Abstract
OBJECTIVE
To determine whether the effects of intensive (<120 mmHg) compared with standard (<140 mmHg) systolic blood pressure (SBP) treatment are different among those with prediabetes versus those with fasting normoglycemia at baseline in the Systolic Blood Pressure Intervention Trial (SPRINT).
RESEARCH DESIGN AND METHODS
This was a post hoc analysis of SPRINT. SPRINT participants were categorized by prediabetes status, defined as baseline fasting serum glucose ≥100 mg/dL versus those with normoglycemia (fasting serum glucose <100 mg/dL). The primary outcome was a composite of myocardial infarction, acute coronary syndrome not resulting in myocardial infarction, stroke, acute decompensated heart failure, or death from cardiovascular causes. Cox regression was used to calculate hazard ratios for study outcomes with intensive compared with standard SBP treatment among those with prediabetes and normoglycemia.
RESULTS
Among 9,361 participants randomized (age 67.9 ± 9.4 years; 35.5% female), 3,898 and 5,425 had baseline prediabetes and normoglycemia, respectively. After a median follow-up of 3.26 years, the hazard ratio for the primary outcome was 0.69 (95% CI 0.53, 0.89) and 0.83 (95% CI 0.66, 1.03) among those with prediabetes and normoglycemia, respectively (P value for interaction 0.30). For all-cause mortality, the hazard ratio with intensive SBP treatment was 0.77 (95% CI 0.55, 1.06) for prediabetes and 0.71 (95% CI 0.54, 0.94) for normoglycemia (P value for interaction 0.74). Effects of intensive versus standard SBP treatment on prespecified renal outcomes and serious adverse events were similar for prediabetes and normoglycemia (all interaction P > 0.05).
CONCLUSIONS
In SPRINT, the beneficial effects of intensive SBP treatment were similar among those with prediabetes and fasting normoglycemia.
Introduction
Prediabetes or diabetes and hypertension frequently coexist. Patients with established diabetes and hypertension have a fourfold greater risk of cardiovascular disease (CVD) compared with those with normoglycemia and normotension (1). Whether prediabetes alone is associated with increased CVD risk remains controversial, but in coexistence with hypertension, it is reported to increase CVD risk by 2.4-fold (2). The optimal systolic blood pressure (SBP) target for initiation and treatment with antihypertensive medications is uncertain, particularly in those with prediabetes and diabetes. The discordant results of the recent Systolic Blood Pressure Intervention Trial (SPRINT) and the Action to Control Cardiovascular Risk in Diabetes Blood Pressure (ACCORD BP) trial add to this uncertainty. SPRINT demonstrated significant reductions in cardiovascular disease (CVD) events and all-cause mortality with more intensive SBP treatment (SBP target <120 mmHg) compared with standard treatment (SBP target <140 mmHg) among U.S. adults at high CVD risk but without a diagnosis of diabetes (3). However, in ACCORD BP, a similar SBP treatment comparison resulted in a nonstatistically significant 12% lower risk of CVD events over 5 years of treatment in older U.S. adults with type 2 diabetes and at high CVD risk (4).
Prediabetes is the term used to identify individuals at high risk for the development of diabetes whose hemoglobin A1c or fasting serum glucose (FSG) levels are higher than normal but do not meet the criteria for clinical diabetes. Determining whether the effects of intensive SBP treatment in SPRINT differ among participants with prediabetes and normoglycemia at baseline may provide valuable information on the SBP treatment target in a broader population with dysglycemia. FSG values are available in SPRINT, but hemoglobin A1c values were not obtained. We, therefore, sought to determine whether the effect of intensive SBP treatment compared with standard SBP treatment is different among participants with prediabetes, defined as baseline FSG ≥100 mg/dL, versus normoglycemia, defined as FSG <100 mg/dL, at baseline in SPRINT. Although the current analysis was not prespecified, the question of the effect of intensive blood pressure treatment in high-risk patients with prediabetes versus normoglycemia became urgent given the inconsistent results of the overall SPRINT and ACCORD trials. We, therefore, hypothesized that the effect of intensive treatment on the primary outcome in SPRINT would be similar among participants with prediabetes or normoglycemia at baseline.
Research Design and Methods
This was a post hoc analysis of SPRINT. The rationale, design, and main results of SPRINT have previously been published (3,5). The study protocol was approved by each site’s institutional review board, and every participant provided written informed consent. The writing committee wrote the manuscript and attests to the completeness and accuracy of the data and analysis. The manuscript was reviewed and approved by the SPRINT Steering Committee and Publications subcommittee. An independent Data and Safety Monitoring Board monitored unblinded study data and provided oversight of participant safety.
Eligibility
Briefly, participants were men and women age 50 years or older, with SBP 130–180 mmHg on no medication or taking a medication from one antihypertensive medication class, 130–170 mmHg on medications from up to two classes, 130–160 mmHg on medications from up to three classes, 130–150 mmHg on medications from up to four classes, and had one or more high–CVD risk conditions. High–CVD risk conditions were defined as a history of clinical or subclinical CVD other than stroke, estimated glomerular filtration rate (eGFR) of 20–59 mL/min/1.73 m2 using the four-variable MDRD equation (6), 10-year risk for CVD ≥15% calculated using the Framingham Risk Score for general clinical practice (7), and/or age ≥75 years. Main exclusion criteria included diabetes, a history of stroke, heart failure, proteinuria ≥1 g/day, and an eGFR <20 mL/min/1.73 m2. Detailed inclusion and exclusion criteria are listed in the SPRINT design paper (5).
Randomization and Interventions
Eligible participants were randomly assigned to either an SBP target of <120 mmHg or <140 mmHg. All major classes of antihypertensive medications were included in the SPRINT formulary. Participants were seen monthly for the first 3 months and then every 3 months thereafter.
Study Measurements
Each participant’s sociodemographic characteristics were collected at baseline. Clinical and laboratory data were collected at baseline and every 3 months thereafter. At each visit, trained clinical staff measured blood pressures with an automated device (Omron-HEM-907 XL) using standardized procedures (3,5,8). Blood pressure measurement requirements included measuring blood pressure early in the visit and not after stressful exam components such as blood draws, proper positioning of the participant in a chair with back support, and proper cuff size determination. The Manual of Procedures stated that participants should be resting, not completing questionnaires, and not speaking with study staff during the 5-min rest period or while blood pressure measurements were being taken. The Manual of Procedures also stated that staff should leave the room during the 5-min rest period and provided a script that staff could use to explain that they would be absent during the 5-min rest period and would then enter the room and obtain the measurements without speaking to the participant. FSG was obtained at baseline and 2 and 4 years postrandomization. Samples were centrifuged and shipped on ice to the central laboratory. Glucose was measured in serum using the hexokinase method on a Roche analyzer. Structured interviews were conducted every 3 months to ascertain self-reported CVD outcomes.
Study Outcomes
The primary outcome was a CVD composite of myocardial infarction, acute coronary syndrome not resulting in myocardial infarction, stroke, acute decompensated heart failure, or death from CVD causes. Secondary outcomes included the individual components of the primary outcome, death from any cause, and the composite of the primary outcome or death from any cause. An adjudication committee blinded to treatment assignment adjudicated all outcomes using a prespecified protocol. Renal outcomes were prespecified and defined differently for those with and without chronic kidney disease (CKD) at baseline as detailed in the SPRINT protocol (8). Among participants with CKD at baseline, the renal outcome was defined by a composite of a decrease in the eGFR of ≥50% or the development of end-stage renal disease requiring long-term dialysis or kidney transplantation. Among those without CKD at baseline, the renal outcome was defined by a decrease in the eGFR ≥30% to <60 mL/min/1.73 m2. Incident albuminuria was defined by a doubling of the ratio of urinary albumin (in milligrams) to creatinine (in grams) from <10 at baseline to >10 during follow-up.
Serious adverse events were defined as those that were fatal or life-threatening, that resulted in clinically significant or persistent disability, that required or prolonged hospitalization, or that were judged by the investigator to represent a clinically significant hazard or harm to the participant that might require medical or surgical intervention to prevent one of the other events listed above. Clinical and laboratory variables, including serum electrolytes, were examined for potential adverse events.
Statistical Analysis
Based on a planned enrollment of 9,250 participants and an event rate in the standard treatment arm of 2.2% per year for the primary outcome, SPRINT had 88.7% power to detect a 20% relative risk reduction in the primary end point between intensive and standard SBP treatment (5). Among those randomized to standard treatment, there were 2,704 and 1,957 in the normoglycemia and prediabetes groups, respectively. In the intensive treatment arm, there were 2,721 and 1,941 in the normoglycemia and prediabetes groups, respectively. The absolute risk of the primary outcome at 3 years (approximately the median follow-up in SPRINT) among those with normoglycemia in the standard arm was 6.3%. Using these numbers and a two-tailed α of 0.05, our design provided 80% power to detect a 55% relative change (in either direction) in the hazard ratio of intensive treatment compared with standard treatment between baseline prediabetes and normoglycemia groups on the additive scale. Baseline characteristics were compared across treatment arms stratified by those with prediabetes, defined as baseline FSG ≥100 mg/dL, versus those with normoglycemia, defined as baseline FSG <100 mg/dL using ANOVA for continuous variables and χ2 tests for categorical variables. Baseline characteristics were also compared between those with prediabetes and normoglycemia at baseline regardless of randomized treatment assignment.
Participants were censored on the date of last event ascertainment prior to 21 August 2015. Using the intention-to-treat approach for all randomly assigned participants with two-sided tests at the 5% level of significance, we used Cox proportional hazards regression with stratification according to clinic and prediabetes versus normoglycemia status at baseline to calculate hazard ratios for the primary outcome associated with intensive SBP treatment versus standard SBP treatment (referent) among those with prediabetes or normoglycemia at baseline. We tested the proportional hazards assumption by modeling the product of SBP treatment arm and the log of follow-up time as an interaction term; no violations were observed. To assess for effect modification of treatment arm among SPRINT participants with prediabetes and normoglycemia, we included the product term (SBP treatment arm × prediabetes or normoglycemia) in the Cox proportional hazards regression in the full sample using a likelihood ratio test. This was repeated for all secondary outcomes separately. Measures of interaction for the primary outcome are presented on both the additive and multiplicative scales (9).
It is known that there is a positive and graded association between increasing FSG as a continuous variable, beginning as low as 85 mg/dL, and impaired glucose tolerance and clinical diabetes (10–12). We, therefore, performed secondary analyses to assess for a graded interaction between baseline FSG and the effect of intensive treatment on the primary and secondary CVD outcomes (10–12). First, we compared hazard ratios of intensive versus standard SBP treatment within quartiles of baseline FSG levels. Second, we examined and graphed the interaction between the effect of intensive treatment on the primary outcome and baseline FSG, modeled as a continuous variable with restricted cubic splines using the mfpi command in Stata (13,14). Several sensitivity analyses were conducted. First, because some people with prediabetes may revert to a fasting normoglycemia over time, we repeated all analyses restricting the prediabetes group (FSG ≥100 mg/dL) to only SPRINT participants with elevated FSG at both baseline and the 2-year SPRINT study visit. Second, we repeated all analyses restricted to those whose blood sample was recorded as fasting at the time of the blood draw by study staff (n = 580 excluded). In order to comply with the strict definition of prediabetes (FSG 100–125 mg/dL), we also repeated analyses excluding SPRINT participants who had a baseline FSG ≥126 mg/dL, the cutoff for a diagnosis of diabetes, at baseline (n = 295).
All analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC), and Stata, version 14.2 (StataCorp, College Station, TX).
Results
Study Participants
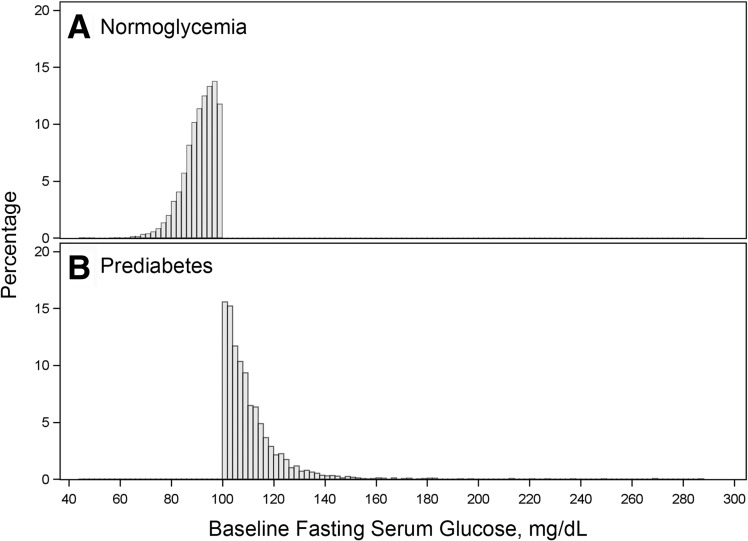
A total of 3,898 participants (41.6%) had prediabetes (FSG ≥100 mg/dL) and 5,425 (58.0%) had normoglycemia (FSG <100 mg/dL) at baseline (Supplementary Fig. 1). The distribution of baseline FSG is shown in Fig. 1. FSG in the two treatment arms over the course of the trial among those with prediabetes and normoglycemia is shown in Supplementary Fig. 2 and Supplementary Tables 1 and 2. The baseline characteristics of the SPRINT study population by treatment arm within prediabetes and normoglycemia strata are shown in Table 1. Table 1 also shows baseline characteristics between prediabetes and normoglycemia regardless of treatment assignments. Overall, the mean ± SD age was 67.9 ± 9.4 years, 35.5% were female, and the mean Framingham Risk score was 24.8%. There were no significant differences in baseline characteristics between treatment arms within the prediabetes or within the normoglycemia strata. The median follow-up was 3.26 years (interquartile range 2.79–3.79).
Figure 1.
Histogram of baseline FSG among those with normoglycemia (A) and prediabetes (B).
Table 1.
Baseline characteristics of SPRINT participants overall and by treatment arm stratified among those with normoglycemia and prediabetes at baseline and by normoglycemia and prediabetes status overall
| Characteristic | Baseline FSG |
Baseline FSG |
Pa | ||||
|---|---|---|---|---|---|---|---|
| Normoglycemia: <100 mg/dL |
Prediabetes: ≥100 mg/dL |
||||||
| Intensive treatment | Standard treatment | Intensive treatment | Standard treatment | Normoglycemia: <100 mg/dL | Prediabetes: ≥100 mg/dL | ||
| n | 2,721 | 2,704 | 1,941 | 1,957 | 5,425 | 3,898 | |
| Age, years | 68.1 ± 9.6 | 68.1 ± 9.7 | 67.6 ± 9.1 | 67.7 ± 9.2 | 68.1 ± 9.6 | 67.6 ± 9.2 | 0.02 |
| Female sex | 1,111 (40.8) | 1,044 (38.6) | 559 (28.8) | 593 (30.3) | 2,155 (39.7) | 1,152 (29.6) | <0.001 |
| Race or ethnic group | |||||||
| Non-Hispanic black | 863 (31.7) | 901 (33.3) | 508 (26.2) | 513 (26.2) | 1,764 (32.5) | 1,021 (26.2) | <0.001 |
| Hispanic | 286 (10.5) | 279 (10.3) | 214 (11) | 199 (10.2) | 565 (10.4) | 413 (10.6) | 0.78 |
| Non-Hispanic white | 1,520 (55.9) | 1,478 (54.7) | 1,173 (60.4) | 1,215 (62.1) | 2,998 (55.3) | 2,388 (61.3) | <0.001 |
| Other | 52 (1.9) | 46 (1.7) | 46 (2.4) | 30 (1.5) | 98 (1.8) | 76 (1.9) | 0.61 |
| Criterion for high CVD risk | |||||||
| Age ≥75 years | 806 (29.6) | 791 (29.3) | 508 (26.2) | 520 (26.6) | 1,597 (29.4) | 1,028 (26.4) | <0.01 |
| CKD | 805 (29.6) | 771 (28.5) | 525 (27) | 544 (27.8) | 1,576 (29.1) | 1,069 (27.4) | 0.09 |
| CVD | 521 (19.1) | 525 (19.4) | 418 (21.5) | 405 (20.7) | 1,046 (19.3) | 823 (21.1) | 0.03 |
| Clinical | 375 (13.8) | 379 (14) | 324 (16.7) | 312 (15.9) | 855 (15.8) | 700 (18) | <0.01 |
| Subclinical | 275 (10.1) | 287 (10.6) | 212 (10.9) | 187 (9.6) | 293 (5.4) | 197 (5.1) | 0.46 |
| FRS ≥15% | 2,022 (74.3) | 1,992 (73.7) | 1,534 (79) | 1,555 (79.5) | 4,014 (74.1) | 3,089 (79.3) | <0.001 |
| SBP, mmHg | 140.3 ± 15.9 | 140.1 ± 15.5 | 138.8 ± 15.6 | 139.1 ± 15.2 | 140.2 ± 15.7 | 138.9 ± 15.4 | <0.001 |
| Diastolic blood pressure, mmHg | 78.6 ± 11.9 | 78.4 ± 11.9 | 77.7 ± 11.9 | 77.6 ± 12.1 | 78.5 ± 11.9 | 77.7 ± 12 | <0.01 |
| Serum creatinine, mg/dL | 1.1 ± 0.4 | 1.1 ± 0.4 | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.4 | 1.1 ± 0.3 | 0.14 |
| eGFR, mL/min/1.73 m2 | 71.1 ± 20.9 | 71.4 ± 20.9 | 72.7 ± 20.3 | 72.1 ± 20.1 | 71.3 ± 20.9 | 72.4 ± 20.2 | <0.01 |
| Urine albumin-to-creatinine ratio, mg/g | 48.1 ± 204.6 | 40.4 ± 137.4 | 38.6 ± 134.1 | 42.0 ± 172.1 | 44.2 ± 174.6 | 40.3 ± 154.4 | 0.27 |
| FSG, mg/dL | 90.5 ± 6.5 | 90.7 ± 6.4 | 110.5 ± 12.6 | 110 ± 12.4 | 90.6 ± 6.5 | 110.2 ± 12.5 | <0.001 |
| Fasting total cholesterol, mg/dL | 192.4 ± 42.3 | 190.5 ± 40.7 | 187.1 ± 39.9 | 189.4 ± 41.3 | 191.5 ± 41.5 | 188.3 ± 40.6 | <0.001 |
| Fasting HDL cholesterol, mg/dL | 55.1 ± 15 | 54.6 ± 15.1 | 49.9 ± 12.7 | 50.4 ± 13.5 | 54.8 ± 15.1 | 50.1 ± 13.1 | <0.001 |
| Smoking status | |||||||
| Never smoked | 1,238 (45.5) | 1,221 (45.2) | 808 (41.6) | 844 (43.1) | 2,459 (45.3) | 1,652 (42.4) | <0.01 |
| Former smoker | 1,072 (39.4) | 1,106 (40.9) | 901 (46.4) | 884 (45.2) | 2,178 (40.1) | 1,785 (45.8) | <0.001 |
| Current smoker | 407 (15) | 373 (13.8) | 231 (11.9) | 227 (11.6) | 780 (14.4) | 458 (11.7) | <0.001 |
| Missing data | 4 (0.1) | 4 (0.1) | 1 (0.1) | 2 (0.1) | 8 (0.1) | 3 (0.1) | 0.33 |
| BMI, kg/m2 | 29.1 ± 5.8 | 29.1 ± 5.7 | 30.9 ± 5.7 | 30.8 ± 5.6 | 29.1 ± 5.7 | 30.9 ± 5.6 | <0.001 |
| Metabolic syndrome | 507 (18.6) | 528 (19.5) | 1,263 (65.1) | 1,283 (65.6) | 1,035 (19.2) | 2,546 (65.5) | <0.001 |
| Statin use, n (%) | 1,084 (39.8) | 1,129 (41.8) | 894 (46.1) | 939 (48) | 2,213 (41.1) | 1,833 (47.3) | <0.001 |
| Aspirin use, n (%) | 1,359 (49.9) | 1,323 (48.9) | 1,045 (53.8) | 1,020 (52.1) | 2,682 (49.6) | 2,065 (53) | <0.01 |
| FRS, % | 24.3 ± 12.6 | 24.1 ± 12.2 | 25.7 ± 12.6 | 25.8 ± 12.8 | 24.2 ± 12.4 | 25.7 ± 12.7 | <0.001 |
| Antihypertensive medication classes | |||||||
| Diuretics | 1,113 (40.9) | 1,176 (43.5) | 926 (47.7) | 967 (49.4) | 2,289 (42.2) | 1,893 (48.6) | <0.0001 |
| Calcium channel blockers | 920 (33.8) | 945 (35.0) | 682 (35.1) | 705 (36.0) | 1,865 (34.4) | 1,387 (35.6) | 0.23 |
| ACE inhibitors | 1,045 (38.4) | 948 (35.1) | 713 (36.7) | 738 (37.7) | 1,993 (36.7) | 1,451 (37.2) | 0.63 |
| Angiotensin II receptor blockers | 564 (20.7) | 586 (21.7) | 426 (22.0) | 399 (20.4) | 1,150 (21.2) | 825 (21.2) | 0.97 |
| β-Blockers | 935 (34.4) | 894 (33.1) | 763 (39.3) | 696 (35.6) | 1,829 (33.7) | 1,459 (37.4) | 0.0002 |
| α-1 blockers | 106 (3.9) | 105 (3.9) | 109 (5.6) | 102 (5.2) | 211 (3.9) | 211 (5.4) | 0.0005 |
| Direct vasodilators | 46 (1.7) | 43 (1.6) | 29 (1.5) | 23 (1.2) | 89 (1.6) | 52 (1.3) | 0.23 |
| Central α-2 agonists or other centrally acting drugs | 59 (2.2) | 50 (1.8) | 49 (2.5) | 38 (1.9) | 109 (2.0) | 87 (2.2) | 0.46 |
| Number of antihypertensive agents | |||||||
| Mean ± SD | 1.8 ± 1.0 | 1.8 ± 1.0 | 1.9 ± 1. | 1.9 ± 1.1 | 1.8 ± 1.0 | 1.9 ± 1.0 | <0.0001 |
| 0 | 260 (9.6) | 276 (10.2) | 171 (8.8) | 173 (8.8) | 344 (8.8) | 536 (9.9) | <0.0001 |
| 1 | 857 (31.5) | 848 (31.4) | 503 (25.9) | 531 (27.1) | 1,034 (26.5) | 1,705 (31.4) | |
| 2 | 954 (35.1) | 932 (34.5) | 703 (36.2) | 689 (35.2) | 1,392 (35.7) | 1,886 (34.7) | |
| 3 | 524 (19.3) | 508 (18.8) | 431 (22.2) | 451 (23.0) | 882 (22.6) | 1,032 (19.0) | |
| 4 | 126 (4.6) | 140 (5.2) | 133 (6.8) | 113 (5.8) | 246 (6.3) | 266 (4.9) | |
Data are means ± SD or n (%) unless otherwise indicated. Race and ethnic group were self-reported. There were no significant differences (P < 0.05) between the two treatment arms in those with baseline FSG <100 mg/dL or ≥100 mg/dL. FRS, Framingham Risk Score.
aP value is for the comparison of normoglycemia vs. prediabetes groups regardless of treatment arm.
Achieved SBP
The mean SBP achieved at 1 year for intensive and standard arms was 120.7 mmHg and 136.2 mmHg, respectively, in those with prediabetes and 121.8 mmHg and 136.2 mmHg, respectively, in those with normoglycemia (Supplementary Fig. 3). The mean ΔSBP achieved between treatment arms among those with prediabetes and normoglycemia was 15.5 mmHg and 14.4 mmHg, respectively. Throughout the 3.26 years of follow-up, the mean number of antihypertensive medications in the intensive and standard arms was 1.9 and 2.8 in the prediabetes strata and 1.8 and 2.6 in the normoglycemia strata.
Clinical Outcomes
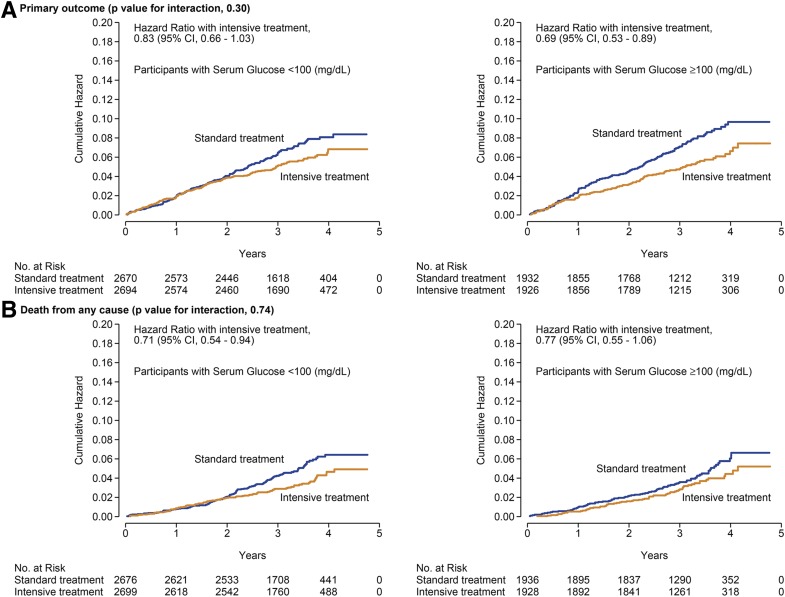
A total of 245 (101 in the intensive arm and 144 in the standard arm) and 316 (142 in the intensive arm and 174 in the standard arm) primary outcome events were observed among those with prediabetes and normoglycemia, respectively (Table 2). The hazard ratio for the primary outcome was 0.69 (95% CI 0.53, 0.89) and 0.83 (95% CI 0.66, 1.03) among those with prediabetes and normoglycemia, respectively (interaction P 0.30) (Fig. 2 and Supplementary Table 3). For all-cause mortality, the hazard ratio for those with prediabetes and normoglycemia was 0.77 (95% CI 0.55, 1.06) and 0.71 (95% CI 0.54, 0.94; interaction P 0.74), respectively. The effects of intensive versus standard SBP treatment were similar among those with prediabetes and normoglycemia across the components of the primary outcome and the other prespecified secondary outcomes (Table 2) (all interaction P > 0.10).
Table 2.
Incidence rates and hazard ratios for the primary and secondary outcomes by treatment arm among those with normoglycemia and prediabetes at baseline
| Baseline FSG |
P for interaction |
||||||
|---|---|---|---|---|---|---|---|
| <100 mg/dL |
≥100 mg/dL |
||||||
| Intensive treatment |
Standard treatment | Hazard ratio (95% CI) | Intensive treatment | Standard treatment | Hazard ratio (95% CI) | ||
| n | 2,721 | 2,704 | 1,941 | 1,957 | |||
| Primary outcome | 142 (1.7) | 174 (2.1) | 0.83 (0.66, 1.03) | 101 (1.6) | 144 (2.3) | 0.69 (0.53, 0.89) | 0.30 |
| Secondary outcomes | |||||||
| Myocardial infarction | 57 (0.7) | 72 (0.8) | 0.80 (0.57, 1.14) | 40 (0.6) | 44 (0.7) | 0.95 (0.61, 1.45) | 0.56 |
| Acute coronary syndrome | 23 (0.3) | 17 (0.2) | 1.32 (0.7, 2.47) | 17 (0.3) | 23 (0.4) | 0.76 (0.4, 1.44) | 0.23 |
| Stroke | 36 (0.4) | 32 (0.4) | 1.19 (0.73, 1.91) | 26 (0.4) | 38 (0.6) | 0.72 (0.44, 1.2) | 0.16 |
| Heart failure | 37 (0.4) | 52 (0.6) | 0.72 (0.47, 1.1) | 25 (0.4) | 48 (0.8) | 0.47 (0.29, 0.76) | 0.19 |
| Death from CVD causes | 21 (0.2) | 37 (0.4) | 0.62 (0.36, 1.06) | 16 (0.3) | 27 (0.4) | 0.56 (0.3, 1.04) | 0.81 |
| Death from any cause | 89 (1.0) | 125 (1.4) | 0.71 (0.54, 0.94) | 65 (1) | 84 (1.3) | 0.77 (0.55, 1.06) | 0.74 |
| Primary outcome or death | 197 (2.3) | 240 (2.9) | 0.82 (0.68, 0.99) | 134 (2.2) | 182 (3) | 0.73 (0.58, 0.91) | 0.42 |
| Participants with CKD at baseline | |||||||
| Composite renal outcome | 8 (0.3) | 10 (0.4) | 0.75 (0.29, 1.93) | 6 (0.4) | 5 (0.3) | 1.38 (0.38, 4.96) | 0.46 |
| Long-term dialysis | 5 (0.2) | 7 (0.3) | 0.75 (0.24, 2.38) | 1 (0.1) | 3 (0.2) | 0.27 (0.03, 2.61) | 0.43 |
| Incident albuminuria | 34 (3.4) | 33 (3.9) | 0.82 (0.49, 1.37) | 15 (2.5) | 26 (3.9) | 0.51 (0.24, 1.08) | 0.32 |
| Participants without CKD at baseline | |||||||
| ≥30% reduction in eGFR to <60 mL/min/1.73 m2 | 73 (1.2) | 23 (0.4) | 3.13 (1.95, 5.01) | 54 (1.2) | 14 (0.3) | 4.15 (2.25, 7.67) | 0.47 |
| Incident albuminuria | 62 (1.9) | 74 (2.3) | 0.78 (0.55, 1.1) | 47 (2) | 59 (2.5) | 0.84 (0.56, 1.25) | 0.78 |
Data are number of patients (% per year) unless otherwise indicated. Numbers are counts and annual rates unless otherwise indicated. The primary outcome was the first occurrence of myocardial infarction, acute coronary syndrome, stroke, heart failure, or death from cardiovascular causes. The composite renal outcome for participants with CKD at baseline was the first occurrence of a reduction in the eGFR of ≥50%, long-term dialysis, or kidney transplantation. Reductions in the eGFR were confirmed by a second laboratory test at least 90 days later. Incident albuminuria was defined by a doubling of the ratio of urinary albumin (in milligrams) to creatinine (in grams) from <10 at baseline to >10 during follow-up. No long-term dialysis or kidney transplantation was reported among participants without CKD at baseline.
Figure 2.
Cumulative incidence of the primary outcome (A) and all-cause mortality (B) by treatment arm stratified by those with normoglycemia and prediabetes at baseline.
Among participants with or without CKD at baseline, the treatment effects of intensive treatment on renal outcomes were consistent among participants with prediabetes and normoglycemia (all P values for interaction >0.30) (Table 2). The numbers of events in the CKD subgroup were small, particularly for the primary composite renal outcome of ≥50% reduction in eGFR or end-stage renal disease.
Serious Adverse Events
Among those with prediabetes and normoglycemia, serious adverse events occurred in 733 (37.5%) and 1,000 (37.0%) in the standard arm and 737 (38.0%) and 1,052 (38.7%) in the intensive arm, respectively (Supplementary Table 4). Hazard ratios for all serious adverse events combined and individual serious adverse events of interest were similar between those with prediabetes and normoglycemia (all P values for interaction >0.05).
Results from sensitivity analyses excluding participants with FSG ≥100 mg/dL who reverted to <100 mg/dL at the 2-year SPRINT study visit were qualitatively similar (Supplementary Table 5). Results were nearly identical when the cohort was restricted to the participants whom the study staff noted to be fasting for the blood draw at the baseline visit (Supplementary Table 6) and, in a separate sensitivity analysis, when the cohort was restricted to those who had an FSG 100 to <126 mg/dL instead of FSG ≥100 mg/dL at baseline (Supplementary Table 7). The quartile analysis investigating a graded interaction between the effect of intensive treatment and FSG revealed that there were no significant interactions for the primary outcome, components of the primary outcomes, and all secondary outcomes, as all P values for interaction were ≥0.10 (Supplementary Table 8). The interaction between intensive treatment and baseline FSG, modeled as a continuous variable, showed only minor variation in the effect of intensive treatment on the primary outcome and all-cause mortality across ranges of FSG (Supplementary Fig. 4). Assessment of interaction at FSG values ≥150 mg/dL is limited given the small sample size (n = 51).
Conclusions
The current SPRINT analysis demonstrates that the beneficial effects of intensive SBP treatment on CVD events and all-cause mortality extend to patients with prediabetes and are similar among those with prediabetes and fasting normoglycemia. Notably, the effect size of intensive SBP treatment in those with prediabetes was similar to the effect in the overall SPRINT population (i.e., an ∼25% relative risk reduction). Moreover, the current analysis indicates that the effect of intensive SBP treatment was consistent across a range of FSG levels (i.e., in quartiles and in the spline analysis), and no significant interaction trend was detected. The effects of intensive SBP treatment on renal and safety outcomes were also not statistically different between those with prediabetes and those with fasting normoglycemia. In totality, the current analysis suggests that the benefits of intensive SBP treatment in high–CVD risk U.S. adults are similar in patients with prediabetes and fasting normoglycemia with no attenuation of effect at higher FSG levels.
Given the apparent discordant results between SPRINT and ACCORD BP, there is some uncertainty as to whether the benefits of intensive SBP lowering extend to those with diabetes. Since the design of SPRINT excluded people with established diabetes, it is difficult to generalize SPRINT results to these patients. The current analysis, however, suggests that an inherent difference in the cardiovascular benefits of intensive SBP treatment across levels of FSG is unlikely and that benefits of intensive SBP treatment extend at least to those with prediabetes, a condition with increased risk for diabetes and cardiovascular events. In the ACCORD BP trial, with SBP targets identical to those in SPRINT, an intensive SBP target goal (SBP <120 mmHg) compared with a standard SBP target goal (SBP < 140 mmHg) did not result in a statistically significant lower risk of CVD events in those with diabetes (4). However, in contrast to SPRINT’s design, ACCORD BP used a complex, double, 2 × 2 factorial design to simultaneously study the effects of blood pressure, lipid, and glycemic control interventions. ACCORD BP was also underpowered to detect a significant cardiovascular protective effect of intensive SBP lowering. It should further be noted that, among ACCORD BP participants in the standard glycemia arm, intensive SBP treatment significantly reduced CVD events compared with standard SBP treatment, with a reduction (26%, P = 0.049) similar to that observed in SPRINT (15). In addition, intensive SBP lowering resulted in a statistically significant (41%) reduction in stroke in ACCORD BP in contrast to SPRINT, which demonstrated a significant reduction in new heart failure events but not stroke events (4). This may be due to differences in the inclusion/exclusion criteria of the trials. For example, people with prior stroke were excluded from SPRINT but not in ACCORD BP. In addition, the stroke difference in ACCORD BP did not emerge until after 3 years of follow-up, while SPRINT was stopped after 3.26 years of follow-up. A beneficial effect on CVD events or mortality of intensive SBP treatment in those with diabetes cannot be ruled out (16).
Findings from several recent meta-analyses of blood pressure–lowering trials suggest that CVD risk reductions with more intensive SBP treatment indeed extend to those with diabetes (16–19). One meta-analysis of 19 trials (N = 44,989) comparing intensive with standard blood pressure treatment found that intensive treatment to a lower SBP goal incrementally lowered CVD risk, especially among high–CVD risk patients with diabetes (18). In another meta-analysis of blood pressure–lowering trials (n = 613,815), each 10 mmHg lower SBP reduced the risk of CVD events by 12% among those with diabetes (17). The CVD or all-cause mortality risk reductions persist <130 mmHg. A recent network meta-analysis of 42 blood pressure–lowering trials (30 of which included patients with type 2 diabetes) including 144,220 patients found significantly lowers risks of CVD events among participants who achieved SBP 120–124 mmHg compared with all other achieved SBP groups including 130–134, 140–144, 150–154, or 160 mmHg or higher (19). In contrast, another meta-analysis of blood pressure trials (n = 73,738) found that effects of treatment were attenuated in people with diabetes when SBP was lowered to <130 mmHg (20). The current analysis adds to this body of literature by demonstrating that intensive SBP treatment reduces CVD events and all-cause mortality in those with prediabetes, and the beneficial effects of intensive treatment did not appear to be attenuated by higher FSG levels. Considering that there are an estimated 82 million U.S. adults with prediabetes and another 23 million U.S. adults with clinical diabetes (21) and that ∼25% of the U.S. adult population with diabetes, or 5.5 million U.S. adults, currently meet the other SPRINT eligibility criteria aside from diabetes (22), the public health implications of the findings of the current study are substantial.
The effects of intensive SBP treatment on renal and safety outcomes were also similar in those with prediabetes and normoglycemia. This included incident albuminuria and a ≥30% reduction in eGFR among participants without CKD at baseline and a ≥50% reduction in patients with eGFR. No patient progressed to long-term hemodialysis or kidney transplantation among patients with CKD at baseline.
A key strength of the current study flows from the rigorously conducted randomized controlled study design with adjudicated outcomes in a large, racially diverse population of SPRINT, thus allowing for large subgroups of those with prediabetes and those with fasting normoglycemia at baseline. In addition, the protocol was successfully implemented, and a large ΔSBP was achieved between treatment arms for the duration of the trial. Adherence to the methods used in SPRINT, including blood pressure measurement, will help realize potential benefits of SPRINT-based intensive treatment in clinical practice (23).
Because SPRINT did not obtain hemoglobin A1c at baseline, our definition of prediabetes and normoglycemia is based on a single FSG measurement, which is more subject to day-to-day variability. However, in sensitivity analysis, with restriction to those whose prediabetes status was unchanged from baseline to year 2, the results were nearly identical. There is also the potential for misclassification of glycemia status owing to inadequate fasting, but sensitivity analyses restricting evaluation to blood samples that were recorded to be fasting yielded nearly identical results. As with even the largest clinical trials, power for interaction and subgroup analyses is small. Therefore, the limited power of our subgroup analysis prevents us from definitely ruling out that the treatment effects cannot differ substantially.
In conclusion, among U.S. adults at high CVD risk but without clinical diabetes, the beneficial effects of intensive SBP treatment to <120 mmHg compared with standard SBP treatment to <140 mmHg on the reduction of CVD events and all-cause mortality were similar among those with prediabetes and fasting normoglycemia.
Supplementary Material
Article Information
Funding. A.P.B. was supported by National Heart, Lung, and Blood Institute (NHLBI) (Bethesda, MD) grant 1K01HL133468-01. SPRINT is funded with Federal funds from the National Institutes of Health (NIH), including the NHLBI, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, and the National Institute of Neurological Disorders and Stroke, under contract nos. HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, and HHSN268200900049C, and is also funded by the NIH Office of the Director Inter-Agency Agreement no. A-HL-13-002-001. The trial was also supported in part with resources and use of facilities through the Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International, Inc. The authors also acknowledge the support from the following Clinical and Translational Science Awards, funded by National Center for Advancing Translational Sciences: Case Western Reserve University, UL1TR000439; Ohio State University, UL1RR025755; University of Pennsylvania, UL1RR024134 and UL1TR000003; Boston University, UL1RR025771; Stanford University, UL1TR000093; Tufts University, UL1RR025752, UL1TR000073, and UL1TR001064; University of Illinois, UL1TR000050; University of Pittsburgh, UL1TR000005; University of Texas Southwestern, 9U54TR000017-06; University of Utah, UL1TR000105-05; Vanderbilt University, UL1TR000445; George Washington University, UL1TR000075; University of California, Davis, UL1TR000002; University of Florida, UL1 TR000064; and University of Michigan, UL1TR000433. The authors also acknowledge funding to Tulane University (P30GM103337, Centers of Biomedical Research Excellence award, National Institute of General Medical Sciences).
All components of the SPRINT protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the U.S. Department of Veterans Affairs, or the U.S. government.
Duality of Interest. A.P.B. has an institutional grant from Novartis not related to the current project. A.K.C. is a consultant to Boehringer Ingelheim as member of the Advisory Committee of a clinical trial, is the Data and Safety Monitoring Board Chair for a Baxter clinical trial, and is a contributor to UpToDate. G.W.E. has an institutional grant from AstraZeneca. W.C.C. has an institutional grant from Eli Lilly and performs uncompensated consulting for Takeda and Novartis. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. A.P.B. contributed to the conception and design of the study, researched data, designed the analysis, and wrote the manuscript. A.P.B., J.B.K., Y.Z., and G.W.E. performed analyses. All authors researched data, reviewed and edited the manuscript, and contributed to the study design, interpretation of data, and discussion. T.G. and D.M.R. contributed statistical expertise. A.P.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 77th Scientific Sessions of the American Diabetes Association, San Diego, CA, 9–13 June 2017.
Footnotes
Clinical trial reg. no. NCT01206062, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-0885/-/DC1.
A complete list of the SPRINT Research Group can be found in the Supplementary Data online.
This article is featured in a podcast available at http://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 1993;16:434–444 [DOI] [PubMed] [Google Scholar]
- 2.Qiu M, Shen W, Song X, et al. Effects of prediabetes mellitus alone or plus hypertension on subsequent occurrence of cardiovascular disease and diabetes mellitus: longitudinal study. Hypertension 2015;65:525–530 [DOI] [PubMed] [Google Scholar]
- 3.Wright JT Jr, Williamson JD, Whelton PK, et al.; SPRINT Research Group . A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med 2015;373:2103–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cushman WC, Evans GW, Byington RP, et al.; ACCORD Study Group . Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambrosius WT, Sink KM, Foy CG, et al.; SPRINT Study Research Group . The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials 2014;11:532–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group . A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999;130:461–470 [DOI] [PubMed] [Google Scholar]
- 7.D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743–753 [DOI] [PubMed] [Google Scholar]
- 8.Systolic Blood Pressure Intervention Trial (SPRINT) protocol version 4.0 [article online], 2012. Available from https://www.sprinttrial.org/public/Protocol_Current.pdf. Accessed 18 October 2015
- 9.Knol MJ, VanderWeele TJ. Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol 2012;41:514–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nichols GA, Hillier TA, Brown JB. Normal fasting plasma glucose and risk of type 2 diabetes diagnosis. Am J Med 2008;121:519–524 [DOI] [PubMed] [Google Scholar]
- 11.Shaw JE, Zimmet PZ, Hodge AM, et al. Impaired fasting glucose: how low should it go? Diabetes Care 2000;23:34–39 [DOI] [PubMed] [Google Scholar]
- 12.Charles MA, Fontbonne A, Thibult N, Warnet J-M, Rosselin GE, Eschwege E. Risk factors for NIDDM in white population. Paris prospective study. Diabetes 1991;40:796–799 [DOI] [PubMed] [Google Scholar]
- 13.Royston P, Sauerbrei W. A new approach to modelling interactions between treatment and continuous covariates in clinical trials by using fractional polynomials. Stat Med 2004;23:2509–2525 [DOI] [PubMed] [Google Scholar]
- 14.Royston P, Sauerbrei W. Interaction of treatment with a continuous variable: simulation study of significance level for several methods of analysis. Stat Med 2013;32:3788–3803 [DOI] [PubMed] [Google Scholar]
- 15.Margolis KL, O’Connor PJ, Morgan TM, et al. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial. Diabetes Care 2014;37:1721–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perkovic V, Rodgers A. Redefining blood-pressure targets--SPRINT starts the marathon. N Engl J Med 2015;373:2175–2178 [DOI] [PubMed] [Google Scholar]
- 17.Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016;387:957–967 [DOI] [PubMed] [Google Scholar]
- 18.Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet 2016;387:435–443 [DOI] [PubMed] [Google Scholar]
- 19.Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol 2017;2:775–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ 2016;352:i717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benjamin EJ, Blaha MJ, Chiuve SE, et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 2017;135:e146–e603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bress AP, Tanner RM, Hess R, et al. Prevalence of eligibility criteria for the Systolic Blood Pressure Intervention Trial in US adults among excluded groups: age <50 years, diabetes mellitus, or a history of stroke. J Am Heart Assoc 2016;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bress AP, Kramer H, Khatib R, et al. Potential Deaths Averted and Serious Adverse Events Incurred From Adoption of the SPRINT (Systolic Blood Pressure Intervention Trial) Intensive Blood Pressure Regimen in the United States: Projections From NHANES (National Health and Nutrition Examination Survey). Circulation 2017;135:1617–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.