Abstract
African Americans (AAs) tend to have higher plasma insulin concentrations than European Americans (EAs); the increased insulin concentrations have been attributed to increased secretion and/or decreased insulin clearance by liver or other tissues. This work characterizes the contributions of hepatic versus extrahepatic insulin degradation related to ethnic differences between AAs and EAs. By using a recently developed mathematical model that uses insulin and C-peptide measurements from the insulin-modified, frequently sampled intravenous glucose tolerance test (FSIGT), we estimated hepatic versus extrahepatic insulin clearance in 29 EA and 18 AA healthy women. During the first 20 min of the FSIGT, plasma insulin was approximately twice as high in AAs as in EAs. In contrast, insulin was similar in AAs and EAs after the 20–25 min intravenous insulin infusion. Hepatic insulin first-pass extraction was two-thirds lower in AAs versus EAs in the overnight-fasted state. In contrast, extrahepatic insulin clearance was not lower in AAs than in EAs. The difference in insulin degradation between AAs and EAs can be attributed totally to liver clearance. The mechanism underlying reduced insulin degradation in AAs remains to be clarified, as does the relative importance of reduced liver clearance to increased risk for type 2 diabetes.
Introduction
African Americans (AAs), particularly AA women, are at increased risk for type 2 diabetes compared with their white counterparts (1,2). Diabetes incidence is estimated to be 2.4-fold higher for AA women and 1.5-fold higher for AA men than for white women and men, respectively (3). The mechanisms underlying increased risk in AAs are unexplained. The elevated insulin in AA has been attributed to both increased insulin secretion (4–6) and decreased clearance (7,8), with these changes already apparent in AA adolescents (5,6,9). Whether hyperinsulinemia in AAs reflects a different metabolic pattern of insulin clearance relative to whites is of interest. Although substantial clearance of insulin occurs in the liver, with a single-pass degradation that can be >50% (10), insulin is also cleared by kidney and muscle tissue. Is it possible that the relative importance of insulin degradation by liver, with regard to other extrahepatic tissues, differs among ethnic groups, and do these divergences contribute to differences in peripheral insulin concentrations?
Methods for estimating hepatic insulin degradation from oral (10,11) and frequently sampled intravenous glucose tolerance (FSIGT) tests (12,13) have been developed on the basis of deconvolution of plasma C-peptide concentrations to calculate the insulin secretion rate (ISR) (14). A recently developed method (13) enables both hepatic and extrahepatic insulin degradation to be estimated from FSIGT data. This new model exploits the fact that during the insulin-modified FSIGT, all the insulin appearing during the first 20 min is from endogenous secretion (and hence is subjected to first-pass hepatic extraction before entering the systemic circulation), whereas the intravenous insulin infusion beginning at 20 min delivers insulin directly into the circulation. This approach was previously used to discriminate hepatic from extrahepatic clearance in a single population (13), but the method has not been used to differentiate patterns of insulin degradation in various ethnic groups. We used this method to compare degradation of insulin in AA versus European American (EA) participants.
Research Design and Methods
Clinical Study
The experimental protocol and enrollment criteria were previously described (15–17). Participants were 23 AA and 30 EA premenopausal women. Exclusion criteria were type 1 or type 2 diabetes, polycystic ovary disease, disorders of glucose or lipid metabolism, use of medication that could affect body composition or glucose metabolism (including antihypertensive medication, oral contraceptives, and postmenopausal hormone replacement therapy), use of tobacco, alcohol consumption >400 g/week, history of hypoglycemic episodes, and/or a medical history that contraindicated inclusion in the study. All participants had normal glucose tolerance. Participants were informed of the experimental design, and written informed consent was obtained. The study was approved by the institutional review board for human use at the University of Alabama at Birmingham (Birmingham, AL).
Protocol
All testing was done on an inpatient basis at the University of Alabama at Birmingham’s General Clinical Research Center (15–17). Participants were asked to consume at least 250 g of carbohydrates for 3 days before admission and were provided with a list of common foods and their carbohydrate content. They came to the General Clinical Research Center the evening before testing. While there, participants were given a standard meal consisting of 50% energy from carbohydrates, 30% from fat, and 20% from protein. No food was consumed for 12 h before the FSIGT, which was performed at 7:00 a.m. the following morning. After completion of the glucose tolerance test, participants were given a late breakfast/lunch.
FSIGT
Insulin sensitivity, insulin secretion, and insulin clearance were determined from the FSIGT, as previously described (15–17). Blood was sampled three times over a 15-min period before glucose delivery. At t = 0, a bolus of glucose (50% dextrose, 300 mg/kg) was injected intravenously. Insulin (0.02 units/kg) was administered intravenously over a 5-min period from 20 to 25 min after the glucose injection. Postinjection blood was sampled (2.0 mL) at 0, 2, 3, 4, 5, 6, 8, 10, 12, 15, 19, 20, 21, 22, 24, 26, 28, 30, 35, 40, 45, 50, 55, 60, 70, 80, 100, 120, 140, 180, 210, and 240 min after glucose administration.
Laboratory Analyses
Concentrations of glucose, insulin, and C-peptide were analyzed in the Metabolism Core Laboratory of the General Clinical Research Center and Nutrition Obesity Research Center. Glucose was measured in 10 μL of sera with an Ektachem DT II System (Johnson & Johnson Clinical Diagnostics, Rochester, NY). This analysis had a mean intra-assay coefficient of variation (CV) of 0.61% and a mean interassay CV of 1.45%. Insulin assay sensitivity was 3.35 μIU/mL, mean intra-assay CV was 3.49%, and mean interassay CV was 5.57%. C-peptide assay sensitivity was 0.318 ng/mL, mean intra-assay CV was 3.57%, and mean interassay CV was 5.59%.
Mathematical Model for Hepatic and Extrahepatic Insulin Clearance
Hepatic and extrahepatic insulin clearance components were calculated as described previously (13). The ISR was calculated by a classic method: C-peptide deconvolution by using a two-compartment representation and assumed kinetic parameters (14). C-peptide and insulin secretion are assumed to be equimolar. Once calculated, the insulin infusion rate is used as input into the model. Hepatic delivery of insulin is given as ISR + HPF ⋅ P where P is the systemic plasma insulin concentration and HPF is the hepatic plasma flow (specified as 0.576 L/min/m2 [18]). Extrahepatic clearance is assumed to be linear with parameter CLP. Hepatic clearance is assumed to be either linear (with parameter FEL describing hepatic fractional extraction) or saturable [with parameters Vmax and Km and hepatic clearance = Vmax ⋅ delivery/(delivery + Km)], and the best fit model is selected for each participant. For the saturable model, the hepatic fractional extraction varies over time during the FSIGT; to provide a single value for comparison with what is obtained from the linear model when depicting the distribution across participants, FEL is calculated in the prechallenge stage (corresponding to overnight-fasted values). The distribution volume for insulin (Vd) is also estimated; thus, a total of three (linear representation) or four (saturable representation) parameters are estimated. Additional details and the full equations can be found in Polidori et al. (13).
Parameter Estimation and Statistical Analysis
The model parameters were estimated by using least squares and a simplex search algorithm (fminsearch in MATLAB, version 9). For each participant, the best-fit parameters for both the linear and the saturable models were determined, and the preferred model was chosen by using the Akaike information criterion (19). The difference between modeled and measured insulin concentration was quantified by using the normalized root mean square error (13). Data are presented as mean (SD) unless otherwise stated. Between-group comparisons were done by using the Wilcoxon rank sum test; P < 0.05 was considered statistically significant.
Results
Six of the 53 participants were not included in the analysis for purely technical reasons. C-peptide measurements were not available for three AA women, and three other women (one EA, two AAs) had plasma insulin concentration profiles indicating that the exogenous infusion was given considerably later than between 20 and 25 min, and the exact timing could not be verified. Therefore, 47 participants were included in the analysis.
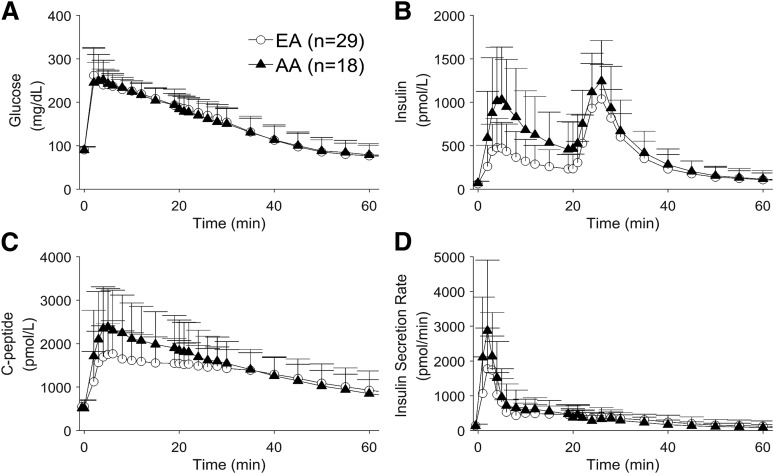
Demographic information and basal plasma concentrations for the 47 participants are shown in Table 1. Insulin sensitivity and acute insulin response from the FSIGT also are summarized; additional information on these parameters can be found in previous publications (15–17). The mean plasma glucose, insulin, and C-peptide concentrations as well as the calculated ISR for both groups are shown in Fig. 1. During the first 20 min (when all insulin comes from endogenous secretion), mean plasma insulin concentrations were approximately twice as high in AA as in EA women as shown by area under the curve (AUC0–20 min = 215 [134] vs. 102 [72] pmol/L ⋅ h, respectively; P = 0.0015). In contrast, after the intravenous insulin infusion at 20–25 min, resulting plasma insulin values were similar between AA and EA participants (AUC20–60 min = 284 [130] vs. 235 [126] pmol/L ⋅ h, respectively; P = 0.09).
Table 1.
Demographic information for both EA and AA participants in the study
| EA | AA | |
|---|---|---|
| n | 29 | 18 |
| Age (years) | 26 (4) | 25 (4) |
| Body weight (kg) | 70 (13) | 74 (16) |
| BMI (kg/m2) | 25 (4) | 27 (6) |
| Fasting plasma glucose (mg/dL) | 91 (6) | 90 (8) |
| Fasting plasma insulin (pmol/L) | 62 (28) | 71 (23) |
| Fasting plasma C-peptide (pmol/L) | 552 (158) | 515 (178) |
| AIRg (μU/mL ⋅ min) | 492 (386) | 1,158 (755) |
| SI (10−4 min−1/μU/mL) | 5 (3) | 4 (4) |
Data are mean (SD). AIRg, acute insulin response; SI, insulin sensitivity.
Figure 1.
Average plasma glucose (A), insulin (B), and C-peptide (C) measurements and calculated ISR (D) for both EA and AA participants in the first 60 min of the test.
Modeling of Plasma Insulin Profiles
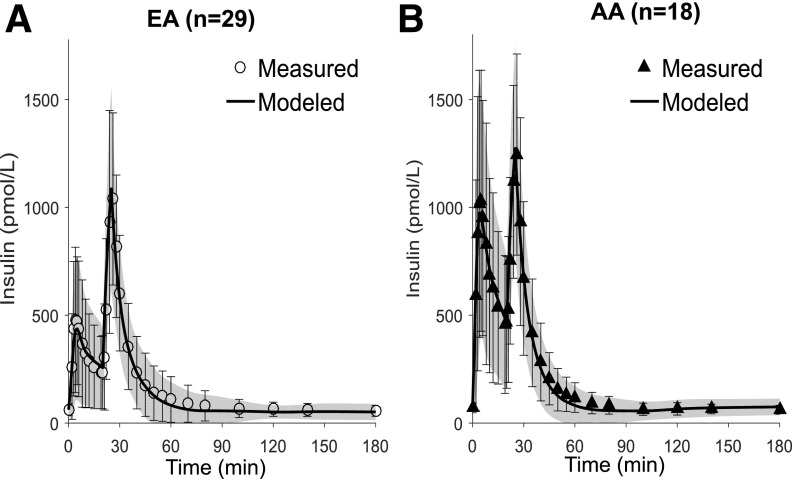
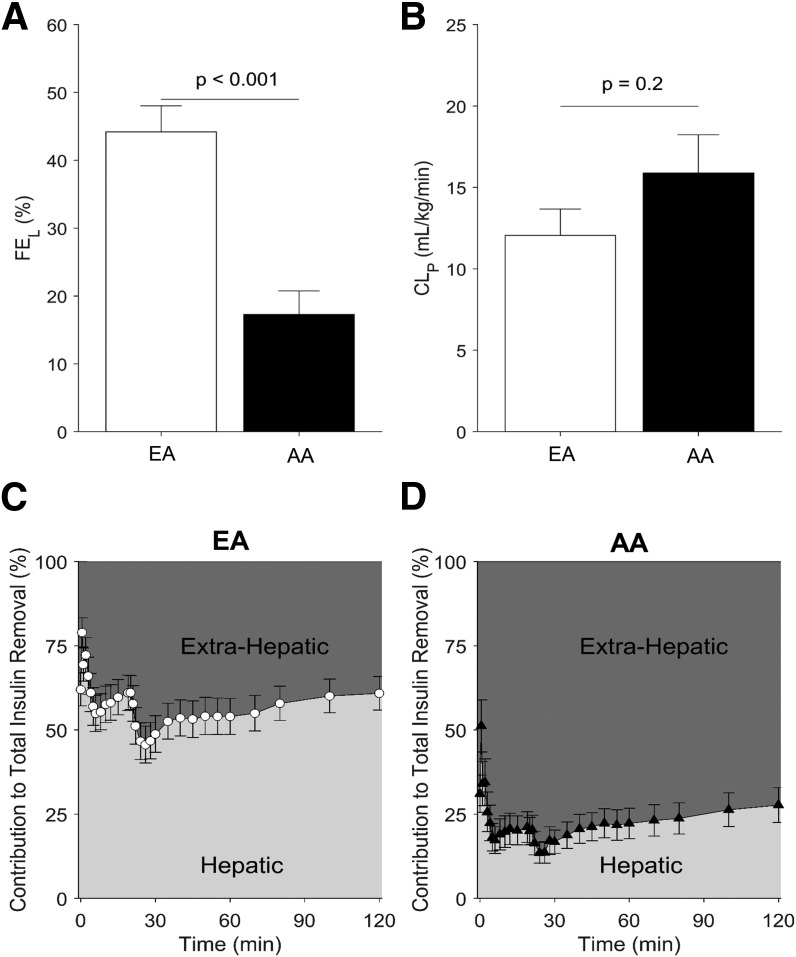
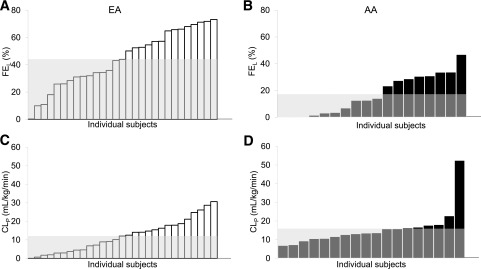
Figure 2 shows the average measured and modeled insulin profiles for EA and AA participants. Plasma insulin was well described by the model in both groups, with mean (SD) normalized root square error = 8% (3%) in EAs and 9% (3%) in AAs. The saturable hepatic clearance model was preferred in 33% (6 of 18) of the AA participants and 14% (4 of 29) of the EA participants; the linear hepatic clearance model was sufficient for the remaining participants. The parameters were estimated with similar precision as in our previous study (13) (median CV values in this study were 10% for Vd, 16% for CLp, 14% for FEL [linear model]) and somewhat higher for the parameters of the saturable model (34% for Vmax and 60% for Km). Hepatic insulin extraction was dramatically lower in AA than in EA participants (Fig. 3A). Hepatic fractional extraction in the overnight-fasted state was only 17% (15%) in AAs compared with 44% (21%) in EAs (P < 0.001). In sharp contrast, extrahepatic clearance was not reduced in AA compared with EA participants (CLP = 15.9 [10.0] vs. 12.1 [8.7] mL/kg/min, respectively; P = 0.20) (Fig. 3B). Thus, 85% of insulin entering the liver escaped first-pass degradation in AA participants, whereas only 55% of entering insulin escaped in EA participants. The relative contribution of hepatic insulin degradation to total insulin clearance was much lower in AAs than in EAs (Fig. 3C and D), with 0–2-h mean values for the percentage of whole-body insulin clearance occurring in the liver of 23% (19%) in AA participants compared with 57% (28%) in EA participants (P < 0.001). Figure 4 shows hepatic first-pass extraction (FEL) (Fig. 4A and B) and extrahepatic clearance (CLP) (Fig. 4C and D) distribution values in all AA and EA participants, respectively.
Figure 2.
Measured and modeled plasma insulin profiles in EA (A) and AA (B) participants. Data are mean (SD) for measured and modeled (line and shaded region) profiles.
Figure 3.
Hepatic insulin fraction (FEL) (A) and extrahepatic clearance indices (CLp) (B) in EA vs. AA participants. Hepatic and extrahepatic contribution to total insulin clearance in the first 120 min of the experiment in EAs (C) and AAs (D). Data are mean + SE. For participants where the saturable hepatic clearance model provided the best fit, FEL values shown in A were calculated in the prechallenge state.
Figure 4.
Distribution of hepatic insulin fractions (FEL) (A and B) and extrahepatic clearance individual indices (CLP) (C and D) in EA and AA participants (white and black bars, respectively) in ascending order with average values shown in gray areas.
Discussion
Previous studies (7) have demonstrated that genetic predisposition and environmental factors can contribute differently to the development of type 2 diabetes in different populations (20,21). Specifically, AAs were discovered to have a greater risk of type 2 diabetes than EAs likely because of genetic inheritance and environmental aspects such as westernization, sedentary lifestyle, and obesity (22).
The rate of degradation of insulin is a significant aspect in the development of type 2 diabetes. It has been demonstrated in Hispanic and AA cohorts that the lower metabolic clearance rate of insulin is associated with a higher risk of type 2 diabetes after adjusting for demographics, lifestyle factors, HDL cholesterol, indexes of obesity and adiposity, and insulin secretion (23). Reduced insulin clearance is important because it is independently associated and inversely correlated with insulin resistance. In animal models with induced obesity (24,25), reduced insulin clearance is accompanied by increased insulin secretion in response to insulin resistance to maintain normoglycemia. In addition, insulin clearance is heritable in Mexican Americans, as confirmed by Guo et al. (26) who also identified regions on chromosomes 15 and 20 that may influence it. To the best of our knowledge, similar genetic studies have not been done in AA populations. Thus, it would be interesting to determine whether genetic factors are likewise relevant to insulin clearance in AAs.
In the current study population of EAs and AAs, the fractional liver insulin extraction is a striking two-thirds lower in AAs than in EAs (15% vs. 44%) (Fig. 3A). This calculated difference is consistent with the observed plasma insulin concentrations (Fig. 1); AAs have a significantly higher insulin concentration immediately after glucose injection (i.e., at the beginning of the test) when circulating insulin is only due to endogenous secretion (mean AUC0–20 min for plasma insulin is 110% higher in AAs than in EAs), whereas the difference between AAs and EAs is minimal after the insulin infusion, confirming previous reports (16,27). Insulin secretion after glucose administration is also higher in AAs than in EAs (mean AUC0–20 min for ISR is 48% higher in AAs than in EAs). Thus, both elevated insulin secretion and decreased hepatic insulin extraction contribute to twofold higher levels of plasma insulin concentrations observed in AAs in the first 20 min compared with EAs.
The etiology of the lower hepatic but not extrahepatic insulin degradation in AAs is not understood. Insulin degradation is a receptor-mediated system: the first step happens when insulin is bound by its receptor, and then, the receptor-bound insulin is internalized into the cellular endosomes with an energy-requiring absorptive endocytosis (28). After their formation, endosomes quickly acidify, and insulin is dissociated from the receptor within the vesicle (28). As a result of the specific insulin-degrading enzyme, insulin degradation begins right before the acidification. In addition, enzyme CEACAM1 promotes insulin clearance by enhancing the rate of uptake of the insulin-receptor complex (29). Dissociated insulin is degraded in endosomes, translocated to cytosol and degraded there, or delivered to other subcellular compartments, such as peroxisomes (28). The receptor is finally recycled in a vesicle back to the plasma membrane (28). Insulin-degrading enzyme and/or CEACAM1 expression might be specifically reduced in AAs. Another possible cause of the impairment of hepatic insulin clearance in AAs is reduced hepatic insulin receptor number or activity (30), making them less available for binding and endocytosis. Recently reviewed evidence that insulin action on glucose output by the liver is mediated by indirect mechanisms (i.e., suppression of adipocyte lipolysis) has indicated that insulin receptor–mediated clearance may be differentiated from metabolic actions of the hormone (31).
The results of the current study confirm previous findings in adults and adolescents (32,33). AA adults and children have lower hepatic insulin clearance, greater acute insulin response, and decreased insulin sensitivity than their EA counterparts. This might be a plausible effect of differences in obesity-related phenotypes between AA and EA populations, such as accumulation of fat depots and levels of IGF-I (34). This work strengthens prior observations that ethnicity and race profoundly affect glucose metabolism, suggesting that insulin clearance is a fundamental component. With regard to other ethnic groups, two studies comparing hepatic insulin extraction between AA and Hispanic girls found mixed results. In one study of overweight children, no between-group differences were seen (35), whereas in a previous separate study from the same group in normal weight children, hepatic extraction was lower in AA children than in Hispanic children (36).
The current study has some limitations. The number of participants analyzed is not large, and the population is made up of healthy women. Additional studies need to include larger populations of both sexes with various levels of glucose tolerance. Another limitation is that the ISRs in EA and AA participants were calculated by using the standard approach in which individual participant C-peptide kinetic parameters are calculated on the basis of demographic information and no ethnic differences in C-peptide kinetics are assumed. If C-peptide kinetics were considerably different in AA women, some differences would be found in the calculated ISRs that would affect the estimated insulin clearance parameters. Although more research into C-peptide kinetics in different groups would be of interest, such differences are unlikely to affect the major conclusions of this work because a sensitivity analysis (data not shown) demonstrated that mean ISRs in AA participants would need to be much higher (∼45%) than estimated for the calculated hepatic extraction to be similar between EAs and AAs. This difference is well outside the range of differences observed in any single participant between ISRs calculated from individually measured C-peptide kinetic parameters and those of the adjusted group parameters (14) and is therefore an unlikely explanation for why insulin clearance per se is lower in AAs. However, a comparison of C-peptide parameters in AAs with those for whites and/or American Indians would be of interest. Finally, future studies could measure liver fat content to determine whether its increase contributes to reduced hepatic insulin clearance in AAs. In the current study population, hepatic lipids were not measured, but the cohort was made up of young, healthy women unlikely to have elevated hepatic fat levels.
In conclusion, we have demonstrated that hepatic insulin clearance is 60% lower in AAs than in EAs. This striking difference may contribute to the pathogenesis of type 2 diabetes, the prevalence of which is greater in AAs and in African populations (15). Reduced insulin clearance could cause peripheral hyperinsulinemia, which could result in insulin resistance. The possibility that lower liver insulin clearance may be an important pathogenic factor in AAs deserves consideration.
No relevant difference in insulin clearance was found with regard to the extrahepatic component per se. Yet, the difference in hepatic extraction alone could help to explain the hyperinsulinemic response to glucose injection observed in AAs. The underlying biochemical mechanisms regulating the ethnic difference in hepatic extraction are not totally clear, and as such, more studies are needed of the mechanisms responsible for liver insulin degradation. Knowledge of these mechanisms could lead to appropriate treatment to improve clearance, if it can be established that altered clearance contributes to the development of type 2 diabetes.
Supplementary Material
Article Information
Funding. This study was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (R01-DK-58278, P30-DK-079626, P30-DK-56336, UL1-RR-02577). B.A.G. is supported by grants from NIDDK (R01-DK-096388, R01-DK-103863, P30-DK-079626, P30-DK-56336) and the American Institute for Cancer Research. R.N.B. is supported by grants from NIDDK (DK-27619, DK-29867).
Duality of Interest. D.C.P. is a full-time employee of Janssen Research & Development. R.N.B. is supported by grants from AstraZeneca and GI Dynamics and is an advisory board member of Ingredion. No other potential conflicts of interest relevant to this article were reported.
Authors Contributions. F.P. contributed to the data analysis and interpretation and wrote the manuscript. D.C.P. analyzed and interpreted data and edited the manuscript. B.A.G. designed the study, contributed to the data interpretation, and edited the manuscript. R.N.B. originated the concept that reduced liver insulin clearance might be an important factor in pathogenesis of type 2 diabetes, contributed to the data interpretation, and edited the manuscript. R.N.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented at the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 10–14 June 2016.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db17-0413/-/DC1.
References
- 1.Harris MI, Hadden WC, Knowler WC, Bennett PH. Prevalence of diabetes and impaired glucose tolerance and plasma glucose levels in U.S. population aged 20-74 yr. Diabetes 1987;36:523–534 [DOI] [PubMed] [Google Scholar]
- 2.Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. JAMA 2000;283:2253–2259 [DOI] [PubMed] [Google Scholar]
- 3.Meier JJ, Veldhuis JD, Butler PC. Pulsatile insulin secretion dictates systemic insulin delivery by regulating hepatic insulin extraction in humans. Diabetes 2005;54:1649–1656 [DOI] [PubMed] [Google Scholar]
- 4.Haffner SM, D’Agostino R, Saad MF, et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes 1996;45:742–748 [DOI] [PubMed] [Google Scholar]
- 5.Arslanian S, Suprasongsin C. Differences in the in vivo insulin secretion and sensitivity of healthy black versus white adolescents. J Pediatr 1996;129:440–443 [DOI] [PubMed] [Google Scholar]
- 6.Jiang X, Srinivasan SR, Radhakrishnamurthy B, Dalferes ERJ, Berenson GS. Racial (black-white) differences in insulin secretion and clearance in adolescents: the Bogalusa Heart Study. Pediatrics 1996;97:357–360 [PubMed] [Google Scholar]
- 7.Osei K, Schuster DP, Owusu SK, Amoah AG. Race and ethnicity determine serum insulin and C-peptide concentrations and hepatic insulin extraction and insulin clearance: comparative studies of three populations of West African ancestry and white Americans. Metabolism 1997;46:53–58 [DOI] [PubMed] [Google Scholar]
- 8.Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J. Hyperinsulinemia in African-American children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes 2002;51:3014–3019 [DOI] [PubMed] [Google Scholar]
- 9.Svec F, Nastasi K, Hilton C, Bao W, Srinivasan SR, Berenson GS. Black-white contrasts in insulin levels during pubertal development. The Bogalusa Heart Study. Diabetes 1992;41:313–317 [DOI] [PubMed] [Google Scholar]
- 10.Campioni M, Toffolo G, Basu R, Rizza RA, Cobelli C. Minimal model assessment of hepatic insulin extraction during an oral test from standard insulin kinetic parameters. Am J Physiol Endocrinol Metab 2009;297:E941–E948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piccinini F, Dalla Man C, Vella A, Cobelli C. A model for the estimation of hepatic insulin extraction after a meal. IEEE Trans Biomed Eng 2016;63:1925–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toffolo G, Campioni M, Basu R, Rizza RA, Cobelli C. A minimal model of insulin secretion and kinetics to assess hepatic insulin extraction. Am J Physiol Endocrinol Metab 2006;290:E169–E176 [DOI] [PubMed] [Google Scholar]
- 13.Polidori DC, Bergman RN, Chung ST, Sumner AE. Hepatic and extrahepatic insulin clearance are differentially regulated: results from a model-based analysis of intravenous glucose tolerance data. Diabetes 2016;65:1556–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992;41:368–377 [DOI] [PubMed] [Google Scholar]
- 15.Ellis AC, Alvarez JA, Granger WM, Ovalle F, Gower BA. Ethnic differences in glucose disposal, hepatic insulin sensitivity, and endogenous glucose production among African American and European American women. Metabolism 2012;61:634–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandler-Laney PC, Phadke RP, Granger WM, et al. Adiposity and β-cell function: relationships differ with ethnicity and age. Obesity (Silver Spring) 2010;18:2086–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandler-Laney PC, Phadke RP, Granger WM, et al. Age-related changes in insulin sensitivity and β-cell function among European-American and African-American women. Obesity (Silver Spring) 2011;19:528–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caumo A, Florea I, Luzi L. Effect of a variable hepatic insulin clearance on the postprandial insulin profile: insights from a model simulation study. Acta Diabetol 2007;44:23–29 [DOI] [PubMed] [Google Scholar]
- 19.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. New York, Springer, 2002 [Google Scholar]
- 20.Dowse GK, Zimmet PZ, Alberti KGMM, et al.; Mauritius NCD Study Group . Serum insulin distributions and reproducibility of the relationship between 2-hour insulin and plasma glucose levels in Asian Indian, Creole, and Chinese Mauritians. Metabolism 1993;42:1232–1241 [DOI] [PubMed] [Google Scholar]
- 21.Dowling HJ, Pi-Sunyer FX. Race-dependent health risks of upper body obesity. Diabetes 1993;42:537–543 [PubMed] [Google Scholar]
- 22.King H, Rewers M; WHO Ad Hoc Diabetes Reporting Group . Global estimates for prevalence of diabetes mellitus and impaired glucose tolerance in adults. Diabetes Care 1993;16:157–177 [DOI] [PubMed] [Google Scholar]
- 23.Lee CC, Haffner SM, Wagenknecht LE, et al. Insulin clearance and the incidence of type 2 diabetes in Hispanics and African Americans: the IRAS Family Study. Diabetes Care 2013;36:901–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strömblad G, Björntorp P. Reduced hepatic insulin clearance in rats with dietary-induced obesity. Metabolism 1986;35:323–327 [DOI] [PubMed] [Google Scholar]
- 25.Mittelman SD, Van Citters GW, Kim SP, et al. Longitudinal compensation for fat-induced insulin resistance includes reduced insulin clearance and enhanced beta-cell response. Diabetes 2000;49:2116–2125 [DOI] [PubMed] [Google Scholar]
- 26.Guo X, Cui J, Jones MR, et al. Insulin clearance: confirmation as a highly heritable trait, and genome-wide linkage analysis. Diabetologia 2012;55:2183–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chow CC, Periwal V, Csako G, et al. Higher acute insulin response to glucose may determine greater free fatty acid clearance in African-American women. J Clin Endocrinol Metab 2011;96:2456–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: progress and potential. Endocr Rev 1998;19:608–624 [DOI] [PubMed] [Google Scholar]
- 29.Heinrich G, Ghadieh HE, Ghanem SS, et al. Loss of hepatic CEACAM1: a unifying mechanism linking insulin resistance to obesity and non-alcoholic fatty liver disease. Front Endocrinol (Lausanne) 2017;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lockwood DH, Hamilton CL, Livingston JN. The influence of obesity and diabetes in the monkey on insulin and glucagon binding to liver membranes. Endocrinology 1979;104:76–81 [DOI] [PubMed] [Google Scholar]
- 31.Bergman RN, Iyer MS. Indirect regulation of endogenous glucose production by insulin: the single gateway hypothesis revisited. Diabetes 2017;66:1742–1747 [DOI] [PubMed] [Google Scholar]
- 32.Faber OK, Hagen C, Binder C, et al. Kinetics of human connecting peptide in normal and diabetic subjects. J Clin Invest 1978;62:197–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goree LL, Darnell BE, Oster RA, Brown MA, Gower BA. Associations of free fatty acids with insulin secretion and action among African-American and European-American girls and women. Obesity (Silver Spring) 2010;18:247–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osei K, Schuster DP. Ethnic differences in secretion, sensitivity, and hepatic extraction of insulin in black and white Americans. Diabet Med 1994;11:755–762 [DOI] [PubMed] [Google Scholar]
- 35.Hasson RE, Adam TC, Davis JN, Watanabe RM, Goran MI. Compensatory responses to insulin resistance in obese African-American and Latina girls. Pediatr Obes 2013;8:e68–e73 [DOI] [PubMed] [Google Scholar]
- 36.Goran MI, Bergman RN, Cruz ML, Watanabe R. Insulin resistance and associated compensatory responses in African-American and Hispanic children. Diabetes Care 2002;25:2184–2190 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.