pitts et al., 2017). However, anxiety and psychosis-like symptoms are also often seen acutely with psychedelics (Carhart-Harris et al., 2016c) and these can also be attenuated by 5-HT2AR antagonism (Vollenweider et al., 1998). Moreover, in studies that found enhanced mood with psychedelics, psychological preparation and support was generally provided, which helps channel the experience in a positive direction. Similarly, volunteers may have had positive expectations about their experience that biased their appraisal of the acute experience. These matters are relevant to our extra-pharmacological model presented above (Figure 2), as well as the enhanced environmental sensitivity model proposed for serotonin itself (Branchi, 2011) and 5-HT2AR signalling more specifically (pathway 2, Figure 3).
One proposed solution to this apparent paradox, is that the acute and longer-term effects of psychedelics are distinct, with the acute effects being marked by emotional arousal and lability (Carhart-Harris et al., 2016c; Kaelen et al., 2015) rather than positive mood per se, and longer-term changes are more reliably biased towards positive mood (perhaps somewhat analogous to near-death experiences (Greyson, 2008)) with improvements in psychological well-being (Griffiths et al., 2006; Hendricks et al., 2015a, 2015b), optimism (Carhart-Harris et al., 2016c) and openness (MacLean et al., 2011). The importance of emotional breakthrough after acute struggle may be highly relevant in this context (Watts et al., 2017), as may the occurrence of peak-type experiences (Roseman et al., 2017a), both topics we intend to study more closely in the future. Agonist-induced 5-HT2AR downregulation may also play a significant role (Buckholtz et al., 1990), at least during the after-glow period 1–2 weeks post exposure (Winkelman, 2014).
This is a complex problem for future studies to dissect. However, one way we may begin to inform on it, is to address the question of whether the acute and longer-term responses to psychedelics relate to each other – and indeed, there is already ample evidence that they do (Carhart-Harris et al., 2017a; Griffiths et al., 2016; Roseman et al., 2017a; Ross et al., 2016). A recent questionnaire study found that the psychological difficulty of an acute psychedelic experience was predictive of longer-term improvements in well-being (Carbonaro et al., 2016), although the same study also found that the duration of such difficulty was predictive of long-term decreases in well-being (Carbonaro et al., 2016). A number of studies have found that especially intense psychedelic experiences predict positive long-term outcomes – particularly if they contain phenomena consistent with so-called ‘mystical’ (Stace, 1961) or ‘peak’ (Maslow, 1970) experiences (Bogenschutz et al., 2015; Griffiths et al., 2008, 2016; Johnson et al., 2016; Ross et al., 2016). Moreover, a recent LSD neuroimaging study by our team found that acute ‘entropic’ brain changes under the drug (Carhart-Harris et al., 2014b) were predictive of long-term increases in the personality trait ‘openness’ (Lebedev et al., 2016). As highlighted in our EP model (Figure 2), it is important that we try to better understand how extra-pharmacological factors may interact with a drug’s direct pharmacological effects to determine the quality of an acute drug experience and ensuing long-term effects – and this is especially pertinent in the context of psychedelics.
The function of brain 5-HT2AR signalling
5-HT2AR mediated plasticity
There is a growing body of evidence that enhanced 5-HT2AR signalling produces a plastic state (in the sense of an enhanced capacity for change), both psychologically (Boulougouris et al., 2008; Carhart-Harris et al., 2015a, 2016c; Clarke et al., 2007; Kaelen et al., 2015; Kuypers et al., 2016) and neurobiologically (Azmitia, 2001; Barre et al., 2016; Carhart-Harris et al., 2012a, 2014b, 2016c; Gewirtz et al., 2002; Lebedev et al., 2016; Tagliazucchi et al., 2016; Vaidya et al., 1997; Yoshinaga et al., 2013). We propose that this 5-HT2AR-mediated plasticity is of fundamental importance to the acute and longer-term action of 5-HT2AR agonist psychedelics, potentially explaining their idiosyncratic phenomenology and remarkable behavioural effects – including their ability to elicit long-term beneficial (Carhart-Harris et al., 2016a; Griffiths et al., 2011; Hendricks et al., 2015a), and (albeit less common) harmful changes (Lerner and Lev-Ran, 2015; Cohen, 1966; Iaria et al., 2010).
Plasticity and the entropic brain
The proposal that psychedelics induce a plastic state is consistent with the ‘entropic brain’ hypothesis, introduced by us in 2014 (Carhart-Harris et al., 2014b). This idea emerged out of observations of consistencies between neuroimaging findings on the action of psychedelics (Carhart-Harris et al., 2014b; Muthukumaraswamy et al., 2013) and a sense that their physical (brain) effects recapitulate their psychological effects – and vice versa. Inspired by Karl Friston’s Free-Energy principle (Friston, 2010), the information theory-based measure of entropy was applied to the psychedelic state in an effort to capture its essential phenomenological and neurophysiological qualities. Entropy is formally both uncertainty and unpredictability (Ben-Naim, 2007) – and not coincidentally, these terms possess meaning in both a mechanistic and subjective sense. A growing number of analyses are now endorsing the principle that the brain exhibits increased entropy under psychedelics (Atasoy, 2017; Carhart-Harris et al., 2014b; Lebedev et al., 2016; Schartner et al., 2017; Tagliazucchi E, 2014; Viol, 2016) (see also Gallimore, 2015) and countless other human and animal studies by independent teams, despite not formally measuring entropy, report findings that are consistent with the entropic brain principle (Celada et al., 2013b; Muthukumaraswamy et al., 2013; Riba et al., 2004, 2014; Wood et al., 2012).
Entropy exists most purely as an index of uncertainty (Ben-Naim, 2007) but its origins lie in thermodynamics (Ben-Naim, 2007, 2008). Entropy is perhaps most familiar to people in the context of thermodynamics and specifically how it relates to the second law: that isolated systems tend towards disorder, or exhibit increased entropy over time (i.e. decay). The relationship between information theory-based entropy and thermodynamic entropy is a formal one, with the latter being merely an applied and contextualised version of the former (Ben-Naim, 2007, 2008).
In the context of 5-HT2AR signalling and how this may inform on the function of brain serotonin, one may think of enhancing 5-HT2AR signalling as analogous to increasing the temperature (or excitability) of the brain; indeed, the excitatory effect of 5-HT2AR signalling has long been recognised (Aghajanian and Marek, 1999; Celada et al., 2013b). Extending this analogy to the process of annealing (i.e. whereby a metal is heated to make it more malleable) – one may think of 5-HT2AR signalling as functioning to induce an entropic state characterised by enhanced flexibility and malleability during which work can be done that, upon cooling, may leave a lasting change (Gopnik, 2010). Viewed through the lens of the popular Bayesian brain model of brain function (Knill and Pouget, 2004), one could see this 5-HT2AR-mediated entropic state as working to ‘reset’ reinforced priors in depression – such as pessimistic beliefs and negative self-perceptions (Moutoussis et al., 2014). See Carhart-Harris et al. (2017b) for recent neurobiological support for this idea.
5-HT2AR induced plasticity mediates environmental sensitivity
The evolutionary value of neural and behavioural plasticity is well recognised (Belsky and Pluess, 2013; Boyce and Ellis, 2005), and in this context, the plasticity-mediating role of serotonin is becoming increasingly well appreciated (Alboni et al., 2017; Belsky et al., 2009; Branchi, 2011; Chiarotti et al., 2017). The importance of plasticity for learning has obvious functional value: in early life, when behaviour and cognition require considerable refinement but also in extreme adversity, when major behavioural change may be necessary for survival.
Serotonin is known to play a vital role in brain development (Azmitia, 2001; Kepser and Homberg, 2015; Lambe et al., 2011) and has been found to reverse processes of maturation, both at the cellular (Kobayashi et al., 2010; Maya Vetencourt et al., 2008) and brain network level (Carhart-Harris et al., 2016d; Tagliazucchi et al., 2016) in both cases likely via 5-HT2AR related mechanisms. Regarding 5-HT2AR signalling, fMRI studies have shown that LSD and psilocybin temporarily reverse processes of network integration and segregation that characterise the developing brain (Wylie et al., 2014), and this ‘brain regression’ is mirrored at the psychological level by a psychological regression that is characteristic of the psychedelic state (Carhart-Harris et al., 2016d; Roseman et al., 2014; Tagliazucchi et al., 2016). Consistently, processes of neuronal differentiation that occur during development were found to be aided by 5-HT1AR signalling but inhibited by 5-HT2AR signalling (Azmitia, 2001).
Crucially, 5-HT2AR signalling has been found to be highly influential during early development (Beique et al., 2004; Zhang, 2003) and to be maximal during key developmental periods (Lambe et al., 2011) suggesting that 5-HT2AR-mediated plasticity facilitates the intense learning that is needed during critical periods. Children have been found to demonstrate superior performance than adults in certain tasks requiring open-mindedness and the ‘de-weighting’ of prior knowledge (Lucas et al., 2014) and psychedelics are strongly associated with unconventional thinking (Harman et al., 1966; Kuypers et al., 2016), vivid imagery and imagination (Carhart-Harris et al., 2012b, 2015a; Kaelen et al., 2016) and suggestibility (Carhart-Harris et al., 2015a).
Trend decreases in openness appear to occur with maturation (Costa and McCrae, 1988) and 5-HT2AR availability is known to markedly decrease once adulthood has been reached (Sheline et al., 2002). Enduring increases in openness have been found after psilocybin (MacLean et al., 2011) and LSD (Carhart-Harris et al., 2016c) – remarkable findings given that personality is normally highly stable in adulthood. Trait absorption, which is related to openness and a susceptibility to become immersed and absorbed in one’s inner or outer world (Ott, 2006; Parsons et al., 2015; Tellegen and Atkinson, 1974), has been found to: (1) predict sensitivity to psilocybin’s acute effects (Studerus et al., 2012), and (2) be associated with a polymorphism linked to stronger 5-HT2AR binding (Ott et al., 2005).
There is likely to be an optimal level of cognitive and psychological flexibility for a given context (Carhart-Harris et al., 2014b) and high doses of psychedelics risk overshooting this through extreme 5-HT2AR signalling causing an excessive flexibility that is unconducive to accurate reality testing and conventional cognition and behaviour (Carhart-Harris et al., 2014b). Interestingly, recent anecdotal reports suggest that semi-regular use of very low doses of psychedelics (referred to colloquially as ‘micro-dosing’) may facilitate creative problem solving and improve mood (Gregoire, 2016; Waldman, 2017) – a claim that urgently requires empirical verification through controlled research. Reports of ‘over-view’ type insights, i.e. an improved ability to see the ‘bigger picture’ under psychedelics, are relatively common among user, participant and patient reports (Sessa, 2008; Harman et al., 1966), and ‘aha’ type insights have been described (Grof, 1975; Sandison and Hopkin, 1964; Watts et al., 2017). Moreover, acute insight experienced during treatment with psilocybin for treatment-resistant depression was recently found to be predictive of positive long-term clinical outcomes (Carhart-Harris et al., 2017a). If evidence for psychedelic-induced insight is substantiated by further research, this will have interesting implications for our understanding of optimal cognition (Carhart-Harris et al., 2014b) and the science of nootropics (Froestl et al., 2014).
Relatedly, more work is required to test the reliability of the recent finding that psychedelics tune the brain closer to criticality (Atasoy et al., 2017), and what the functional and therapeutic implications of this might be. Critical systems are known to be maximally sensitive to perturbation (Bak, 1997), and although speculative, this could account for the high sensitivity to the environment that is characteristic of the psychedelic state (Hartogsohn, 2016).
Much has been written about differential vulnerability to stress in medicine and psychiatry, e.g. the so-called stress-diathesis model of mental illness (Morley, 1983). However, recent revisions of this model possess considerable appeal, particularly when applied to the context of brain serotonin (Belsky and Pluess, 2009; Branchi, 2011). According to these revised models, greater sensitivity to the environment may translate into greater well-being if conditions be favourable, or vulnerability to mental illness if conditions be adverse (Belsky and Pluess, 2009; Branchi, 2011). The involvement of serotoninergic mechanisms in mediating sensitivity to the environment is supported by gene-environment interaction studies that have linked certain serotonin genotypes to greater susceptibility to stress (Caspi et al., 2003). Particular focus has been placed on a serotonin transporter (5-HTT) gene polymorphism, and the finding that the (s/s) allele, associated with lower re-uptake and thus higher synaptic 5-HT (Lesch et al., 1996) is associated with a greater likelihood of depressive symptoms in response to stress (Caspi et al., 2003; Karg et al., 2011; Zammit and Owen, 2006) – although see (Mirkovic et al., 2016). Evidence of increased plasticity with SSRIs and increased 5-HT transmission more generally (Mattson et al., 2004) has been used to endorse a view that serotonin mediates susceptibility to the environment (Branchi, 2011) but little has been written about how specific 5-HT receptors mediate this effect.
Crucially, 5-HT2AR polymorphisms have been associated with: (1) increased sensitivity to stressful and enriching environments (Dressler et al., 2016; Fiocco et al., 2007;Jiang et al., 2016; Jokela et al., 2007; Lebe et al., 2013; Salo et al., 2011); (2) early life stress and maternal deprivation increases the availability of 5-HT2ARs and their sensitivity to excitation (Benekareddy et al., 2010; Vazquez et al., 2000); (3) time-dependent sensitisation stress which models post-traumatic stress disorder (PTSD) in rodents, increases 5-HT2AR affinity (Harvey et al., 2003); (4) chronic glucocorticoid administration to rodents increases 5-HT2AR densities (Katagiri et al., 2001); (5) 5-HT2AR availability is highest during critical development periods (Sheline et al., 2002); (6) 5-HT2AR signalling mediates behavioural responses to stress in non-human animals (Aloyo and Dave, 2007) and (7) humans experiencing psychedelic drug trips are, like children, exquisitely sensitive to their environment (Eisner, 1997; Hartogsohn, 2016), with the provision of a supportive, nurturing environment being strongly advocated for psychedelic ‘trippers’ (Johnson et al., 2008) – as for children. In summary, all these findings lend support to the notion that a key function of 5-HT2AR signalling is to mediate plasticity and associated change, especially in situations where change would be functionally advantageous.
5-HT2AR-mediated plasticity: an adaptive response to extreme adversity
As discussed earlier (Section 3.2.2), anxiety and stress are potent non-pharmacological inducers of 5-HT release (Bland et al., 2003b; Fujino et al., 2002; Rex et al., 2005). Anxiety and stress are most intensely evoked when survival is threatened, and accordingly, massive 5-HT release (~250 fold vs. baseline) has been detected in the rodent brain during asphyxiation and cardiac arrest, and although other neurotransmitters also show a marked increase, the increase in 5-HT release was especially marked (Li et al., 2015). It has been speculated that elevated levels of the endogenous 5-HT2AR agonist psychedelic DMT (Barker et al., 2012) may account for spontaneously occurring psychedelic-like states such as occur in near-death experiences (Strassman, 2000) but to our knowledge, empirical evidence for this theory has yet to published. Functionally, there is no more extreme condition than being proximal to death (if still fully alert). It is intriguing to consider what role may be served by enhanced serotonergic functioning during the perceived threat of death, and particularly 5-HT2AR signalling.
Indeed, similarities between the phenomenology of near-death experiences and the psychedelic state (e.g. disturbed time perception, reliving/autobiographical memory recollection, sudden insight, a sense of peace, a sense of interconnectedness and unity, a sense of other-worldliness, religious and/or mystical-type feelings – which may include a sense of presence or an encounter with (a perceived) person or deity of significance, and a message or instruction (Greyson, 2008; Greyson, 1993; Greyson and Bush, 1992; Greyson, 1983)) may rest on similarities in their pharmacology – i.e. extreme 5-HT2AR signalling. Similarly, the incipient phase of a psychosis – which may be associated with a ‘psychedelic-like’ phenomenology (e.g. a fragmenting self/ego, muddled thinking, bizarre thought content, de-realisation, mystical-type experiences and/or religious conversation or epiphany, putative insight, magical thinking, perceptual disturbances and a sense of dread etc. (Bowers and Freedman, 1966; Chapman, 1966)) may also involve exceptionally high 5-HT2AR transmission (Gouzoulis-Mayfrank et al., 1998, 2005;Gouzoulis et al., 1994).
Consistent with the bipartite model we present here (Figure 3), a stress-induced upregulation of 5-HT2AR signalling as an adaptive response to extreme adversity (Benekareddy et al., 2010; Berton et al., 1998; Harvey et al., 2003; Katagiri et al., 2001), could be an unacknowledged factor in the pathogenesis of psychosis (Gouzoulis-Mayfrank et al., 1998; Gouzoulis et al., 1994; Holloway et al., 2013). Indeed, if this hypothesis was to hold up to empirical testing, it would have important implications for how we understand and perhaps treat psychosis. For example, it could: (1) imply a role for 5-HT1AR agonism and 5-HT2AR antagonism in the moderation of the ‘prodromal state’, and/or (2) endorse the importance of providing a highly supportive environment for the at-risk individual – as is provided for actual drug-induced psychedelic experiences (Johnson et al., 2008).
Earlier (Section 3.2.2), we discussed the hypothesis that 5-HT mediates passive coping (or an improved ability to tolerate stress) under adverse conditions via postsynaptic 5-HT1AR signalling. Enhanced coping via a moderation of stress may be advantageous during difficult conditions but it may also be counterproductive, e.g. if it promotes a ready acceptance of these conditions and a compromised ability to learn from or strive to change them. Conversely, if learning and adaptability are enhanced (e.g. via 5-HT2AR signalling), then this may confer significant evolutionary advantages. Moreover, humans’ remarkable adaptability is one of our defining traits – being fundamental to our development and thriving as a species (Anton et al., 2014; Stini, 1975).
We propose that an enhanced ability to tolerate stress, mediated by enhanced postsynaptic 5-HT1AR signalling, may be a logical, adaptive response to moderate levels of adversity but that enhanced adaptability and capacity for change (e.g. in outlook and behaviour) mediated by 5-HT2AR signalling may be optimal when the level of adversity reaches a critical point – e.g. when one’s life is in danger. A number of different experiments could be performed to test this hypothesis, with its basic tenets being: (1) extremely adverse conditions evoke enhanced 5-HT2AR signalling, plasticity and propensity for change; (2) 5-HT1AR signalling dominates serotonin functioning during normal conditions and during mild-moderate adversity but 5-HT2AR plays an increasingly prominent role as the level of adversity increases to a critical point, and concentrations of synaptic 5-HT do similarly (e.g. as can be achieved experimentally with extreme stress (Amat et al., 2005), or with raphe stimulation (Amargos-Bosch et al., 2004; Puig et al., 2005)).
The rapid and transient downregulation of hippocampal 5-HT1ARs seen with severe acute stress may be one mechanism by which this hypothesised transition to 5-HT2AR dominance under extreme adversity occurs (Berton et al., 1998; Lopez et al., 1999), and increased limbic 5-HT release with mild stress (Bekris et al., 2005) compared with cortical 5-HT release with more intense stress (Amat et al., 2005) may be another. Another possibility is that 5-HT2ARs switch from their default low-affinity state to a high-affinity state (Glennon et al., 1988) under conditions of extreme stress. Indeed, this may explain why 5-HT2AR density (Katagiri et al., 2001) and affinity (Harvey et al., 2003) are increased under extreme stress in rodents. The development of 5-HT2AR agonist radioligands (Ettrup et al., 2014, 2016; Jorgensen et al., 2016) may help us to better test for altered 5-HT2AR availability in the human brain during extreme stress and to correlate this with state and trait psychological variables. Our firm hypothesis would be that 5-HT2AR binding would be significantly increased in highly stressed individuals and that this may relate to psychological and neurobiological measures of plasticity.
Serotonin and positive mood
According to the central hypothesis of this paper, the principal function of brain serotonin is to facilitate adaptive responses to adverse conditions via two distinct pathways. Consequently, like much of the literature on the function of brain 5-HT, this paper has concentrated on adversity. This approach can be defended on a number of grounds: (1) there are plenty of relevant data on adversity because it is relatively easy to experimentally induce; (2) adverse conditions provide models from which to test experimental treatments; (3) adversity and its behavioural and biological corollaries are of central relevance to medicine and psychiatry; and, perhaps most critically, (4) negotiating adversity is of fundamental evolutionary importance.
Regarding the pharmacology of positive mood, the reliability with which potent 5-HT releasers such as MDMA and mephedrone induce marked positive mood (Carhart-Harris et al., 2011, 2015b) could be seen as supportive of the (albeit disputed (Andrews et al., 2015)) association between enhanced serotonergic functioning and positive mood. The euphoria associated with these compounds is distinct from that associated with other stimulants that have more pronounced catecholamineric releasing effects – such as methamphetamine (Bedi et al., 2014). It is intriguing to consider how much of a contribution 5-HT2AR agonism makes to the euphoric effects of MDMA and mephedrone (Schmid et al., 2015), particularly since 5-HT2AR antagonism significantly attenuates the positive mood effects of MDMA (van Wel et al., 2012) and 5-HT1AR antagonism does this less reliably (van Wel et al., 2012). PET imaging work utilising potent 5-HT releasers and receptor-selective ligands sensitive to this release (Paterson et al., 2010, 2013; Tyacke and Nutt, 2015) may be able to shed new light on the association between enhanced 5-HT transmission and positive mood (Jorgensen et al., 2016) that may help to disambiguate this matter (Andrews et al., 2015).
It would also be relevant to better understand why more selective 5-HT releasers such as fenfluramine do not produce the same kind of euphoria associated with MDMA and mephedrone, e.g. increased anxiety and decreased positive mood were seen with high doses of fenfluramine (Brauer et al., 1996), although reduced anxiety has also been observed with lower doses (Hetem et al., 1996). The contribution of catecholamine release to the MDMA and mephedrone ‘high’ may be an important factor, as may the remarkable potency of their 5-HT release, which is comparatively much greater for MDMA and mephedrone (Golembiowska, et al., 2016) than for fenfluramine (Zolkowska et al., 2008). It is also possible that the pharmacology of fenfluramine’s metabolite, norfenfluramine, which is different to that of its parent compound, e.g. norfenfluramine has greater 5-HT2C receptor agonism (Miller, 2005), may account for some of its aversive effects. Relatedly, it is known that the 5-HT2C receptor agonist mCPP tends to induce anxiety and panic (Wood, 2003).
It is important to state that 5-HT2AR agonist psychedelics are not hedonic drugs in the classic sense (Carhart-Harris and Nutt, 2013). Psychedelics are not habit forming in animals or humans (Bogenschutz and Johnson, 2016) and typical patterns of use are relatively sporadic, with protracted periods of abstinence (Nichols, 2004). However, very low (‘micro-doses’) are reportedly being taken regularly for (putative) mood and cognition enhancement (Gregoire, 2016; Waldman, 2017) and states of extreme positive mood are not infrequently reported with larger doses of psychedelics (Schmid et al., 2015), particularly when taken in supportive environments (Studerus et al., 2012) – although marked anxiety and/or dysphoria can also occur (Carbonaro et al., 2016). As highlighted in our EP model (Figure 2), context is likely to play an important role in determining the quality of a psychedelic experience (Hartogsohn, 2016; Roseman et al., 2017a) – and positive mood associated with 5-HT2AR agonist psychedelics may have much to do with positive expectations and environmental factors.
This said, it is intriguing to consider the possibility that a ‘loosened mind’, whether subsequent to enhanced 5-HT2AR signalling or not, may be a non-negligible component of the neurobiology of positive mood itself (Ashby et al., 1999). Blocking the 5-HT2AR has been found to significantly attenuate the positive mood effects of three different classic psychedelics (Kometer et al., 2012; Preller, 2016; Valle et al., 2016) and MDMA (van Wel et al., 2012), and it is intriguing to consider whether 5-HT2AR-mediated plasticity may be an underappreciated component of the antidepressant action of SSRIs (Chamberlain et al., 2006; Petit et al., 2014; Qesseveur et al., 2016). Several studies have demonstrated a relationship between positive mood and creative thinking (De Dreu et al., 2008; Hirt et al., 2008), with the elation, flight of ideas and general hyper-creativity of manic states being relevant in this context (Jamison, 1994).
‘The secret to happiness is freedom’. (Thucydides c. 450BC)
It is presumed that the brain (like the mind) functions in a freer, less constrained manner during creative states, as during positive mood (Martindale, 2007) – although this hypothesis needs to be better tested (although see Atasoy et al., 2017) – and imaging studies with potent serotonergic compounds may help in this regard (Carhart-Harris et al., 2012a, 2014d, 2015b, 2016d; Heifets and Malenka, 2016; Roseman et al., 2014). It is commonplace to refer to depressive states as excessively rigid (Holtzheimer and Mayberg, 2011); being characterised by emotional withdrawal and anhedonia, and impaired and pessimistically biased cognition (Berman et al., 2011; Holtzheimer and Mayberg, 2011), whereas the psychedelic experience is often described as psychologically liberated (Turton et al., 2014; Watts et al., 2017) and functional neuroimaging findings support such a description (e.g. Petri et al., 2014). A recent qualitative analysis of treatment responses to psilocybin for depression suggested that successful treatment response is characterised by a sense of having been psychologically ‘reset’, with renewed feelings of ‘connection’ and emotional ‘acceptance’ post-treatment (Roseman et al., 2017b; Watts et al., 2017). Moreover, pre- versus post-treatment fMRI data from our psilocybin for treatment-resistant depression trial suggest a potential neurobiological counterpart to the psychological notion of ‘reset’ (Carhart-Harris et al., 2017b).
Limitations
It is appropriate to acknowledge some of limitations of this review. Only two serotonin receptor subtypes have been discussed in depth and it would be wrong to dismiss the contribution of the others. For example, some relatively new antidepressants have an important (antagonist) action at 5-HT2C receptors (which has secondary faciliatory effects on DA transmission) (MacIsaac et al., 2014) and others, such as vortioxetine, have appreciable affinities for several other 5-HT receptors (Riga et al., 2016; Thase et al., 2016) – perhaps most notably, the 5-HT6 receptor (Karila et al., 2015)). Similarly, we did not address literature on functional selectivity or agonist trafficking (Berg et al., 1998; Gray and Roth, 2001; Meana, 2013) and neither have we discussed the role of heterodimers in serotonergic and particularly 5-HT2AR functioning (Gonzalez-Maeso, 2011, 2014; Gonzalez-Maeso and Sealfon, 2012), nor the role of glutamatergic mechanisms that follow 5-HT2AR signalling and how these are involved in plasticity (Aghajanian and Marek, 1999). It should also be acknowledged that much importance has been ascribed to psychedelics’ 5-HT2AR agonist properties but many of the psychedelic compounds featured also possess considerable actions at other 5-HT receptors, including the 5-HT1AR (Nichols, 2004). Although we acknowledge this limitation, we also wish to emphasise that the evidence is compelling that 5-HT2AR agonism is key to psychedelics’ most characteristic effects (Halberstadt, 2015), 5-HT1AR agonism attenuates rather than augments these effects (Pokorny et al., 2016; Strassman, 1996) and more selective 5-HT2AR agonists appear to have the same quintessential psychological effects as the less selective psychedelics (Halberstadt, 2017).
We acknowledge that what is presented here is a simplified and therefore incomplete picture of brain serotonin function. This was an intentional approach (and compromise) however, as our main aim was not to produce an exhaustive review of serotonin transmission at its many receptors but rather distil it down to some key principles. We chose to focus on the 5-HT1A and 2A receptors because we felt that the functions associated with their signalling give the most comprehensive perspective of the general functioning of brain serotonin transmission. These two receptors are more implicated in the pharmacology of major psychiatric disorders than any of the other 5-HT receptor subtypes (Artigas et al., 2013b; Azmitia, 2007) – although others have highlighted the 5-HT1B receptor using a similar argument (Nautiyal and Hen, 2017) and it must be conceded that wealth of data does not necessarily imply strength of relationship. However, that the 5-HT1A and 2A receptors have opposite effects on single cell activity has long been a matter of intrigue (Araneda and Andrade, 1991). Crucially, that these receptors also seem to subserve distinct functions (Table 1) implies that the 5-HT system is not just diverse, but adaptive. We propose that the 5-HT system is specifically adaptive to the severity of adversity and whether it is better to passively tolerate it (with the assistance of 5-HT1AR signalling) or more actively respond it via a major change in perspective and/or behaviour (with the assistance of 5-HT2AR signalling).
Another criticism of this paper is that it has focused too much on 5-HT2AR agonist psychedelics and MDMA, rather than on classical preclinical behavioural literature and less potent serotonergic manipulations. In defence of our approach, the primacy we have given to research on psychedelics has allowed us to conceive a truly novel model of brain serotonin function. The most unique component of our model is pathway 2 (Figure 3), i.e. that 5-HT2AR signalling mediates plasticity related processes in aid of active coping. That this pathway has not previously been emphasised in models of serotonin function may have been due to a historical focus on the association between 5-HT2AR agonism and pathology and an insufficient willingness to acknowledge and endeavour to study these drugs’ complex subjective effects. We share the view of others (Grof, 1979; Heifets and Malenka, 2016) that 5-HT2AR agonist psychedelics and MDMA are remarkably powerful tools for studying the human brain and mind – and their scientific and medicinal value has not yet been properly appreciated (Carhart-Harris and Goodwin, 2017). We also believe that human studies with these compounds can be done safely if appropriate safeguards are heeded (Johnson et al., 2008).
It could be argued that too much emphasis has been placed on extreme states in this paper that are not relevant to normal physiological conditions. Basal 5-HT2AR signalling has shown to be important for the maintenance of normal levels of cognitive flexibility (Boulougouris et al., 2008; Clarke et al., 2004, 2007) and may also account for traits such as high ‘absorption’ (Ott et al., 2005). We subscribe to the principle that challenging a system with an extreme perturbation can yield especially valuable insights about its normal functioning, by pushing it to and beyond its limits. Moreover, given that evolutionary pressures are major drivers of adaptation and change, understanding how a particular function operates during extreme conditions (e.g. when one’s life is in danger), may be particularly informative about why that function exists at all. It seems reasonable to infer that states induced by MDMA and 5-HT2AR agonist psychedelics may be possible to achieve without these drugs, if only at an attenuated level. These drugs may therefore justifiably be considered ‘unveilers of function’. Note: the term ‘psychedelic’ literally means ‘mind-revealing’.
Relatedly, it is intriguing to speculate that 5-HT2AR signalling may have played an important role in human evolutionary as well as ontogenetic development, perhaps through enhancing plasticity and adaptability during extreme conditions. The 5-HT2AR is densest in evolutionary recent brain regions (Beliveau et al., 2016; Erritzoe et al., 2009; Ettrup et al., 2014, 2016; Varnas et al., 2004; ). Indeed, it is readily apparent in Figure 1 that 5-HT2AR expression is especially dense in regions of the so-called default-mode network, which is associated with especially high-level psychological functions, such as self-consciousness and the ‘self’ or ‘ego’ itself (Carhart-Harris and Friston, 2010) as well as the acute network level effects of psychedelics, as determined by human neuroimaging studies (Carhart-Harris et al., 2014). By body weight, humans have vastly more cortex than other species (MacLean, 1990; Molnar et al., 2014) (where 5-HT2ARs are densest (Ettrup et al., 2014)) and our remarkable adaptability is one of our most defining species traits (Anton et al., 2014) – as is our sense of self. It has been hypothesised by a popular proponent of psychedelic drug-use (Terrence McKenna) that ingestion of naturally occurring psychedelics (e.g. psilocybe mushrooms) catalysed the evolution of the human neocortex (Abraham et al., 1998). A perhaps more plausible (and less psychedelic-centric) alternative however, is that non-linearities evolved in the serotonergic system (Erritzoe et al., 2010; Jansson et al., 2001) that conferred optimal adaptability, including a capacity to switch to greater 5-HT2AR signalling when conditions demand it (such as during extreme adversity). Future work may endeavour to test the hypothesis that 5-HT2AR signalling serves an exceptional function in humans. The vastness of our 5-HT2AR dense cortex suggests that this hypothesis is worth exploring, and the development of agonist radioligands that can label the 5-HT2AR in its high affinity state may help us in this regard (Ettrup et al., 2014, 2016; Jorgensen et al., 2016).
Regarding neuroimaging the psychedelic state, this is a nascent and fast-moving field and it would be beyond the scope of this article to discuss the relevant published findings in detail (this area is deserving of its own review paper). Suffice to say that an emergent principle from the various studies is that the brain is uncharacteristically ‘entropic’ in the psychedelic state (Carhart-Harris et al., 2014), reflecting a greatly heightened plasticity in which old material may be unlearned (consistent with the principles of extinction learning) and new ideas and associations learned.
It might be argued (unfairly in our view) that the present contribution on the function of brain serotonin has not added anything new to previous models (Andrews et al., 2015; Azmitia, 2007; Branchi, 2011; Dayan and Huys, 2009; Deakin, 1998). We acknowledge that the model presented here has been much inspired by previous attempts to resolve this enigma but feel it also significantly advances on them and is entirely novel in its own right. It integrates findings that were inspirational for previous models but also assimilates recent and (perhaps somewhat overlooked) data on the brain and behavioural effects of potent serotonergic drugs such as MDMA and the 5-HT2AR agonist psychedelics. Previous models acknowledged the role of hippocampal 5-HT1AR signalling in resilience (Deakin, 2013; Deakin and Graeff, 1991) but we have significantly extended on this by our thorough coverage of 5-HT2AR functioning and its mediation of plasticity in aid of optimal adaptability.
Regarding specific past contributions, we acknowledge the work of Deakin and Graeff (Deakin, 2013; Deakin and Graeff, 1991) and others (Cools et al., 2008; Crockett et al., 2009; Wise et al., 1970) concerning the role of 5-HT in aversive processing, plus the increasingly compelling work on serotonin’s role in promoting patience (Fonseca et al., 2015; Miyazaki et al., 2012, 2014) and collectively relate these to our hypothesis that postsynaptic 5-HT1AR signalling mediates passive coping in response to adversity. It is worth commenting on a nuance here: in Deakin and Graeff’s model, 5-HT1AR signalling is linked to chronic adversity – which we do not dispute; however, we would argue that 5-HT2AR signalling becomes increasingly relevant as the severity of adversity reaches a critical point. Indeed, we have emphasised the importance of the severity of adversity in our model – but it may be worthwhile to also consider the role of the chronicity of adversity in determining the differential engagement of 5-HT receptor subtypes (Cohen et al., 2015; Dayan and Huys, 2009).
We also acknowledge the increasingly appealing perspectives of Branchi (2011), Belsky et al. (2009) and others (Homberg, 2012) concerning serotonin and plasticity, and relate this to our hypothesis that 5-HT2AR signalling mediates plasticity in aid of optimal adaptability. We acknowledge Andrew et al.’s hypothesis of serotonin mediating an adaptive homeostasis (Andrews et al., 2015) (see also Hale et al. (2013)) and believe this could be broadly related to our bipartite model. However, we feel our model is more psychologically focused, receptor specific, and consistent with the classical view that enhanced 5-HT transmission (within certain bounds and contexts) is conducive to positive mood. Perspectives such as Andrews and colleagues (2015) that challenge this view, cite, among other things, the relationship between punishment, 5-HT release and depression – to endorse the perspective that serotonergic functioning is elevated in depression (Barton et al., 2008)). Consistent with the classical (Wise et al., 1970) and arguably still dominant perspective (Cowen and Browning, 2015) however, our view is that increased 5-HT release in response to adversity is functional rather than pathological, serving to moderate stress via postsynaptic 5-HT1AR signalling, and in extreme cases, initiate a rapid plasticity in the service of major change – via 5-HT2AR signalling.
Conclusions: the function of brain serotonin
This paper has sought to address a major unresolved problem in neuropsychopharmacology, namely what is the function of brain serotonin? It proposes that the principal function of brain serotonin is to enhance adaptive responses to adverse conditions via two distinct pathways: (1) a passive coping pathway which improves stress tolerability; and (2) an active coping pathway associated with heightened plasticity, which, with support, can improve an organism’s ability to identify and overcome source(s) of stress by changing outlook and/or behaviour. Crucially, we propose that these two functions are mediated by signalling at postsynaptic 5-HT1A and 5-HT2A receptors respectively, with 5-HT1AR signalling dominating under ordinary conditions but 5-HT2AR signalling becoming increasingly operative as the level of adversity reaches a critical point.
We suggest that the two functions of interest (5-HT1AR-mediated stress relief and 5-HT2AR-mediated plasticity) are sufficiently distinct – and may even be mutually oppositional in certain contexts (see also Azmitia, 2001), evoking dilemmas over whether it is better to passively endure or actively approach, and in so doing, initiate some sort of fundamental change – with the potential for major resolution. This rule may not be absolute however, and the two functions may also be complementary, e.g. in the case of enhanced serotonin functioning with chronic SSRI use – or indeed with normal basal 5-HT functioning, facilitating improved endurance and plasticity (Clarke et al., 2004, 2007; Mithoefer et al., 2011, 2016; van Apeldoorn et al., 2008).
Despite this complementarity, we do anticipate that conventional serotonergic antidepressants such as the SSRIs and classic psychedelics such as psilocybin may become competitive options for the treatments of certain disorders such as depression; most fundamentally because they work via distinct pathways (i.e. 5-HT1AR versus the 5-HT2AR signalling) – but also because they cannot easily be taken in combination, i.e. conventional antidepressants attenuate the characteristic psychological effects of psychedelics (Bonson et al., 1996; Bonson and Murphy, 1996). SSRIs are established evidence-based treatments for anxiety and major depression (Baldwin et al., 2016; Hieronymus et al., 2016), whereas psychedelics are experimental medicines in an early phase of development (Carhart-Harris and Goodwin, 2017; Carhart-Harris et al., 2016). However, if evidence supporting the therapeutic value of psychedelics accrues – as we anticipate, and it is increasingly shown that their therapeutic mechanisms are significantly distinct from those of conventional medications, then this will open-up new and potentially empowering options for patients and clinicians (as well as a real potential for resistance – however it may arise). For the brave new psychiatry of the future – that many would like to see (Miller, 2010) – decisions about whether to passively endure or actively address, may become increasingly pertinent.
‘Progress is impossible without change, and those who cannot change their minds cannot change anything’. (George Bernard Shaw)
Acknowledgments
RCH would like to thank Samantha Strong for the illustrations in Figure 3 and David Erritzoe for intellectual input. This article’s central thesis was conceived of by RCH and he wrote the paper. DJN provided intellectual and editorial support
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr Robin Carhart-Harris is supported by the Alex Mosley Charitable Trust.
References
- Abraham R, McKenna T, Sheldrake R. (1998) The Evolutionary Mind: Trialogues at the EDGE OF THE UNThinkable, Santa Cruz, California: Trialogue. [Google Scholar]
- Achor RW, Hanson NO, Gifford RW., Jr (1955) Hypertension treated with Rauwolfia serpentina (whole root) and with reserpine; controlled study disclosing occasional severe depression. J Am Med Assoc 159: 841–845. [DOI] [PubMed] [Google Scholar]
- Adell A, Carceller A, Artigas F. (1991) Regional distribution of extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in the brain of freely moving rats. J Neurochem 56: 709–712. [DOI] [PubMed] [Google Scholar]
- Adell A, Casanovas JM, Artigas F. (1997) Comparative study in the rat of the actions of different types of stress on the release of 5-HT in raphe nuclei and forebrain areas. Neuropharmacology 36: 735–741. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. (1999) Serotonin and hallucinogens. Neuropsychopharmacology 21: 16S-23S. [DOI] [PubMed] [Google Scholar]
- Alboni S, van Dijk RM, Poggini S, et al. (2017) Fluoxetine effects on molecular, cellular and behavioral endophenotypes of depression are driven by the living environment. Mol Psychiatry 22: 635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloyo VJ, Dave KD. (2007) Behavioral response to emotional stress in rabbits: role of serotonin and serotonin2A receptors. Behav Pharmacol 18: 651–659. [DOI] [PubMed] [Google Scholar]
- Amargos-Bosch M, Bortolozzi A, Puig MV, et al. (2004) Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cerebral Cortex (New York, NY: 1991) 14: 281–299. [DOI] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, et al. (2005) Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nature Neuroscience 8: 365–371. [DOI] [PubMed] [Google Scholar]
- Amat J, Matus-Amat P, Watkins LR, et al. (1998) Escapable and inescapable stress differentially and selectively alter extracellular levels of 5-HT in the ventral hippocampus and dorsal periaqueductal gray of the rat. Brain Res 797: 12–22. [DOI] [PubMed] [Google Scholar]
- Anastasio NC, Stutz SJ, Fink LH, et al. (2015) Serotonin (5-HT) 5-HT2A Receptor (5-HT2AR):5-HT2CR Imbalance in Medial Prefrontal Cortex Associates with Motor Impulsivity. ACS Chem Neurosci 6: 1248–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson IM, Parry-Billings M, Newsholme EA, et al. (1990) Decreased plasma tryptophan concentration in major depression: relationship to melancholia and weight loss. J Affect Disord 20: 185–191. [DOI] [PubMed] [Google Scholar]
- Andrade R. (2011) Serotonergic regulation of neuronal excitability in the prefrontal cortex. Neuropharmacology 61: 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PW, Bharwani A, Lee KR, et al. (2015) Is serotonin an upper or a downer? The evolution of the serotonergic system and its role in depression and the antidepressant response. Neuroscience and Biobehavioral Reviews 51: 164–188. [DOI] [PubMed] [Google Scholar]
- Anisman H, Du L, Palkovits M, et al. (2008) Serotonin receptor subtype and p11 mRNA expression in stress-relevant brain regions of suicide and control subjects. Journal of Psychiatry & Neuroscience: JPN 33: 131–141. [PMC free article] [PubMed] [Google Scholar]
- Antkiewicz-Michaluk L, Wasik A, Mozdzen E, et al. (2014) Antidepressant-like effect of tetrahydroisoquinoline amines in the animal model of depressive disorder induced by repeated administration of a low dose of reserpine: behavioral and neurochemical studies in the rat. Neurotox Res 26: 85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton SC, Potts R, Aiello LC. (2014) Human evolution. Evolution of early Homo: an integrated biological perspective. Science (New York, NY) 345: 1236828. [DOI] [PubMed] [Google Scholar]
- Araneda R, Andrade R. (1991) 5-Hydroxytryptamine2 and 5-hydroxytryptamine 1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience 40: 399–412. [DOI] [PubMed] [Google Scholar]
- Arnone D, McKie S, Elliott R, et al. (2012) Increased amygdala responses to sad but not fearful faces in major depression: relation to mood state and pharmacological treatment. Am J Psychiatry 169: 841–850. [DOI] [PubMed] [Google Scholar]
- Artigas F. (2013. a) Future directions for serotonin and antidepressants. ACS Chem Neurosci 4: 5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artigas F. (2013. b) Serotonin receptors involved in antidepressant effects. Pharmacol Ther 137: 119–131. [DOI] [PubMed] [Google Scholar]
- Artigas F. (2015) Developments in the field of antidepressants, where do we go now? Eur Neuropsychopharmacol 25: 657–670. [DOI] [PubMed] [Google Scholar]
- Asberg M, Traskman L, Thoren P. (1976) 5-HIAA in the cerebrospinal fluid. A biochemical suicide predictor? Arch Gen Psychiatry 33: 1193–1197. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Isen AM, Turken, et al. (1999) A neuropsychological theory of positive affect and its influence on cognition. Psychol Rev 106: pp. [DOI] [PubMed] [Google Scholar]
- Atasoy SR R, Kaelen M, Kringelbach M, et al. (2017) The neural correlates of LSD experience with connectome-specific harmonic waves. In preparation. [Google Scholar]
- Attar-Levy D, Martinot JL, Blin J, et al. (1999) The cortical serotonin(2) receptors studied with positron-emission tomography and [F-18]-setoperone during depressive illness and antidepressant treatment with clomipramine. Biol Psychiatry 45: 180–186. [DOI] [PubMed] [Google Scholar]
- Audero E, Mlinar B, Baccini G, et al. (2013) Suppression of serotonin neuron firing increases aggression in mice. J Neurosci 33: 8678–8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod J, Inscoe JK. (1963) The uptake and binding of circulating serotonin and the effect of drugs. J Pharmacol Exp Ther 141: 161–165. [PubMed] [Google Scholar]
- Azmitia EC. (2001) Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res Bull 56: 413–424. [DOI] [PubMed] [Google Scholar]
- Azmitia EC. (2007) Serotonin and brain: evolution, neuroplasticity, and homeostasis. International review of neurobiology 77: 31–56. [DOI] [PubMed] [Google Scholar]
- Bak P. (1997) How nature works: the science of self-organized criticality, Oxford: Oxford University Press. [Google Scholar]
- Baldwin DS, Asakura S, Koyama T, et al. (2016) Efficacy of escitalopram in the treatment of social anxiety disorder: A meta-analysis versus placebo. Eur Neuropsychopharmacol 26: 1062–1069. [DOI] [PubMed] [Google Scholar]
- Barker SA, McIlhenny EH, Strassman R. (2012) A critical review of reports of endogenous psychedelic N, N-dimethyltryptamines in humans: 1955-2010. Drug Test Anal 4: 617–635. [DOI] [PubMed] [Google Scholar]
- Barre A, Berthoux C, De Bundel D, et al. (2016) Presynaptic serotonin 2A receptors modulate thalamocortical plasticity and associative learning. Proc Natl Acad Sci USA 113: E1382–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett FS, Bradstreet MP, Leoutsakos JS, et al. (2016) The challenging experience questionnaire: characterization of challenging experiences with psilocybin mushrooms. J Psychopharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth M, Kriston L, Klostermann S, et al. (2016) Efficacy of selective serotonin reuptake inhibitors and adverse events: meta-regression and mediation analysis of placebo-controlled trials. Br J Psychiatry 208: 114–119. [DOI] [PubMed] [Google Scholar]
- Barton DA, Esler MD, Dawood T, et al. (2008) Elevated brain serotonin turnover in patients with depression: effect of genotype and therapy. Arch Gen Psychiatry 65: 38–46. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Rothman RB. (2008) Locomotor stimulation produced by 3,4-methylenedioxymethamphetamine (MDMA) is correlated with dialysate levels of serotonin and dopamine in rat brain. Pharmacol Biochem Behav 90: 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister D, Barnes G, Giaroli G, et al. (2014) Classical hallucinogens as antidepressants? A review of pharmacodynamics and putative clinical roles. Ther Adv Psychopharmacol 4: 156–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Cecchi GA, Slezak DF, et al. (2014) A window into the intoxicated mind? Speech as an index of psychoactive drug effects. Neuropsychopharmacology 39: 2340–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Hyman D, de Wit H. (2010) Is ecstasy an ‘empathogen’? Effects of +/-3,4-methylenedioxymethamphetamine on prosocial feelings and identification of emotional states in others. Biol Psychiatry 68: 1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Phan KL, Angstadt M, et al. (2009) Effects of MDMA on sociability and neural response to social threat and social reward. Psychopharmacology (Berl) 207: 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beheydt LL, Schrijvers D, Docx L, et al. (2015) Cognitive and psychomotor effects of three months of escitalopram treatment in elderly patients with major depressive disorder. J Affect Disord 188: 47–52. [DOI] [PubMed] [Google Scholar]
- Beique J-C, Campbell B, Perring P, et al. (2004) Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors. J Neurosci 24: 4807–4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekris S, Antoniou K, Daskas S, et al. (2005) Behavioural and neurochemical effects induced by chronic mild stress applied to two different rat strains. Behavioural brain research 161: 45–59. [DOI] [PubMed] [Google Scholar]
- Beliveau V, Ganz M, Feng L, et al. (2016) A high-resolution in vivo atlas of the human brain’s serotonin system. J Neurosci 37:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, et al. (2009) Vulnerability genes or plasticity genes? Mol Psychiatry 14: 746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pluess M. (2009) Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull 135: 885–908. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. (2013) Beyond risk, resilience, and dysregulation: phenotypic plasticity and human development. Dev Psychopathol 25: 1243–1261. [DOI] [PubMed] [Google Scholar]
- Ben-Naim A. (2007) Entropy Demystified: The Second Law Reduced to Plain Common Sense, Hackensack, N.J.: World Scientific. [Google Scholar]
- Ben-Naim A. (2008) A Farewell to Entropy: Statistical Thermodynamics Based on Information : S=logW, Hackensack, NJ; London.: World Scientific. [Google Scholar]
- Benekareddy M, Goodfellow NM, Lambe EK, et al. (2010) Enhanced function of prefrontal serotonin 5-HT(2) receptors in a rat model of psychiatric vulnerability. J Neurosci 30: 12138–12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneytez ME, Lopez Rodriguez ML, Rosado ML, et al. (1998) Preclinical pharmacology of B-20991, a 5-HT1A receptor agonist with anxiolytic activity. Eur J Pharmacol 344: 127–135. [DOI] [PubMed] [Google Scholar]
- Berg KA, Maayani S, Goldfarb J, et al. (1998) Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol Pharmacol 54: 94–104. [PubMed] [Google Scholar]
- Berman MG, Peltier S, Nee DE, et al. (2011) Depression, rumination and the default network. Soc Cogn Affect Neurosci 6: 548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. (1998) What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev 28: 309–369. [DOI] [PubMed] [Google Scholar]
- Berton O, Aguerre S, Sarrieau A, et al. (1998) Differential effects of social stress on central serotonergic activity and emotional reactivity in Lewis and spontaneously hypertensive rats. Neuroscience 82: 147–159. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Hinz R, Taylor M, et al. (2006) Increased 5-HT(2A) receptor binding in euthymic, medication-free patients recovered from depression: a positron emission study with [(11)C]MDL 100,907. Am J Psychiatry 163: 1580–1587. [DOI] [PubMed] [Google Scholar]
- Blanco C, Bragdon LB, Schneier FR, et al. (2013) The evidence-based pharmacotherapy of social anxiety disorder. Int J Neuropsychopharmacol 16: 235–249. [DOI] [PubMed] [Google Scholar]
- Bland ST, Hargrave D, Pepin JL, et al. (2003. a) Stressor controllability modulates stress-induced dopamine and serotonin efflux and morphine-induced serotonin efflux in the medial prefrontal cortex. Neuropsychopharmacology 28: 1589–1596. [DOI] [PubMed] [Google Scholar]
- Bland ST, Twining C, Watkins LR, et al. (2003. b) Stressor controllability modulates stress-induced serotonin but not dopamine efflux in the nucleus accumbens shell. Synapse 49: 206–208. [DOI] [PubMed] [Google Scholar]
- Blier P, Bergeron R, de Montigny C. (1997) Selective activation of postsynaptic 5-HT1A receptors induces rapid antidepressant response. Neuropsychopharmacology 16: 333–338. [DOI] [PubMed] [Google Scholar]
- Blier P, Ward NM. (2003) Is there a role for 5-HT1A agonists in the treatment of depression? Biological Psychiatry 53: 193–203. [DOI] [PubMed] [Google Scholar]
- Blue ME, Yagaloff KA, Mamounas LA, et al. (1988) Correspondence between 5-HT2 receptors and serotonergic axons in rat neocortex. Brain Res 453: 315–328. [DOI] [PubMed] [Google Scholar]
- Bogenschutz MP, Forcehimes AA, Pommy JA, et al. (2015) Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol 29: 289–299. [DOI] [PubMed] [Google Scholar]
- Bogenschutz MP, Johnson MW. (2016) Classic hallucinogens in the treatment of addictions. Prog Neuropsychopharmacol Biol Psychiatry 64: 250–258. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Hen R, Underwood MD, et al. (2012) Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression. Biol Psychiatry 72: 562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Hen R, et al. (2009) Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology 34: 2376–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwig TG. (2014) Neuroimaging and electroconvulsive therapy: a review. J ECT 30, 138–142. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Buckholtz JW, Murphy DL. (1996) Chronic administration of serotonergic antidepressants attenuates the subjective effects of LSD in humans. Neuropsychopharmacology 14: 425–436. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Murphy DL. (1996) Alterations in responses to LSD in humans associated with chronic administration of tricyclic antidepressants, monoamine oxidase inhibitors or lithium. Behav Brain Res 73: 229–233. [DOI] [PubMed] [Google Scholar]
- Boothman LJ, Allers KA, Rasmussen K, et al. (2003) Evidence that central 5-HT2A and 5-HT2B/C receptors regulate 5-HT cell firing in the dorsal raphe nucleus of the anaesthetised rat. Br J Pharmacol 139: 998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose SK, Mehta MA, Selvaraj S, et al. (2011) Presynaptic 5-HT1A is related to 5-HTT receptor density in the human brain. Neuropsychopharmacology 36: 2258–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouckaert F, Sienaert P, Obbels J, et al. (2014) ECT: its brain enabling effects: a review of electroconvulsive therapy-induced structural brain plasticity. J ECT 30(2):143–51. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Glennon JC, Robbins TW. (2008) Dissociable effects of selective 5-HT2A and 5-HT2C receptor antagonists on serial spatial reversal learning in rats. Neuropsychopharmacology 33: 2007–2019. [DOI] [PubMed] [Google Scholar]
- Bouso JC, Gonzalez D, Fondevila S, et al. (2012) Personality, psychopathology, life attitudes and neuropsychological performance among ritual users of Ayahuasca: a longitudinal study. PLoS One 7: e42421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MB, Jr., Freedman DX. (1966) ‘Psychedelic’ experiences in acute psychoses. Archives of General Psychiatry 15: 240–248. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. (2005) Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev Psychopathol 17: 271–301. [DOI] [PubMed] [Google Scholar]
- Bradbury S, Bird J, Colussi-Mas J, et al. (2013) Acquisition of MDMA self-administration: pharmacokinetic factors and MDMA-induced serotonin release. Addict Biol. [DOI] [PubMed] [Google Scholar]
- Branchi I. (2011) The double edged sword of neural plasticity: increasing serotonin levels leads to both greater vulnerability to depression and improved capacity to recover. Psychoneuroendocrinology 36: 339–351. [DOI] [PubMed] [Google Scholar]
- Brauer LH, Johanson CE, Schuster CR, et al. (1996) Evaluation of phentermine and fenfluramine, alone and in combination, in normal, healthy volunteers. Neuropsychopharmacology 14: 233–241. [DOI] [PubMed] [Google Scholar]
- Bremner JD. (1984) Fluoxetine in depressed patients: a comparison with imipramine. J Clin Psychiatry 45: 414–419. [PubMed] [Google Scholar]
- Bressa GM, Marini S, Gregori S. (1987) Serotonin S2 receptors blockage and generalized anxiety disorders. A double-blind study on ritanserin and lorazepam. Int J Clin Pharmacol Res 7: 111–119. [PubMed] [Google Scholar]
- Brown GL, Goodwin FK, Ballenger JC, et al. (1979) Aggression in humans correlates with cerebrospinal fluid amine metabolites. Psychiatry Res 1: 131–139. [DOI] [PubMed] [Google Scholar]
- Brown GL, Linnoila MI. (1990) CSF serotonin metabolite (5-HIAA) studies in depression, impulsivity, and violence. J Clin Psychiatry 51 Suppl: 31–41; discussion 42-33. [PubMed] [Google Scholar]
- Buchborn T, Schroder H, Hollt V, et al. (2014) Repeated lysergic acid diethylamide in an animal model of depression: Normalisation of learning behaviour and hippocampal serotonin 5-HT2 signalling. J Psychopharmacol 28: 545–552. [DOI] [PubMed] [Google Scholar]
- Buckholtz NS, Zhou DF, Freedman DX, et al. (1990) Lysergic acid diethylamide (LSD) administration selectively downregulates serotonin2 receptors in rat brain. Neuropsychopharmacology 3: 137–148. [PubMed] [Google Scholar]
- Bui E, Orr SP, Jacoby RJ, et al. (2013) Two weeks of pretreatment with escitalopram facilitates extinction learning in healthy individuals. Hum Psychopharmacol 28: 447–456. [DOI] [PubMed] [Google Scholar]
- Burnet PWJ, Mead A, Eastwood SL, et al. (1995) Repeated ECS differentially affects rat brain 5-HT1A and 5-HT2A receptor expression. NeuroReport 6: 901–904. [DOI] [PubMed] [Google Scholar]
- Burnet PWJ, Sharp T, LeCorre SM, et al. (1999) Expression of 5-HT receptors and the 5-HT transporter in rat brain after electroconvulsive shock. Neurosci Lett 277: 79–82. [DOI] [PubMed] [Google Scholar]
- Busch AK, Johnson WC. (1950) L.S.D. 25 as an aid in psychotherapy; preliminary report of a new drug. Dis Nerv Syst 11: 241–243. [PubMed] [Google Scholar]
- Butler MO, Morinobu S, Duman RS. (1993) Chronic electrovonvulsive seizurs increase the expression of serotonin2 receptor mRNA in rat frontal cortex. J Neurochem 61: 1270–1276. [DOI] [PubMed] [Google Scholar]
- Butler T, Schofield PW, Greenberg D, et al. (2010) Reducing impulsivity in repeat violent offenders: an open label trial of a selective serotonin reuptake inhibitor. Aust N Z J Psychiatry 44: 1137–1143. [DOI] [PubMed] [Google Scholar]
- Cahir M, Ardis T, Reynolds GP, et al. (2007) Acute and chronic tryptophan depletion differentially regulate central 5-HT1A and 5-HT 2A receptor binding in the rat. Psychopharmacology (Berl) 190: 497–506. [DOI] [PubMed] [Google Scholar]
- Carbonaro TM, Bradstreet MP, Barrett FS, et al. (2016) Survey study of challenging experiences after ingesting psilocybin mushrooms: Acute and enduring positive and negative consequences. J Psychopharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris R, Brugger S, Nutt D, et al. (2013. a) Psychiatry’s next top model: cause for a re-think on drug models of psychosis and other psychiatric disorders. J Psychopharmacol 27: 771–778. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Bolstridge M, Rucker J, et al. (2016. b) Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry 3: 619–627. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Erritzoe D, Williams T, et al. (2012. a) Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci U S A 109: 2138–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Friston KJ. (2010) The default-mode, ego-functions and free-energy: a neurobiological account of Freudian ideas. Brain 133: 1265–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Kaelen M, Bolstridge M, et al. (2016. c) The paradoxical psychological effects of lysergic acid diethylamide (LSD). Psychol Med: 1–12. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Kaelen M, Whalley MG, et al. (2015. a) LSD enhances suggestibility in healthy volunteers. Psychopharmacology (Berl) 232: 785–794. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, King LA, Nutt DJ. (2011) A web-based survey on mephedrone. Drug Alcohol Depend 118: 19–22. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Leech R, Erritzoe D, et al. (2013. b) Functional connectivity measures after psilocybin inform a novel hypothesis of early psychosis. Schizophr Bull 39: 1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Leech R, Hellyer PJ, et al. (2014. b) The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci 8: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Leech R, Williams TM, et al. (2012. b) Implications for psychedelic-assisted psychotherapy: functional magnetic resonance imaging study with psilocybin. Br J Psychiatry 200: 238–244. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Murphy K, Leech R, et al. (2015. b) The effects of acutely administered 3,4-methylenedioxymethamphetamine on spontaneous brain function in healthy volunteers measured with arterial spin labeling and blood oxygen level-dependent resting state functional connectivity. Biol Psychiatry 78: 554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Muthukumaraswamy S, Roseman L, et al. (2016. d) Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc Natl Acad Sci U S A 113: 4853–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Nutt DJ. (2013) Experienced drug users assess the relative harms and benefits of drugs: a web-based survey. J Psychoactive Drugs 45: 322–328. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Wall MB, Erritzoe D, et al. (2014. d) The effect of acutely administered MDMA on subjective and BOLD-fMRI responses to favourite and worst autobiographical memories. Int J Neuropsychopharmacol 17: 527–540. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Goodwin GM. (2017) The therapeutic potential of psychedelic drugs: past, present and future. Neuropsychopharmacology. Epub [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, et al. (2017. a) Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology (Berlin). Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, et al. (2017. b) Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms. Nature Scientific Reports. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carli M, Baviera M, Invernizzi RW, et al. (2006) Dissociable contribution of 5-HT1A and 5-HT2A receptors in the medial prefrontal cortex to different aspects of executive control such as impulsivity and compulsive perseveration in rats. Neuropsychopharmacology 31: 757–767. [DOI] [PubMed] [Google Scholar]
- Carlsson A. (1981) Some current problems related to the mode of action of antidepressant drugs. Acta Psychiatr Scand Suppl 290: 63–66. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Jocic Z, Hall JM, et al. (1999) Mirtazapine augmentation in the treatment of refractory depression. J Clin Psychiatry 60: 45–49. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, et al. (2003) Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301: 386–389. [DOI] [PubMed] [Google Scholar]
- Catlow BJ, Song S, Paredes DA, et al. (2013) Effects of psilocybin on hippocampal neurogenesis and extinction of trace fear conditioning. Exp Brain Res 228: 481–491. [DOI] [PubMed] [Google Scholar]
- Cavus I, Duman RS. (2003) Influence of estradiol, stress, and 5-HT2A agonist treatment on brain-derived neurotrophic factor expression in female rats. Biol Psychiatry 54: 59–69. [DOI] [PubMed] [Google Scholar]
- Celada P, Bortolozzi A, Artigas F. (2013. a) Serotonin 5-HT1A receptors as targets for agents to treat psychiatric disorders: rationale and current status of research. CNS Drugs 27: 703–716. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig M, Amargos-Bosch M, et al. (2004) The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J Psychiatry Neurosci: JPN 29: 252–265. [PMC free article] [PubMed] [Google Scholar]
- Celada P, Puig MV, Artigas F. (2013. b) Serotonin modulation of cortical neurons and networks. Front Integr Neurosci 7: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Muller U, Blackwell AD, et al. (2006) Neurochemical modulation of response inhibition and probabilistic learning in humans. Science 311: 861–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J. (1966) The early symptoms of schizophrenia. Br J Psychiatry 112: 225–251. [DOI] [PubMed] [Google Scholar]
- Charig W, AI, Robinson JM, Nutt DJ, et al. (1986) L-typtophan and prolactin release: evidence for interaction between 5HT1 and 5HT2 receptors. Human Psychopharmacology 1: 93–97. [Google Scholar]
- Chattopadhyay A. (2007) Serotonin Receptors in Neurobiology. Boca Raton: CRC Press. [PubMed] [Google Scholar]
- Chepenik LG, Cornew LA, Farah MJ. (2007) The influence of sad mood on cognition. Emotion 7: 802–811. [DOI] [PubMed] [Google Scholar]
- Cherek DR, Lane SD. (2001) Acute effects of D-fenfluramine on simultaneous measures of aggressive escape and impulsive responses of adult males with and without a history of conduct disorder. Psychopharmacology (Berl) 157: 221–227. [DOI] [PubMed] [Google Scholar]
- Chiarotti F, Viglione A, Giuliani A, et al. (2017) Citalopram amplifies the influence of living conditions on mood in depressed patients enrolled in the STAR*D study. Transl Psychiatry 7(3):e1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilmonczyk Z, Bojarski AJ, Pilc A, et al. (2015) Functional selectivity and antidepressant activity of serotonin 1A receptor ligands. Int J Mol Sci 16: 18474–18506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, et al. (2004) Cognitive inflexibility after prefrontal serotonin depletion. Science 304: 878–880. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Dalley JW, et al. (2007) Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex 17: 18–27. [DOI] [PubMed] [Google Scholar]
- Cohen JY, Amoroso MW, Uchida N. (2015) Serotonergic neurons signal reward and punishment on multiple timescales. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. (1966) A classification of LSD complications. Psychosomatics 7: 182–186. [DOI] [PubMed] [Google Scholar]
- Cools R, Roberts AC, Robbins TW. (2008) Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn Sci 12: 31–40. [DOI] [PubMed] [Google Scholar]
- Coppen A. (1967) The biochemistry of affective disorders. Br J Psychiatry 113: 1237–1264. [DOI] [PubMed] [Google Scholar]
- Coppen A, Shaw DM, Farrell JP. (1963) Potentiation of the antidepressive effect of a monoamine-oxidase inhibitor by tryptophan. Lancet 1: 79–81. [DOI] [PubMed] [Google Scholar]
- Coppen AJ. (1969) Biochemical aspects of depression. Int Psychiatry Clin 6: 53–81. [PubMed] [Google Scholar]
- Corchs F, Nutt DJ, Hood S, et al. (2009) Serotonin and sensitivity to trauma-related exposure in selective serotonin reuptake inhibitors-recovered posttraumatic stress disorder. Biol Psychiatry 66: 17–24. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr., McCrae RR. (1988) Personality in adulthood: a six-year longitudinal study of self-reports and spouse ratings on the NEO Personality Inventory. J Pers Soc Psychol 54: 853–863. [DOI] [PubMed] [Google Scholar]
- Cowen PJ, Browning M. (2015) What has serotonin to do with depression? World Psychiatry 14: 158–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett MJ, Clark L, Robbins TW. (2009) Reconciling the role of serotonin in behavioral inhibition and aversion: acute tryptophan depletion abolishes punishment-induced inhibition in humans. J Neurosci 29: 11993–11999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran HV, D’Souza DC, Robbins TW, et al. (2009) Modelling psychosis. Psychopharmacology (Berl) 206: 513–514. [DOI] [PubMed] [Google Scholar]
- da Cunha-Bang S, Stenbaek DS, Holst K, et al. (2013) Trait aggression and trait impulsivity are not related to frontal cortex 5-HT2A receptor binding in healthy individuals. Psychiatry Res 212: 125–131. [DOI] [PubMed] [Google Scholar]
- Dayan P, Huys Q. (2015) Serotonin’s many meanings elude simple theories. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan P, Huys QJ. (2009) Serotonin in affective control. Annu Rev Neurosci 32: 95–126. [DOI] [PubMed] [Google Scholar]
- de Almeida J, Mengod G. (2007) Quantitative analysis of glutamatergic and GABAergic neurons expressing 5-HT(2A) receptors in human and monkey prefrontal cortex. J Neurochem 103: 475–486. [DOI] [PubMed] [Google Scholar]
- de Boer SF, Koolhaas JM. (2005) 5-HT1A and 5-HT1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis. Eur J Pharmacol 526: 125–139. [DOI] [PubMed] [Google Scholar]
- De Dreu CKW, Baas M, Nijstad BA. (2008) Hedonic tone and activation level in the mood-creativity link: Toward a dual pathway to creativity model. J Personality Social Psychol 94: 739–756. [DOI] [PubMed] [Google Scholar]
- Deakin J. (1998) The role of serotonin in depression and anxiety. Eur Psychiatry 13 Suppl 2: 57s–63s. [DOI] [PubMed] [Google Scholar]
- Deakin J. (2013) The origins of ‘5-HT and mechanisms of defence’ by Deakin and Graeff: a personal perspective. J Psychopharmacol 27: 1084–1089. [DOI] [PubMed] [Google Scholar]
- Deakin JB, Rahman S, Nestor PJ, et al. (2004) Paroxetine does not improve symptoms and impairs cognition in frontotemporal dementia: a double-blind randomized controlled trial. Psychopharmacology (Berl) 172: 400–408. [DOI] [PubMed] [Google Scholar]
- Deakin JF, Graeff FG. (1991) 5-HT and mechanisms of defence. J Psychopharmacol 5: 305–315. [DOI] [PubMed] [Google Scholar]
- Dean B, Tawadros N, Seo MS, et al. (2014) Lower cortical serotonin 2A receptors in major depressive disorder, suicide and in rats after administration of imipramine. Int J Neuropsychopharmacology 17: 895–906. [DOI] [PubMed] [Google Scholar]
- Dong J, de Montigny C, Blier P. (1998) Full agonistic properties of BAY x 3702 on presynaptic and postsynaptic 5-HT1A receptors electrophysiological studies in the rat hippocampus and dorsal raphe. J Pharmacol Expl Therapeutics 286: 1239–1247. [PubMed] [Google Scholar]
- Dougherty DM, Moeller FG, Bjork JM, et al. (1999) Plasma L-tryptophan depletion and aggression. Adv Exp Med Biol 467: 57–65. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Richard DM, James LM, et al. (2010) Effects of acute tryptophan depletion on three different types of behavioral impulsivity. Int J Tryptophan Res 3: 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolder PC, Schmid Y, Müller F, et al. (2016) LSD acutely impairs fear recognition and enhances emotional empathy and sociality. Neuropsychopharmacology 41:2638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler WW, Balieiro MC, Ferreira de Araujo L, et al. (2016) Culture as a mediator of gene-environment interaction: Cultural consonance, childhood adversity, a 2A serotonin receptor polymorphism, and depression in urban Brazil. Soc Sci Med 161: 109–117. [DOI] [PubMed] [Google Scholar]
- Duke AA, Begue L, Bell R, et al. (2013) Revisiting the serotonin-aggression relation in humans: a meta-analysis. Psychol Bull 139: 1148–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebdrup BH, Rasmussen H, Arnt J, et al. (2011) Serotonin 2A receptor antagonists for treatment of schizophrenia. Expert Opin Investig Drugs 20: 1211–1223. [DOI] [PubMed] [Google Scholar]
- Eisner B. (1997) Set, setting, and matrix. J Psychoactive Drugs 29: 213–216. [DOI] [PubMed] [Google Scholar]
- Engel K, Bandelow B, Gruber O, et al. (2009) Neuroimaging in anxiety disorders. J Neural Transm 116: 703–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erritzoe D, Frokjaer VG, Haugbol S, et al. (2009) Brain serotonin 2A receptor binding: relations to body mass index, tobacco and alcohol use. Neuroimage 46: 23–30. [DOI] [PubMed] [Google Scholar]
- Erritzoe D, Holst K, Frokjaer VG, et al. (2010) A nonlinear relationship between cerebral serotonin transporter and 5-HT(2A) receptor binding: an in vivo molecular imaging study in humans. J Neurosci 30: 3391–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettrup A, da Cunha-Bang S, McMahon B, et al. (2014) Serotonin 2A receptor agonist binding in the human brain with [C]Cimbi-36. J Cereb Bloodflow Metab 1188–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettrup A, Svarer C, McMahon B, et al. (2016) Serotonin 2A receptor agonist binding in the human brain with [(11)C]Cimbi-36: Test-retest reproducibility and head-to-head comparison with the antagonist [(18)F]altanserin. Neuroimage 130: 167–174. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Melega WP, Jorgensen MJ, et al. (2001) Social impulsivity inversely associated with CSF 5-HIAA and fluoxetine exposure in vervet monkeys. Neuropsychopharmacology 24: 370–378. [DOI] [PubMed] [Google Scholar]
- Ferres-Coy A, Santana N, Castane A, et al. (2013) Acute 5-HT(1)A autoreceptor knockdown increases antidepressant responses and serotonin release in stressful conditions. Psychopharmacology (Berl) 225: 61–74. [DOI] [PubMed] [Google Scholar]
- File SE, Gonzalez LE, Andrews N. (1996) Comparative study of pre- and postsynaptic 5-HT1A receptor modulation of anxiety in two ethological animal tests. J Neurosci 16: 4810–4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiocco AJ, Joober R, Poirier J, et al. (2007) Polymorphism of the 5-HT(2A) receptor gene: association with stress-related indices in healthy middle-aged adults. Front Behav Neurosci 1: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PJ, Tampakeras M, Sinyard J, et al. (2007) Opposing effects of 5-HT(2A) and 5-HT(2C) receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology (Berl) 195: 223–234. [DOI] [PubMed] [Google Scholar]
- Fonseca MS, Murakami M, Mainen ZF. (2015) Activation of dorsal raphe serotonergic neurons promotes waiting but is not reinforcing. Current Biology: CB 25: 306–315. [DOI] [PubMed] [Google Scholar]
- Frankel PS, Cunningham KA. (2002) The hallucinogen d-lysergic acid diethylamide (d-LSD) induces the immediate-early gene c-Fos in rat forebrain. Brain Res 958: 251–260. [DOI] [PubMed] [Google Scholar]
- Frecska E, More CE, Vargha A, et al. (2012) Enhancement of creative expression and entoptic phenomena as after-effects of repeated ayahuasca ceremonies. J Psychoactive Drugs 44: 191–199. [DOI] [PubMed] [Google Scholar]
- Friston K. (2010) The free-energy principle: a unified brain theory? Nature Rev Neurosci 11: 127–138. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Grasby PM, Frith CD, et al. (1991) The neurotransmitter basis of cognition: psychopharmacological activation studies using positron emission tomography. Ciba Found Symp 163: 76–87; discussion 87-92. [DOI] [PubMed] [Google Scholar]
- Froestl W, Muhs A, Pfeifer A. (2014) Cognitive enhancers (Nootropics). Part 1: drugs interacting with receptors. Update 2014. J Alzheimer’s Disease: JAD 41: 961–1019. [DOI] [PubMed] [Google Scholar]
- Frokjaer VG, Mortensen EL, Nielsen FA, et al. (2008) Frontolimbic serotonin 2A receptor binding in healthy subjects is associated with personality risk factors for affective disorder. Biol Psychiatry 63: 569–576. [DOI] [PubMed] [Google Scholar]
- Frye CG, Wardle MC, Norman GJ, et al. (2014) MDMA decreases the effects of simulated social rejection. Pharmacol Biochem Behav 117: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino K, Yoshitake T, Inoue O, et al. (2002) Increased serotonin release in mice frontal cortex and hippocampus induced by acute physiological stressors. Neurosci Lett 320: 91–95. [DOI] [PubMed] [Google Scholar]
- Furr A, Lapiz-Bluhm MD, Morilak DA. (2012) 5-HT2A receptors in the orbitofrontal cortex facilitate reversal learning and contribute to the beneficial cognitive effects of chronic citalopram treatment in rats. Int J Neuropsychopharmacol 15: 1295–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddum JH. (1953) Antagonism between lysergic acid diethylamide and 5-hydroxytryptamine. J Physiol 121: 15P. [PubMed] [Google Scholar]
- Gaddum JH. (1957) Serotonin-LSD interactions. Ann N Y Acad Sci 66: 643–647; discussion, 647-648. [DOI] [PubMed] [Google Scholar]
- Gallimore AR. (2015) Restructuring consciousness -the psychedelic state in light of integrated information theory. Front Hum Neurosci 9: 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamma A, Buck A, Berthold T, et al. (2000) 3,4-Methylenedioxymethamphetamine (MDMA) modulates cortical and limbic brain activity as measured by [H(2)(15)O]-PET in healthy humans. Neuropsychopharmacology 23: 388–395. [DOI] [PubMed] [Google Scholar]
- Gasser P, Holstein D, Michel Y, et al. (2014) Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis 202: 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser P, Kirchner K, Passie T. (2015) LSD-assisted psychotherapy for anxiety associated with a life-threatening disease: a qualitative study of acute and sustained subjective effects. J Psychopharmacol 29: 57–68. [DOI] [PubMed] [Google Scholar]
- Gee P, Schep LJ, Jensen BP, et al. (2016) Case series: toxicity from 25B-NBOMe–a cluster of N-bomb cases. Clin Toxicol (Phila) 54: 141–146. [DOI] [PubMed] [Google Scholar]
- Gerber DJ, Tonegawa S. (2004) Psychotomimetic effects of drugs–a common pathway to schizophrenia? N Engl J Med 350: 1047–1048. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Chen AC, Terwilliger R, et al. (2002) Modulation of DOI-induced increases in cortical BDNF expression by group II mGlu receptors. Pharmacol Biochem Behav 73: 317–326. [DOI] [PubMed] [Google Scholar]
- Gimpl MP, Gormezano I, Harvey JA. (1979) Effects of LSD on learning as measured by classical conditioning of the rabbit nictitating membrane response. J Pharmacol Exp Ther 208: 330–334. [PubMed] [Google Scholar]
- Glennon RA, Dukat M, Westkaemper RB (2000) Serotonin receptor subtypes and ligands. Neuropsychopharmacology – the Fourth generation of progress. [Google Scholar]
- Glennon RA, Seggel MR, Soine WH, et al. (1988) [125I]-1-(2,5-dimethoxy-4-iodophenyl)-2-amino-propane: an iodinated radioligand that specifically labels the agonist high-affinity state of 5-HT2 serotonin receptors. J Med Chem 31: 5–7. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, McKenney JD. (1984) Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci 35: 2505–2511. [DOI] [PubMed] [Google Scholar]
- Golembiowska K, Jurczak A, Kaminska K, et al. (2016) Effect of some psychoactive drugs used as ‘legal highs’ on brain neurotransmitters. Neurotox Res 29: 394–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J. (2011) 5HT(2A)-mGlu2 receptor heterocomplex: a new target for antipsychotic drugs. Current Neuropharmacology 9: 25–26. [Google Scholar]
- Gonzalez-Maeso J. (2014) Family a GPCR heteromers in animal models. Front Pharmacol 5: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Sealfon SC. (2012) Functional selectivity in GPCR heterocomplexes. Mini-Rev Medicinal Chem 12: 851–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopnik A. (2010) How babies think. Sci Am 303(1):76–81. [DOI] [PubMed] [Google Scholar]
- Gordon JA, Hen R. (2004) The serotonergic system and anxiety. Neuromolecular Med 5: 27–40. [DOI] [PubMed] [Google Scholar]
- Gould E. (1999) Serotonin and hippocampal neurogenesis. Neuropsychopharmacology 21: 46S–51S. [DOI] [PubMed] [Google Scholar]
- Gouzoulis E, Hermle L, Sass H. (1994) [Psychedelic experiences at the onset of productive episodes of endogenous psychoses]. Psychedelische Erlebnisse zu Beginn produktiver Episoden endogener Psychosen. Der Nervenarzt 65: 198–201. [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Heekeren K, Neukirch A, et al. (2005) Psychological effects of (S)-ketamine and N,N-dimethyltryptamine (DMT): a double-blind, cross-over study in healthy volunteers. Pharmacopsychiatry 38: 301–311. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Hermle L, Thelen B, et al. (1998) History, rationale and potential of human experimental hallucinogenic drug research in psychiatry. Pharmacopsychiatry 31 Suppl 2: 63–68. [DOI] [PubMed] [Google Scholar]
- Gray JA. (1983) A theory of anxiety: the role of the limbic system. Encephale 9: 161B–166B. [PubMed] [Google Scholar]
- Gray JA, Roth BL. (2001) Paradoxical trafficking and regulation of 5-HT(2A) receptors by agonists and antagonists. Brain Res Bull 56: 441–451. [DOI] [PubMed] [Google Scholar]
- Gregoire C. (2016) Everything you wanted to know about microdosing (but were afraid to ask). The Huffinton Post. [Google Scholar]
- Greyson B. (1983) The near-death experience scale. Construction, reliability, and validity. J Nervous Mental Dis 171: 369–375. [DOI] [PubMed] [Google Scholar]
- Greyson B. (1993) Varieties of near-death experience. Psychiatry 56: 390–399. [DOI] [PubMed] [Google Scholar]
- Greyson B. (2008) The near-death experience. Alternative therapies in health and medicine 14: 14; author reply 14-15. [PubMed] [Google Scholar]
- Greyson B, Bush NE. (1992) Distressing near-death experiences. Psychiatry 55: 95–110. [DOI] [PubMed] [Google Scholar]
- Griffiths R, Richards W, Johnson M, et al. (2008) Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. J Psychopharmacol 22: 621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Grob CS. (2010) Hallucinogens as medicine. Scientific American 303: 76–79. [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Carducci MA, et al. (2016) Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J Psychopharmacol 30: 1181–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Richards WA, et al. (2011) Psilocybin occasioned mystical-type experiences: immediate and persisting dose-related effects. Psychopharmacology (Berl) 218: 649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, McCann U, et al. (2006) Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl) 187: 268–283; discussion 284–292. [DOI] [PubMed] [Google Scholar]
- Grinspoon L, Bakalar JB. (1979) Psychedelic Drugs Reconsidered. New York: Basic Books. [Google Scholar]
- Grob CS, Danforth AL, Chopra GS, et al. (2011) Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry 68: 71–78. [DOI] [PubMed] [Google Scholar]
- Grof S. (1975) Realms of Human Unconscious: Observations from LSD Research, [S.l.]: Viking Press. [Google Scholar]
- Grof S. (1979) Realms of the Human Unconscious: Observations from LSD Research. London: Souvenir Press. [Google Scholar]
- Gronli J, Fiske E, Murison R, et al. (2007) Extracellular levels of serotonin and GABA in the hippocampus after chronic mild stress in rats. A microdialysis study in an animal model of depression. Behav Brain Res 181: 42–51. [DOI] [PubMed] [Google Scholar]
- Gross C, Zhuang X, Stark K, et al. (2002) Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature 416: 396–400. [DOI] [PubMed] [Google Scholar]
- Gross-Isseroff R, Salama D, Israeli M, et al. (1990) Autoradiographic analysis of age-dependent changes in serotonin 5-HT2 receptors of the human brain postmortem. Brain Res 519: 223–227. [DOI] [PubMed] [Google Scholar]
- Gur E, Lerer B, Newman ME. (1999) Chronic clomipramine and triiodothyronine increase serotonin levels in rat frontal cortex in vivo: relationship to serotonin autoreceptor activity. J Pharmacol Exp Ther 288: 81–87. [PubMed] [Google Scholar]
- Hajos M, Gartside SE, Varga V, et al. (2003) In vivo inhibition of neuronal activity in the rat ventromedial prefrontal cortex by midbrain-raphe nuclei: role of 5-HT1A receptors. Neuropharmacology 45: 72–81. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL. (2015) Recent advances in the neuropsychopharmacology of serotonergic hallucinogens. Behav Brain Res 277:99–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL. (2017) Pharmacology and toxicology of N-Benzylphenethylamine (‘NBOMe’) hallucinogens. Curr Top Behav Neurosci 32:283–311. [DOI] [PubMed] [Google Scholar]
- Hale MW, Raison CL, Lowry CA. (2013) Integrative physiology of depression and antidepressant drug action: implications for serotonergic mechanisms of action and novel therapeutic strategies for treatment of depression. Pharmacol Ther 137: 108–118. [DOI] [PubMed] [Google Scholar]
- Hall H, Farde L, Halldin C, et al. (2000) Autoradiographic localization of 5-HT(2A) receptors in the human brain using [(3)H]M100907 and [(11)C]M100907. Synapse 38: 421–431. [DOI] [PubMed] [Google Scholar]
- Harman WW, McKim RH, Mogar RE, et al. (1966) Psychedelic agents in creative problem-solving: a pilot study. Psychol Rep 19: 211–227. [DOI] [PubMed] [Google Scholar]
- Hartogsohn I. (2016) Set and setting, psychedelics and the placebo response: An extra-pharmacological perspective on psychopharmacology. J Psychopharmacol 30: 1259–1267. [DOI] [PubMed] [Google Scholar]
- Harvey BH, Naciti C, Brand L, et al. (2003) Endocrine, cognitive and hippocampal/cortical 5HT 1A/2A receptor changes evoked by a time-dependent sensitisation (TDS) stress model in rats. Brain Res 983: 97–107. [DOI] [PubMed] [Google Scholar]
- Harvey JA. (1996) Serotonergic regulation of associative learning. Behav Brain Res 73: 47–50. [DOI] [PubMed] [Google Scholar]
- Harvey JA. (2003) Role of the serotonin 5-HT(2A) receptor in learning. Learn Mem 10: 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey JA, Quinn JL, Liu R, et al. (2004) Selective remodeling of rabbit frontal cortex: relationship between 5-HT2A receptor density and associative learning. Psychopharmacology (Berl) 172: 435–442. [DOI] [PubMed] [Google Scholar]
- Harvey JA, Schlosberg AJ, Yunger LM. (1975) Behavioral correlates of serotonin depletion. Fed Proc 34: 1796–1801. [PubMed] [Google Scholar]
- Harvey ML, Swallows CL, Cooper MA. (2012) A double dissociation in the effects of 5-HT2A and 5-HT2C receptors on the acquisition and expression of conditioned defeat in Syrian hamsters. Behav Neurosci 126: 530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler F, Studerus E, Lindner K, et al. (2009) Investigation of serotonin-1A receptor function in the human psychopharmacology of MDMA. J Psychopharmacol 23: 923–935. [DOI] [PubMed] [Google Scholar]
- Heifets BD, Malenka RC. (2016) MDMA as a probe and treatment for social behaviors. Cell 166: 269–272. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, et al. (1998) Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci U S A 95: 15049–15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks PS, Johnson MW, Griffiths RR. (2015. a) Psilocybin, psychological distress, and suicidality. J Psychopharmacol 29: 1041–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks PS, Thorne CB, Clark CB, et al. (2015. b) Classic psychedelic use is associated with reduced psychological distress and suicidality in the United States adult population. J Psychopharmacol. [DOI] [PubMed] [Google Scholar]
- Hess SM, Doepfner W. (1961) Behavioral effects and brain amine content in rats. Arch Int Pharmacodyn Ther 134: 89–99. [PubMed] [Google Scholar]
- Hetem LA, de Souza CJ, Guimaraes ES, et al. (1996) Effect of d-fenfluramine on human experimental anxiety. Psychopharmacology (Berl) 127: 276–282. [PubMed] [Google Scholar]
- Hieronymus F, Emilsson JF, Nilsson S, et al. (2016) Consistent superiority of selective serotonin reuptake inhibitors over placebo in reducing depressed mood in patients with major depression. Mol Psychiatry 21: 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt ER, Devers EE, McCrea SM. (2008) I want to be creative: Exploring the role of hedonic contingency theory in the positive mood-cognitive flexibility link. J Personality Soc Psychol 94: 214–230. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Sharp T. (1991) Effect of the 5-HT1A receptor agonist 8-OH-DPAT on the release of 5-HT in dorsal and median raphe-innervated rat brain regions as measured by in vivo microdialysis. Life Sci 48: 1779–1786. [DOI] [PubMed] [Google Scholar]
- Hofmann A. (1980) LSD: My Problem Child. NY: McGraw-Hill. [Google Scholar]
- Holloway T, Moreno JL, Umali A, et al. (2013) Prenatal stress induces schizophrenia-like alterations of serotonin 2A and metabotropic glutamate 2 receptors in the adult offspring: role of maternal immune system. J Neurosci 33: 1088–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzheimer PE, Mayberg HS. (2011) Stuck in a rut: rethinking depression and its treatment. Trends Neurosci 34: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg JR. (2012) Serotonin and decision making processes. Neurosciand BiobehavRev 36: 218–236. [DOI] [PubMed] [Google Scholar]
- Horder J, Matthews P, Waldmann R. (2011) Placebo, prozac and PLoS: significant lessons for psychopharmacology. J Psychopharmacol 25: 1277–1288. [DOI] [PubMed] [Google Scholar]
- Hornung JP. (2003) The human raphe nuclei and the serotonergic system. J Chem Neuroanat 26: 331–343. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, et al. (1994) International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol Rev 46: 157–203. [PubMed] [Google Scholar]
- Hoyer D, Engel G, Kalkman HO. (1985) Molecular pharmacology of 5-HT1 and 5-HT2 recognition sites in rat and pig brain membranes: radioligand binding studies with [3H]5-HT, [3H]8-OH-DPAT, (-)[125I]iodocyanopindolol, [3H]mesulergine and [3H]ketanserin. Eur J Pharmacol 118: 13–23. [DOI] [PubMed] [Google Scholar]
- Huang GJ, Herbert J. (2005) The role of 5-HT1A receptors in the proliferation and survival of progenitor cells in the dentate gyrus of the adult hippocampus and their regulation by corticoids. Neuroscience 135: 803–813. [DOI] [PubMed] [Google Scholar]
- Hunt GE, McGregor IS, Cornish JL, et al. (2011) MDMA-induced c-Fos expression in oxytocin-containing neurons is blocked by pretreatment with the 5-HT-1A receptor antagonist WAY 100635. Brain Res Bull 86: 65–73. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Domes G, Liechti ME. (2012) MDMA enhances ‘mind reading’ of positive emotions and impairs ‘mind reading’ of negative emotions. Psychopharmacology (Berl) 222: 293–302. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Schmid Y, Simmler LD, et al. (2014. a) MDMA enhances emotional empathy and prosocial behavior. Soc Cogn Affect Neurosci 9: 1645–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Simmler LD, Schillinger N, et al. (2014. b) Pharmacokinetic and pharmacodynamic effects of methylphenidate and MDMA administered alone or in combination. Int J Neuropsychopharmacol 17: 371–381. [DOI] [PubMed] [Google Scholar]
- Iaria G, Fox CJ, Scheel M, et al. (2010) A case of persistent visual hallucinations of faces following LSD abuse: a functional Magnetic Resonance Imaging study. Neurocase 16: 106–118. [DOI] [PubMed] [Google Scholar]
- Idzikowski C, Cowen PJ, Nutt D, et al. (1987) The effects of chronic ritanserin treatment on sleep and the neuroendocrine response to L-tryptophan. Psychopharmacology (Berl) 93: 416–420. [DOI] [PubMed] [Google Scholar]
- Imoto Y, Kira T, Sukeno M, et al. (2015) Role of the 5-HT4 receptor in chronic fluoxetine treatment-induced neurogenic activity and granule cell dematuration in the dentate gyrus. Mol Brain 8: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbell H, Miner EJ, Logan CR. (1959) Relationships of psychotomimetic to anti-serotonin potencies of congeners of lysergic acid diethylamide (LSD-25). Psychopharmacologia 1: 20–28. [DOI] [PubMed] [Google Scholar]
- Ivgy-May N, Roth T, Ruwe F, et al. (2015) Esmirtazapine in non-elderly adult patients with primary insomnia: efficacy and safety from a 2-week randomized outpatient trial. Sleep Med 16: 831–837. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. (1992) Structure and function of the brain serotonin system. Physiolog Rev 72: 165–229. [DOI] [PubMed] [Google Scholar]
- Jamison KR. (1994) Touched with Fire: Manic-depressive Illness and the Artistic Temperament. New York; London: Free Press Paperbacks. [Google Scholar]
- Janiger O, Dobkin de Rios M. (1989) LSD and creativity. J Psychoactive Drugs 21: 129–134. [DOI] [PubMed] [Google Scholar]
- Jansson A, Tinner B, Bancila M, et al. (2001) Relationships of 5-hydroxytryptamine immunoreactive terminal-like varicosities to 5-hydroxytryptamine-2A receptor-immunoreactive neuronal processes in the rat forebrain. J Chem Neuroanat 22: 185–203. [DOI] [PubMed] [Google Scholar]
- Jarema M. (2007) Atypical antipsychotics in the treatment of mood disorders. Curr Opin Psychiatry 20: 23–29. [DOI] [PubMed] [Google Scholar]
- Jennings KA. (2013) A comparison of the subsecond dynamics of neurotransmission of dopamine and serotonin. ACS Chem Neurosci 4: 704–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings KA, Sheward WJ, Harmar AJ, et al. (2008) Evidence that genetic variation in 5-HT transporter expression is linked to changes in 5-HT2A receptor function. Neuropharmacology 54: 776–783. [DOI] [PubMed] [Google Scholar]
- Jha S, Rajendran R, Fernandes KA, et al. (2008) 5-HT2A/2C receptor blockade regulates progenitor cell proliferation in the adult rat hippocampus. Neurosci Lett 441: 210–214. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Cui C, Ge H, et al. (2016) Effect of 5-HT2A receptor polymorphisms and occupational stress on self-reported sleep quality: a cross-sectional study in Xinjiang, China. Sleep Med 20: 30–36. [DOI] [PubMed] [Google Scholar]
- Johnson M, Richards W, Griffiths R. (2008) Human hallucinogen research: guidelines for safety. J Psychopharmacol 22: 603–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Garcia-Romeu A, Griffiths RR. (2016) Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokela M, Keltikangas-Jarvinen L, Kivimaki M, et al. (2007) Serotonin receptor 2A gene and the influence of childhood maternal nurturance on adulthood depressive symptoms. Arch Gen Psychiatry 64: 356–360. [DOI] [PubMed] [Google Scholar]
- Jolas T, Schreiber R, Laporte AM, et al. (1995) Are postsynaptic 5-HT1A receptors involved in the anxiolytic effects of 5-HT1A receptor agonists and in their inhibitory effects on the firing of serotonergic neurons in the rat? J Pharmacol Exp Ther 272: 920–929. [PubMed] [Google Scholar]
- Jones KA, Srivastava DP, Allen JA, et al. (2009) Rapid modulation of spine morphology by the 5-HT2A serotonin receptor through kalirin-7 signaling. Proc Natl Acad Sci USA 106: 19575–19580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen LM, Weikop P, Villadsen J, et al. (2016) Cerebral 5-HT release correlates with [11C]Cimbi36 PET measures of 5-HT2A receptor occupancy in the pig brain. J Cereb Blood Flow Metab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi SH, Espinoza RT, Pirnia T, et al. (2016) Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry 79:282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelen M, Barrett FS, Roseman L, et al. (2015) LSD enhances the emotional response to music. Psychopharmacology (Berl) 232: 3607–3614. [DOI] [PubMed] [Google Scholar]
- Kaelen M, Roseman L, Kahan J, et al. (2016) LSD modulates music-induced imagery via changes in parahippocampal connectivity. Eur Neuropsychopharmacol. [DOI] [PubMed] [Google Scholar]
- Kamboj SK, Kilford EJ, Minchin S, et al. (2015) Recreational 3,4-methylenedioxy-N-methylamphetamine (MDMA) or ‘ecstasy’ and self-focused compassion: Preliminary steps in the development of a therapeutic psychopharmacology of contemplative practices. J Psychopharmacol 29: 961–970. [DOI] [PubMed] [Google Scholar]
- Kankaanpaa A, Meririnne E, Lillsunde P, et al. (1998) The acute effects of amphetamine derivatives on extracellular serotonin and dopamine levels in rat nucleus accumbens. Pharmacol Biochem Behav 59: 1003–1009. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, et al. (2011) The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited evidence of genetic moderation. Arch Gen Psychiatry 68: 444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karila D, Freret T, Bouet V, et al. (2015) Therapeutic potential of 5-HT6 receptor agonists. J Med Chem 58: 7901–7912. [DOI] [PubMed] [Google Scholar]
- Katagiri H, Kagaya A, Nakae S, et al. (2001) Modulation of serotonin2A receptor function in rats after repeated treatment with dexamethasone and L-type calcium channel antagonist nimodipine. Prog Neuropsychopharmacol Biol Psychiatry 25: 1269–1281. [DOI] [PubMed] [Google Scholar]
- Kawahara H, Yoshida M, Yokoo H, et al. (1993) Psychological stress increases serotonin release in the rat amygdala and prefrontal cortex assessed by in vivo microdialysis. Neurosci Lett 162: 81–84. [DOI] [PubMed] [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, et al. (2011) Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. Br J Pharmacol 164: 1949–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepser L-J, Homberg JR. (2015) The neurodevelopmental effects of serotonin: a behavioural perspective. Behav Brain Res 277: 3–13. [DOI] [PubMed] [Google Scholar]
- King AR, Martin IL, Melville KA. (1974) Reversal learning enhanced by lysergic acid diethylamide (LSD): concomitant rise in brain 5-hydroxytryptamine levels. Br J Pharmacol 52: 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Allen AR, Lucki I. (1995) Regional differences in the effects of forced swimming on extracellular levels of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res 682: 189–196. [DOI] [PubMed] [Google Scholar]
- Kirilly E, Benko A, Ferrington L, et al. (2006) Acute and long-term effects of a single dose of MDMA on aggression in Dark Agouti rats. Int J Neuropsychopharmacol 9: 63–76. [DOI] [PubMed] [Google Scholar]
- Kishi T, Yoshimura R, Kitajima T, et al. (2010) HTR2A is associated with SSRI response in major depressive disorder in a Japanese cohort. Neuromolecular Med 12: 237–242. [DOI] [PubMed] [Google Scholar]
- Knill DC, Pouget A. (2004) The Bayesian brain: the role of uncertainty in neural coding and computation. Trends Neurosci 27: 712–719. [DOI] [PubMed] [Google Scholar]
- Knorr U, Vinberg M, Gade A, et al. (2011) A randomized trial of the effect of escitalopram versus placebo on cognitive function in healthy first-degree relatives of patients with depression. Ther Adv Psychopharmacol 1: 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knorr UB. (2012) The effect of selective serotonin reuptake inhibitors in healthy first-degree relatives of patients with major depressive disorder – an experimental medicine blinded controlled trial. Dan Med J 59: B4426. [PubMed] [Google Scholar]
- Kobayashi K, Ikeda Y, Sakai A, et al. (2010) Reversal of hippocampal neuronal maturation by serotonergic antidepressants. Proc Natl Acad Sci USA 107: 8434–8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W, Patoiseau JF, Assie MB, et al. (1998) F 11440, a potent, selective, high efficacy 5-HT1A receptor agonist with marked anxiolytic and antidepressant potential. J Pharmacol Exp Ther 287: 266–283. [PubMed] [Google Scholar]
- Kometer M, Schmidt A, Bachmann R, et al. (2012) Psilocybin biases facial recognition, goal-directed behavior, and mood state toward positive relative to negative emotions through different serotonergic subreceptors. Biol Psychiatry 72: 898–906. [DOI] [PubMed] [Google Scholar]
- Kraehenmann R, Schmidt A, Friston K, et al. (2016) The mixed serotonin receptor agonist psilocybin reduces threat-induced modulation of amygdala connectivity. Neuroimage Clin 11: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus C, Castren E, Kasper S, et al. (2017) Serotonin and neuroplasticity – links between molecular, functional and structural pathophysiology in depression. Neurosci Biobehav Rev. [DOI] [PubMed] [Google Scholar]
- Kuhn R. (1958) The treatment of depressive states with G 22355 (imipramine hydrochloride). Am J Psychiatry 115: 459–464. [DOI] [PubMed] [Google Scholar]
- Kuypers KP, Riba J, de la Fuente Revenga M, et al. (2016) Ayahuasca enhances creative divergent thinking while decreasing conventional convergent thinking. Psychopharmacology (Berl) 233: 3395–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Fillman SG, Webster MJ, et al. (2011) Serotonin receptor expression in human prefrontal cortex: balancing excitation and inhibition across postnatal development. PLoS One 6: e22799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfumey L, Hamon M. (2000) Central 5-HT(1A) receptors: regional distribution and functional characteristics. Nucl Med Biol 27: 429–435. [DOI] [PubMed] [Google Scholar]
- Le Poul E, Laaris N, Doucet E, et al. (1995) Early desensitization of somato-dendritic 5-HT1A autoreceptors in rats treated with fluoxetine or paroxetine. Naunyn Schmiedebergs Arch Pharmacol 352: 141–148. [DOI] [PubMed] [Google Scholar]
- Lebe M, Hasenbring MI, Schmieder K, et al. (2013) Association of serotonin-1A and -2A receptor promoter polymorphisms with depressive symptoms, functional recovery, and pain in patients 6 months after lumbar disc surgery. Pain 154: 377–384. [DOI] [PubMed] [Google Scholar]
- Lebedev AV, Kaelen M, Lovden M, et al. (2016) LSD-induced entropic brain activity predicts subsequent personality change. Hum Brain Mapp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner GA, Lev-Ran S. (2015) LSD-associated “Alice in Wonderland Syndrome” (AIWS): A Hallucinogen Persisting Perception Disorder (HPPD) Case Report. Isr J Psychiatry Relat Sci 52:67–68. [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, et al. (1996) Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274: 1527–1531. [DOI] [PubMed] [Google Scholar]
- Li D, Mabrouk OS, Liu T, et al. (2015) Asphyxia-activated corticocardiac signaling accelerates onset of cardiac arrest. Proc Natl Acad Sci USA 112: E2073–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Inoue T, Abekawa T, et al. (2006) 5-HT1A receptor agonist affects fear conditioning through stimulations of the postsynaptic 5-HT1A receptors in the hippocampus and amygdala. Eur J Pharmacol 532: 74–80. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Vollenweider FX. (2000) The serotonin uptake inhibitor citalopram reduces acute cardiovascular and vegetative effects of 3,4-methylenedioxymethamphetamine (‘Ecstasy’) in healthy volunteers. J Psychopharmacol 14: 269–274. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Vollenweider FX. (2001) Which neuroreceptors mediate the subjective effects of MDMA in humans? A summary of mechanistic studies. Hum Psychopharmacol 16: 589–598. [DOI] [PubMed] [Google Scholar]
- Lopez JF, Liberzon I, Vazquez DM, et al. (1999) Serotonin 1A receptor messenger RNA regulation in the hippocampus after acute stress. Biol Psychiatry 45: 934–937. [DOI] [PubMed] [Google Scholar]
- Lord L, San Juan AT, Roseman L, et al. (2017) Expanded breadth of neural communication in psychedelic induced altered states of consciousness. In preparation. [Google Scholar]
- Lucas CG, Bridgers S, Griffiths TL, et al. (2014) When children are better (or at least more open-minded) learners than adults: developmental differences in learning the forms of causal relationships. Cognition 131: 284–299. [DOI] [PubMed] [Google Scholar]
- Lucki I. (1991) Behavioral studies of serotonin receptor agonists as antidepressant drugs. J Clin Psychiatry 52 Suppl: 24–31. [PubMed] [Google Scholar]
- Ma Y. (2015) Neuropsychological mechanism underlying antidepressant effect: a systematic meta-analysis. Mol Psychiatry 20: 311–319. [DOI] [PubMed] [Google Scholar]
- MacIsaac SE, Carvalho AF, Cha DS, et al. (2014) The mechanism, efficacy, and tolerability profile of agomelatine. Expert Opinion Pharmacother 15: 259–274. [DOI] [PubMed] [Google Scholar]
- MacLean KA, Johnson MW, Griffiths RR. (2011) Mystical experiences occasioned by the hallucinogen psilocybin lead to increases in the personality domain of openness. J Psychopharmacol 25: 1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean PD. (1990) The Triune Brain in Evolution: Role in Paleocerebral Functions. New York: Plenum Press. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, et al. (2000) Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20: 9104–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangell LB, Johnson CR, Kertz B, et al. (2002) Olanzapine in the treatment of apathy in previously depressed participants maintained with selective serotonin reuptake inhibitors: an open-label, flexible-dose study. J Clin Psychiatry 63: 391–395. [DOI] [PubMed] [Google Scholar]
- Martindale C. (2007) Creativity, primordial cognition, and personality. Pers Individ Dif 43: 1777–1785. [Google Scholar]
- Maslow AH. (1970) Religions, Values and Peak Experiences, [S.l.]: Viking Press. [Google Scholar]
- Matias S, Lottem E, Dugué GP, Mainen ZF. (2017) Activity patterns of serotonin neurons underlying cognitive flexibility. Elife. 6 pii: e20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos FF, Rollema H, Basbaum AI. (1990) Characterization of monoamine release in the lateral hypothalamus of awake, freely moving rats using in vivo microdialysis. Brain Res 528: 39–47. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. (2004) BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci 27: 589–594. [DOI] [PubMed] [Google Scholar]
- Maya Vetencourt JF, Sale A, Viegi A, et al. (2008) The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science 320: 385–388. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, et al. (2000) Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry 48: 830–843. [DOI] [PubMed] [Google Scholar]
- McCabe C, Mishor Z, Cowen PJ, et al. (2010) Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biol Psychiatry 67(5):439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannald MA. (2015) Serotonin: waiting but not rewarding. Curr Biol 25: R103–104. [DOI] [PubMed] [Google Scholar]
- McGlothlin W, Cohen S, McGlothlin MS. (1967) Long lasting effects of LSD on normals. Arch Gen Psychiatry 17: 521–532. [DOI] [PubMed] [Google Scholar]
- McMahon FJ, Buervenich S, Charney D, et al. (2006) Variation in the gene encoding the serotonin 2A receptor is associated with outcome of antidepressant treatment. Am J Hum Genet 78: 804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meana JJ. (2013) Agonist signal trafficking at serotonin 5-Ht2a receptor in human brain: implications for schizophrenia and antipsychotic treatment. Bas Clin Pharmacol Toxicol 113: 6–6. [Google Scholar]
- Meller R, Babity JM, Grahame-Smith DG. (2002) 5-HT2A receptor activation leads to increased BDNF mRNA expression in C6 glioma cells. Neuromolecular Med 1: 197–205. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. (2012) Serotonergic mechanisms as targets for existing and novel antipsychotics. Handb Exp Pharmacol: 87–124. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Kapur S, Eisfeld B, et al. (2001) The effect of paroxetine on 5-HT(2A) receptors in depression: an [(18)F]setoperone PET imaging study. Am J Psychiatry 158: 78–85. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Kapur S, Wilson AA, et al. (1996) Neuromodulation of frontal and temporal cortex by intravenous d-fenfluramine: an [15O]H2O PET study in humans. Neurosci Lett 207: 25–28. [DOI] [PubMed] [Google Scholar]
- Meyer JH, McMain S, Kennedy SH, et al. (2003) Dysfunctional attitudes and 5-HT2 receptors during depression and self-harm. Am J Psychiatry 160: 90–99. [DOI] [PubMed] [Google Scholar]
- Mirkovic B, Laurent C, Podlipski MA, et al. (2016) Genetic Association studies of suicidal behavior: a review of the past 10 years, progress, limitations, and future Directions. Front Psychiatry 7:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. (2010) Is pharma running out of brainy ideas? Science 329: 502–504. [DOI] [PubMed] [Google Scholar]
- Miller KJ. (2005) Serotonin 5-HT2C receptor agonists: For the treatment of obesity. Mol Intervent 5: 282–291. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Einstein EB, Bentham MP, et al. (2015) Expression of the 5-HT1A serotonin receptor in the hippocampus is required for social stress resilience and the antidepressant-like effects induced by the nicotinic partial agonist cytisine. Neuropsychopharmacology 40: 938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PJ. (2005) Antidepressant treatment and rodent aggressive behaviour. Eur J Pharmacol 526(1-3):147–62. [DOI] [PubMed] [Google Scholar]
- Mithoefer MC, Grob CS, Brewerton TD. (2016) Novel psychopharmacological therapies for psychiatric disorders: psilocybin and MDMA. Lancet Psychiatry 3: 481–488. [DOI] [PubMed] [Google Scholar]
- Mithoefer MC, Wagner MT, Mithoefer AT, et al. (2011) The safety and efficacy of {+/-}3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: the first randomized controlled pilot study. J Psychopharmacol 25: 439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithoefer MC, Wagner MT, Mithoefer AT, et al. (2013) Durability of improvement in post-traumatic stress disorder symptoms and absence of harmful effects or drug dependency after 3,4-methylenedioxymethamphetamine-assisted psychotherapy: a prospective long-term follow-up study. J Psychopharmacol 27: 28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki K, Miyazaki KW, Doya K. (2012) The role of serotonin in the regulation of patience and impulsivity. Mol Neurobiol 45: 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki KW, Miyazaki K, Tanaka KF, et al. (2014) Optogenetic activation of dorsal raphe serotonin neurons enhances patience for future rewards. Curr Biol 24: 2033–2040. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Kaas JH, de Carlos JA, et al. (2014) Evolution and development of the mammalian cerebral cortex. Brain, Behav Evolution 83: 126–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morairty SR, Hedley L, Flores J, et al. (2008) Selective 5HT2A and 5HT6 receptor antagonists promote sleep in rats. Sleep 31: 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley S. (1983) The stress-diathesis model of illness. J Psychosomatic Res 27: 86–87. [DOI] [PubMed] [Google Scholar]
- Moorman JM, Grahame-Smith DG, Smith SE, et al. (1996) Chronic electroconvulsive shock enhances 5-HT2 receptor-mediated head shakes but not brain C-fos induction. Neuropharmacology 35(3):303–313. [DOI] [PubMed] [Google Scholar]
- Mosienko V, Beis D, Pasqualetti M, et al. (2015) Life without brain serotonin: reevaluation of serotonin function with mice deficient in brain serotonin synthesis. Behav Brain Res 277: 78–88. [DOI] [PubMed] [Google Scholar]
- Moutoussis M, Fearon P, El-Deredy W, et al. (2014) Bayesian inferences about the self (and others): a review. Consciousness Cognition 25: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muguruza C, Miranda-Azpiazu P, Diez-Alarcia R, et al. (2014) Evaluation of 5-HT2A and mGlu(2/3) receptors in postmortem prefrontal cortex of subjects with major depressive disorder: Effect of antidepressant treatment. Neuropharmacology 86: 311–318. [DOI] [PubMed] [Google Scholar]
- Mulders P, vanEijndhoven P, Pluijmen J, et al. (2016) Default mode network coherence in treatment-resistant major depressive disorder during electroconvulsive therapy. J Affective Disorders 130–137. [DOI] [PubMed] [Google Scholar]
- Muller CP, Homberg JR. (2015) Serotonin revisited. Behav Brain Res 277: 1–2. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Carhart-Harris RL, Moran RJ, et al. (2013) Broadband cortical desynchronization underlies the human psychedelic state. J Neurosci 33: 15171–15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nautiyal KM, Hen R. (2017) Serotonin receptors in depression: from A to B. F1000Res 6:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE. (2004) Hallucinogens. Pharmacol Ther 101: 131–181. [DOI] [PubMed] [Google Scholar]
- Niitsu Y, Hamada S, Hamaguchi K, et al. (1995) Regulation of synapse density by 5-HT2A receptor agonist and antagonist in the spinal cord of chicken embryo. Neurosci Lett 195: 159–162. [DOI] [PubMed] [Google Scholar]
- Nour MM, Evans L, Nutt D, Carhart-Harris RL. (2016) Ego-Dissolution and psychedelics: validation of the ego-dissolution inventory (EDI). Front Hum Neurosci 10:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour MM, et al. (2017). J Psychoactive Drugs. In press. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, King LA, Phillips LD, et al. (2010) Drug harms in the UK: a multicriteria decision analysis. Lancet 376: 1558–1565. [DOI] [PubMed] [Google Scholar]
- Ogren SO, Eriksson TM, Elvander-Tottie E, et al. (2008) The role of 5-HT(1A) receptors in learning and memory. Behav Brain Res 195: 54–77. [DOI] [PubMed] [Google Scholar]
- Oleskevich S, Leck KJ, Matthaei K, et al. (2005) Enhanced serotonin response in the hippocampus of Galphaz protein knock-out mice. Neuroreport 16: 921–925. [DOI] [PubMed] [Google Scholar]
- Olivier B, Mos J, van der Heyden J, et al. (1989) Serotonergic modulation of social interactions in isolated male mice. Psychopharmacology (Berl) 97: 154–156. [DOI] [PubMed] [Google Scholar]
- Olivier B, Mos J. (1990) Serenics, serotonin and aggression. Prog Clin Biol Res 361:203–30. [PubMed] [Google Scholar]
- Osorio Fde L, Sanches RF, Macedo LR, et al. (2015) Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a preliminary report. Rev Bras Psiquiatr 37: 13–20. [DOI] [PubMed] [Google Scholar]
- Ostroff RB, Nelson JC. (1999) Risperidone augmentation of selective serotonin reuptake inhibitors in major depression. J Clin Psychiatry 60: 256–259. [DOI] [PubMed] [Google Scholar]
- Ott U. (2006) States of absorption: in search of neurobiological foundations. In: Jamieson GA. (ed) Hypnosis and Consciousness States: The Cognitive Neuroscience Perspective. New York: Oxford University Press, pp. 257–270. [Google Scholar]
- Ott U, Reuter M, Hennig J, et al. (2005) Evidence for a common biological basis of the Absorption trait, hallucinogen effects, and positive symptoms: epistasis between 5-HT2a and COMT polymorphisms. Am J Med Genet B Neuropsychiatr Genet 137B: 29–32. [DOI] [PubMed] [Google Scholar]
- Pandey DK, Mahesh R, Kumar AA, et al. (2010) A novel 5-HT(2A) receptor antagonist exhibits antidepressant-like effects in a battery of rodent behavioural assays: approaching early-onset antidepressants. Pharmacol Biochem Behav 94: 363–373. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Rizavi HS, et al. (2002) Higher expression of serotonin 5-HT(2A) receptors in the postmortem brains of teenage suicide victims. Am J Psychiatry 159: 419–429. [DOI] [PubMed] [Google Scholar]
- Pare CM. (1965) Treatment of depression. Lancet 1: 923–925. [PubMed] [Google Scholar]
- Parks CL, Robinson PS, Sibille E, et al. (1998) Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci U S A 95: 10734–10739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons TD, Barnett M, Melugin PR. (2015) Assessment of personality and absorption for mediated environments in a college sample. Cyberpsychol Behav Soc Netw 18: 752–756. [DOI] [PubMed] [Google Scholar]
- Paterson LM, Kornum BR, Nutt DJ, et al. (2013) 5-HT radioligands for human brain imaging with PET and SPECT. Med Res Rev 33: 54–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson LM, Tyacke RJ, Nutt DJ, et al. (2010) Measuring endogenous 5-HT release by emission tomography: promises and pitfalls. J Cereb Blood Flow Metab 30: 1682–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos A, Palacios JM. (1985) Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res 346: 205–230. [DOI] [PubMed] [Google Scholar]
- Pazos A, Probst A, Palacios JM. (1987) Serotonin receptors in the human brain–III. Autoradiographic mapping of serotonin-1 receptors. Neuroscience 21: 97–122. [DOI] [PubMed] [Google Scholar]
- Pedigo NW, Yamamura HI, Nelson DL. (1981) Discrimination of multiple [3H]5-hydroxytryptamine binding sites by the neuroleptic spiperone in rat brain. J Neurochem 36: 220–226. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ, Snyder SH. (1979) Multiple serotonin receptors: differential binding of [3H]5-hydroxytryptamine, [3H]lysergic acid diethylamide and [3H]spiroperidol. Mol Pharmacol 16: 687–699. [PubMed] [Google Scholar]
- Petit AC, Quesseveur G, Gressier F, et al. (2014) Converging translational evidence for the involvement of the serotonin 2A receptor gene in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 54: 76–82. [DOI] [PubMed] [Google Scholar]
- Petri G, Expert P, Turkheimer F, et al. (2014) Homological scaffolds of brain functional networks. J R Soc Interface 11: 20140873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piszczek L, Piszczek A, Kuczmanska J, et al. (2015) Modulation of anxiety by cortical serotonin 1A receptors. Front Behav Neurosci 9: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts EG, Minerva AR, Oliver EB, et al. (2017) 3,4-Methylenedioxymethamphetamine increases affiliative behaviors in squirrel monkeys in a serotonin 2A receptor-dependent manner. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaznik A, Kostowski W, Stefanski R. (1994) Limbic mechanisms of anxiolytics acting on 5-HT receptors. Pol J Pharmacol 46: 473–477. [PubMed] [Google Scholar]
- Pletscher A. (1991) The discovery of antidepressants: a winding path. Experientia 47: 4–8. [DOI] [PubMed] [Google Scholar]
- Pletscher A, Shore PA, Brodie BB. (1955) Serotonin release as a possible mechanism of reserpine action. Science 122: 374–375. [DOI] [PubMed] [Google Scholar]
- Pokorny T, Preller KH, Kraehenmann R, et al. (2016) Modulatory effect of the 5-HT1A agonist buspirone and the mixed non-hallucinogenic 5-HT1A/2A agonist ergotamine on psilocybin-induced psychedelic experience. Eur Neuropsychopharmacol 26: 756–766. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. (1992) Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J Neurosci 12: 440–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova NK, Naumenko VS, Plyusnina IZ. (2007) Involvement of brain serotonin 5-HT1A receptors in genetic predisposition to aggressive behavior. Neurosci Behav Physiol 37: 631–635. [DOI] [PubMed] [Google Scholar]
- Popovic D, Vieta E, Fornaro M, et al. (2015) Cognitive tolerability following successful long term treatment of major depression and anxiety disorders with SSRi antidepressants. J Affect Disord 173: 211–215. [DOI] [PubMed] [Google Scholar]
- Preller KH, Herdener M, Pokorny T, et al. (2016) The role of the serotonin 2A receptor in the fabric and modulation of personal meaning in LSD-induced states. ECNP abstract P.1.i.003. [Google Scholar]
- Preller KH, Pokorny T, Hock A, et al. (2016) Effects of serotonin 2A/1A receptor stimulation on social exclusion processing. Proc Natl Acad Sci U S A 113: 5119–5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J, Cole V, Goodwin GM. (2009) Emotional side-effects of selective serotonin reuptake inhibitors: qualitative study. Br J Psychiatry 195: 211–217. [DOI] [PubMed] [Google Scholar]
- Puglisi-Allegra S, Andolina D. (2015) Serotonin and stress coping. Behav Brain Res 277: 58–67. [DOI] [PubMed] [Google Scholar]
- Puig MV, Artigas F, Celada P. (2005) Modulation of the activity of pyramidal neurons in rat prefrontal cortex by raphe stimulation in vivo: involvement of serotonin and GABA. Cereb Cortex 15: 1–14. [DOI] [PubMed] [Google Scholar]
- Puig MV, Gulledge AT. (2011) Serotonin and prefrontal cortex function: neurons, networks, and circuits. Mol Neurobiol 44: 449–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pum ME, Huston JP, Muller CP. (2009) The role of cortical serotonin in anxiety and locomotor activity in Wistar rats. Behav Neurosci 123: 449–454. [DOI] [PubMed] [Google Scholar]
- Qesseveur G, Petit AC, Nguyen HT, et al. (2016) Genetic dysfunction of serotonin 2A receptor hampers response to antidepressant drugs: A translational approach. Neuropharmacology 105: 142–153. [DOI] [PubMed] [Google Scholar]
- Quesseveur G, Reperant C, David DJ, et al. (2013) 5-HT(2)A receptor inactivation potentiates the acute antidepressant-like activity of escitalopram: involvement of the noradrenergic system. Exp Brain Res 226: 285–295. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kuypers KP. (2006) Acute effects of 3,4-methylenedioxymethamphetamine (MDMA) on behavioral measures of impulsivity: alone and in combination with alcohol. Neuropsychopharmacology 31: 1048–1055. [DOI] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, et al. (1998) Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci U S A 95: 14476–14481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramboz S, Saudou F, Amara DA, et al. (1996) 5-HT1B receptor knock out–behavioral consequences. Behav Brain Res 73(1-2):305–12. [DOI] [PubMed] [Google Scholar]
- Ranade S, Pi HJ, Kepecs A. (2014) Neuroscience: waiting for serotonin. Curr Biol 24: R803–805. [DOI] [PubMed] [Google Scholar]
- Rapport MM, Green AA, Page IH. (1948) Serum vasoconstrictor, serotonin; isolation and characterization. J Biol Chem 176: 1243–1251. [PubMed] [Google Scholar]
- Resnick O, Krus DM, Raskin M. (1965) Accentuation of the psychological effects of LSD-25 in normal subjects treated with reserpine. Life Sci 4: 1433–1437. [DOI] [PubMed] [Google Scholar]
- Rex A, Voigt JP, Fink H. (2005) Anxiety but not arousal increases 5-hydroxytryptamine release in the rat ventral hippocampus in vivo. Eur J Neurosci 22: 1185–1189. [DOI] [PubMed] [Google Scholar]
- Riba J, Anderer P, Jane F, et al. (2004) Effects of the South American psychoactive beverage ayahuasca on regional brain electrical activity in humans: a functional neuroimaging study using low-resolution electromagnetic tomography. Neuropsychobiology 50: 89–101. [DOI] [PubMed] [Google Scholar]
- Riga MS, Sanchez C, Celada P, et al. (2016) Involvement of 5-HT3 receptors in the action of vortioxetine in rat brain: Focus on glutamatergic and GABAergic neurotransmission. Neuropharmacology 108: 73–81. [DOI] [PubMed] [Google Scholar]
- Riga MS, Soria G, Tudela R, et al. (2014) The natural hallucinogen 5-MeO-DMT, component of Ayahuasca, disrupts cortical function in rats: reversal by antipsychotic drugs. Int J Neuropsychopharmacology 17: 1269–1282. [DOI] [PubMed] [Google Scholar]
- Romano AG, Hood H, Harvey JA. (2000) Dissociable effects of the 5-HT(2) antagonist mianserin on associative learning and performance in the rabbit. Pharmacol Biochem Behav 67: 103–110. [DOI] [PubMed] [Google Scholar]
- Romano AG, Quinn JL, Li L, et al. (2010) Intrahippocampal LSD accelerates learning and desensitizes the 5-HT(2A) receptor in the rabbit, Romano et al. Psychopharmacology (Berl) 212: 441–448. [DOI] [PubMed] [Google Scholar]
- Romano AG, Quinn JL, Liu R, et al. (2006) Effect of serotonin depletion on 5-HT2A-mediated learning in the rabbit: evidence for constitutive activity of the 5-HT2A receptor in vivo. Psychopharmacology (Berl) 184: 173–181. [DOI] [PubMed] [Google Scholar]
- Rosell DR, Thompson JL, Slifstein M, et al. (2010) Increased serotonin 2A receptor availability in the orbitofrontal cortex of physically aggressive personality disordered patients. Biol Psychiatry 67: 1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman L, Leech R, Feilding A, et al. (2014) The effects of psilocybin and MDMA on between-network resting state functional connectivity in healthy volunteers. Front Hum Neurosci 8: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman L, et al. (2017. a) Peak experience predicts therapeutic success of psilocybin for treatment-resistant depression. Psychopharmacology (Berlin). Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman L, et al. (2017. b) Increased amygdala responses to emotional faces with psilocybin for treatment-resistant depression. Neuropharmacology Under review. [DOI] [PubMed] [Google Scholar]
- Ross S, Bossis A, Guss J, et al. (2016) Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol 30: 1165–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueter LE, Jacobs BL. (1996) A microdialysis examination of serotonin release in the rat forebrain induced by behavioral/environmental manipulations. Brain Res 739: 57–69. [DOI] [PubMed] [Google Scholar]
- Russ SL, Elliott MS. (2017) Antecedents of mystical experience and dread in intensive meditation psychology of consciousness. Theory, Res Practice 4:38–53. [Google Scholar]
- Salo J, Jokela M, Lehtimaki T, et al. (2011) Serotonin receptor 2A gene moderates the effect of childhood maternal nurturance on adulthood social attachment. Genes Brain Behav 10: 702–709. [DOI] [PubMed] [Google Scholar]
- Samuels BA, Anacker C, Hu A, et al. (2015) 5-HT1A receptors on mature dentate gyrus granule cells are critical for the antidepressant response. Nat Neurosci 18: 1606–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanches RF, de Lima Osorio F, Dos Santos RG, et al. (2016) antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: A SPECT study. J Clin Psychopharmacol 36: 77–81. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Hyttel J. (1994) Isolation-induced aggression in mice: effects of 5-hydroxytryptamine uptake inhibitors and involvement of postsynaptic 5-HT1A receptors. Eur J Pharmacol 264: 241–247. [DOI] [PubMed] [Google Scholar]
- Sandison RA. (1954) Psychological aspects of the LSD treatment of the neuroses. J Ment Sci 100: 508–515. [DOI] [PubMed] [Google Scholar]
- Sandison RA, Hopkin I. (1964) Psychotherapy using LSD. Nurs Times 60: 529–532. [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, et al. (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301: 805–809. [DOI] [PubMed] [Google Scholar]
- Schartner MM, Carhart-Harris RL, Barrett AB, et al. (2017) Increased spontaneous MEG signal diversity for psychoactive doses of ketamine, LSD and psilocybin. Sci Rep 7:46421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid Y, Enzler F, Gasser P, et al. (2015) Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry 78: 544–553. [DOI] [PubMed] [Google Scholar]
- Schmid Y, Hysek CM, Simmler LD, et al. (2014) Differential effects of MDMA and methylphenidate on social cognition. J Psychopharmacol 28: 847–856. [DOI] [PubMed] [Google Scholar]
- Schreiber R, De Vry J. (1993) 5-HT1A receptor ligands in animal models of anxiety, impulsivity and depression: multiple mechanisms of action? Prog Neuropsychopharmacol Biol Psychiatry 17: 87–104. [DOI] [PubMed] [Google Scholar]
- Schultz W. (2010) Dopamine signals for reward value and risk: basic and recent data. Behav Brain Funct 6: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartenbeck P, FitzGerald TH, Mathys C, et al. (2014) The dopaminergic midbrain encodes the expected certainty about desired outcomes. Cereb Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa B. (2008) Is it time to revisit the role of psychedelic drugs in enhancing human creativity? J Psychopharmacol 22: 821–827. [DOI] [PubMed] [Google Scholar]
- Sessa B. (2016) MDMA and PTSD treatment: ‘PTSD: From novel pathophysiology to innovative therapeutics’. Neurosci Lett. [DOI] [PubMed] [Google Scholar]
- Seymour B, Daw ND, Roiser JP, et al. (2012) Serotonin selectively modulates reward value in human decision-making. J Neurosc 32: 5833–5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp T, Boothman L, Raley J, et al. (2007) Important messages in the ‘post’: recent discoveries in 5-HT neurone feedback control. Trends Pharmacol Sci 28: 629–636. [DOI] [PubMed] [Google Scholar]
- Shaw E, Woolley DW. (1956) Some serotoninlike activities of lysergic acid diethylamide. Science 124: 121–122. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Mintun MA, Moerlein SM, et al. (2002) Greater loss of 5-HT(2A) receptors in midlife than in late life. Am J Psychiatry 159: 430–435. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Papakostas GI. (2008) Augmentation of antidepressants with atypical antipsychotics for treatment-resistant major depressive disorder. Acta Psychiatr Scand 117: 253–259. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Sanders-Bush E, Manier DH, et al. (2009) Elevated 5-HT 2A receptors in postmortem prefrontal cortex in major depression is associated with reduced activity of protein kinase A. Neuroscience 158: 1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siepmann M, Grossmann J, Muck-Weymann M, et al. (2003) Effects of sertraline on autonomic and cognitive functions in healthy volunteers. Psychopharmacology (Berl) 168: 293–298. [DOI] [PubMed] [Google Scholar]
- Sijbesma H, Schipper J, de Kloet ER, et al. (1991) Postsynaptic 5-HT1 receptors and offensive aggression in rats: a combined behavioural and autoradiographic study with eltoprazine. Pharmacol Biochem Behav 38: 447–458. [DOI] [PubMed] [Google Scholar]
- Skinbjerg M, Sibley DR, Javitch JA, et al. (2012) Imaging the high-affinity state of the dopamine D2 receptor in vivo: fact or fiction? Biochem Pharmacol 83: 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleight AJ, Stam NJ, Mutel V, et al. (1996) Radiolabelling of the human 5-HT2A receptor with an agonist, a partial agonist and an antagonist: effects on apparent agonist affinities. Biochem Pharmacol 51: 71–76. [DOI] [PubMed] [Google Scholar]
- Smith KA, Fairburn CG, Cowen PJ. (1997) Relapse of depression after rapid depletion of tryptophan. Lancet 349: 915–919. [DOI] [PubMed] [Google Scholar]
- Smith TD, Kuczenski R, George-Friedman K, et al. (2000) In vivo microdialysis assessment of extracellular serotonin and dopamine levels in awake monkeys during sustained fluoxetine administration. Synapse 38: 460–470. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Price JC, Meltzer CC, et al. (2007) 5HT2A receptor binding is increased in borderline personality disorder. Biol Psychiatry 62: 580–587. [DOI] [PubMed] [Google Scholar]
- Soubrie P. (1986) [Serotonergic neurons and behavior]. J Pharmacol 17: 107–112. [PubMed] [Google Scholar]
- Stace WT. (1961) Mysticism and Philosophy. London: Macmillan. [Google Scholar]
- Stanley M, Mann JJ. (1983) Increased serotonin-2 binding sites in frontal cortex of suicide victims. Lancet (London, England) 1: 214–216. [DOI] [PubMed] [Google Scholar]
- Stefanski R, Palejko W, Bidzinski A, et al. (1993) Serotonergic innervation of the hippocampus and nucleus accumbens septi and the anxiolytic-like action of midazolam and 5-HT1A receptor agonists. Neuropharmacology 32: 977–985. [DOI] [PubMed] [Google Scholar]
- Stewart LH, Ferguson B, Morgan CJ, et al. (2014) Effects of ecstasy on cooperative behaviour and perception of trustworthiness: a naturalistic study. J Psychopharmacol 28: 1001–1008. [DOI] [PubMed] [Google Scholar]
- Stini WA. (1975) Ecology and human adaptation, Dubuque, Iowa: W. C. Brown Co. [Google Scholar]
- Strassman R. (2000) DMT: The Spirit Molecul : A Doctor’s Revolutionary Research into the Biology of Near-death and Mystical Experiences. Rochester, Vt.: Park Street Press. [Google Scholar]
- Strassman RJ. (1996) Human psychopharmacology of N,N-dimethyltryptamine. Behav Brain Res 73: 121–124. [DOI] [PubMed] [Google Scholar]
- Strauss CV, Vicente MA, Zangrossi H., Jr (2013) Activation of 5-HT1A receptors in the rat basolateral amygdala induces both anxiolytic and antipanic-like effects. Behav Brain Res 246: 103–110. [DOI] [PubMed] [Google Scholar]
- Strome EM, Clark CM, Zis AP, et al. (2005) Electroconvulsive shock decreases binding to 5-HT2 receptors in nonhuman primates: an in vivo positron emission tomography study with [18F]setoperone. Biol Psychiatry 57(9):1004–10 [DOI] [PubMed] [Google Scholar]
- Studerus E, Gamma A, Kometer M, et al. (2012) Prediction of psilocybin response in healthy volunteers. PLoS One 7: e30800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada K, Kasamo K, Suzuki T, et al. (2004) Endogenous 5-HT inhibits firing activity of hippocampal CA1 pyramidal neurons during conditioned fear stress-induced freezing behavior through stimulating 5-HT1A receptors. Hippocampus 14: 143–147. [DOI] [PubMed] [Google Scholar]
- Tagliazucchi E C-HR, Nutt DJ, Chialvo D. (2014) Enhanced repertoire of brain dynamical states during the psychedelic experience. Hum Brain Mapp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E, Roseman L, Kaelen M, et al. (2016) Increased global functional connectivity correlates with LSD-induced ego dissolution. Curr Biol 26: 1043–1050. [DOI] [PubMed] [Google Scholar]
- Tauscher J, Bagby RM, Javanmard M, et al. (2001) Inverse relationship between serotonin 5-HT(1A) receptor binding and anxiety: a [(11)C]WAY-100635 PET investigation in healthy volunteers. A J Psychiatry 158: 1326–1328. [DOI] [PubMed] [Google Scholar]
- Teegarden BR, Al Shamma H, Xiong Y. (2008) 5-HT(2A) inverse-agonists for the treatment of insomnia. Curr Top Med Chem 8: 969–976. [DOI] [PubMed] [Google Scholar]
- Tellegen A, Atkinson G. (1974) Openness to absorbing and self-altering experiences (‘absorption’), a trait related to hypnotic susceptibility. J Abnorm Psychol 83: 268–277. [DOI] [PubMed] [Google Scholar]
- Thase ME, Mahableshwarkar AR, Dragheim M, et al. (2016) A meta-analysis of randomized, placebo-controlled trials of vortioxetine for the treatment of major depressive disorder in adults. European Neuropsychopharmacology 26: 979–993. [DOI] [PubMed] [Google Scholar]
- Toth M. (2003) 5-HT1A receptor knockout mouse as a genetic model of anxiety. Eur J Pharmacol 463: 177–184. [DOI] [PubMed] [Google Scholar]
- Tu W, Cook A, Scholl JL, et al. (2014) Serotonin in the ventral hippocampus modulates anxiety-like behavior during amphetamine withdrawal. Neuroscience 281C: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turecki G, Briere R, Dewar K, et al. (1999) Prediction of level of serotonin 2A receptor binding by serotonin receptor 2A genetic variation in postmortem brain samples from subjects who did or did not commit suicide. Am J Psychiatry 156: 1456–1458. [DOI] [PubMed] [Google Scholar]
- Turton S, Nutt DJ, Carhart-Harris RL. (2014) A qualitative report on the subjective experience of intravenous psilocybin administered in an FMRI environment. Curr Drug Abuse Rev 7: 117–127. [DOI] [PubMed] [Google Scholar]
- Twarog BM, Page IH. (1953) Serotonin content of some mammalian tissues and urine and a method for its determination. Am J Physiol 175: 157–161. [DOI] [PubMed] [Google Scholar]
- Tyacke RJ, Nutt DJ. (2015) Optimising PET approaches to measuring 5-HT release in human brain. Synapse 69: 505–511. [DOI] [PubMed] [Google Scholar]
- Udenfriend S, Weissbach H, Bogdanski DF. (1957) Effect of iproniazid on serotonin metabolism in vivo. J Pharmacol Exp Ther 120: 255–260. [PubMed] [Google Scholar]
- UK ECT Review Group (2003) Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet 361(9360):799–808. [DOI] [PubMed] [Google Scholar]
- Urban DJ, Zhu H, Marcinkiewcz CA, et al. (2016) Elucidation of the behavioral program and neuronal network encoded by Dorsal Raphe serotonergic neurons. Neuropsychopharmacology 41: 1404–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya VA, Marek GJ, Aghajanian GK, et al. (1997) 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J Neurosci 17: 2785–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle M, Maqueda AE, Rabella M, et al. (2016) Inhibition of alpha oscillations through serotonin-2A receptor activation underlies the visual effects of ayahuasca in humans. Eur Neuropsychopharmacol 26: 1161–1175. [DOI] [PubMed] [Google Scholar]
- van Amsterdam J, Nutt D, Phillips L, et al. (2015) European rating of drug harms. J Psychopharmacol 29: 655–660. [DOI] [PubMed] [Google Scholar]
- van Apeldoorn FJ, van Hout WJPJ, Mersch PPA, et al. (2008) Is a combined therapy more effective than either CBT or SSRI alone? Results of a multicenter trial on panic disorder with or without agoraphobia. Acta Psychiatrica Scand 117: 260–270. [DOI] [PubMed] [Google Scholar]
- van Heeringen C, Audenaert K, Van Laere K, et al. (2003) Prefrontal 5-HT2a receptor binding index, hopelessness and personality characteristics in attempted suicide. J Affect Disord 74: 149–158. [DOI] [PubMed] [Google Scholar]
- van Wel JH, Kuypers KP, Theunissen EL, et al. (2012) Effects of acute MDMA intoxication on mood and impulsivity: role of the 5-HT2 and 5-HT1 receptors. PLoS One 7: e40187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanover KE, Davis RE. (2010) Role of 5-HT2A receptor antagonists in the treatment of insomnia. Nat Sci Sleep 2: 139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnas K, Halldin C, Hall H. (2004) Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Hum Brain Mapp 22: 246–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez DM, Lopez JF, Van Hoers H, et al. (2000) Maternal deprivation regulates serotonin 1A and 2A receptors in the infant rat. Brain Res 855: 76–82. [DOI] [PubMed] [Google Scholar]
- Vazquez-Borsetti P, Cortes R, et al. (2009) Pyramidal neurons in rat prefrontal cortex projecting to ventral tegmental area and dorsal raphe nucleus express 5-HT2A receptors. Cereb Cortex 19: 1678–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viol A, Palhano-Fontes F, Onias H, et al. (2016) Shannon entropy of brain functional complex networks under the influence of the psychedelic Ayahuasca. Cornell University Library. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgin DV, Fay R, Kubin L. (2003) Postnatal development of serotonin 1B, 2 A and 2C receptors in brainstem motoneurons. Eur J Neurosci 17: 1179–1188. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, et al. (1998) Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 9: 3897–3902. [DOI] [PubMed] [Google Scholar]
- Waldman A. (2017) A Really Good Day: How Microdosing made a Mega Difference in my Mood, My Marriage, and My Life. New York: Knopf Publishing Group. [Google Scholar]
- Watanabe N, Omori IM, Nakagawa A, et al. (2008) Mirtazapine versus other antidepressants in the acute-phase treatment of adults with major depression: systematic review and meta-analysis. J Clin Psychiatry 69: 1404–1415. [DOI] [PubMed] [Google Scholar]
- Watts R, Day C, Krzanowski J, et al. (2017) Patients’ accounts of increased ‘connection’ and ‘acceptance’ after psilocybin for treatment-resistant depression. J Humanist Psychol Epub: 1–45. [Google Scholar]
- Weber ET, Andrade R. (2010) Htr2a gene and 5-HT(2A) receptor expression in the cerebral cortex studied using genetically modified mice. Front Neurosci 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisstaub NV, Zhou M, Lira A, et al. (2006) Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science 313: 536–540. [DOI] [PubMed] [Google Scholar]
- Welsh SE, Romano AG, Harvey JA. (1998) Effects of serotonin 5-HT(2A/2C) antagonists on associative learning in the rabbit. Psychopharmacology (Berl) 137: 157–163. [DOI] [PubMed] [Google Scholar]
- White SM, Kucharik RF, Moyer JA. (1991) Effects of serotonergic agents on isolation-induced aggression. Pharmacol Biochem Behav 39: 729–736. [DOI] [PubMed] [Google Scholar]
- Wichers MC, Koek GH, Robaeys G, et al. (2005) IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol Psychiatry 10: 538–544. [DOI] [PubMed] [Google Scholar]
- Wilkie MJV, Smith G, Day RK, et al. (2009) Polymorphisms in the SLC6A4 and HTR2A genes influence treatment outcome following antidepressant therapy. Pharmacogenomics Journal 9: 61–70. [DOI] [PubMed] [Google Scholar]
- Winkelman M. (2014) Psychedelics as medicines for substance abuse rehabilitation: evaluating treatments with LSD, Peyote, Ibogaine and Ayahuasca. Curr Drug Abuse Rev 7(2):101–16. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, et al. (2004) 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology (Berl) 176: 376–385. [DOI] [PubMed] [Google Scholar]
- Wise CD, Berger BD, Stein L. (1970) Serotonin: a possible mediator of behavioral suppression induced by anxiety. Dis Nerv Syst 31: Suppl:34–37. [PubMed] [Google Scholar]
- Wolff MC, Leander JD. (2002) Selective serotonin reuptake inhibitors decrease impulsive behavior as measured by an adjusting delay procedure in the pigeon. Neuropsychopharmacology 27: 421–429. [DOI] [PubMed] [Google Scholar]
- Wood J, Kim Y, Moghaddam B. (2012) Disruption of prefrontal cortex large scale neuronal activity by different classes of psychotomimetic drugs. J Neurosci 32: 3022–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MD. (2003) Therapeutic potential of 5-HT2C receptor antagonists in the treatment of anxiety disorders. Curr Drug Targets CNS Neurol Disord 2(6):383–387. [DOI] [PubMed] [Google Scholar]
- Woolley DW, Shaw E. (1954) A biochemical and pharmacological suggestion about certain mental disorders. Proc Natl Acad Sci USA 40: 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie KP, Rojas DC, Ross RG, et al. (2014) Reduced brain resting-state network specificity in infants compared with adults. Neuropsychiatr Dis Treat 10: 1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanowitch R, Coccaro EF. (2011) The neurochemistry of human aggression. Adv Genet 75: 151–169. [DOI] [PubMed] [Google Scholar]
- Yatham LN, Liddle PF, Lam RW, et al. (2010) Effect of electroconvulsive therapy on brain 5-HT2 receptors in major depression. Br J Psychiatry 196: 474–479. [DOI] [PubMed] [Google Scholar]
- Yatham LN, Liddle PF, Dennie J, et al. (1999) Decrease in brain serotonin 2 receptor binding in patients with major depression following desipramine treatment – A positron emission tomography study with fluorine-18-labeled setoperone. Arch Gen Psychiatry 56: 705–711. [DOI] [PubMed] [Google Scholar]
- Yoshinaga N, Niitsu T, Hanaoka H, et al. (2013) Strategy for treating selective serotonin reuptake inhibitor-resistant social anxiety disorder in the clinical setting: a randomised controlled trial protocol of cognitive behavioural therapy in combination with conventional treatment. BMJ Open 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka M, Matsumoto M, Togashi H, et al. (1995) Effects of conditioned fear stress on 5-HT release in the rat prefrontal cortex. Pharmacol Biochem Behav 51: 515–519. [DOI] [PubMed] [Google Scholar]
- Zammit S, Owen MJ. (2006) Stressful life events, 5-HTT genotype and risk of depression. Br J Psychiatry 188: 199–201. [DOI] [PubMed] [Google Scholar]
- Zanoveli JM, Nogueira RL, Zangrossi H., Jr (2005) Chronic imipramine treatment sensitizes 5-HT1A and 5-HT 2 A receptors in the dorsal periaqueductal gray matter: evidence from the elevated T-maze test of anxiety. Behav Pharmacol 16: 543–552. [DOI] [PubMed] [Google Scholar]
- Zhang G, Asgeirsdottir HN, Cohen SJ, et al. (2013) Stimulation of serotonin 2A receptors facilitates consolidation and extinction of fear memory in C57BL/6J mice. Neuropharmacology 64: 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Cinalli D, Cohen SJ, et al. (2016) Examination of the hippocampal contribution to serotonin 5-HT2A receptor-mediated facilitation of object memory in C57BL/6J mice. Neuropharmacology 109: 332–340. [DOI] [PubMed] [Google Scholar]
- Zhang G, Stackman RW., Jr (2015) The role of serotonin 5-HT2A receptors in memory and cognition. Front Pharmacol 6: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZW. (2003) Serotonin induces tonic firing in layer V pyramidal neurons of rat prefrontal cortex during postnatal development. J Neurosci 23: 3373–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Cao X, Mar AC, et al. (2014) Activation of postsynaptic 5-HT1A receptors improve stress adaptation. Psychopharmacology (Berl) 231: 2067–2075. [DOI] [PubMed] [Google Scholar]
- Zhou JS, Li L, Cao X, et al. (2008) [Effect of 5-HT and postsynaptic 5-HT1 A on the mood and recognition of the repeated restraint stress in rats]. Zhong Nan Da Xue Xue Bao Yi Xue Ban 33: 305–311. [PubMed] [Google Scholar]
- Zis AP, Nomikos GG, Brown EE, et al. (1992) Neurochemical effects of electrically and chemically induced seizures: an in vivo microdialysis study in the rat hippocampus. Neuropsychopharmacology 7: 189–195. [PubMed] [Google Scholar]
- Zolkowska D, Baumann MH, Rothman RB. (2008) Chronic fenfluramine administration increases plasma serotonin (5-hydroxytryptamine) to nontoxic levels. J Pharmacol Exp Ther 324: 791–797. [DOI] [PubMed] [Google Scholar]