Abstract
Background
Cardiac arrest is a common presentation to intensive care units. There is evidence that management protocols between hospitals differ and that this variation is mirrored in patient outcomes between institutions, with standardised treatment protocols improving outcomes within individual units. It has been postulated that regionalisation of services may improve outcomes as has been shown in trauma, burns and stroke patients, however a national protocol has not been a focus for research. The objective of our study was to ascertain current management strategies for comatose post cardiac arrest survivors in intensive care in the United Kingdom.
Method
A telephone survey was carried out to establish the management of comatose post cardiac arrest survivors in UK intensive care units. All 235 UK intensive care units were contacted and 208 responses (89%) were received.
Results
A treatment protocol is used in 172 units (82.7%). Emergency cardiology services were available 24 hours a day, 7 days a week in 54 (26%) hospitals; most units (123, 55.8%) transfer patients out for urgent coronary angiography. A ventilator care bundle is used in 197 units (94.7%) and 189 units (90.9%) have a policy for temperature management. Target temperature, duration and method of temperature control and rate of rewarming differ between units. Access to neurophysiology investigations was poor with 91 units (43.8%) reporting no availability.
Conclusions
Our results show that treatments available vary considerably between different UK institutions with only 28 units (13.5%) able to offer all aspects of care. This suggests the need for ‘cardiac arrest care bundles’ and regional centres to ensure cardiac arrests survivors have access to appropriate care.
Keywords: Cardiac arrest, resuscitation, intensive care, therapeutic hypothermia
Background
Out-of-hospital cardiac arrest is common in the United Kingdom (UK) with an incidence of 123 cases per 100,000 population per annum;1 emergency medical services (EMS) personnel attempt resuscitation in approximately 30,000 patients a year. The incidence of in-hospital cardiac arrest treated by a resuscitation team is 1.6 per 1000 hospital admissions.2 In the period 1995 to 2005, mechanically ventilated survivors of cardiac arrest accounted for 5.8% of admissions to UK Intensive care units (ICUs).3 There is evidence that high-quality post-resuscitation care improves the likelihood of survival with good functional outcome;3 however, protocols and patient numbers differ between hospitals and this variation is mirrored in patient outcomes between institutions.4,5 The introduction of standardised treatment protocols improves outcomes within individual units6 and it is postulated that regionalisation of services may also improve outcomes as has been shown in trauma, burns and stroke patients;7 however, a national protocol has not been a focus for research.
The objective of our study was to ascertain current management strategies for comatose post-cardiac arrest survivors in intensive care in the United Kingdom.
Methods
All UK ICUs with entries in the 2008 UK Directory of Critical Care8 were contacted by telephone between October 2013 and March 2014. The consultant in charge of the unit that day was asked questions using a standardised questionnaire (online appendix). If the consultant in charge of the unit was unavailable after a repeat phone call the senior nurse or another member of the medical team (registrar or staff grade) was interviewed. Data were collated, anonymised and analysed using a Microsoft EXCEL spreadsheet (Microsoft Corporation, Reading, UK). Ethical committee approval was not required for the study.
Results
All 235 UK ICUs were contacted and 208 responses (89%) were received. All these units admitted comatose survivors of in- or out-of-hospital cardiac arrest. Most units (172 units, 82.7%) follow a protocol for the management of these patients.
Access to emergency cardiology services varied (Figure 1), with percutaneous coronary interventions (PCIs) available 24 hours a day, 7 days a week in only 54 (26%) hospitals. A further 6.7% (16 hospitals) had PCI available during working hours Monday to Friday, whereas most hospitals (123, 55.8%) transferred patients to other units for urgent PCI. Four (1.9%) hospitals had another arrangement to access PCI and 11 (5.3%) hospitals reported no access to PCI.
Figure 1.
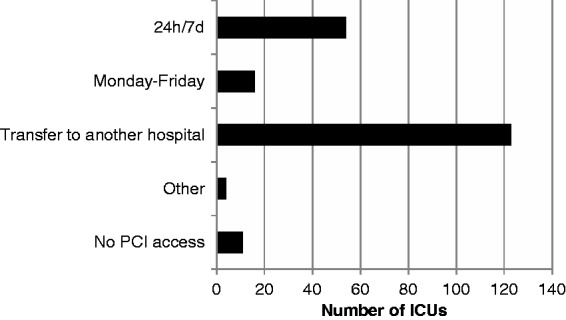
Access to emergency cardiology services for percutaneous coronary intervention (PCI).
Nearly all units use a ventilator care bundle (197 units (94.7%)) and control blood sugar (204 units (98.1%)) with 202 units (97.1%) aiming for blood sugar <10 mmol l−1.
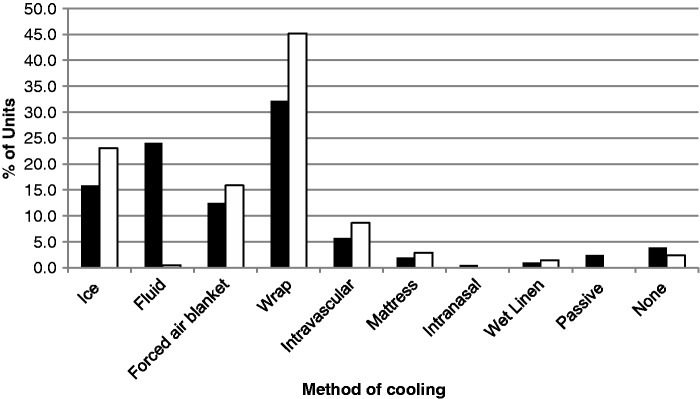
There is a policy for temperature management in 189 units (90.9%) for those who are comatose and require ventilatory support (Table 1). Temperature management is case-by-case depending on consultant preference in 12 (5.8%) units, and 7 units (3.4%) do not have a specific cooling policy. The target temperature varies between units with 24 units (11.5%) aiming for normothermia (36–37℃), 16 units (7.7%) aiming for 35℃, 159 units (76.9%) aiming for 32–34℃ and only 1 unit (0.5%) aiming for less than 32℃. Cooling duration was generally 24 hours (159 units (76.9%)) but ranged from 12 hours (7 units (3.4%)) to 72 hours (3 units, (1.4%)). The method of cooling initiation and maintenance of target temperature was dependent on equipment availability and geographical location (Figure 2). A surface-cooling device was the commonest modality for both initiation (67 units (32.2%)) and maintenance (94 units (45.2%)). Forced air blankets were also frequently used (26 units (24%) for initiation and 33 units (15.9%) for maintenance). Ice was used more commonly for maintenance of hypothermia (48 units (23.1%)) than for initiation (33 units (15.9%)). Cold fluid was used in 50 units (24%) for initiation of cooling, but only one unit (0.5%) for maintenance. Intravenous cooling devices were used by only 12 units (5.8%) for initiation and 18 units (8.7%) for maintenance. A number of units use multiple methods to lower temperature depending on the number of patients requiring therapy at any given time. Rewarming protocols depend largely on the method of cooling used, with most adopting a rate of 0.5℃ h−1 (76 units (36.5%)) or using passive rewarming (64 units (30.8%)). The fastest active rewarming rate reported was 2℃ h−1 (two units (1.0%)) and slowest 0.25℃ h−1 (13 units (6.3%)).
Table 1.
Details of hospital temperature control policies for the 208 ICUs that responded. Values are number (proportion).
| Temperature control policy | |
| Unit temperature control policy | 189 (90.9%) |
| Consultant decision regarding temperature control | 12 (5.8%) |
| No temperature control policy | 7 (3.4%) |
| Target temperature | |
| <32℃ | 1 (0.5%) |
| 33–34℃ | 159 (76.9%) |
| 35℃ | 16 (7.7%) |
| 36–37℃ | 24 (11.5%) |
| Temperature control duration | |
| 0 hours | 13 (6.3%) |
| 12 hours | 7 (3.4%) |
| 18 hours | 1 (0.5%) |
| 24 hours | 160 (76.9%) |
| 36 hours | 1 (0.5%) |
| 48 hours | 16 (7.7%) |
| 72 hours | 3 (1.4%) |
| Don’t know | 7 (3.4%) |
| Rate of rewarming | |
| 0.25℃ h−1 | 13 (6.3%) |
| 0.3℃ h−1 | 2 (1%) |
| 0.5℃ h−1 | 76 (36.5%) |
| 1℃ h−1 | 18 (8.7%) |
| 2℃ h−1 | 2 (1%) |
| Passive (uncontrolled) | 64 (30.8%) |
| Don’t know | 33 (15.8%) |
Figure 2.
Methods used for inducing (▪) and maintaining (□) hypothermia by the ICUs that used therapeutic hypothermia or active temperature control. Some ICUs used more than one method depending on resource availability.
A seizure protocol was followed in 28 units (13.5%) and 31 units (14.9%) stated that they followed a protocol for the withdrawal of treatment in post-cardiac arrest patients. The use and availability of electroencephalography (EEG) and somatosensory-evoked potentials (SSEPs) varied considerably (Figure 3). Only seven hospitals (3.4%) had continuous EEG monitoring for patients receiving neuromuscular blockers, 21 units (10.1%) used intermittent EEG on comatose patients (usually in response to clinical suspicion of seizure activity). Eighty units (38.5%) used EEG for prognostication with only nine (4.3%) using SSEPs; 91 units (43.8%) reported no availability or no use of EEG or SSEPs.
Figure 3.
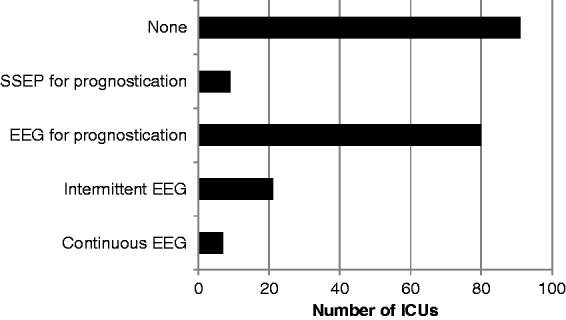
Access to neurophysiology investigations including electroencephalography (EEG) and somatosensory-evoked potentials (SSEPs).
Overall only 28 units (13.5%) are able to offer the full range of care with a protocol; round-the-clock emergency PCI; temperature management; a ventilator care bundle and access to neurophysiology investigations. A further 61 units (29.3%) are able to offer all intensive care support including neurophysiology investigations but rely on transferring patients for emergency cardiology. The majority, 119 units (57.2%), are unable to offer all components.
Discussion
Our survey has shown that the management of post-cardiac syndrome varies considerably between different UK institutions, with only 28 units (13.5%) able to offer all aspects of care.
Our results are consistent with other studies showing that treatment varies considerably between different institutions.5,9–11 A recent study from Copenhagen documented better risk-adjusted outcomes among non-ST elevation myocardial infarction survivors of out-of-hospital cardiac arrest who were transferred directly to one of two tertiary heart centres.12 Other studies have found hospital factors such as size, volume of post-cardiac arrest survivors, teaching hospital status and resources5,13,14 to be linked to patient outcome particularly in patients with intermediate severity illness as measured by Simplified Acute Physiologic (SAPS II) scores.4 The results of these studies have been contradictory however and it is still uncertain which specific hospital characteristics are associated with increased survival amongst cardiac arrest survivors.15 There has been discussion about implementing cardiac arrest care bundles16,17 and regionalising post-cardiac arrest care with the creation of cardiac arrest centres. These would emulate the regionalisation of trauma18,19 and stroke care,20 which has already been shown to improve outcomes from these conditions.6,21–24
Improving post-cardiac arrest care will contribute to reducing premature mortality from cardiovascular disease in the UK.25 Cardiac arrest secondary to myocardial infarction is common. Current UK guidance from the National Institute of Health and Care Excellence (NICE) recommends that post-cardiac arrest patients (including those that are comatose and ventilated) with ST-elevation myocardial infarction (STEMI) have early coronary angiography and, when appropriate, primary PCI.26 Our survey shows that many UK hospitals cannot achieve this because they lack 24/7 PCI facilities requiring secondary transfer of patients to other institutions introducing delays to this time-critical treatment. This supports regionalisation of post-cardiac arrest care to those centres that offer the key components of post-cardiac arrest care including primary PCI with initial transport redirected to these centres by the ambulance service.
Nearly all ICUs used some form of temperature management and this has been described in a previous survey of UK ICUs.27 During the conduct of our survey, the Targeted Temperature Management (TTM) trial was published, and showed no difference in outcome when using a target temperature of 33℃ or 36℃.28 This would explain why some units in our survey were targeting a temperature of 36℃.
Close neurological monitoring with specialised investigations such as EEG and SSEPs can help guide prognostication and inform decisions on withdrawal of life-sustaining treatment (WLST). Recent guidelines on prognostication after out-of-hospital cardiac arrest emphasise the importance of using multiple techniques to prognosticate and in particular highlights the potential value of SSEPs and EEG.29 That only 4.3% of our respondents stated that they used SSEPs for prognostication is a concern.
A strength of our study is that we were able to achieve an 89% response rate and used a standardised questionnaire for the survey. A potential weakness of our study is that the consultant in charge of the ICU may have described their personal practice rather than the policy of the ICU as a whole, and individual responders may not have had detailed knowledge of the availability of ancillary services such as PCI and neurophysiology investigations. The publication of the TTM trial28 in 2013 during the time our survey was conducted and the subsequent publication of the Guidelines on Provision of Intensive Care Services in 201530 may mean that the core temperature for targeted temperature management has changed and that uptake of neurophysiology investigations for prognostication has increased since our survey was completed.
We did not ask each unit for an estimate of how many post-cardiac arrest patients were admitted each year or what proportion of these were in- or out-of-hospital cardiac arrest survivors. There is likely to be a considerable variation in this number, and there has already been some regionalisation of care in some parts of the UK.31
Conclusion
Although we have not documented the treatments patients actually receive, we have shown that the availability of key components of post-cardiac arrest care varies significantly in the UK and that only a minority of units have access to the full range of care cardiac arrest survivors might need. This suggests the need for ‘cardiac arrest care bundles’ and regional centres to ensure cardiac arrests survivors have access to appropriate care. Prospective randomised trials are unlikely to be feasible and we will probably have to rely on high-quality observational studies to assess the impact of ‘cardiac arrest care bundles’ and regionalisation of care.
Authors’ Contributions
AF, MT, JS, JB and MT designed the study and questionnaire. AF, TC, ER, LS, KS, and CR collected the data. AF analysed the data. All authors contributed to, read and approved the final manuscript.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This article summarises independent research supported by the Cardiac Arrest Individual Registry and Outcomes (CAIRO) Programme, funded by a National Institute for Health Research (NIHR) under its Programme Development Grant Programme (Reference Number RP-DG-0612-10004) and the David Telling Charitable Trust. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health or the David Telling Charitable Trust.
References
- 1.Woollard M. Public access defibrillation: a shocking idea? J Public Health Med 2001; 23: 98–102. [DOI] [PubMed] [Google Scholar]
- 2.Nolan JP, Soar J, Smith GB, et al. Incidence and outcome of in-hospital cardiac arrest in the United Kingdom National Cardiac Arrest Audit. Resuscitation 2014; 85: 987–992. [DOI] [PubMed] [Google Scholar]
- 3.Nolan JP, Laver SR, Welch CA, et al. Outcome following admission to UK intensive care units after cardiac arrest: a secondary analysis of the ICNARC Case Mix Programme Database. Anaesthesia 2007; 62: 1207–1216. [DOI] [PubMed] [Google Scholar]
- 4.Schober A, Holzer M, Hochroeser H, et al. Effect of intensive care after cardiac arrest on patient outcome: a database analysis. Crit Care 2014; 18: R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr BG, Kahn JM, Merchant RM, et al. Inter-hospital variability in post-cardiac arrest mortality. Resuscitation 2009; 80: 30–34. [DOI] [PubMed] [Google Scholar]
- 6.Sunde K, Pytte M, Jacobsen D, et al. Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation 2007; 73: 29–39. [DOI] [PubMed] [Google Scholar]
- 7.Donnino MW, Rittenberger JC, Gaieski D, et al. for the National Post Arrest Research Forum (NPARC). The development and implementation of cardiac arrest centres. Resuscitation 2011; 82: 974–978. [DOI] [PubMed] [Google Scholar]
- 8.Anonymous. Directory of critical care. Loughborough: CMA Medical Data, 2008.
- 9.Engdahl J, Abrahamsson P, Bång A, et al. Is hospital care of major importance for outcome after out-of-hospital cardiac arrest? Experience acquired from patients with out-of-hospital cardiac arrest resuscitated by the same Emergency Medical Service and admitted to one of two hospitals over a 16-year period in the municipality of Göteborg. Resuscitation 2000; 43: 201–211. [DOI] [PubMed] [Google Scholar]
- 10.Langhelle A, Tyvold SS, Lexow K, et al. In-hospital factors associated with improved outcome after out-of-hospital cardiac arrest: a comparison between four regions in Norway. Resuscitation 2003; 56: 247–263. [DOI] [PubMed] [Google Scholar]
- 11.Callaway CW, Schmicker R, Kampmeyer M, et al. Investigators TROC: Receiving hospital characteristics associated with survival after out-of-hospital cardiac arrest. Resuscitation 2010; 81: 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Søholm H, Wachtell K, Nielsen SL, et al. Tertiary centres have improved survival compared to other hospitals in the Copenhagen area after out-of-hospital cardiac arrest. Resuscitation 2013; 84: 162–167. [DOI] [PubMed] [Google Scholar]
- 13.Carr Bg, Goyal M, Band RA, et al. A national analysis of the relationship between hospital factors and post cardiac arrest mortality. Intens Care Med 2009; 35: 505–511. [DOI] [PubMed] [Google Scholar]
- 14.Stub D, Smith K, Bray JE, et al. Hospital characteristics are associated with patient outcomes following out-of-hospital cardiac arrest. Heart 2011; 97: 1489–1494. [DOI] [PubMed] [Google Scholar]
- 15.Cudnik MT, Sasson C, Rea TD, et al. Increasing hospital volumes is not associated with improved survival in out of hospital cardiac arrest of cardiac aetiology. Resuscitation 2012; 83: 862–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nolan JP, Soar J. Post resuscitation care: time for a care bundle? Resuscitation 2008; 76: 161–162. [DOI] [PubMed] [Google Scholar]
- 17.Intensive Care Society. Standards for the management of patients after cardiac arrest. London: Intensive Care Society, 2008.
- 18.MacKenzie EJ, Rivara FP, Jurkovich GJ, et al. A national evaluation of trauma-center care on mortality. New Engl J Med 2006; 354: 366–378. [DOI] [PubMed] [Google Scholar]
- 19.McCullough AL, Haycock JC, Forward DP, et al. II Major trauma networks in Britain. Brit J Anaesth 2014; 113: 202–206. [DOI] [PubMed] [Google Scholar]
- 20.Morris S, Hunter R, Ramsey AIG, et al. Impact of centralising acute stroke services in English metropolitan areas on mortality and length of hospital stay: difference-in-differences analysis. Brit Med J 2014; 349: g4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis DP, Fisher R, Aguilar S, et al. The feasibility of a regional cardiac arrest receiving system. Resuscitation 2007; 74: 44–51. [DOI] [PubMed] [Google Scholar]
- 22.Lurie KG, Idris A, Holcomb JB. Level 1 cardiac arrest centers: learning from the trauma surgeons. Acad Emerg Med 2005; 12: 79–80. [DOI] [PubMed] [Google Scholar]
- 23.Bobrow BJ, Kern KB. Regionalization of postcardiac arrest care. Curr Opin Crit Care 2009; 15: 221–227. [DOI] [PubMed] [Google Scholar]
- 24.Nichol G, Aufderheide TP, Eigel B, et al. American Heart Association Emergency Cardiovascular Care Committee, Council on Arteriosclerosis, Thrombosis, Vascular Biology, Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation, Council on Cardiovascular Nursing, Council on Clinical Cardiology, Advocacy Committee, Council on Quality of Care and Outcomes Research. Regional systems of care for out-of-hospital cardiac arrest: a policy statement from the American Heart Association. Circulation 2010; 121: 709–729. [DOI] [PubMed] [Google Scholar]
- 25.Department of health cardiovascular disease team Cardiovascular Disease Outcomes Strategy. Improving outcomes for people with or at risk of cardiovascular disease. London: Department of Health, 2013.
- 26.National Institute for Health and Care Excellence. Clinical Guideline 167: Myocardial infarction with ST-segment elevation: the acute management of myocardial infarction with ST segment elevation. London: NICE, 2013. [PubMed]
- 27.Binks AC, Murphy RE, Prout RE, et al. Therapeutic hypothermia after cardiac arrest- implementation in UK intensive care units. Anaesthesia 2010; 65: 260–265. [DOI] [PubMed] [Google Scholar]
- 28.Neilson N, Wettersley J, Cronberg T, et al. Targeted temperature management at 33C versus 36C after cardiac arrest. New Eng J Med 2013; 369: 2197–2206. [DOI] [PubMed] [Google Scholar]
- 29.Sandroni C, Cariou A, Cavallaro F, et al. Prognostication in comatose survivors of cardiac arrest. An Advisory Statement from the European Resuscitation Council and the European Society of Intensive Care Medicine. Resuscitation 2014; 85: 1779–1789. [DOI] [PubMed] [Google Scholar]
- 30.Nolan JP and Soar J. Section 4.1.8 Post cardiac arrest management. Guidelines for the provision of intensive care services. 1st ed. London: Faculty of Intensive Care Medicine / Intensive Care Society, 2015.
- 31.Fothergill RT, Watson LR, Virdl GK, et al. Survival of resuscitated cardiac arrest patients with ST-elevation myocardial infarction (STEMI) conveyed directly to a Heart Attack Centre by ambulance clinicians. Resuscitation 2014; 85: 96–98. [DOI] [PubMed] [Google Scholar]