Abstract
Acute right ventricular failure remains an immensely challenging clinical condition associated with a high mortality rate. In this narrative review, we highlight the pathophysiological mechanisms underlying right ventricular failure and suggest an initial diagnostic approach to this critically ill group of patients. Based on the best available evidence and our cumulative clinical experience as a national cardiothoracic centre, we summarize the basic principles of medical management and mechanical salvage therapy, finalizing with a series of recommendations for the management of right ventricular failure in specific clinical scenarios.
Keywords: Extra-corporeal membrane oxygenation, heart-assist devices, right-sided heart failure, shock, cardiogenic
Introduction
The onset of right ventricular failure (RVF) can be acute, subacute or chronic, and right ventricular dysfunction is associated with a wide range of clinical conditions1 (Table 1). Although the initial clinical signs of acute right heart failure can be subtle and easily overlooked, as the condition deteriorates, the characteristic findings become evident: lower limb oedema, tender hepatomegaly, distended jugular veins, the presence of a third heart sound and a regurgitant murmur in the tricuspid area.
Table 1.
Causes of acute right ventricular failure.
| Pulmonary venous hypertension (left ventricular failure) |
| Right ventricular ischaemia |
| Exacerbation of chronic obstructive airway disease |
| Pulmonary arterial hypertension (due to hypoxic vasoconstriction or primary pulmonary vascular disease) |
| Post-cardiotomy |
| Massive pulmonary embolism |
| Post-LVAD insertion |
| Cardiac allograft rejection |
LVAD: left ventricular assist device.
The first challenge posed by acute RVF is the timely identification in a complex critically ill patient of a condition that mimics several other more common disease processes. The second challenge concerns the delicate therapeutic management required to optimize cardiac function in the face of rapidly evolving systemic hypo-perfusion and multi-organ failure.
In this narrative review, we will focus on the pathophysiological mechanisms underlying primary acute RVF, on the initial and ongoing medical management of this condition in the intensive care unit (ICU), and we will discuss available mechanical salvage therapies.
Pathophysiology
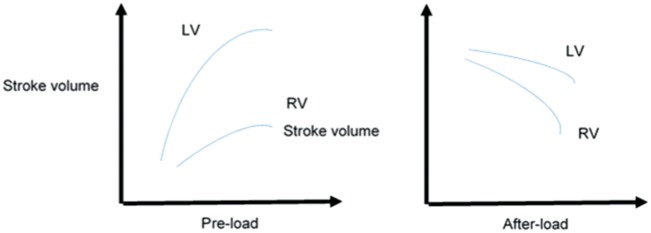
Under normal physiological circumstances, the right ventricular stroke volume must equal the left ventricular stroke volume. RVF develops when the right ventricle’s diastolic and/or systolic function is compromised beyond the capacity of the intrinsic myocardial compensatory mechanisms to maintain an adequate stroke volume. The ensuing multi-organ failure syndrome is due to impaired end-organ perfusion in the face of reduced anterograde blood flow and, perhaps more importantly, due to increased retrograde venous pressures.2 The three main factors that regulate ventricular ejection are pre-load, after-load and contractility. The relationship between stroke volume and pre-load is described by an inverted U-curve (Frank–Starling curve), and the relationship between stroke volume and after-load corresponds to a down-sloping curve (Figure 1). The right ventricular wall is significantly thinner than the left ventricular wall, and this makes the right ventricle less capable of managing pressure after-load and more capable of tolerating volume over-load. Overall, the right ventricle displays a phenomenal capacity for recovery and plastic remodelling in response to changing hemodynamic conditions.3
Figure 1.
Differences in right and left ventricular response to increases in pre-load and after-load.
Although to an extent the right and left ventricles operate independently, their function is linked via the interventricular septum. As the failing right ventricle progressively dilates, the interventricular septum is displaced medially and bulges into the left ventricular cavity, jeopardizing left ventricular diastolic filling and thus initiating a self-perpetuating cascade leading to refractory cardiogenic shock4 (Figure 2). This ventricular inter-dependence effect is greatly magnified in the face of rising intra-pericardial pressures, such as seen in the context of pericardial effusion.
Figure 2.
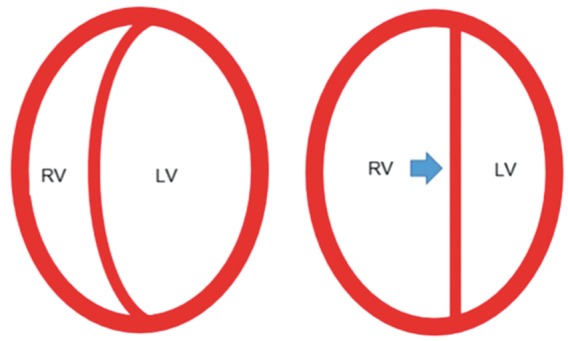
Ventricular interdependence. As the falling right ventricle progressively dilates, the interventricular septum is displaced medially and bulges into the left ventricular cavity, jeopardizing left ventricular diastolic filling and thus initiating a self-perpetuating cascade leading to refractory cardiogenic shock.
Initial work-up
Mandatory investigations for all patients presenting with acute RVF include chest radiograph, arterial blood gas analysis, serum troponin and BNP levels, ECG and an echocardiogram.5 Further investigation for specific underlying conditions will be guided by the patient’s clinical presentation and the findings on examination.
Echocardiography provides invaluable information regarding right ventricular structure and function, pulmonary and tricuspid valve competency and can also offer a reasonable estimation of pulmonary artery pressures. There is ongoing debate regarding the best echocardiographic measure of right ventricular function, but the assessment of the tricuspid annulus plane systolic excursion (TAPSE) seems to be sufficiently specific and reproducible, with a TAPSE value of less than 2 cm as the cut-off value to define right ventricular dysfunction.6 Right ventricular free wall hypertrophy is a marker of chronicity, and the degree of right ventricular dilatation has important prognostic implications.7 If further anatomical detail is required or the acoustic windows are sub-optimal, then a trans-oesophageal echocardiogram should be obtained.
Following the publication of several trials that failed to demonstrate a survival benefit for its routine use, the pulmonary artery catheter (PAC) has fallen out of grace in general ICUs. However, in patients with advanced structural heart disease and profound cardiogenic shock, the PAC remains the standard of care, enabling continuous monitoring of cardiac output and careful titration of inotropes and pulmonary vasodilators in response to dynamic variation in pulmonary vascular resistance and left atrial filling pressure.8 Moreover, the trans-pulmonary pressure gradient, which is an essential index of the reversibility of pulmonary hypertension in congestive heart failure patients undergoing transplant assessment, can only be reliably obtained with the use of the PAC.
Patients presenting with acute RVF of suspected ischaemic aetiology should undergo urgent coronary angiography, which is not only diagnostic but also potentially therapeutic.
Although cardiac magnetic resonance imaging is rapidly evolving as the gold standard for the assessment of the finer details of right ventricular structure, the intrinsic physiological instability of patients with acute RVF makes this investigation impractical in the acute setting.9
General management
Optimization of pre-load
Although initially stroke volume may increase in response to fluid loading, once the inflection point of the Frank–Starling curve is reached, further volume loading over-distends the right ventricle, impairing optimal coupling of myofibrils and causing a drop in right stroke volume. The challenge with fluid management in acute right heart failure is finding the optimal filling point. This point represents a moving target, varying with time and with changing physiological stimuli.
The accurate bedside assessment of fluid responsiveness in RVF patients can be surprisingly complex. Traditionally, static measurements such as right atrial pressure and pulmonary artery wedge pressure have been used to predict fluid responsiveness, but the positive predictive value of such static haemodynamic indices remains well below 50%.10 In patients with normal bi-ventricular function, dynamic monitoring techniques based on stroke volume variation (SVV) or pulse pressure variation (PPV) have proven to be more reliable, with PPVs consistently above 85%.11 However, recent research has demonstrated that the use of PPV and SVV for pre-load optimization in RVF patients may be unreliable,12,13 and importantly, a large proportion of patients in this population will not be in sinus rhythm, rendering dynamic monitoring tools based on respiratory variation in stroke volume less useful.
Our approach to predicting fluid responsiveness in this population relies heavily on the monitoring of real-time changes in cardiac output in response to fluid challenges as measured with the PAC and echocardiography.
At this point in time, there is no strong evidence to recommend the use of one particular fluid over the other for the resuscitation of patients with cardiogenic shock, and crystalloids remain the default choice. However, the cumulative results of recent trials would suggest starches should be avoided and that the use of albumin is safe.14,15
Finally, every effort should be made to ensure unimpeded flow of the venous return into the right heart. This entails regular echocardiographic monitoring to rule out pericardial effusion and the monitoring of intra-abdominal pressures – values higher than 15 mmHg should prompt investigation for the underlying causes.
Optimization of after-load
The resistance of the pulmonary circulation, which is normally one-third of the systemic vascular resistance, represents the right ventricular after-load. Pulmonary vascular resistance is at its lowest at functional residual capacity, increasing exponentially as lung volumes change in either sense. As the lungs inflate, pulmonary vascular resistance increases due to compression of alveolar capillaries and as lungs deflate the resistance increases due to compression of the parenchymal capillaries. Patients with acutely impaired right ventricular function are exquisitely sensitive to increases in pulmonary vascular resistance, and therefore, lung under-recruitment can be as harmful as lung over-recruitment. Although lung-protective ventilation at 6 mL/kg of tidal volume can generate hypercapnia and relative hypoventilation, increasing pulmonary vascular resistance, since the introduction of this strategy the incidence of acute RVF in the acute respiratory distress syndrome has decreased from 60% to 25%.16
The application of positive end-expiratory pressure (PEEP) can have variable effects on right heart function – on the one hand, PEEP compresses the pulmonary vasculature increasing right ventricular after-load, but on the other, it improves alveolar recruitment and counteracts hypoxic pulmonary vasoconstriction. Multiple techniques for PEEP titration have been described, and in this particular challenging patient population, there might be a specific role for oesophageal pressure-guided PEEP titration.17
Positive pressure ventilation increases intra-thoracic pressures and right ventricular after-load and generally has a deleterious effect on right ventricular stroke volume. Intubation and ventilation in a patient with critically impaired right ventricular function can precipitate cardiac arrest and should be undertaken with great caution and using induction agents that have minimal impact on cardiac function.
The pulmonary vascular tone is exquisitely responsive to a variety of physiological stimuli – pulmonary vasoconstriction is promptly triggered by hypoxemia, hypercapnia and acidemia. In this sense, a right ventricular protective strategy should always entail optimization of oxygenation, avoidance of hypercapnia and strict maintenance of pH above at least 7.20.18
There are a variety of agents that exhibit pulmonary vasodilatory activity, with variable degrees of pulmonary selectivity.19 The main groups of intravenous pulmonary vasodilators include inodilators like dobutamine and the phosphodiesterase III inhibitors (milrinone, enoximone), angiotensin inhibitors (bosentan), endothelin receptor antagonists (ambrisentan) and selective phosphodiesterase V inhibitors (sildenafil, tadanafil).20,21 The main inhaled vasodilator is nitric oxide (NO). Prostacyclins can be delivered intravenously (epoprosterenol) or in nebulized form (iloprost). The choice of drug should be based on the individual responsiveness profile of each patient. The potential for synergistic interaction amongst these agents should always be taken into consideration, e.g. inhaled NO displays synergistic interaction with both dobutamine and prostacyclins.22,23 The continuous monitoring of pulmonary vascular resistance with the PAC plays an important part in this tailoring process. Although these agents exhibit dose-effect pharmacokinetics, the limiting factor for most of them is systemic vasodilatation leading to worsening hypotension. Inhaled NO, which is quickly inactivated and not absorbed systemically, is the notable exception.24
At our centre, ventilated patients with RVF are usually managed initially with milrinone and NO, and once they are ready for extubation, NO is replaced with sildenafil.
Optimization of contractility
As an initial measure, ensuring a normal concentration of all electrolytes participating in the generation of membrane potential is important to optimize right ventricular performance.
The right ventricle is predominantly perfused during diastole, and the right ventricular perfusion pressure is effectively the difference between the mean arterial pressure (MAP) and the intra-ventricular pressure. From this point of view, extreme tachycardia should be avoided to enable maximum pressure generation in the right ventricle. If a vasopressor is required to support the MAP, its differential effects on the systemic and pulmonary circulations should be taken into account. In this sense, vasopressin is less of a pulmonary vasoconstrictor than noradrenaline in most circumstances, and therefore, it may be of benefit in the context of acute RVF.25
In patients presenting with an acute coronary syndrome, emergency angiographic revascularization is indicated to ensure that oxygen delivery matches myocardial oxygen demand. If the patient’s coronary anatomy is not suited for angioplasty and stenting, surgical coronary artery bypass grafting may be necessary.26
Inotropes increase cardiac contractility by augmenting the intra-cellular availability of calcium at the expense of increased myocardial oxygen consumption. There is significant regional variability in the choice of inotropes, and no particular agent has been demonstrated superiority. Trials comparing dobutamine vs. milrinone and adrenaline vs. dobutamine plus noradrenaline have found no demonstrable survival benefit.27 The post-hoc analysis of patients the cardiogenic shock sub-group in the SOAP II study found a slight survival benefit for noradrenaline over dopamine.28 Due to its adverse effects on multiple endocrine axis and its tendency to induce tachy-arrhythmias, dopamine is now rarely used as a first-line inotrope.
Levosimendan is a unique agent in that it exerts a positive inotropic effect on the ventricles without increasing myocardial oxygen consumption. It has pleiotropic effects, also acting as a pulmonary and systemic vasodilator.29 These effects are mediated through interaction with troponin-C, inhibition of phosphodiesterase III and modulation of potassium channels. Levosimendan has the potential to improve right ventricular stroke volume in a variety of clinical scenarios, but it should be used with extreme caution in patients with reduced peripheral vascular resistance due to the risk of worsening hypotension.30,31
There is currently insufficient evidence to make definitive recommendations regarding the choice of inotropes in acute RVF. At our institution, we tend to use interchangeably either adrenaline or the combination of milrinone and noradrenaline. The main limiting factor for the use of adrenaline is tachy-arrhythmias and lactic acidosis, and the main limiting factor for milrinone is peripheral vasodilatation. We use levosimendan adjunctively in selected cases.
Mechanical support
When patients continue to deteriorate in spite of all efforts to optimize pre-load, after-load and contractility, the only therapeutic option left to support the failing circulation is extra-corporeal life support (ECLS).
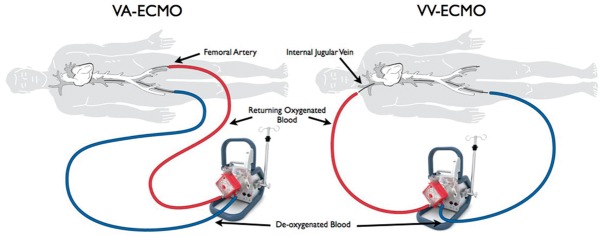
The most readily available form of ECLS in this context is veno-arterial extra-corporeal membrane oxygenation (VA-ECMO), an abbreviated cardio-pulmonary bypass circuit capable of providing varying degrees of cardiac and respiratory support (Figure 3). VA-ECMO improves perfusion of vital organs, off-loads the right heart and provides relative stability, allowing enough time for the right ventricle to heal (bridge to recovery) for a right ventricular assist device (RVAD) to be implanted (bridge to bridge) or for a heart transplant (bridge-to-transplant) to become available.32 Percutaneous cannulation of the femoral vessels, simplified heparin-bonded circuits and portable pump-oxygenator units have facilitated the rapid delivery of VA-ECMO at the bedside and the development of ECMO retrieval programmes. However, initiating ECMO still requires careful consideration of the risk-benefit balance, since this salvage therapy puts patients at considerable risk of catastrophic haemorrhage, embolic events and lower limb ischaemia. Even in the most favourable circumstances, the mortality for patients with cardiogenic shock requiring VA-ECMO remains very high, usually in excess of 50%.33 Of note, in some situations where RVF is driven mainly by an increase in pulmonary vascular resistance due to profound hypoxia or hypercapnia, isolated respiratory support with veno-venous ECMO may be sufficient to improve haemodynamics and stabilize the patient (Figure 3).
Figure 3.
Modes of ECMO support. VV-ECMO provides only respiratory support while VA-ECMO provides full cardiovascular and respiratory support.
Right ventricular assist devices (RVADs) are internal continuous flow pumps that bypass the failing right ventricle, propelling blood from the right atrium directly into the pulmonary artery and allowing time for the failing right ventricle to recover and remodel.34 If the right ventricle is expected to heal within the timeframe of a few weeks, percutaneously implanted temporary RVADs are the support device of choice – a variety of designs are commercially available, and clinical data on their applicability and safety profile are rapidly emerging35,36 (Figure 4). On the contrary, if right ventricular recovery is expected to require longer than a few weeks, then a longer term surgically implanted RVAD will be required. In this case, sternotomy and cardio-pulmonary bypass will be required for implantation, and long-term systemic anticoagulation is mandatory, putting patients at significant risk of hemorrhagic complications (Figure 5). Although some health systems use VADs as destination therapy, in the UK, they are currently approved only as bridge-to-transplant, i.e. only patients that would otherwise be eligible for transplantation are candidates for VADs.37
Figure 4.
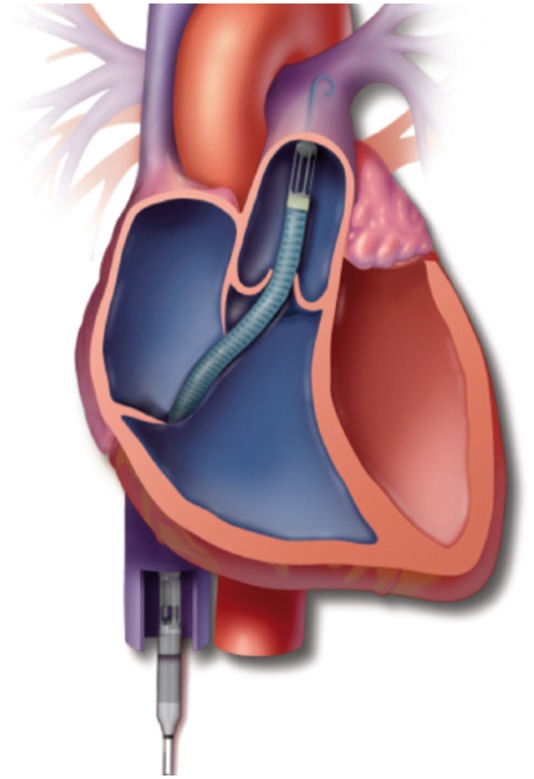
The Impella RP is temporary RVAD inserted percutaneously through the femoral vein. It pumps blood from the inferior vena cava through the tricuspid and pulmonic valves directly into the pulmonary artery.
Figure 5.
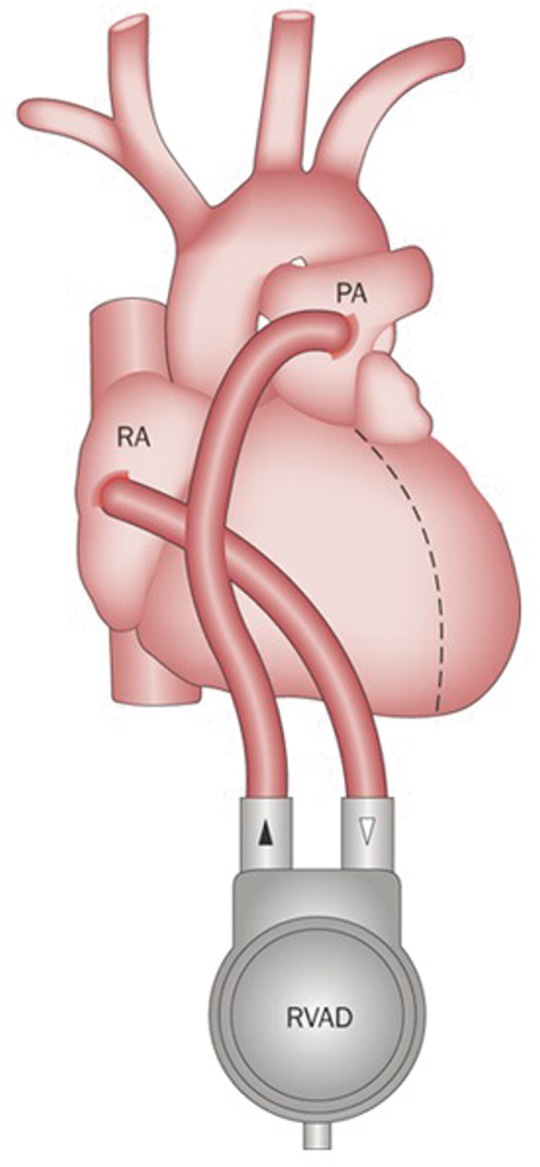
Implantable RVADs are appropriate for long-term therapy. The pump blood from the right atrium directly flows into the pulmonary artery, bypassing the falling right ventricle.
Management of specific conditions
Beyond the general optimization of pre-load, after-load and contractility and the consideration of mechanical support, some conditions leading to acute RVF require specific interventions.
Right ventricular infarction remains the most common cause of isolated RVF in the general population. In this scenario, the priority is urgent revascularization to minimize the extent of myocardial necrosis. Usually this can be achieved via angiography, but if the coronary anatomy is unfavourable, surgical revascularization may be necessary. Although two large clinical trials have failed to demonstrate a survival benefit, the intra-aortic balloon pump (IABP) is still widely indicated for cardiogenic shock associated with left ventricular infarction.38 There is much less experience with the use of IABP in right ventricular infarction, but several groups have suggested improved haemodynamics after insertion of the device in this context.39
In massive pulmonary embolism, the right ventricle strains against a hyper-acute increase in after-load which causes it to dilate and eventually collapse – the mainstay of treatment for this situation is thrombolysis.40 Thrombolysis is usually achieved via the administration of systemic thrombolytics, but if this is unsuccessful or contra-indicated, local mechanical thrombolysis may be attempted. The use of VA-ECMO as a bridging strategy to stabilize the patient while effective mechanical thrombolysis is achieved has been well documented, with most specialist centres reporting a survival rate of around 70%.41
Patients presenting with acute exacerbation of idiopathic pulmonary arterial hypertension will have a chronically hypertrophied, debilitated right ventricle, and this demographic group will require careful titration of pulmonary vasodilators and inotropic support. The reversibility of right ventricular dysfunction following the correction of chronic pressure over-load and the surprising re-modelling potential of the right ventricle warrants evaluation for lung transplantation in all patients and consideration of ECLS in selected cases.42 Pulmonary to left atrium bypass with and intercalated oxygenator as pre-conditioning for lung transplantation is a promising new therapy for this challenging condition.43
RVF complicating acute exacerbation of chronic obstructive pulmonary disease (COPD) represents a situation of combined respiratory and cardiovascular collapse. In COPD, the increase in pulmonary vascular resistance is due to structural lung changes as much as hypoxic vasoconstriction and unlike primary arterial pulmonary hypertension, this increase in resistance is poorly responsive to pharmacological manipulation. Initiation of positive pressure ventilation in this context elevates right ventricular after-load to critical levels and can lead to cardiovascular collapse. Although currently the standard management of severe COPD exacerbations relies on non-invasive ventilation, bronchodilators and inotropic support, novel management strategies based on extra-corporeal CO2 removal hold promise for the future.44
A significant percentage of patients undergoing left ventricular assist device implantation for left ventricular failure develop post-operative RV failure. This is due to acute right ventricular volume over-load in the context of improved left ventricular output and to dynamic changes in right ventricular architecture.45 A similar situation can be encountered in patients with advanced left ventricular failure receiving a heart transplant in which the implanted right ventricle struggles to cope with the chronically elevated pulmonary pressures and eventually collapses.46 If medical management does not rapidly reverse the situation, urgent RVAD support is indicated.47
Conclusion
The management of patients with acute RVF poses a unique clinical challenge. Timely identification and careful physiological management are necessary to improve chances of survival. The initial approach should focus on establishing an aetiological diagnosis and optimizing pre-load, after-load and contractility. If patients fail to respond to the initial intensive care stabilization and specific medical therapies, extra-corporeal support should be promptly considered.
Due to the inherent complexity of these cases, we recommend that patients with acute RVF are managed by a multi-disciplinary team and preferably in tertiary cardiac centres experienced in ECLS and heart transplantation.
Authors’ contributions
IA: Manuscript idea; work plan; literature search; selection of relevant references; text for sections: Introduction, pathophysiology, initial work-up and general management. AR: Overall senior supervision; text for sections: Specific conditions; proofreading; final revision of text.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Ramu B, Thenappan T. Evolving concepts of pulmonary hypertension secondary to left heart disease. Curr Heart Fail Rep 2016; 13: 92–102. [DOI] [PubMed] [Google Scholar]
- 2.Ventetuolo C, Klinger J. Management of acute right ventricular failure in the intensive care unit. Ann Am Thorac Soc 2014; 11: 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poels EM, da Costa Martins PA, van Empel VP. Adaptive capacity of the right ventricle: why does it fail? Am J Physiol Heart Circ Physiol 2015; 308: H803–H813. [DOI] [PubMed] [Google Scholar]
- 4.Jardin F. Ventricular interdependence: how does it impact on hemodynamic evaluation in clinical practice? Int Care Med 2003; 29: 361–363. [DOI] [PubMed] [Google Scholar]
- 5.Troisi F, Greco S, Brunetti ND, et al. Right heart dysfunction assessed with echography, B-type natriuretic peptide and cardiopulmonary test in patients with chronic heart failure. J Cardiovasc Med 2008; 9: 672–676. [DOI] [PubMed] [Google Scholar]
- 6.Scrutinio D, et al. Tricuspid annular plane systolic excursion in acute decompensated heart failure: relevance for risk stratification. Can J Cardiol 2016; 32: 963–969. [DOI] [PubMed] [Google Scholar]
- 7.Cho JH, Kutti Sridharan G, Kim SH, et al. Right ventricular dysfunction as an echocardiographic prognostic factor in hemodynamically stable patients with acute pulmonary embolism: a meta-analysis. BMC Cardiovasc Disord 2014; 14: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marik PE. Obituary: pulmonary artery catheter 1970 to 2013. Ann Intensive Care 2013; 3: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caudron J, et al. Cardiac MRI assessment of right ventricular function in acquired heart disease: factors of variability. Acad Radiol 2012; 19: 991–1002. [DOI] [PubMed] [Google Scholar]
- 10.Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med 2013; 41: 1774–1781. [DOI] [PubMed] [Google Scholar]
- 11.Pinsky MR, Payen D. Functional hemodynamic monitoring. Crit Care 2005; 9: 566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daudel F, et al. Pulse pressure variation and volume responsiveness during acutely increased pulmonary artery pressure: an experimental study. Crit Care 2010; 14: R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wyler von Ballmoos M, et al. Pulse-pressure variation and hemodynamic response in patients with elevated pulmonary artery pressure: aclinical study. Crit Care 2010; 14: R111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meybohm P, et al. Re-evaluating currently available data and suggestions for planning randomised controlled studies regarding the use of hydroxyethyl starch in critically ill patients – a multidisciplinary statement. Crit Care 2013; 17: R166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vincent J-L, et al. Albumin administration in the acutely ill: what is new and where next? Crit Care 2014; 18: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price LC, et al. Pulmonary vascular and right ventricular dysfunction in adult critical care: current and emerging options for management: a systematic literature review. Crit Care 2010; 14: R169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talmor D, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med 2008; 359: 2095–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kummer W. pulmonary vascular innervation and its role in responses to hypoxia. Pro Am Thor Soc 2011; 8: 471–476. [DOI] [PubMed] [Google Scholar]
- 19.Rich S. The effects of vasodilators in pulmonary hypertension. Circ Heart Fail 2009; 2: 145–150. [DOI] [PubMed] [Google Scholar]
- 20.Lowson S. Alternatives to nitric oxide. Br Med Bull 2004; 70: 119–131. [DOI] [PubMed] [Google Scholar]
- 21.Rich S, McLaughlin VV. Endothelin receptor blockers in cardiovascular disease. Circulation 2003; 108: 2184–2190. [DOI] [PubMed] [Google Scholar]
- 22.Vizza CD, et al. Acute hemodynamic effects of inhaled nitric oxide, dobutamine and a combination of the two in patients with mild to moderate secondary pulmonary hypertension. Crit Care 2001; 5: 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuhlen R, et al. Combination of inhaled nitric oxide and intravenous prostacyclin for successful treatment of severe pulmonary hypertension in a patient with acute respiratory distress syndrome. Intensive Care Med 1999; 25: 752–754. [DOI] [PubMed] [Google Scholar]
- 24.Griffiths MJ, Evans TW. Inhaled nitric oxide therapy in adults. N Engl J Med 2005; 353: 2683–2695. [DOI] [PubMed] [Google Scholar]
- 25.Gordon AC, Wang N, Walley KR, et al. The cardiopulmonary effects of vasopressin compared with norepinephrine in septic shock. Chest 2012; 142: 593–605. [DOI] [PubMed] [Google Scholar]
- 26.Inohara T, Kohsaka S, Fukuda K, et al. The challenges in the management of right ventricular infarction. Eur Heart J Acute Cardiovasc Care 2013; 2: 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francis GS, Bartos JA, Adatya S. Inotropes. J Am Coll Cardiol 2014; 63: 2069–2078. [DOI] [PubMed] [Google Scholar]
- 28.De Backer D, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010; 362: 779–789. [DOI] [PubMed] [Google Scholar]
- 29.Rehberg S, et al. Role of Levosimendan in intensive care treatment of myocardial insufficiency. Anaesthesist 2007; 56: 30–43. [DOI] [PubMed] [Google Scholar]
- 30.Duygu H, et al. Effect of levosimendan on right ventricular systolic and diastolic functions in patients with ischaemic heart failure. Int J Clin Pract 2008; 62: 228–233. [DOI] [PubMed] [Google Scholar]
- 31.Morais RJ. Levosimendan in severe right ventricular failure following mitral valve replacement. J Cardiothorac Vasc Anesth 2006; 20: 82–84. [DOI] [PubMed] [Google Scholar]
- 32.Kar B, et al. Percutaneous circulatory support in cardiogenic shock. Circulation 2012; 125: 1809–1817. [DOI] [PubMed] [Google Scholar]
- 33.Takayama H, et al. Clinical outcome of mechanical circulatory support for refractory cardiogenic shock in the current era. J Heart Lung Transplant 2013; 32: 106. [DOI] [PubMed] [Google Scholar]
- 34.Hsu PL, et al. Mechanical circulatory support for right heart failure: current technology and future outlook. Artif Organs 2012; 36: 332–347. [DOI] [PubMed] [Google Scholar]
- 35.Aggarwal V, Einhorn BN, Cohen HA. Current status of percutaneous right ventricular assist devices: first-in-man use of a novel dual lumen cannula. Catheter Cardiovasc Interv 2016; 88: 390–396. [DOI] [PubMed] [Google Scholar]
- 36.Anderson MB, et al. Benefits of a novel percutaneous ventricular assist device for right heart failure: the prospective RECOVER RIGHT study of the Impella RP device. J Heart Lung Transplant 2015; 34: 15498–15560. [DOI] [PubMed] [Google Scholar]
- 37.Miller LW, Guglin M. Patient selection for ventricular assist devices: a moving target. J Am Coll Cardiol 2013; 61: 1209–1221. [DOI] [PubMed] [Google Scholar]
- 38.Sjauw KD, et al. A systematic review and meta-analysis of intra-aortic balloon pump therapy in ST-elevation myocardial infarction: should we change the guidelines? Eur Heart J 2009; 30: 459–468. [DOI] [PubMed] [Google Scholar]
- 39.Arafa OE, Geiran OR, Andersen K, et al. Intraaortic balloon pumping for predominantly right ventricular failure after heart transplantation. Ann Thorac Surg 2000; 70: 1587–1593. [DOI] [PubMed] [Google Scholar]
- 40.Wang TF, Squizzato A, Dentali F, et al. The role of thrombolytic therapy in pulmonary embolism. Blood 2015; 125: 2191–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yusuf HO, et al. Extracorporeal membrane oxygenation in acute massive pulmonary embolism: a systematic review. Perfusion 2015; 30: 611–616. [DOI] [PubMed] [Google Scholar]
- 42.Machuca T, et al. Interventional therapies for right ventricular failure secondary to precapillary pulmonary hypertension. Adv Pulmonary Hypertension 2015; 13: 197–201. [Google Scholar]
- 43.Mayes J, Niranjan G, Dark J, et al. Bridging to lung transplantation for severe pulmonary hypertension using dual central Novalung lung assist devices. Interact Cardiovasc Thorac Surg 2016; 22: 677–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Del Sorbo L, et al. Extracorporeal CO2 removal in hypercapnic patients at risk of noninvasive ventilation failure: a matched cohort study with historical control. Crit Care Med 2015; 43: 120–127. [DOI] [PubMed] [Google Scholar]
- 45.Cheung AW, et al. Short-term mechanical circulatory support for recovery from acute right ventricular failure: clinical outcomes. J Heart Lung Transplant 2014; 33: 794–799. [DOI] [PubMed] [Google Scholar]
- 46.Klotz S, et al. Reversible pulmonary hypertension in heart transplant candidates-pretransplant evaluation and outcome after orthotopic heart transplantation. Eur J Heart Fail 2003; 5: 645–653. [DOI] [PubMed] [Google Scholar]
- 47.Ochiai Y, et al. Predictors of severe right ventricular failure after implantable left ventricular assist device insertion: analysis of 245 patients. Circulation 2002; 106: I198–I202. [PubMed] [Google Scholar]