Abstract
There is interest in introducing generic antiretroviral drugs (ARVs) into high-income countries in order to maximise efficiency in health care budgets. Studies examining patients’ and providers’ knowledge and attitudes to generic substitution in HIV are few. This was a cross-sectional, observational study with a convenience sample of adult HIV-infected patients and health care providers (HCPs). Data on demographics, knowledge of generic medicine and facilitators of generic substitution were collected. Descriptive and univariate analysis was performed using SPSS V.23™. Questionnaires were completed by 66 patients. Seventy-one per cent would have no concerns with the introduction of generic ARVs. An increase in frequency of administration (61%) or pill burden (53%) would make patients less likely to accept generic ARVs. There were 30 respondents to the HCP survey. Concerns included the supply chain of generics, loss of fixed dose combinations, adherence and use of older medications. An increase in dosing frequency (76%) or an increase in pill burden (50%) would make HCPs less likely to prescribe a generic ARV. The main perceived advantage was financial. Generic substitution of ARVs would be acceptable to the majority of patients and HCPs. Reinvesting savings back into HIV services would facilitate the success of such a programme.
Keywords: HIV, AIDS, treatment, generic antiretrovirals, Ireland, antiretrovirals, patient opinion, health care provider opinion, antiretroviral therapy
Introduction
Chronic disease is responsible for significant morbidity and mortality in developed societies and an increasing proportion of health budgets worldwide are being transferred from the management of acute care into that of chronic disease.1,2 Given the global burden and economic impact of chronic disease, efforts are refocussing onto strategies to prevent or ameliorate ill health and disability using primary, secondary and tertiary disease prevention strategies. Health care medication budgets are increasingly under strain with more people living longer and requiring long-term medication to maintain their continued good health.1,2 HIV medicine is an area that demonstrates admirably this shift in focus from short-term acute care to longer term chronic disease management.3 Over the last 20 years, the success of HIV therapeutics has transformed this once fatal infection into a chronic disease. Patients with HIV infection who are engaged in care and on active antiretroviral (ARV) regimens are now expected to have close to normal lifespans.4 To date, the role of ARVs has been primarily in tertiary prevention of disease progression, with a small role in post-exposure prophylaxis.5 Recent guidance, utilising ARVs in primary and secondary prevention strategies for HIV (Pre-Exposure Prophylaxis [PreP] and Treatment as Prevention [TasP]), ensures that this will expand.6–9 However, the funding for such strategies at a time of ongoing fiscal constraint has been challenging in many health care systems.10,11 One strategy to optimise resource utilisation in order to facilitate service expansion in the setting of a fixed medication budget is generic substitution. Generic substitution has delivered significant cost savings to the Irish health service in recent years but it has not been introduced into routine HIV clinical care and there are no national guidelines in relation to their introduction (Barry M, 2016, personal communication). Generic ARVs have been widely used in low- and middle-income countries and are now being considered in high-income countries as many of the most commonly used ARVs have come off patent or are due to come off patent in the near future.12–14 This offers the opportunity to introduce generic substitution in this disease area.15 It has been calculated that generic substitution of ARVs would result in cost savings to the NHS of £1.25 billion16 and it has been modelled to be cost saving in other health services.17,18 However, it remains a controversial strategy with conflicting results presented in published cost-effectiveness analysis.19,20
There are many well-recognised barriers to generic substitution such as concerns about bioequivalence, quality and the robustness of the supply chain.21–27 In HIV medicine specifically, there is concern that patients’ virological outcomes and quality of life would be adversely affected by a switch from a single daily tablet regimen (STR) to a once-daily regimen of three pills.28–30 STRs have non-nucleotide reverse transcriptase inhibitors or integrase inhibitors as part of their active components. As yet, there is no equivalent STR with a protease inhibitor (PI) which mandates that patients on PI-based regimens have greater than one pill a day. To date there have been no data to suggest that patients on PI-based regimens are adversely affected by the lack of STR. Studies examining patients’ knowledge and attitudes to pill burden and generic substitution in HIV are few despite deeply held provider beliefs that pill burden concerns are of key importance to patients.29,31,32 This study aims to add to the knowledge base in order to inform policy with regards to the acceptability of generic substitution to patients and providers in the area of HIV.
Methods
Quantitative survey methodology was used to achieve the study end points
Surveys were tailored for patients and health care providers (HCP) and were broadly adapted from a survey that had been previously published by the group33 (Supplemental Appendix 1). They included both structured and unstructured questions. Surveys were piloted on a sample of all stakeholders prior to wider dissemination. Surveys contained 27 items in total. Surveys consisted of three domains: (i) demographic features of stakeholders; (ii) knowledge of generic medicine and (iii) perspectives on generic substitution, willingness to accept substitution and perceived facilitators and barriers to generic substitution. Ethical approval for the study was obtained through the institution’s Research Ethics Committee.
This was a cross-sectional observational study. Survey administration and recruitment methods were stakeholder specific. One-to-one interviews were conducted with a convenience sample of adult patients attending outpatients for routine HIV follow-up during an eight-week period over July and August 2015. The Genitourinary Medicine and Infectious Diseases clinic is a department within the university teaching hospital of St. James Hospital. It currently provides HIV care to over 2000 adult patients and is the largest service of its kind in Ireland.34 All patients gave written consent prior to participation. There were no exclusion criteria. Health care professionals were identified from the mailing list of a multidisciplinary continuing professional education forum focussed on HIV medicine and asked to complete an online version of the survey administered using Survey Monkey™. The initial survey was emailed on 26 August 2015, two reminder emails were sent and the survey was closed off on 31 October 2015. The survey mailing list was made up of 30 specialists (21 infectious diseases [ID], five genitourinary medicine [GU] and four virologists), 12 doctors-in-training (11 ID and one GU), 13 nurse specialists in HIV and four pharmacists and comprehensively covered the multidisciplinary caregivers involved in HIV care provision in Ireland in Autumn 2015.
Data collection and analysis
Patient hand-completed surveys were entered into a coded database by one study investigator, while HCP survey data were downloaded from the online website (Survey Monkey™). The patient dataset was screened for implausible data entry errors. Anomalous data were checked with raw questionnaire forms and subsequently corrected. To validate the categorical variable data, a random sample of the patient questionnaires (10%) were selected, and a second study investigator undertook a double data entry exercise. The datasets were matched and an error difference of <1% was determined correlating with an acceptable difference. Narrative data received in response to open-ended questions in the two surveys underwent a process of enrichment where applicable, and answers were categorised based on identified themes. This was conducted by the senior author who read and reread the narratives to capture an overall sense. Themes and sub-themes were then established and checked by the first and senior authors until consensus was reached.
Final datasets were imported into SPSS V.23™ for descriptive analysis. Univariate analysis was performed on categorical variables using Chi square test. All tests were double sided and a p-value of <0.05 was taken to indicate statistical significance.
Results
Patient survey
Cohort description
Questionnaires were completed by 66 patients. The sample was ethnically diverse and broadly reflective of the ethnicity of the patients attending the clinic (Table 1). Sixty-eight per cent (n = 45/65) were in current employment, 29% (n = 19/65) were jobseekers and two were in full-time education. Ninety-four per cent (n = 62/65) were currently receiving ARVs of whom 95% (n = 59/62) were taking a once-daily regimen and 50% (n = 31/62) were on a STR. Thirty-five per cent (n = 22/66) were prescribed medication other than ARVs. In those receiving additional medications, the median number of medications was 2 (range 1–8).
Table 1.
Demographic details and ARV prescriptions of the survey respondents, the overall HIV clinic cohort in St. James Hospital and the Irish national cohort.
| Survey respondents N (%) | Overall cohort N (%) | National cohort 2010 N (%) | |
|---|---|---|---|
| Total | 66 | 2174 | 3254 |
| Male | 54 (82) | 1565 (72) | 2023 (62) |
| Country of birth | |||
| Ireland | 37 (56) | 1090 (50) | 1761 (51) |
| Europe (excluding Ireland) | 12 (27) | 304 (14) | 240 (8) |
| South America | 5 (8) | 179 (8) | n/a |
| Africa | 9 (14) | 502 (23) | 1048 (33) |
| Asia | 2 (3) | 52 (2) | 36 (1) |
| US | 1 (1) | 21 (1) | n/a |
| Receiving ARVs | 62 (94) | n/a | 2574 (80) |
| Once-daily regimen | 59 (90) | n/a | n/a |
| Single tablet regimen | 31 (47) | n/a | 822 (32) |
ARV: antiretroviral.
Knowledge of generic medicines
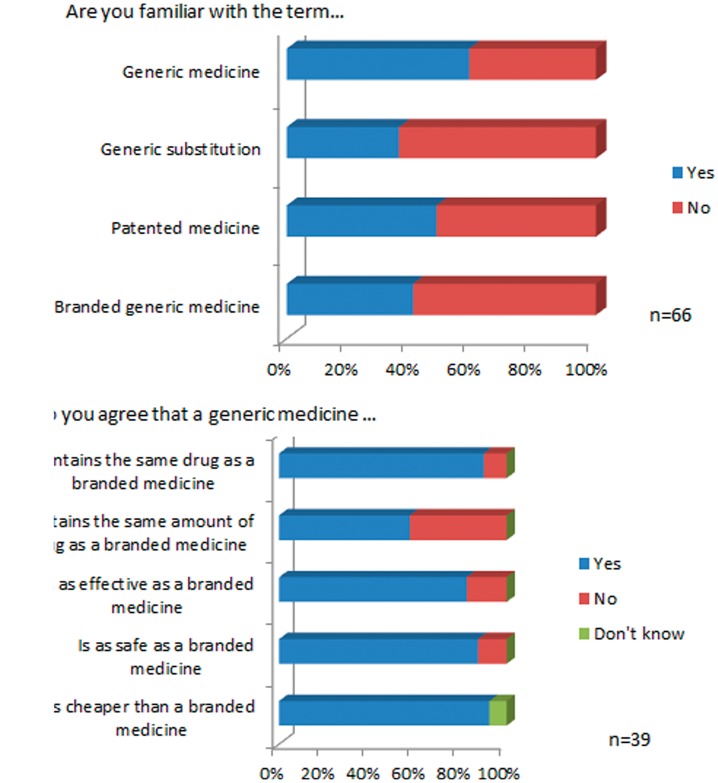
Thirty-nine patients (59%) reported that they were familiar with the term ‘generic medicine’. Knowledge of other terms such as ‘generic substitution’, ‘patented medicine’ and ‘branded generic medicine’ was lower and the results are summarised in Figure 1(a). Prior formal education of patients with regards to generic medicine was low, with only 21% (n = 14/66) having ever discussed generic medications with a HCP.
Figure 1.
Patient knowledge (a) and attitudes (b) to generic medicines.
Only patients who were familiar with the term ‘generic medicine’ were asked to complete the questions on their attitude to generic medicines. The majority of patients agreed that a generic medicine contained the same drug as a branded medicine (n = 36/39) and was as safe (n = 35/39) and effective (n = 33/39) as a branded medicine (Figure 1(b)).
When asked if they would have any specific concerns if generic ARVs were introduced, 71% (n = 47/66) said that they would not have any concerns. Six per cent (n = 4/66) said they were not sure and 23% (n = 15/66) said that they would be concerned. Concerns included effectiveness of generics, potential side effects, quality of generics and questions around why they were cheaper. Some patients said that they would want more information before accepting generic medications or would prefer to remain on medication they were familiar with.
Sixty-one per cent of patients (n = 40/60) reported that an increase in frequency of administration would affect them while 24% (n = 17/66) felt it would not affect them and 15% (n = 10/66) did not know. Fifty-three per cent (n = 35/66) reported an increase in pill burden would affect them while just over one-third of patients (n = 23/66) reported that an increase in pill burden would not affect them with the remainder (n = 8/66) unsure of its impact.
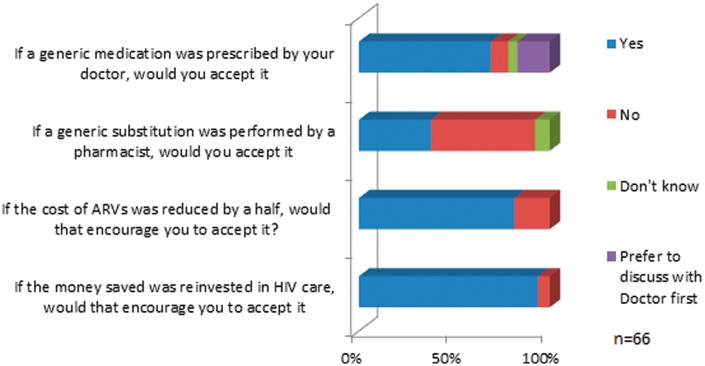
Patients were more willing to accept a generic substitution introduced by their physician (n = 45/66, 68%) than one introduced by their pharmacist (n = 25/66, 39%).
The approximate cost of a one-month supply of ARVs was correctly estimated at €1000 by 23% of patients (n = 15/66), while 12% estimated that it cost €50 or less (n = 8/66) per month and a further quarter (n = 24/66) of patients estimated that it cost €500/month. Eleven per cent (n = 7/66) estimated the cost at greater than €1000 and 14 patients (24%) could not provide an estimate of cost (Figure 2).
Figure 2.
Patients’ attitudes to generic substitution of ARVs. ARV: antiretroviral.
Univariate analysis
We did not detect a significant difference in willingness to accept generic substitution between those who were currently receiving a single tablet regimen (STR) versus those not prescribed a STR (p = 0.56). Similarly, there was no difference detected in their willingness to accept an increase in dosing frequency (p = 0.23). No significant difference was found in willingness to accept generic substitution between those currently receiving a once-daily regimen and those receiving a twice-daily regimen (p = 0.54) nor was there any significant relationship detected in their willingness to accept an increase in dosing frequency (p = 0.52).
There was no difference detected in willingness to accept generic substitution (p = 0.64), an increase in dosing frequency (p = 0.84) or an increase in pill burden (p = 0.44) depending on whether or not patients were receiving medications other than ARVs.
HCP survey
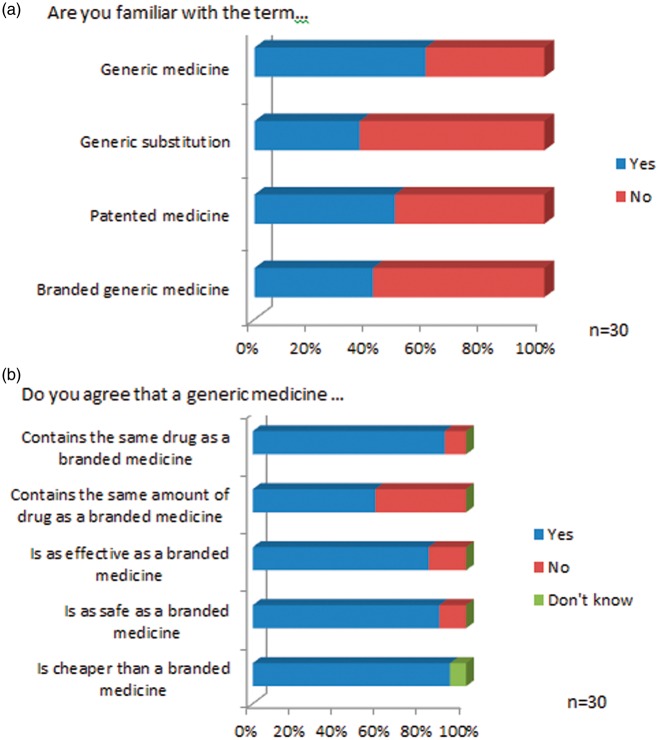
There were 30 respondents to the HCP survey giving a response rate of 51% (n = 30/59). Twenty-two (71%) were physicians, four (13%) were nurse specialists and four (13%) were pharmacists. Sixty-six per cent of respondents (n = 20/30) had been working in HIV medicine for greater than five years (range 1 to greater than 20 years, mode 10–15 years). Of the physician respondents, 12 of 19 IDs specialists (63%), four of five GU consultants (80%) and six doctors in training responded (50%). Seven HCPs (24%) responded that they had never received formal education with regard to generic medicines. Knowledge of the terms associated with generic medicines was good (Figure 3(a)). The majority of HCPs agreed that generic medicines were as effective as branded medication (n = 23/30, 77%), were as safe as branded medicines (n = 25/30, 64%) and were of the same quality as branded medicines (n = 19/30, 63%). Attitudes to generic substitution are summarised in Figure 3(b).
Figure 3.
Healthcare providers’ (a) knowledge of the terminology associated with generic medicines (b) and their attitudes towards generic medicines.
Concerns around the supply chain of generics were prominent among the cohort surveyed, with a small number (n = 11, 37%) stating that they would have no concerns. The main advantage to a generic switch was felt to be financial, while the disadvantages fell into three broad themes relating to (a) loss of fixed dose combinations (FDCs), (b) potential effects on adherence and (c) the use of older medications. When asked if generic medications became available would they be willing to prescribe them, there was broad acceptance with 30% (n = 9/30) stating ‘Yes’, 43% (n = 13/30) stating ‘Yes in some cases’ and 27% (n = 8/30) stating they would ‘on a case-by-case basis’.
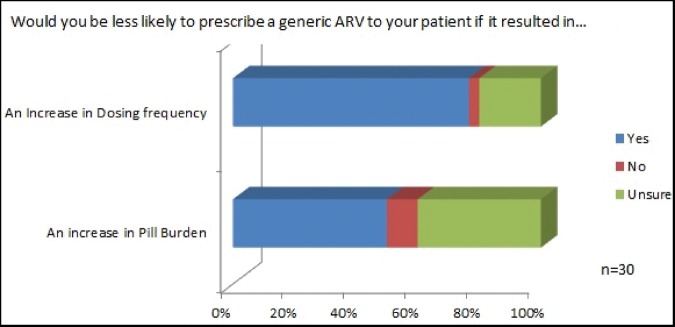
Seventy-six per cent (n = 23/30) responded that an increase in dosing frequency would affect their willingness to prescribe a generic ARV. Fifty per cent (n = 15/30) would be less willing to prescribe a generic if it resulted in an increase in pill burden (Figure 4(b)).
Figure 4.
Health care providers’ responses to the importance of an increase in dosing frequency or pill burden as a result of a generic substitution.
There was good awareness of the cost of ARVs with 50% (n = 15/30) correctly stating the total national annual cost of ARVs and 77% aware of the monthly cost per prescription. Seventy-seven per cent were agreeable to generic substitution if it halved the cost of ARVs and 93% would prescribe generics if the money saved could be reinvested in HIV care.
Discussion
Generic substitution of ARVs presents an opportunity to redirect money from tertiary prevention of HIV progression and reinvest it into primary and secondary prevention strategies. The direct medical cost of HIV care for an individual patient in France had been estimated at €20,000 per year of which 73% was accounted for by the cost of ARVs. Greater than €8 billion was spent on ARVs in Germany between 1999 and 2012.13,18 A recent UK study demonstrated a modelled lifetime cost of HIV care as €246,000 per patient, with the introduction of generic ARVs resulting in significant cost savings.35 The establishment of best practice, evidence-based prevention programmes for HIV guarantee that this cost will increase as newer, more expensive ARVs are introduced, increasing numbers of HIV-infected patients are diagnosed and strategies such as PreP–TasP are expanded.5,7–9,36 The results of this study show that generic substitution would be acceptable to the majority of patients and HCPs in Ireland. They broadly mirror those of a larger study of HIV-infected patients and their HCPs in France, which demonstrated that generic ARVs would be acceptable to most patients and physicians but loss of the FDC and an increase in pill burden reduced acceptability.32
Barriers to generic substitution for both patients and providers were unsurprisingly focussed around any potential increase in pill burden or dosing frequency. This has been previously shown to be important to patients and physicians when questioned about the introduction of generic ARVs.29,32 In high-income countries, generic ARVs are not available as FDCs. The introduction of generic ARVs to patients currently in receipt of STRs of non-nucleotide reverse transcriptase inhibitor (NNRTI)-based treatment would result in an increase in pill burden. The patient cohort predominantly received a once-daily regimen, with the majority receiving STRs. As such they are representative of the patients that would be considered for a generic substitution of their ARVs.36 For these patients a change in dosing frequency was more of a concern than a modest increase in pill burden. Despite these concerns, the majority would be willing to accept generic substitution, especially if proposed by their medical practitioner. In a national survey of HIV patients in Ireland in 2010, 42% were receiving a PI-based therapy and therefore would have been taking more than a STR. There was no evidence in that study that using a multi-tablet combination resulted in a reduction in virological outcome.36 There was little knowledge of generic medicines among patients and some of the concerns voiced with regard to changes in side effects or an increase in the amount of medication taken would be easily alleviated with targeted education by HCPs. In line with this, facilitators of generic substitution included positive messages from doctors with regards to their safety and advisability, demonstrating the importance of buy-in from medical personnel in ensuring the success of a generic switch strategy.
It is clear from this study that concerns around the quality and supply chain consistency of the generic ARVs would need to be alleviated before HCPs would embrace a generic switch strategy. Generic ARVs have been successfully implemented in low- and middle-income countries where the procurement and supply chain challenges have been more significant than those that would be anticipated in a high-income country with existing robust medicines regulatory bodies.37,38 It is likely that the main challenge to the introduction of generic ARVs in high-income countries will be the lack of generic FDCs and the unavailability of generic integrase inhibitor therapy rather than difficulty with the quality and supply of the available generic products. With over a quarter of HCP replying that they had never received any education with regard to generic medicine, an information programme would be helpful to increase knowledge and reduce concerns around the quality of generic ARVs. As the cost of ARVs in Ireland is state funded, it is perhaps unsurprising that knowledge of the cost of ARVs was poor among patients. Despite this, when faced with a hypothetical question regarding a reduction in the cost of ARVs and reinvestment into other HIV services, financial motivators were powerful tools driving acceptability. This demonstrates that even in health care services where the cost of ARVs is not borne directly by the patients, education with regards to the cost of the health service provided can act as a facilitator to change in behaviour. Knowledge of the cost of ARVs was high among HCP and was again the main driver for a switch to generic medicines. Demonstrating predicted savings to the health service and ring fencing those savings for reinvestment into HIV medicine-related services would result in acceptance of generic ARVs for nearly all respondents.
Limitations
The patient survey was performed in a single centre and it is possible that it is not representative of patients attending other HIV centres. However, the centre is the largest of its kind in Ireland with a HIV-infected patient cohort of over 2000, and as such, is likely to be representative of the patient cohorts attending smaller centres.36 The sample size was small and therefore it was not appropriate to undertake extensive statistical tests on the responses. We did not detect any significant difference in willingness to accept generic substitution for any patient demographic variable or between those currently receiving a STR and those not receiving a STR, but it is possible that this is down to the size of our sample.
Conclusion
Generic substitution of ARVs would be acceptable to the majority of patients and HCPs and given the potential saving to the health service it should be considered in the Irish setting. Targeted education programmes for both patients and HCPs would alleviate misapprehensions and concerns surrounding generic substitution and should be included as part of any implementation process. Financial considerations were powerful motivators and commitment to reinvesting savings generated from generic substitution back into HIV services would facilitate the success of a ARV generic substitution programme.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by the Research Summer School of the Royal College of Surgeons of Ireland.
References
- 1.Busse R, Blumel M, Scheller-Kreinsen D, et al. Tackling Chronic Disease in Europe; Strategies, Interventions and Challenges, Geneva: WHO, 2010, pp. 127–127. Available at: http://www.euro.who.int/en/health-topics/Life-stages/healthy-ageing/publications/2010/tackling-chronic-disease-in-europe-strategies,-interventions-and-challenges-2010 (accessed 21 February 2017). [Google Scholar]
- 2.Department of Health and Children in Ireland. Tackling chronic disease: a policy framework for the management of chronic disease, Dublin: Department of Health and Children in Ireland, 2014, pp. 26–26. Available at: http://health.gov.ie/wp-content/uploads/2014/03/tackling_chronic_disease.pdf (accessed 21 February 2017). [Google Scholar]
- 3.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet 2013; 382: 1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakagawa F, May M, Phillips A. Life expectancy living with HIV: recent estimates and future implications. Curr Opin Infect Dis 2013; 26: 17–25. [DOI] [PubMed] [Google Scholar]
- 5.Fisher M, Briggs E, Cresswell F, et al. UK guideline for the use of post-exposure prophylaxis following sexual exposure. Int J STD AIDS 2016; 27: 713–738. [DOI] [PubMed] [Google Scholar]
- 6.Society EAC. Guidelines Version 8, 2015.
- 7.Churchill D, Waters L, Ahmed N, et al. British HIV Association Guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy. HIV Med 2016; 17(Suppl 4): s2–s104. [DOI] [PubMed] [Google Scholar]
- 8.US Public Health Service. Pre-exposure prophylaxis for the prevention of HIV infection in the United States, Atlanta, GA: CDC, 2014. [Google Scholar]
- 9.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Washington DC: Department of Health and Human Services. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf (2016, accessed 21 February 2017).
- 10.Gomez GB, Borquez A, Case KK, et al. The cost and impact of scaling up pre-exposure prophylaxis for HIV prevention: a systematic review of cost-effectiveness modelling studies. PLoS Med 2013; 10: e1001401–e1001401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenyon CR, Osbak K. How many MSM in Europe could benefit from PrEP – a 9 billion Euro question? Int J STD AIDS 2015; 26: 988–990. [DOI] [PubMed] [Google Scholar]
- 12.Wormser GP, Lappas T. Is there a role for generic antiretroviral drugs in the United States? Expert Rev Anti Infect Ther 2014; 12: 897–899. [DOI] [PubMed] [Google Scholar]
- 13.Yazdanpanah Y, Schwarzinger M. Generic antiretroviral drugs and HIV care: an economic review. Med Mal Infect 2016; 46: 67–71. [DOI] [PubMed] [Google Scholar]
- 14.Beck EJ, Passarelli C, Lui I, et al. Scaling-up the use of generic antiretrovirals in resource-limited countries: generic drugs for health. Antivir Therapy 2014; 19: 117–123. [DOI] [PubMed] [Google Scholar]
- 15.Gazzard B, Moecklinghoff C, Hill A. New strategies for lowering the costs of antiretroviral treatment and care for people with HIV/AIDS in the United Kingdom. Clinicoecon Outcomes Res 2012; 4: 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill A, Hill T, Jose S, et al. Predicted savings to the UK National Health Service from switching to generic antiretrovirals, 2014–2018. J Int AIDS Soc 2014; 17: 19497–19497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Restelli U, Scolari F, Bonfanti P, et al. New highly active antiretroviral drugs and generic drugs for the treatment of HIV infection: a budget impact analysis on the Italian National Health Service (Lombardy Region, Northern Italy). BMC Infect Dis 2015; 15: 323–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoll M, Kollan C, Bergmann F, et al. Calculation of direct antiretroviral treatment costs and potential cost savings by using generics in the German HIV ClinSurv cohort. PLoS One 2011; 6: e23946–e23946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sweet DE, Altice FL, Cohen CJ, et al. Cost-effectiveness of single- versus generic multiple-tablet regimens for treatment of HIV-1 infection in the United States. PLoS One 2016; 11: e0147821–e0147821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walensky RP, Sax PE, Nakamura YM, et al. Economic savings versus health losses: the cost-effectiveness of generic antiretroviral therapy in the United States. Ann Intern Med 2013; 158: 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chong CP, Hassali MA, Bahari MB, et al. Exploring community pharmacists’ views on generic medicines: a nationwide study from Malaysia. Int J Clin Pharm 2011; 33: 124–131. [DOI] [PubMed] [Google Scholar]
- 22.Dunne S, Shannon B, Dunne C, et al. Patient perceptions of generic medicines: a mixed-methods study. Patient 2014; 7: 177–185. [DOI] [PubMed] [Google Scholar]
- 23.Dunne SS, Shannon B, Cullen W, et al. Perceptions and attitudes of community pharmacists towards generic medicines. J Manag Care Spec Pharm 2014; 20: 1138–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunne SS, Shannon B, Cullen W, et al. Beliefs, perceptions and behaviours of GPs towards generic medicines. Fam Pract 2014; 31: 467–474. [DOI] [PubMed] [Google Scholar]
- 25.Heikkila R, Mantyselka P, Hartikainen-Herranen K, et al. Customers’ and physicians’ opinions of and experiences with generic substitution during the first year in Finland. Health Policy 2007; 82: 366–374. [DOI] [PubMed] [Google Scholar]
- 26.Himmel W, Simmenroth-Nayda A, Niebling W, et al. What do primary care patients think about generic drugs? Int J Clin Pharmacol Ther 2005; 43: 472–479. [DOI] [PubMed] [Google Scholar]
- 27.Keenum AJ, Devoe JE, Chisolm DJ, et al. Generic medications for you, but brand-name medications for me. Res Social Adm Pharm 2012; 8: 574–578. [DOI] [PubMed] [Google Scholar]
- 28.Ramjan R, Calmy A, Vitoria M, et al. Systematic review and meta-analysis: patient and programme impact of fixed-dose combination antiretroviral therapy. Trop Med Int Health 2014; 19: 501–513. [DOI] [PubMed] [Google Scholar]
- 29.Engelhard E, Smith C, Vervoort S, et al. Patients’ willingness to take separate component antiretroviral therapy regimens for HIV in the Netherlands. J Int AIDS Soc 2014; 17: 19536–19536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramiro MA, Llibre JM. Legal, ethical, and economic implications of breaking down once-daily fixed-dose antiretroviral combinations into their single components for cost reduction. Enferm Infecc Microbiol Clin 2014; 32: 598–602. [DOI] [PubMed] [Google Scholar]
- 31.Allavena C, Jacomet C, Pereira B, et al. Acceptability and confidence in antiretroviral generics of physicians and HIV-infected patients in France. J Int AIDS Soc 2014; 17: 19608–19608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacomet C, Allavena C, Peyrol F, et al. Perception of antiretroviral generic medicines: one-day survey of HIV-infected patients and their physicians in France. PLoS One 2015; 10: e0117214–e0117214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Leary A, Usher C, Lynch M, et al. Generic medicines and generic substitution: contrasting perspectives of stakeholders in Ireland. BMC Res Notes 2015; 8: 790–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuite H, Horgan M, Mallon PW, et al. Patients accessing ambulatory care for HIV-infection: epidemiology and prevalence assessment. Irish Med J 2015; 108: 199–202. [PubMed] [Google Scholar]
- 35.Nakagawa F, Miners A, Smith CJ, et al. Projected lifetime healthcare costs associated with HIV infection. PLoS One 2015; 10: e0125018–e0125018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Connell S, Lillis D, Cotter A, et al. Opt-out panel testing for HIV, Hepatitis B and Hepatitis C in an urban emergency department: a pilot study. PLoS One 2016; 11: e0150546–e0150546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ripin DJ, Jamieson D, Meyers A, et al. Antiretroviral procurement and supply chain management. Antivir Therapy 2014; 19: 79–89. [DOI] [PubMed] [Google Scholar]
- 38.Rago L, Sillo H, t Hoen E, et al. Regulatory framework for access to safe, effective quality medicines. Antivir Therapy 2014; 19: 69–77. [DOI] [PubMed] [Google Scholar]