Abstract
Many physiological and cellular processes cycle with time, with the period between one peak and the next being roughly equal to 24 h. These circadian rhythms underlie ‘permissive homeostasis’, whereby anticipation of periods of increased energy demand or stress may enhance the function of individual cells, organ systems or whole organisms. Many physiological variables related to survival during critical illness have a circadian rhythm, including the sleep/wake cycle, haemodynamic and respiratory indices, immunity and coagulation, but their clinical significance remains underappreciated. Critically ill patients suffer from circadian dysrhythmia, manifesting overtly as sleep disturbance and delirium, but with widespread covert effects on cellular and organ function. Environmental and pharmacological strategies that ameliorate or prevent circadian dysrhythmia have demonstrated clinical benefit. Harnessing these important biological phenomena to match metabolic supply to demand and bolster cell defenses at the apposite time may be a future therapeutic strategy in the intensive care unit.
Keywords: Chronobiology disorders, circadian rhythms, biological clocks, critical illness, physiology
‘Rhythms of life’
The earth completes one rotation on its axis every 24 h, exposing its surface inhabitants to predictable periods of light and darkness. This influences the temporal organisation of behaviour, such as physical activity, feeding and sleeping, each of which imposes specific demands on cells and physiological systems, such as exposure to pathogens, oxidative stress or surges in energy requirements. Diverse biological processes, from global haemodynamics to intracellular protein levels – even the composition of the microbiome1 – also demonstrate distinct temporal oscillations with a period of approximately 24 h between one peak and the next.2 These cycles are termed circadian rhythms and have been observed in almost every living creature on Earth.3 They support ‘predictive homeostasis’ by upregulating metabolic capacity and self-defense mechanisms in anticipation of periods of increased demand. In critical illness, where support of physiological processes stands between life and death, there is a pressing need to understand the biological significance of these rhythms and how to circumvent the negative consequences of their disruption.
Molecular basis of circadian rhythms
Circadian rhythmicity has been demonstrated in physiological indices (core body temperature, brain wave activity, cardiovascular and respiratory function, coagulation and immunity)4 and intracellular processes (mitochondrial metabolism, protein expression, enzyme activity, redox cycles, DNA repair and cell regeneration).5,6 Rhythmicity is generated at the level of the individual cell by a complex series of positive and negative feedback loops, controlled by a set of circadian transcription factors, 10 of which have been identified so far, including: circadian locomotor output cycles kaput (CLOCK), period (PER),1–3 cryptochrome circadian clock (CRY)1,2 and brain and muscle ARNT-like 1 (BMAL1).7 It is estimated that one-third of all gene activity is regulated by this intracellular ‘clock’.8 Polymorphisms in clock genes determine important phenotypes, such as sleeping behaviour, which varies between individuals across a spectrum from ‘morningness’ (early birds) to ‘eveningness’ (night owls).9 Such chronotypes also appear to influence the time of peak performance in athletes,10 and may even determine the most likely hour of death.9
Entrainment and synchronisation of many clocks
Whilst every cell has rhythms driven by its own ‘clock’, in humans a central pacemaker located in the suprachiasmatic nucleus (SCN) of the hypothalamus co-ordinates the clocks in many diverse tissues through neural and hormonal pathways.11 The SCN controls the secretion of the chief circadian hormone, melatonin, through sympathetic projections to the pineal gland. During a typical light/dark cycle, pineal melatonin secretion begins between 9 and 11 pm, reaches a peak between 1 and 3 am and falls to baseline again between 7 and 9 am.12,13 Melatonin acts at cell surface receptors within the central nervous system, where it regulates the sleep/wake cycle. It also acts on peripheral tissues, stabilising their circadian rhythms and aligning the phase relationships of different peripheral clocks.11 Through projections to other hypothalamic centres, the SCN also drives circadian fluctuation in sympathetic and parasympathetic tone, as well as the release of cortisol and growth hormone.14 Circadian rhythms persist in the absence of external stimuli, but they may be modulated over time by external cues, or ‘Zeitgebers’ (‘time givers’), the most important of which is light.15 It was recently discovered that a third type of photoreceptor, the retinal ganglion cell, projects non-visual light information to the SCN, tuning the central pacemaker to seasonal changes in the light/dark cycle (entrainment).16 Other ‘Zeitgebers’ capable of resetting the clock include ambient temperature, feeding and social interaction.17
Circadian rhythms in the intensive care unit
The chief tenet of intensive care medicine is the support of organ function and cell viability during episodes of severe pathophysiological stress. Physiological indices are measured continuously, and interventions undertaken to maintain them within the ranges observed in health. However, the function of various systems, including the autonomic nervous system, solid organs, coagulation and immune systems, displays circadian rhythmicity4 (summarised in Figure 1). Familiarity with ‘normal’ patterns of variation in haemodynamic indices over 24 h would assist clinicians in distinguishing these natural fluctuations (illustrated schematically in Figure 2) from a change in the patient’s clinical status. Cycles of activity place patients at differential risk of adverse events at different times of day. Nocturnal decrease in blood pressure increases the risk of haemodynamic compromise, particularly in shocked patients, or those with coronary insufficiency. The morning triad of rising heart rate, persistently high systemic vascular resistance and peak in platelet aggregability could explain the three-fold increase in incidence of myocardial ischaemia observed between the hours of 6 am and noon.18 Pharmacologically blunting circadian peaks in heart rate and haemostasis with beta-blockers and aspirin alter this pattern of morbidity and mortality.19,20 Respiratory function, from lung mechanics to the secretion of surfactant, have circadian rhythms, with surfactant nadir at 10 pm and peak 12 h later, theoretically influencing susceptibility to acute lung injury.21 Targeted monitoring and intervention could reduce the harm associated with periods of heightened ‘chronorisk’.
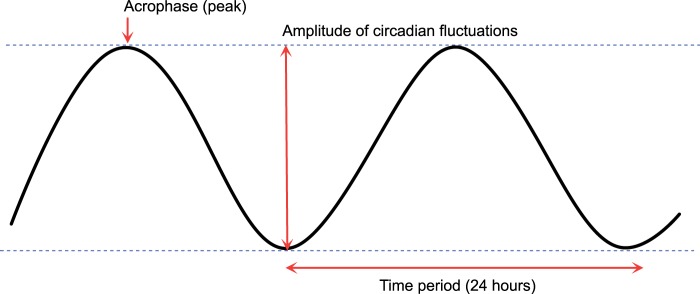
Figure 3.
Morphology of circadian rhythms can be described by the amplitude (difference between the peak and trough) and the time at which the peak (acrophase) occurs within the cycle. The time period from one peak to the next is 24 h.
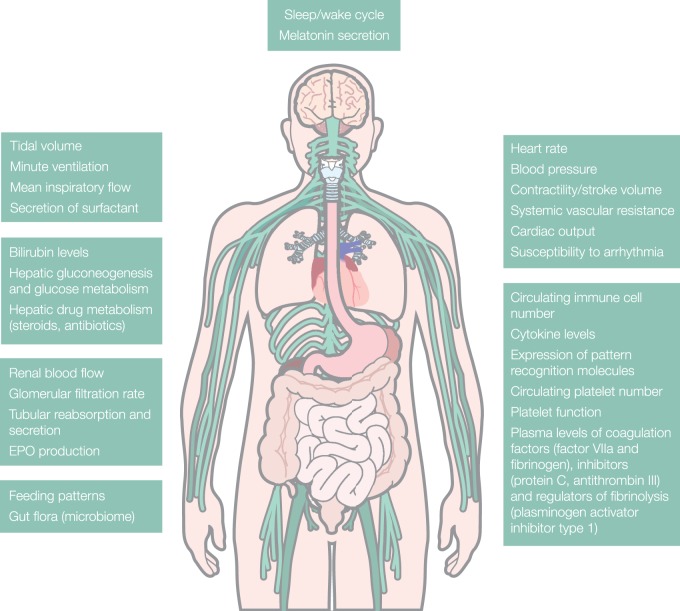
Figure 1.
Summary of different organ functions relevant to critical illness which have been found to demonstrate circadian rhythms.5
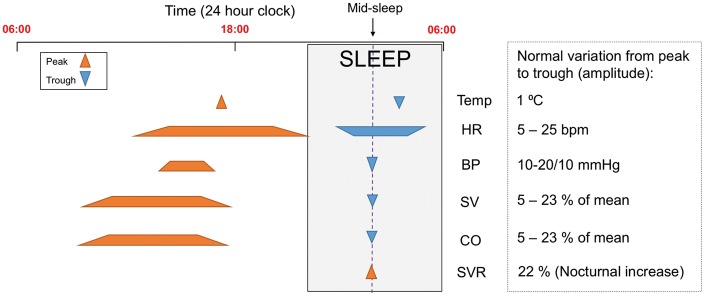
Figure 2.
Schematic representation of time points (or time ranges) at which peaks and troughs of haemodynamic indices occur during a typical 24 h period. Normal degree of variation between peak and trough indicated.
Circadian variation in the systems which we cannot visualise on a bedside monitor may be just as influential. Cycles in liver and kidney function markedly influence the metabolism and excretion of many drugs, generating significant variation in circulating drug concentrations according to the time of administration.22 Chronopharmacology is an emerging field dedicated to optimising the timing of drug delivery to enhance efficacy and minimise toxicity, through improved understanding of circadian pharmacokinetics and pharmacodynamics.23 This is particularly important for drugs with a narrow therapeutic index and significant circadian fluctuation, many of which are in common use in the intensive care unit, including antibiotics, steroids, anticoagulants and antihypertensives.24–26 Immune function ebbs and flows over a 24-h period, as demonstrated by the levels of circulating immune cells and cytokines and the expression of pattern recognition molecules.2,27 The clinical manifestations of rheumatoid arthritis follow the pattern of interleukin-6 (IL-6) expression, peaking in the morning, while mortality rates following administration of endotoxin depend upon the time of exposure.28
The amplitude of circadian variation (the difference between the peak and trough) itself appears to play an important physiological function and has been proposed to represent capacity for adaptation. In healthy subjects, resting heart rate varies between 5 and 25 beats per minute, and decreased heart rate variability is a poor prognostic factor in critical illness.29,30 The nocturnal dip in blood pressure observed in healthy subjects appears to be protective, as patients with hypertension or autonomic neuropathy, in whom this circadian trough is diminished or abolished, suffer a greater degree of end-organ damage than those in whom it is preserved.31 There is, as yet, no evidence base for deliberately emulating the circadian rhythms of physiological indices on the intensive care unit, but this concept deserves further thought and investigation.
Critical illness causes circadian dysrhythmia
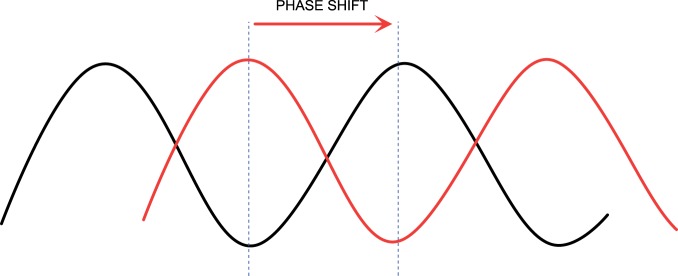
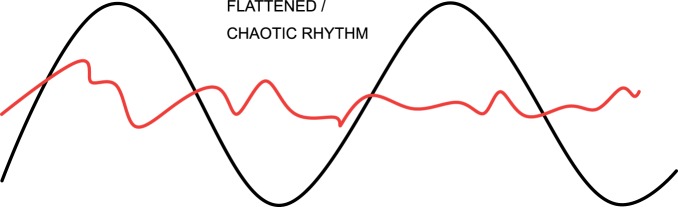
Circadian rhythms in critically ill patients are severely disrupted, with alterations in the normal 24-h patterns of melatonin and cortisol secretion, brain wave and spontaneous motor activity, blood pressure, heart rate and core temperature.32 Circadian dysrhythmia involves a change in one or more of the aspects of the morphology of a normal cycle (illustrated in Figure 3). This may be a change in amplitude (exaggeration or flattening of the normal degree of fluctuation from the mean, Figure 4), a phase shift (such that peak function or ‘acrophase’ no longer occurs at the usual time of day, Figure 5), or disintegration of a defined cycle into a chaotic pattern33 (Figure 6). It may also describe an uncoupling of one or more peripheral rhythms from either the central pacemaker or from each other.34 Critical illness circadian dysrhythmia often takes the form of decreased amplitude and phase delay, with progressive degradation over time to erratic fluctuation or complete flattening.4 In health, core temperature has one of the most stable rhythms, with nadir consistently around 5 am, but in chronic critical illness, nadir becomes widely dispersed throughout day or night, and variation between peaks and troughs diminishes.35 Loss of amplitude may impair the capacity for adaptation, while phase shifts uncouple maximum function from peak demand. Severity of illness has been shown to correlate with degree of circadian disruption.36
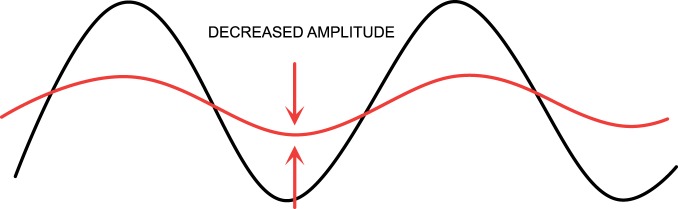
Figure 4.
Circadian dysrhythmia may take the form of a change in the amplitude (difference between peak and trough).
Figure 5.
Circadian dysrhythmia may take the form of a shift of the peak (acrophase) to a different time point within the 24-h period.
Figure 6.
Circadian dysrhythmia may take the form of loss of organisation of the usual pattern.
Aetiology of circadian dysrhythmia on the intensive care unit
Circadian dysrhythmia in the intensive care unit results from both environmental and internal disturbance. There is loss of distinction between day and night, with the greatest light intensity in an average intensive care unit 10 to 50 times less than that outdoors on even an overcast day (and up to 1000 times less than a sunny day).37,38 Continuous glare from monitors and side lights also diminishes nocturnal darkness. Entrainment by alternative ‘Zeitgebers’ is also lost, with daytime meals, physical activity and social interactions replaced with continuous feeding regimes, bed rest and sedation. Feeding mice according to a reversed schedule (during sleep rather than the active phase), uncouples their liver circadian rhythms from the master pacemaker, and results in higher mortality rates on subsequent induction of sepsis.39 Systemic inflammation also interferes with circadian rhythms. The rhythm of the urinary metabolite of melatonin has diminished amplitude and delayed acrophase in septic patients compared to non-septic critically ill patients.34 This effect appears to be mediated by decreased clock gene expression (CRY1 and PER2), in the presence of high levels of circulating cytokines (IL-6 and tumour necrosis factor alpha (TNF alpha)), and can be induced by infusion of endotoxin in healthy humans.40 Peripheral secretion of melatonin by macrophages, in response to inflammation, generates high local concentrations (millimolar), which drown out the subtle fluctuations (nanomolar to picomolar) from the pineal gland, such that central communication with peripheral clocks is lost in inflammatory states.41 In this context, melatonin appears to have a secondary function as an immunomodulator (enhancing phagocytosis initially, and later inducing production of anti-inflammatory cytokines).42 The uncoupling of peripheral clocks from the master pacemaker has been proposed to play a role in the pathogenesis of multiple organ failure.43 Impairments in liver and kidney function will alter metabolism and secretion of melatonin, while opiates and benzodiazepines enhance its day time production in a dose-dependent manner, through induction of a key enzyme in its biosynthetic pathway.44,45 Pineal secretion will be stimulated by beta-receptor agonists and endogenous catecholamines.32 Other intensive care interventions, even oxygen itself, may contribute to circadian dysrhythmia, with hyperoxia shown to disrupt circadian gene expression and proposed to contribute to the development of acute lung injury.46 Melatonin secretion appears to be lower during periods of mechanical ventilation,47 and of all the ventilation modes, pressure support results in the greatest degree of sleep disturbance.48
Ramifications of circadian dysrhythmia
‘Jet lag’ denotes the overt symptoms of circadian disruption, but we now know that effects extend deeper, impairing physiological and cellular processes. Functional impairment is mediated through anomalies of clock gene expression and the downstream effects on the multiple other genes whose expression they control. For example, alternating light and dark exposure every 10 h (rather than every 12) decreases levels of BMAL1 and PER2, and subsequently downregulates target genes which include those crucial to hypertrophic remodelling.49 This manifests clinically as impaired compensation in a mouse model of pressure overload cardiomyopathy.50 Circadian dysrhythmia has been implicated in the pathogenesis of cardiac arrhythmias and sudden cardiac death.51 Disturbance in renal circadian rhythms is a risk factor for hypertension, polyuria and fibrosis.52,53 Simulated jet lag increases the rate of tumour progression in mice,54,55 corresponding to the increased risk of breast cancer in shift workers.56 Altered circadian expression of a major regulator of fibrinolysis appears to influence susceptibility to myocardial infarction.57 Circadian dysrhythmia alters metabolic function, with derangements in lipid and glucose homeostasis leading to obesity and glucose intolerance.58 A mutation in one clock gene triggers a metabolic switch towards fatty acid oxidation and increased myocardial oxygen consumption, inhibiting the ability of cells to meet metabolic demands under stress conditions.5 Immunity could also be weakened by disruption of the circadian patterns required for the temporal organisation of its many different steps.59 Reduction of heart rate variability is a poor prognostic sign in critically ill patients, correlating with severity of organ dysfunction and predicting ability to wean from mechanical ventilation.29,30 Finally, the most visible corollaries of circadian dysrhythmia on the intensive care unit are sleep fragmentation and delirium. Polysomnography in critically ill patients reveals severe disruption in brain wave circadian rhythms, with loss of slow wave and rapid eye movement (REM) sleep, a shift towards light sleep, or complete abolition of the recognisable stages of sleep and wakefulness.60 There is a complex relationship between circadian dysrhythmia, sleep disturbance and delirium, with delirium suggested to be the visible embodiment of circadian dysrhythmia. Delirium occurs in up to 80% of critically ill patients and is associated with striking increases in morbidity and mortality.61 Any of these circadian dysrhythmias has the potential to threaten survival in critical illness. Disrupting circadian rhythms in septic mice increases their mortality rates, and severity of illness has been shown to correlate with degree of circadian disruption.36,62
Chronotherapy
In animal models of circadian dysrhythmia-induced pathology, restoration of normal circadian rhythms attenuates or reverses symptoms.49 Proposed chronotherapies in intensive care include re-entrainment with bright light or restoration of dark periods, and pharmacological intervention with night-time administration of melatonin or its agonists (ramelteon).63
Light is the most potent cue for the entrainment of circadian rhythms and may boost flagging circadian rhythms in patients. Lower mortality rates (7% compared to 12%) and shorter lengths of stay have been reported in brighter intensive care rooms (average intensity 1000–2500 lux compared to <400 lux).64 Lack of visible daylight is a risk factor for developing delirium, while windows appear to be protective.65,66 Several randomised control trials (RCTs) have demonstrated that morning light exposure in acutely ill patients reduces delirium prevalence (from 40–42% in controls to 0–16% in the light therapy groups) and duration.63 The impact of light therapy on other sequelae of circadian dysrhythmia, such as autonomic, cardiovascular, metabolic or immune dysfunction, remains largely unexplored in this cohort, but as targets they may prove to be equally relevant. The optimum timing, intensity, wavelength and duration of light exposure is yet to be defined,67 with the SCN in septic patients less sensitive to light than in health.68 With evidence accumulating for marked clinical benefit from a strategy as non-invasive and inexpensive as light, monitoring light intensity on the intensive care unit and setting morning targets constitutes a simple standard of care. Other environmental strategies to entrain circadian rhythms include minimisation of noise and sleep interruption and early mobilisation. In animal models, restricting feeding to the active phase amplifies circadian rhythms and improves metabolic disorders.69,70 Abandoning 24-h continuous feeding regimes, particularly during the night, and investing in chrononutritional research will be an important aspect of the multi-modal environmental approach to treating circadian dysrhythmia.
The pharmacological strategy for enhancing circadian rhythmicity has so far focused on night-time administration of melatonin or its agonists. Several RCTs have demonstrated their effectiveness in reducing delirium rates: in the post-operative period,71 in medical inpatients72 and in the intensive care setting73 – with incidence two to four times lower in the treatment groups compared to controls. Doses of up to 10 mg of melatonin were not associated with any ill effects, and in several studies the time of administration was as early as 6 to 8 pm.63 Melatonin may exert beneficial effects during critical illness through several pathways, dampening the hyperinflammatory response during sepsis in neonates and animal models and demonstrating in vitro antimicrobial activity against multi-drug-resistant bacteria.74 The discovery that melatonin is produced within every cell capable of oxidative phosphorylation highlights its vital role as an antioxidant.75 It defends against oxidative damage by scavenging reactive oxygen and nitrogen species and by promoting the activities of antioxidant enzymes. Its activity has been shown to protect kidney grafts and gut mucosa from the harmful effects of ischaemia reperfusion injury, and thus demonstrates potential in combating the oxidative stress compounding critical illness.76
Future chronotherapies may need to take account of the genetically determined differences in circadian phenotypes (chronotypes) between individuals. Clock gene polymorphisms linked to different sleep/wake patterns are likely to influence the timing of the whole gamut of systems under circadian control.77 There may be a role for personalised medicine in enhancing the efficacy of chronotherapy, with prior knowledge of an individual’s genetic and phenotypic circadian profile informing decisions about the optimum dose or timing of chronotherapies.
Future directions
With 24-h continuous monitoring, intensive care units are an opportune setting for chronobiological surveillance, to characterise periods of increased risk and to administer medications or interventions at times most conducive to achieving their desired effect. Collaboration between physicians, scientists and mathematicians will be required to navigate this field, to combine computational analysis of vast time-series data sets (‘big data’) with molecular processes and clinical outcomes. Knowledge of circadian cycles involved in immunity and haemostasis may allow us to schedule surgery at favourable times, a so-called ‘rhythm strategy’ already utilised in the administration of chemotherapy.78 Chronobiological interventions have shown promise in improving clinical outcomes through amelioration of delirium and sleep disturbance, but further work is required to determine their effectiveness in combating the covert dysfunction in other organ systems associated with circadian dysrhythmias.
Conclusions
Circadian dysrhythmia should be recognised as a pathological syndrome that has a significant impact on the outcome of critically ill patients79 but also the clinical staff who take care of them. The overt symptoms of circadian dysrhythmia, such as sleep disturbance, inattention and dysphoria, challenge the work performance and mental health of shift workers in the intensive care unit, with long-term health consequences ranging from metabolic dysfunction to cancer.80 Intensive care physicians are dedicated to identifying and treating physiological disturbances, but the monitoring and restoration of circadian rhythms have not yet been introduced to clinical practice. In the future, various indices, from serum melatonin to heart rate variability to core temperature, may be used as biomarkers to diagnose circadian disturbance and may one day represent key therapeutic targets.
Key points
Circadian rhythms synchronise biological function with anticipated demand.
Circadian dysrhythmia is a common affliction in the intensive care unit, both in patients and in staff.
Circadian dysrhythmia directly contributes to disease, and will exacerbate cell and organ dysfunction in critical illness, creating a vicious circle.
Morning bright light is a harmless and powerful way of bolstering failing circadian rhythms.
Melatonin may improve outcomes in critical illness through its chronobiological, anti-inflammatory, antimicrobial and antioxidant properties.
Acknowledgements
HM thanks the London Clinic for its support.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Thaiss CA, Levy M, Korem T, et al. Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell 2016; 167: 1495–1510. [DOI] [PubMed] [Google Scholar]
- 2.Esquifino AI, Cano P, Jiménez-Ortega V, et al. Neuroendocrine-immune correlates of circadian physiology: studies in experimental models of arthritis, ethanol feeding, aging, social isolation, and calorie restriction. Endocrine 2007; 32: 1–19. [DOI] [PubMed] [Google Scholar]
- 3.Dunlap JC. Molecular bases for circadian clocks. Cell 1999; 96: 271–290. [DOI] [PubMed] [Google Scholar]
- 4.Chan MC, Spieth PM, Quinn K, et al. Circadian rhythms: from basic mechanisms to the intensive care unit. Crit Care Med 2012; 40: 246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bray MS, Shaw CA, Moore MW, et al. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am J Physiol Heart Circ Physiol 2008; 294: H1036–H1047. [DOI] [PubMed] [Google Scholar]
- 6.Peek CB, Affinati AH, Ramsey KM, et al. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science 2013; 342: 1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kusanagi H, Hida A, Satoh K, et al. Expression profiles of 10 circadian clock genes in human peripheral blood mononuclear cells. Neurosci Res 2008; 61: 136–142. [DOI] [PubMed] [Google Scholar]
- 8.Bozek K, Relógio A, Kielbasa SM, et al. Regulation of clock-controlled genes in mammals. PLoS One 2009; 4: e4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim AS, Chang AM, Shulman JM, et al. A common polymorphism near PER1 and the timing of human behavioral rhythms. Ann Neurol 2012; 72: 324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Facer-Childs E, Brandstaetter R. The impact of circadian phenotype and time since awakening on diurnal performance in athletes. Curr Biol 2015; 25: 518–522. [DOI] [PubMed] [Google Scholar]
- 11.Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev 2010; 90: 1063–1102. [DOI] [PubMed] [Google Scholar]
- 12.Pevet P, Challet E. Melatonin: both master clock output and internal time-giver in the circadian clocks network. J Physiol Paris 2011; 105: 170–182. [DOI] [PubMed] [Google Scholar]
- 13.Brzezinski A. Melatonin in humans. N Engl J Med 1997; 336: 186–195. [DOI] [PubMed] [Google Scholar]
- 14.Buijs RM, la Fleur SE, Wortel J, et al. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J Comp Neurol 2003; 464: 36–48. [DOI] [PubMed] [Google Scholar]
- 15.Roenneberg T, Kumar CJ, Merrow M. The human circadian clock entrains to sun time [letter]. Curr Biol 2007; 17: R44–R45. [DOI] [PubMed] [Google Scholar]
- 16.Brainard GC, Hanifin JP, Greeson JM, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci 2001; 21: 6405–6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hastings MH, Duffield GE, Ebling FJ, et al. Non-photic signalling in the suprachiasmatic nucleus. Biol Cell 1997; 89: 495–503. [DOI] [PubMed] [Google Scholar]
- 18.Khan MS, Ahmad SI. Circadian variation – increased morning incidence of acute myocardial infarction in patients with coronary artery disease. J Pak Med Assoc 2003; 53: 84–87. [PubMed] [Google Scholar]
- 19.Manfredini R, Boari B, Salmi R, et al. Twenty-four-hour patterns in occurrence and pathophysiology of acute cardiovascular events and ischemic heart disease. Chronobiol Int 2013; 30: 6–16. [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM, Manson JE, Buring JE, et al. Circadian variation of acute myocardial infarction and the effect of low-dose aspirin in a randomized trial of physicians. Circulation 1990; 82: 897–902. [DOI] [PubMed] [Google Scholar]
- 21.Mortola JP. Breathing around the clock: an overview of the circadian pattern of respiration. Eur J Appl Physiol 2004; 91: 119–129. [DOI] [PubMed] [Google Scholar]
- 22.Beauchamp D, Labrecque G. Chronobiology and chronotoxicology of antibiotics and aminoglycosides. Adv Drug Deliv Rev 2007; 59: 896–903. [DOI] [PubMed] [Google Scholar]
- 23.Dallmann R, Brown SA, Gachon F. Chronopharmacology: new insights and therapeutic implications. Annu Rev Pharmacol Toxicol 2014; 54: 339–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beauchamp D, Labrecque G. Chronobiology and chronotoxicology of antibiotics and aminoglycosides. Adv Drug Deliv Rev 2007; 59: 896–903. [DOI] [PubMed] [Google Scholar]
- 25.Schved JF, Gris JC, Eledjam JJ. Circadian changes in anticoagulant effect of heparin infused at a constant rate [letter]. Br Med J (Clin Res Ed) 1985; 290: 1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hermida RC, Ayala DE, Mojón A, et al. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int 2010; 27: 1629–1651. [DOI] [PubMed] [Google Scholar]
- 27.Dengler V, Westphalen K, Koeppen M. Disruption of circadian rhythms and sleep in critical illness and its impact on innate immunity. Curr Pharm Des 2015; 21: 3469–3476. [DOI] [PubMed] [Google Scholar]
- 28.Halberg F, Johnson EA, Brown BW, et al. Susceptibility rhythm to E. coli endotoxin and bioassay. Proc Soc Exp Biol Med 1960; 103: 142–144. [DOI] [PubMed] [Google Scholar]
- 29.Buchman TG, Stein PK, Goldstein B. Heart rate variability in critical illness and critical care. Curr Opin Crit Care 2002; 8: 311–315. [DOI] [PubMed] [Google Scholar]
- 30.Huang CT, Tsai YJ, Lin JW, et al. Application of heart-rate variability in patients undergoing weaning from mechanical ventilation. Crit Care 2014; 18: R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueda Y, Aoi W, Yamachika S, et al. Loss of nocturnal decline in blood pressure in diabetic patients with autonomic neuropathy. Jpn Heart J 1992; 33: 801–815. [DOI] [PubMed] [Google Scholar]
- 32.Paul T, Lemmer B. Disturbance of circadian rhythms in analgosedated intensive care unit patients with and without craniocerebral injury. Chronobiol Int 2007; 24: 45–61. [DOI] [PubMed] [Google Scholar]
- 33.Papaioannou V, Mebazaa A, Plaud B, et al. Chronomics’ in ICU: circadian aspects of immune response and therapeutic perspectives in the critically ill. Intensive Care Med Exp 2014; 2: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li CX, Liang DD, Xie GH, et al. Altered melatonin secretion and circadian gene expression with increased proinflammatory cytokine expression in early-stage sepsis patients. Mol Med Rep 2013; 7: 1117–1122. [DOI] [PubMed] [Google Scholar]
- 35.Tweedie IE, Bell CF, Clegg A, et al. Retrospective study of temperature rhythms of intensive care patients. Crit Care Med 1989; 17: 1159–1165. [DOI] [PubMed] [Google Scholar]
- 36.Gazendam JA, Van Dongen HP, Grant DA, et al. Altered circadian rhythmicity in patients in the ICU. Chest 2013; 144: 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verceles AC, Silhan L, Terrin M, et al. Circadian rhythm disruption in severe sepsis: the effect of ambient light on urinary 6-sulfatoxymelatonin secretion. Intensive Care Med 2012; 38: 804–810. [DOI] [PubMed] [Google Scholar]
- 38.Turner PL, Van Someren EJ, Mainster MA. The role of environmental light in sleep and health: effects of ocular aging and cataract surgery. Sleep Med Rev 2010; 14: 269–280. [DOI] [PubMed] [Google Scholar]
- 39.Oyama Y, Iwasaka H, Koga H, et al. Uncoupling of peripheral and master clock gene rhythms by reversed feeding leads to an exacerbated inflammatory response after polymicrobial sepsis in mice. Shock 2014; 41: 214–221. [DOI] [PubMed] [Google Scholar]
- 40.Haimovich B, Calvano J, Haimovich AD, et al. In vivo endotoxin synchronizes and suppresses clock gene expression in human peripheral blood leukocytes. Crit Care Med 2010; 38: 751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pontes GN, Cardoso EC, Carneiro-Sampaio MM, et al. Injury switches melatonin production source from endocrine (pineal) to paracrine (phagocytes) – melatonin in human colostrum and colostrum phagocytes. J Pineal Res 2006; 41: 136–141. [DOI] [PubMed] [Google Scholar]
- 42.Carrillo-Vico A, Lardone PJ, Alvarez-Sánchez N, et al. Melatonin: buffering the immune system. Int J Mol Sci 2013; 14: 8638–8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Godin PJ, Buchman TG. Uncoupling of biological oscillators: a complementary hypothesis concerning the pathogenesis of multiple organ dysfunction syndrome. Crit Care Med 1996; 24: 1107–1116. [DOI] [PubMed] [Google Scholar]
- 44.Esposti D, Esposti G, Lissoni P, et al. Action of morphine on melatonin release in the rat. J Pineal Res 1988; 5: 35–39. [DOI] [PubMed] [Google Scholar]
- 45.Morera AL, Abreu-Gonzalez P, Henry M. Zaleplon increases nocturnal melatonin secretion in humans. Prog Neuropsychopharmacol Biol Psychiatry 2009; 33: 1013–1016. [DOI] [PubMed] [Google Scholar]
- 46.Czachor A, Krueger Z, Cho Y, et al. Disruption of circadian rhythms in critical illness – a role of hyperoxia-induced lung injury. Curr Pharm Des 2015; 21: 3489–3495. [DOI] [PubMed] [Google Scholar]
- 47.Frisk U, Olsson J, Nylén P, et al. Low melatonin excretion during mechanical ventilation in the intensive care unit. Clin Sci (Lond) 2004; 107: 47–53. [DOI] [PubMed] [Google Scholar]
- 48.Boyko Y, Ording H, Jennum P. Sleep disturbances in critically ill patients in ICU: how much do we know. Acta Anaesthesiol Scand 2012; 56: 950–958. [DOI] [PubMed] [Google Scholar]
- 49.Martino TA, Tata N, Belsham DD, et al. Disturbed diurnal rhythm alters gene expression and exacerbates cardiovascular disease with rescue by resynchronization. Hypertension 2007; 49: 1104–1113. [DOI] [PubMed] [Google Scholar]
- 50.Scheer FA, Hilton MF, Mantzoros CS, et al. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA 2009; 106: 4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jeyaraj D, Haldar SM, Wan X, et al. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature 2012; 483: 96–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koch BC, Nagtegaal JE, Kerkhof GA, et al. Circadian sleep-wake rhythm disturbances in end-stage renal disease. Nat Rev Nephrol 2009; 5: 407–416. [DOI] [PubMed] [Google Scholar]
- 53.Bonny O, Firsov D. Circadian regulation of renal function and potential role in hypertension. Curr Opin Nephrol Hypertens 2013; 22: 439–444. [DOI] [PubMed] [Google Scholar]
- 54.Filipski E, Delaunay F, King VM, et al. Effects of chronic jet lag on tumor progression in mice. Cancer Res 2004; 64: 7879–7885. [DOI] [PubMed] [Google Scholar]
- 55.Van Dycke KC, Rodenburg W, van Oostrom CT, et al. Chronically alternating light cycles increase breast cancer risk in mice. Curr Biol 2015; 25: 1932–1937. [DOI] [PubMed] [Google Scholar]
- 56.He C, Anand ST, Ebell MH, et al. Circadian disrupting exposures and breast cancer risk: a meta-analysis. Int Arch Occup Environ Health 2015; 88: 533–547. [DOI] [PubMed] [Google Scholar]
- 57.Oishi K, Uchida D, Ohkura N, et al. Ketogenic diet disrupts the circadian clock and increases hypofibrinolytic risk by inducing expression of plasminogen activator inhibitor-1. Arterioscler Thromb Vasc Biol 2009; 29: 1571–1577. [DOI] [PubMed] [Google Scholar]
- 58.Garaulet M, Gómez-Abellán P. Chronobiology and obesity. Nutr Hosp 2013; 28(Suppl 5): 114–120. [DOI] [PubMed] [Google Scholar]
- 59.Dimitrov S, Lange T, Tieken S, et al. Sleep associated regulation of T helper 1/T helper 2 cytokine balance in humans. Brain Behav Immun 2004; 18: 341–348. [DOI] [PubMed] [Google Scholar]
- 60.Gehlbach BK, Chapotot F, Leproult R, et al. Temporal disorganization of circadian rhythmicity and sleep-wake regulation in mechanically ventilated patients receiving continuous intravenous sedation. Sleep 2012; 35: 1105–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scott BK. Disruption of circadian rhythms and sleep in critical illness and its impact on the development of delirium. Curr Pharm Des 2015; 21: 3443–3452. [DOI] [PubMed] [Google Scholar]
- 62.Perras B, Kurowski V, Dodt C. Nocturnal melatonin concentration is correlated with illness severity in patients with septic disease [letter]. Intensive Care Med 2006; 32: 624–625. [DOI] [PubMed] [Google Scholar]
- 63.Oldham MA, Lee HB, Desan PH. Circadian rhythm disruption in the critically ill: an opportunity for improving outcomes. Crit Care Med 2016; 44: 207–217. [DOI] [PubMed] [Google Scholar]
- 64.Beauchemin KM, Hays P. Dying in the dark: sunshine, gender and outcomes in myocardial infarction. J R Soc Med 1998; 91: 352–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Rompaey B, Elseviers MM, Schuurmans MJ, et al. Risk factors for delirium in intensive care patients: a prospective cohort study. Crit Care 2009; 13: R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keep P, James J, Inman M. Windows in the intensive therapy unit. Anaesthesia 1980; 35: 257–262. [DOI] [PubMed] [Google Scholar]
- 67.Skene DJ. Optimization of light and melatonin to phase-shift human circadian rhythms. J Neuroendocrinol 2003; 15: 438–441. [DOI] [PubMed] [Google Scholar]
- 68.Perras B, Meier M, Dodt C. Light and darkness fail to regulate melatonin release in critically ill humans. Intensive Care Med 2007; 33: 1954–1958. [DOI] [PubMed] [Google Scholar]
- 69.Hatori M, Vollmers C, Zarrinpar A, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab 2012; 15: 848–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tahara Y, Shibata S. Circadian rhythms of liver physiology and disease: experimental and clinical evidence. Nat Rev Gastroenterol Hepatol 2016; 13: 217–226. [DOI] [PubMed] [Google Scholar]
- 71.Sultan SS. Assessment of role of perioperative melatonin in prevention and treatment of postoperative delirium after hip arthroplasty under spinal anesthesia in the elderly. Saudi J Anaesth 2010; 4: 169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Al-Aama T, Brymer C, Gutmanis I, et al. Melatonin decreases delirium in elderly patients: a randomized, placebo-controlled trial. Int J Geriatr Psychiatry 2011; 26: 687–694. [DOI] [PubMed] [Google Scholar]
- 73.de Jonghe A, van Munster BC, Goslings JC, et al. Effect of melatonin on incidence of delirium among patients with hip fracture: a multicentre, double-blind randomized controlled trial. CMAJ 2014; 186: E547–E556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tekbas OF, Ogur R, Korkmaz A, et al. Melatonin as an antibiotic: new insights into the actions of this ubiquitous molecule. J Pineal Res 2008; 44: 222–226. [DOI] [PubMed] [Google Scholar]
- 75.Manchester LC, Coto-Montes A, Boga JA, et al. Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J Pineal Res 2015; 59: 403–419. [DOI] [PubMed] [Google Scholar]
- 76.Bubenik GA. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci 2002; 47: 2336–2348. [DOI] [PubMed] [Google Scholar]
- 77.Lim AS, Chang AM, Shulman JM, et al. A common polymorphism near PER1 and the timing of human behavioral rhythms. Ann Neurol 2012; 72: 324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cancer and rhythm. [editorial]. Cancer Causes Control 2006; 17: 483. [DOI] [PubMed]
- 79.Madrid-Navarro CJ, Sanchez-Galvez R, Martinez-Nicolas A, et al. Disruption of circadian rhythms and delirium, sleep impairment and sepsis in critically ill patients. Potential therapeutic implications for increased light-dark contrast and melatonin therapy in an ICU environment. Curr Pharm Des 2015; 21: 3453–3468. [DOI] [PubMed] [Google Scholar]
- 80.Editorial: health impact and management of a disrupted circadian rhythm and sleep in critical illnesses [editorial]. Curr Pharm Des 2015; 21: 3428. [DOI] [PMC free article] [PubMed]